?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The great majority of the rural populations in developing countries relies on traditional medicinal plants for treating different diseases. This study investigated the antibacterial activity of Calpurnia aurea, Vernonia amygdalina, and Rumex nepalensis collected from Goba District, Bale Zone, southeastern Ethiopia against five human bacterial test pathogens (Escherichia coli, Salmonella typhi, Staphylococcus aureus, Shigella dysenteriae and Pseudomonas aeruginosa). Plant specimens were extracted with 70% ethanol, 80% methanol, petroleum ether, and distilled water. The crude extracts were evaluated against test pathogens using agar well diffusion and micro dilution methods. The highest yield was achieved by aqueous extract of C. aurea leaf (16.6 mg/ml). The antibacterial activity of the plants’ extracts ranged from a zone of inhibition of 5.4 ± 0.4 mm to 23.0 ± 0.3 mm. Overall, the highest zone of inhibition (23 ± 0.3 mm) and the highest activity index (AI) comparable to 80% of inhibition by standard antibiotic ciprofloxacin (5 µg/disc) were revealed by the methanol extract of C. aurea leaves against S. aureus. The smallest minimum inhibitory concentration (0.78 mg/ml) was achieved by 80% methanol extracts of C. aurea roots and V. amygdalina leaves against S. aureus and E. coli, respectively. Our study revealed promising antibacterial activity of the evaluated plants.. Further studies are required to evaluate the active ingredients at the in-vitro and in-vivo levels.
Introduction
Globally, diarrhea diseases are among the leading infectious causes of morbidity and mortalities. The use of plants in maintaining human health began as folk medicine and through the years has been incorporated into traditional and allopathic medicine [Citation1]. Medicinal plants serve as leads for new drug development [Citation2]. About 80% of people in developing countries totally rely on herbal drugs for their primary healthcare, and more than 25% of prescribed medicines in developed countries are derived from wild plant species [Citation3]. In Ethiopia, 95% of traditional medicine preparations are made from plants [Citation4].
Multidrug-resistant strains of bacteria have become a major public health problem in recent years [Citation5]. Their spread may potentially be curtailed by the use of plant-based antimicrobials, which are safer than synthetic alternatives, accessible in local communities, inexpensive to purchase, easy to administer, and more affordable [Citation6]. Ethno-botanically derived compounds have greater antimicrobial activity and potential for development of novel products than compounds derived from random screening [Citation7].
Ethiopia is endowed with great plant biodiversity that is associated with the vast use of plant-based medicines for treating many human and animal diseases [Citation4]. Calpurnia aurea, V. amygdalina, and R. nepalensis are medicinal plants endemic to Bale Zone in southeastern Ethiopia that are used to treat diarrhea diseases and other gastrointestinal disorders; C. aurea, locally called cheketa or cheka in Afan Oromo and digita in Amharic, belongs to the family Fabaceae and is mostly found in Bale Zone [Citation8]. The leaves, seeds, and/or roots of C. aurea are used to treat diarrhea, dysentery and abdominal pain, syphilis, malaria, rabies, diabetes, pulmonary TB, hypertension, leishmaniasis, trachoma, elephantiasis, fungal diseases, swellings, stomachache, abscesses, and bladder disorders as well as to kill maggots, lice and relieve itching [Citation8–11].
V. amygdalina is a perennial shrub belonging to the family Asteraceae [Citation12] that grows throughout tropical Africa. It is commonly called bitter leaf due to its bitter taste [Citation13]. The plant is locally named girawa and ebicha in the Amharic and Afan Oromo languages, respectively [Citation14]. It has wide spectrum potencies, being used for the treatment of stomachache, wounds and infections, swelling, diabetes, insomnia, tooth ache, acne, pneumonia, stroke, arthritis, fatigue, cough, diarrhea, scabies, hepatitis, ascariasis, tonsillitis, fever, mastitis, tapeworm, and worm infection [Citation15,Citation16]. Rumex nepalensis, commonly known as Nepal Dock, belongs to the family Polygonaceae [Citation17]. It is locally known as tult in Amharic and shabbe in Oromic [Citation8] also lut in Amharic [Citation18] and grows abundantly in many parts of the country. In Ethiopia, the pounded roots of R. nepalensis are given to humans with diarrhea, stabbing pain, colic, and other intestinal problems [Citation8,Citation10].
Several ethnomedicinal survey of C. aurea, V. amygdalina, and/or R. nepalensis were conducted in south east Ethiopia [Citation8], in Ada’a District, East Shewa Zone, Ethiopia [Citation14], in Bale Mountains National Park, Ethiopia [Citation15,Citation19], and in Goba District of Bale Zone, Southeast Ethiopia [Citation20]. Laboratory-based studies of the antibacterial activity of extracts of C. aurea were reported from Ethiopia and elsewhere. The methanol extract of C. aurea leaf exhibited a promising antibacterial against skin disease causing bacterial pathogens [Citation9]. The ethyl acetate and dichloromethane extract of C. aurea leaf from Libo Kemkem District, Ethiopia revealed significant antibacterial effects growth of pathogenic bacteria [Citation21]. Melese et al. [Citation22] evaluated the antibacterial activity of C. aurea (leaf, bark) from Wonsho and Shebedino districts, southern Ethiopia found that the methanol extract of C. aurea bark to have higher antibacterial activity than the leaf extract. The ethanol extracts of C. aurea bark, leaf, stem and root samples from Guder town, West Shewa Zone, Ethiopia showed a high antibacterial activity against several bacterial pathogens [Citation23]. Umer et al. [Citation24] reported the methanol extract of C. aurea leaf to have a good antimicrobial activity.
The methanolic and ethanolic crude extracts of V. amygdalina leaves collected from Haramay University, Eastern Ethiopia showed the highest inhibitory effects against all bacterial pathogens compared with the water crude extracts on all bacterial pathogens [Citation25]. Phytochemical analysis of the ethanolic extracts of V. amygdalina revealed the presence of flavonoids, alkaloids, steroids, terpenoids, glycosides, tannins, phenols, saponins, and the absence of anthraquinones [Citation26] which have different activities. Various secondary metabolites were isolated from root and aerial parts of R. nepalensis which have pharmacological activities such as anti-inflammatory, antioxidant, antimicrobial, wound healing, and anti-plasmodial activities [Citation18]. Antibacterial activity of crude extract of R. abyssinicus root, a species related to R. nepalensis showed a high antibacterial activity with its acetone extract from Ethiopia [Citation27]. But studies in-vitro antimicrobial study of these plants particularly in Goba District of Bale Zone are absent. Evaluation of the efficacy of plants in different agroecology could potentially affect the accumulation of bioactive pharmaceutical ingredients that are found in plants [Citation28]. The purpose of this study was therefore to investigate the antibacterial activities of the solvent extracts of some plant parts of C. aurea, V. amygdalina, and R. nepalensis on some bacterial pathogens in-vitro.
Methods and materials
Study area
The study was done on plant samples collected from Goba district. Bale Zone, southeastern Ethiopia. Goba District lies between 6°36’00’’ N and 7°10’00’’N latitude and between 39°35’00’’E and 40°15’00’’ E longitude (). The altitude of the district ranges from about 2,400 to 4,377 m. Mean monthly temperatures of the district range from 4°C to 25°C. Mean annual rainfall varies from 900 mm in the lowlands to 1,400 mm in highlands. The district is endowed with many plants used to treat human diseases [Citation15,Citation20].
Collection of plant materials
Fresh C. aurea leaves and roots, V. amygdalina leaves and R. nepalensis roots () were collected from fields and forests of Goba District (). These plants were botanically identified at the National Herbarium, Addis Ababa University. The selection of the plants was based mainly on the presence of ethno-medicinal knowledge and their use to treat gastrointestinal disorders [Citation15].
Preparation of plant extracts
Each plant part was washed thoroughly with tap water and then rinsed three times with sterile distilled water. The plant part was then air-dried on sterile blotter under the laminar flow hood for a week. The dried plant materials were ground into a powdered mass using mortar and pestle and then sieved using a sieve with 0.5 mm pore size. The powder was stored in a sterile bottle at room temperature until it was extracted with organic and aqueous solvents. Fifty grams of powdered mass of each plant material was macerated for 48 h [Citation29] in 250 ml of 70% ethanol (v/v), 80% methanol (v/v), and petroleum ether (all from Sigma Aldrich) and in distilled water in a 500 ml sterile conical flask covered with a cotton wool plug and wrapped with aluminum foil. The filtrate was separated from debris using Whatman No. 1 filter paper and then concentrated by rotary evaporator (Büchi® rotary evaporator 200, Switzerland) at a temperature of 40 C and 40 revolutions per minute (rpm). The concentrated (crude) extract was transferred to a pre-weighed sterile glass vial, packed into a glass vial, and stored in desiccators over silica gel until use. Yield of each plant extract was calculated as yield percentage:
The working stock solution was obtained by re-dissolving the crude extract of the individual extract in 10% DMSO [Citation30] to yield a final concentration of 200 mg/ml for each extract. Each extract was stored at 4°C in sterile airtight containers in the dark for further studies.
Test organisms
Standard isolates of Gram-positive bacteria (Staphylococcus aureus ATCC 25,923) and Gram-negative bacteria (E. coli ATCC 25,922, Salmonella typhi ATCC 13,311, P. aeruginosa ATCC 27,853, and S. dysenteriae), all obtained from the Ethiopian Public Health Institute, were used for antimicrobial assay. The pure bacterial cultures were maintained on Nutrient Agar Slant (Oxoid, UK) and was sub-cultured regularly to obtain pure culture of the same medium and stored at 4°C as stock culture for future use.
Preparation of inocula
Pure cultures of each bacterial test pathogen were suspended in nutrient broth, streaked onto nutrient agar (Oxoid, UK), and incubated overnight at 37°C. Two to three colonies of pure cultures of the test bacterium were selected and transferred with a sterilized wire loop to a sterile test tube containing 4–5 ml of sterile Muller Hinton broth (MHB) medium (Oxoid, UK). The broth culture was incubated at 35°C until it reached the 0.5 McFarland turbidity standard to prepare 1 × 108 CFU/ml inocula by using UV-Visible spectrophotometer at 625 nm [Citation31,Citation32].
Antibacterial activity through agar well diffusion method
The antibacterial activity of the plant extracts was evaluated using the agar well diffusion method, following standard protocols [Citation31–33]. A sterile cotton swab with a wooden applicator stick was dipped into the standardized bacterial suspension (1–2 × 108 cfu/ml) [Citation31] of the bacterial test pathogen prepared in Muller-Hinton broth (MBH) in 10 ml sterile test tubes. The swab was then evenly streaked in three directions over the entire surface of the Muller Hinton Agar (MHA) (Oxoid, UK) plates to obtain uniform inocula. Four equidistant wells of 5 mm were punched into a MHA plate using a sterile cork borer of 5 mm diameter [Citation34], after which 100 μl of 200 mg/ml plant extract was dispensed into each of the four wells. Ciprofloxacin (Sigma-Aldrich) (5 µg/disc) was used as positive control and 5% DMSO was used as the negative control. The inoculated Mueller-Hinton Agar plates were kept in the refrigerator for 30 min to allow the extracts to diffuse into the agar and were then incubated at 37°C for 16–20 h. The zones of inhibition (hereafter as ZOI) of the tested bacterial test pathogens were recorded.
Determination of activity indices (AIs)
The activity index (AI) of the extract of each plant (at 200 mg/ml) against individual bacterial test pathogens were calculated according to the method of Dharajiya et al. [Citation35] as
Determination of minimum inhibitory concentration (MIC)
The MIC was determined by a broth micro-dilution method on a sterile 96-well microtiter plate (Greiner Bio-One, Germany) following the standard procedures [Citation31–33]. A stock solution of 200 mg/ml was prepared by dissolving 20 mg of the plant material into 100 µl ml of 5% DMSO and was transferred to the well in the first column of the microtiter plate. Fifty μl of sterile MHB was added to the remaining wells on the microtiter plate. From a stock solution (200 mg/ml) of crude extract of each plant, 50 μl were transferred to the well of the second column to yield a 100 mg/ml of plant extract [Citation36]. A double-fold serial dilution was made by transferring 50 μl of the plant extract from the wells in the second column to the subsequent wells to obtain the final testing concentrations of 50 mg/ml to 0.78 gm/ml for MIC. Each plate had a set of two controls. The wells in 10th column contained all solutions (50 μl of sterile MHB and 5% dimethylsulphoxide (DMSO) plus ciprofloxacin (5 μg/ml) as a positive control); the wells in the 11th column contained 100 μl of MHB and 5% DMSO as a negative control [Citation21,Citation31,Citation36]. The density of microbial inoculum was adjusted to McFarland 0.5 turbidity units (1.5 × 108 CFU/ml) [Citation31–33], and 50 μl of the standardized inoculum of the test bacterium was inoculated into every well containing the plant extract, the positive control, and the negative control. The plate was loosely covered with parafilm and incubated at 37°C for 16–20 h [Citation31,Citation32]. Each test was done in triplicate. After incubation, 30 μl of the aqueous solution of 2.5 mg/ml of 2,5-diphenyl tetrazolium chloride (UNI CHEM, India) were added and mixed in each well and further incubated for 30 min. The MIC value for each extract was visually assessed and recorded. MIC was defined as the lowest concentration of the extracts in which no color change was recorded [Citation37].
Statistical analysis
The mean diameter of ZOI produced by each plant extract were expressed as means and standard deviation (mean ± SD) and analyzed using Statistical 7.0.61.0 software (Stat Soft, Inc., 2004) [Citation38], including factorial ANOVA with general linear model (GLM). The normality of the data distribution was checked using Kolmogorov–Smirnov and Shapiro–Wilk tests. Analysis of variance (ANOVA) and the least signifcant difference (LSD) test for multiple means comparison (post-hoc test) were employed to compare the mean ± SD within and between groups of means and the differences were considered statistically significant at p < 0.05.
Results
Yield percentage of plant crude extracts
Yield percentage varied significantly among extracts of the plants and solvents of extraction (p < 0.0001) (). The highest and the lowest yield percentage were obtained by an aqueous extract of C. aurea leaves and the petroleum ether extract of R. nepalensis roots, respectively ().
Table 1. Yield percentage of the solvent extracts of C. aurea, V. amygdalina and R. nepalensis in Goba district, Bale Zone, Southeastern Ethiopia performed at MWU, 2017.
In-vitro antibacterial activity (agar well diffusion method)
The solvent extracts of the three medicinal plants had inhibitory activity against 52.5% of the bacterial test pathogens (). The methanol, ethanol and aqueous extracts of C. aurea leaves showed antibacterial activity against all bacterial test pathogens, but the Petroleum ether extract was inhibitory to only two test pathogens. In extracts of C. aurea leaves, the highest and the lowest pairs of ZOI were achieved by methanol extracts against S. aureus (23.1 ± 0.3 mm) and P. aeruginosa (6.0 ± 0.1 mm). The ethanol and the aqueous extracts exhibited low to moderately high antibacterial activity but they exhibited the inhibitory highest activity against P. aeruginosa and S. dysentriae, respectively. The 80% methanol extract of C. aurea roots exhibited antibacterial activity against three bacterial test pathogens. The highest diameter of ZOI was exhibited against E. coli (21.2 ± 0.41 mm) followed by S. aureus (20.2 ± 0.2 mm). Only P. aeruginosa and S. typhi were moderately inhibited by the 70% ethanol extract. In aqueous extracts, only S. aureus was inhibited but no antibacterial activity was recorded by petroleum ether extracts (; ).
Figure 3. In-vitro antibacterial activity of crude extracts of C. aurea (leaves), C. aurea (roots), V. amygdalina (leaves) and R. nepalensis (roots) against human bacterial test pathogens (at 200 mg/ml); solvent extracts of C. aurea leaf against S. aureus (a) and P. aeruginosa (b); solvent extracts of C. aurea root against E. coli (c) and S. aureus (d); solvent extracts of V. amygdalina leaf against E. coli (e) and S. aureus (f); solvent extracts of R. nepalensis root against S. aureus (g) and E. coli (h); zone inhibition exhibited by the standard antibiotic (ciprofloxacin, 5 µg/ml) against the bacterial test pathogens s: E. coli (i), Shigella dysenteriae (j) Pseudomonas aeruginosa (k), S. aureus (l), salmonella typhi (m); M, E, a and P represents plant crude extracts by 80% methanol, 70% ethanol, aqueous and petroleum ether, respectively.
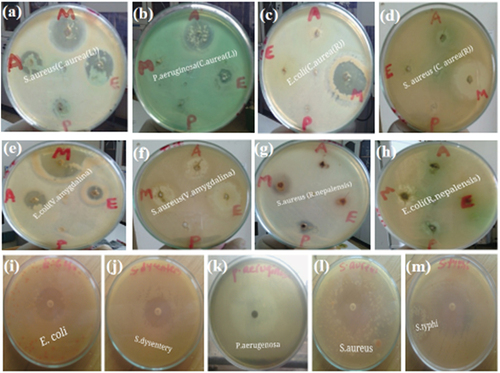
Table 2. Frequency and percentage of inhibitory effects of solvent extracts of C. aurea, V. amygdalina, and R. nepalensis against five bacterial test pathogens in Goba district, Bale Zone, Southeastern Ethiopia performed at MWU, 2017.
Table 3. In-vitro antibacterial activity of solvent extracts of C. aurea, V. amygdalina, and R. nepalensis against five bacterial test pathogens (at 200 mg/ml) in Goba district, Bale Zone, Southeastern Ethiopia performed at MWU, 2017.
In the crude extracts of V. amygdalina, the 80% methanol extracts showed inhibitory activity against all bacterial test pathogens except S. typhi; the highest and the lowest diameter of ZOI were recorded against E. coli (19.2 ± 0.47 mm) and P. aeruginosa (6.0 ± 0.36 mm), respectively. The 70% ethanol extract was inhibitory to all test pathogen extracts and exhibited the highest diameter of ZOI against S. aureus and the lowest against P. aeruginosa. No antibacterial activity was displayed by the petroleum ether extracts (; ). The extracts of R. nepalensis roots generally exhibited low antibacterial activity (; ). The 80% methanol extract was inhibitory to three test pathogens only and the highest diameter of ZOI was detected against S. aureus. The 70% ethanol extract of this plant showed antibacterial activity only against E. coli, but the petroleum extract against two test pathogens (E. coli and S. aureus). The aqueous extract failed to show any inhibition against all bacterial test pathogens (; ).
Activity indices (AIs) of solvent extracts of the plants
As compared to the positive control (ciprofloxacin, 5 μg/disc), the activity indices (AIs) of the solvent extracts of C. aurea leaves, C. aurea roots, V. amygdalina leaves, and R. nepalensis roots against the five bacterial test pathogens ranged from 0.17 to 0.80. The highest AI was recorded by the 80% methanol extracts of C. aurea leaves, C. aurea roots, V. amygdalina leaves, and R. nepalensis roots against S. aureus (AI = 0.80), S. aureus (AI = 0.69), E. coli (AI = 0.62), and S. aureus (AI = 0.41), respectively ().
Table 4. Activity indices of solvent extracts of C. aurea, V. amygdalina, and R. nepalensis against five bacterial test pathogens at concentration of 200 mg/ml using ciprofloxacin as standard antibiotics.
Minimum inhibitory concentration (MIC) of solvent extracts of the plants
Only the extracts of the 80% methanol, 70% ethanol and the aqueous extracts were used for MIC testing. In C. aurea leaves, the smallest MIC was exhibited by the 70% ethanol extract against S. aureus (at 1.56 mg/ml). At 3.125 mg/ml, the smallest MIC was attained by the 80% methanol extract against S. dysenteriae and S. aureus, by 70% ethanol extract against S. dysenteriae and by aqueous extract against E. coli. In the extracts of C. aurea roots, the lowest MIC was attained by the 80% methanol extract against S. aureus (0.78 mg/ml), followed by the same extract against E. coli and S. dysenteriae (1.56 mg/ml). In the leaf extracts of V. amygdalina, the lowest MIC was recorded for 80% methanol against E. coli (0.78 mg/ml), followed by S. aureus (3.125 mg/ml). The 70% ethanol extracts showed the lowest MIC against S. dysenteriae and S. typhi (both at 1.56 mg/ml). The root extracts of R. nepalensis achieved the lowest MIC with the 80% methanol extract against S. aureus (at 3.125 mg/ml) and against E. coli (at 6.25 mg/ml) ().
Table 5. MIC of solvent extracts of C. aurea, V. amygdalina, and R. nepalensis against five bacterial test pathogens in Goba district, Bale Zone, Southeastern Ethiopia performed at MWU, 2017.
Discussion
The present study represents the first study to report the antimicrobial potential of the extracts of C. aurea leaves and roots, V. amygdalina leaves and R. nepalensis roots in the study area. In C. aurea leaves, the yield percentages with 80% methanol, 70% ethanol, and aqueous extracts were lower than in a study in southern Ethiopia [Citation22] that reported yields of 26.2%, 20.2%, and 30.4%, respectively. The yield percentage of extracts of C. aurea roots was high with the 70% ethanol; this result was higher than the yield percentage from West Shewa, Ethiopia (5.6 mg/ml) [Citation23]. The yield percentage with 70% ethanol extract of V. amygdalina leaves was the highest as compared to other extracts higher than results (5.37%) from Ondo State, Nigeria [Citation39]. The yield percentage of the extracts of R. nepalensis roots was higher than the results from Bhopal, India [Citation40] for methanol (3.5%) and aqueous extracts (4.8%). Organic solvents with high polarities were reportedly found to produce a high yield [Citation22], in agreement with our study.
The methanol, ethanol, and aqueous extracts of C. aurea leaves showed inhibitory activity against all bacterial test pathogens. It was reported that the extracts of C. aurea leaves displayed antibacterial activity against many Gram-positive and Gram-negative bacteria [Citation22–24], in line with the results of our study. The highest and lowest antibacterial activities were exhibited by the methanol extracts of C. aurea leaves against S. aureus (23.1 ± 0.3 mm) and P. aeruginosa (6.0 ± 0.1 mm), respectively. Using methanol extract of C. aurea leaves (at 250 mg/ml), Umer et al. [Citation24] reported a lower ZOI against S. aureus (11 mm) and a higher ZOI against E. coli (14 mm) and P. aeruginosa (9 mm each), than in our study. The high antimicrobial activity of the methanol extract against S. aureus consolidates the potential role of this extract in treating skin infections [Citation9].
The ZOI exhibited by the 70% ethanolic extracts of C. aurea leaves were higher than those reported by Melese et al. [Citation22] against P. aeruginosa (8.72 ± 0.2 mm) and S. aureus (9.27 ± 1.47 mm). However, at 75, 50 and 25 mg/ml of ethanolic extracts of C. aurea leaves, Wasihun et al. [41] reported a ZOI against P. aerugionosa (22, 19 and 17 mm) and S. aureus (30, 23 and 19 mm), higher than in our study (at 200 mg/ml). The variation may be due to difference in the extraction method, test concentration and the method of antimicrobial assay. Phytochemical screening of the methanol and ethanol extracts of C. aurea leaves revealed the presence of cardiac glycosides, phytosteroids, flavonoids, alkaloids, phenols, tannins, saponins, and terpenoids [Citation24,Citation41,Citation42], possibly causing the inhibitory activity in our study.
The 80% methanol extract of C. aurea roots exhibited moderately high to high antibacterial activity against three bacterial test pathogens. Such activity was lower than the ZOI against E. coli (25 mm), but similar with the ZOI against S. aureus (20 mm) with study from western Ethiopia [Citation42]. The 70% ethanol extracts of C. aurea roots showed moderate to high antimicrobial activity only against P. aeruginosa and S. typhi, but Mulatu [Citation23] reported antimicrobial activity against S. aureus, E. coli in addition to S. typhi despite concentration difference. Petroleum ether extracts failed to produce antibacterial activity against any of the test pathogens. The extracts of C. aurea root including the ethanol and methanol extract were reported to contain metabolites such as cardiac glycosides, flavonoids, tannins, terpenoids, saponins, steroids, alkaloids, and phenolic compounds with antimicrobial activity [Citation23,Citation42,Citation43], which also supports the result of our study. Phenols and polyphenols were strongly present in both the ethanol and methanol extracts of C. aurea root extract [Citation43].
The extracts of V. amygdalina leaves were inhibitory to 65% of the test bacterial pathogens. Several multi-drug resistant Gram positive and Gram negative bacteria were reportedly found to be affected by the extract of V. amygdalina leaves [Citation25,Citation44]. The 80% methanol extracts of V. amygdalina leaf was inhibitory to all except S. typhi; It produced the highest and the lowest diameter of ZOI against E. coli and P. aeruginosa. The presence of active substances such as saponins, flavonoids, glycosides, alkaloids, tannins, phenolics, anthracenosides, terpenes, and phytosteroids was reported [Citation45], and probably accounted for wider range of antibacterial activity noted in our methanol extracts.
The 70% ethanol extract of V. amygdalina leaves exhibited the highest and the lowest diameter of ZOI against S. aureus and P. aeruginosa, respectively. Our results opposed the findings of Evbuomwan et al. [Citation44] who reported lower ZOI against S. aureus (9.0 ± 2.0 mm), but higher ZOI against P. aeruginosa (16.0 ± 5.0 mm), than our study. The aqueous extracts of V. amygdalina leaves had low to moderate inhibitory activity against most test pathogens. Flavonoids, alkaloids, steroids, terpenoids, glycosides, tannins, phenols, and saponins were reported from both the ethanol and aqueous extracts of V. amygdalina leaves [Citation26,Citation44,Citation46] and some level of anthocyanin, quinones, and coumarins reported from the aqueous extracts of V. amygdalina leaves [Citation46] which may account for the high antimicrobial activity in our study.
The solvent extracts of R. nepalensis roots exhibited the lowest antibacterial activity and were effective only against 30% of the test pathogens. A relatively higher antibacterial activity was obtained by the methanol extracts than the other solvents. Its antibacterial activity was moderately higher against S. aureus and E. coli than against P. aeruginosa and S. typhi, corroborating the study of Hussain et al. [Citation47]. Various metabolites from the methanol extracts of R. nepalensis roots such as emodin, endocrocin, chrysophanol, neopodin, physcion, torachrysone, aloesin, catechin, quercetin, resveratrol, and their derivatives which were identified for their antimicrobial, antidiabetic, anti-inflammatory, antioxidant, and antitumor activities [Citation17,Citation18,Citation40].
Compared to the positive control (ciprofloxacin, 5 μg/disc), the highest AI was recorded by the 80% methanol extracts of C. aurea leaves against S. aureus (AI = 0.80), a result higher than from southern Ethiopia (AI = 0.56) [Citation22]. The C. aurea root extract exhibited the highest AI by its methanol extract against S. aureus (AI = 0.69), a result lower than a methanol extract of C. aurea root bark against E. coli (AI = 1.0) and S. aureus (AI = 0.8) [Citation42]. The low AI in our study could be due to higher breakpoint rate of susceptibility to ciprofloxacin against E. coli and S. aureus [Citation33] but not due to lower efficacy of our root extract of C. aurea. The extract of V. amygdalina leaves exhibited the highest AI against E. coli (AI = 0.62), smaller than the results from the same plant extract (AI = 0.76) in Nigeria [Citation48]. In the extracts of R. nepalensis roots, the highest AI was attained by the methanol extract of R. nepalensis roots against S. aureus (AI = 0.41) nearly approaches the AI of methanol extract of the related species Rumex abyssinicus in western Ethiopia (AI = 0.47) [Citation27]. Such indices could be useful for determining the relative antimicrobial activity of individual plant extract which could help the decision to extract active compounds from the individual plant extract.
The MICs of the four plant extracts ranged from 0.78 mg/ml to 200 mg/ml. In C. aurea leaves, the smallest MIC was exhibited by the 70% ethanol extract against S. aureus (at 1.56 mg/ml), which was smaller than the result reported from western Shewa Regions, Ethiopia (12.5 mg/ml) [Citation23]. The MIC values of 80% methanol extracts of C. aurea leaves was far smaller than the MIC values against E. coli (31.25 mg/ml), Shigella spp. (125 mg/ml), P. aeruginosa (125 mg/ml), S. aureus (125 mg/ml), and S. typhi (31.25 mg/ml) from study result in Addis Ababa, Ethiopia [Citation24]. The MIC of the aqueous extract of C. aurea leaf against S. aureus, E. coli and P. aeruginosa were smaller than the MIC against S. aureus (100 mg/ml), E. coli and P. aeruginosa (200 mg/ml each) in Sidama Zone, southern Ethiopia [Citation22]. This indicates a high antibacterial activity of the C. aurea leaf extracts in our study with the three solvents of extraction.
In the extracts of C. aurea roots, the smallest MIC was attained by the 80% methanol extract against S. aureus (0.78 mg/ml), followed by the same extract against E. coli and S. dysenteriae (1.56 mg/ml). Such result was lesser than the MIC of C. aurea roots bark extract from Wollega, West Ethiopia against S. aureus (50 mg/ml) and against E. coli (6.25 mg/ml) [Citation42], and indicating higher antibacterial efficacy of the methanol extract in our study. The smallest MIC of ethanol extract of C. aurea roots was attained against S. typhi (at 3.125 mg/ml), but it attained the lowest MIC at 12.5 mg/ml against S. aureus (); these MIC values are smaller or comparable with the result from West Shewa, Ethiopia on the same extract [Citation23].
The MIC values of the extracts of V. amygdalina leaves ranged from 0.78 mg/ml to 50 mg/ml; the smallest MIC was recorded for 80% methanol against E. coli (0.78 mg/ml), followed by S. aureus (3.125 mg/ml). Such values were lower than the result from Haramaya University, Eastern Ethiopia against E. coli (100 mg/ml) and S. aureus (50 mg/ml) [Citation25]. The 70% ethanol extracts of extracts of V. amygdalina showed the smallest MIC against S. dysenteriae and S. typhi (both at 1.56 mg/ml), but at 12.5 mg/ml, 25 mg/ml and 50 mg/ml against P. aeruginosa, S. aureus and E. coli, respectively, in conformity with the result of Akinduti et al. [Citation48], who reported the same. The MIC of aqueous extract against E. coli (50 mg/ml) was in conformity with the result of Ghamba et al. [Citation49] who reported the same MIC. The ethanol extracts exhibited lower MICs on most of the isolates, which may be due to the high capacity of ethanol extract to destroy the cell wall/membrane of bacterial isolates [Citation39].
The 80% methanol extract of R. nepalensis roots achieved the smallest MIC against S. aureus (at 3.125 mg/ml) and against E. coli (at 6.25 mg/ml) (). A related species to R. nepalesnis from southeastern Ethiopia exhibited a low MIC against S. aureus (at 16 μg/ml) and E. coli (at 128 μg/ml) [Citation36], in line with the high antibacterial efficacy of the methanol extract in our study. The ethanol extract of R. nepalensis attained its low MIC in one test only (S. aureus at 12.5 mg/ml). In a study of R. nepalensis in central Ethiopia, the MIC of the ethanol extract against S. aureus, E. coli, P. aeruginosa and S. enteritidis was high (>512 μg/ml) [Citation50], showing weaker activity of the ethanol extract against many pathogens, in agreement with our study. The limitations of this study include lack of reagents and laboratory facilities to evaluate the bioactive metabolites responsible for antibacterial activity of the evaluated medicinal plants, and hence any interpretation of the results of this study has been made solely on in-vitro tests.
Conclusions
This study evaluated the antibacterial activity of extracts of C. aurea, V. amygdalina and R. nepalensis leaves and/or roots against bacterial pathogens causing enteric infections. Overall, more than half (52.5%) of the solvent extracts of four plant parts of three plant species had inhibitory effects against the bacterial test pathogens. In each plant extract, the highest inhibitory activity and AI were recorded by the 80% methanol extracts. The MICs of solvent extracts of plant parts ranged from 0.78 mg/ml to 200 mg/ml. The methanol and/or ethanol extracts generally exhibited the lowest MICs compared to the other solvent extracts. Plant extracts with a high in-vitro inhibitory activity and AIs were also related with a low MIC indicating the potential use of such extracts for treatment of human bacterial pathogens after identification of active metabolite in such extracts. The results of this study contribute to identifying new antibacterial compounds from extracts used in folk medicine. Further investigations should focus on isolating chemical constituents responsible for the antibacterial activities observed in these plants.
Ethical statement
Ethical clearance letter (approval number: CNS/004/2016 and date: August 20/2016) was obtained from the ethics committee of the College of Natural and Computational Science, Madda Walabu University. This study involved neither human nor animal subjects. It employed well identified and characterized standard test pathogens from the Ethiopian Public Health Institute. All experimental procedures on medicinal plants and plant parts were conducted in accordance to national and international guidelines.
Acknowledgments
We would like to thank the Department of Biology and the School of Graduate Studies of Madda Walabu University for their administrative and material support. We are also grateful to the Ethiopian Public Health Institute for providing us the bacterial test pathogens used in this study. We also thank Ann Byers for editing the manuscript at short notice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dubey R, Dubey K, Sridhar C, et al. Human vaginal pathogen inhibition studies on aqueous, methanolic and saponins extracts of stem barks of ziziphus mauritiana. Int J Pharm Sci Res. 2011;2(3):659–663. doi: 10.13040/IJPSR.0975-8232
- Akinjogunla OJ, Adegoke AA, Udokang IP, et al. Antimicrobial potential of Nymphaea lotus (Nymphaeaceae) against wound pathogens. J Med Plants Res. 2009;3(3):138–141. doi: 10.5897/JMPR.9000177
- Hamilton AC. Medicinal plants, conservation and livelihoods. Biodivers. 2004;13(8):1477–1517. doi: 10.1023/B:BIOC.0000021333.23413.42
- Dawit A. Traditional medicine in Ethiopia: the attempts being made to promote it for effective and better utilization. SINET: Eth J Sci. 1986;9:61–69.
- Carlet J, Jarlier V, Harbarth S, et al. Participants of the 3rd World Healthcare-Associated infections Forum. Ready for a world without antibiotics? The pensières antibiotic resistance call to action. Antimicrob Resist Infect Control. 2012;1(1):11. doi: 10.1186/2047-2994-1-11
- Vaou N, Stavropoulou E, Voidarou C, et al. Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms. 2021;9(10):2041. doi: 10.3390/microorganisms9102041
- Ayaz M, Ali T, Sadiq A, et al. Editorial: Current trends in medicinal plant research and neurodegenerative disorders. Front Pharmacol. 2022;13:922373. doi: 10.3389/fphar.2022.922373
- Belay S, Assefa A, Tedila H. A review on medicinal plants against some human pathogenic bacteria (E. coli, S.Dysentery, S. typhi, P.Aeruginosa and S. aureus) in South East Ethiopia. J Biol, Agri Healthcare. 2019;9(19):16–26.
- Tadeg H, Mohammed E, Asres K, et al. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol. 2005;100(1–2):168–175. doi: 10.1016/j.jep.2005.02.031
- d’Avigdor E, Wohlmuth H, Asfaw Z, et al. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):38.
- Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi: 10.1186/1746-4269-11-4
- Ijeh II, Ejike CECC. Current perspectives on the medicinal potentials of Vernonia amygdalina del. J Med Plant Res. 2011;5(7):1051–1061. doi: 10.5897/JMPR.9000004
- Alara OR, Abdurahman NH, Abdul Mudalip SK, et al. Phytochemical and pharmacological properties of Vernonia amygdalina: a review. J Chem Eng Ind Biotechnol. 2017;2(1):80–96.
- Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia Regional State, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25. doi: 10.1186/s13002-015-0014-6
- Yineger H. A Study on the ethnobotany of medicinal plants and floristic composition of the dry Afromontane forest at Bale Mountains National Park, Ethiopia [ M.Sc Thesis]. Addis Ababa: Addis Ababa University, Ethiopia; 2005.
- Misha G, Yarlagadda R, Wolde-Mariam M. Knowledge, attitude, practice and management of traditional medicine among people of Shopa Bultum, Southeast Ethiopia. Res J Pharm Biol Chem Sci. 2014;5(5):152–170.
- Shaikh S, Shriram V, Srivastav A, et al. A critical review on Nepal dock (Rumex nepalensis): a tropical herb with immense medicinal importance. Asian Pac J Trop Med. 2018;11(7):405–414. doi: 10.4103/1995-7645.237184
- Gonfa YH, Beshah F, Tadesse MG, et al. Phytochemical investigation and potential pharmacologically active compounds of Rumex nepalensis: an appraisal. Beni-Suef Univ J Basic Appl Sci. 2021;10(1):18. doi: 10.1186/s43088-021-00110-1
- Yinger H, Kelbessa E, Bekele T, et al. Ethnoveterinary medicinal plants at Bale Mountain National Park. J Ethnopharmacol. 2007;112(1):55–70. doi: 10.1016/j.jep.2007.02.001
- Tegene AS. An ethnobotanical study of traditionally used medicinal plants for treatment of human diseases in Goba District of Bale Zone, Southeast Ethiopia. Adv Life Sci Technol. 2018;68:1–7.
- Belay D, Kenubih A, Yesuf M, et al. Antioxidant and antimicrobial activity of solvent fractions of Calpurnia aurea (Ait.) Benth. (Fabaceae). J Exp Pharmacol. 2021;13:499–509. doi: 10.2147/JEP.S285872
- Melese A, Dobo B, Mikru A. Antibacterial activities of Calpurnia aurea and Ocimum lamiifolium extracts against selected gram positive and gram-negative bacteria. Ethiop J Sci Technol. 2019;12(3):203–220. doi: 10.4314/ejst.v12i3.2
- Mulatu G. Antibacterial activities of Calpurnia aurea against selected animal pathogenic bacterial strains. Adv Pharmacol Pharm Sci. 2020:2020:8840468. doi: 10.1155/2020/8840468
- Umer S, Tekewe A, Kebede N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement Altern Med. 2013;13(21). doi: 10.1186/1472-6882-13-21
- Habtom S, Gebrehiwot S. In vitro antimicrobial activities of crude extracts of Vernonia amygdalina and Croton macrostachyus against some bacterial and fungal test pathogens. J Phytopharmacol. 2019;8(2):57–62. doi: 10.31254/phyto.2019.8206
- Alara OR, Abdurahman NH, Ukaegbu CI, et al. Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave assisted extraction techniques. J Taibah Univ Sci. 2019;13(1):414–422. doi: 10.1080/16583655.2019.1582460
- Fufa FM, Padmanabhan R, Gurmessa GT. Phytochemical investigation and in vitro antibacterial evaluation on root extracts of Rumex abyssinicus. Nat Prod Chem Res. 2016;4(6):239. doi: 10.4172/2329-6836.1000239
- Ren G, Li L, Hu H, et al. Influence of the environmental factors on the accumulation of the bioactive ingredients in Chinese rhubarb products. Plos One. 2016;11(5):e0154649. doi: 10.1371/journal.pone.0154649
- Yeo YL, Chia YY, Lee CH, et al. Effectiveness of maceration periods with different extraction solvents on in-vitro antimicrobial activity from fruit of Momordica charantia L. J Appl Pharm Sci. 2014;4(10):016–023.
- Shiromi PSA, Hewawasam RP, Jayalal RGU, et al. Chemical composition and antimicrobial activity of two Sri Lankan Lichens, parmotrema rampoddense, and parmotrema tinctorum against methicillin-sensitive and methicillin-resistant staphylococcus aureus. J Evid Based Complementary Altern Med. 2021;2021:9985325. doi: 10.1155/2021/9985325
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. doi: 10.1038/nprot.2007.521
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005
- CLSI, Performance standards for antimicrobial susceptibility testing. 28th. ( CLSI supplement M100). Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2018.
- Moussa A, Noureddine D, Abdelmelek M, et al. Antibacterial activity of various honey types of Algeria against pathogenic gram-negative bacilli: Escherichia coli and Pseudomonas aeruginosa. Asian Pac J Trop Dis. 2012;2(3):211–214. doi: 10.1016/S2222-1808(12)60048-6
- Dharajiya D, Jasani H, Khatrani T, et al. Evaluation of antibacterial and antifungal activity of fenugreek (Trigonella foenum-graecum) extracts. Int J Pharm Pharm Sci. 2016;8(4):212–217.
- Kebede T, Gadisa E, Tufa A, et al. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. PloS One. 2021;16(3):e0249253. doi: 10.1371/journal.pone.0249253
- Molla Y, Nedi T, Tadesse G, et al. Evaluation of the in vitro antibacterial activity of the solvent fractions of the leaves of Rhamnus prinoides L’Herit (Rhamnaceae) against pathogenic bacteria. BMC Complement Altern Med. 2016;16(1):287. doi: 10.1186/s12906-016-1279-6
- Statistica. Statistica. Data analysis software system (version 7.0.61.0). Oklahoma: Stat Soft Inc; 2004.
- OlusolaMakinde O, Olabanji OB, Ibisanmi TA. Evaluation of the bioactive compounds of Vernonia amygdalina delile extracts and their antibacterial potentials on water-related bacteria. Bull Natl Res Cent. 2021;45(1):191. doi: 10.1186/s42269-021-00651-6
- Khan AN, Bhat I. Extraction, qualitative and quantitative determination of secondary metabolites of Rumex nepalensis roots. J Drug Deliv Ther. 2018;8(6–s):97–00. doi: 10.22270/jddt.v8i6-s.2092
- Wasihun Y, Habteweld HA, Ayenew KD. Antibacterial activity and phytochemical components of leaf extract of Calpurnia aurea. Sci Rep. 2023;13(1):9767. doi: 10.1038/s41598-023-36837-3
- Kebede GT, Negero ZA. Phytochemical investigation and assessment of antibacterial activities of Calpurnia aurea root bark. Nat Prod Chem Res. 2021;9(8):383.
- Dula DE, Zelalem A. Phytochemical screening of Calpurnia aurea root extract. Kenkyu J Pharm Pract Health Care. 2018;4:61–68. doi: 10.31872/2018/KJPHC-100116
- Evbuomwan L, Chukwuka EP, Obazenu EI, et al. Antibacterial activity of Vernonia amygdalina leaf extracts against multidrug resistant bacterial isolates. J Appl Sci Environ Manage. 2022;22(1):17–21.
- Eleyinmi AF, Sporns P, Bressler DC, et al. Nutritional composition of Gongronema latifolium and Vernonia amygdalina. Nutr Food Sci. 2008;38(2):99–109. doi: 10.1108/00346650810862975
- Akinduti PA, Emoh-Robinson V, Obamoh-Triumphant HF, et al. Antibacterial activities of plant leaf extracts against multi-antibiotic resistant staphylococcus aureus associated with skin and soft tissue infections. BMC Complement Med Ther. 2022;22(1):47.
- Hussain F, Ahmad B, Hameed I, et al. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of polygonaceae. Afr J Biotechnol. 2010;9(31):5032–5036.
- Ali M, Diso SU, Waiya SA, et al. Phytochemical screening and antibacterial activity of bitter leaf (Vernonia amygdalina). Ann Microbiol Infect Dis. 2019;2(4):01–07.
- Ghamba PE, Balla H, Goje LJ, et al. In vitro antimicrobial activities of Vernonia amygdalina on selected clinical isolates. Int J Curr Microbiol App Sci. 2014;3(4):1103–1113.
- Lulekal E, Rondevaldova J, Bernaskova E, et al. Antimicrobial activity of traditional medicinal plants from Ankober District, North Shewa Zone, Amhara region, Ethiopia. Pharm Biol. 2014;52(5):614–20. doi: 10.3109/13880209.2013.858362