ABSTRACT
Thymosin beta-10 (TMSB10) and Endocan (END) are key regulators of tumorigenesis. However, limited studies have assessed the effect of chrysin on TMSB10 and END in breast cancer (BC) cell lines. This research aimed to evaluate TMSB10 and END serum levels in BC patients. Moreover, it aimed to evaluate the effect of Chrysin on these tumor markers in MCF-7 cells. Therefore, samples were collected from 50 BC patients to evaluate TMSB10 and END serum levels as a clinical estimation trail using ELISA followed by evaluation for chrysin effect on TMSB10 and END levels as antiproliferative treatment in MCF-7 cells. Serum TMSB10 and END were significantly elevated (P = 0.002 and 0.015) in BC patients than in controls. TMSB10 and END demonstrated a sensitivity of 62% and 60%, and proved to be a prognostic indicator for metastasis at a cut-off value ≥535.6 ng/mL and 720.83 pg/mL in BC. Chrysin inhibited the growth of MCF-7 cells and suppressed the TMSB10 and END levels. Accordingly, TMSB10 and END could be prospective serum biomarkers for tumorigenesis and development of BC, while chrysin might be a promising treatment agent to halt the progression of cancer whose effectiveness needs to be further investigated.
Introduction
Breast cancer (BC) is the second most prevalent malignancy in women worldwide and the second leading cause of cancer-related deaths, with an age-dependent risk increase [Citation1,Citation2]. Survival is the primary concern for BC patients owing to distant metastasis or poor progression, the leading cause of mortality in those patients [Citation3].
Currently, early diagnosis through mammographic screening and the use of systemic adjuvants, besides increased awareness, have reduced the rate of mortality and even metastasis. However, the patient’s quality of life is greatly impacted by the chemotherapy [Citation4]. The risk of metastasis is difficult to be predicted in patients. Nearly 40% of BC patients relapse and eventually die of metastasis. Therefore, new diagnostic and prognostic biomarkers are crucially needed for the early detection of patients at high risk of developing metastasis and thus would aid oncologists in improving the clinical management of the disease [Citation5,Citation6].
The beta-thymosin family mainly includes thymosin beta-4 (TMSB4), thymosin beta-10 (TMSB10), and thymosin beta-15 (TMSB15), which function as intracellular actin-sequestering peptides to hinder the polymerization of actin and disrupt the F-actin formation [Citation7].
TMSB10 regulates cell proliferation and motility, acts as an oncogene and has been confirmed to be overexpressed in furthermost human cancer types [Citation8]. TMSB10 is strongly linked to high-grade aggressive BC, as it promotes tumor progression and metastasis, but the underlying mechanism remains unclear [Citation9]. Furthermore, endocan (END), an endothelial dysfunction biomarker, is a protein produced by endothelial cells, a soluble dermatan sulfate proteoglycan (DSPG). END secretion is managed through the impact of inflammatory mediators and cytokines such as TNFα, IL-1, VEGF-A, and FGF-2 [Citation10–12]. END serum levels have been elevated in some cancers [Citation13,Citation14], and its level correlates positively with tumor relapse and progression. However, there are limited studies on END in BC patients [Citation15].
Several studies have demonstrated that chrysin hinders cancer cell proliferation via cell cycle apoptosis and alteration, microRNA modulation, inhibition of angiogenesis and invasion, metastasis without prompting undesirable side effects and toxicity to normal cells [Citation16,Citation17]. Chrysin is a natural polyphenolic flavone, found in propolis and honey [Citation18], and it plays plentiful bioactivities including anti-allergic, antibacterial, antioxidant, antidiabetic, and anticancer [Citation19–23]. Chrysin also shows selective regulation of cellular pathways correlated to survival, growth, angiogenesis, inflammation, invasion, and metastasis of cancer cells [Citation24]. Hence, it can be involved in BC prevention and therapy as a natural compound, overcoming the acute and long-term side effects caused by chemotherapy that considerably affect the patients’ well-being [Citation25]. Chemo-preventive agents derived from natural source compounds rich in chrysin were investigated as a follow-up treatment for the females with predisposing factors for attaining breast cancer disease [Citation26]. In this study, we aimed to evaluate TMSB10 and END serum levels in BC patients and determine their diagnostic and prognostic values in BC. Moreover, this study, we aimed to evaluate the effect of chrysin on these tumor markers in MCF-7 cell line.
Materials and methods
Aim, design, and setting of the study
This study design is a prospective, consecutive entry. Between February 2018 and March 2019, blood samples were collected from the Oncology Centre. A total of 50 newly diagnosed BC female patients were randomly included in the study. Twelve age-matched healthy women served as controls. Patients with elevated creatinine levels, autoimmune diseases, liver dysfunction or other malignant diseases were excluded from the study. We investigated the role of chrysin as a natural flavone on cell viability to illuminate its antitumor effect using the MCF-7 cell line. Moreover, we evaluated the levels of both TMSB10 and END in MCF-7 cell line after inhibition with chrysin. A comparison was made between the effect of chrysin and a standard chemotherapeutic agent (doxorubicin).
The study purpose as well as its procedures were elucidated to all subjects, and then the informed consent was obtained prior to blood sample collection. All procedures involving human participants in this study were in accordance with the 1964 Helsinki declaration code of ethics, and its later amendments.
Characteristics of participants
Demographic data for all patients were collected at baseline, including age at diagnosis, menstrual status, and contraception use. Clinical data involved date of diagnosis, clinical stage, primary tumor size, number of lymph nodes involved, biopsy, histological sub-type, histological grade, molecular luminal subtype (A and B), estrogen receptor status (ER), progesterone receptor status (PR), HER2 status, Ki-67, surgery, chemotherapy, radiotherapy, and hormonal therapy. Correspondingly, routine biochemical markers were assessed.
Materials and methods description
A standard form was used for all participants to record the history and physical examination findings. Seven milliliters of peripheral blood samples were collected from both patients and controls. Blood samples were centrifuged at 3500 rpm for 20 min to separate serum and then stored at −80°C.
The serum TMSB10 and END levels were measured using a specific quantitative sandwich enzyme linked-immunosorbent assay kit (ELISA) according to the manufacturer’s standard protocol using Human TMSB10 ELISA kit catalog number MBS015949 (MyBiosource, San Diego, CA, USA) and Human Endocan ELISA kit with catalog number #MBS824849 (MyBiosource, San Diego, CA, USA). END levels were expressed as pg/mL, while TMSB10 levels were expressed as ng/mL.
Doxorubicin (Dox 50 mg/25 mL) intravenous vial was purchased from Ebewe Pharma (Unterach, am Attersee, Austria). The tetrazolium dye assay was performed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium-bromide (MTT). It was purchased from SERVA Electrophoresis GmbH (Heidelberg, Germany). DMSO (dimethyl sulfoxide) was obtained from Sigma–Aldrich (St. Louis, MO, USA). Trypsin-Versene (EDTA) Mix (1×), PBS-1× (Phosphate buffered Saline), penicillin–streptomycin stock antibiotic and DMEM (Dulbecco’s Modified Eagle’s Medium) were obtained from Lonza Verviers SPRL, Belgium. Sterile-filtered fetal bovine serum (FBS) was obtained from Life Science Group, UK. All other chemicals were of high analytical grade. Chrysin was purchased from Sigma Aldrich (Darmstadt, Germany) with CAS number c80105-25 G. It was weighted and dissolved in DMSO to prepare a concentration of 6.25, 12.5, 25, and 50 µg/mL.
Breast Cancer Cell Line and cell viability assay:
The MCF-7 cells (Michigan Cancer Foundation-7) were obtained from VACSERA (Vaccine and Sera) holding company, Dokki, Giza, Egypt. Tissue culture flasks, dishes, cryo vials, sterile pipettes and sterile DNase-free tubes were purchased from Greiner Bio-One, Germany. MCF-7 cells were sustained to grow as confluent monolayers in DMEM containing FBS (10%) enhanced with 1% v/v penicillin – streptomycin and incubated at 37°C, 5% CO2, and 95% humidity.
For cell viability assay, MTT assay was performed to determine cell viability according to the cell metabolic ability of tetrazolium salt reduction. In a 96-well plate, MCF-7 cells were seeded at a density of 5 × 103 cells per well. After 24 hours of incubation, media was removed, and a fresh serum-free one which contains 0.1% DMSO was added.
Different concentrations of chrysin were added (6.25, 12.5, 25, and 50 µg/mL). Dox dose was (100 µg/mL) as standard treatment and then a combination of Dox (dose 100 µg/mL) with Chrysin dose (50 µg/mL), and each concentration was done in seven replicates and the whole experiment was repeated three times. After overnight incubation, MTT solution (20 µl) was added to each well. Furthermore, after 4 hours of incubation, MTT-containing medium was carefully aspirated from each well, then cells were treated with 100 μl of DMSO for 90 min. The optical density of solubilized formazan product was determined at wavelength of 450 nm in a microplate Reader (Bio-Tek ELX800, USA). The cell viability was determined compared to that of the control cells.
ELISA Assay for cells: Confluent MCF-7 cells were treated with DMSO as vehicle control, serial concentrations of chrysin (6.25, 12,5, 25, and 50 µg/mL) were used, and standard chemotherapeutic treatment Dox with dose (100 µg/mL), each concentration was done as seven replicates. After incubation for 24 h, cells were trypsinized, then rinsed with PBS, and centrifuged. The cell supernatants were used to determine TMSB10 and Endocan levels using the ELISA technique by following the kits’ instructions.
Statistical analysis
The statistical analysis was conducted using version 23 of the SPSS statistics software (SPSS, Inc., Chicago, IL). In the applied significance statistical tests, two-sided Ps below 0.05 were considered statistically significant. The Kolmogorov–Smirnov test was used to verify the normality of quantitative data. Non-parametric data were expressed as the median (minimum-maximum), whereas normally distributed data were expressed as mean ± standard deviation (SD±). The receiver operating characteristic was carried out using MedCalc statistical software version 12.3, and the Youden index was used to find the cutoff point. The analysis of relapse-free survival was performed using Kaplan–Meier analysis for a total of 50 months of data collection and follow-up for the cases in this study.
Trial registration: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Mansoura University, Faculty of Pharmacy (protocol code 2018–04 and date of approval 28 January 2018).
Results
Clinical part
We measured serum TMSB10 and END levels in both patients with breast cancer and healthy controls, then evaluated the value of these markers. The main tumor characteristics are summarized in the following:
Study Population
Fifty patients with BC were randomly recruited in the current study, along with 12 age-matched healthy female volunteers who served as the control group. Serum levels of ALP and CA 15–3 were measured in healthy controls and BC patients. ALP and CA 15–3 levels showed a significant increase in BC patients than in healthy controls, with the mean values of 73.45 and 60.56 ng/mL for ALP (P = 0.003), as well as median values of 27 and 14 U/mL for CA 15–3 (P = 0.004), respectively, using the independent t-test (.
Table 1. Serum levels of ALK and CA 15–3 biomarkers in BC patients compared to healthy controls.
Tumor characteristics, Tumor therapy and surgical procedure
The included 50 patients were diagnosed with advanced invasive BC, 27 cases (54%) with early stage (Group A: clinical stage I, II) and 23 cases (46%) with advanced stage (Group B: clinical stage III, IV). The mean age of Group A cases was 51.25 years (SD ± 10.14), while Group B cases had a mean age of 53.39 years (SD ± 16.59), using an independent t-test.
As shown in , 25 cases (92.6%) of Group A and 22 cases (95.7%) of Group B had infiltrating duct carcinoma, respectively. Advanced clinical stages (clinical stage III and IV) were found in 52.17% (12 cases) and 47.82% (11 cases) of group B patients, while stages I and II were found in 22.22% (6 cases) and 21 cases (77.7%) of group A patients, respectively. The mean diameter of the largest primary tumor in Group A cases was 2.70 cm (SD ± 1.06); in contrast, Group B subjects had a mean diameter of the largest primary tumor of 3.74 cm (SD ± 2.25). The median number of lymph nodes was found to be three in Group B with a range of (0–24). Using the Pearson chi-square test, the lympho-vascular invasion was present in 14.8% (4 cases) of Group A and in 34.8% (eight cases) of Group B. Regarding the type of therapy, 11 cases in Group A (40.7%) underwent modified radical mastectomy, whilst nine cases in Group B (39.1%) had only diagnostic biopsy. The majority of Group A patients received adjuvant therapy as determined by the Pearson chi-square test.
Table 2. Tumor pathological characteristics, receptor status (ER, PR, HER2neu and Ki-67) and subtypes of early and advanced stages and metastatic patterns in patients.
Recurrence and metastatic pattern, Receptor status and cancer subtypes
Eleven cases in Group B developed local or systemic recurrence with distant metastasis, while none of the Group A patients had distant metastasis. Her2 enriched subgroup was the most prevalent subtypes in both groups; 44.4% (12 cases) in Group A, and 52.2% (12 cases) in Group B cases, Luminal B subtype was 40.7% (11 cases) in Group A and 34.8% (8 cases) in Group B, using the Pearson chi-square test. Receptor status (ER, PR, HER2neu, and Ki-67) and subtypes are summarized in .
Clinical Biomarkers in study cases
The BC patients were classified according to metastatic status; 39 cases showed non-metastatic (78%), and 11 cases showed metastasis (22%). Using the Pearson chi-square test, the median ALP and Ki 67% in sera were considerably higher in the metastatic group than the non-metastatic (P = 0.646 and 0.343, respectively). There was a significant increase in the serum levels of CA15–3 in the metastatic group (166 U/mL) than in the non-metastatic (26 U/mL), with P = 0.037.
Serum Thymosin β-10 (TMSB10)
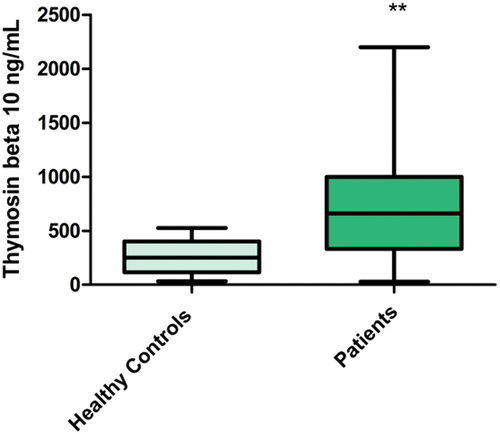
Serum levels of TMSB10 showed a highly significant increase in BC patients (727.52 ng/mL, SD ± 503.93) than in the control group (256.10 ng/mL, SD ± 152.96) with P = 0.002 (mean 727.52, 256.10 ng/mL respectively), . Serum TMSB10 level was considerably higher in Group B (advanced-stage group) (747.60 ng/mL, SD ± 535.47) compared to Group A (early-stage group) (710.41 ng/mL, SD ± 485.08), (P = 0.798). Serum TMSB10 level was higher in metastatic group (mean = 901.23 ng/mL, SD ± 537.58) compared to the non-metastatic group (mean = 678.52 ng/mL, SD ± 490.11), with P = 0.199.
Figure 1. Serum TMSB10 levels in control and BC cases. (**p ≤ 0.01 is considered highly significant compared to control group).
Diagnostic and Prognostic value of TMSB10 in BC patients: Evaluation of the diagnostic and prognostic roles of serum TMSB10 as a biomarker was done by adopting the ROC analysis. Results revealed that serum TMSB10 level could differentiate the BC patients from the healthy individuals at an optimal cutoff point ≥525.6 ng/mL. The TMSB10 diagnostic specificity and sensitivity were found to be 100% and 62%, respectively. The area under the curve (AUC) was 0.827, with P = 0.0001, 95% CI = 0.709 to 0.911, and Z = 6.089, as demonstrated in .
Figure 2. (a) Receiver operating characteristic curve for serum TMSB10 levels (diagnostic curve) in the 50 BC patients, AUC was 0.827, P = 0.0001. (b) Receiver operating characteristic curve for serum TMSB10 levels (prognostic curve comparing patients who achieve partial or complete remission (non-metastatic) and patients who relapsed and have metastasis, AUC was 0.705, P value was 0.0134.
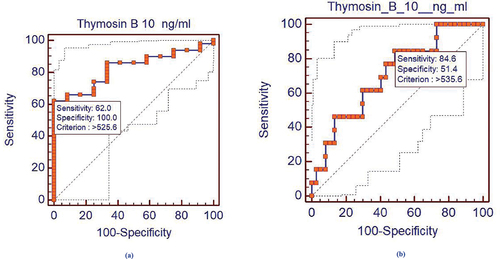
The prognostic cutoff point was evaluated, and the BC patients were classified as having metastasis below or above the TMSB10 prognostic cutoff point (found to be ≥535.6 ng/mL), shown in . Eighteen patients (46.2%) demonstrated partial/complete response (no metastasis), and two patients (18.2%) showed relapse and metastasis. Above the prognostic cutoff value of TMSB10 in sera, 21 patients (53.8%) exhibited partial or complete response (no metastasis), and nine patients (81.8%) demonstrated relapse and metastasis. There was a significant increase in the above cutoff value compared to the below cutoff value groups (P = 0.0134), 95% CI = 0.559 to 0.825, Z = 2.472; .
Table 3. Metastatic compared to non-metastatic cases regarding the prognostic cutoff value of TMSB-10 (ng/ml).
Serum endocan (END)
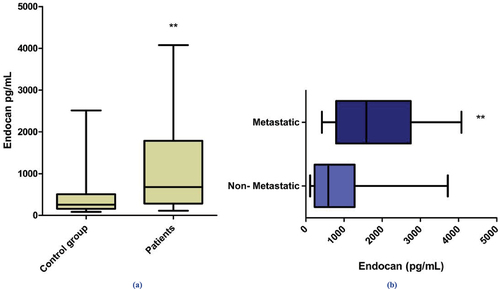
Serum Endocan levels of BC patients (median = 1156.77 pg/mL) showed a highly significant increase compared to that in healthy controls (median = 494.17 pg/mL) with (P = 0.015), . Serum Endocan level was significantly higher in the metastatic group (median = 1585.83 pg/mL, range 420.83–4077.5) compared to the non-metastatic group (median = 587.50 pg/mL, range = 112.5–3717.5), and P = 0.013, using the Mann–Whitney test; .
Figure 3. (a) Serum Endocan levels in breast cancer patients compared to healthy control group. (**p ≤ 0.01 was considered highly significant as compared to control group). (b) Serum Endocan Levels in metastatic patients compared to non-metastatic group. (**p ≤ 0.01 was considered highly significant as compared to non-metastatic group).
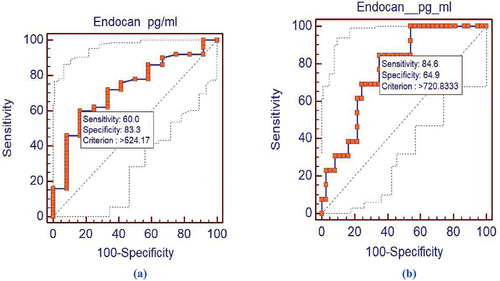
Diagnostic and prognostic value of END in BC patients: The ROC analysis was performed to evaluate the diagnostic and prognostic values of serum END as a biomarker in BC. Results revealed that serum END level could differentiate the BC patients from the healthy individuals at an optimal cutoff point ≥524.17 pg/mL. The END diagnostic specificity and sensitivity were found to be 83.3% and 60%, respectively; the AUC was 0.727, P value = 0.0043, 95% CI = 0.599 to 0.832, and Z = 2.854; .
Figure 4. (a) Receiver operating characteristic curve for serum endocan levels (diagnostic curve) in the 50 BC patients, AUC = 0.727, P value: 0.0043. (b) Receiver operating characteristic curve for serum Endocan levels (prognostic curve for patients who achieve partial or complete remission and patients who relapsed and showed metastasis where AUC = 0.771, P = 0.017.
The BC patients were classified as having metastasis below or above the END prognostic cutoff point, 23 patients (59%) showed partial with no metastasis or complete response, while two patients (18.2%) showed metastasis and relapsed. According to the prognostic cutoff point, the BC patients were classified into two groups regarding metastatic status either below or above the Endocan prognostic cutoff point (≥720.83 pg/mL), as shown in . Above this cutoff value of END in sera, 16 patients (41%) showed partial/complete response, and nine patients (81.8%) showed relapse and metastasis. There was a higher significant value in the above cutoff value compared to the below cutoff value groups (P = 0.017, AUC = 0.771, 95% CI = 0.631 to 0.878, Z = 3.952; .
Table 4. Metastatic compared to non-metastatic cases regarding the prognostic cutoff value of Endocan (pg/ml).
Survival analysis
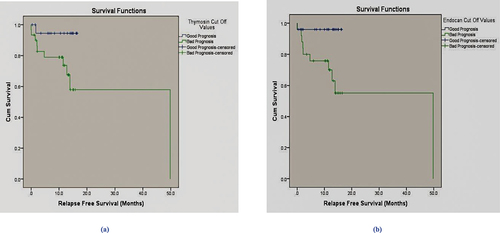
The relapse-free survival was calculated based on the serum TMSB10 and END prognostic cutoff values. Patients with serum levels ≤731.6 ng/mL for TMSB10 and 1099.17 pg/mL for END at diagnosis showed a significant difference in RFS when compared to those with serum levels higher than the corresponding cutoff value (50-month RFS: 78%, P = 0.034 and 0.012 for TMSB10 and END), respectively, using Log Rank (Mantel-Cox), with Chi-square values = 4.509 and 6.331; .
Figure 5. (a) Kaplan Meier relapse free survival analysis (RFS) for serum Thymosin beta-10 (ng/mL), the increase in relapsed cases was significant compared to patients who achieved remission (P = 0.034). (b) Kaplan Meier relapse free survival analysis (RFS) for serum Endocan (pg/mL) overall RFS 78%, the increase in relapsed cases was significant compared to patients who achieved remission (P = 0.012).
In-vitro results: breast cell line part
Cell Viability
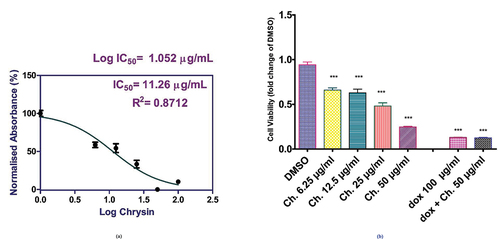
Chrysin was applied to MCF-7 cells in a variety of doses (6.25, 12.5, 25, and 50 µg/mL), along with 100 µg/mL of doxorubicin, to test the viability of the cells. Moreover, a combination of Dox 100 µg/mL with chrysin dose 50 µg/mL and when compared to DMSO-treated cells, the DMSO group mean value was 0.9420- SD ± 0.0750, and in the chrysin groups according to doses, mean values were 0.6590 µg/mL – SD ± 0.0652, 0.6286 µg/mL – SD ± 0.107, 0.4811 µg/mL – SD ± 0.093 and 0.2467 µg/mL – SD ± 0.018, respective to chrysin doses. The Dox group (100 µg/mL) mean value was 0.1293 µg/mL- SD ± 0.0054, while regarding its combination with a chrysin of dose 50 µg/mL, the mean value was 0.1243 µg/mL- SD ± 0.0127. The cell viability was significantly inhibited in chrysin-treated cells in a dose-dependent manner, with a P value <0.0001. The cytotoxic effects of chrysin on MCF-7 cells reduced the cancer cell viability to 50% (IC50 value = 11.26 μg/ml), .
Figure 6. (a) Potency of chrysin in different doses on MCF-7 cell viability (IC50). (b) Cell viability assay (effect of chrysin treatment with different doses or in combination with doxorubicin (Dox) compared to DMSO on MCF-7 cellular viability. (DMSO: dimethyl sulfoxide; Ch: chrysin; Dox: doxorubicin). *p < 0.05 was considered significant. ***p < 0.001 was considered highly significant.
The TMSB-10 and END levels in MCF-7 cancer cells
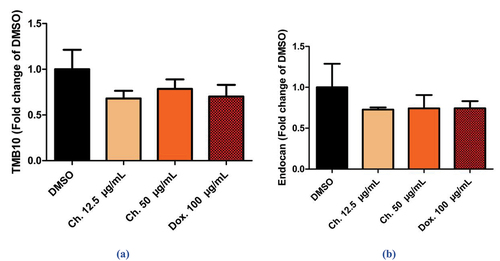
The results revealed that the decrease in levels of TMSB10 and END in cells treated with Chrysin was equivalent to that observed in Dox-treated cells. MCF-7 cells were treated with 100 µg/mL of Dox dose for 24 h (mean = 0.7025 µg/mL, SD ± 0.2544). Cells exhibited a decrease in the TMSB10 levels, as fold decrease when compared to vehicle-treated control cells.
As regard treated cells with chrysin doses of 12.5 and 50 µg/mL for 24 h showed a dose-dependent inhibition by 68.044% and 78.51% in TMSB10 levels as fold decrease, and this was equal to the decrease (70.24%) caused by Dox dose when compared to control cells with mean = 0.6804 µg/mL, SD ± 0.1689, and = 0.7851 µg/mL, SD ± 0.2088 respectively, with P value = 0.3980, . Chrysin also showed a dose-dependent decrease in END levels by 72.80% and 74.26%, comparable to that decrease observed in Dox-treated cells (74.47%) when compared to control cells (mean = 0.7280 µg/mL, SD ± 0.0529, = 0.7427 µg/mL, SD ± 0.3264, 0.7448 µg/mL, SD ± 0.1712) upon treatment with chrysin doses (12.5 and 50 µg/mL) and Dox dose of 100 µg/mL, respectively, P value = 0.6365, .
Discussion
Finding a medication that has the lowest amount of toxicity and the highest level of effectiveness is the most significant challenge in BC management regimens. Plenty of research works are now focusing on the naturally derived and dietary compounds in an effort to develop a novel and more potent treatment approach for patients with BC. Natural compounds can combat BC aggressiveness, modulate cancer-related pathways, and suppress the proliferation of cancerous cell [Citation25].
The effects of serum TMSB10 and END levels on RFS, as well as their sensitivity and specificity, diagnostic value, and estimation of response outcomes in BC patients, were examined in this study. Chrysin’s potential as a natural anticancer drug for the treatment of MCF-7 breast cell line was another goal of the study.
TMSB10 overexpression is typically associated with some cancers, for instance BC, hepatocellular carcinoma, and thyroid carcinoma. Therefore, TMSB10 May be considered a potential prognostic biomarker [Citation27]. According to Santelli et al. study’s findings, high amounts of TMSB10 were discovered in the sera of patients who had been exposed to a variety of carcinogens and tumor cell lines [Citation28]. In this study, serum TMSB10 levels were significantly higher in BC patients than in the control group, and higher in the advanced-stage group compared to the early-stage group, also in the metastatic group compared to the non-metastatic group. These findings corroborated a prior study finding that serum TMSB10 levels were elevated in BC patients and linked favorably with clinical stages, prognosis, and distant site metastases in BC patients [Citation27].
In cancer patients, the serum protein END exists in the polysaccharide form. END holds a core protein that composed of 165 amino acids and a mono-mucopolysaccharides chain linked covalently to the 137th serine residue (DS chains), which is a vital constituent of the functional structure of END [Citation29,Citation30]. Numerous studies have identified high END expression in uterine cancer, BC, and other tumors. Furthermore, prognosis of cancer, angiogenesis and metastasis was confirmed to be allied with END expression. Most studies showed that END modulates cancer by tumor-related inflammation, angiogenesis, lymph-angiogenesis and the tumor cells themselves. Subsequently, Endocan may be a promising target for cancer prognosis and diagnosis [Citation12,Citation31–33]. Consistent with previous researches, this study demonstrated that END levels in the sera of BC patients were significantly higher than those of healthy controls.
To the best of our knowledge, no previous research has studied diagnostic and prognostic cutoff values of TMSB10 and END in BC. Therefore, TMSB10 and END were selected for subsequent exploration in the current study. According to the current study, the diagnostic cutoff value of serum TMSB10 levels in BC patients was ≥525.6 ng/mL, with 62% selectivity and 100% specificity. It also showed that evaluating the serum level of END was considered an excellent diagnostic marker in BC, as a serum level ≥524.17 pg/mL was considered a cutoff point with 60% selectivity and 83% specificity. The test proved that evaluating TMSB10 and END levels in sera is highly significant in the diagnosis of BC patients. The optimal cutoff level that harvests the optimum classification of BC patients according to the response to therapy in the two main groups was recognized at serum levels ≥731.6 ng/mL for TMSB10 and ≥720.83 pg/mL for END. We found that levels above the cutoff value of TMSB10 and END were negatively associated with response outcomes. These findings indicated that more research into the predictive significance of TMSB10 and END levels on the response outcome is necessary.
The number of relapsed cases that were above the prognostic cutoff values of TMSB10 and END and whom developed metastases with an overall RFS of 78% significantly increased when we evaluated the link between Relapse-free Survival Analysis and the prognostic cutoff values.
A study by Zhang et al. [Citation28] found that TMSB10 was upregulated in BC cells. As well, overexpression of TMSB10 stimulates, while silencing of it suppresses proliferation, invasion, and migration of BC cells in in-vitro and in-vivo. Moreover, Samarghandian et al. study [Citation34] found that chrysin had an antiproliferative effect on human MCF-7 cells. The study found that chrysin may have strong anticancer action because it showed growth inhibition of the BC cells and, according to flow cytometry, caused cancer cells to undergo apoptosis.
The current results from the in-vitro MCF-7 cells study confirmed the potent antitumor activity of chrysin, causing a significant dose-dependent reduction in cellular viability. Chrysin treatment demonstrated that in the combination of Dox with a chrysin dose of 50 µg/mL, the viability was inhibited significantly compared to the vehicle-treated cells. Furthermore, chrysin treatment and Dox treatment independently inhibited MCF-7 cell growth and caused comparable suppression in both TMSB10 and END levels. Likewise, the decrease caused by treatment with chrysin was equivalently effective as that caused by Dox-treated cells when compared to vehicle-treated control cells. All these results were further reinforced by the dose-dependent cytotoxic activity of chrysin treatment in MCF-7 cells, as indicated by MTT assay.
A recent study (2022) proposed the use of chrysin as a combination with metformin drug in different ratios in micellar form for the treatment of BC to achieve a synergistic delivery of this combination. The results showed suppression of proliferation and a significant increase in the apoptosis rate in T47D cells (a human breast cancer cell line). The combination with chrysin effectively downsized the tumor volume and mass, suggesting a promising treatment procedure for BC [Citation35].
Consequently, the current findings suggest that chrysin was able to reduce the levels of TMSB10 and END in MCF-7 cells, to the same extent as the traditional chemotherapy (Doxorubicin). Both are tumorigenic biomarkers and are associated with high-grade aggressive BC. Likewise, chrysin effectively decreased cancer viability in MCF-7 cells, which may be due to multiple mechanisms including antiproliferative activity, reduction of both oncogenes TMSB10 and END levels, suggesting that it may be a promising BC therapy with the minimal side effects than chemotherapeutics.
In conclusion, the current study first demonstrated the diagnostic value of TMSB10 and END in the development and progression of BC tumors. The findings showed that MCF-7 cells and the sera of BC patients both had considerably higher amounts of TMSB10 and END serum. Poor prognosis, tumor growth and metastasis were enhanced by high serum levels of TMSB10 and END in BC patients. TMSB10 and END may also be thought of as potential therapeutic targets in the future. This study suggests that chrysin, a natural flavone with antiproliferative properties, could have therapeutic benefits in BC and might be considered in future studies. To confirm these findings, nevertheless, longer follow-up clinical trials and future multicentric investigations with larger sample sizes are needed.
Author contributions
N. H., Y. S., Z. E. and A. E. contributed to the conceptualization and study design and performed investigation and validation. N. H. and Z. E. performed the analysis and interpretation of the patients’ data regarding breast cancer disease; N.H. and Y. S. performed analysis, data curation, formal analysis and interpretation of data regarding the cell-line part.
N. H., Y. S. and A. E. performed the methodology. N. H. provided and funded the study resources, writing and editing the original draft. Y. S., Z. E. and A. E. performed the research supervision, reviewing the draft writing. All authors read and approved the final manuscript.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Mansoura University, Faculty of Pharmacy (protocol code 2018–04 and date of approval 28 January 2018).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [N.E.H.], upon reasonable request.
Additional information
Funding
References
- Sharma GN, Dave R, Sanadya J, et al. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Ca A Cancer J Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254
- Gerber B, Freund M, Reimer T. Recurrent breast cancer: treatment strategies for maintaining and prolonging good quality of life. Dtsch Arztebl Int. 2010;107(6):85. doi: 10.3238/arztebl.2010.0085
- Weigelt B, Peterse JL, van’t Veer LJ. Van’t Veer LJJNrc: Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670
- Milas L, Peters LJ, Ito H. Spontaneous metastasis: random or selective? Clinical & experimental metastasis. Clin Exp Metastasis. 1983;1(4):309–315. doi: 10.1007/BF00121193
- Giavazzi R, Alessandri G, Spreafico F, et al. Metastasizing capacity of tumour cells from spontaneous metastases of transplanted murine tumours. Br J Cancer. 1980;42(3):462–472. doi: 10.1038/bjc.1980.259
- Safer D, Golla R, Nachmias VT. Isolation of a 5-kilodalton actin-sequestering peptide from human blood platelets. Proc Nat Acad Sci. 1990;87(7):2536–2540. doi: 10.1073/pnas.87.7.2536
- Sribenja S, Li M, Wongkham S, et al. Advances in thymosin β10 research: differential expression, molecular mechanisms, and clinical implications in cancer and other conditions. Cancer Invest. 2009;27(10):1016–1022. doi: 10.3109/07357900902849640
- Bouchal P, Dvořáková M, Roumeliotis T, et al. Combined proteomics and transcriptomics identifies carboxypeptidase B1 and nuclear factor κB (NF-κB) associated proteins as putative biomarkers of metastasis in low grade breast cancer. Molecular & Cellular Proteomics. 2015;14(7):1814–1830. doi: 10.1074/mcp.M114.041335
- Lassalle P, Molet S, Janin A, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271(34):20458–20464. doi: 10.1074/jbc.271.34.20458
- Sarrazin S, Adam E, Lyon M, et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta (BBA)- Rev Cancer. 2006;1765(1):25–37. doi: 10.1016/j.bbcan.2005.08.004
- Yang J, Yang Q, Yu S, et al.: Endocan: A new marker for cancer and a target for cancer therapy. Biomedical reports 2015, 3(3):279–283.
- Lv Z, Fan Y, Chen H, et al. Endothelial cell-specific molecule-1: a potential serum marker for gastric cancer. Tumor Biol. 2014;35(10):10497–10502. doi: 10.1007/s13277-014-2319-9
- El Behery MM, Seksaka MA, Ibrahiem MA, et al. El Alfy Y: Clinicopathological correlation of endocan expression and survival in epithelial ovarian cancer. Arch Gynecol Obstetrics. 2013;288(6):1371–1376. doi: 10.1007/s00404-013-2863-3
- Ates O, Gedik E, Sunar V, et al. Serum endocan level and its prognostic significance in breast cancer patients. J Oncological Sci. 2018;4(1):15–18. doi: 10.1016/j.jons.2017.11.004
- Mohammadian F, Abhari A, Dariushnejad H, et al. Upregulation of Mir-34a in AGS gastric cancer cells by a PLGA-PEG-PLGA chrysin nano formulation. Asian Pac J Cancer Prev. 2016;16(18):8259–8263. doi: 10.7314/APJCP.2015.16.18.8259
- Mohammadian F, Pilehvar-Soltanahmadi Y, Alipour S, et al. Chrysin alters microRnas expression levels in gastric cancer cells: possible molecular mechanism. Drug Res (Stuttg). 2017;67(9):509–514. doi: 10.1055/s-0042-119647
- Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nanomedicine, biotechnology: An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artific Cells Nanomed Biotechnol. 2018;46(4):706–716. doi: 10.1080/21691401.2017.1337022
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artificial cells, nanomedicine, and biotechnology. Artific Cells Nanomed Biotechnol. 2017;45(6):1201–1206. doi: 10.1080/21691401.2016.1216854
- Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artific Cells Nanomed Biotechnol. 2018;46(4):706–716. doi: 10.1080/21691401.2017.1337022
- Deldar Y, Zarghami F, Pilehvar-Soltanahmadi Y, et al. Antioxidant effects of chrysin-loaded electrospun nanofibrous mats on proliferation and stemness preservation of human adipose-derived stem cells. Cell and tissue banking. Cell Tissue Bank. 2017;18(4):475–487. doi: 10.1007/s10561-017-9654-1
- Chen Y-H, Yang Z-S, Wen C-C, et al. Evaluation of the structure–activity relationship of flavonoids as antioxidants and toxicants of zebrafish larvae. Food Chem. 2012;134(2):717–724. doi: 10.1016/j.foodchem.2012.02.166
- Ahad A, Ganai AA, Mujeeb M, et al. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol. 2014;279(1):1–7. doi: 10.1016/j.taap.2014.05.007
- Rasouli S, Zarghami N. Synergistic growth inhibitory effects of chrysin and metformin combination on breast cancer cells through hTERT and cyclin D1 suppression. Asian Pac J Cancer Prev: APJCP. 2018;19(4):977. doi: 10.22034/APJCP.2018.19.4.977
- Abotaleb M, Samuel SM, Varghese E, et al. Flavonoids in cancer and apoptosis. Cancers. 2019;11(1):28. doi: 10.3390/cancers11010028
- Balam FH, Ahmadi ZS, Ghorbani AJH. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): a systematic review. Heliyon. 2020;6(3):e03557. doi: 10.1016/j.heliyon.2020.e03557
- Zhang X, Ren D, Guo L, et al. Thymosin beta 10 is a key regulator of tumorigenesis and metastasis and a novel serum marker in breast cancer. Breast Cancer Res. 2017;19(1):15. doi: 10.1186/s13058-016-0785-2
- Santelli G, Califano D, Chiappetta G, et al. Thymosin β-10 gene overexpression is a general event in human carcinogenesis. Am J Pathol. 1999;155(3):799–804. doi: 10.1016/S0002-9440(10)65178-4
- Leroy X, Aubert S, Zini L, et al. Vascular endocan (ESM‐1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology. 2010;56(2):180–187. doi: 10.1111/j.1365-2559.2009.03458.x
- Sarrazin S, Lyon M, Deakin JA, et al. Characterization and binding activity of the chondroitin/dermatan sulfate chain from Endocan, a soluble endothelial proteoglycan. Glycobiology. 2010;20(11):1380–1388. doi: 10.1093/glycob/cwq100
- Celik ZE, Demir F, Yonar H, et al. Association of endocan expression with clinicopathological prognostic parameters in breast carcinoma. Cancer Biomarkers. 2021;2021(4):1–7. doi: 10.3233/CBM-201026
- Basim P, Argun D. A comparison of the circulating endocan levels between the inflammatory and malignant diseases of the same organ: the breast. J Invest Surg. 2021;2020(11):1–7. doi: 10.1080/08941939.2020.1792008
- Yang Y-C, Pan K-F, Lee W-J, et al. Circulating proteoglycan endocan mediates EGFR-driven progression of non–small cell lung cancer. Cancer Res. 2020;80(16):3292–3304. doi: 10.1158/0008-5472.CAN-20-0005
- Samarghandian S, Azimi-Nezhad M, Borji A, et al. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Phcog Mag. 2016;12(Suppl 4):S436. doi: 10.4103/0973-1296.191453
- Luo D, Wang X, Zhong X, et al. MPEG-PCL nanomicelles platform for synergistic metformin and chrysin delivery to breast cancer in mice. Anticancer Agents Med Chem. 2022;22(2):280–293 doi: 10.2174/1871520621666210623092725