ABSTRACT
Infectious diseases caused by bacteria, fungi and viruses are globally of major concern. Plant essential oils produced by various differentiated structures possess varied properties as significant antiseptic, antibacterial, antifungal, antiviral, antioxidant, anti-parasitic and insecticidal activities. The essential oil of three lemon odor plants; Cupressus macrocarpa, Cymbopogon citratus and Citrus limon were isolated and evaluated for their antimicrobial activities. The analysis of essential oil components of C. macrocarpa by GC-MS revealed D-limonene (38.00%) as the major component followed by citral (9.72%), carveol (6.86%) and citronellal (5.35%). Analyses of the essential oil of C. citratus resulted in the identification of pseudolimonene (19.2%) as the most abundant component followed by D-limonene (12.34%), Ɣ-terpinene (10.89%), citronellol (9.58%), sabinene hydrate (9.24%), (+)-2-bornanone (8.29%) and α-terpinolene (5.53%). Also, GC-MS detected neral dimethyl acetal (41.56%) was the main component of C. limon EO with carveol (12.39%) and citral (11.21%). A study of the antimicrobial activity of the three essential oils against E. coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus. and Candida albicans has been evaluated and revealed that C. citratus EO had antibacterial activity against gram-positive organisms and C. limon EO exhibited the strongest antifungal activity against Candida albicans.
Introduction
In recent years, there has been a growing interest in researching and developing new antimicrobial agents from various sources to combat microbial resistance. Plants and other natural sources can provide a huge range of complex and structurally diverse compounds. Plant essential oils are produced by various differentiated structures, especially the number and characteristics of which are highly variable. Essential oils possess varied properties as significant antiseptic, antibacterial, antifungal, antiviral, antioxidant, anti-parasitic and insecticidal activities. They have been screened as potential sources of novel antimicrobial compounds, agents promoting food preservation, and alternatives to treat infectious diseases. Thus, essential oils can serve as a powerful tool to reduce bacterial resistance [Citation1,Citation2].
Three lemon odor plants were collected from Egypt to evaluate the antimicrobial activities of their essential oils. The plants with lemon odor seemed to have antimicrobial activity. The lemon-like scent of plants from varied families could be ascribed to diverse compounds such as D-limonene, Ɣ-terpinene, β-pinene, α-pinene and citral [Citation3,Citation4]. Also, these components were reported to possess antimicrobial activities [Citation5–9].
The essential oils of Cupressus macrocarpa, Cymbopogon citratus and Citrus limon plants having lemon odor were isolated and characterization of their varied components. Moreover, a comparative study of the antimicrobial activity of the three essential oils against varied microorganism as E. coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus and Candida albicans, amongst some of the main bacteria with multidrug resistance and are included in the category of community and hospital acquired pathogens [Citation2], has been evaluated.
The ‘Cypress’ plants belong to the family Cupressaceae and are grown in many subtropical areas for commercial purposes, such as ornamentation, and as a source of wood-building material. Cupressus macrocarpa (Hartweg. Ex. Gordon) as a medicinal plant belongs to this family and commonly known as Monterey Cypress. It is an evergreen tree up to 23-m tall with horizontal branches and it is widely distributed throughout the tropical and temperate regions around the world, i.e., Mexico, North America Asia, and North Africa. It is native to the Californian coast and has been planted in some countries including South Africa as it is relatively fast-growing and have dimensionally stable timber, with an attractive grain that is naturally durable and suited to many high value appearance grade purposes. Also, it was famous for the treatment of various ailments, e.g. eliminates fluid retention, styptic problem, rheumatism and whooping cough. It received little concern regarding its phytochemical constituents, also it was reported the isolation of ten sesquiterpenes from its foliage. It was reported that large amounts of monoterpenes, as compared to sesquiterpenes or diterpenes, were detected in the EOs of the branchlets of several Cupressus species fresh and dried leaves. It's worth noting that the C. macrocarpa essential oil showed remarkable antimicrobial, antioxidant and strong antifungal activities against specific dermal fungi [Citation10–15].
Genus Cymbopogon (family: Poaceae) represents an important genus of about 120 species that grows in tropical and subtropical regions around the world. On account of their diverse uses in pharmaceutical, cosmetics, food, flavor, and agriculture industries, Cymbopogon citratus is popularly known as citronella grass or lemongrass. It is a tufted perennial grass growing to a height of 1 m with numerous stiff leafy stems arising from short rhizomatous roots. It is native to tropical areas of Africa, America, Asia, South and Central America. It possesses strong lemony taste and odor due to its high content of the aldehyde citral. In addition to citral, the essential oil of Cymbopogon spp. consists of small quantities of geraniol, geranyl acetate and monoterpene olefins, such as limonene (in C. flexuosus) and myrcene (in C. citratus). C. citratus is commonly used in folk medicine for treatment of coughs, consumption, elephantiasis, malaria, ophthalmia, pneumonia as well as vascular, nervous and gastrointestinal disturbances. Studies on this plant have demonstrated as antispasmodic, analgesic, anti-inflammatory, anti-pyretic, diuretic, antidepressant, antioxidant, antiseptic, astringent, bactericidal, fungicidal, nervine and sedative properties [Citation16–19].
Genus Citrus belonging to Rutaceae family includes a great number of species; most of them are evergreen aromatic shrubs to small size trees. Citrus plants are well known as one of the world’s major fruit crops grown in many countries all over the world. They are widely cultivated mainly for their highly appreciated tasty fresh fruits as well as their processed products which have a large market worldwide. Egypt is ranked among the top 10 largest citrus producers in the world. Citrus essential oils much needed in the pharmaceutical industry to mask the unpleasant tastes of drugs via their strong flavoring effect. Citrus limon (Lemon) is among the important species of genus Citrus. Its essential oils were composed of mixtures of hydrocarbons, terpenes, sesquiterpenes, aldehydes, alcohols, esters and sterols. Citrus plants constitute one of the main sources of essential oil, which are extensively studied for their potential uses in the food industry. The main components of its essential oils were limonene and β-Pinene. Moreover, it was reported that essential oil of this plant showed antioxidant and antimicrobial activities [Citation20–22].
In the framework of continuing research for green sources used as safe antimicrobial agents, this study is concerned with analyzing the essential oils isolated from Cupressus macrocarpa, Cymbopogon citratus and Citrus limon, besides discovering their potential antimicrobial activity.
Materials and methods
Plant material
The fresh healthy leaves of Cupressus macrocarpa, Cymbopogon citratus. and Citrus limon were collected early in the morning in February 2022 from trees growing in the Fruit Experimental Station, Mansoura University. The identity of plants was kindly authenticated by Dr. Mohsen Fahmy, Prof. of Pomology, Faculty of Agriculture, Mansoura University, Egypt. Voucher specimens were coded as Cm-1–2022, Cc-1–2022 & Cl-1–2022 for Cupressus macrocarpa, Cymbopogon citratus & Citrus limon, respectively, and were kept in Pharmacognosy Department, Faculty of Pharmacy, Mansoura University, Egypt.
Essential oil isolation
Fresh leaves (700 g of each plant) separately subjected, immediately after collection, to hydro distillation for 3 h. using a Clevenger-type apparatus. The oils were dried over anhydrous sodium sulfate then stored at + 4°C in the dark until tested. Cupressus macrocarpa yielded 3.3 mL of EO, Cymbopogon citratus yielded 2.0 mL of EO and Citrus limon yielded 2.7 mL of EO.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC/MS analysis of the purified oils were performed using an Agilent-mass spectrometer (5977B MSD) coupled to an Agilent-gas chromatograph 7890B GC System). Injection of an aliquots of diluted oils (1 mL of 1 ppm concentration) was done then injected into the GC/MS apparatus Autosampler. Using split-less mode, Samples were injected. Analysis was performed on GC/MS (Agilent Model 7890 MSD) equipped with a HP-5 MS capillary column (30 m × 0.25 mm i.d., 0.25 μm coating) and the gas chromatograph was operated under the following conditions: temperature programming was performed as column temperature was 70°C for 5 min, and programmed at a rate of 5°C/min to 290°C, and finally held isothermally for 5 min. The detector and injector temperatures were 290 and 280°C, respectively. Helium (99.999% purity) was used as the carrier gas at a flow rate of 1.0 mL/min. in addition to significant quadrupole MS operating parameters: Electrospray ionization at 70 eV with scan mass range of 30 to 600 m/z. The components were identified by comparing their mass spectra with those in the National Institute of Standards and Technology library (NIST 2017) stored in the MASSHUNTER software.
Antimicrobial activity of the essential oils
In this study, two different methods were used to assess the antimicrobial activity of oils: agar disc diffusion method and broth microdilution methods [Citation23,Citation24].
Test organisms and media
The activity of oils was tested microbial strains obtained from microbiology department at faculty of Pharmacy, Mansoura hospitals strains of (E. coli ATCCBAA196, Klebsiella pneumoniae ATCC4352, Pseudomonas aeruginosa PAO1, Proteus mirabilis clinical strain, Staphylococcus aureus Newman, Bacillus cereus clinical strain and Candida albicans Sc5314. Clinical strains were confirmed by standard biochemical tests. Muller Hinton agar/broth was used for bacterial cultures and Sabouraud Dextrose Agar/broth for C. albicans growth.
Agar diffusion method
On the surface of solid media, microbial cultures (10 [Citation5] cfu/ml) were spread. Filter paper discs of 6 mm diameter were placed on the surface and soaked with 4 µl of tested oil. plates were incubated at 37°C for 24 h for bacteria and at 30°C for 48 h for C. albicans. The zones of growth inhibition were measured to the nearest millimeter.
Micro-broth microdilution method
One milliliter of oil was solubilized in petroleum ether and diluted to 10 ml with sterile distilled water. To disperse oils in distilled water, the mixtures were shaken for few minutes. This solution mother was further tenfold diluted (10−1 to 10−5). Thirty microliters of each dilution and 10 µl of culture (10 [Citation5] cfu/ml) were added to wells of 96-well microtitre plates containing 160 µl of Muller Hinton broth (for S. aureus and B. cereus) or Sabouraud dextrose broth (for C. albicans). Incubation of plates were done at 37°C for 24 h for bacteria and at 30°C for 48 h for C. albicans. Wells containing petroleum ether plus distilled water and growth media with or without culture were included as positive and negative controls, respectively. Wells were examined for visible turbidity after incubation and plated on their respective media. The antimicrobial activity of standard antimicrobials ampicillin and amphotericin B was tested against bacteria and fungi, respectively.
Results and discussion
Identification of the essential oil constituents
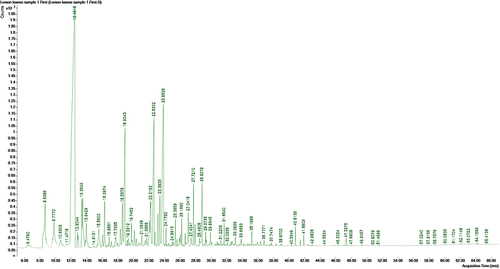
The analysis of the essential oil of the three plants and the identification of their components were carried out depending on their retention times and the average area under the peaks. The analysis of the essential oil of C. macrocarpa resulted in the identification of 36 major components representing 90.18% of the total oil composition (, ). The non-oxygenated fraction of this oil is (53.9%). Monoterpenes hydrocarbons (50.68%) are the most abundant non-oxygenated components and D-limonene (38.00%) is the main constituent followed by trans-β-Ocimene (4.58%), pseudolimonene (3.81%) in addition to other minors. The oxygenated fraction contributes about 36.2% represented in a descending order by aldehydes (17.58%), alcohols (12.54%) then esters (3.48%), acetals (2.08%), ketones (0.41%) and ethers (0.11%). Citral (9.72%) is the dominant aldehyde component followed by citronellal (5.35%). Carveol is the major alcohol, while linalyl acetate is the major esters present. To the best of our knowledge, it was reported that the major compounds identified in essential oil from C. macrocarpa Hartwig cone present in Nilgiris, India were dinopol, terpinel-4-ol, β-pinene and α-pinene. Some studies revealed that the EOs composition of the genus Cupressus can be affected by many factors such as genetics (species or variety) or origin, nutritional conditions such as pesticide use, fertilization, latitude, drying and harvest time. Moreover, the essential oil yield and composition may be affected by the distillation time [Citation13]. So, this may explain the difference of some components and percentages of some other components of oil in this study compared to other previous reported analysis of C. macrocaroa essential oil.
Table 1. Chemical compositions of the essential oil of C. macrocarpa leaves.
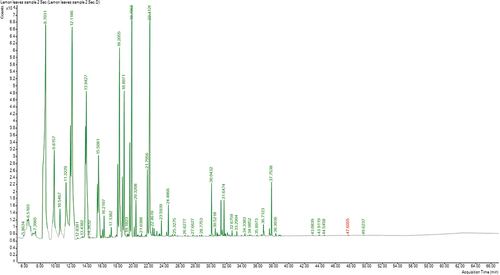
Also, 29 major components were identified in C. citratus EO representing 98.56% of the total oil composition (, ), included 63.11% non-oxygenated fraction, 29.85% oxygenated fraction and 5.60% unknown component. Monoterpenes hydrocarbons (60.03%) are the most abundant non-oxygenated components and pseudolimonene (19.2%) is the main constituent followed by D-limonene (12.34%), Ɣ-Terpinene (10.89%), α-Terpinolene (5.53%), M-Cymene (3.81%), β-Myrcene (2.87%) with other components. Alcohols (19.81%) are the main components of the oxygenated fraction followed by ketones (9.64%), aldehydes (0.28%) and ether (0.12%). The most abundant alcohols are citronellol and sabinene hydrate (9.58%& 9.24%, respectively). The major component of ketones is (+)-2-Bornanone (8.29%).
Table 2. Chemical compositions of the essential oil of C. citratus leaves.
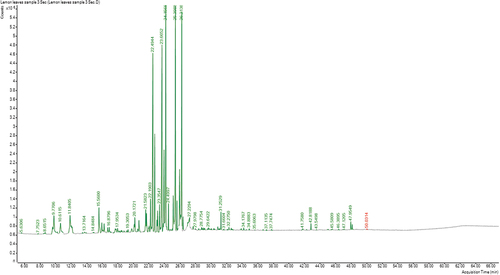
The essential oil of C. limon resulted in the identification of 29 major components representing 86.23% of the total oil composition (. ), the oxygenated fraction is the most abundant with percentage of 76.56%, while the non-oxygenated fraction represents (9.67%). Acetals (42.59%) represent the major components of the oxygenated fraction followed by alcohols (13.27%), aldehydes (12.87%), epoxy and diepoxy (4.67%), ketones (1.79%), ether and acid (0.7%) and esters (0.67%). Neral dimethyl acetal (41.56%) is the main acetal component. The most abundant alcohol is carveol (12.39%). Citral (11.21%) is the major aldehyde, while epoxy-linalool oxide (4.5%) is the major diepoxy component.
Table 3. Chemical compositions of the essential oil of C. limon leaves.
It is worth noting that some studies revealed that the composition of the essential oil may vary with the extraction method, developmental stage of the plant, the solvent used for extraction and the drying of the plant materials under varied conditions, thus it can exert significant effect on the chemical profile of the essential oils derived [Citation19,Citation20].
As reported in previous literature, compounds as limonene, Ɣ-terpinene, β-pinene and α-pinene were the influential terpenes responsible for the distinctive odor of the lemon juice [Citation3]. Also, the lemon-like scent could be ascribed to the existence of a cyclic monoterpene (citral) [Citation4]. Herein as shown in (), there is a comparison between the percentages of these varied components in each plant which explains the presence of the lemon odor in these three plants.
Table 4. Comparison between the percentages of compositions responsible for the lemon odor of three plants.
Antimicrobial activity
The results of agar disc diffusion method revealed that C. citratus EO had antibacterial activity against gram-positive organisms (B. cereus and S. aureus) and B. cereus bacteria was more sensitive to it with 45 mm inhibition zone diameter. This may be confirmed with some previous reports that revealed that the gram-positive bacteria are more sensitive to C. citratus essential oil than the gram-negative bacteria [Citation16]. Regarding antifungal activity, both C. citratus and C. limon had antifungal activity against C. albicans and it was noted that C. limon EO exhibited higher antifungal activity with 20 mm inhibition zone diameter compared to 9 mm inhibition zone diameter by C. citratus EO ().
Table 5. Antimicrobial activities of the essential oil from C. macrocarpa, C. citratus and C. limon using agar diffusion method.
The micro-broth dilution method confirmed the previous results of the inhibitory activity of C. citratus EO on B. cereus and C. albicans, as no growth was observed with solution mother compared to controls (). Also, lower dilutions of this oil (10−1, 10−2) decreased the growth of B. cereus without inhibiting it. On the other hand, the strongest antifungal activity exhibited by C. limon against C. albicans as the growth of it was inhibited by the dilution 10−2.
Table 6. Evaluation of antimicrobial activity of C. citratus and C. limon EOs by micro-broth dilution method against S. aureus and C. albicans.
Although pseudo-limonene, the main component present in C. citratus EO, is known for its low antibacterial activity, its EO exhibited significant antibacterial activity. This may be attributed to the synergistic effect of this component with other antibacterial components present as D-limonene, Ɣ-terpinene, citronellol, sabinene hydrate and other components [Citation5,Citation6,Citation25–27]. Also, the antifungal activity of C. citratus and C. limon EOs may be due to the presence of some antifungal components as D-limonene, Ɣ-terpinene & citronellol and citral & carveol present in C. citratus and C. limon EOs, respectively [Citation6–9].
The EOs of the three plants did not show any activities against Gram-negative organisms, also the essential oil of C. macrocarpa had no antimicrobial activities against any of the tested microorganisms. This may be explained by varying of the chemical compositions of EOs due to different extraction method, developmental stage of the plant, the solvent used for extraction and thus different biological attributes of the essential oils derived may be occurred [Citation19,Citation20].
Conclusion
In order to find explore highly effective and safer natural organic compounds as antimicrobial agents struggle against infectious disease, the present work reported the chemical compositions and antimicrobial activities of the three lemon odor essential oils from Cupressus macrocarpa, Cymbopogon citratus and Citrus limon. The chemical composition of C. macrocarpa EO revealed that D-limonene, citral, carveol and citronellal were the main components. Pseudolimonene, D-limonene, Ɣ-terpinene, citronellol, sabinene hydrate, (+)-2-bornanone and α-terpinolene were the most abundant components of C. citratus EO. The major components found in C. limon were neral dimethyl acetal, caveol and citral. Regarding the antimicrobial activity of the three essential oils, C. citratus EO exhibited significant antibacterial activity against B. cereus bacteria. The EO of both C. limon and C. citratus showed antifungal activity, but the C. limon EO showed more potency than the C. citratus EO against candida albicans fungus. Therefore, widening the scope of action of the three essential oils and suggesting their use in phytotherapy as antimicrobial activity or even as natural food preservatives.
Abbreviations
C. macrocarpa: Cupressus macrocarpa, C. citratus: Cymbopogon citratus, C. limon: Citrus limon, E.O: Essential oils, GC-MS: Gas Chromatography-Mass Spectroscopy, E. coli: Escherichia coli, S. aureus: Staphylococcus aureus, B. cereus: Bacillus cereus, C. albicans: Candida albicans
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dhifi W, Bellili S, Jazi S, et al. Essential oils’ Chemical characterization and investigation of some biological activities: a critical review. Medicines. 2016;3(4):25. doi: 10.3390/medicines3040025
- Chouhan S, Sharma K, Guleria S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines. 2017;4(3):58. doi: 10.3390/medicines4030058
- Yetisen M, Guclu G, Kelebek H, et al. Elucidation of key aroma enhancement in cloudy lemon juices by the addition of peel oil using GC–MS-Olfactometry. Int J Food Sci Technol. 2022;57(8):5280–5288.
- Oladeji OS, Adelowo FE, Ayodele DT, et al. Phytochemistry and pharmacological activities of Cymbopogon citratus: a review. Scientific African. 2019;6:e00137. doi: 10.1016/j.sciaf.2019.e00137
- Sousa LGV, Castro J, Cavaleiro C, et al. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci Rep. 2022;12(1):4417. doi: 10.1038/s41598-022-08217-w
- Han Y, Sun Z, Chen W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules. 2022;25(1):33. doi: 10.3390/molecules25010033
- Couto CSF, Raposo NRB, Rozental S, et al. Chemical composition and antifungal properties of essential oil of origanum vulgare Linnaeus (lamiaceae) against sporothrix schenckii and sporothrix brasiliensis. Trop J Pharm Res. 2015;14(7):1207.
- Pereira FO, Mendes JM, Lima IO, et al. Antifungal activity of geraniol and citronellol, two monoterpenes, alcohols, against Trichophyton rubrum involves inhibition of ergosterol biosynthesis. Pharm Biol. 2014;53(2):228–234. doi: 10.3109/13880209.2014.913299
- Lim AC, Tang SGH, Zin NM, et al. Chemical composition, antioxidant, antibacterial, and antibiofilm activities of Backhousia citriodora essential oil. Molecules. 2022;27(15):4895. doi: 10.3390/molecules27154895
- Farjon A. Nomenclature of the Mexican cypress or “cedar of goa”, Cupressus lusitanica Mill. (cupressaceae). Taxon. 1993;42(1):81–4. doi: 10.2307/1223306
- Graniti A. Cypress canker: a pandemic in progress. Annu Rev Phytopathol. 1998;36(1):91–114. doi: 10.1146/annurev.phyto.36.1.91
- Manivannan R, Kumar MS, Jawahar N, et al. A comparative antimicrobial study on the essential oil of the leaves of various species of Cupressus. Ancient Science Of Life. 2005;24(3):131–133.
- Saad AM, Mohammed MMD, Ghareeb MA, et al. Chemical composition and antimicrobial activity of the essential oil of the leaves of Cupressus macrocarpa Hartweg. ex-gordon. J Appl Pharm Sci. 2017;7(9):207–212.
- Salem MZM, Elansary HO, Ali HM, et al. Bioactivity of essential oils extracted from Cupressus macrocarpa branchlets and corymbia citriodora leaves grown in Egypt. BMC Complement Med Ther. 2018;18(1):23. doi: 10.1186/s12906-018-2085-0
- Watt MS, Kimberley MO, Steer BSC, et al. Spatial comparisons of productivity and carbon sequestration for Cupressus lusitanica and macrocarpa within New Zealand. For Ecol Manage. 2023;536:120829. doi: 10.1016/j.foreco.2023.120829
- Naik MI, Fomda BA, Jaykumar E, et al. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pac J Trop Med. 2010;3(7):535–538. doi: 10.1016/S1995-7645(10)60129-0
- Hanaa ARM, Sallam YI, El-Leithy AS, et al. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann Agric Crop Sci. 2012;57(2):113–116.
- Manvitha K, Bidya B. Review on pharmacological activity of Cymbopogon citratus. Int J Herb Med. 2014;1(6):5–7.
- Mukarram M, Choudhary S, Khan MA, et al. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants. 2022;11(1):20. doi: 10.3390/antiox11010020
- Kamal GM, Anwar F, Hussain AI, et al. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int Food Res J. 2011;18(4):1275–1282.
- Sherif AE, Marzouk AM, Zaghloul MG, et al. Chemical composition and cytotoxic activity of petitgrain essential oil of Citrus aurantium L. “Russian colon. J Am Sci. 2015;11(8):64–68.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus limon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16(1):146. doi: 10.1186/s12944-017-0487-5
- Tepe B, Donmez E, Unlu M, et al. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of salvia cryptantha (montbret et aucher ex Benth.) and salvia multicaulis (vahl). Food Chem. 2014;84(4):519–525. doi: 10.1016/S0308-8146(03)00267-X
- Bachir RG, Benali M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac J Tropical Biomedicine. 2012;2(9):739–742. doi: 10.1016/S2221-1691(12)60220-2
- Heni S, Bennadja S, Djahoudi A. Chemical composition and antibacterial activity of the essential oil of Thymus ciliatus growing wild in Northeastern Algeria. J Appl Pharm Sci. 2015;5(12):56–60. doi: 10.7324/JAPS.2015.501209
- Lopez-Romero JC, González-Ríos H, Borges A, et al. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015;2015:1–9. Article ID 795435. doi: 10.1155/2015/795435
- Ramos S, Rojas LB, Lucena ME, et al. Chemical composition and antibacterial activity of Origanum majorana L. essential oil from the Venezuelan Andes. J Essent Oil Res. 2011;23(5):45–49. doi: 10.1080/10412905.2011.9700481