ABSTRACT
Nanotechnology is a growing advanced field with immense applications in new cancer diagnostic platforms. Nanoparticles have been utilized for improving diagnosis treatment of various disease like cancer. Nanotechnology has enabled an approach utilizing nanoparticles with an ability to passively accumulate at tumor sites, making them a perfect alternative to conventional approaches in cancer treatment. Nanoparticles were examined for varieties of clinical uses, which include drug transporter, gene transport to tumors, imaging contrasting agents on basis of its most subtle selectivity and multitudinous estimation capacity. Nanoparticles can be utilized in targeted drug delivery with an advantage such as biocompatibility and reduced toxicity. Nanoparticles have various effects such as high surface to volume ratio that helps in its binding, absorption and transportation of small biomolecules, such as deoxyribonucleic acid, ribonucleic acid drugs and protein molecules to the targeted site, thereby increasing the effect of therapeutic agents. Above advantages making them much distinct and effective in comparison to conventional approaches in cancer treatment. This review will summarize about nanoparticles for cancer detection and nano vehicles used in drug delivery.
Introduction
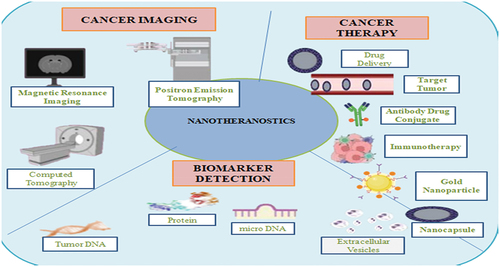
According to data reports generated by the WHO – World Health Organization – cancer is known as the most prevalent issues of global death, resulting around 10 million deceased in 2020, which means about one in every six deaths, among them breast, lung, colon, and rectal cancers, are the vogue cancers. With reference to the data collected yearly, about 400,000 youth diagnosed with cancer. One of the most frequent cancer types is cervical cancer in 23 nations. Early detection is an important key factor to fight cancer [Citation1]. Precision nanodevices targeting carcinoma cells and endothelial tumor cells with tumor-specific ligands could improve the diagnosis, such as Quantum dots (QDs), gold nanoparticles (AuNPs), and polymer dots, and are the most prevalent nanoparticle used in research applied for analyzing cancer [Citation2]. Nanotechnology is the uttermost advanced and evolving area of science. It deals with the engineering manipulation of nanosize particle of various matters on basis of atomic and molecular scale. Nanotechnology deals with science, technology and engineering so it link the gap connecting biological and physical sciences using nanostructures, nanoparticles and nanophases in various scientific domains. Nanoscale ranges from 1 to 100 nanometers. Nanostructures construction mainly include two methods for its synthesis, i.e. top down and bottom up. Top down approaches that a bulk size material is broken down to get nanosized particles. The bottom-up technique have reference to the assembling of nanoshape construction starting with the bottom up, molecule by molecule or atom by atom using nanoscale (1–100 nm), phenomenal and chemical processes and organizing shape of atom and molecule self-assembly [Citation3]. Various forces help in self assembly of nanomedicine and drug molecule non-covalent interactions like hydrogen bonds, van der Waals and hydrophobic interactions. Nanotechnology has enormous amount of potential applications like in cancer therapy, drug delivery, in food and agriculture, nanobiosensors, theragnostics, metal nanoparticles and nanomedicine. Nanooncology is another application of nanosciences, which helps in reduction of toxicity in existing chemotherapy techniques. Large size is the major issue for targeted drug delivery. New drug delivery systems that target pharmaceuticals to specific bodily areas may be a possibility for resolving these key challenges. Target therapies are effective in contrast to available therapies like chemotherapy and with reduced undesirable side effects or complication. Nanoparticle-based therapies have numerous varieties of physicochemical and biological attributes like high surface area to volume ratio, relevant organizational membrane or blood–brain barriers or tissue barriers; they have various capabilities like carrying specified agent on the surface and to conquer cellular or tissue barriers for circulating within the blood stream for extended period of time. These properties are not present in other particles. This property allows for thick coating nanoparticles surface, such as immunoglobulin, small sized molecules, aptamers, peptides and other components surfaces [Citation1]. With help of nanotechnology-assisted molecular diagnostics, cancer biomarker diagnostics become easy when compared with other methods. Nanobiosensor makes it possible to identify several protein biomarkers with limited time or within seconds. Different nanotechnology-associated cancer therapy helps in medication distribution and also increased drug potency and minimizing their harmful effects. These nanoparticles can be occupied as vehicles that can easily overcome a variety of biological barriers [Citation4]. For cancer therapy, various tools like liposome, polymeric micelles, carbon nanotubes (CNTs) are used to deliver anticancer drug. DNA-based targeting can be active targeting and passive targeting. Nanoparticles are mostly organic or inorganic; they are unique depending on their size, texture, physical and chemical properties [Citation5]. As shown in , the three different ways in which nanotheranostics can be used are cancer imaging, cancer management and through biomarker detection [Citation6].
Nanoparticles types
Nanoparticles types have been given in given below:
Table 1. Variety of nanoparticles used for drug delivery system.
Carbon based – These nanoparticles are carbon-based nanoparticles because they are made from carbon component which is 100 times robust than steel, and they include carbon made nanotubes, carbon QDs, graphene QDs, graphene, graphene oxide and CNTs. CNT consists of graphene sheets which are rolled into cylindrical forms or tube. CNTs can be used for delivering drugs as well as bioimaging and tracking, because they showed strong optical near-infrared absorption, Raman scattering and photo-acoustic properties [Citation7]. Fullerenes are basically the allotropes of carbon form, which consists of 60 or some more carbon atoms.
Semiconductor based – Semiconductor includes properties similar to both metals and non-metals. Strong characteristics of semiconductor QDs include good photo-stability, a limited and controllable emission wavelength, and relatively high two-photon absorption cross-section [Citation8]. Due to their unique wide band gaps property, they can be used for photo-catalysis, electronic devices and photo-optics.
Ceramic based – Nanoparticles which includes inorganic solids are known as ceramic-based nanoparticles that are manufactured with help of different oxides, carbides, carbonates and phosphates. Ceramic nanoparticles can be used for drug transport and also as imaging agent because of their high heat resistant property and also chemical inertness. Ceramic nanoparticles offer total protection to molecules such as proteins, enzymes, and medicines that are entrapped against the denaturing effects of environmental temperature and pH [Citation9].
Metal based – Preparation of these nanoparticles is done from metals using chemical and electrochemical processes. Gold and silver nanoparticles are known as noble metal-based nanoparticles, and they can be used as probes to generate signal in disease diagnosis [Citation17]. Their localized surface plasmon resonance makes them more efficient in detection and imaging of bio-molecules. Because of AuNPs coating in SEM sample, high-quality microscopic image produced.
Polymer based – Polymer nanoparticles are organic-based materials shaped like nanocapsules or nanosphere. Drug delivery done with polymer nanoparticles is highly biodegradable and can be used for entrapping both hydrophobic and hydrophilic drugs [Citation18]. They ensure control release and protection of drug molecules within internal and external environment.
Lipid based - Lipid nanoparticles are composed of solid core lipid as well as matrix with lipophilic molecules for external core, and its stabilization can be done by surface-active agents such as detergents or emulsifiers. Applications of lipid nanoparticles are drug carrier and delivering nucleic acids in gene therapy. Use of nanoparticles improves site-specific delivery, reduction of toxic side effects, overcoming physical barriers, cost reduction and bioavailability [Citation19].
Cancer diagnosis
Cancer is caused by changes in our genes that control how our cells work and function. Cancer is a rapid development of atypical cells or cancer cells, which is later going to pass or spread in other parts known as metastasis. The most common cause for death is cancer metastasis. Different tests used for diagnosis of cancer are laboratory test, imaging test and biopsy. In laboratory test, urine and blood test is done and blood test is done for leukemia patient. Imaging tests include X-rays, MRIs, positron emission tomography scans, computerized tomography or CT scans [Citation20]. In biopsy, a sample of tissue is taken which is further analyzed. Presence of cancer biomarkers in our body indicates its existence. Due to high selectivity and sensitivity of nanoparticles, we can measure multiple targets. Through nanotechnology, we can improve biosensors and enable selective targeting. Nanoparticles used in cancer diagnosis are colloidal AuNPs, nanoshells, microRNA detection,and DNA methylation detection as given in [Citation21].
Colloidal AuNPs – Gold nanoparticles are inorganic nanoparticles designed to create particles with specific size, shape and structure, which determines unique optical and electrochemical property. Properties of AuNPs like sensing, high compatibility, less toxic, electronic, sensing and reactivity make them most potentially productive in medical imaging, cancer detection and drug delivery. Physical absorption, ionic or covalent bonding, or both, can be employed to attach anticancer medications to the exterior of AuNPs, which can be controlled by external activation or biological stimuli [Citation22]. Detection of cervical and pancreatic tumor by biopsy can be done by AuNPs associated with antibodies [Citation2].
Nanoshell – Nanoshells are spherical nanoparticles of 10–300 nm size made up of dielectric core enveloped in a thin metallic shell, most commonly gold. Plasmon resonance can be tailored to any wavelength area either near visible or infrared by altering relative size of inner and outer shell layer. Nanoshells with resonance near infrared between 700 and 1100 nm in wavelength range of spectrum depending on their size can act like well build structures with absorption and scattering of mid-infrared light [Citation23]. Gold nanoshells are mostly preferred for cancer treatment and bio-sensing. Nanoshells when linked with antibody for specific delivery into cell-line model has proved desired tumor death only and does not intact normal cell [Citation24].
microRNA Detections – For early detection of cancer, compact non-coded single-stranded ribonucleic acid molecules with composition of 19-23 nucleotides microRNA are recognized as non-invasive biomarker. miRNAs play significant part in the development of cancer like proliferation, programmed cell death (apoptosis) and metastatic cancer [Citation25]. Over expression of miRNA appeared in both oncogenes and cellular regulation process. In accordance with research, miR-196a/b-3p and miR-106a-5p were circulating and their over expressions were shown in the metastasis cancer stage. mir-21 is a diagnostic biomarker for several types of solid cancers, because it is an anti-apoptotic factor which prevents apoptosis specifically up regulated and these are also linked to abnormal growth in tumors [Citation26]. miRNA is used as therapeutic target. Fluorescence methods, like FRET, electrochemical method and colorimetric-based biosensors, can be used for identification of miRNA.
DNA methylation detection – It is a cell-specific marker important for transcriptional regulation and genomic imprinting [Citation27]. Most of the deoxyribonucleic acid methylation molecular markers are identified or measured for various cancers, including lung, prostate, breast and colorectal cancers [Citation28]. Various alterations like hypermethylation linked to the deactivation of tumor suppressor genes (loss of function), cell cycle genes and deoxyribonucleic acid damage repair genes and hypomethylation causes tumor formation through the unusual switching on proto-oncogenes, loss of imprinting, or genetic instability, which can be used for therapeutic monitoring as well as cancer detection, classification and prognosis. DNA methylation is measured by fluorimetric nanobiosensors [Citation29]. Detection through nanobiosensors proved to be quick and sensitive for diagnosing early stage in cancer. Most nanomedicines are self assembled and can carry maximum drug proportions.
Table 2. Approved nanotherapeutics for cancer diagnosis.
Nanovehicles in cancer therapy
Liposome – It is an organic nanocarrier, biocompatible and sturdy in colloidal solution with less or equal to 400 nm vesicles. Liposomes are bi-layer vesicles, which are soluble fractions of lipid. Liposome membrane consists of phospholipids which can be natural or manmade. They are very viable andamphiphilic (both hydrophilic and hydrophobic parts) in nature, and their surfaces are easy to modify because of which they are considered appropriate for drug delivery and treating various infectious diseases and cancer. When liposome is functionalized with polyethylene glycol, they tend to increase half-life circulation and shield residual charge on surface. For liposome, carrier’s size is important because it involved an important function as encapsulating the drugs into liposome or their half-life circulation. Small-sized liposome showed higher chance of evading phagocyte ingestion [Citation36]. Liposome showed less toxicity when compared with other free drugs. Doxil was approved in 1995 by FDA, and this nanodrug is used to treat a variety of malignancies, from acquired immune deficiency syndrome-related Kaposi’s sarcoma to metastatic ovarian cancer. Doxil works through two different mechanisms: intercalation into DNA molecules, which prevent topoisomerase and nucleic acid repair, intracellular formation of reactive oxygen species and free radicals, which cause lipid oxidative degradation and damage cellular membrane. Both Doxil and Lipodox follow a passive targeting method to accumulate into tumors by the enhanced permeability retention (EPR) effect [Citation37]. An antifungal medication used in treating the several fungal infections is called amphotericin B (AmB) or L-AmB. It specifically targets cell membranes, showing a stronger affinity for the fungal cells membrane that contains ergosterol, when compared with mammalian cell membranes that include cholesterol. Unlike other antifungal medicines, AmB does not cause the establishment of resistant fungus species as it does not operate by inhibiting enzymes. Hybrid lipid nanoparticles act as theranostics, and it can do both labeling and killing cancer cells.
CNTs – CNTs are nanometer-sized carbon tubes. CNT dimensions are reported in a range of ratios with sizes between 10 and 15 nm. CNTs are tubular structures creation done by wrapping a graphene thick film into cylindrical form in nanometer range [Citation38]. According to the number of sheets of carbon atoms, CNTs are often divided into single-wall NTs (SWNTs) and multiwall NTs (MWNTs) and both of them are essential for cancer research and have unique capabilities [Citation39]. SWCNTs are composed of a single film of graphite curved into a tube shape, while MWCNTs are made of a densely packed assembly of SWCNTs to mimic the rings of a trunk of the tree. SWCNTs are used to make MWCNTsfunctionalized with polyethylene glycol to improve biocompatibility. Medicines must contain much more storing capacities and prolonged half-lives during circulation, and SWCNTs can spread throughout the cell membrane more easily. Because of these types of unique properties, SWCNTs are micro/nanocarrier helpful in chemotherapeutic and detecting cancer. SWCNTs possess electric properties that MWCNT variations cannot share. It is more difficult to produce SWCNTs when compared with MWCNTs. Thermal effect property of CNTs in which they absorb infrared region light results in heating of nanotubes. SWCNTs are bounded with anticancer drug Paclitaxel. These types of nanostructures have proved to be more systematically enhanced and can cause the death of malignant cells A549 and NCI-H460. A study conducted in 2019 found that employing survivin siRNA including doxorubicin coupled SWCNT transporter like two agents enhancing programmed cell death and also generating reduced survivin for inhibiting apoptosis gene [Citation40]. As a carbon-based nanomaterial that causes hyperthermia, MWNTs can be employed for photothermal therapy to eliminate cancer cells. An active targeted-cum-pH responsive method for DOX administration to malignant cells has been created; synthetic folic acid (FA) has been linked to MWCNTs. For the manufacturing of FA-bound multipurpose MWCNTs, chemical-treated MWCNTs are being covalently coupled with polyethyleneimine followed by sequential change with FA, fluorescein isothiocyanate, triethylamine and acetic anhydride. The resulting nanocarriers demonstrated enhanced biological compatibility and colloidal strength. FA-bound MWCNTs demonstrated pH-sensitive DOX discharge in acid settings as well as relatively greater DOX loading of up to 70.4%. CNTs could improve treatment efficiency by focusing specific targeted site by accumulating inside diseased area like sites related with therapeutic effect. Other features of CNTs include Raman and photoluminescence, and these properties help in improving nanotubes.
Polymer Micelles – Polymeric micelles are nanoscopic core or spheroid nanomicelles generated in an aqueous solution from the buildup of amphiphilic block copolymers. The hydrophobic core and hydrophilic surface of micelles have allowed poorly soluble drug loading, alteration and conjugation for selective brain medication delivery. Micelles are also a type of nanomaterial that can be exploited to create effective cancer-targeting therapies treatment [Citation41]. The core-shell construction is done by self-building of amphipathic block copolymers in saturated fluid and distinguishes these nanoconstructs, enhancing the dissolving nature for transporting moieties, increasing pharmaceutical therapeutic potency, and lessen adverse effects on healthy tissues. Micelles were synthesized using di-block or tri-block homo-chiral and hetero-chiral block copolymers. Micelles are certainly composed of amphipathic block copolymers along photodegradable linkers that act like crossover, linking chains of hydrophilic and hydrophobic groups. The polymer units were mainly composed of PEG and polyamino acid units with poly(aspartic acid) as well as poly (glutamic acid-hydroxamate) [Citation42]. By using TEM, the micelles’ sizes were determined to be between 19 and 200 nm. The critical micelle concentration of a copolymer is the amount required for micelles to form. The required quantity for micelle needs to be minimum so that it stops micelles from dissociating after being diluted in the bloodstream [Citation43]. Micelles can range between 10 and 100 nm, which is ideal for EPR process in proximity to cancer area, and it also shows long-lasting circulation time in blood. Two processes are used by polymeric micelles to transport therapeutic load to carcinoma. The therapeutic agents can either be internally absorbed as pharmaceuticals consolidated inside micelles for its expression in cells, or the drug might be released from the micelles to act on cancer cells. Physically entrapped pharmaceuticals are primarily released by diffusion, whereas chemically associated drugs are discharged via micelle bulk degradation or surface erosion [Citation44]. Micelles also have the potential to create effective phototherapy nanomaterials. According to Deng et al.’s reports, micelles synthesis consists of amphipathic iridium-based photo-sensitizer (i.e. C14-IP2000) packed along (zinc (II)-2,9,16,23-(tetra-t-butyl)phthalocyanine) photothermal drug, used for the creation of micelles. These nanoconstructs were suited for combination of tumor ablation because they had excellent photodynamic altering capability, considerable photo-thermal changing capacity, efficacious in blood passage and passive tumor attacking ability [Citation45]. Liquid-filled polyurea microcapsules, single-molecular micelles including PMMA and poly(ethylene glycol) methyl ether methacrylate (PEGMA), nanomicelles constructed with polyethylene glycol coupled to doxorubicin by a ultraviolet-sensitive amide associations are some examples of photo-cleavable polymeric arranged designs [Citation46]. Micelle-based transport methods that potentially function as effective and tumor-targeting nanoplatforms, include telmisartan, camptothecin, gemcitabine, etoposide phosphate, deoxycholate acid and tretinoin called as all-trans retinoic acid.
QDs – QDs are extremely tiny semiconductor-based nanocrystals ranging between 1 and15 nm with remarkable optical capabilities, and particle size of QDs determine their optical properties. They were first discovered by analyst Alexei Ekimov, and he did focused study about semiconductors in the 1980s. Tetravalent elements like carbon, silicon, and germanium, with four electrons within its protective shell, make up a QD. These elements also share other physical–chemical characteristics like their metalloid composition or semiconducting electrical capabilities like small particle size, tunable structure and wide range of qualities with attractive functions, including solar cells, LED technology and other biomedical ones like imaging, drug transport, and photodynamic therapy to treat cancer. According to Abdellatif et al. (2022) in 2021, the market for QDs is expected to be worth USD 4 billion, and in the following 5 years, it is expected to increase to USD 8.6 billion [Citation47]. LED and display device technologies occupied QDs market. Market products, such as VIVODOTS®, uses QDs to map tumors tissues intra-operatively and also prevented the needless excision of healthy tissues. Another market participant controlled by NNCrystal US CorporationTM is NN-Labs®. Cadmium-based and cadmium-free QDs both used for cellular imaging, in vivo imaging, or like probes in fluorescent labeling are the products of biomedical importance.
Nanorobots
Nanorobots are small robots that can precisely construct and operate items at the nanoscale. The first generation of miniature robots were known as ‘micromotor’ or ‘nanoengines’, which are characterized like micro-level devices ability to transport different energy forms into motion or control. Micro/nanorobot, in contrast very compact forms, can carry out preplanned operations by automatic input. Because of their micro/small size and controlled direction abilities, micro/nanorobots have found extensive use in a wide range of areas, which would include therapeutic target transport, cell capture along with dissociation, analyzing, identification, environmental biological treatment, cancer treatment and diagnostics. Research is progressing in movement control techniques for micro-nanorobots. As an illustration, the directional movement of micro-nanorobots probably carried out by applying electromagnetic field; magnetic field, ultrasonic field and optical field. They can play significant function for the development of minimally invasive diagnostic and therapeutic procedures for a range of illnesses, including cancer, diabetic, gene therapy, surgery, breaking kidney stones, neurological and cardiovascular ailments. Due to their nanoscale, they behave as nanosurgeons, finding a direct path to the cell and being able to direct medication delivery to the location of action. Although the technology for antiviral diagnostics and therapies has not yet been fully explored, the future will undoubtedly see study in this area. Numerous microbots or nanorobots have been thoroughly created consequently for advancements in micro-nanomaterial synthesis technology, and these particles possess numerous potential functions, particularly for the biomedical industry. The main components of biomedicine are diagnosis and treatment [Citation48]. These nanobots with help of ultrasonic signals and lasers can attack tumor at its site or deliver accurate drug at cancer site. Drug naoparticle carriers must be used to ensure successful and targeted cancer diagnosis by delivering drug within acceptable period or distance from targeted locations. As a result, the cells can take in and release the preloaded medications. Potential to avoid impediments, elude from the immune response, and exhibit regulated motion are hence requirements for optimal drug carriers. Energy supply source, sensors, actuators, embedded computers, pumps and structural strength are the main components of nanorobotic systems. It also features a payload chamber for the medicine to be loaded and a tiny camera to travel through the bloodstream. Earlier diagnosis of cancer in patient increases the survival rate.
Robotic Engines – Microrobots can also be powered by ultrasound, an outside source of energy. First ultrasound-powered microbots or nanorobots were build by metallic asymmetric nanowires that were propelled through waveguide [Citation49]. Targeted auditory streaming stress that was generated onto surface of asymmetric nanowires served as the motor that propelled the devices forward. More recent research has used traveling waves to cause bubble entrapped inside microrobots model to oscillate and high-intensity focused ultrasound to cause chemical fuels to vaporize quickly, causing microrobots to move in a bullet-like manner. Developing nanorobots, sometimes referred to as active colloids and micro/nanomotors, are potent tools at the micro/nanoscale which can translate a range of energy forms into mechanical movement. The propelled force allows micro/nanorobots to independently penetrate biological membrane barriers such as the blood–tumor barrier, blood–brain barrier and thick extracellular matrix rather than passively moving through blood flow. Nanorobots’ ability to deliver drugs directly to tumors can significantly increase medication bioavailability and lower dosage requirements, hence lowering adverse effect risks [Citation50]. There are two categories of nanorobots: outfield drive and self drive. Outfield drives, including optical drives, sound field drives, magnetic field drives, and so forth, typically have outfield sources for their driving power. Self-driving nanorobots can generate energy from fluid environments via enzymes or chemical processes. Bio-hybrid nanorobots are also under study.
Magnetic Nanorobots – As the name suggest, magnetic field is used in these types of nanorobots. Magnetic nanorobots have the advantage of enhancing drug penetration in solid tumors. A nanospinner consisting of magnetic nanoparticles may boost level of drug invading by using mechanical power on a revolving electromagnetic field also focusing it onto mitochondria underlying tumor cells. The magnetic nanorobots must be supervised in order for them to be effective. According to a paper study (Lu et al., 2021), they designed magnetic nanorobot which may alter its form to travel across bent and narrow bloodstream [Citation51]. Nanoparticles easily travel inside body blood system like fluid moves, responding to fluctuating electromagnetic field around it.
Bio-hybrid Nanorobots – It is possible to create biohybrid micro/nanorobots which have preferable functions by combining the positive qualities of complex biological components and includes increased energy efficiency, more power-to-weight ratio, adequate power capacity, biocompatibility, self-repair and self-assembly, with non-living systems at different range of particles, cells, organisms and tissues. Motile microorganisms, which are in charge of locomotion, are combined with synthetic structures to create bio-hybrid robots, which have other functionalities. Recent developments in synthetic biology have made it possible to improve bacterium that moves powers not with usage of synthetic elements. As instance, genetically modified microbes are being designed for producing variety of active components, including medicinal payload, gas-filled microstructures and magnetic particles. It is necessary to use powerful actuators, such as living cells or creatures, as well as appropriate structure design in order to create bio-hybrid MNRs which can exhibit biomaterials behavior along with carry out on-demand activities. Bioimaging and cancer therapy are some applications for biohybrid robots that show promise [Citation52]. In bio-hybrid nanorobots, chemotherapeutic drug delivery and bioimaging capabilities are combined. Through the site-specific delivery of biohybrid nanorobots to the cancerous cell area, the therapeutic medicine is delivered to destroy the malignant cells and the fluorescence signal can be used to detect the significant impact.
DNA Nanorobots – Biological processes in nature normally use the DNA molecule to store and transfer genetic information. Deoxyribonucleic acid can act as best drug carrier. DNA is biological a material found in body and also biodegradable so less immune response with more targeting capacity. DNA nanotechnology has developed a method for customizing the biological availability and activity of nanostructures by removing the DNA molecule from organic environment and utilizing essential constructing component for creating nanostructures of well-defined but still seemingly limitless sizes and shapes [Citation53]. Massive advancements in the construction of useful DNA nanostructures have combined fundamental functionalities and then prompted a variety of stimulus-responsive processes for completing challenging jobs in a predetermined way along biochemical precision. DNA nanostructures were evolved from a stable to a more specific form which is increasingly capable. DNA may attach elements having high-negative phosphate groups by destroying extremely coordinated onto exterior water surface that is essential for its durability. Nanoparticle self-assembly and drug delivery can both be accomplished using DNA origami. Bottom up process is used for making nanomedicines smaller in size and efficient. The latter can cause the target to move voluntarily in vivo by coupling the aptamers. The substances that are absorbed by antibodies, or environmental conditions (including pH or temperature) trigger them to respond (like nucleic acid strands and small molecule immunoglobulin). According to paper (Lu et al., 2021) in year 2018, Li et al. created autonomous DNA nanorobot technique capable of detecting malignant endothelial cells and cause tumor vascular embolism [Citation51]. As a result, the cancer cells blood circulation cut off while circulatory network of healthy tissues remained unharmed.
DNA origami – Rothemund developed the DNA origami method in 2006. A lengthy scaffold of single-stranded DNA is folded into the appropriate DNA origami structure utilizing more than hundred staple strands to maintain the scaffold order. By using the DNA origami approach, DNA nanostructures with clearly specified homogeneous geometries, exact spatial addressability and noticeable biocompatibility can be rationally designed and produced. DNA origami nanostructures or nanoscale folding of DNA created two- or three-dimensional shapes within nanoscale demonstrated EPR properties. The blank canvas of DNA origami can hold a variety of therapeutic payloads as well as tumor-targeting molecules along logically planned codes or designs can be found over the accessible nanostructure. Because of its distinctive advantages, DNA origami nanostructures include variety of biomedical uses. At the cellular level, DNA origami owns capability to function like intelligent drug delivering technique and biological molecule machines. Particularly, DNA origami with administered structure, biocompatibility, as well as to other biological molecules (including such proteins, liposomes and nucleic acids) allows versatile environment considering designing molecular interfaces also improving functionality for hybrid nanostructures [Citation54]. Examples include DNA-based nanorobots which control cell signaling and DNA origami transporters packed with medicines, CpG sequences, enzymes, and tiny interfering RNA. DNA nano-based delivery of thrombin using the DNA origami approach helped in creating tube-shaped DNA nanorobot for delivering thrombin to tumor arteries only, inducing thrombosis for the treatment of tumors. The development of nanorobots that contain thrombin is poised to provide a tumor infarction method that is therapeutically promising [Citation55]. Thrombin placed within the nanorobot’s inner cavity protecting extremely responsive molecular cargo from interactions with the environment. Being used on control mouse models with tumor growth, thrombin in unwrapped nanorobot stimulated concentrated coagulation against precisely occludes tumor blood arteries. This caused tumor to ‘starve to death’.
In a paper study (Aye & Sato, 2022), DNA origami constructed forms were used for delivering anticancer drug DOX to the MCF7 cell line in breast cancer. Double helices of Watson-Crick nucleotides pairs are used as docking sites for doxorubicin inserts [Citation56]. After 24 hours of treatment, the internalization of DOX-origami nanostructures and the co-localization indicators between drug as well as transporters were discovered on cytoplasm by confocal fluorescence microscopy analysis. In a normal cell line, origami-coupled medicines efficiently caused cell degradation. Drug-resistant MCF7 cells could not be killed by free drug or loaded drugs double-stranded DNA, but the origami-bound drug resulted in the death of cancer cells, showing how transporter-coupled medication can control drug resistance. For doxorubicin delivery tests, DNA origami nanostructures in the shapes of a triangle, a tube, and a ribbon have been employed. Few other studies demonstrated increased amount of medications quite possibly placed within 3D DNA origami; however 2D structures released them more quickly. It is possible to employe DNA nanostructures as imaging agents for cellular identification by chemically modifying them with fluorescent chemicals. Fluorescent probes like cyanine dye molecules may be covalently integrated onto DNA origami strands and used for direct visualization within living cells [Citation57]. DNA origami nanostructures linked efficiently to infrared-emitting QDs which continue to be steady around increased salt concentrations.
Conclusion
Nanotechnology is an advanced field with numerous applications. In nanotechnology, lot of progress is going on for improving cancer detection. Number of disease exists having no proper cure but in future with help of nanotechnology chances in finding solutions are positive. Using nanomedicine in targeting cancer is a potentially good alternative. Nanomedicines have advanced significantly in several areas, particularly in the treatment of cancer. High therapeutic efficacy, reduced toxicity, site-specific delivery, tailored binding with the ligand, and cost-effectiveness are all benefits of nanoparticle-mediated anticancer drug delivery, and their extraordinary ability to penetrate tissue, specifically retain, and kill tumor cells, nanomedicines are helpful for treating and preventing several types of cancer. Nanocarriers or nanomedicines directly go to targeted cancer site. Nanocarriers or nanomedicine used in drug delivery should be used carefully. Nanosciences can help in improving personalized cancer therapy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Zhang Y, Li M, Gao X, et al. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12. doi: 10.1186/s13045-019-0833-3
- Chaturvedi V, Singh A, Singh V, et al. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab. 2019;20(6):416–429. doi: 10.2174/1389200219666180918111528
- Bayda S, Adeel M, Tuccinardi T, et al. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2019;25(1):112. doi: 10.3390/molecules25010112
- Brignole C, Pastorino F. Special issue “Recent advances in precision nanomedicine for cancer”. Molecules. 2020;25(18):4148. doi: 10.3390/molecules25184148
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011
- Chavda VP, Khadela A, Shah Y, et al. Current status of cancer Nanotheranostics: emerging strategies for cancer management. Nanotheranostics. 2023;7(4):368. doi: 10.7150/ntno.82263
- Sajjadi M, Nasrollahzadeh M, Jaleh B, et al. Carbon-based nanomaterials for targeted cancer nanotherapy: recent trends and future prospects. J Drug Targeting. 2021;29(7):716–741. doi: 10.1080/1061186X.2021.1886301
- Kim D, Lee N, Park YI, et al. Recent advances in inorganic nanoparticle-based NIR luminescence imaging: semiconductor nanoparticles and lanthanide nanoparticles. Bioconjugate Chem. 2017;28(1):115–123. doi: 10.1021/acs.bioconjchem.6b00654
- Singh D, Dubey P, Pradhan M, et al. Ceramic nanocarriers: versatile nanosystem for protein and peptide delivery. Expert Opin Drug Delivery. 2013;10(2):241–259. doi: 10.1517/17425247.2012.745848
- Aghebati‐Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2019;235(3):1962–1972. doi: 10.1002/jcp.29126
- Yih TC, Al‐Fandi M. Engineered nanoparticles as precise drug delivery systems. J Cell Biochem. 2006;97(6):1184–1190. doi: 10.1002/jcb.20796
- Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147–166. doi: 10.1016/j.nano.2011.05.016
- Fan J, Cheng Y, Sun M. Functionalized gold nanoparticles: synthesis, properties and biomedical applications. Chem Rec. 2020;20(12):1474–1504.
- Sakthi Devi R, Girigoswami A, Siddharth M, et al. Applications of gold and silver nanoparticles in theranostics. Appl Biochem Biotechnol. 2022;194(9):4187–4219. doi: 10.1007/s12010-022-03963-z
- Ahmadi F, Sodagar-Taleghani A, Ebrahimnejad P, et al. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int J Pharmaceut. 2022;625:122099. doi: 10.1016/j.ijpharm.2022.122099
- Mathur P, Jha S, Ramteke S, et al. Pharmaceutical aspects of silver nanoparticles. Artific Cells Nanomed Biotechnol. 2018;46(sup1):115–126. doi: 10.1080/21691401.2017.1414825
- Markwalter CF, Kantor AG, Moore CP, et al. Inorganic complexes and metal-based nanomaterials for infectious disease diagnostics. Chem Rev. 2018;119(2):1456–1518. doi: 10.1021/acs.chemrev.8b00136
- Borel T, Sabliov CM. Nanodelivery of bioactive components for food applications: types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Annu Rev Food Sci Technol. 2014;5:197–213. doi: 10.1146/annurev-food-030713-092354
- Bockamp E, Rosigkeit S, Siegl D, et al. Nano-enhanced cancer immunotherapy: immunology encounters nanotechnology. Cells. 2020;9(9):2102. doi: 10.3390/cells9092102
- Baetke S, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. British J Radiol. 2015;88(1054):20150207. doi: 10.1259/bjr.20150207
- Zhang Y, Li M, Gao X, et al. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12(1). doi: 10.1186/s13045-019-0833-3
- Montané X, Bajek A, Roszkowski K, et al. Encapsulation for cancer therapy. Molecules. 2020;25(7):1605. doi: 10.3390/molecules25071605
- Lal S, Clare S, Halas N. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851. doi: 10.1021/ar800150g
- Majoros I, Ward B, Lee K, et al. Progress in cancer nanotechnology. Prog Mol Biol Transl Sci. 2010;95:193–236.
- Saliminejad K, Khorram Khorshid H, Soleymani Fard S, et al. An overview of microRnas: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2018;234(5):5451–5465. doi: 10.1002/jcp.27486
- Rezayi M, Farjami Z, Hosseini Z, et al. MicroRNA-based biosensors for early detection of cancers. Curr Pharm Des. 2019;24(39):4675–4680. doi: 10.2174/1381612825666190111144525
- Sina AAI, Carrascosa LG, Trau M. DNA methylation-based point-of-care cancer detection: challenges and possibilities. Trends Mol Med. 2019;25(11):955–966. doi: 10.1016/j.molmed.2019.05.014
- Syedmoradi L, Esmaeili F, Norton ML. Towards DNA methylation detection using biosensors. Analyst. 2016;141(21):5922–5943. doi: 10.1039/C6AN01649A
- Dadmehr M, Hosseini M, Hosseinkhani S, et al. DNA methylation detection by a novel fluorimetric nanobiosensor for early cancer diagnosis. Biosens Bioelectron. 2014;60:35–44. doi: 10.1016/j.bios.2014.03.033
- Klochkov SG, Neganova ME, Nikolenko VN, et al. Implications of nanotechnology for the treatment of cancer: recent advances. In: Seminars in cancer biology. Vol. 69. 2021 February. pp. 190–199.
- Kopeckova K, Eckschlager T, Sirc J, et al. Nanodrugs used in cancer therapy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2019;163(2):122–131. doi: 10.5507/bp.2019.010
- Ali ES, Sharker SM, Islam MT, et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: current status and future perspectives. In: Seminars in cancer biology. Vol. 69. Academic Press; 2021 February. pp. 52–68.
- Krukiewicz K, Zak JK. Biomaterial-based regional chemotherapy: local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater Sci Eng C. 2016;62:927–942. doi: 10.1016/j.msec.2016.01.063
- Halwani AA. Development of pharmaceutical nanomedicines: from the bench to the market. Pharmaceutics. 2022;14(1):106. doi: 10.3390/pharmaceutics14010106
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108
- Tenchov R, Bird R, Curtze AE, et al. Lipid nanoparticles─ from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi: 10.1021/acsnano.1c04996
- Farjadian F, Ghasemi A, Gohari O, et al. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. doi: 10.2217/nnm-2018-0120
- Rahamathulla M, Bhosale RR, Osmani RA, et al. Carbon nanotubes: Current perspectives on diverse applications in targeted drug delivery and therapies. Materials. 2021;14(21):6707. doi: 10.3390/ma14216707
- Tang L, Xiao Q, Mei Y, et al. Insights on functionalized carbon nanotubes for cancer theranostics. J Nanobiotechnol. 2021;19(1):1–28. doi: 10.1186/s12951-021-01174-y
- Sheikhpour M, Naghinejad M, Kasaeian A, et al. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: a critical review. Int J Nanomed. 2020;15:7063. doi: 10.2147/IJN.S263238
- Mehrabian A, Mashreghi M, Dadpour S, et al. Nanocarriers call the last shot in the treatment of brain cancers. Technol Cancer Res Treat. 2022;21:15330338221080974. doi: 10.1177/15330338221080974
- Perumal S, Atchudan R, Lee W. A review of polymeric micelles and their applications. Polymers. 2022;14(12):2510. doi: 10.3390/polym14122510
- Glavas L, Olsén P, Odelius K, et al. Achieving micelle control through core crystallinity. Biomacromolecules. 2013;14(11):4150–4156. doi: 10.1021/bm401312j
- Fatfat Z, Fatfat M, Gali-Muhtasib H. Micelles as potential drug delivery systems for colorectal cancer treatment. World J Gastroenterol. 2022;28(25):2867. doi: 10.3748/wjg.v28.i25.2867
- Niculescu AG, Grumezescu AM. Novel tumor-targeting nanoparticles for cancer treatment—A review. Int J Mol Sci. 2022;23(9):5253. doi: 10.3390/ijms23095253
- KubiakA T. Polymeric capsules and micelles as promising carriers of anticancer drugs Kapsułki i micele polimerowe jako nośniki leków przeciwnowotworowych. Polymers In Medicine. 2022;52(1):37–50. doi: 10.17219/pim/145513
- Abdellatif AA, Younis MA, Alsharidah M, et al. Biomedical applications of quantum dots: overview, challenges, and clinical potential. Int J Nanomed. 2022;17:1951. doi: 10.2147/IJN.S357980
- Zhang Y, Zhang Y, Han Y, et al. Micro/Nanorobots for medical diagnosis and disease treatment. Micromach. 2022;13(5):648. doi: 10.3390/mi13050648
- Soto F, Wang J, Ahmed R, et al. Medical micro/nanorobots in precision medicine. Adv Sci. 2020;7(21):2002203. doi: 10.1002/advs.202002203
- Zhang D, Liu S, Guan J, et al. “Motile-targeting” drug delivery platforms based on micro/nanorobots for tumor therapy. Front Bioeng Biotechnol. 2022;10. doi: 10.3389/fbioe.2022.1002171
- Lu W, Yao J, Zhu X, et al. Nanomedicines: Redefining traditional medicine. Biomed Pharmacother. 2021;134:111103. doi: 10.1016/j.biopha.2020.111103
- Sun L, Yu Y, Chen Z, et al. Biohybrid robotics with living cell actuation. Chem Soc Rev. 2020;49(12):4043–4069. doi: 10.1039/D0CS00120A
- Hu Y. Self-assembly of DNA molecules: towards DNA nanorobots for biomedical applications. Cyborg And Bionic Systems. 2021;2021. doi: 10.34133/2021/9807520
- Wang ST, Gray MA, Xuan S, et al. DNA origami protection and molecular interfacing through engineered sequence-defined peptoids. Proc Nat Acad Sci. 2020;117(12):6339–6348. doi: 10.1073/pnas.1919749117
- Bell TH, Kaminsky LM, Gugino BK, et al. Factoring ecological, societal, and economic considerations into inoculant development. Trends Biotechnol. 2019;37(6):572–573. doi: 10.1016/j.tibtech.2019.02.009
- Aye SL, Sato Y. Therapeutic applications of programmable DNA nanostructures. Micromach. 2022;13(2):315. doi: 10.3390/mi13020315
- Udomprasert A, Kangsamaksin T. DNA origami applications in cancer therapy. Cancer Sci. 2017;108(8):1535–1543. doi: 10.1111/cas.13290