ABSTRACT
Probiotics are beneficial for certain illnesses, and a variety of clinical studies have reported remarkable effects in the cutaneous system. The objective of current study was to test the antimicrobial impact of Lactobacillus plantarum-ATCC8014 filtrate and lyophilized cells on various pathogenic bacterial strains isolated from skin. The filtrate and lyophilized cell solution of L. plantarum-ATCC8014 showed various impacts on isolated bacteria from the skin. L. plantarum-ATCC8014 filtrate has the highest inhibition zones versus all tested bacteria compared to lyophilized cell solution, whereas it showed the highest action toward S. aureus which had been selected to induce infection, as well as comparing the anti-inflammatory impact of both forms. Five animal groups were tested after the induction of a Staphylococcus aureus wound infection model and monitored for comparative evaluation of the beneficial impact of L. plantarum-prepared forms in the healing process. L. plantarum filtrate has higher antibacterial action compared to lyophilized cells as well as an anti-inflammatory value of 12.7 ± 2.1 µg/ml. Furthermore, L. plantarum filtrate accelerates wound healing, which is indirectly related to animals’ body weight. Microbiological counting of bacterial load in skin and internal organs, including the liver and spleen, revealed that treatment with L. plantarum filtrate led to a notable reduction in bacterial load and prevented its dissemination. Histological examination confirmed the impact of the prepared filter in controlling scars and normalized mast cells, which regulate inflammation. Inflammatory cytokines and oxidative enzyme testing in all groups reflected the protective roles of L. plantarum filtrate with minimal burden on body functions.
Introduction
Lactobacillus plantarum is a probiotic strain with a diverse range of health advantages. Lactobacillus plantarum has various beneficial health effects, such as antimicrobial activity, anti-obesity, and can also improve skin health or combat disease, immune-boosting and anti-inflammatory effects [Citation1]. Inflammation is an immunity-mediated response to viral or pathogenic infection, toxic compounds, or irradiation. Probiotics has ability to regulate it by pro-inflammatory mediators (e.g. COX-2) and cytokines (e.g. TNF-α, IL-1β, and IL-6), resulting in the recruitment of immune cells (e.g. macrophages) and systemic responses. Probiotics are frequently delicate to changes in temperature, humidity, and high oxygen levels, which could significantly degrade their effectiveness while being stored or delivered. Earlier research has raised questions about the safety of probiotic usage in persons with weakened immune systems. Alternatives like prebiotics, synbiotics, and paraprobiotics have lately been proposed as a means of addressing these worries [Citation2]. Particularly, interest has been focused on paraprobiotics, which are immobilized microbiological cells or cell fragments that can benefit the host. Postbiotics, which concentrate on the constituents (components or decomposition products), generated by living bacteria or produced after bacterial disintegration, have lately evolved and share nomenclature with Para probiotics. Recent research has shown that probiotic lysates primarily affect the skin, such as reducing atopic dermatitis. For psychological health and an overall higher quality of life, skin health is crucial. Additionally, it is essential for preserving healthy skin functions and avoiding a number of skin illnesses [Citation3–8].
Staphylococcus aureus is one of the most common and significant bacterial species of Gram-positive bacteria that are present in skin and can enter the bloodstream [Citation9]. Staphylococcus spp. can tolerate the salt content of sweat and ingest urea as a nitrogen source, and they have a variety of strategies to stay on the skin. Various Staphylococcus spp. may also release enzymes that destroy skin layers and adherents that strengthen their adherence to the skin [Citation10,Citation11]. Thirty percent of people worldwide have skin infections from Staphylococcus aureus. In clinical settings, it is a relatively prevalent bacterium among patients. Getting rid of S. aureus in surgical patients reduces susceptibility to invasive infections. Due to the bacterium’s antimicrobial resistance, alternative treatments are a growing strategy in this field of study [Citation12].
The biological mechanism of wound healing has several facets and involves both extracellular and intracellular components in large numbers. Hemostasis, inflammation, proliferation, and maturation are the four stages of healing that play a crucial role in skin remodeling after injury [Citation13]. According to the statistical assessment of chronic wounds, caring for and treating the injury takes a lot of time and money each year Although there are antibiotic medicines available for normal wound care and maintenance, they do not address all aspects of healing [Citation14]. Six different LAB, named Lactobacillus paracasei SGL 04, Lactobacillus plantarum SGL 07, Lactobacillus fermentum SGL 10, Lactobacillus brevis SGL 12, Lactobacillus casei SGL 15 and Lactobacillus salivarius SGL 19 May 2001improve the wound healing process and exert anti-inflammatory and anti-pathogenic effects. On the other hand, it improves skin barrier function in a reconstructed human epidermis model [Citation15]. In the current report antibacterial, anti-inflammatory and wound healing processes have been tested for Lactobacillus plantarum-ATCC8014 lyophilized form as well as its filtrate.
Materials and methods
Microbiological cultures
Lactobacillus plantarum ATCC8014 were purchased from American-type culture collection and it cultured in MRS broth at 37°C for 24 hr. Filtrates were obtained and sterilized in 0.01µ membrane filter (Millipore, Sigma, USA), Lactobacillus plantarum ATCC8014 was also lyophilized and kept in −80°C. Thus, both filtrate and lyophilized cells were kept for further examination. The lyophilized solution was prepared by dissolving 50 mg of lyophilized cells in 1 ml distilled water.
A total of 50 specimens were collected from small breaks of skin of patients admitted to El Mansoura University Hospital between August 2021 and December 2021. The bacterial cultures were incubated at 37°C and differentiated using gram staining and further identified using Vitek-2 system.
Antibacterial activity of lactobacillus plantarum ATCC8014
The agar well diffusion experiment was used to evaluate Lactobacillus plantarum ATCC8014 filtrate and lyophilized cells solution against isolated and identified bacterial cells including Escherichia coli, Bacillus subtilis, Micrococcus luteus, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhimurium [Citation16,Citation17].
Anti-inflammatory assay
The anti-inflammatory capacities of the solution of lyophilized cells of Lactobacillus plantarum ATCC8014 as well as its filtrate against reference drug (ibuprofen) were determined according to Granica et al. [Citation18] with some adjustments, by inhibiting the L.O.X enzyme (lipoxygenase enzyme) from Glycine max (type I-B). Briefly, 1.0 to 120 g/ml of the sample were combined with 100 l of soybean L.O.X. solution (1000 U/ml in borate buffer solution, pH 9) and 200 l of borate buffer in 96-well plates for 10 min at 30°C. To begin the reaction, 100 l of linoleic acid (the substrate) was pre-incubated with the samples. Using a microplate reader, the inhibitory action was discovered by observing the rise in absorbance at 234 nm (BioTek, U.S.A.).
Animals
Nine-week-old rats weight (120–150 gm weight) were purchased from Animals house in Faculty of Science-Al Azhar University. Animals were placed in separately ventilated cages with regulated moisture (30–70%), light cycle (12:12), and temperatures (24–25°C). Pelletized food and water were available at all times. The animals were randomly assigned before the experiment began, and the rats were kept for a week before the trial to acclimate. The animal study was approved by ethical committee in The Regional center for Mycology and Biotechnology – Al azhar University (RCMB01082021).
Testing for infected wound healing
A wound healing assay for infected wound was used to assess the safety of both filtrate and solution of lyophilized cells [Citation19]. Twenty-four hours before the test, the animals’ dorsal backs were shaved to eliminate hair without harming the outer layer of skin using isoflurane as gas anesthesia. In accordance with the treatment plan, the rats were separated into five groups (N = 3), each of which had three rats: Group I: contains 10% sodium lauryl sulfate as a negative control.
Wound
Rats were anesthetized by ketamine hydrochloride (50 mg/kg body weight), a part of the dorsal hair has been shaven each animal. Using a sterile biopsy punch, a full-thickness excision wound of 3 cm in diameter was made in the animal’s dorsal portion skin).
Bacteria
Staphylococcus aureus has been selected as the most affected bacterial isolate by Lactobacillus plantarum filtrate and lyophilized cells solution.
Group II
The wound was performed and infected with 0.5 ml of 1 × 103 Staphylococcus aureus as a positive control, Group III: where wound was performed and infected with 0.5 ml of 1 × 103 Staphylococcus aureus and surface treated with 1 ml Lactobacillus plantarum-ATCC8014 1 ml of filtrate were applied twice daily for 21 days, and Group IV: where the wound was performed and infected with 0.5 ml of 1 × 103 Staphylococcus. aureus and surface treated with Lactobacillus plantarum- ATCC8014, 1 ml solution (0.5 gm of lyophilized cells dissolved in 1 ml deionized sterile water) of lyophilized cells twice daily for 21 days. Group V: where wound was performed and infected with 1 × 103 Staphylococcus aureus given surface treatment by chloramphenicol (2.8 mg/kg) treatment and given individual housing. Photographs of the injuries were obtained at 21 days, and image was used to compute the wound area. Additionally, body weights were recorded at 7, 14 and 28 days.
Bacterial count
To check bacterial load: at the end of experiment animals were anesthetized and sterile swabs were used to collect samples from skin-infected wound areas of the different groups and cultured on mannitol salt agar. Furthermore, animals were sacrificed using cervical dislocation liver and spleen from different animal’s groups were collected and processed then cultured on mannitol salt agar [Citation20,Citation21].
Histopathological analysis
The tissues from formalin-fixed wounds were then cleaned in xylene, molded in paraffin, and dehydrated with graded alcohol. Hematoxylin and eosin was used to produce stain sections that were 4–5 µm thick (H&E) general skin structure, and histological changes in the epidermis and dermis as well as toluidine blue dye were used to examine mast cell variations in different groups [Citation22,Citation23].
Mediators analysis
Before killing the rats were anaesthetized, and blood samples were collected from the retro-orbital plexus and allowed to clot and centrifuged at 6000 rpm for 10 min at 5°C in heparinized Eppendorf tubes. Serum was then gathered and stored at −80°C pending analysis. IFN-γ, IL-4, TNF-α, and IL-17 serum levels were measured using kits from Abcam (U.S.A.) in accordance with the manufacturer’s instructions [Citation24].
Enzymes assay
At the end of the experiment, antioxidant enzyme of superoxide dismutase (SOD), and Lipid peroxidation of Malondialdehyde (MDA) were measured in serum of different animal groups [Citation25].
Biochemical analysis
The blood samples were collected from different groups at the end of experiment and serum were obtained and stored −80°C until ready for further analysis. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine and Blood Urea Nitrogen (BUN) were measured according to the instructions of the manufacturer’s kits (Human diagnostic company, Germany) [Citation26].
Statistical analysis
The mean and standard deviation of mean (SEM) of the results are given. One-way ANOVA was applied to analyze statistical variances, and Tukey’s test was used for post-hoc analysis with a significance level of p ≤ 0.05. The Graph pad prism Version 5 was used to conduct statistical analysis (San-Francisco, USA).
Results
Bacterial isolates and antimicrobial results
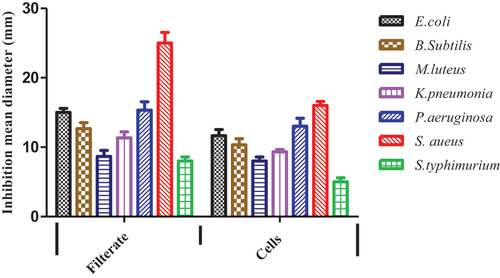
Seven different bacterial species were obtained from skin breaks of patients and have been identified as following: Escherichia coli, Bacillus subtilis, Micrococcus Letus, Klebsiella pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhimurium Furthermore, tested filtrate and lyophilized cells solution of L. plantarum-ATCC8014 showed various impacts toward isolated bacteria from skin. It could be noticed that L. plantarum-ATCC8014 filtrate has the highest inhibition zones versus all tested bacteria compared to lyophilized cells solution whereas it showed the highest action toward S. aureus which had been selected to induce infection in vivo studies as depicted in ().
Testing of L. plantarum-ATCC8014 lyophilized cells solution and filtrate on lipoxygenase enzymatic action
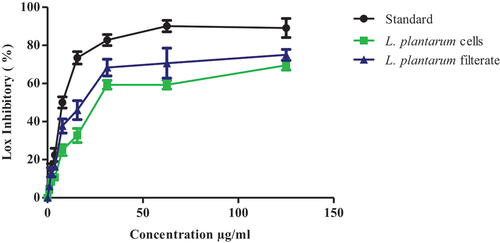
Anti-inflammatory action of Lactobacillus plantarum ATCC8014 samples was tested using lipoxygenase enzymatic inhibition where L. plantarum-ATCC8014 lyophilized cells solution showed good inhibition of lipoxygenase action with IC50 value 13.3 ± 2.1 µg/ml, while IC50 = 12.7 ± 2.1 µg/ml for L. plantarum-ATCC8014 filtrate versus standard dug IC50 value of 1.3 ± 1.1 µg/ml as shown in ()
Figure 2. Line chart of in vitro anti-inflammatory test of L. plantarum-ATCC8014 filtrate and L. plantarum-ATCC8014 cells solution versus ibuprofen as standard drug. Results are expressed as a mean ± SEM (n = 3), where, (IC50 =14.3±2.1µg/ml for L. plantarum-cells; IC50 =12.7±2.1µg/ml for L. plantarum-ATCC8014 filtrate while, IC50 =1.3±1.1 µg/ml for standard).
Effectiveness of various treatments in body weights and wound healing
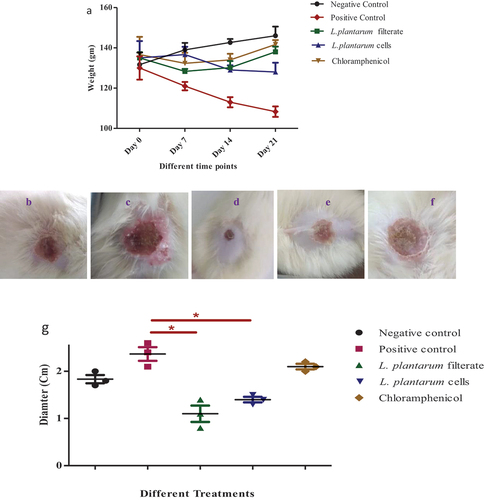
The results revealed variations in body weights in tested animals’ groups where infected wound with S. aureus showed a significant decrease in body weight relative to negative control group especially at day 21. While, application of various treatments led to enhancement of weights of animals relative to uninfected wound group. Furthermore, application of Lactobacillus. plantarum-ATCC8014 filtrate and solution of Lactobacillus. plantarum-ATCC8014 lyophilized cells solution showed a successive wound healing where administration of Lactobacillus. plantarum-ATCC8014 filtrate showed best healing activity as shown in ().
Figure 3. (a) various weights of rats in regular wound and in infected wound by S. aureus and upon using L. plantarum-ATCC8014 filtrate, L. plantarum-ATCC8014 lyophilized cells solution and chloramphenicol respectively. Wound diameter(cm) in (b) uninfected wound; (c) infected wound by S. aureus; (d) infected wound treated by L. plantarum-ATCC8014 filtrate; (e) infected wound treated by L. plantarum-ATCC8014 solution of lyophilized cells; (f) infected wound treated by chloramphenicol; (g) statistical analysis illustrating differences in wound diameter between various tested groups (n=3). Results are represented as mean ± SEM. Where (*) p < 0.5 considered as significant.
Testing bacterial load in skin and different organs
While the uninfected group has no bacterial growth, a dramatic elevation of bacterial count could be seen upon infection of wound using S. aureus. Samples collected from the third group where L. plantarum-ATCC8014 filtrate was used on wound infection showed significant reduction in bacterial count. In the fourth group, upon administration of a solution of L. plantarum-ATCC8014 lyophilized form a reduction in bacterial count (p ≤ 0.05) relative to second infection group and also as shown below application of chloramphenicol has a successive reduction of bacterial count in skin infection relative to the second group as shown in .
Figure 4. S. aureus colony count (cfu/ml) skin (1:10 dilution); in different groups of animals where: 1st group represent negative control where sample collected from a sterile wound; 2nd group represent positive control with 0.5 ml x103 S. aureus used to induce infection in wound; while 3th, 4th and 5th groups were treated with 1ml of L. plantarum-ATCC8014 filtrate, 1 ml of L. plantarum-ATCC8014 lyophilized cells suspension and chloramphenicol respectively. Furthermore, to detect bacterial count in spleen (1:1 dilution) as well as liver (1:1 dilution) in samples from different groups at the end of experiment. (data were represented as means ± SEM; n= 3 where *P≤ 0.05 considered as significant relative to negative control; ***P≤ 0.001 considered as significant as highly significant relative to other treatments i.e. A dramatic difference among positive control and other groups of animals).
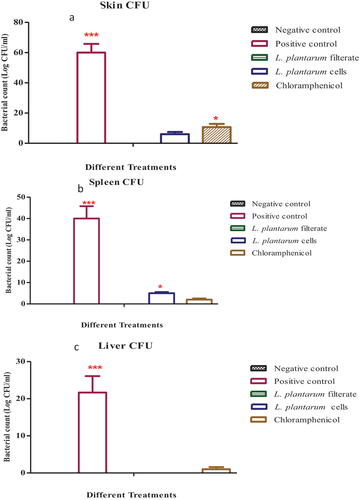
In an attempt to ascertain the proliferation or spread of bacterial infection to significant bacterial count could be detected in the second infected group. Meanwhile, samples cultured from third groups of spleen and liver where using L. plantarum-ATCC8014 filtrate no bacterial culture could be seen (p ≤ 0.001). Furthermore, samples cultured from fourth and fifth groups of spleen and liver where using solution of L. plantarum-ATCC8014 lyophilized form as well as chloramphenicol respectively, a notable reduction in bacterial count relative to second group (p ≤ 0.05) could be seen as shown in ().
Histology of wounds
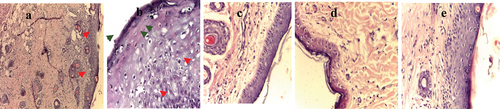
Examination of stained sections by hematoxylin and eosin at uninfected wound sites from various rats’ groups revealed that animals in the uninfected wound group had slow wound healing over time while inflammation could be detected clearly in the tissue. However, upon infection using S. aureus, there was accumulation of bacteria and irregularities in different skin layers could be seen. Tissue proliferation, collagen accumulation, and re-epithelization of layers of skin could be detected with treatments using filtrate of L. plantarum-ATCC8014, solution of L. plantarum-ATCC8014 lyophilized form as well as chloramphenicol, respectively; However, using filtrate of probiotic bacteria showed better healing relative than using lyophilized cells suspension as depicted in .
Figure 5. Histopathological examination of core injuries stained by hematoxylin and eosin (H&E) stain) (x=40) after sacrificing of rats’ and the end of experiment (a) Uninfected wound containing mild inflammation and rupture of epidermal layer (green arrow) and many blood vessels (red arrows) could be seen; (b) infected wound by S. aureus showing accumulation of bacteria along the surface of skin (green arrows) and severe irregularities and vacuoles in epidermal layer (red arrows); (c) infected wound by S. aureus treated with L. plantarum-ATCC8014 filtrate where regular surface layer of healed wound with slight irregularities in dermal layer; (d) infected wound by S. aureus treated by solution of L. plantarum-ATCC8014 lyophilized cells showing shrink healed epidermal layer as well as irregular dermal layer (e) infected wound by S. aureus treated with chloramphenicol showing regular structure of skin.
The infiltration of mast cells has been observed in different groups upon staining using toluidine blue and it could be noticed that there was a dramatic increase (p ≤ 0.05) upon treatment using L. plantarum-ATCC8014 filtrate as well as solution of L. plantarum-ATCC8014 lyophilized form in wound infection model relative to untreated group as depicted in ()
Figure 6. Toluidine blue-stained histopathological slices of rat skin from all the study groups are shown in microstructural analysis (x=40). (a) lesion with no infection has a considerable number of mast cells (red arrows); (b) lesion with bacterial infection with a minimal number of mast cells (red arrows); (c)&; (d)&; (e) lesions with bacterial infection and treated by L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 and chloramphenicol respectively, has a good number of mast cells (red arrows). Where in all groups the mast cells (red arrow) are primarily found in the perivascular space and are crimson purple with several vesicles. Collagen fibers are blue throughout all areas.
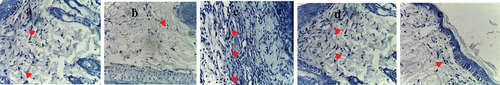
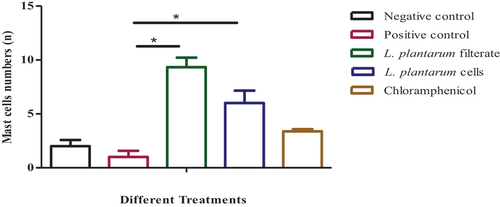
Figure 7. A bar graph (n=8 sections/group) displaying the overall percentages of mast cells in the skin of rats in all treatments. The data is shown as (means SEM); where*: significant where P≤0.05 significant difference among positive control and animals treated by L. plantarum filtrate and L. plantarum lyophilized cells respectively.
Levels of oxidative stress enzymes
The concentrations of SOD in various groups at the end of experiment were detected as depicted in (). There is a dramatic reduction in SOD level in the second group in infected wound group. While treatments using filtrate of L. plantarum-ATCC8014, solution of lyophilized form as well as chloramphenicol and elevation of SOD levels could be seen (p ≤ 0.05). There is no statistical difference between various treatment groups and negative control indicating that SOD slightly retrieves its regular values in various treatments.
Figure 8. Oxidative enzymes in serum of various groups of animals. SOD levels in serum of animal group with uninfected wound; rat group with infected wound showed significant reduction in level (P≤ 0.05) relative to negative control; rat groups with infected wounds and treated with L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol respectively. While, MDA concentrations in serum of rat group with uninfected wound; rat group with infected wound showed dramatic elevation level (P≤ 0.05) relative to 1st uninfected wound group and various treatments groups. Significant difference among positive control and animals treated by L. plantarum filtrate and L. plantarum lyophilized cells respectively.
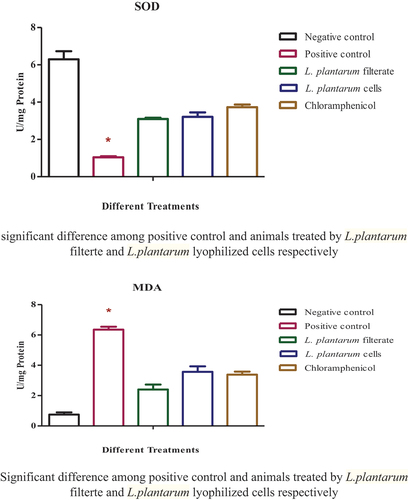
The results of the MDA activity in various treatments in serum of sacrificed animals at the end of experiment and are represented in . S. aureus infection upregulated MDA level in the 2nd group of wound infection (p ≤ 0.05), while treatment using L. plantarum-ATCC8014 filtrate, lyophilized form as well as chloramphenicol with slight differences between treatment groups and recover normal MDA level in different treatments relative to control group.
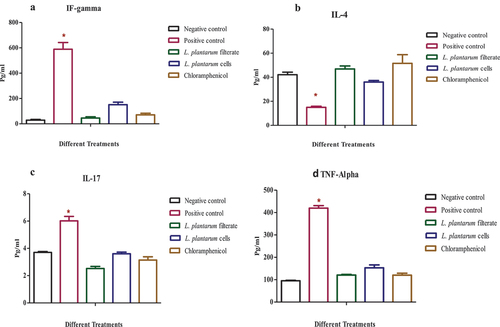
Inflammatory mediators’ levels
Various cytokines levels including IFN-γ, IL-4, TNF-α, and IL-17 were assessed in Rats control group which contained uninfected wound, infected wound with S. aureus only as second positive control and upon treatment using either filtrate of L. plantarum-ATCC8014, lyophilized cells and chloramphenicol, respectively.
It could be noticed that infection of wound using S. aureus significantly upregulates pro-inflammatory cytokines including IFN-γ, TNF-α, and IL-17 (p ≤ 0.05) relative to control uninfected group. While using either L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol lead to derive pro-inflammatory mediators’ levels into values slightly similar to the first group and in most cases levels mediators of L. plantarum-ATCC8014 filtrate have the closest values relative to control and treatment using standard drug. While, anti-inflammatory mediator IL-4 showed dramatic decrease in the second group of animals relative to the first control group (p ≤ 0.05). Furthermore, application of either L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol lead to protective up regulation of IL-4 anti-inflammatory mediator to values near to control group as shown in () (*) means significant difference among positive control and negative control compared with treated groups.
Figure 9. Determination serum inflammatory mediators (A) IFN‐γ, (B) IL-4, (C) IL-17, (D) TNF-α, in control uninfected wound, infected wound with S. aureus, and upon treatment with L. plantarum filtrate-ATCC8014, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol respectively. Data are means ± SEM of 3 rats per group where (*P < 0.05).
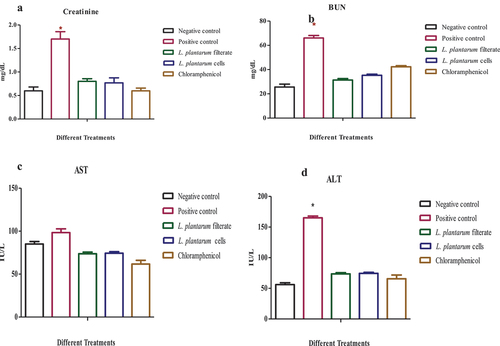
Observations of biochemical parameters
Wound infected rats showed a considerable rise in ALT, creatinine, and BUN levels in contrast to other groups (p ≤ 0.05). However, AST in wound infected rats was not affected by L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol relative uninfected wound and infected wound with S. aureus as depicted in ().
Figure 10. Screening of biochemical functions in different rat groups (A) creatinine, (B) BUN (C), AST and (D)ALT, (, in uninfected wound, infected wound with S. aureus, and after application of L. plantarum-ATCC8014 filtrate, solution of L. plantarum-ATCC8014 lyophilized form and chloramphenicol respectively. Data are means ± SEM of 3 rats per group where (*P < 0.05). Significant difference among positive control and negative control as well as other treated.
Discussion
A lactic acid bacteria called L. plantarum is generally considered as safe and has a beneficial effect on inflammatory processes [Citation27–32]. Bacterial cell-wall elements or released bacterial products interact with immune cells to control them. According to earlier research, L. plantarum can create peptides that are antimicrobial toward Staphylococcus sp. and certain other bacteria [Citation33].
In the present study, antimicrobial activity of both L. plantarum-ATCC8014 filtrate and lyophilized cells exhibited a significant effect on tested bacterial stains, especially S. aureus. In accordance with Muhammad et al. [Citation34] who reported efficient L. plantarum successive effect on rotting and dangerous bacteria including S. aureus and L. monocytogenes. Furthermore, Zeng et al. [Citation35] used Chinese homemade pickles to explore the antipathogenic function and probiotics capability of native lactic acid bacteria, and it was found that L. plantarum offered some interesting probiotic prospects and may be suitable for the production of food preserves.
In the present study, anti-inflammatory action of L. plantarum-ATCC8014 where compared to ibuprofen where filtrate illustrates higher activity than lyophilized cells. There has been a few research on the cell-free supernatants of probiotics’ anti-inflammatory properties. The minimal NO production of Bifidobacterium bifidum was noted by Kang et al. [Citation36]. Another study found that L. acidophilus’ cell-free supernatant had anti-inflammatory characteristics and controlled the inflammatory reaction [Citation37]. The permanence of the proportion of lactic acid in the specimen was strongly influenced by the time it took to extract the cultured probiotic as well as how long it was stored, according to the findings of long-term stability studies [Citation38].
In the current study L. plantarum-ATCC8014 filtrate and lyophilized cells solution were applied in infected wound with S. aureus to compare their healing activities versus chloramphenicol. It could be noticed that both filtrate and lyophilized cells solution has a successive impact after application to infected wound compared to treatment using chloramphenicol which enhances clinical status of animals and positively impacts body weights as well as examined healing activity using diameter of wound. In same line with research groups reported the role of L. plantarum to enhance clinical symptoms on infected wound [Citation39]. Furthermore, Elebeedy et al. [Citation40] reported successive probiotics’ antimicrobial properties of L. plantarum when used alone or together as a synergistical combination, with ethanol extracts of Lawsonia inermis leaves have an additive effect on the healing of infected wounds.
In the present work after application of filtrate and lyophilized cells solution of L. plantarum-ATCC8014 in infected wound bacterial load in skin were disappeared completely in skin, liver and spleen of animals treated with filtrate, while a minimal bacterial load could be seen in skin and disseminated to internal organs upon using lyophilized cells solution in a similar level relative to using chloramphenicol. In accordance with Ong et al. [Citation41] reported that using portion of L. plantarum USM8613 that is high in protein significantly decreases bacterial count throughout duration, along with higher wound closure percentages in comparison to controls.
According to histopathology research, uninfected rats had a healing capacity of roughly 90%. However, only the L. plantarum filtrate group and the solution of lyophilized cells group were able to accomplish about 90% and 80% healing, respectively, in wound-infected animals. This suggested that L. plantarum filtrate might function as a dual mechanism to aid in skin regeneration and infection eradication. These findings are supported by numerous investigations that found L. plantarum filtrate might speed up wound healing by improving tissue repair and structural rigidity [Citation42–45].
It could be noticed that continued application of the L. plantarum-ATCC8014 filtrate and lyophilized cells solution to a lesion infection model led to boost oxidation reactions in the animals and suppress peroxidase enzyme activity, which in turn increases oxidative stress and speeds up healing process. It has been reported that oxidative stress serves as an important modulator in physiological activities and that it may be a mediator of the pathological development of inflammatory skin disorders. Numerous investigations revealed that oxidative stress reactions may be triggered by endogenous and external stimulatory aspects such levels of biochemical components (oxygen, nitrogen, and Sulphur), which can have activating consequences on the growth of cellular metabolism [Citation46–49].
Immune cells called mast cells (MCs) are found in almost all tissues, but they are particularly abundant in organs like the skin that are susceptible to the outside atmosphere. MCs have a key role in a variety of physiological and inflammatory processes, including tumor development, angiogenesis, and wound healing. In a study employing large excisional wounds on MC deficient animals, decreased re-epithelialization was noted [Citation50–53]. In this study, treatment using L. plantarum-ATCC8014 filtrate and lyophilized cells solution to enhanced mast cells is a lesion infection model and may enhance healing process.
Inflammatory mediators apparently aid in the closure of acute wounds by encouraging cellular proliferation and the synthesis of bioactive peptides [Citation54,Citation55]. In this study using either L. plantarum-ATCC8014 filtrate, or lyophilized cells solution, or chloramphenicol in various treatments lead to optimize levels of protective mediators including IFN-γ, TNF-α, and IL-17 as well as up regulation of level of anti-inflammatory cytokine (IL-4) which reported to have roles in both autocrine and paracrine mechanisms, encourage the recruitment, growth, and development during healing process [Citation56].
The results in this report suggest the possibility of using L. plantarum-ATCC8014 filtrate or its solution of lyophilized form in wound infection model with effective antibacterial, anti-inflammatory actions and minimal side effects on kidney and liver functions to be produced on a large scale after result verifications.
Conclusion
This work suggests the possible multifunctional impact of L. plantarum filtrate in animal wound infection that has antimicrobial and anti-inflammatory activity against wide spread of pathogenic micro-organisms. At the same time, its activity in wound healing that enhances the growth of cells and increases the formation of granulation and fibrous tissues, by accelerating re-epithelialization of keratinocyte monolayers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ayichew T, Belete A, Alebachew T, et al. Bacterial probiotics their importance and limitations: a review. J Nutri Health Sci. 2017;4(2):202–209. doi: 10.15744/2393-9060.4.202
- Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, et al. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009
- Kim H, Kim HR, Kim N-R, et al. Oral administration of Lactobacillus plantarum lysates attenuates the development of atopic dermatitis lesions in mouse models. J Microbiol. 2015;53(1):47–52. doi: 10.1007/s12275-015-4483-z
- Lee SH, Yoon JM, Kim YH, et al. Therapeutic effect of tyndallized Lactobacillus rhamnosus IDCC 3201 on atopic dermatitis mediated by down-regulation of immunoglobulin E in NC/Nga mice. Microbiol Immunol. 2016;60(7):468–476. doi: 10.1111/1348-0421.12390
- Im A-R, Lee B, Kang D-J, et al. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J Med Food. 2018;21(10):1016–1023. doi: 10.1089/jmf.2017.4100
- Ogawa M, Saiki A, Matsui Y, et al. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: a randomized, double-blind, placebo-controlled study. Exp Ther Med. 2016;12(6):3863–3872. doi: 10.3892/etm.2016.3862
- Szyszkowska B, Lepecka-Klusek C, Kozlowicz K, et al. The influence of selected ingredients of dietary supplements on skin condition. Postepy Dermatologii i Alergologii. 2014;3:174–181. doi: 10.5114/pdia.2014.40919
- Choi E-J, Iwasa M, Han K-I, et al. Heat-killed Enterococcus faecalis EF-2001 ameliorates atopic dermatitis in a murine model. Nutrients. 2016;8(3):146. doi: 10.3390/nu8030146
- Cervera C, Almela M, Martínez-Martínez JA, et al. “Risk factors and management of gram-positive bacteraemia”. Int J Antimicrob Agents. 2009;34(Suppl 4):S26–30. doi: 10.1016/S0924-8579(09)70562-X
- Scharschmidt TC, Fischbach MA. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome? 1 Drug Discov Today Dis Mech. 2013;10(3–4):e83–e89. doi: 10.1016/j.ddmec.2012.12.003
- Grice EA. The intersection of microbiome and host at the skin interface: genomic- and metagenomic-based insights. Genome Res. 2015;25(10):1514–1520. doi: 10.1101/gr.191320.115
- Zipperer A, Konnerth MC, Laux C, et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535(7613):511–516. doi: 10.1038/nature18634
- Vaid B, Chopra BS, Raut S, et al. Antioxidant and wound healing property of Gelsolin in 3T3-L1 cells. Oxid Med Cel Longev. 2020;2020:1–7. doi: 10.1155/2020/4045365
- Mousavi SM, Zarei M, Hashemi SA, et al. Asymmetric membranes: a potential scaffold for wound healing applications. Symmetry. 2020;12(7):1100. doi: 10.3390/sym12071100
- Nussbaum SR, Fife MJ, DaVanzo CEJ, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27–32. doi: 10.1016/j.jval.2017.07.007
- Brandi J, Cheri S, Manfredi M, et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci Rep. 2020;10(1):11572.2020. doi: 10.1038/s41598-020-68483-4
- Dahiya P, Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J Pharm Sci. 2012;74(5):443–450. doi: 10.4103/0250-474X.108420
- Granica S, Czerwińska ME, Piwowarski JP, et al. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa hudziok obtained after seeds cultivation. J Agric Food Chemistry. 2013;61(4):801–810.2013. doi: 10.1021/jf304002h
- Bosshard E. Review on skin and mucous-membrane irritation tests and their application. Food Chem Toxicol. 1985;23(2):149–154. doi: 10.1016/0278-6915(85)90007-9
- Awad M, Yosri M, Abdel-Aziz MM, et al. Assessment of the antibacterial potential of biosynthesized silver nanoparticles combined with vancomycin against methicillin-resistant Staphylococcus aureus–induced infection in rats. Biol Trace Elem Res. 2021;199(11):4225–4236.2021. doi: 10.1007/s12011-020-02561-6
- Shatzkes K, Singleton E, Tang C, et al. Predatory bacteria attenuate Klebsiella pneumoniae burden in rat lungs. M Bio. 2016;7(6):e01847–e01816. doi: 10.1128/mBio.01847-16
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Philadelphia: Churchill Livingstone; 2008.
- Saddik MS, Elsayed MMA, El-Mokhtar MA, et al. Tailoring of novel azithromycin-loaded zinc oxide nanoparticles for wound healing. Pharmaceutics. 2022;14(1):111.2022. doi: 10.3390/pharmaceutics14010111
- Yosri M, Elaasser MM, Abdel-Aziz MM, et al. Determination of therapeutic and safety effects of zygophyllum coccineum extract in induced inflammation in rats. Biomed Res Int. 2022;2022:1–17. doi: 10.1155/2022/7513155
- Chang D, Zhang X, Rong S, et al. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid Med Cell Longev. 2013;587826. doi: 10.1155/2013/587826
- Zhao Y, Sun Y, Wang G, et al. Dendrobium officinale polysaccharides protect against MNNG-induced PLGC in rats via activating the NRF2 and antioxidant enzymes HO-1 and NQO-1. Oxid Med Cell Longevity. 2019;2019:1–11. doi: 10.1155/2019/9310245.9310245
- Smelt MJ, de Haan BJ, Bron PA, et al. The impact of Lactobacillus plantarum WCFS1 teichoic acid D-alanylation on the generation of effector and regulatory T-cells in healthy mice. PLoS One. 2013;8(4):e63099. doi: 10.1371/journal.pone.0063099
- Meijerink M, van Hemert S, Taverne N, et al. Identification of genetic loci in Lactobacillus plantarum that modulate the immune response of dendritic cells using comparative genome hybridization. PLoS One. 2010;5(5):e10632. doi: 10.1371/journal.pone.0010632
- van Hemert S, Meijerink M, Molenaar D, et al. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010;10(1):293. doi: 10.1186/1471-2180-10-293
- Yaqoob P. Ageing, immunity and influenza: a role for probiotics? Proc Nutr Soc. 2014;73(2):309–317. doi: 10.1017/S0029665113003777
- van Baarlen P, Troost FJ, van Hemert S, et al. Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A. 2009;106(7):2371–2376. doi: 10.1073/pnas.0809919106
- Seddik HA, Bendali F, Gancel F, et al. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicro Prot. 2017;9(2):111–122. doi: 10.1007/s12602-017-9264-z
- Bai Y, Yu L, Zhou H, et al. High cell density culture of Lactobacillus plantarum Zhang-LL. Chinese Agri Sci Bulletin (In Chinese). 2015;31(31):285–290.
- Muhammad Z, Ramzan R, Abdelazez A, et al. Assessment of the antimicrobial potentiality and functionality of Lactobacillus plantarum strains isolated from the conventional inner Mongolian fermented cheese against foodborne pathogens. Pathogens. 2019;8(2):71. doi: 10.3390/pathogens8020071
- Zeng Y, Li Y, Wu QP, et al. Evaluation of the antibacterial activity and probiotic potential of Lactobacillus plantarum isolated from Chinese homemade pickles. Can J Infect Dis Med Microbiol. 2020;2020(11):1–11. Article ID 8818989. doi: 10.1155/2020/8818989
- Kang CH, Kim JS, Park HM, et al. Antioxidant activity and short-chain fatty acid production of lactic acid bacteria isolated from Korean individuals and fermented foods. 3 biotech. 3 Biotech. 2021;11(5):217.2021. doi: 10.1007/s13205-021-02767-y
- Maghsood F, Mirshafiey A, Farahani MM, et al. Dual effects of cell free supernatants from lactobacillus acidophilus and lactobacillus rhamnosus gg in regulation of MMP-9 by up-regulating TIMP-1 and down-regulating CD147 in PMA differentiated THP-1 cells. Cell J. 2018;19(4):559–568.2018. doi: 10.22074/cellj.2018.4447
- Saadatzadeh A, Fazeli MR, Jamalifar H, et al. Probiotic properties of lyophilized cell free extract of Lactobacillus casei. Jundishapur J Nat Pharm Prod. 2013;8(3):131–137. doi: 10.17795/jjnpp-8564
- Peral MC, Martinez MA, Aldez VJC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009;6(1):73–81. doi: 10.1111/j.1742-481X.2008.00577.X
- Elebeedy D, Ghanem A, El-Sayed M, et al. Synergistic antimicrobial effect of Lactiplantibacillus plantarum and Lawsonia inermis against Staphylococcus aureus. Infect Drug Resist. 2022;15:545–554. doi: 10.2147/IDR.S342976
- Ong JS, Taylor TD, Yong CC, et al. Lactobacillus plantarum USM8613 aids in wound healing and suppresses Staphylococcus aureus infection at wound sites. Probiotics Antimicro Prot. 2020;12(1):125–137. doi: 10.1007/s12602-018-9505-9
- Gupta A, Kumar P. Assessment of the histological state of the healing wound. Plast Aesthet Res. 2015;2(5):239–242. doi: 10.4103/2347-9264.158862
- Cocetta V, Catanzaro D, Borgonetti V, et al. A fixed combination of probiotics and herbal extracts attenuates intestinal barrier dysfunction from inflammatory stress in an in vitro model using caco-2 cells. Recent Pat Food Nutr Agric. 2019;10(1):62–69. doi: 10.2174/2212798410666180808121328
- Ostad S, Salarian A, Ghahramani M, et al. Live and heat-inactivated lactobacilli from feces inhibit Salmonella typhi and Escherichia coli adherence to caco-2 cells. Folia Microbiol. 2009;54(2):157–160. doi: 10.1007/s12223-009-0024-7
- Onbas T, Osmanagaoglu O, Kiran F. Potential properties of Lactobacillus plantarum F-10 as a bio-control strategy for wound infections. Probiotics Antimicro Prot. 2019;11(4):1110–1123.2019. doi: 10.1007/s12602-018-9486-8
- Zhao J, Tian F, Zhao N, et al. Effects of probiotics on d‐galactose‐induced oxidative stress in plasma: a meta‐analysis of animal models. J Funct Foods. 2017;39:44–49. doi: 10.1016/j.jff.2017.09.055
- Zhao J, Tian F, Yan S, et al. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d -galactose-induced aging mice. Food Funct. 2018;9(2):917–924. doi: 10.1039/C7FO01574G
- Zhou Y, Xu Q, Dong Y, et al. Supplementation of mussel peptides reduces aging phenotype, lipid deposition and oxidative stress in d‐galactose‐induce aging mice. J Nutr Health Aging. 2017;21(10):1314–1320. doi: 10.1007/s12603-016-0862-3
- Ni Q, Zhang P, Li Q, et al. Oxidative stress and gut microbiome in inflammatory skin diseases. Front Cell Dev Biol. 2022;10:849985. doi: 10.3389/fcell.2022.849985
- Weller K, Foitzik K, Paus R, et al. Mast cells are required for normal healing of skin wounds in mice. FASEB J. 2006;20(13):2366–2368. doi: 10.1096/fj.06-5837fje
- Ammendola M, Zuccalà V, Patruno R, et al. Tryptase-positive mast cells and angiogenesis in keloids: a new possible post-surgical target for prevention. Updates Surg. 2013;65(1):53–57. doi: 10.1007/s13304-012-0183-y
- Yang HW, Liu XY, Shen ZF, et al. An investigation of the distribution and location of mast cells affected by the stiffness of substrates as a mechanical niche. Int J Biol Sci. 2018;14(9):1142–1152. doi: 10.7150/ijbs.26738
- Church MK, Kolkhir P, Metz M, et al. The role and relevance of mast cells in urticaria. Immunol Rev. 2018;282(1):232–247. doi: 10.1111/imr.12632
- Patel S, Srivastava S, Singh MR, et al. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi: 10.1016/j.biopha.2019.108615
- Xiao T, Yan Z, Xiao S, et al. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11(1):232. doi: 10.1186/s13287-020-01755-y
- Rodrigues M, Kosaric N, Bonham CA, et al. Wound healing: a cellular perspective. Physiol Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017