ABSTRACT
Probiotics (PR) and intermittent fasting (IF) are believed to alleviate dysbiosis, which contributes to Non-alcoholic fatty liver disease (NAFLD) pathogenesis. Our study investigated the effect of PR, IF, and their combination (IP) on fatty liver in a rat model induced by a high-fat diet (HFD) We used 40 male rats, which were randomly assigned to one of five groups: control (fed standard chow), HFD (fed HFD for 12 weeks), PR (HFD for 12 weeks + probiotics (a daily dose of 10 × 109 CFU orally in the last 4 weeks), IF (HFD for 12 weeks + alternate day fasting (ADF) in the last 4 weeks), and IP (HFD for 12 weeks + probiotics (a daily dose of 10 × 109 CFU orally) + ADF in the last 4 weeks). Serum liver enzymes, lipids, and the HOMA-IR score were considerably lower in the IF and IP groups than in the HFD group. These changes were reflected in the pathological view. PR, IF, and IP noticeably upregulated serum FGF19, serum and hepatic FGF21, and reduced Lipopolysaccharides (LPS) and flavin containing dimethylaniline monoxygenase 3 (FMO3), indicating improvement in gut dysbiosis suggestive a hepatoprotective impact of IF and PR on NAFLD via modulating.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has received worldwide attention as the most common liver condition. Its incidence is increasing due to overweight and related metabolic diseases [Citation1]. NAFLD encompasses a diverse range of clinical conditions, from simple steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis [Citation2]. NAFLD’s underlying mechanisms are complex and unclear. However, the multiple hits theory explains its complicated molecular pathophysiology [Citation3].
There is currently no approved pharmaceutical treatment for NAFLD, while various substances are being studied [Citation4]. The gut microbiota (GM) is thought to contribute to NAFLD pathogenesis as it influences lipogenesis, apoptosis, bile acid metabolism, inflammation, and fibrosis. Lipopolysaccharides (LPS) and trimethylamine N-oxide (TMAO), two gut microbial metabolites, have been linked to host immunity and inflammation [Citation5]. LPS directly damages hepatocytes via activation of hepatic stellate and Kupffer cells, resulting in the release of numerous inflammatory mediators [Citation6] and thus promoting the development of NAFLD [Citation7].
Unbalances in the GM lead to increased conversion of choline to trimethylamine (TMA), which is then converted to TMAO by flavin-containing dimethylaniline monoxygenase 3 (Fmo3) (the main enzyme converting TMA to TMAO in the liver). Modification of TMAO levels seems to affect insulin and inflammatory signaling, bile acid metabolism, and lipogenesis, leading to fat deposition in the liver, hepatotoxicity, and liver function impairment [Citation8]. FMO3 and TMAO have been linked to NAFLD because of their involvement in glucose and lipid metabolism [Citation2].
Thus, it seems reasonable that compounds that reverse gut dysbiosis in NAFLD, such as probiotics, symbiotics, and prebiotics, may be effective treatments [Citation9]. Furthermore, probiotics (PR) can prevent the translocation of microbial pathogens and endotoxins by decreasing inflammatory responses through the production of antimicrobial peptides and restricting the translocation of LPS, thereby ameliorating dysbiosis, enhancing gut bacterial ecology, and adjusting immune functions [Citation10, Citation11].
Nonetheless, dietary restriction and physical activity-based lifestyle interventions remain the first-line management for NAFLD [Citation12]. Because of its benefits for weight and insulin sensitivity, intermittent fasting (IF) is regarded as one of the most promising lifestyle modifications; however, little is known about IF’s role in NAFLD management [Citation13]. Zhang et al., 2020, observed that 4 weeks of alternate-day fasting (ADF) improved insulin sensitivity, remodeled the GM, and modified microbial metabolites. Furthermore, ADF appears to enhance FGF21 and bile acid (BA) metabolism pathways. Since BAs are significant microbiota metabolites, it is particularly critical to investigate the molecules and mechanisms via which ADF affects the ‘gut microbiota-liver axis’ [Citation14].
FGF19 and FGF21 analogues are being investigated in clinical trials for the management of NAFLD. They have different target tissues and physiological functions [Citation15]. No previous research, to the best of our knowledge, compared the effect of PR, IF, and their combination IP on GM and NAFLD, nor the function of FGF19 and FGF21 in their effect.
Materials and methods
Experimental animals
Male Sprague-Dawley rats, aged 6–8 weeks and weighing 150–200 g, were used in this study. Under a 12-hour light/dark cycle, with free access to water and full veterinary care, the temperature stayed around 23°C. Animals were bred and housed at Mansoura University’s Medical Experimental Research Center (MERC). The local ethics committee authorized all experimental protocols (MDP.21.06.69).
Experimental design
Rats were randomly assigned to one of five groups (eight rats each): the Control group (C): fed on a standard chow diet (48.8% carbohydrate, 21% protein, 3% fat, fibers, minerals, and water) [Citation16], the High fat diet (HFD) group: fed on HFD for 12 weeks (60.98% fat: beef tallow, lard, corn oil, 14.47% protein, 24.55% carbohydrate) [Citation17], the probiotic (PR) group: rats fed on HFD for 12 weeks + probiotics in the last 4 weeks; the intermittent fasting (IF) group: rats fed on HFD for 12 weeks + ADF in the last 4 weeks; and the mixed probiotic and intermittent fasting (IP) group: rats fed on HFD for 12 weeks + both administrations of PR and ADF in the last 4 weeks. MERC provided the diets (standard and high-fat diets), and Bio Schwartz provided the probiotics (40 billion CFU). The capsule was dissolved in distilled water and administered every day for the final 4 weeks of the experiment (1 mL for each rat in the PR and IP groups in the morning with a daily dose of 10 × 109 CFU orally by gastric gavage [Citation18]).
Alternate day fasting protocol
To implement alternate periods of 24-hour fasting and feeding, the intermittent fasting regimen comprises total food deprivation and ad libitum access to water from 10:00 a.m. to 10:00 a.m. the following day [Citation19].
Animal euthanasia and collection of samples
After 12 weeks, 12-hour-fasted rats were sacrificed via thiopental anesthesia overdose. Then, blood samples were collected by cardiac puncture. Following blood collection in test tubes, blood samples were kept at room temperature for 2 h to clot before centrifugation at 1000 r.p.m. for 20 minutes to extract serum samples, which were frozen and maintained at 20 until biochemical analysis. The following tests were performed: lipid profile (cholesterol, triglycerides), liver enzymes [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], fasting insulin, fasting glucose, the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index, LPS, FGF19, and FGF21 levels. Livers were excised, and a very small part of the liver was excised and kept frozen at 80 for liver FGF21 and FMO3 analysis. The rest of the hepatic tissue was fixed in 10% formalin for histopathological examination.
Serum analysis
a) Assay of serum levels of liver enzymes, lipid profile, glucose, insulin, and HOMA index
Aspartate and alanine aminotransferases (AST and ALT) were determined following an enzymatic colorimetric method using a Randox reagent kit (Biomed Diagnostics). Serum triglycerides and total cholesterol concentrations were measured using commercial kits (Biomed Diagnostics). Serum glucose concentration was determined by the glucose oxidase method using an enzymatic kit from Biomed Diagnostics [Citation20]. Serum insulin levels were measured by ELISA insulin kits in rats treated with Abclonal. Homeostatic model of assessment of insulin resistance (HOMA-IR index) = (fasting plasma glucose (mg/dl) × fasting insulin (IU/L))/405.
b) Measurement of serum levels of FGF21, FGF19 and lipopolysaccharides (LPS)
FGF21, FGF19, and LPS concentrations were measured in serum by ELISA kits for rats from Quantikine, Elabscience, and Biorbyt, respectively, following the manufacturer’s instructions.
Tissue analysis
a) Histopathological assessment (hematoxylin and eosin stain)
The liver was dissected into serial sections of 5 μ after being placed in 10% neutral buffered formalin and processed by standard procedure for paraffin embedding. The serial sections were then stained with hematoxylin and eosin (H&E) and examined blindly by a pathologist and scored as follows: lobular inflammation (overall assessment of all inflammatory foci; no foci, score = 0; 2 foci per 200 magnification field, score 1; 2–4 foci per 200 magnification field, score 2; >4 foci per 200 magnification field, score 3); steatosis (<5%, score 0; 5–33%, score 1; >33–66%, score 2; >66% score 3); and hepatocellular ballooning (none, score 0; few balloon cells, score 1; numerous cells/prominent ballooning, score 2) [Citation21].
b) Quantitative real-time PCR of FGF21
Total RNA was extracted from liver tissue lysate using Direct-zol RNA Miniprep Plus (Cat# R2072, ZYMO RESEARCH CORP., USA), and quantity and quality were determined using a Beckman dual spectrophotometer (USA). Thermo Fisher Scientific’s SuperScript IV One-Step RT-PCR Kit (Cat# 12594100, Waltham, MA, USA) was used for reverse transcription of extracted RNA followed by PCR in one step. The primers used are shown in .
Table 1. Primers used in the study for real-time qPCR.
Western blot analysis
Western blot analysis was done using an a polyclonal anti-FMO3 antibody kit purchased from Bio Basic Inc. (Markham, Ontario, L3R 8T4 Canada) and was performed according to the manufacturer’s instructions.
Statistical analysis:
Collected data were represented as Mean ± SD or medians (interquartile range). The analysis of variance (ANOVA) was used to compare parametric data, followed by Tukey’s post hoc analysis. P < 0.05 was considered significant. The Kruskal-Wallis test was used on non-parametric data.
Results
Effect of probiotics and intermittent fasting on body weights, serum liver enzymes, lipid profiles, and insulin resistance
Body weights in rats fed HFD were significantly higher than those on an ordinary chow diet. In comparison to the HFD group, all treated groups had marked reductions in body weight; moreover, intermittent fasting reduced body weights significantly more than probiotics.
HFD significantly increased the serum ALT and AST levels by percentages of 48.4% and 69.2%, respectively, when compared to the (C) group. In comparison to the HFD group, all treated groups had a marked decrease in ALT and AST levels, with no remarkable change between PR, IF, and IP as regards the levels of ALT and AST as shown in and .
Figure 1. Effect of probiotics and Intermittent fasting on HOMA Ir (A), lipid profile (B) and liver enzymes (C) in different groups. C, control group; HFD, high fat diet group; PR, probiotic group; IF, intermittent fasting group; IP, combined intermittent fasting and probiotics group. Parameters described as mean ±SD, Probability significance <0.05. Test used: One way ANOVA followed by Tukey post hoc test except for Homa IR, Kruskal – Wallis was used. asignificance with control group, bsignificance with HFD group, cSignificance with PR group, dSignificance with if group.
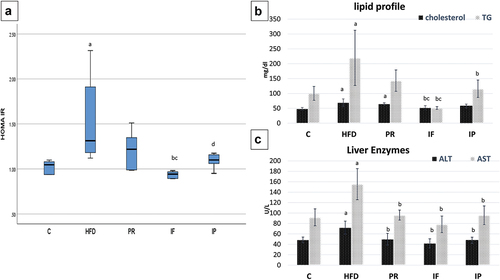
Table 2. Effect of probiotics and intermittent fasting on liver enzymes, lipid profile, insulin resistance in different groups.
Moreover, HFD significantly raised the serum cholesterol (chol) and triglyceride (TG) levels by percentages of 42.6% and 119%, respectively, when compared to the (C) group. Unexpectedly, probiotics didn`t improve chol and TG. Both chol and TG were significantly reduced in the IF group by percentage change (24.9 and 76.7) in relation to HFD. When compared to the HFD group, the IP group had a significant reduction in TG but no discernible change in cholesterol level as shown in and .
HFD significantly increased HOMA-IR when compared to the (C) group by 24.7%. Compared to HFD, Probiotics didn`t improve Homa IR, while IF markedly reduced it by −28.2% percent as shown in and .
Effect of PR, IF and IP on serum levels of LPS, FGF19 and FGF21
LPS were considerably increased with the HFD, three folds as compared to the C group. Compared to the HFD group, PR, IF, and IP reduced LPS level of −36.6%, −42.2%, and −59.6%, respectively. Yet, PR and IF groups had significantly higher LPS levels than C group. Interestingly, their combination IP significantly opposed the HFD impact on LPS level as shown in table [Citation3] and .
Figure 2. Effect of probiotics and intermittent fasting on serum LPS (A), FGF19 (B), FGF 21 (C), FGF21 gene expression (D) and western blotting of FMO3 in liver (E and F) in different groups.C, control group; HFD, high fat diet group; PR, probiotic group; IF, intermittent fasting group; IP, combined intermittent fasting and probiotics group. Parameters described as mean ±SD, Probability significance <0.05. Test used: One way ANOVA followed by Tukey post hoc test. asignificance with control group, bsignificance with HFD group, cSignificance with PR group.
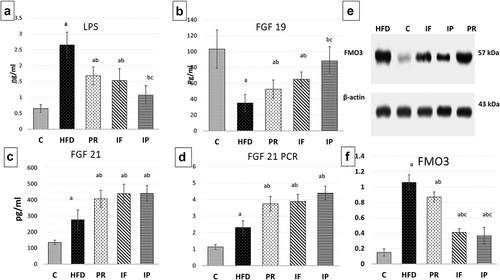
As regards serum FGF19 levels, it was noticeably decreased in the HFD, PR, and IF groups by −66%, −49%, and 37%, respectively, when compared to the C group, with no significant difference between the C and IP groups. The decrease of FGF19 that occurred with HFD was partially counteracted by PR, IF, and, more efficiently, their combination in IP group ( and ).
Table 3. Effect of probiotics and intermittent fasting on LPS, FGF19, and FGF21 in serum, FGF21 gene expression in liver tissues, and western blotting of FMO3 in liver tissues.
There was a significant rise in serum FGF21 levels in HFD, PR, IF, and IP (104%, 200%, 224%, and 224%, respectively) when compared to the C group. PR, IF and IP groups had higher levels of serum FGF21 by percentages of 47%, 59%, and 59%, respectively, in comparison to HFD, with no significant difference among them as shown in Table [Citation3] and .
Effect of PR, IF and IP on FGF21 gene expression in Liver
Using Quantitative Real Time PCR, HFD increased liver FGF21 gene expression one-fold. Surprisingly, the PR, IF, and IP groups increased it by 228%, 240%, and 285%, respectively, compared to normal as shown in Table [Citation3] and .
Effect of PR, IF and IP on FMO3 protein levels in liver
FMO3 protein levels in liver tissues were examined in all studied groups using the western blot method. When compared to the (C) group, HFD increased its level by a factor of six. FMO3 levels in all groups that underwent HFD were still higher than control group. Though PR, IF, and IP partially counteracted the effect of HFD on FMO3 protein levels and reduced its levels by −18%, −61%, and −65%, respectively. There was a significant decrease in FMO3 protein levels in both IF and IP when compared to the PR group, with no obvious difference between the IF and IP groups as shown in table [Citation3] and .
Histopathological study of the liver tissue (H&E)
Microscopic images of H&E-stained hepatic slices from control rats indicate that the hepatic parenchyma, hepatic cords, central veins (CV), portal regions, and sinusoids are normal, as shown in . As shown in ), microscopic images of H&E-stained hepatic sections from HFD rats reveal diffuse ballooning degeneration in hepatocytes (opened arrowhead), focal macrovesicular steatosis (thin arrows), infiltration of many inflammatory cells (mononuclear cells and eosinophils) in portal areas (thick arrow), and aggregates in sinusoids cells (arrowhead). Microscopic images of H&E-stained hepatic sections from (Pr) group rats, as shown in , demonstrate diffuse mild hydropic degeneration in hepatocytes (yellow arrows) and localized ballooning degeneration (opened arrowhead). Microscopic images of H&E-stained hepatic slices from the IF and IP groups indicate almost normal hepatic parenchyma, normal radially organized hepatic cords, and normal CV, as shown in respectively.
Figure 3. Microscopic pictures of H&E-stained hepatic sections from C group (A), HFD group (B,C), Pr group (D), if group (E) and IP group (F). Hepatic sections from control group (A) indicates that the hepatic parenchyma, hepatic cords, central veins (CV), portal regions, and sinusoids are normal. Hepatic sections from HFD group (B,C) show diffuse ballooning degeneration in hepatocytes (opened arrowhead), focal macrovesicular steatosis (thin arrows), infiltration of many inflammatory cells (mononuclear cells and eosinophils) in portal areas (thick arrow), and aggregates in sinusoids cells (arrowhead). Hepatic sections from Pr group (D) show diffuse mild hydropic degeneration in hepatocytes (yellow arrows) and localized ballooning degeneration (opened arrowhead). Microscopic images of H&E-stained hepatic slices from the if and IP groups (E and F respectively) show almost normal hepatic parenchyma, normal radially organized hepatic cords, and normal CV. The HFD group shows a significant increase in NAS which is improved markedly by PR, if and IP. IF and IP show more improvement in NAS than PR group. C, control group; HFD, high fat diet group; Pr, probiotic group; IF, intermittent fasting group; IP, combined intermittent fasting and probiotics group; CV, central veins..
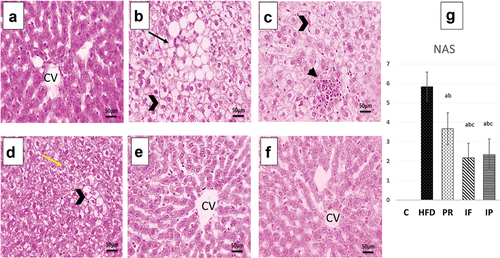
NAFLD activity Score (NAS): The HFD group showed a significant increase in NAS, which was then markedly improved by PR, IF, and IP as shown in .
Discussion
In a rat model fed HFD for 12-week to induce fatty liver, we tried lifestyle intervention via intermittent fasting (IF), gut microbiota modification via probiotics (PR), and their combination (IP) to investigate their effects on NAFLD parameters and their impact on FGF19 and FGF21. The results of this study showed that the 12-week HFD model caused changes in Sprague-Dawley rats that mimicked the full spectrum of histological and metabolic features of human NAFLD [Citation7].
HFD led to a significant rise in the HOMA IR score with no obvious effect of PR, which is consistent with Duseja et al., 2019 [Citation22]. However, HOMA IR was improved after IF, in line with a previous study by Baumeier et al. (2015) [Citation23]. Fasting is thought to be similar to aerobic exercise in many physiological effects [Citation24], as it regulates insulin and glucose tolerance despite the continuation of the diet by increasing insulin receptor sensitivity and stimulating glucose uptake by muscle and liver cells by insulin [Citation25]. Increased β cell survival, enhanced nuclear expression of the pancreatic regeneration marker, attenuated hepatic insulin signaling, and decreased expression of glycogen phosphorylase may all contribute to this impact via restoring autophagic flux in islets [Citation26]. Unexpectedly, IP showed no significant improvement as compared to either IF or PR alone, in agreement with previous studies by Tay et al., 2020, and Mohamad Nor et al., 2021 [Citation27,Citation28].
The results of this study revealed a significant rise in TG and CHOL following HFD that constitutes a typical biochemical picture of dyslipidemia, which can be explained by increased dietary lipid consumption, increased supply of FFAs from insulin-resistant adipose tissue, or accelerated lipogenesis in response to IR [Citation29]. When compared to HFD, PR did not improve cholesterol or TG levels; nevertheless, prior research indicated that lactobacillus strains dramatically lowered blood levels of chol and TG in rats fed HFD, implying that probiotics could treat metabolic problems depending on strain specificity [Citation30]. IF and IP similarly improved the lipid profile; however, adding Probiotics to fasting had no meaningful impact. Many earlier studies reported that IF improved the lipid profile independently of diet [Citation14,Citation26], with no additional benefit of probiotic supplementation [Citation27,Citation28]. One explanation may be that fasting inducs PPARα/FGF21, which have pleiotropic effects on sugar and lipid metabolism, reduces fat accumulation, and improves gut microbiota [Citation31,Citation32].
In the present study, serum ALT and AST, which are important markers of liver injury, were markedly elevated with HFD. The effect of HFD on liver enzymes was markedly counteracted by PR, IF, and IP. Having a protective effect on liver injury, different types of probiotics mitigated the HFD-induced increase of ALT and AST serum levels [Citation33,Citation34] by reducing the effect of pathogenic bacteria on NAFLD development [Citation35]. The beneficial effect of fasting on liver enzymes was documented by earlier studies [Citation14,Citation26], via enhancement of cellular resistance to disease through modulating pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, tumor necrosis factor-α, oxidative stress markers (C-reactive protein and malondialdehyde), and circulating ketones during fasting [Citation36], besides the direct hepatic benefit of fasting, which is closely related to its effect on the gut microbiome [Citation37]. Interestingly, there was no extra advantage to probiotic supplementation with fasting supported by results from previous studies [Citation27,Citation28].
As a low-grade inflammatory state, LPS levels were significantly increased in HFD rats, which were then reduced by PR, IF, and IP. The best effect was in the combined group. It was stated that some species of probiotics significantly reduced the LPS by preventing HFD/LPS-induced systemic inflammation [Citation38]. However, IF is thought to lower plasma LPS through restructuring gut microbiota and producing beneficial metabolites, therefore improving the gut barrier function and alleviating systemic inflammation [Citation5].
As regards FMO3 and its pivotal role in transforming the gut metabolite TMA into TMAO, its tissue expression was markedly increased with HFD, which was improved by PR, IF, and IP with the effect noted more in the combined group. Previous studies revealed that probiotics reduced serum TMA and TMAO via changes in intestinal flora [Citation39]. According to Gnoni et al., 2021, the IF decreased TMAO levels by activating carbohydrate metabolism and glycolysis via modulating the gut microbiome and reversing metabolic disorders [Citation5,Citation40].
FGF19 has an insulin-sensitizing effect, regulates hepatic bile acid, and controls lipid and carbohydrate metabolism [Citation41]; the results of this study showed that HFD significantly decreased FGF19, which was improved by PR, IF, and IP. Interestingly, fasting, whether alone or with PR had the best effect. FGF-19 deficiency in NASH may be due to an unfavorable bile acid composition [Citation42].
FGF21 has direct anti-inflammatory and anti-fibrotic effects and regulates the activation of hepatic stellate cells independent of either insulin resistance or obesity [Citation43]. In this study, both serum FGF21 levels and FGF21 gene expression in liver tissue were in close correlation with each other; FGF21 was significantly increased with HFD, which suggests that FGF21 action may be impaired as a previous study stated that FGF21 receptors were downregulated, like states of hormone resistance in NAFLD [Citation44]. Upregulation of FGF21 is likely an adaptive response to energy disruption in this metabolic state [Citation45]. Surprisingly, FGF21 levels increased more and more in the PR, IF, and IP groups when compared to HFD, with no significant difference among them. In consistent with this result, a study conducted in 2019 by Zhao and his colleagues stated a rise in FGF21 after fructose induced NAFLD especially after probiotics application in the last 4 weeks of the experiment. They proved that FGF21 is required for the beneficial effects of probiotics in regulating the gut-adipose-liver axis to reduce NAFLD [Citation45]. Zhang et al., 2020 declared that IF improved insulin sensitivity via upregulating FGF21 secretion, which downregulates inflammation signaling and activates insulin signaling [Citation14]. Moreover, PPARα plays a central role in the fasting response by directly stimulating the transcription of genes involved in fatty acid oxidation and ketone-body production and inducing the hormone FGF21 in liver [Citation46].
In the present work, HFD for 12 weeks led to a histopathological pattern of NAFLD, where there was mild amelioration in liver histopathology by ingestion of probiotics, indicating a hepatoprotective effect of PR in accordance with previous studies [Citation30,Citation44]. However, IF and IP significantly improved histopathological results as compared to both HFD and PR.
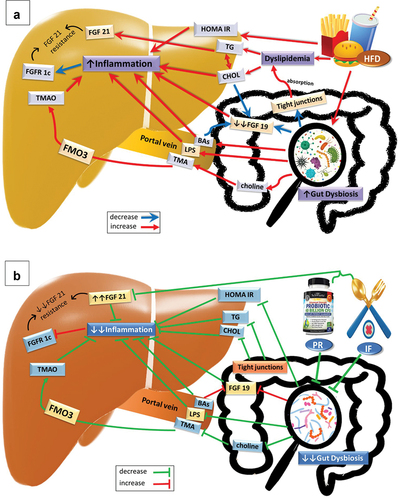
Insulin resistance plays a critical role in the pathophysiology of NAFLD via augmenting fatty acids flow to hepatocytes and increasing hepatic lipogenesis, resulting in mitochondrial damage, endoplasmic reticulum stress, autophagy, the release of pro-inflammatory cytokines and a rise in peroxidation processes [Citation8]. These effects cause hepatic stellate cell activation, which contributes to fibrosis and hepatocellular ballooning. These histopathological changes can lead to fibrosis, cirrhosis, hepatocellular carcinoma, and an increase in mortality from liver-related causes [Citation47]. IF was stated to improve the accumulation of hepatic TG and liver steatosis independent of the diet [Citation14,Citation26], which may be due to improved oral glucose tolerance, restoring peripheral insulin sensitivity and glucose homeostasis, normalizing adipokine and cytokine secretion from adipose tissue, altering the frequency and amount of fatty acid delivery to the liver, and changing the composition of the gut microbiota in a beneficial way, which can reduce inflammatory immune responses [Citation48]. Interestingly, there was no extra benefit from probiotics in addition to fasting; Mohamad Nor et al., 2021, revealed that supplementation with probiotics did not elicit any improvement in the NAS [Citation28]. The underlying NAFLD mechanisms that our study investigated and the effect of PR and IF on them are summarized in .
Figure 4. Graphical abstract of the effect of (A) High Fat Diet (HFD), (B) probiotics (PR) and intermittent fasting (IF) on gut microbiota, FGF19, FGF21 and different parameters in the experiment. Abbreviations; BAs: bile acids, CHOL: cholesterol, FGF19: fibroblast growth factor 19, FGF21: fibroblast growth factor 21, FGFR1c: fibroblast growth factor receptor 1c, FMO3: flavin containing dimethylaniline monoxygenase 3, HFD: high fat diet, HOMA IR: homeostasis model assessment-estimated insulin resistance, IF: intermittent fasting, LPS: lipopolysaccharides, PR: probiotics, TG: triglyceride, TMA: trimethylamine and TMAO: trimethylamine N-oxide..
Conclusions
This study might open new insight into usage of intermittent fasting as an easy, cheap, and noninvasive tool for induction of FGF21 and FGF19 which could be applied widely in different therapeutic fields especially fatty liver and insulin resistance associated with obesity without the need of addition of probiotics.
Acknowledgments
Great thanks to Dr. Walaa Fekry Awadin for the pathological examination and valuable comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466
- Iruzubieta P, Medina JM, Fernández-López R, et al. A role for gut microbiome fermentative pathways in fatty liver disease progression. J Clin Med. 2020;9(5):1369. doi: 10.3390/jcm9051369
- Ni Y, Ni L, Zhuge F, et al. The gut microbiota and its metabolites, novel targets for treating and preventing non‐alcoholic fatty liver disease. Mol Nutr Food Res. 2020;64(17):2000375. doi: 10.1002/mnfr.202000375
- Eslam M, Sarin SK, Wong V-S, et al. Change in antibiotic regimen for emerging multidrug resistance in nosocomial ascitic fluid infection. Hepatol Int. 2020;14(1):1–4. doi: 10.1007/s12072-019-10000-5
- Guo Y, Luo S, Ye Y, et al. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106(1):64–79. doi: 10.1210/clinem/dgaa644
- Trikudanathan G, Vege SS. Current concepts of the role of abdominal compartment syndrome in acute pancreatitis–an opportunity or merely an epiphenomenon. Pancreatology. 2014;14(4):238–243. doi: 10.1016/j.pan.2014.06.002
- Wang Y, Cui S, Zheng J, et al. Berberine ameliorates intestinal mucosal barrier dysfunction in nonalcoholic fatty liver disease (NAFLD) rats. J King Saud Univ. 2020;32(5):2534–2539. doi: 10.1016/j.jksus.2020.03.019
- Carpi RZ, Barbalho SM, Sloan KP, et al. The effects of probiotics, prebiotics and synbiotics in non-alcoholic fat liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review. Int J Mol Sci. 2022;23(15):8805. doi: 10.3390/ijms23158805
- Khan A, Ding Z, Ishaq M, et al. Understanding the effects of gut microbiota dysbiosis on nonalcoholic fatty liver disease and the possible probiotics role: recent updates. Int J Biol Sci. 2021;17(3):818. doi: 10.7150/ijbs.56214
- Moossavi S, Miliku K, Sepehri S, et al. The prebiotic and probiotic properties of human milk: implications for infant immune development and pediatric asthma. Front Pediatr. 2018;197. doi: 10.3389/fped.2018.00197
- Gupta H, Youn GS, Shin MJ, et al. Role of gut microbiota in hepatocarcinogenesis. Microorganisms. 2019;7(5):121. doi: 10.3390/microorganisms7050121
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001
- Tsai M-C, Liu Y-Y, Lin C-C, et al. Gut microbiota dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: a cross-sectional study in Taiwan. Nutrients. 2020;12(3):820. doi: 10.3390/nu12030820
- Zhang H, Zhang W, Yun D, et al. Alternate-day fasting alleviates diabetes-induced glycolipid metabolism disorders: roles of FGF21 and bile acids. J Nutr Biochem. 2020;83:108403. doi: 10.1016/j.jnutbio.2020.108403
- Sciarrillo CM, Keirns BH, Koemel NA, et al. Fibroblast growth factor 19: potential modulation of hepatic metabolism for the treatment of non‐alcoholic fatty liver disease. Liver Int. 2021;41(5):894–904. doi: 10.1111/liv.14802
- Abdul Kadir NAA, Rahmat A, Jaafar HZE. Protective effects of tamarillo (Cyphomandra betacea) extract against high fat diet induced obesity in Sprague-Dawley rats. J Obes. 2015;2015:1–8. doi: 10.1155/2015/846041
- Andrade JMO, Paraíso AF, de Oliveira MVM, et al. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30(7–8):915–919. doi: 10.1016/j.nut.2013.11.016
- Skrypnik K, Bogdański P, Łoniewski I, et al. Effect of probiotic supplementation on liver function and lipid status in rats. Acta Sci Pol Technol Aliment. 2018;17(2):185–192. doi: 10.17306/J.AFS.0554
- Liu H, Javaheri A, Godar RJ, et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy. 2017;13(11):1952–1968. doi: 10.1080/15548627.2017.1368596
- Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22(2):246. doi: 10.1136/jcp.22.2.246-b
- Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x
- Duseja A, Acharya SK, Mehta M, et al. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6(1):e000315. doi: 10.1136/bmjgast-2019-000315
- Baumeier C, Kaiser D, Heeren J, et al. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta (BBA)-Molecular Cell Biol Lipids. 2015;1851(5):566–576. doi: 10.1016/j.bbalip.2015.01.013
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008
- Sharma N, Arias EB, Sequea DA, et al. Preventing the calorie restriction-induced increase in insulin-stimulated Akt2 phosphorylation eliminates calorie restriction’s effect on glucose uptake in skeletal muscle. Biochim Biophys Acta (BBA)-Molecular Basis Dis. 2012;1822(11):1735–1740. doi: 10.1016/j.bbadis.2012.07.012
- de Souza Marinho T, Ornellas F, Barbosa-da-Silva S, et al. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition. 2019;65:103–112. doi: 10.1016/j.nut.2019.02.020
- Tay A, Pringle H, Penning E, et al. PROFAST: a randomized trial assessing the effects of intermittent fasting and Lacticaseibacillus rhamnosus probiotic among people with prediabetes. Nutrients. 2020;12(11):3530. doi: 10.3390/nu12113530
- Mohamad nor MH, Ayob N, Mokhtar NM, et al. The effect of probiotics (MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. 2021;13(9):3192. doi: 10.3390/nu13093192
- Luci C, Bourinet M, Leclère PS, et al. Chronic inflammation in non-alcoholic steatohepatitis: molecular mechanisms and therapeutic strategies. Front Endocrinol. 2020;11:597648. doi: 10.3389/fendo.2020.597648
- Pant R, Sharma N, Kabeer SW, et al. Selenium-enriched probiotic alleviates western diet-induced non-alcoholic fatty liver disease in rats via modulation of autophagy through AMPK/SIRT-1 pathway. Biol Trace Elem Res. 2022;201:1344–1357. doi: 10.1007/s12011-022-03247-x
- Liu X, Zhang Y, Ma C, et al. Alternate-day fasting alleviates high fat diet induced non-alcoholic fatty liver disease through controlling PPARα/Fgf21 signaling. Mol Biol Rep. 2022;49(4):3113–3122. doi: 10.1007/s11033-022-07142-5
- Deng Y, Liu W, Wang J, et al. Intermittent fasting improves lipid metabolism through changes in gut microbiota in diet-induced obese mice. Med Sci Monit Int Med J Exp Clin Res. 2020;26:e926789–1. doi:10.12659/MSM.926789
- Zhao L, Shen Y, Wang Y, et al. Lactobacillus plantarum S9 alleviates lipid profile, insulin resistance, and inflammation in high-fat diet-induced metabolic syndrome rats. Sci Rep. 2022;12(1):15490. doi: 10.1038/s41598-022-19839-5
- Long X, Zeng X, Tan F, et al. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 2020;11(6):5460–5472. doi: 10.1039/D0FO00519C
- Nabavi S, Rafraf M, Somi MH, et al. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97(12):7386–7393. doi: 10.3168/jds.2014-8500
- Faris M, Jahrami H, Abdelrahim D, et al. The effects of Ramadan intermittent fasting on liver function in healthy adults: a systematic review, meta-analysis, and meta-regression. Diabet Res Clin Pract. 2021;178:108951. doi: 10.1016/j.diabres.2021.108951
- Yin C, Li Z, Xiang Y, et al. Effect of intermittent fasting on non-alcoholic fatty liver disease: systematic review and meta-analysis. Front Nutr. 2021;8:709683. doi: 10.3389/fnut.2021.709683
- Zeng Y, Zhang H, Tsao R, et al. Lactobacillus pentosus S-PT84 prevents low-grade chronic inflammation-associated metabolic disorders in a lipopolysaccharide and high-fat diet C57/BL6J mouse model. J Agric Food Chem. 2020;68(15):4374–4386. doi: 10.1021/acs.jafc.0c00118
- Liang X, Zhang Z, Lv Y, et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition. 2020;79:110941. doi: 10.1016/j.nut.2020.110941
- Gnoni M, Beas R, Raghuram A, et al. Potential role of intermittent fasting on decreasing cardiovascular disease in human immunodeficiency virus patients receiving antiretroviral therapy. World J Exp Med. 2021;11(5):66. doi: 10.5493/wjem.v11.i5.66
- Henriksson E, Andersen B. FGF19 and FGF21 for the treatment of NASH—two sides of the same coin? Differential and overlapping effects of FGF19 and FGF21 from mice to human. Front Endocrinol. 2020;11:601349. doi: 10.3389/fendo.2020.601349
- Jiao N, Baker SS, Chapa-Rodriguez A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67(10):1881–1891. doi: 10.1136/gutjnl-2017-314307
- Maratos-Flier E. Fatty liver and FGF21 physiology. Exp Cell Res. 2017;360(1):2–5. doi: 10.1016/j.yexcr.2017.05.006
- Altamimy KM, Alshammari GM, Yagoub AEA, et al. Saudi traditional fermented goat milk protects against experimental non-alcoholic fatty liver disease by hypoglycaemic and antioxidant potentials. Fermentation. 2022;8(12):735. doi: 10.3390/fermentation8120735
- Zhao C, Liu L, Liu Q, et al. Fibroblast growth factor 21 is required for the therapeutic effects of Lactobacillus rhamnosus GG against fructose-induced fatty liver in mice. Mol Metab. 2019;29:145–157. doi: 10.1016/j.molmet.2019.08.020
- Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003
- Montemayor S, Bouzas C, Mascaró CM, et al. Effect of dietary and lifestyle interventions on the amelioration of NAFLD in patients with metabolic syndrome: the FLIPAN study. Nutrients. 2022;14(11):2223. doi: 10.3390/nu14112223
- Morales-Suarez-Varela M, Collado Sanchez E, Peraita-Costa I, et al. Intermittent fasting and the possible benefits in obesity, diabetes, and multiple sclerosis: a systematic review of randomized clinical trials. Nutrients. 2021;13(9):3179. doi: 10.3390/nu13093179
