ABSTRACT
Diabetes mellitus (DM) a metabolic disorder resulting in myocardial injury and diabetic cardiomyopathy (DCM) in association with oxidative stress induction. This study was therefore, investigated the potential protective effect of Tempol (TPL) on oxidative cardiac damage in experimental diabetic rats. Rats were assigned to three equal groups: group (NC); normal control rats received citrate buffer injections. Group (DC): diabetic control rats received a single i.p. STZ injection (60 mg/kg body weight), and Group (D/TPL) diabetic rats treated with TPL (10 mg/kg b.w; by i.p. injection) for four weeks. Our results showed a significant increment in plasma creatine phosphokinase (CPK), lactate dehydrogenase (LDH) activities, cholesterol, triglycerides, Nitric oxide (NO), and lipid peroxidation (LPO), In addition, GSH content and antioxidant enzyme activity were significantly deceased in DC group compared to normal control. While TPL treatment significantly ameliorated the observed biochemical abnormalities. Further, TPL reduced number of dead myocardial cells in diabetic-treated rats and, remarkably improved the histopathological alterations of the aorta. The results proved the protective effect of tempol on STZ-induced diabetic cardiomyopathy by reducing myocardial cell death through reducing oxidative stress and inhibition of lipid peroxidation.
Introduction
Diabetes mellitus (DM) is a metabolic disorder due to deficiency in the secretion and/or action of insulin, and leads to chronic high blood glucose level resulting in various health conditions and diseases [Citation1,Citation2]. Cardiovascular diseases (CVD) are the leading cause of death of diabetic patients, where the prevention strategies become a great public health concern [Citation3,Citation4]. Hyperglycemia is an important risk factor for the development of cardiovascular disease in DM. The relationship between diabetes and early cardiovascular disease is well-established [Citation5].
As previously reported [Citation6–9], DM mediated hyperglycemia can lead to induction of oxidative stress generating reactive oxygen species (ROS) responsible for the oxidation of cell macromolecules, including DNA, proteins, and lipids [Citation7,Citation10]. In this regard, Vanessa Fiorentino et al. [Citation7] reported the involvement of glycoxidative stress (GOS) in several diabetes complications, especially disturbance of cellular homeostasis and cardiovascular damage [Citation10], and ROS in endothelial dysfunction [Citation11,Citation12]. This dysfunction is associated with reduced NO bioavailability, which could be due to impaired NO production by the endothelium and/or increased NO inactivation by ROS [Citation13,Citation14].
The superoxide anion (O2-) has been identified as the key mediator in numerous deleterious effects of oxygen derivatives [Citation12], whose overproduction of O2- promotes the interaction of O2- with NO created by the eNOS enzyme, leading to the formation of peroxynitrite (ONOO-), and reduction of NO bioavailability for other biological responses, like endothelial-dependent vasorelaxation. Moreover, ONOO- can stimulate lipid peroxidation and thus contributes to the oxidized LDL generation, whose activity leads to the development of atheromatous plaque and vascular dysfunction [Citation15]. As a result, cells have antioxidant defense systems, such as superoxide dismutase, catalase, glutathione peroxidase, glutathione, and vitamins act effectively in maintaining the balance between the endogenous or exogenous ROS at optimal physiological levels [Citation6]. In this regard, SOD, enzymes catalyzing the dismutation of O2- to O2 and H2O2, was reported to have key role in maintaining vascular reactivity [Citation12,Citation16] and protecting the vascular cells against O2- anions. Hyperglycemia can elevate the quantity of produced O2- up than the cell antioxidant capacity, and subsequently causes oxidative endothelial cell damages [Citation10,Citation16], This causes oxidative stress in endothelial cells, affecting their normal function [Citation12,Citation16]. Thus, inhibiting or delaying oxidative chain reactions might be promising therapeutic strategies for more effective protection against oxidative stress damages. Recent research has revealed an inverse relationship between antioxidant consumption and the severity of oxidative stress damage [Citation14,Citation17]. Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a piperidine nitroxide, and of the SOD mimetic type, permeable to cell membranes with strong antioxidant properties owed to its efficient scavenging ability to nitroxide radicals [Citation12,Citation17,Citation18, Citation19].
The current research aimed to investigate the effect of tempol on glycoxidative stress (GOS) and cardiac damage in diabetic rats. In this context, we investigated the hypothesis that treatment with SOD-mimetic antioxidants, such as TPL may reverse this condition and restore the defense capabilities of endogenous antioxidants.
Materials and methods
Chemicals and reagents
Streptozotocin and Tempol were obtained from Sigma-Aldrich Chemicals (St. Louis, MO, USA). All other chemical products employed in this study were of highest purity and analytical grade.
Experimental animals
Twenty-four Wistar male rats weighing 200 ± 10 g were obtained from the Pasteur Institute (Algiers, Algeria). They were maintained under laboratory conditions at a temperature of 22 ± 2°C, with a photoperiod of 12 h/12 h (light/dark) and relative humidity (50–55%). The animals were given a standard pellet diet (ONAB; El Harrouch, Algeria) and clean tap water ad libitum. Rats were included in the experiment after three weeks of adaptation.
Induction of diabetes and experimental procedure
Experimental diabetes design was performed using one dose of a freshly prepared solution of streptozotocin dissolved in cold citrate buffer (0.1 M, pH 4.5) and administered intra-peritoneally (at a dose of 60 mg/kg b.w after fasting for 12 h). To limit severe complications of hypoglycemia, STZ injected rats were given 5% w/v glucose solution as a drink for the next 24 hours. Three days after post-STZ injection, blood glucose was measured by a glucometer (Accu-check Performa). Fasting blood glucose (FBG) > 350 mg/dl indicates diabetes. Animals were divided into three experimental groups of six each:
Group (NC) was the normal control; rats were intraperitoneally injected with citrate buffer.
Group (DC) served as a diabetic control; received one intraperitoneal (i.p) injection of streptozotocin (STZ, 60 mg/kg b.w) was given to rats.
Group (D/TPL) Diabetic rats received STZ and treatment with tempol (TPL) via intraperitoneal injection (10 mg/kg b.w/day/30 days) after 4 weeks of diabetes induction.
The doses of streptozotocin and tempol were based on the previous studies of Kherouf et al. [Citation6] and Kwon et al. [Citation18], respectively.
At the end of the treatments, the body weights of animals were recorded. Rats were killed by decapitation and the heart was immediately excised and washed in NaCl solution (0.9%), which was used for the preparation of heart homogenates and histopathological study.
Blood collection
Blood samples were collected in heparinized sample tubes and plasma was separated by centrifuged at 3000 g for 15 min. The collected plasma (the supernatant) was stored at −20°C for the biochemical parameters.
Heart tissue homogenate preparation
1 g of heart samples were homogenized in two volumes of buffer solution of phosphate-buffered saline (PBS: pH 7.4). After centrifugation (10,000 g for 15 min at 4°C), the resulting supernatant was used for the determination of reduced glutathione (GSH) and protein contents and antioxidant enzyme activities.
At the end of the treatments, the body weights of the animals were measured. Rats were killed by decapitation and the heart was immediately excised and washed in NaCl solution (0.9%), which was used for the preparation of heart homogenates and histopathologic study.
Biochemical parameters
Biochemical parameters were estimated by colorimetric method using the available Spinreact Reagents Kits (Spain, refs. No: glucose (GLU)-1001190, total cholesterol (TC)- 1001090, triglycerides (TG)- 41031, lactate dehydrogenase (LDH)- 1001260 and creatine phosphokinase (CPK)- 1001050)
Reduced glutathione contents
Heart GSH content was assessed by a colorimetric method as described by Ellman (1959), modified by Jollow et al [Citation20], based on the reaction of DTNB (5,5′-dithiobis-2-nitrobenzoic acid) with compounds having sulfhydryl groups and, resulting in a yellow color change. The absorbance was read at 412 nm. The total GSH level was expressed as nmol/mg protein.
Lipid peroxidation levels
Lipid peroxidation (LPO) levels in the heart homogenates were determined using a commercial kit (Bioxytech LPO-586™). Malondialdehyde (MDA), which is considered the terminal product of LPO, reacts with thiobarbituric acid and the absorbance was determined at 532 nm. The level of lipid peroxidation was expressed as nmol of MDA/mg of protein.
Nitric oxide levels
Plasma nitric oxide (NO) levels were quantified by the method of Miranda et al [Citation21], based on the reduction of nitrate in the presence of vanadium (III) coupled with the detection by acid Griess reaction. The absorbance was recorded at 540 nm.
Superoxide dismutase activity
Superoxide dismutase (SOD) activity in cardiac tissue homogenate was measured following the method of Beyer and Fridovich [Citation22]. Optical density was recorded at 560 nm. SOD unit activity was defined as the quantity of enzyme required to inhibit the reduction of nitro blue tetrazolium (NBT) by 50% and SOD activity was expressed and reported as unit/mg protein.
Catalase activity
Catalase (CAT) activity in heart homogenates was estimated according to the method of Aebi [Citation23], using hydrogen peroxide (H2O2) as a substrate. The decrease in absorbance due to H2O2 disappearance was monitored spectrophotometrically at 240 nm for 1 min and the Catalase activity was expressed as µmol H2O2 /min/mg protein.
Glutathione S-transferase activity
Glutathione S-transferase (GST) activity was evaluated in cardiac homogenates using the method of Habig et al. [Citation24]. The absorbance was recorded at 340 nm. GST activity was calculated as nmol of GST/min/mg protein.
Histopathological studies
Aortic samples were fixed in Bouin’s solution. After 24 hours of fixation, the specimens were washed, dehydrated with a graduated ethanol series, and embedded in paraffin. Serial sections of 5 µm thickness were cut and then stained with routine eosin and hematoxylin ((E&H) [Citation25], The preparations were then examined under a Leica DM 710 optical microscope and a Leica microsystem camera (image processing software, LAZ EZ version 3) and photographed using a digital camera (Leica ICC50 W).
Analysis of cardiomyocyte apoptosis
Tissue section pre-processing
Tissue fragments were dehydrated in a succession of graded alcohols baths (100, 90, 80, and 70%) for 5 minutes. After a single wash in PBS, the samples were incubated at 37°C for 30 min in a proteinase K solution, followed by 5 min in a H2O2 3% blocking solution.
Detection
We employed the TUNEL Apoptosis detection Kit for Paraffin-embedded Tissue Sections (Biotin labeled POD) (Cat. No. L00297) is one of GenScript’s newly introduced products. The kit can detect fragmented DNA in the nucleus during apoptosis. In this modified TUNEL assay kit, biotinylated nucleotide is labeled at the DNA 3´-OH ends using the natural or recombinant terminal deoxynucleotidyl transferase (TdT or rTdT). Then, horseradish peroxidase-labeled streptavidin (streptavidin-HRP) is bound to these biotinylated nucleotides, which are detected using the peroxidase substrate, hydrogen peroxide, and 3,3’-diaminobenzidine (DAB)-Solution, a stable chromogen. Using this procedure, apoptotic nuclei are stained dark brown. Optical microscope Leica DM 710 was used to examine the different slides at × 350 magnification. The slide were analyzed with QuPath-0.3.2 software to calculate the positive cell number [Citation26].
Statistical analysis
The experimental values represent the mean of six independent assays + SD (standard deviations). Comparison between different groups was determined using the Student’s t-test. Statistical tests were performed using GraphPad Prism (Ver 7, California, USA). The significance level was accepted at p < 0.05 for all tests.
Results
Effect of diabetes and tempol treatment on body, absolute and relative heart weights
In , the diabetic control group (DC) showed a significant decrease in body weight compared to the normal control group (NC) (p < 0.01).The observed loss of body weight in DC was significantly ameliorated following tempol treatment (D/TPL).
Table 1. Effect of diabetes and tempol treatment on body, absolute and relative heart weights.
In diabetic rats, absolute and relative heart weights were significantly decreased (p < 0.05) when compared with the NC. In contrast, the diabetic group treated with tempol showed a marked recovery in heart weight compared to the DC group ().
Effect of diabetes and tempol treatment on biochemical parameters
As indicated in , a significant rise (p < 0.01) in the concentrations of glucose, triglycerides, cholesterol, and the activities of plasma LDH and CPK was observed in the DC group compared to the normal control group (p < 0.01). However, the (D/TPL) group showed a significant decrease (p < 0.05) in the concentration of glucose and the activity of LDH compared to the DC group. In addition, the TPL treatment reduced the raised levels of triglycerides, cholesterol, and CPK activity in diabetic rats.
Table 2. Effect of diabetes and tempol treatment on biochemical parameters.
Effect of diabetes and tempol treatment on nitric oxide and lipid peroxidation levels
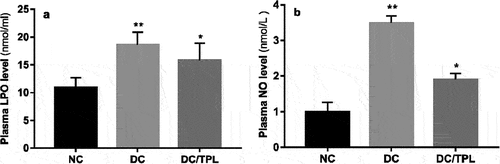
As shown in , a marked depletion in plasma antioxidant status was registered in the DC group, as indicated by a significant increase (p < 0.01) of LPO and NO levels when compared to the NC group. Interestingly, treatment with Tempol in diabetic rats (D/TPL) significantly reduced plasma LPO and NO (p < 0.05) compared to DC rats.
Figure 1. Effect of diabetes and tempol treatment on the LPO (lipid peroxides) (a) and NO(nitric oxide) (b) levels in the plasma of normal control rats (NC) and treated group (NC: normal control; DC: diabetic control; DC/TPL: diabetic rat treated with tempol after 8 weeks of post-diabetes induction. Values are presented as means ± SD of six (n = 6) rats in each group. Significant differences: DC, DC/TPL versus NC group (**p < 0.01; *p < 0.05).
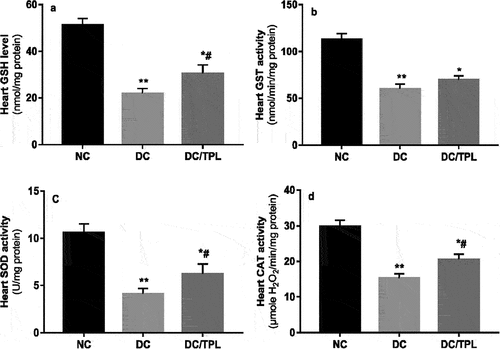
Effect of diabetes and tempol treatment on heart oxidative stress parameters
The oxidative stress effects were revealed by a significant decrease in GSH content and antioxidant enzyme activities (SOD, CAT, and GST) in DC rats compared to normal control rats (p < 0.01). Interestingly, treatment with tempol in the DC group significantly (p < 0.05) increased the GSH content and activities of antioxidant enzymes compared to the DC group ().
Figure 2. Effect of diabetes and tempol treatment on heart tissue oxidant/antioxidant status of normal control rats (NC) and treated group (NC: normal control; DC: diabetic control; DC/TPL: diabetic rat treated with tempol). GSH: reduced glutathione (a); GST: glutathione S-transferase (b); SOD: superoxide dismutase (c); CAT: catalase (d); Significant differences: DC, DC/TPL versus NC group (** p < 0.01; *p <0.05). DC/TPL versus DC group (#p <0.05).
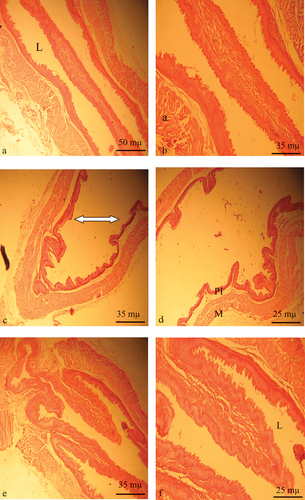
Histopathological findings
Histological studies of Aorta sections of diabetic animals revealed arterioplasty with thinning of the inner wall and resorption of the media compared to the normal structure of the control rats (). In contrast, TPL administration in diabetic-treated group restored the caliber of the lumen and structure of the intima ( and ).
Figure 3. Aorta histological sections of control, diabetic and diabetic-tempol treated rats stained with HE. a and b control: show intact structure of the aorta L artery lumen, PI inner wall intima, M media. c and d diabetic rat: show arterioplasty (white arrow) with thinning of the inner wall and resorption of the media. e and f diabetic treated with tempol restoration of L lumen caliber and intima structure (150X and 300X).
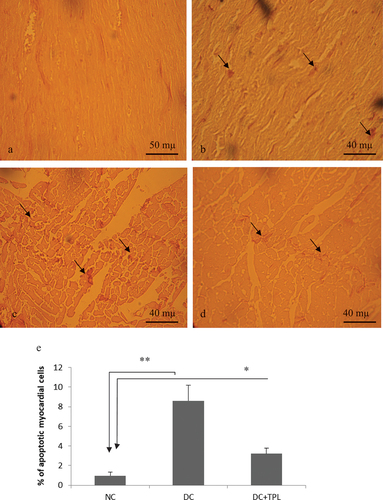
Effect of diabetes and tempol treatment on cardiomyocyte apoptosis
Application of the TUNEL (Terminal deoxynucleotidyl Transferase Biontin-dUTP nick and labeling) method allowed us to detect apoptotic cardiomyocytes and estimate their frequency. depicts histological slices of the myocardium demonstrating the appearance of apoptotic cardiomyocytes with nuclei positively stained brown by DAB. The number of labeled cells and their intensity of labeling vary from one group to another ( and ). Compared to the control group and diabetic rats treated with tempol (DC: 8.6 ± 1.6 and DC+TPL: 3.2 ± 0.96 vs. T: 0.96 ± 0.21 P < 0.001 and P < 0.01), diabetic rats exhibit a higher frequency of apoptosis. The addition of TPL significantly decreased the frequency of myocyte death cells ().
Figure 4. Histology sections of cardiac muscle showing apoptotic myocytes (TUNEL-positive cells DAB-stained in black, ×350). a and b myocardium of control rats. d and c myocardium of diabetic rats, showing significantly dense stains and significantly high counts of TUNEL-positive cells (inset). c. Myocardium of diabetic rats treated with tempol for four weeks, showing less dense stains and lower counts of apoptotic cells compared to diabetic non-treated rat (D). e frequency of apoptotic myocardial cells assess by QuPath bio-image analysis software 3.2, in groups of control rats, diabetic rats and tempol-treated diabetic rats (*p < 0,01 vs NC and **p < 0.001 vs NC).
Discussion
Endothelial dysfunction resulting from insulin resistance, is an early vascular change that promotes the development of cardiovascular disease [Citation27]. In fact, endothelial abnormalities lead to a deficit of endothelium-dependent vasorelaxant ability in the blood vessels, in association with a decreased NO bioavailability [Citation28].
Hyperglycemia is one of the major factors that contributes to the incidence of cardiovascular disease in DM [Citation5]. and overall is paired with the activation of several ROS overproduction pathways and enhanced oxidant generation in endothelial cells [[Citation29]. Oxidative stress may contribute to endothelial malfunctions by decreasing the bioavailability of NO [Citation30].
Several studies have investigated the positive implications of antioxidants like polyphenols, polysaccharides, rutin, quercetin, anthocyanins, and epigallocatechin gallate on vascular health [Citation31,Citation32], as well as vitamins and their metabolites [Citation33]. In this study, we examined the prophylactic effectiveness of TPL on metabolic responses and cardiac antioxidant status during experimental glucotoxicity in rats. Our results clearly showed a decrease in body and absolute and relative heart weights, which can be attributed to loss of appetite, disturbances in the metabolism of carbohydrates, proteins, and/or lipids [Citation34], and decreased mass of adipose tissue [Citation35] The lipid profile results of diabetic rats demonstrated a significant increase in plasma cholesterol and triglyceride levels compared with normal controls. These metabolic alterations can be attributed to the increased degeneration of pancreatic β-cells after injecting STZ, which can lead to a reduction in insulin secretion. Furthermore, Mollica et al. [Citation35] as reported the experimentally induced diabetes revealed elevated plasma cholesterol and triglyceride levels can be due to hormone-sensitive lipase activation, which releases free fatty acids from adipose tissue after a deficiency of insulin. While, compared to diabetic controls, TPL treatment significantly reduced plasma glucose levels in STZ-induced diabetic rats. The mechanism for this antihyperglycemic activity could be due to the stimulation of the islets of Langerhans to secrete insulin, activation of insulin receptors [Citation36], and increasing the translocation of the glucose transporter-1 from intracellular compartments to the cell surface, leading to increased glucose cellular uptake [Citation37]
Diabetes induced biochemical alterations were significantly correlated with an induction in the activities of CPK and LDH [Citation38] showed that the CPK is one of the enzymatic markers of cardiac muscle damage and that serum CPK elevation is associated with cardiomyopathy in diabetic rats.
In addition, Badole et al. [Citation39] and Li et al. [Citation40] found that necrosis in the heart of diabetic rats was accompanied by an increase in the aspartate transaminase AST and cardiac LDH activities.; hence, these two enzymes might be used as specific markers of myocardial necrosis. It has been shown that NF-κB, a transcription factor implicated in a wide range of cellular responses (inflammatory response, growth response, and apoptosis), and can be activated under oxidative conditions [Citation41]. The activation of NF-κB can be initiated by very small quantities of pro-oxidant agents, up to about one µmol of H2O2 in certain cell types, and also by an imbalance between GSH and GSSG [Citation41]. NF-κB activation can be triggered by very small amounts of pro-oxidant agents, down to approximately one µmol of H2O2 in certain cell types, as well as by GSH/GSSG imbalance [Citation42]. A four-week chronic administration of TPL restored the activities of LDH and CPK to normal levels while simultaneously reducing the rate of apoptosis in diabetic animals. This suggests that the anti-apoptotic effect of TPL is mainly due to a local antioxidant effect rather than a hypoglycemic effect [Citation18]. TPL significantly improved CAT activity, cardiac GSH levels, and especially the activity of SOD in diabetic rats, whereas its effects on blood glucose were not significant, indicating the anti-apoptotic effect of TPL likely implicates other mechanisms to regulate blood glucose. Diabetes is linked to elevated free fatty acids [Citation43] which induce apoptosis in beta cells of the pancreas and vascular endothelial cells in humans [Citation44,Citation45]. Keep in mind that, as stated in the results section above, plasma LPO indicators of lipid peroxidation increased in diabetic rats because of an excess of free radical generation [Citation46]. According to our findings, i.p diabetic rats, TPL had lower LPO levels and better cardiac-muscle antioxidant status (GSH, catalase, and SOD). Therefore, the beneficial effects of TPL may arise from reactive oxygen species scavenging, lipid peroxidation prevention, and enhancement of the redox system in the heart [Citation18].
Conclusion
In conclusion, the results of this study proved Tempol as an effective agent in preventing damage caused by free radicals owed to its antioxidant properties, reducing the apoptotic process, and lowering the risk of diabetic cardiomyopathy in rats. Tempol modulates apoptosis indirectly by decreasing NF-κB activation, reducing pro-oxidants, and increasing antioxidants (SOD, CAT, and GSH).
Availability of data and material
The corresponding author can provide the data that supports the study’s conclusions upon request
Author contribution
Tichati L is the investigator, performed the animal experiments, data analyses and paper writing. Lakbar C immunohistochemistry investigation. Trea F involved in the determination of the antioxidant markers, histopathological analyses, and discussion of results. Messaoudi A contributed to the completion of the experimental and laboratory analysis. Ouali Kh supervised the project, wrote and submitted the manuscript, as well as responded to the reviewer’s comments and suggestions.
Ethics approvals
All the protocols used in this study were conducted according to the International Guidelines for Laboratory Animal Care and Use (Council of European Communities) (JO86/609/CEE) and approved by the Ethical Committee of Directorate General for Scientific Research and Technological Development at Algerian Ministry of Higher Education and Scientific Research, permit no PNR/SF 08/2012.
Acknowledgments
This research is supported by the General Direction of Scientific Research and Development of Technology and Ministry of Higher Education and Scientific Research, DGRSDT-MESRS Algeria.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Leader steering committee; LEADER trial investigators, Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):311–322. doi: 10.1056/NEJMoa1603827
- Mahmoud AM, Hernandez Bautista RJ, Sandhu MA, et al. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longevity. 2019;2019:19. doi: 10.1155/2019/5484138
- Huxley RR, Peters SA, Mishra GD, et al. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(3):198–206. doi: 10.1016/S2213-8587(14)70248-7
- Satta S, Mahmoud AM, Wilkinson FL, et al. The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longevity. 2017;2017:1–18. doi: 10.1155/2017/9237263
- Papatheodorou K, Papanas N, Banach M, et al. Complications of diabetes. J Diabetes Res. 2016;2016:1–3. doi: 10.1155/2016/6989453
- Kherouf A, Aouacheri O, Tichati L, et al. Potential antioxidant properties and anti-diabetic and hepatic/pancreatic protective effects of dietary boswellia serrata gum resin powder against oxidative damage in streptozotocin‐induced diabetic rats. Comparative Clinical Pathology2021. 2021;30(6):891–904. doi: 10.1007/s00580-021-03284-3
- Vanessa Fiorentino T, Prioletta A, Zuo P, et al. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005
- Karasu Ç. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J. 2010;4(1):240–256. doi: 10.2174/1874192401004010240
- Mahmoud AM. Exercise ameliorates metabolic disturbances and oxidative stress in diabetic cardiomyopathy: possible underlying mechanisms. Exercise for cardiovascular disease prevention and treatment: from molecular to clinical, part 1. 2017;207–230. doi: 10.1007/978-981-10-4307-9_12
- Negre-Salvayre A, Salvayre R, Augé N, et al. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signaling. 2009;11(12):3071–3109. doi: 10.1089/ars.2009.2484
- Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012. doi: 10.1155/2012/918267
- Nassar T, Kadery B, Lotan C, et al. Effects of the superoxide dismutase-mimetic compound tempol on endothelial dysfunction in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2002;436(1–2):111–118. doi: 10.1016/S0014-2999(01)01566-7
- Witting PK, Rayner BS, Wu BJ, et al. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell Physiol Biochem. 2007;20(5):255–268. doi: 10.1159/000107512
- Kaneto H, Katakami N, Matsuhisa M, et al. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;11:453892. doi: 10.1155/2010/453892
- Jiang H, Zhou Y, Nabavi SM, et al. Mechanisms of oxidized LDL-Mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovasc Med. 2022;9:925923. doi: 10.3389/fcvm.2022.925923
- Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44(4):381–386. doi: 10.1161/01.HYP.0000142232.29764.a7
- Calabrese G, Ardizzone A, Campolo M, et al. Beneficial effect of tempol, a membrane-permeable radical scavenger, on inflammation and osteoarthritis in in vitro models. Biomolecules. 2021;11(3):352. doi: 10.3390/biom11030352
- Wang M, Li K, Zou Z, et al. Piperidine nitroxide Tempol enhances cisplatin‑induced apoptosis in ovarian cancer cells. Oncol Lett. 2018;16(4):4847–4854. doi: 10.3892/ol.2018.9289
- Kwon TH, Chao DL, Malloy K, et al. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. J Neurotrauma. 2003 Apr;20(4):337–345. doi: 10.1089/089771503765172291
- Jollow DJ, Mitchell JR, Zampaglione NA, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity. Anal Biochem. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal Of Biological Chemistry. 1974;249(22):7130–7139. doi: 10.1016/S0021-9258(19)42083-8
- Haoult R. Techniques d’histopathologie et de cytopathologie. Ed Maloine. 1984;19:225–227.
- Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-17204-5
- Blandin A, Le Lay S. Extracellular vesicles and metabolic diseases: Dangerous liaisons. Med Sci. 2021;37(12):1125–1132. doi: 10.1051/medsci/2021209
- Domagala TB, Szeffler A, Dobrucki LW, et al. Nitric oxide production and endothelium-dependent vasorelaxation ameliorated by N 1-methylnicotinamide in human blood vessels. Hypertension. 2012;59(4):825–832. doi: 10.1161/hypertensionaha.111.183210
- Gero D. Hyperglycemia-induced endothelial dysfunction. In: Endothelial dysfunction—old concepts and new challenges. London, UK: IntechOpen; 2007. pp. 179–206.
- Montiel V, Lobysheva I, Gérard L, et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine. 2022;77:103893. doi: 10.1016/j.ebiom.2022.103893
- Zhou DD, Luo M, Shang A, et al. Antioxidant food components for the prevention and treatment of cardiovascular diseases: effects, mechanisms, and clinical studies. Oxid Med Cell Longevity. 2021;2021:1–17. doi: 10.1155/2021/6627355
- Al-Dujaili EA, Casey C, Stockton A. Antioxidant properties and beneficial cardiovascular effects of a natural extract of pomegranate in healthy volunteers: a randomized preliminary single-blind controlled study. Antioxidants. 2022;11(11):2124. doi: 10.3390/antiox11112124
- Shah AK, Dhalla NS. Effectiveness of some vitamins in the prevention of cardiovascular disease: a narrative review. Front Physiol. 2021;12:729255. doi: 10.3389/fphys.2021.729255
- Juárez-Rojop IE, Díaz-Zagoya JC, Ble-Castillo JL, et al. Hypoglycemic effect of Carica papaya leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-236
- Mollica A, Zengin G, Locatelli M, et al. Anti-diabetic and anti-hyperlipidemic properties of Capparis spinosa L.: in vivo and in vitro evaluation of its nutraceutical potential. J Funct Foods. 2017;35:32–42. doi: 10.1016/j.jff.2017.05.001
- Ghadermazi R, Khoshjou F, Hossini Zijoud SM, et al. Hepatoprotective effect of tempol on oxidative toxic stress in STZ-induced diabetic rats. Toxin Reviews. 2018;37(1):82–86. doi: 10.1080/15569543.2017.1313277
- Shahidi S, Jabbarpour Z, Saidijam M, et al. The effects of the synthetic antioxidant, tempol, on serum glucose and lipid profile of diabetic and non-diabetic rats. Avicenna J Med Biochem. 2016;4(2016):1–7. doi: 10.17795/ajmb-31043
- Nzekwe S, Morakinyo A, Ntwasa M, et al. Influence of flavonoid-rich fraction of monodora tenuifolia seed extract on blood biochemical parameters in streptozotocin-induced diabetes mellitus in male Wistar rats. Metabolites. 2023;13(2):292. doi: 10.3390/metabo13020292
- Badole SL, Chaudhari SM, Jangam GB, et al. Cardioprotective activity of pongamia pinnata in streptozotocin-nicotinamide induced diabetic rats. Bio Med Res Int. 2015;2015:403291. doi: 10.1155/2015/403291
- Li H, Liu Z, Wang J, et al. Susceptibility to myocardial ischemia-reperfusion injury at early stage of type 1 diabetes in rats. Cardiovasc Diabetol. 2013;12(1):1–11. doi: 10.1186/1475-2840-12-133
- Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–86. doi: 10.1016/j.cotox.2017.11.002
- Rajlic S, Treede H, Münzel T, et al. Early detection is the best prevention-characterization of oxidative stress in diabetes mellitus and its consequences on the cardiovascular system. Cells. 2023;12(4):583. doi: 10.3390/cells12040583
- Li Q, Zhao M, Wang Y, et al. Associations between serum free fatty acid levels and incident diabetes in a 3-year cohort study. Diabetes Metab Syndr Obes. 2021;2743–2751. doi: 10.2147/DMSO.S302681
- Krümmel B, von Hanstein AS, Plötz T, et al. Differential effects of saturated and unsaturated free fatty acids on ferroptosis in rat β-cells. J Nutr Biochem. 2022;106:109013. doi: 10.1016/j.jnutbio.2022.109013
- Zhang D, Li J, Li T. Agmatine mitigates palmitate (PA)-induced mitochondrial and metabolic dysfunction in microvascular endothelial cells. Hum Exp Toxicol. 2022;41:09603271221110857. doi: 10.1177/09603271221110857
- Sinaei N, Jafari E, Najafi A, et al. Hepatic oxidative damages and glucose tolerance in diabetic rats exposed to repeated oral doses of diazinon. Iran J Toxicol. 2022;16(3):221–228. doi: 10.32598/IJT.16.3.950.1
