ABSTRACT
This study investigated the toxicity of lipopolysaccharides (LPS) and the therapeutic role of zinc oxide nanoparticles (ZnO NPs) on the optic nerve of mother rats and their offspring treated with LPS. This study used eight male and forty female rats (Rattus norvegicus). Following mating, pregnant rats were separated into four groups: controls, LPS, ZnO NPs and LPS and ZnO NP-treated groups. LPS was injected twice during pregnancy. ZnO NPs were injected three times duringpregnancy, and a dose after birth. Mother rats and their offspring at 21daysold were sacrificed and separated from their optic nerve for histological and immunohistochemical examinations. Collected blood was collected from the mother rat for biochemical examination to evaluate superoxide dismutase-1 (SOD-1), catalase and malondialdehyde (MDA). This study discovered that ZnO NPs improved the histology of the optic nerve, decreased Cyclooxygenase-2 (COX-2) immunostaining, which overexpressed in the optic nerve of rats receiving LPS treatment and increased MDA. ZnO NPs also decreased the levels of the antioxidant enzymes such as SOD-1 and catalase, which had increased in the LPS-treated group. In conclusion, ZnO NPs injection into mother rats treated with LPS alleviated the markers of optic nerve injury in its offspring due to their antioxidant and neuroprotective effect.
Introduction
Lipopolysaccharides (LPS) are a toxic component in Gram-negative bacteria’s cell walls [Citation1]. The hydrophilic core polysaccharide chain, the repetitive hydrophilic O-antigenic oligosaccharide side chain and the hydrophobic lipid portion that is known as lipid A are the three structural elements that make up the macromolecules of intact bacterial LPS [Citation2]. The intrauterine infection brought on by bacterial LPS exposure causes preterm delivery, neurological damage and fetal inflammation in the retina and optic nerve, which manifests in their structure and neurochemistry. It also results in a decrease in tyrosine hydroxylase immunoreactive (TH-IR), dopaminergic amacrine cells and all of which can impair vision. When compared to control fetuses, myelin sheath thickness decreased in LPS-exposed fetuses at a critical time for optic nerve development [Citation3]. According to several studies, LPS causes systemic inflammation and affects directly placental cells causing the release of pro-inflammatory mediators that activate microglia. In addition, astrocytes cause the production of cytokines in the developing fetal brain [Citation4,Citation5].
The optic nerve (cranial nerve II) is a central nervous system (CNS) tract that passes through the optic canal to leave the orbit. It is made up of the retinal ganglion cells (RGCs) axons. It allows vision by transmitting neural impulses from the retina to the brain. It is divided into four sections: the intraocular nerve head, the intraorbital, the intracanalicular and the intracranial [Citation6]. The types of glial cells in the optic nerve are oligodendrocytes, astrocytes and microglia. Oligodendrocytes are responsible for producing the myelin sheaths that protect the CNS axons and contact nodes of Ranvier as well as they are the locations where action potentials are propagated and axonal integrity. Astrocytes are responsible for numerous physiological and pathological activities such as potassium homeostasis and metabolism as well as reactive astrogliosis in response to CNS trauma. Microglia are immune cells in CNS and have a significant impact on inflammation and infections [Citation7].
Sherwin and Fern showed that LPS induced white matter glial cell death and the ultra-structural examination clarified oligodendroglial cell death, whereas astrocytes and axons were unchanged in the optic nerves of rats [Citation8]. Microglial activation is provoked by LPS; consequently, the elevation of TLR4 signaling in the retina and optic nerve [Citation9] determines LPS produced by Gram-negative bacteria and mediates the inflammatory response to these bacteria [Citation10]. In addition, microglial activation leads to the release of pro-inflammatory cytokines, free radicals and nitric oxide from microglia and astrocytes. All these molecules cause the death of retinal ganglion cells (RGCs), optic nerve fibers, other astrocytes and oligodendrocytes. Death of all prior cells leads to Glaucoma [Citation11].
Many studies demonstrated that injecting bacterial LPS into the optic nerve of rats induced optic neuritis (ON). ON is characterized by a disruption of the blood-brain barrier (BBB) and leukocyte infiltration, as well as unilateral visual loss, afferent pupillary deficiency, abnormal visual evoked potentials (VEPs), periocular or retro-orbital pain in conjunction with eye movement, astrocytosis, demyelination, axon degeneration and RGC degeneration [Citation12]. RGC oxidative damage is caused by increasing reactive oxygen species (ROS), and it is associated with numerous diseases such as glaucoma, hereditary optic atrophy, ischemic optic neuropathy, Traumatic Optic Neuropathy and Optic Neuritis. LPS injections also increased the levels of inducible nitric oxide synthase, COX-2, interleukin-1β and TNFα mRNA levels as well as increased the production of retinal superoxide, decreased activity of superoxide dismutase 2 and activated the inflammasome. All previous findings have been associated with optic nerve damage [Citation13].
In recent days, life is made easier by advances in nanoscience and nanotechnologies. Nanotechnology is used widely in research areas due to the arrangement of their atoms on the 1–100 nm scale [Citation14]. Nanomedicine is used to treat cerebral ischemia by active targeting (using targeting ligands) or passive targeting (using enhanced permeability and retention effect) due to an increase in the number of patients with neurodegenerative diseases [Citation15]. ZnO NPs play an essential role in neuroprotection against 6-hydroxydopamine-induced cell injury in vitro by preventing apoptosis, decreasing reactive oxygen species and improving mitochondrial membrane potential [Citation16].
It was reported that 50 µg/mL of ZnO NPs plays a therapeutic role in SH-SY5Y cells resulting in a clear recovery from neurological damage. This leads to Alzheimer’s disease (AD) and Parkinson’s Disease (PD), which are neurodegenerative diseases that cause progressive malfunction and death of neurons. It results in acute brain injury and a sequence of neural damaging events indicating that ZnO NPs can be used as a new gateway in the treatment of neurodegenerative diseases16. Ashraf et al. proved that ZnO NPs made from aloe vera leaf extract have anti-oxidant and anti-glycation properties and they are used in neurodegenerative disease prevention and reversal therapies [Citation17].
Materials and methods
Chemicals
LPS
LPS was purchased from Sigma-Aldrich, Egypt. 1.5 mg of LPS dissolved in 30 ml distilled water. On days 7 and 9, respectively, two doses of LPS (150 µg/kg) were intraperitoneally injected [Citation18].
ZnO NPs
ZnO NPs are also purchased from Sigma-Aldrich; They are a white fine powder, and 300 mg of ZnO NPs is dissolved in 50 ml of distilled water. On the 14th, 17th and 20th days of gestation, with one dose after birth, ZnO NPs (20 mg/kg) were given intraperitoneally [Citation19,Citation20].
Transmission electron microscopy of ZnO NPs
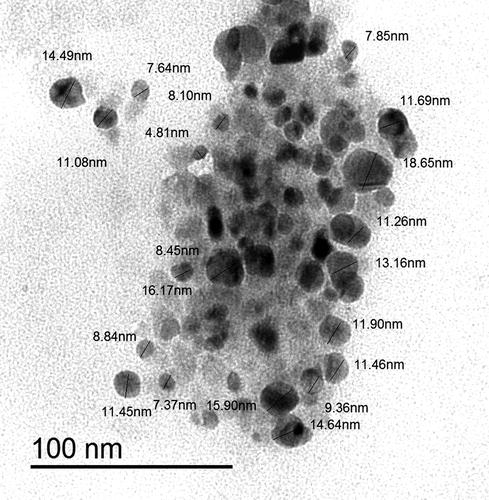
A morphological examination of ZnO NPs was examined by TEM (JEOL JEM-2100, JEOL Ltd., Tokyo, Japan). After diluting a sample of ZnO NP dispersion with deionized water, a single drop of the diluted sample was put onto a copper grid coated with carbon, dried for 10 minutes at room temperature and then examined through a TEM without any staining. Digital Micrograph and Soft Imaging Viewer Software (Gatan Microscopy Suite Software, version 2.11.14044.0) were used for the image acquisition and analysis process.
Zeta size of ZnO NPs
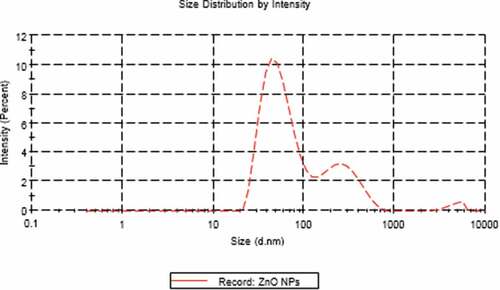
Zeta average size of ZnO NPs and polydispersity index (PDI) were measured using a Zetasizer Nano ZS analyzer (Malvern Instruments, Malvern, UK) and the dynamic light scattering (DLS) technique according to DeLoid et al. [Citation21].
Zeta potential of ZnO NPs
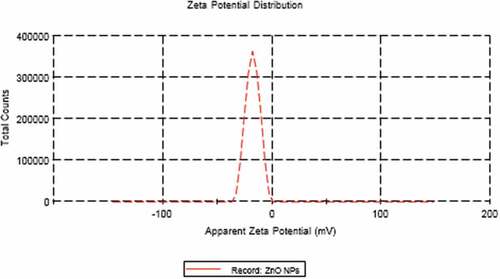
Zeta potential of ZnO NPs was evaluated in triplicates, using the same apparatus and the same dilution in deionized water, as mentioned before in the zeta average size measurement.
Ethical approval
The study received approval from the Ethical Committee for Animal Experimentation at the Faculty of Science, Mansoura University, Egypt. Approval number is Sci-Z-ph-2021–29.
Experimental work
This study used eight male and forty female rats (Rattus norvegicus) weighing 120-150 g obtained from Egypt’s Ministry of Health’s Hellwan Breeding Farm. After mating in special cages (3 females/1 male), the existence of spermatozoa in the vaginal smear in the next morning was considered the first day of gestation. Pregnant rats were divided into four groups: control, ZnO NPs, LPS and LPS and ZnO NP-treated groups. Each group has 10 pregnant rats. Mother rats and their five offspring at 21 days post-partum in the study groups were slaughtered by cervical dislocation after anesthesia with 50 mg/kg phenobarbitone given intraperitoneally [Citation22]. Collected blood was collected for biochemical examination. The intraorbital portion from the optic nerve of the mother and 21-day-old offspring was separated and was fixed in 10% phosphate-buffered formalin (pH 7.4) and prepared for histological examinations. Other intraorbital parts of the optic nerve were maintained in phosphate-buffered 2.5% glutaraldehyde for transmission electron microscopy examination. For biochemical investigations, the serum of mother rats was maintained in the refrigerator at −4°C.
Light microscopy
As aforementioned, the intraorbital portion of the right optic nerve of mother rats and their offspring at 21 days post-partum were incised from five rats and immediately preserved in 10% phosphate-buffered formalin (pH 7.4) before being processed for histological examinations, and serial 5 µm thick cross-sections of the optic nerve were stained with hematoxylin and eosin. Finally, slides were investigated using a bright-field light Olympus microscope using an optiKa vision pro camera [Citation23].
Transmission electron microscopy
The intraorbital portion of the left optic nerve from five mothers and five offspring rats at 21 days was fixed in phosphate-buffered 2.5% glutaraldehyde (pH 7.4) and then post-fixed in phosphate-buffered 1% osmium tetraoxide at 4°C for 1.5 hours. They were dehydrated in ethyl alcohol in ascending grades and then cleaned in acetone before being embedded in epoxy resin. By using an LKB Ultratome IV, ultrathin slices were cut and placed on grids, stained with uranyl acetate and lead citrate and seen on a Joel 100C×l transmission electron microscope (Mansoura University, Egypt) [Citation24].
Immunohistochemical staining
Five µm histological cross-sections of formalin-fixed, paraffin-embedded right optic nerve sections from five mothers and five offsprings at 21 days were mounted on polylysine-coated glass slides and stored at room temperature. Optic nerve sections (dilution of 1/100) were incubated with Monoclonal Mouse Anti-Human COX-2 antibody (Clone CX-294) for an hour. After washing with PBS buffer, the sections were treated with HRP-conjugated secondary antibody (Code-No. RP 111; Diagnostic Biosystems) for 30 minutes to detect the immune response. Data were obtained using a bright-field light Olympus microscope and an image analyzer computer system Ltd. (Cambridge, UK). The measurements of the mean area % of COX were performed in five non-overlapping fields at a magnification of x400.
Biochemical assays
The following biochemical assays were used to present the findings
Determination of SOD-1 activity
SOD-1 activity was measured by ELISA kit (Catalog # E4584–100, 100 assays, stored at 4°C). One hundred µL of the serum was added into appropriate wells. Then, wells were covered and incubated for 1.5 hr at 37°C. After that, the cover was removed and the wells’ content was discarded without letting the wells completely dry. 0.1 ml of Biotin-detection antibody work solution was added into the above wells and incubated at 37°C for 60 min. The wells were washed 3 times with 1× wash solution. 0.1 ml of SABC solution was added into each well, incubated at 37°C for 30 min and then washed five times with 1× wash solution. Ninety micoliters of the TMB substrate was added and incubated at 37°C in the dark within 15–30 min when blue color appeared. Finally, 50 µL of stop solution was added and the SOD-1 activity was measured at 450 nm within 20 min.
Determination of catalase activity
The activity of catalase was measured spectrophotometrically using the Koroliuk et al. technique [Citation25]. Ten microliters of the sample was incubated in 0.05 µmol/L Tris-HCI buffer pH = 7 with 100 mol/mL of H2O2 for 10 min. The process was stopped by quickly adding 50 µL of 4% ammonium molybdate. The ammonium molybdate and H2O2 yellow complex was detected at 410 nm.
Determination of lipid peroxidation end product MDA (Thiobarbituric acid reactive substances, TBARS) level in the serum
The thiobarbituric acid reaction method of Placer et al. was used to determine MDA levels in the serum [Citation26]. The thiobarbituric acid reactive substance was measured at 532 nm by comparing the absorption to the standard curve of MDA equivalents. A working solution containing 15% trichloroacetic acid, 0.375% thiobarbituric acid and 0.25N hydrochloric acid was prepared to determine MDA levels, 250 µL of serum and 500 µL of working solution, combined for each sample and put in boiling water for 10 minutes. After cooling, the samples were centrifuged for 10 minutes at 3000 rpm. After that, 200 µL of each supernatant was transferred to microplates and the optical density of the samples was determined at 535 nm. The amount of enzyme required to break down 1 µmol H2O2 per minute is defined as one unit of catalase activity. Finally, MDA concentrations were measured.
Statistical analysis
The current study used one-way ANOVA to illustrate the statistical analysis between study groups that were chosen on SPSS (version 13). Data are presented as means ± standard error (SE). The multiple range comparisons were selected from the Post Hoc tests (Tukey HSD) on the same statistical package to detect the variances between different groups.
Results
Characterization of ZnO NPs
TEM technique was showing that ZnO NPs are homogenous and well-arranged particles with spherical shape and size ranging from 7 nm to 15 nm (). The average particle size distribution of ZnO NPs was 62.91 ± 0.47 nm with a PDI value of 0.34 ± 0.01 (). The zeta potential for ZnO NPs was approximately−17.8 ± 0.4 mV ().
Histopathological observations
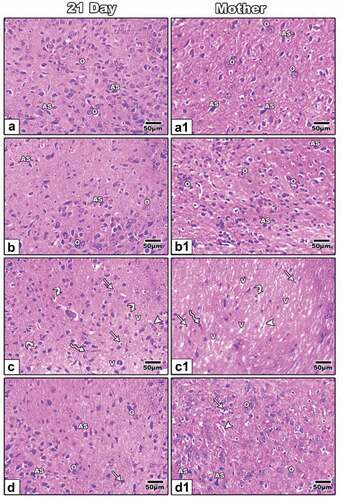
Mother rats and 21-day-old offspring in the control and ZnO NP treatment groups had standard oligodendrocytes with round shapes and dark nuclei and standard astrocytes with oblong shapes and irregular-shaped nuclei in their optic nerve ( (a&a1, b&b1)). The histopathological findings of this study showed that LPS had a significant impact on mother rats and their offspring’s optic nerve structure, which is accompanied by vacuoles, cytoplasmic vacuolated cells, pyknotic cells, degenerated oligodendrocytes and dilated blood vessels ( (c&c1)). In contrast to the LPS and ZnO NP-treated group, which showed histological improvement that appeared in the presence of normal oligodendrocytes, normal astrocytes and less pyknotic cells than in the LPS-treated group, dilated blood vessels are still visible in the mother treated with LPS and ZnO NPs ( (d&d1)).
Figure 4. Photomicrographs cross-histological sections of the optic nerve of 21-day-old offspring (a-d) and mother (a1-d1) rats; Control (a&a1) and ZnO NP-treated group (b&b1) showing normal oligodendrocytes and normal astrocyte; 21-day old offspring rats and their mothers treated with LPS showing cytoplasmic vacuolated cells, pyknotic cells, degenerated oligodendrocytes, dilated blood vessels and vacuoles (c&c1); 21-day-old offspring and their mother rats treated with LPS and ZnO NPs showing structural improvement (d&d1). H&E. Abbreviations: AS indicating astrocyte, O indicating oligodendrocyte, V-vacuole; Arrow indicating pyknotic cells; Arrowhead indicating dilated blood vessels; Curved arrow indicating degenerated oligodendrocytes; Zigzag arrow indicating cells with cytoplasmic vacuolation.
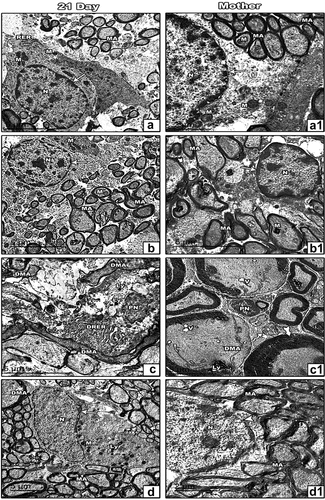
Ultrastructural observations
In the control and ZnO NP-treated group, this study observed normal nuclei and firmly packed myelinated axons of various sizes and normal mitochondria in the optic nerve of both mother rats and their 21-day-old offspring ( (A&A1, B&B1)). It revealed oligodendrocytes with pyknotic nuclei, degenerated myelinated axons, dilated rough endoplasmic reticulum and the presence of lysosomes in the optic nerve of 21-day-old offspring maternally treated with LPS ( (c)). Vacuoles, increasing myeline sheath thickness and demyelination as well as separation between nerve fibers appeared in mothers treated with LPS as well as the presence of the phagosomes (1)). In LPS and ZnO NP-treated group, the improvement was seen in the presence of normal mitochondria, normal myelinated axons and normal nuclei in the 21-day-old offspring, but lysosomes and degenerated myelinated axons were still present ( (d)). In addition, the mother’s improvement was seen in the presence of normal myelinated axons and normal nuclei, but its membrane was irregular ( (d1)).
Figure 5. Transmission electron micrographs of the optic nerve of 21-day-old offspring (a-d) and mother (a1-d1) rats; Control (a&a1) and ZnO NPs treated group (b&b1) showing normal nucleus, normal myelinated axons, normal mitochondria; 21-day-old offspring rats their mothers treated with LPS showing degenerated myelinated axons, pyknotic nucleus, dilated rough endoplasmic reticulum and lysosomes (c). Mother treated with LPS showing vacuoles, demyelination, increasing thickness of myelin sheath and pyknotic nucleus (c1). 21-day-old offspring and their mother rats treated with LPS and ZnO NPs showed structural improvement (d&d1). Abbreviations: DMA indicating degenerated myelinated axon; DRER indicating dilated rough endoplasmic reticulum; LY indicating lysosomes; M indicating mitochondria; MA indicating myelinated axon; N indicating nucleus; PN indicating pyknotic nucleus; RER indicating rough endoplasmic reticulum; V indicating vacuoles; Arrow indicating regular nuclear membrane; Arrowhead indicating irregular nuclear membrane; Wavy arrow indicating phagosomes; Tailed arrow indicating thick myelin sheath.
Immunohistochemistry of COX-2
Compared to control and ZnO NP-treated mother rats ( (a&a1, b&b1) and ), it is found that overexpression of COX-2 immunostaining is associated with inflammation in the optic nerve of 21-day-old offspring and their mothers of LPS-treated group ( (c&c1) and ). It is decreasing in LPS and ZnO NPs treated group ( (d) and ).
Figure 6. Photomicrographs of formalin-fixed histological sections of the optic nerve immunohistochemical stained with COX-2 of 21-day-old offspring (a-d) and mother (a1-d1) rats. Control (a-a1) and ZnONPs treated group (b-b1) showed a low immunohistochemical reaction of COX-2. 21-day-old offspring and mother rats treated with LPS showed an increased dark brown immunohistochemical reaction of COX-2 (c&c1). 21-day-old offspring and mother rats treated with LPS plus ZnO NPs showed a decreased immunohistochemical reaction of COX-2 (d&d1). Arrowhead indicating the reaction of COX-2.
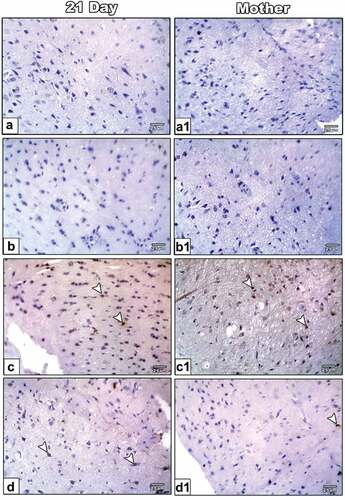
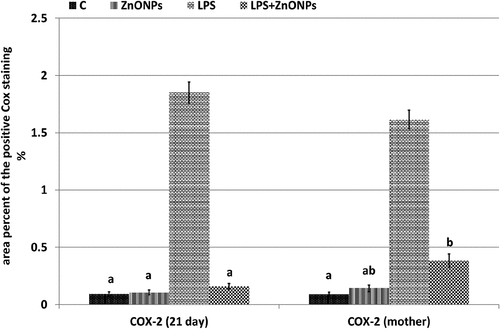
Figure 7. Histogram illustrating the area percent of immunohistochemical reaction of COX-2 in the optic nerve of 21-day-old offspring and their mother rats treated with LPS with or without ZnO NPs. Means with different letters indicate the variations between the groups within the same row using Tukey’s honestly significant difference (p ≤ 0.05) test. Note overexpression of COX-2 in the optic nerve of 21-day-old rats and their mother rats treated with LPS and improved in that treated with ZnO NPs plus LPS. .
Biochemical investigation
This study showed that antioxidant enzymes such as SOD-1 and catalase significantly decreased, while lipid peroxidation product (MDA) significantly increased in the serum of mother rats treated with LPS compared with the control and ZnO NPs treated group. In contrast to LPS plus ZnO NPs treated group, SOD-1 significantly increased and catalase non-significantly increased in addition to MDA non-significantly decreased compared with LPS treated group ().
Table 1. Biochemical markers of serum of mother rats treated with LPS with or without ZnO NPs.
Discussion
Both the mother rats treated with LPS and their offspring had histological abnormalities in their optic nerve. These abnormalities were characterized by vacuoles that appeared in the degenerated optic nerve, cytoplasmic vacuolation and pyknotic cells that accompany apoptosis, degenerated oligodendrocytes and dilated blood vessels that take place during the inflammatory reaction to enhance blood flow to the injured area. In addition, ultrastructural examination showed numerous histopathological changes as oligodendrocytes with pyknotic nuclei indicated that LPS-induced oligodendrocytes programmed cell death, the presence of atrophied mitochondria that is linked with neuro-degeneration and decreasing ATP that is essential for neurotransmission process [Citation27], In addition, damaged nerve fiber and demyelination that is caused by macrophages in a process called myelin phagocytosis [Citation28] occurred because of inflammation in the optic nerve of offspring and their mother treated with LPS. Also, the presence of phagosomes was observed in the optic nerve of mothers treated with LPS to move and migrate across tissues to engulf and eliminate both microorganisms and cell debris.
The present study can conclude that LPS maternally injection has negative effects on their offspring because LPS can pass through the placental barrier and interact with functional TLR4 on fetal/placental tissues, which can raise the levels of cytokines in the amniotic fluid, have a detrimental effect on embryo development, and even result in fetal brain injury [Citation29]. This study is consistent with other previous investigations, reporting that microinjection of LPS into the optic nerve of Sprague Dawley rats showed demyelination and changes in optic nerve structure due to oxidative stress and increase in CD68, GFAP, pro-inflammatory factors, protein expression levels in the signal transducer and activator of transcription (STAT) and nuclear factor-B (NF-B) translocation [Citation30,Citation31]. As a result of chronic experimental hypoxia/ischemia, LPS exposure during the prenatal period delays oligodendrocyte maturation into the mature myelinating phenotype [Citation32]. Its exposure also triggered retinal ganglion cell death by reducing the number of optic nerve axons [Citation33].
At the immunohistochemical level, COX-2 overexpression was found in optic nerve tissue in mother rats and their offspring treated with LPS, which was also reported by Anrather et al. in cultured microglia 8 hours after LPS administration expressed more COX-2 [Citation34]. This result confirmed that LPS affected the optic nerve due to COX-2 overexpression induced by proinflammatory cytokines at the inflammation site that is associated with inflammation and cancer. COX-2 overexpression enhances angiogenesis, prevents apoptosis, elevates metastatic potential and promotes cancer cell proliferation [Citation35].
Biochemical investigation in sera of mother rats treated with LPS showed increased oxidative stress by significantly diminished antioxidant enzymes such as SOD-1 and catalase as well as increasing MDA, which is a lipid peroxidation product. SOD, glutathione peroxidase (GPX), catalase and its supporting enzymes such as glutathione reductase are antioxidant defense systems that neutralize reactive oxygen species [Citation36]. Alternations in the redox system play a key role in the pathogenesis of numerous optic neuropathies accompanied by the neurodegeneration of the inner-most retinal neurons, the retinal ganglion cells (RGCs) and their axons, which form the optic nerve causing glaucoma, ischemic optic neuropathy, optic neuritis and hereditary optic neuropathies [Citation37].
On the other hand, in this study, ZnO NPs improve the histological structure of the optic nerve by reducing COX-2 immunostaining, which means decreasing inflammation caused by LPS as well as lower oxidative stress that appeared in increasing SOD-1 and catalase levels with lowering MDA level. This is because the characterization of ZnO NPs is supported by this study. This characterization includes their large zeta potential indicating good physical stability and their small size, so they can penetrate blood arteries and reach the interstitial spaces, where they can affect different cells in various tissues [Citation38] and they can pass through the placental barrier, and affect the fetus [Citation39]. The anti-inflammatory action of ZnO NPs is coinciding with the report of Mohapatra et al. proving that ZnO NPs mixed with clove and cinnamon have anti-inflammatory and antioxidant properties. The pro-inflammatory cytokines, mast cell proliferation and LPS-induced COX-2 expression are all inhibited by ZnO NPs [Citation40].
These findings are consistent with other studies that reported that ZnO NPs increase antioxidant activity, diminish free radical levels produced by Aflatoxin B1 and haves teratogenic, carcinogenic, mutagenic and immunosuppressive effects in humans and animals when giving Aflatoxins-contaminated feedstuff [Citation41]. In addition, studies showed that ZnO NPs have anticancer effects that can kill cancer cells by inducing oxidative stress in the cancer cells [Citation42], by the activation of DNA repair and by inhibiting both apoptosis and cancer cell growth [Citation43]. In vitro, nanoscale zinc oxide organizes neuronal excitability, enhances glutamate secretion and alters the balance of neurotransmitter and neuronal excitability in the CNS after LPS injections; so ZnO NPs therapy can improve behavioral and electrophysiological abnormalities induced by LPS [Citation44].
In conclusion, this study showed the therapeutic role of ZnO NPs on the optic nerve of mother rats and their offspring treated with LPS due to their antioxidant activities. Compared to the LPS-treated group, the improvement was accompanied by an increase in the number of microglial cells, astrocytes and oligodendrocytes, which indicates less cell death as well as normal mitochondria and nuclei, which are degenerated in the LPS-treated group, as well as a decrease in COX-2 immunostaining. The increase in SOD-1 and catalase along with a decrease in lipid peroxidation product (MDA), which indicates a reduction in oxidative stress, indicated an improvement in the sera mother.
Acknowledgments
The authors dedicate this work to the soul of Prof. Hassan I El-Sayyad who passed away of COVID-19 in November 2021.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi:10.1146/annurev.biochem.71.110601.135414.
- Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40(4):480–493.
- Loeliger M, Duncan J, Cock M, et al. Vulnerability of dopaminergic amacrine cells and optic nerve myelination to prenatal endotoxin exposure. Invest Ophthalmol Vis Sci. 2007;48(1):472–478.
- Zakharova LA. Perinatal stress in brain programming and pathogenesis of psychoneurological disorders. Izv Akad Nauk Ser Biol. 2015;1(1):17–26. PMID: 25872396. 10.1134/S1062359015010124.
- Silva MG, Daros GC, Santos GM, et al. Impact of prenatal lipopolysaccharide exposure on the development of rats. An Acad Bras Cienc. 2020;92(4):e20200837.
- Salazar JJ, Ramírez AI, Hoz RD, et al. Optic nerve.Anatomy of the human optic nerve: structure and function. IntechOpen. 2018; (online). https://www.intechopen.com/chapters/6
- Butt AM, Pugh M, Hubbard P, et al. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye (Lond). 2004;18(11):1110–1121.
- Sherwin C, Fern R. Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-alpha, IL-1beta, and calcium. J Immunol. 2005;175(1):155–161.
- Astafurov K, Elhawy E, Ren L, et al. Oral microbiome link to neurodegeneration in glaucoma. PLoS ONE. 2014;9(9):e104416.
- Koike N. Stem cells and cancer in hepatology. In Chapter 11 The Role of Stem Cells in the Hepatobiliary System and in Cancer Development: a Surgeon’s Perspective. Acad Press; 2018. p. 211–253. DOI:10.1016/C2016-0-03399-5
- Bochang LV, Fuquan H, Zhongqiao Z, et al. Crocin upregulates CX3CR1 expression by suppressing NF-[kappa]b/yy1 signaling and inhibiting lipopolysaccharide-induced microglial activation. Neurochem Res. 2016;41(8):1949–1957.
- Ma S, Zhang K, Zhu Y, et al. Effect of papaverine on axonal outgrowth of primary retinal ganglion cells of Sprague Dawley rats. Exp Eye Res. 2021;212:108797.
- Kang EYC, Liu PK, Wen YT, et al. Role of oxidative stress in ocular diseases associated with retinal ganglion cells degeneration. Antioxidants. 2021;10(12):1948.
- Bayda S, Adeel M, Tuccinardi T, et al. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112.
- Zhang T-T, Li W, Meng G, et al. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci. 2016;4(2):219–229.
- Ding X, Lin K, Li Y, et al. Synthesis of biocompatible zinc oxide (ZnO) nanoparticles and their neuroprotective effect of 6-OHDA induced neural damage in SH-SY 5Y cells. J Clust Sci. 2020;31(4):1315–1328.
- Ashraf JM, Ansari MA, Fatma S, et al. Inhibiting effect of zinc oxide nanoparticles on advanced glycation products and oxidative modifications: a potential tool to counteract oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2018;55(9):7438–7452.
- Bo QL, Chen YH, Yu Z, et al. Rosiglitazone pretreatment protects against lipopolysaccharide-induced fetal demise through inhibiting placental inflammation. Mol Cell Endocrinol. 2016;423:51–59.
- Torabi M, Kesmati M, Harooni HE, et al. Effects of nano and conventional zinc oxide on anxiety-like behavior in male rats. Indian J Pharmacol. 2013;45(5):508–512.
- Aboelmaaty AM, Omara ST, Aly MS, et al. The antibacterial and anti-inflammatory effects of zinc oxide nanoparticles synthesized by Thymus vulgaris medicinal plant against Escherichia coli and Escherichia coli lipopolysaccharides. Egypt Pharmaceut J. 2022;21(2):153–166.
- DeLoid GM, Cohen JM, Pyrgiotakis G, et al. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat Protoc. 2017 Feb;12(2):355–371. DOI:10.1038/nprot.2016.172.
- Laferriere CA, Pang DS. Review of intraperitoneal injection of sodium pentobarbital as a method of euthanasia in laboratory rodents. J Am Assoc Lab Anim Sci. 2020;59(3):254–263.
- Feldman AT, Wolfe D. Tissue processing and hematoxylin and eosin staining. Methods Mol Biol. 2014;1180:31–43.
- Doig NM, Magill PJ Quantitative analyses of the ultrastructural features of dopaminergic axon terminals. Protocol #1: tissue preparation for electron microscopy. 2022. DOI: 10.17504/protocols.io.j8nlkw55wl5r/v1.
- Koroliuk MA, Ivanova LI, Maiorova IG. A method of determining catalase activity. Laboratornoe Delo. 1998;1:16–19. PMID: 2451064.
- Placer ZA, Cushman LL, Jhonson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–364.
- Muench NA, Patel S, Maes ME, et al. The Influence of mitochondrial dynamics and function on retinal ganglion cell susceptibility in optic nerve disease. Cells. 2021;10(7):1593.
- Koike H, Katsuno M. Macrophages and Autoantibodies in Demyelinating Diseases. Cells. 2021;10(4):844.
- Brown AG, Maubert ME, Anton L, et al. The tracking of lipopolysaccharide through the feto-maternal compartment and the involvement of maternal TLR4 in inflammation-induced fetal brain injury. Am J Reprod Immunol. 2019;82(6):e13189.
- Wang F, Dang Y, Wang J, et al. Gypenosides attenuate lipopolysaccharide-induced optic neuritis in rats. Acta Histochem. 2018;120(4):340–346.
- Aranda ML, Guerrieri D, Piñero G, et al. Critical role of monocyte recruitment in optic nerve damage induced by experimental optic neuritis. Mol Neurobiol. 2019;56(11):7458–7472.
- Segovia KN, McClure M, Moravec M. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63(4):520–530.
- Sharangpani A, Takanohashi A, Bell MJ. Caspase activation in fetal rat brain following experimental intrauterine inflammation. Brain Res. 2008;1200:138–145.
- Anrather J, Gallo EF, Kawano T, et al. Purinergic signaling induces cyclooxygenase-1-dependent prostanoid synthesis in microglia: roles in the outcome of excitotoxic brain injury. PLoS ONE. 2011;6(10):e25916.
- Desai SJ, Prickril B, Rasooly A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr Cancer. 2018;70(3):350–375.
- Ji LL, Yeo D. Oxidative stress: an evolving definition. Fac Rev. 2021;10(13). DOI:10.12703/r/10-13
- Sanz-Morello B, Ahmadi H, Vohra R, et al. Oxidative stress in optic neuropathies. Antioxidants (Basel). 2021;10(10):1538.
- Choi HS, Ashitate Y, Lee JH, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28(12):1300–1303.
- Chen B, Hong W, Yang P, et al. Nano zinc oxide induced fetal mice growth restriction, based on oxide stress and endoplasmic reticulum stress. Nanomaterials (Basel). 2020;10(2):259.
- Mohapatra S, Leelavathi L, Rajeshkumar S, et al. Assessment of cytotoxicity, Anti-Inflammatory and antioxidant activity of zinc oxide nanoparticles synthesized using clove and cinnamon formulation - an in-vitro study. J Evol Med Dent Sci. 2020;9(25):1859–1864.
- Atef HA, Mansour MK, Ibrahim EM, et al. Efficacy of zinc oxide nanoparticles and curcumin in amelioration the toxic effects in aflatoxicated rabbits. Int J Curr Microbiol Appl Sci. 2016;5(12):795–818.
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063–1077.
- Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15(10):572–578.
- Xie Y, Wang Y, Zhang T, et al. Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J Biomed Sci. 2012;19(1):14.