ABSTRACT
Polyvinylpyrrolidone-capped gold nanorods (PVP-capped AuNRs) are emerging as promising agents for cancer therapy. With the widespread utilization of nanoparticles, some is known about the therapeutic effect of PVP-capped AuNRs on oviduct and endometrium adenocarcinomas resulting from 7,12-dimethylbenz[a]anthracene (DMBA)-induced carcinogenesis. Twenty-four female Wister albino rats (Rattus norvegicus) were categorized into four groups: negative control, PVP-capped AuNRs positive control, DMBA-treatment and DMBA plus PVP-capped AuNRs. A fabrication method of PVP-capped AuNRs and their characterization was assessed using ultraviolet spectra (UV), ultrastructure analysis, dynamic light scattering (DLS) and zeta potential (ɳ). Morphometric assessments and the mortality rate after induction with DMBA carcinogenesis were recorded. The oviducts and uteri were dissected and subjected to histopathological and immunohistochemical analysis. PVP-capped AuNRs treatment enhanced the histological features of the DMBA carcinogenesis-dependent oviduct and endometrium adenocarcinomas. In addition, there was a reduced immune reaction of caspase-3 and Ki-67 markers after the treatment, with PVP-capped AuNRs referring to its improvement effect. In conclusion, PVP-capped AuNRs showed therapeutic potential against oviduct and endometrium adenocarcinoma by reaching tumors via enhancing permeability and retention effect. The proposed methodology suggests that PVP-capped AuNRs target the site and the boundaries of cancer, opening new therapeutic options.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a family of structurally related compounds and a significant class of environmental carcinogens. Both epidemiological and laboratory investigations have linked these carcinogens and associated halogenated chemicals to the development of mammary tumors. DMBA is an example of PAH that has been used to encourage tumor growth in laboratory animals (7,12-dimethylbenz[a]anthracene) [Citation1]. Recently, the administration of DMBA to mice’s female reproductive tracts has resulted in a significant upsurge in the incidence of squamous cell carcinomas [Citation2]. Surgery, radiation therapy, hormone therapy and chemotherapy are used concurrently or separately as part of the current endometrial cancer treatment protocol [Citation3]. This protocol highlights the demand for novel therapeutic strategies that have been researched recently. Noble metal-based nanoparticles are created using various physical and chemical processes, and their uses in health care and remedy are unceasingly expanding [Citation4]. The toxicity of nanoparticles made from these noble metals must be understood as concerns about human safety grow. The creation of gold nanoparticles via a bio-based method has been emphasized by life sciences researchers as being urgently essential for use in medication, health care, biolabeling, targeted medication delivery, hyperthermia and biosensors, among other applications [Citation5]. All parameters must be studied before application in vivo for their internal use to ensure safety precautions.
By lowering toxicity, the curative usage of nanomaterials including medications has enhanced the activities [Citation6]. Due to their improved biocompatibility and sensitivity, biofunctionalized noble metal nanoparticles as beneficial mediators have attracted attention in a recent study [Citation7]. In preclinical research, these noble metal nanoparticles have demonstrated promising outcomes as therapeutic carriers in drug delivery systems [Citation8]. It was not well established to balance the development of undesirable consequences with therapeutic characteristics [Citation9]. In standard nanoparticle manufacturing procedures, PVP is one of the most significant capping agents handled in nanotechnology to get around issues like toxicity, size and agglomeration. PVP is therefore employed to generate environmentally acceptable nano-formulations with wider use [Citation10,Citation11]. This research summarizes the impact of PVP-capped AuNRs on oviductal and endometrial adenocarcinomas. Meanwhile, this study focuses on the problems that will require additional investigation in the future [Citation12]. This study examined the present level of information regarding the impact of PPVP-capped AuNRs on oviduct and endometrial structure. It is found that PVP-capped AuNRs inhibit the growth of oviductal and endometrial adenocarcinomas.
Materials and methods
Animal studies and ethical guidelines
Mansoura University Experimental Animal Ethical Committee uses laboratory animals in this work in obedience to the standards of the National Institute of Health (NIH Publication No. 8523, modified 1996). The Ministry of Health of Egypt’s Helwan Breeding Farm provided a group of 24 virgin female Wister albino rats weighing roughly 100 ± 10 g and used for experiments. They had 15 days to adjust before the experiment. They had unrestricted access to food and drink, and a 12-hour light/dark cycle kept them well ventilated.
Induction of female reproductive tract adenocarcinomas
The induction was provided using 7,12-dimethylbenz[a]anthracene (DMBA) (Sigma-Aldrich Company), the most broadly used active chemical inductors of mammary carcinogenesis. The dose was prepared using 250 mg of DMBA powder dissolved in 1 ml dimethyl sulfoxide (DMSO, D2650, Sigma) and 4 ml olive oil as previously reported by ref. [Citation12]. The applied dose was 50 mg/rat; one dose was administered subcutaneously and followed up for 3 weeks [Citation13].
Synthesis of gold nanoparticles
Groundwork of colloidal gold nanorods (AuNrs)
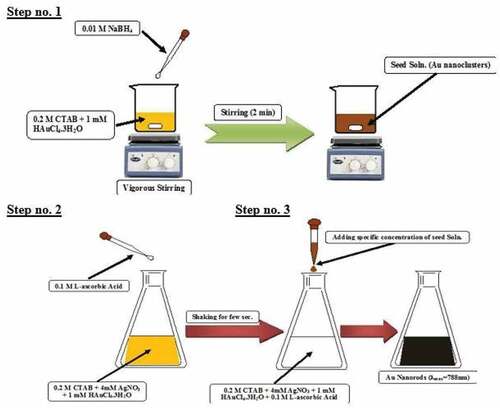
According to earlier publications, colloidal CTAB-capped Au nanorods were created using a seed-mediated approach [Citation14–16]. Seed solutions from gold nanoclusters were fabricated typically via chemical reduction of an aqueous solution of gold ions precursor (i.e. 2.5 ml of 1 mM HAuCl4.3H2O, Au3+ ions) in the existence of an aqueous solution of surfactant (i.e. 2.5 ml of 0.2 M of CTAB) using mild ice cooled reducing agent of sodium borohydride (i.e. 0.6 ml of 0.01 M of NaBH4). NaBH4 is added dropwise creating a reddish-brown colloidal solution that ages 8–12 minutes during vigorously stirring before being injected into the growth fluid. Additionally, 100 ml of a growth solution was created by vigorously swirling 50 ml of 0.2 M CTAB with 1 ml of an aqueous AgNO3 solution.
After that, under vigorous stirring, 50 ml of 1 mM HAuCl4.3 H2O as a precursor for gold ions (Au3+) was added resulting in a yellowish-brown solution. Finally, enough L-ascorbic acid was quickly augmented to the mixture, while it was gently shaken until the yellowish-brown color vanished. A sufficient volume (1.2 ml) of Au0 seed solution was rapidly added to the remedy to flourish to produce NIR-absorbed gold nanorods. The combination was then left uninterrupted for an hour. The solution’s hue changed over time representing the evolution of the gold nanorods. The generated elements and excess CTAB were isolated from the growth fluid by centrifugation at 10 degree Celsius.
Preparation of PVP-capped gold nanoparticles
The surface-modified gold nanorods with PVP were fabricated as follows [Citation17]; about 5 g of PVP (30K, 0.05 mg/mL) was added to as-purified gold nanorods under vigorous stirring for 2 hours and kept at room temperature overnight.
Characterization of gold nanoparticles
The TG-80 twin-beam spectrometer captured the produced samples’ UV–Visible absorption spectra. Over an appropriate scan range of 200 to 900 nm, the absorbance was measured in increments of 5 nm.
Analysis of zeta potential and zeta average
The surface charges of AuNPs and PVP-AuNPs were determined using an American-made Malvern Zeta-size Nano-zs90 Zeta Potential Analyzer.
Transmission electron microscope (TEM) analysis
On the campus of Mansoura University, the electron microscope unit, Egypt, TEM (TEM, JEOL JEM-100C×, USA) was used to analyze the size and form of AuNPs and AuNPs with PVP caps.
Experimental animals
Twenty-four female Wister albino rats (Rattus norvegicus) weighing about 100 g were distributed into four groups (n = 6) such as negative control, PVP-capped AuNRs positive control, DMBA-treatment and DMBA plus PVP-capped AuNRs. After 3 weeks of induction oviduct and endometrial adenocarcinomas using DMBA, treatment was conducted with PVP-capped AuNRs (400 µl/rat) using a single dose. After an extra week of treatment, the animals were sacrificed after being eradicated with chloral hydrate (300 mg/kg body weight).
Histological investigation
The tissues from the oviducts and uteri were washed with xylene, fixed for 10 minutes in phosphate-buffered formalin (pH 7.4), dehydrated with increasing marks of ethyl alcohol and mounted in melted paraplast at 58–62 degree Celsius. Hematoxylin and eosin was then used to stain 5 mm slices of histology tissue, which were then examined using a bright field light microscope.
Immunohistochemistry for caspase-3, Ki-67
Five-micrometer thin sections of formalin-fixed, paraffin-embedded oviduct and uterus samples were placed on polylysine-coated glass slides. These samples were processed in a series of alcohols after dewaxing, xylene development and rehydration. For 10 minutes, tissue slices were exposed to 3% H2O2 to lower endogenous peroxidase activity. After being incubated for 15 minutes at 37°C in processed media containing 0.05% trypsin, the tissue slices were treated with the primary monoclonal mouse antibody against caspase-3 (DAKO, clone MIB5, 1:50, mouse) and the primary antibody against (Ki-67) antibody at 1:50. (pH 7.8). The slides were cleaned and then incubated with a secondary biotin-linked anti-mouse antibody for 50 minutes at room temperature before being exposed to the streptavidin-peroxidase mixture for 50 minutes. Hematoxylin was used to counterstain the sections after they had been washed and developed with diaminobenzidine hydrogen peroxide (DAKO).
Brown nuclear or cytoplasmic labeling was counterstained with hematoxylin to demonstrate the immune response. Sections received a treatment of 1% nonimmune serum phosphate buffer solution (PBS) solution as a negative control. The histology sections were examined using an Olympus bright field light microscope and a digital Canon camera. A digital Olympus camera mounted on an Olympus microscope with a 1/2 × photo adapter and a 40 × objective was also used to take the pictures of the slides used for image analysis. On a computer with an Intel® Core 5® processor, the collected images were processed using the Video Test morphology®software (Russia) and the % area was computed and recorded.
Statistical analyses
Statistical analyses including one-way ANOVA and post hoc analysis between the control and studied groups were carried out using SPSS (version 13). Data are presented as means ± standard error (SE). Data were considered statistically significant at p < 0.05.
Results
Synthesis and characterization of bare and PVP-capped gold nanorods
Gold nanorods (Au-NRs) have been prepared via wet chemicals based on the seed-mediated method as previously reported. Such fabrication method consists of three steps as revealed in ; the first step was the fabrication of gold nanoclusters through the chemical reduction of gold ions (Au3+) to tiny gold nanoclusters (Au0) in the existence of robust reducing agents such as BH4- ions, CTAB as a surfactant and the second step is based on the preparation of growth solution with colorless solution through the reduction of (Au3+ → Au+ → Au0). The last reduction occurs due to the formation [AuCl2]- upon adding a mild reducing agent, such as L-ascorbic acid. The critical factor in easing the creation of gold nanorods is the presence of metallic impurities, such as silver ions (Ag1+), which have redox potential that is lower than Au3+ ions. The final step is the instant addition of an appreciable volume of seed solution to a growth solution to form Au-NRs.
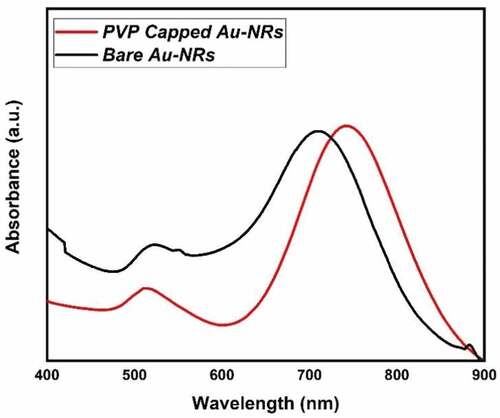
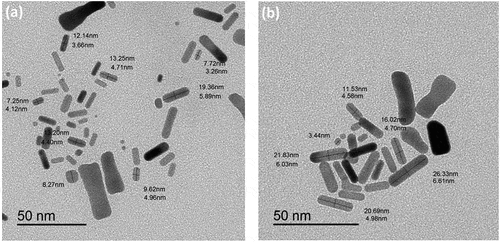
UV-V is a spectroscopy and transmission electron microscopy technique that has explored the photophysical and morphological properties. (black line) depicts the absorption spectra for bare gold nanorods. Two distinct features were observed at a short wavelength of 518 nm due to the incidence of transverse surface plasmon resonance (T-SPR). In contrast, the second band at a longer wavelength of 713 nm is due to the longitudinal surface plasmon resonance (L-SPR). The aspect ratio of bare nanorods has been calculated according to Link et al.’s (2013) empirical formula, where the aspect ratio (R) is ~3, which was in contact with the TEM images, as revealed in (). The average dimensions of bare gold nanorods were 13.25 ± 6.11 nm (Length) and 4.71 ± 1.18 nm (width), whereas the corresponding aspect ratio ranged from 2.5 to 3.
However, the bare gold nanorods have a toxic effect due to the excess cationic surfactant CTAB. Therefore, PVP has been used as a biocompatible capping agent to replace through ligand exchange with CTAB. The photophysical, morphological and colloidal stability properties were tested to ensure the successful exchange of CTAB with PVP (See (red line), 2b and 3, respectively). The red shift in the absorption spectrum of PVP gold nanorods has a longer wavelength than bare-gold nanorods. The longitudinal surface plasmon band (L-SPR) was at 745 nm compared to 713 nm as in bare gold nanorods (see , red line). In addition, the calculated aspect ratio via the empirical formula by Link et al. (2013) was about 3 to 3.4. Moreover, the average dimensions of bare gold nanorods were 19.25 ± 8.11 nm (Length) and 4.71 ± 2.18 nm (width), whereas the corresponding aspect ratio ranged from 3 to 3.7. Furthermore, the dynamic light scattering (DLS) and zeta-potential are investigated for the colloidal stability of PVP-capped Au-NRs as shown in . The average hydrodynamic diameter (HD) was about 77.47 nm with a polydispersity index (PDI) ~0.887, and the corresponding zeta potential (ɳ) was about 23.3 mV.
Morphometric assessment of DMBA-induced rats
Clinical signs from DMBA administration were decreased activity, weakness, decreased food intake, yellowed and dirty fur and diarrhea (). The mortality rate of rats was at a late stage of the experiment. In , rats generally died at a rate of 16.6% (2/12) in the DMBA-induced groups, and no rats died at 0% (0/12) in the control groups. Most dead rats had conspicuously dilated bowels, which was thought to be an intestinal obstruction. Only a few animals showed intestinal adhesions. Bloody ascites, hematemesis and jaundice were discovered, and the tumor had attached itself to the tissues around it ().
Figure 4. (a) A photograph showing the morphological characteristics of the DMBA-treated rat. Notice, ascites due to liver cirrhosis and general animal weakness. (b) Adhesion of internal organs and markedly dilated bowel.
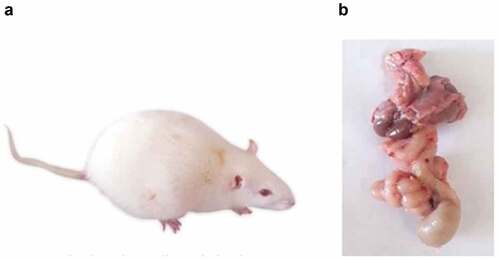
Table 1. Mortality rate of female rats in 7,12-dimethylbenz(a)anthracene-induced groups and control groups, each column represents the number of dead rats (n) divided by the whole number of the group and the percentage, each group consisting of 12 rats during 21 daya from inducing DMBA.
Histopathological observations
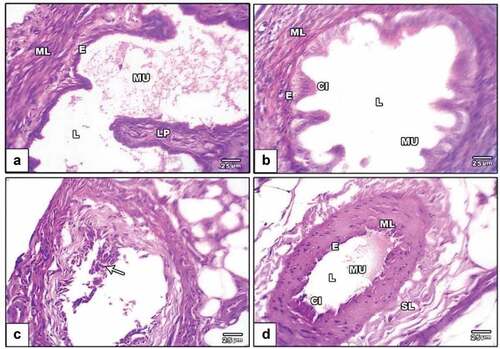
The oviduct from the negative control group was examined histologically. It revealed normal mucosa in the proximal portion of the oviduct that was flung into a sequence of branched longitudinal folds and extended past the oviduct’s open cranial end as finger-like processes known as fimbria. As one gets closer to the uterus, the creases become less noticeable. The mucosa is a straightforward columnar epithelium with secretory cells that are both ciliated and unciliated. Large lymphatic capillaries are found in the highly vascular lamina propria that lies beneath the epithelium (). An acquired circular or spiral layer of smooth muscle (the muscular) and a less well-improved outer layer of longitudinally oriented smooth muscle were visible in the oviduct of the PVP-capped AuNRs positive control group (). In the DMBA carcinogenesis group, the oviduct revealed degenerated mucosal folds with loss of both ciliated and unciliated secretory cells. Palpable abnormality in lamina propria and disorganization of circular and longitudinal smooth muscle is presented in . The DMBA carcinogenesis group treated with PVP-capped AuNRs exhibited modest improvement, but the mucosal folds remained aberrant in morphology and lacked vascular lamina propria differentiation ().
Figure 5. Photomicrographs of a histological cross-section of rat oviduct. (a) Negative control viewing standard oviduct structure with mucosa layer lining with simple epithelium made up of columnar ciliated and non-ciliated cells and is the same as that of the uterine tube. The muscular layers of the lamina propria resemble those of the uterine tube, and it lacks glands. (b) PVP-capped AuNRs positive control showing mucosal folds and developed muscular layers. (c) DMBA carcinogenesis showing degenerated mucosal folds and palpable abnormality in lamina propria (arrowheads). (d) DMBA carcinogenesis treated with PVP-capped AuNRs showing abnormal mucosal folds (arrowheads) and regular muscle cells. The magnification power used is 400 × . Abbreviations; LP, lamina propria; L, lumen; epithelial cell; CI, cilia; MU, mucosa layer; ML, muscular layer; SL, serosa layer.
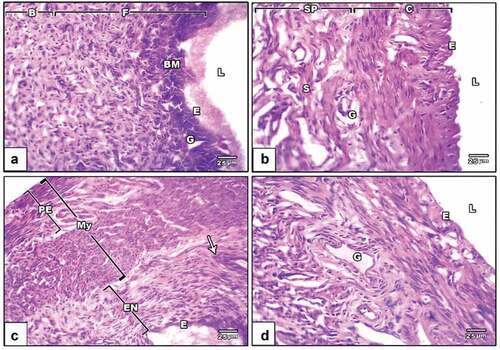
Rat endometrial layer has histological slices from the negative control group, revealing a typical architecture. Tubular glands in the endometrium protrude into the lamina propria beneath the endometrial epithelium. The endometrial epithelium is a straightforward columnar epithelium whose height changes depending on the phases of the estrous cycle. Large numbers of leukocytes and significant vascularity can be found in the lamina propria (). The endometrial layer of the PVP-capped AuNRs positive control group revealed an intact endometrium containing uterine glands surrounded by a compact, highly cellular connective tissue stroma, a simple, high-columnar, somewhat ciliated epithelium with basally situated oval nuclei and an endometrial layer of standard thickness (). In the DMBA carcinogenesis group, the endometrial layer revealed different layers: endometrium with leukocytic infiltration and abnormal thickness, myometrium and perimetrium (). PVP-capped AuNRs treatment of the DMBA carcinogenesis group showed whole endometrium with the usual epithelial lining and stroma containing many uterine glands ().
Figure 6. Photomicrographs of a histological cross-section of rat uterine. (a) Negative control displaying uterine architecture with a broad uterine Lumen and undamaged endothelium. (b) PVP-capped AuNRs positive control displaying an intact endometrium with a typical thickness endometrial layer bordered by a simple, high columnar, somewhat ciliated epithelium with basally situated oval nuclei and an underlying dense, highly cellular connective tissue stroma encircling the uterine glands. (c) DMBA carcinogenesis shows different layers; endometrium with leukocytic infiltration (arrowhead) and abnormal thickness, myometrium and perimetrium. (d) DMBA carcinogenesis treated with PVP-capped AuNRs showing the whole endometrium with standard epithelial lining and stroma containing many uterine glands. The magnification power used is 400 × . Abbreviations; BM, basement membrane; L, lumen; E, epithelial cells; G, zona granulosa; F, functional zone; B, basal zone; Sp, spongy zone; C, compact zone; ST, stroma; PE, perimetrium; My, myometrium; EN, the endometrium.
Immunohistochemical observations
Caspase-3 expression
Oviduct cells in a group of oviducts treated with DMBA carcinogenesis were shown to overexpress caspase-3, a cell death marker. Caspase-3 immunostaining was reduced in the DMBA carcinogenesis group after administering PVP-capped AuNRs (). Compared to the other investigated groups, image analysis revealed a significantly higher caspase-3 in the DMBA carcinogenesis group (). The endometrial stroma of negative control rats possessed weak immunoreactivity of caspase-3 (). In the DMBA carcinogenesis group, strong immunoreactivity was observed in the endometrial epithelial cells and the surrounding glands () and decreased in the PVP-capped AuNRs-treated group (). Compared to the other investigated groups, the DMBA carcinogenesis group’s caspase-3 image analysis showed a considerable rise ().
Figure 7. Photomicrographs of formalin-fixed oviduct immunohistochemical stain with Caspase-3, (a) negative control, (b) PVP-capped AuNRs positive control, (c) DMBA carcinogenesis, D. DMBA carcinogenesis treated with PVP-capped AuNRs. Note increased immunostaining of Caspase-3 in DMBA carcinogenesis and improved in PVP-capped AuNRs-treated studied group. The arrow heads show the caspase-3 immunoreactivity. The magnification power used 400 × .
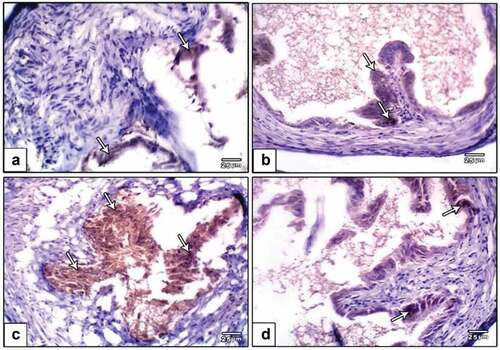
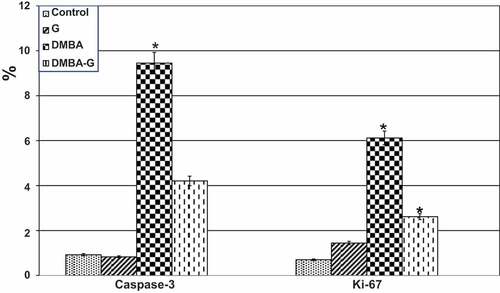
Figure 8. Image analysis of female oviduct exposed to DMBA carcinogenesis and treated with PVP-capped AuNRs. In DMBA carcinogens, caspase-3 expressing crucial mediators of programmed cell death (apoptosis) showed a considerable rise in image analysis. Ki-67 reflecting proliferative activity displaying meaning increased image analysis in both DMBA carcinogens and DMBA carcinogens and PVP-capped AuNRs treatment. Star means significant at P ‹ 0.05.
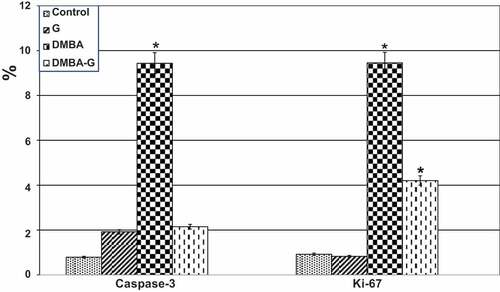
Figure 9. Photomicrographs of formalin-fixed rat uterine immunohistochemical stain with caspase-3, (a) negative control, (b) PVP-capped AuNRs positive control, (c and d) DMBA carcinogenesis and (e and f) DMBA carcinogenesis treated with PVP-capped AuNRs. Note that increased immunostaining of caspase-3 in DMBA carcinogenesis and improved in PVP-capped AuNRs-treated studied groups. The arrowheads show the degree of immunoreactivity.
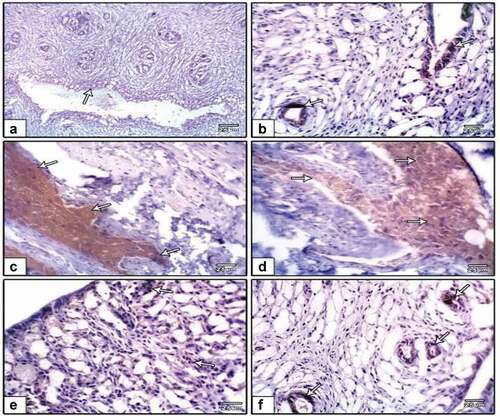
Ki-67 expression
In the oviduct, Ki-67 immunohistochemistry revealed higher immunostaining in the DMBA carcinogenesis group, indicating more unusual proliferative activity (). However, when the DMBA carcinogenesis group was treated with PVP-capped AuNRs, the Ki-67 immunohistochemical reactivity was reduced (). Following image analysis, Ki-67 showed that the immune response to DMBA carcinogenesis was significantly higher than in the other groups that had been studied (). Also, the uterine endometrium of negative control possessed weak-to-moderate positive reaction for Ki-67 () compared to more reaction post-DMBA carcinogenesis injection (). In contrast, improved weak reaction was remarked in DMBA carcinogenesis treated with PVP-capped AuNRs (). Ki-67 displayed an essential increase in the immune reaction in the DMBA carcinogenesis, improved in DMBA carcinogenesis treated with PVP-capped AuNRs group but still did not match with control values ().
Figure 10. Photomicrographs of formalin-fixed oviduct immunohistochemical stain with ki-67, (a) negative control, (b and c) PVP-capped AuNRs positive control, (d and e) DMBA carcinogenesis, (f) DMBA carcinogenesis treated with PVP-capped AuNRs. Note overexpression in immunostaining of ki-67 in DMBA carcinogenesis and amended in PVP-capped AuNRs -treated studied group. The degree of ki-67 immunoreactivity is shown by the arrowheads. The magnification power used 400 × .
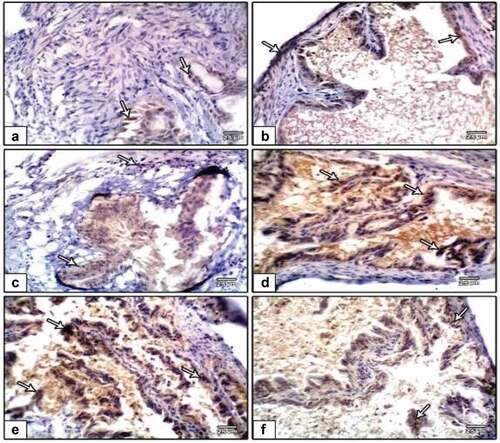
Figure 11. Photomicrographs of formalin-fixed rat uterine immunohistochemical stain with ki-67 (a), negative control (b), PVP-capped AuNRs positive control (c and d) and DMBA carcinogenesis (e and f). DMBA carcinogenesis treated with PVP-capped AuNRs. Note that increased immunostaining of ki-67 in DMBA carcinogenesis and improved in PVP-capped AuNRs-treated studied groups the degree of ki-67 immunoreactivity is shown by the arrowheads.
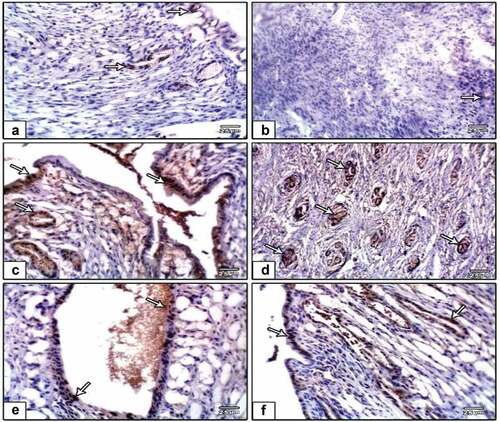
Figure 12. Image analysis of female endometrium exposed to DMBA carcinogenesis and treated with PVP-capped AuNRs. In DMBA carcinogens and DMBA carcinogens and PVP-capped AuNRs-treatment, caspase-3 expressing apoptosis showed a considerable rise in image analysis. Ki-67 reflects a pivotal role in maintaining cell proliferation demonstrating a large increase in image analysis in both DMBA carcinogens and DMBA carcinogens and PVP-capped AuNRs treatment. The star means significant at P ‹ 0.05.
Discussion
Polycyclic aromatic hydrocarbons (PAHs), a type of atmospheric pollutant, are widely dispersed in the environment and enter human bodies through air, water and food. Environmental pollutants made of PAHs are called 7 and 12-dimethylbenz[a]anthracene (DMBA) that have several harmful and carcinogenic effects [Citation18]. DMBA is widely known for its cytotoxicity, carcinogenicity, mutagenicity and immunosuppressive properties [Citation19]. Similar to earlier studies with DMBA-induced mammary tumors, it is concluded that they are nearly identical [Citation20,Citation21]. Since ancient times, gold has been utilized as medicine, particularly in India and China, where it was associated with fertility and long life [Citation22,Citation23]. Gold has been utilized to treat rheumatic and TB ailments throughout the twentieth century [Citation24]. Francisci Antonii details gold nanoparticles’ potential medical applications [Citation25]. They have consistently been shown to be the least harmful and safest metallic nanoparticles [Citation26].
Gold nanoparticles (AuNPs) were discovered to suppress the term of stem cell markers, and a decrease in the number of stem cell phenotype (SP) cells was also documented. Discovering the signaling mechanisms that regulate the suppression of stemness caused by AuNP will aid in discovering vital players elaborate in drug resistance [Citation27]. The current study is the first study that describes the preventative effect of PVP-capped AuNRs against oviduct and endometrial adenocarcinomas brought on by DMBA. The body state of experimental animals is typically regarded as crucial in determining how toxic/carcinogenic chemicals affect human health and how they can be used as anticancer medicines [Citation28].
The present study agrees with ref [Citation29] which found that morphometric symptoms of DMBA administration included decreased activity, weakness, decreased intake of food, yellow and dirty fur and diarrhea. Rat mortality occurred during the last stages of the experiment as well. Most of the dead rats had conspicuously dilated bowels, which was thought to be an intestinal obstruction.
In addition, clinical symptoms were detected during the collection of samples. Only a few animals showed intestinal adhesions. The tumors were discovered to be attached to the tissues around them. This study showed that the female reproductive tract cancer rat model created by DMBA had several traits similar to those of uterine cancer patients. Cells from uterine cancer shed into the ascites and spread locally, mainly to the small bowel mesentery, omentum, peritoneal wall and diaphragm. The rat model used in this study likewise demonstrated this diffuse metastasis [Citation30]. In individuals with advanced cancer, ascites are associated with a bad prognosis. Later, these rats experienced cachexia, weight loss, bloody ascites and abdominal mass. This study established that histological mammary lesions appeared in mice exposed to DMBA.
Rats’ female reproductive tracts could be shown to have both benign and malignant tumors due to large susceptibility of the oviduct layers to DMBA carcinogenesis, the mucosal folds degenerate and both ciliated and unciliated secretory cells are lost. Lamina propria anomaly can be felt with a disarray of the circular and longitudinal smooth muscles. It was discovered that DMBA carcinogenesis had created endometrial adenocarcinomas.
Several layers were visible including perimetrium, myometrium with aberrant thickness and endometrium with leukocytic infiltration. The observed results above for the oviduct have been supported by an increase in the immunohistochemical reactivity of the apoptosis marker caspase-3 and the proliferative activity marker Ki-67 [Citation31,Citation32]. All layers showed improvement following treatment with PVP-capped AuNRs. Also, the current study observed increased uterine levels of caspase-3 immunohistochemistry and Ki-67, which is also employed as a possible lively intermediary or replacement marker for therapy success signaling the activation of uterine wall damage. In the current investigation, PVP-capped AuNRs in the oviduct and endometrium of female rats subjected to DMBA carcinogenesis had a protective effect.
These findings are consistent with Aune et al. [Citation32], who discovered that HeLa cells could be made to undergo apoptosis by GNPs, which can suppress cell multiplying in a dose-reliant way. Therefore, GNPs have the potential to treat cancer and combat medication resistance [Citation33]. They can also be used to deliver large doses of chemotherapy to cancer cells, either locally or intravenously.
Those with uterine cancer also experience this. Furthermore, the bulk of the tumors that advanced in this rat example were adenocarcinomas as shown by these findings and past research [Citation34–37], a well-known pathogenic subtype of human uterine cancer. In this rat model, some pathogenic features of human uterine cancer are duplicated. Many problems still need to be resolved in the future. These elements impact the pharmacokinetics, biodistribution and toxicity of GNP.
In conclusion, adult female albino rats are exposed to gold nanoparticles, which cause histological alterations and metal deposition at numerous places. This treatment was done via binding to VEGF165, and it is a crucial endothelial cell function and angiogenesis regulator, decreasing endothelial cell survival (EC). This binding lessens the proliferation, toxicity and stimulatory effects of VEGF165. Future treatments for oviduct and endometrial cancer, as well as other cancers, may employ GNPs alone or in combination with specific antibodies or anticancer medications to avoid side effects associated with chemotherapy and conventional radiation techniques.
Acknowledgments
The authors dedicate this work to the soul of Prof. Hassan I. El-Sayyad, who passed away due to COVID-19 in September 2021.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Currier N, Solomon SE, Demicco EG, et al. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33(6):726–737. DOI:10.1080/01926230500352226
- Abba MC, Zhong, Y., Lee, J., Kil, H., Lu, Y., Takata, Y. and Aldaz, C. M DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function PTEN mutations. Oncotarget. 2016;7(39):64289. DOI:10.18632/oncotarget.11733
- Roque DR, Dizon DS. New advances in the treatment of endometrial cancer. Clin Investig (Lond). 2011;1(3):413–422.
- Klębowski B, Depciuch J, Parlińska-Wojtan M, et al. Applications of noble metal-based nanoparticles in medicine. Int J Mol Sci. 2018;19(12):4031.
- Parveen A, Malashetty VB, Mantripragada B, et al. Bio-functionalized gold nanoparticles: benign effect in Sprague-Dawley rats by intravenous administration. Saudi J Biol Sci. 2017;24(8):1925–1932.
- Gamal H, Tawfik W, Fahmy HM, et al. Breakthroughs of using photodynamic therapy and gold nanoparticles in cancer treatment. in 2021 IEEE International Conference on Nanoelectronics, Nanophotonics, Nanomaterials, Nanobioscience & Nanotechnology (5NANO). 2021;1–4.
- Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–1671.
- Liu SH, Han MY. Synthesis, functionalization, and bioconjugation of monodisperse, Silica‐Coated gold nanoparticles: robust bioprobes. Adv Funct Mater. 2005;15(6):961–967.
- Hornos Carneiro MF, Barbosa F. Gold nanoparticles: a critical review of therapeutic applications and toxicological aspects. J Toxicol Environ. 2016;19(3–4):129–148.
- Goodarz Naseri M, Saion E, Khalil Zadeh N. The amazing effects and role of PVP on the crystallinity, phase composition and morphology of nickel ferrite nanoparticles prepared by thermal treatment method. Int Nano Lett. 2013;3(1):1–8.
- Pandey G, Singh S, Hitkari G. Synthesis and characterization of polyvinyl pyrrolidone (PVP)-coated Fe3O4 nanoparticles by chemical co-precipitation method and removal of Congo red dye by adsorption process. Int Nano Lett. 2018;8(2):111–121.
- Hou CC, Zhu JQ. Nanoparticles and female reproductive system: how do nanoparticles affect oogenesis and embryonic development. Oncotarget. 2017;8(65):109799.
- Gueyo TN, Zingue, S., Mvondo, M. A., Mmutlane, E., Ndinteh, D. T., Pieme, C. A. and Njamen, D. Cytotoxic and cancer chemopreventive potentials of the Anthonotha macrophylla P. Beauv aqueous extract on 7, 12-dimethylbenz [a] anthracene-induced breast cancer in rats. Biologia. 2021;76(2):729–739. DOI:10.2478/s11756-020-00607-7
- Nikoobakht ESM, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15(10):1957–1962.
- Johnson CJ, Dujardin E, Davis SA, et al. Growth and form of gold nanorods prepared by seed-mediated, surfactant-directed synthesis. J Mater Chem. 2002;12(6):1765–1770.
- Emam AN, Mohamed MB, Girgis E, et al. Hybrid magnetic–plasmonic nanocomposite: embedding cobalt clusters in gold nanorods. RSC Adv. 2015;5(44):34696–34703.
- Requejo KI, Liopo AV, Zubarev ER. Synthesis of gold nanorods using poly (vinylpyrrolidone) of different molecular weights as an additive. ChemistrySelect. 2018;3(43):12192–12197.
- Da Silva Junior FC, Mbmc F, de Castro DEF, et al. A look beyond the priority: a systematic review of the genotoxic, mutagenic, and carcinogenic endpoints of non-priority PAHs. Environ Pollut. 2021;278:116838.
- Veyrand B, Sirot V, Durand S, et al. Human dietary exposure to polycyclic aromatic hydrocarbons: results of the second French total diet study. Environ Int. 2013;54:11–17.
- Kang JS, Jung NJ, Kim S, et al. Downregulation of estrogen receptor alpha and beta expression in carcinogen-induced mammary gland tumors of rats. Exp Oncol. 2004;26(1):31–35.
- Russo J. Significance of rat mammary tumors for human risk assessment. Toxicol Pathol. 2015;43(2):145–170.
- Jaiswal YS, Williams LL. A glimpse of Ayurveda–The forgotten history and principles of Indian traditional medicine. J Tradit Complement Med. 2017;7(1):50–53.
- Pandey MM, Rastogi S, Rawat AKS. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Altern Med. 2013;2013:1–12.
- Balfourier A, Kolosnjaj-Tabi J, Luciani N, et al. Gold-based therapy: from past to present. Proc Natl Acad Sci. 2020;117(37):22639–22648.
- Anik MI, Mahmud N, Al Masud A, et al. Gold nanoparticles (GNPs) in biomedical and clinical applications: a review. Nano Sel. 2022;3(4):792–828.
- Rai M, Deshmukh SD, Ingle AB, et al. Metal nanoparticles: the protective nanoshield against virus infection. Crit Rev Microbiol. 2016;42(1):46–56.
- Xiong X, et al. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget. 2014;5(15):6453. DOI:10.18632/oncotarget.2203
- Lindell AE, Zimmermann-Kogadeeva M, Patil KR Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat Rev Microbiol. 2022; 1.
- Abdelmeguid NE, Khalil MI, Badr NS, et al. Ameliorative effects of colostrum against DMBA hepatotoxicity in rats. Saudi J Biol. 2021;28(4):2254–2266.
- Penet MF, Krishnamachary B, Wildes FB, et al. Ascites volumes and the ovarian cancer microenvironment. Front Oncol. 2018;8:595.
- Kritpracha K, Hanprasertpong J, Chandeying V, et al. Survival analysis in advanced epithelial ovarian carcinoma in relation to proliferative index of MIB‐1 immunostaining. J Obstet Gynaecol Res. 2005;31(3):268–276.
- Aune G, Stunes AK, Tingulstad S, et al. The proliferation markers Ki-67/MIB-1, phosphohistone H3, and survivin may contribute in the identification of aggressive ovarian carcinomas. Int J Clin Exp Pathol. 2011;4(5):444.
- Baharara J, Ramezani T, Divsalar A, et al. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J Med Biotechnol. 2016;8(2):75.
- Me S, Aa H. Gold nanoparticles induce apoptosis in MCF-7 human breast cancer cells. Asian Pac J Cancer Prev. 2012;13(4):1617–1620.
- Archid R et al. Cachexia anorexia syndrome and associated metabolic dysfunction in peritoneal metastasis. Int J Mol Sci. 2019;20(21):5444. DOI:10.3390/ijms20215444
- Crist KA, et al. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7, 12-dimethylbenz [a] anthracene (DMBA) coated suture. Carcinogenesis. 2005;26(5):951–957. DOI:10.1093/carcin/bgi039
- Khan AA. Pro-apoptotic activity of nano-escheriosome based oleic acid conjugate against 7, 12-dimethylbenz (a) anthracene (DMBA) induced cutaneous carcinogenesis. Biomed Pharmacother. 2017;90:295–302.