ABSTRACT
Euclea natalensis is a commonly used plant in traditional African medicine for treating several diseases, including diabetes. The objective of the present study was to evaluate the antidiabetic effects of the leaf extracts of Euclea natalensis and elucidate some of its mechanisms of action. High-fat diet – low Streptozotocin-induced diabetic Sprague Dawley rats were orally treated with the methanol and dichloromethane extracts (100 and 200 mg/kg bw), and their fasting blood glucose levels, body weights, and food and water intake were measured every seven days for 21 days. Biomarkers of oxidative stress, lipid profile, gluconeogenic enzyme activities, glucose transporter 4 (GLUT 4) protein expression, and AMP-activated protein kinase (AMPK) gene expression were also determined. The 200 mg/kg bw of methanol extracts significantly reduced fasting plasma glucose, glycated hemoglobin (HbAc1), triglycerides, and low-density lipoprotein levels. The extract increased insulin sensitivity and hepatic glycogen synthesis, decreased hepatic glucose output, and increased the activities of antioxidant enzymes in diabetic animals after 21 days of treatment. The methanol extract also significantly upregulated the relative expression of GLUT 4 protein via the promoted expression of skeletal muscle and hepatic AMPK. Histological investigation showed ameliorated pancreatic degeneration and expedited regeneration of islets of Langerhans in diabetic rats treated with the methanol extract compared with the dichloromethane extract. The findings demonstrated that Euclea natalensis has beneficial effects on the biochemical and cell abnormalities of type 2 diabetes, partly via the modulation of the AMPK – GLUT 4 pathway.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease with debilitating consequences. It is a non-communicable disease with high mortality rates across the globe. Its increasing global prevalence is linked to sedentary lifestyles, unhealthy diets, obesity, and physical inactivity [Citation1]. It is characterized by impaired glucose homeostasis, culminating in chronic hyperglycemia. This dysregulated glucose metabolism and hyperglycemia are caused by either the inability of the pancreas to produce and secrete insulin or the ability of body tissues to respond effectively to insulin [Citation2]. The failure of the body to react accordingly to insulin is termed insulin resistance, and it is the hallmark of type 2 diabetes mellitus, the most common type of diabetes, accounting for more than 90% of all diabetes cases [Citation3]. Insulin resistance may result from defects in insulin signal transduction pathways, dysfunction of glucose-transporting proteins, and dysregulated glycogen synthesis [Citation4,Citation5].
Glucose transporter protein 4 (GLUT4) is the main glucose-transporting protein highly expressed in muscle and fat tissues. Under normal conditions, when insulin binds to the insulin receptors on the surface of cells, a cascade of phosphorylation reactions of the insulin signal transduction pathway occurs, resulting in the translocation of GLUT4 to the cell membrane for increased uptake of glucose into the cells for energy metabolism [Citation6]. However, under diabetic conditions, binding of insulin to its receptors fails to initiate the translocation of GLUT4 to the cell membrane for the transport of glucose into the cells, resulting in chronic accumulation of glucose in the bloodstream [Citation6]. This has placed GLUT4 as a potential therapeutic target for developing antihyperglycemic drugs.
GLUT4 is, in part, regulated by AMPK, a serine/threonine kinase responsible for maintaining normal energy homeostasis in eukaryotic cells [Citation7]. It is also believed that drug molecules that can upregulate AMPK may be beneficial in managing hyperglycemia, as AMPK regulates gluconeogenesis and lipogenesis [Citation7,Citation8]. On the other hand, the management of type 2 diabetes is by insulin and other antidiabetic drugs, such as metformin. However, many antidiabetic drugs have been associated with severe side effects such as weight gain, flatulence, hypoglycemia, and gastrointestinal problems that limit their use [Citation9,Citation10]. Therefore, there is a need to find antidiabetic agents with fewer or no side effects. Natural products have shown promising results in managing diabetes with fewer side effects, hence the need for continued bioprospecting of natural medicines to discover plant metabolites that can be harnessed in efficacious drugs for managing diabetes mellitus and its associated complications.
Euclea natalensis A. DC, locally known as Motlhakola in Botswana, is a woody plant in the EBENACEACE (ebony) family that is traditionally used for the treatment of many conditions, including headaches, sexually transmitted infections, tuberculosis, skin infections, and diabetes [Citation11]. It has evergreen leaves and small spherical red berries which turn black when ripe. Extracts from the plant have been shown to have antidiabetic effects by inhibiting α-amylase and α-glucosidase activities [Citation11–13]. Extracts of Euclea natalensis are rich in naphthoquinones and pentacyclic tannins [Citation14]. Through scientific research, Euclea natalensis was found to possess antibacterial [Citation14], anti-inflammatory [Citation11], antifungal [Citation15], antioxidant [Citation16], antidiabetic [Citation16,Citation17] and hepatoprotective effects [Citation13]. However, the in vivo effect of the plant on diabetes has not yet been explored. The present study, therefore, evaluates the effect of Euclea natalensis leaf extracts on the biochemical and cellular abnormalities in type 2 diabetic rats. The effects on glucose and lipid metabolism, gene and protein expression, and histological examination were used to elucidate the potential mechanisms of action.
Materials and methods
Chemicals and reagents
Organic solvents (hexane, dichloromethane, methanol) were procured from Merck (South Africa). Metformin (Glucophage) was purchased from Merck (South Africa), streptozotocin was bought from Sigma Aldrich (Germany), and the kits were from different manufacturers. All other reagents were of analytical grade.
Preparation of extracts
The leaves of Euclea natalensis were collected from Matlhakola village (22°43’09.7“S 27°18’39.7“E) in Central Botswana and authenticated at the University’s herbarium and given voucher number BAKW-01/2020. The plant name was checked with The Plant List’s online website (http://www.theplantlist.org/tpl1.1/record/kew-2802685). Dried leaves were ground to a fine powder using a household blender (Ottimo, South Africa). They were sequentially extracted [Citation18] with different solvents in increasing polarity order (hexane > dichloromethane > dichloromethane: methanol (1:1) > 70% methanol > water). Extracts were then filtered using Whatman filter paper (No. 1), and the filtrate was evaporated to dryness on a rotary evaporator. The obtained crude extracts were dried and stored at − 20°C until needed.
Experimental animals
Male Sprague Dawley rats aged 8–10 weeks and weighing between 150 and 180 g were housed at the university’s animal house under a 12 h dark/12 h light cycle and a temperature of 24 ± 1°C. They were fed standard rodent feed (Epol, South Africa), had free access to distilled water ad libitum, and acclimatized for 2 weeks. The Human Subject Research Department of the Ministry of Health and Wellness, Botswana, approved the experimental protocol and gave code: HPDME 13/18/1.
Acute oral toxicity study (LD50 determination)
The acute oral toxicity of Euclea natalensis extracts was determined in Sprague‒Dawley rats according to the Organization for Economic Co-operation and Development [Citation19]. Overnight-fasted rats of 3 rats per group were given a single dose of 2000 mg/kg body weight of the extracts, while normal control rats received the vehicle only by oral gavage. Animals were observed for 30 minutes after dosing, followed by hourly observation for 8 hours and then daily for 14 days for symptoms such as mortality, behavioral patterns, changes in physical appearance, injury, weight, and any signs of illness.
In vivo antidiabetic activities
Oral glucose tolerance test (OGTT) in normal rats
To determine potent doses and potentially effective extracts, an OGTT was performed using normal rats loaded with 2 g/kg bw glucose solution [Citation20]. Overnight-fasted rats were grouped into 6 groups of five rats in each group and treated as follows for each extract. The sample size was determined using the resource equation method [Citation21].
Group 1: (negative control): 2 g/kg bw glucose +2 ml distilled water.
Group 2: (positive control): 2 g/kg bw glucose +200 mg/kg bw metformin.
Group 3: 2 g/kg bw glucose +50 mg/kg bw Hexane extract (HEX).
Group 4: 2 g/kg bw glucose +100 mg/kg bw Hexane extract (HEX).
Group 5: 2 g/kg bw glucose +200 mg/kg bw Hexane extract (HEX).
Group 6: 2 g/kg bw glucose +400 mg/kg bw Hexane extract (HEX).
A metformin dose of 200 mg/kg bw was adopted from Horakova et al. [Citation22] and dissolved in a vehicle (distilled water). Animals were given their treatments immediately after an oral gavage of glucose solution at a concentration of 2 g/kg bw. All animals were assessed for blood glucose concentration at 0, 30, 60, 120, and 180-minute time points by pricking the tail and measuring glucose on a handheld glucometer (Accu-Chek Active, Roche, India). Then, blood glucose levels were plotted against time, and the area under the curve (AUC) was determined using GraphPad Prism. The same experiment was repeated for the other extracts: dichloromethane-methanol extract (DCM), 70% methanol extract (MeOH), and water extract (AQE).
Induction of type 2 diabetes mellitus
Type 2 diabetes mellitus was induced in male Sprague Dawley rats by feeding a high-energy diet (containing 37.5% beef tallow, 25% white sugar, and 37.5% powdered standard rat pellets) for 14 days followed by an intraperitoneal injection of 35 mg/kg bw Streptozotocin [Citation23] (Sigma Aldrich, Germany) dissolved in freshly prepared 0.1 M citrate buffer (pH 4.5). Rats were monitored, and after 72 hours, their fasting blood glucose level was determined by tail pricking and measuring using a handheld glucometer (Accu-Chek Active, Roche, India). Rats with fasting blood glucose levels of more than 7 mmol/L were considered diabetic and used for further experiments.
Subchronic antidiabetic activity in the HFD-STZ diabetes model
The effect of Euclea natalensis leaf extracts on diabetic rats over a prolonged time was evaluated by giving animals an oral dose of the extract daily for 21 days with measurements of blood glucose levels by tail prick being performed every 7 days using a glucometer (Accu-Chek Active, Roche, India). Two potent extracts and two of their doses determined from the OGTT and 200 mg/kg bw metformin [Citation22] were orally administered for this study. Rats were grouped (n = 5 rats per group) as follows.
Group 1: Normal control + distilled water (NC)
Group 2: Diabetic control + distilled water (DC)
Group 3: Diabetic +100 mg/kg bw dichloromethane extract (DCM100)
Group 4: Diabetic +200 mg/kg bw dichloromethane extract (DCM200)
Group 5: Diabetic +100 mg/kg bw methanol extract (MeOH100)
Group 6: Diabetic +200 mg/kg bw methanol extract (MeOH200)
Group 7: Diabetic positive control +200 mg/kg bw metformin (MET200)
The amount of food and water consumed by rats was measured daily, while body weights were measured every 7 days. After the 21st day, animals were anesthetized under diethyl ether and sacrificed. The kidneys, pancreas, and half of the liver were stored in formalin for histological analysis. Half of the liver was kept in normal saline at − 20°C. Muscle tissue was immediately snap-frozen using liquid nitrogen and prepared for protein analysis. Blood was collected by cardiac puncture in EDTA tubes and centrifuged at 10,000 × g for 5 minutes. The separated plasma was collected in sterile microtubes and stored at − 20°C until analysis.
Determination of hepatic glucose output inhibition using the oral pyruvate tolerance test
Overnight fasted diabetic Sprague Dawley rats grouped as in the subchronic study were administered vehicle (saline), plant extracts, metformin as a positive control, and normal saline as a negative control by oral gavage. Fifteen minutes later, they were injected intraperitoneally with normal saline (vehicle) or sodium pyruvate (2 g/kg bw) [Citation24] dissolved in normal saline. Sodium pyruvate (Sigma Aldrich, Germany) is a gluconeogenic substrate that increases blood glucose levels when administered in vivo [Citation25], and failure to increase blood glucose levels implies inhibited gluconeogenesis. Then, blood samples were taken from the tail vein, and glucose levels were measured just before pyruvate administration (time 0) and 30, 60, 90, and 120 min later using a glucometer.
Determination of liver glycogen and the activities of glycolytic enzymes
Determination of hepatic glycogen content was based on a method that uses concentrated potassium hydroxide to digest glycogen into glucose, which is then reacted with the Anthrone reagent (Sigma Aldrich, Germany) and measured at 625 nm as described by Aba [Citation26]. The glucose concentration per 0.5 g of liver tissue was deduced from the glucose standard curve. The liver supernatant was used to determine the activity of glucose 6 phosphatase, measured at 660 nm, and the ATPase activity was calculated as the amount of inorganic phosphate (Pi) released/minute/mg protein [Citation27]. Fructose-1,6-bisphosphatase activity was also measured in the liver supernatant at 680 nm, and the enzyme activity was calculated as the amount of inorganic phosphate (Pi) released/minute/mg protein [Citation27].
Estimation of biochemical parameters and biomarkers of oxidative stress
Plasma glycated hemoglobin was also determined using a standard kit (Elabscience, USA) following the manufacturer’s instructions. The hormones insulin (Rat INS ELISA kit, Elabscience), leptin (Human LEP ELISA kit, Elabscience), and adiponectin (Rat ADP/Acrp30 ELISA kit, Elabscience) were measured following the manufacturer’s instructions. Insulin resistance (homeostasis model assessment of insulin resistance, HOMA-IR), insulin sensitivity (quantitative insulin sensitivity check index, QUICKI), and pancreatic beta-cell function (HOMA – β) were calculated using standard equations. Following the manufacturer’s instructions, the estimation of high-density lipoprotein (HDL) cholesterol, triglycerides, and total cholesterol in plasma was determined using ELISA kits (Agappe Diagnostics, Switzerland). Low-density lipoprotein (LDL) was calculated using the Friedewald equation [Citation28]: LDL-C = (Total Cholesterol) − (HDL-C) − (Triglycerides/5), very low-density lipoprotein (VLDL) cholesterol using the equation, VLDL = Triglycerides/5 [Citation28], and the atherogenic index (AI) using the equation, AI = log(TG/HDL-C) [Citation29]. The antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) were determined using specific ELISA kits (Elabscience, USA) following the manufacturer’s instructions. Following the manufacturer’s instructions, lipid peroxidation was estimated as TBARS (Elabscience, USA).
Estimation of GLUT4 protein expression by multiplex western blot
The expression of GLUT4 protein in muscle tissues was estimated by Western blot following a method described by [Citation30]. Briefly, snap-frozen muscle tissues were ground and subsequently lysed in sucrose lysis buffer containing a protease inhibitor cocktail (1 phenylmethanesulfonylfluoride fluoride and phosphatase inhibitors (1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride)). The protein concentration of the lysate was estimated by the Bradford method (Bradford, 1976). Then, 1 mg/ml total protein was boiled for 5 minutes with 2× Laemmli buffer (Bio-Rad Laboratories, Singapore), and 40 µl of each sample was separated on 10% polyacrylamide mini-gels and transferred to a PDVF membrane (Immobilon -P Transfer Membranes, Millipore, Ireland) by the semidry method using the Bio-Rad Turbo transfer system. The membrane was blocked for 2 hours at room temperature with tween-20 and 5% skim milk in TBS (20 mM Tris-HCL, pH 7.6, and 137 mM NaCL). The membrane was then incubated overnight at 4°C in a blocking buffer with a mixture of mouse anti-GLUT 4 (1F8) and β-actin (8H10D10) mouse monoclonal antibodies (Abcam, USA) diluted at 1:1000 for multiplex blotting. Then, the membrane was washed 5 times for 10 minutes per wash using wash buffer (20 mM Tris, pH 7.5, 150 mM NaCl with 0.05% Tween 20) and incubated for 1.5 h at room temperature in blocking buffer with anti-mouse IgG and horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, USA) at a 1:1300 dilution in blocking buffer. The membrane was then incubated in 10 mL of ECL reagent (Abcam, USA) solution for 5 minutes, and imaging was performed using the ChemiDoc MP Imaging System (Bio-Rad Laboratories, Singapore). The band intensity on the blot images was determined by densitometry using Image Lab Software (Bio-Rad Laboratories, Singapore). The protein expression of GLUT 4 was then calculated relative to the expression of the housekeeping gene β-actin based on band intensity.
AMPK gene expression by quantitative reverse transcriptase polymerase chain reaction (qRT‒PCR)
Approximately 1 g of muscle and liver tissue from the treated and normal animal groups was cut into small pieces and homogenized. Total RNA was isolated using an RNeasy miniprep kit (Zymo Research) following the manufacturer’s protocol. RNA was quantified using Nanodrop One spectroscopy (Thermo Fisher Scientific, USA) at 260 nm, and its quality was determined by agarose gel electrophoresis. Then, 250 ng of total RNA was synthesized into cDNA using the ProtoScript first strand cDNA synthesis kit (New England BioLabs) according to the manufacturer’s instructions. Then, quantitative polymerase chain reaction (qPCR) was performed using Luna Universal qPCR master mix (New England BioLabs) and suitable primers (AMPK1-F: 5’-TGAGAACGTCCTGCTTGAATG-3’; AMPK1-R: 5’-ATCATTGGCTGAGCCACAGC-3’; housekeeping gene, β-actin-F: 5’- CGT AAA GAC CTC TAT GCC AA-3“; β-actin-R: 5”- AGC CAT GCC AAA TGT GTC AT-3’) using an Applied Biosystems 7500 Fast Cycler (Life Technologies, Singapore). PCR conditions were set to initial denaturation: 95°C for 2 minutes, denaturation: 95°C for 20 seconds, and extension at 60°C for 30 seconds. The melt curve was run from 60–95°C. The expression level of the genes was normalized to the housekeeping gene β-actin according to the 2−ΔΔCT method [Citation31].
Histological evaluation of the pancreas
For histological analysis of the effect of Euclea natalensis leaf extracts on the architecture of the pancreas, pancreas tissues stored in 10% formalin were used. The tissues were sectioned, dehydrated on graded ethanol concentrations, and embedded in paraffin. The sectioned tissues were then mounted on glass slides and stained with hematoxylin and eosin stains for visualization. Slides were observed under a light microscope (Leica DM500, Leica Systems, Germany), and pictures were taken with the camera (BHC4-1080P, Bestscope, China) attached to the microscope.
Statistical analysis
Data are presented as the mean ± standard error (SE) of the mean. Statistical significance of the results was determined using one-way analysis of variance (ANOVA) with Tukey‒Kramer’s range test using GraphPad Prism 9.0, and differences were considered significant at p < 0.05.
Results
Acute oral toxicity study (LD50)
All extracts did not elicit mortality, clinical signs of toxicity, or gross behavior in rats administered a high dose of 2 g/kg bw. This dose is recommended for toxicity testing by the Organization for Economic Co-operation and Development (OECD), and this implies that the lethal dose (LD50) of the extracts is higher than 2 g. kg.bw.
Euclea natalensis improved oral glucose tolerance in rats
OGTT was performed in normoglycemic rats to determine the acute effect of the extracts on blood glucose levels and determine the potent extracts and doses. As shown in , all extracts showed some glucose-lowering effect, especially with the 100 and 200 mg/kg body weight doses, except for the water extract, which only exhibited a glucose-lowering effect at the highest concentration of 400 mg/kg body weight. Comparison of the extracts was by the area under the curve (AUC) in , and it was determined that the extracts performed best in the order MeOH > DCM > AQE > HEX. Therefore, MeOH and DCM extracts were chosen for further studies at 100 and 200 mg/kg body weight doses.
Figure 1. Effects of different doses of the extracts on glucose tolerance in normal rats. The results are presented as the mean of five (5) replicates. NC: negative (vehicle): HEX: hexane extract; MeOH: 70% methanol extract; DCM: dichloromethane-methanol (1:1) extract; AQE: aqueous extract at different concentrations in mg/kg bw; MET200: metformin at 200 mg.Kb.bw.
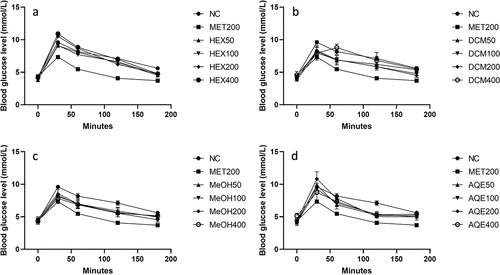
Table 1. Glucose area under the curve, AUC (mmol/l/h) for different treatments.
Euclea natalensis alleviated fasting blood glucose levels in diabetic rats
Induction of diabetes in rats resulted in significantly elevated FBG levels compared to the normal control, as shown in . FBG in diabetic rats increased by 55.2% from 18.22 ± 0.83 to 28.27 ± 0.76 mmol/L from day 1 to day 21, and the area under the curve in was significantly (p < 0.05) higher than that in all other groups except the DCM100 group. FBG levels in groups treated with extracts or metformin decreased compared to the diabetic control (DC) after 21 days. The most effective extract and dose was MeOH at 200 mg/kg bw, as it reduced FBG by 51% from 20.06 ± 0.65 mmol/L to 9.85 ± 0.66 mmol/L after 21 days of treatment. Its effectiveness was not significantly different from metformin’s when using the 21-day timepoint and the AUC, as shown in . This was followed by DCM200 and MeOH100, with 30.2% and 27.9% decreases in FBG, respectively. On the other hand, DCM100 failed to decrease FBG, as it increased by 37.2% from day 1 to day 21 compared to the other extracts.
Figure 2. Effects of Euclea natalensis leaf extracts on fasting blood glucose levels (a), area under the curve of FBG (b), body weight (c), food intake (d), and water intake (e) in diabetic rats over 21 days of treatment. The results are the mean (n = 5) ± standard deviation (SD). Bars with different letters (a, b, c, d) are significantly (p < 0.05) different. STZ: streptozotocin; NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
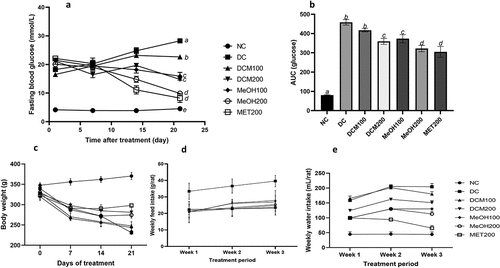
Euclea natalensis improved body weight and increased food and water intake in diabetic rats
Body weights, food and water intake were measured during the treatment period. There was a significant decline (p < 0.05) in the body weight of diabetic rats compared to normal rats from day 1 of treatment to the end of the treatment period, as shown in . However, treatment with extracts and MET200 failed to increase body weight to the initial levels, and MET200 and DCM200 maintained their weights from day 7 to day 21. The effect of food intake, as shown in , indicates that there was a significant (p < 0.05) and consistent increase in food intake by the diabetic control (DC) group after 3 weeks. Food intake was slightly elevated in the other groups treated with extracts or MET200 compared to the NC group. Water intake was significantly elevated in all diabetic groups compared to the normal rats (NC), as indicated in . However, from week 2 to week 3, water intake decreased across the diabetic groups, with a significant decrease in MET200 and MeOH200, while it remained high in the diabetic control (DC) group.
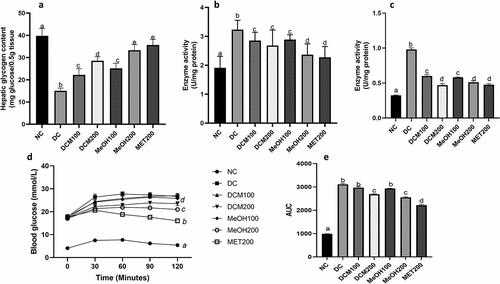
Euclea natalensis ameliorates hepatic glycogen content and activities of gluconeogenic enzymes and hepatic glucose output
shows that the level of glycogen in the livers of diabetic rats (DC) was significantly (p < 0.05) decreased when compared to normal rats (NC). Administration of DCM, MeOH extracts, and metformin significantly (p < 0.05) increased the hepatic glycogen levels of rats, with MET200 recording the highest increase in hepatic glycogen, which was 35.7 ± 1.3 mg glucose/0.5 g tissue. The treatments were evaluated for their effect on gluconeogenic enzymes that affect hepatic glucose output, as shown in . The activities of both hepatic glucose 6 phosphatase (G6P) and fructose 1,6-bisphosphatase (F16P) in , respectively, were significantly (p < 0.05) increased in diabetic rats (DC) compared with normal rats. The F16P activity was decreased in groups treated with the extracts, and metformin with DCM200, MeOH200, and MET200 significantly decreased the F16P activity. On the other hand, G6P activity significantly (p < 0.05) declined in the MeOH200 and MET200 groups compared with the diabetic control. When subjected to an oral pyruvate tolerance test, the DCM200 and MeOH200 extracts significantly reduced hepatic glucose output after 2 hours in diabetic rats. The MeOH200 extract was the most effective in reducing hepatic glucose output after 2 hours; however, based on the area under the curve in , there was no significant difference between its effect and that of DCM200.
Figure 3. Effects of Euclea natalensis leaf extracts on liver glycogen content (a), glucose 6 phosphatase (b), fructose 1,6-bisphosphatase (c), oral pyruvate tolerance curve (d), and area under the curve of the oral pyruvate tolerance test of diabetic rats after the treatment period. The results are the mean (n = 5) ± standard deviation (SD). Bars with different letters (a, b, c, d) are significantly (p < 0.05) different. NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
Euclea natalensis alleviated plasma glycated hemoglobin levels in diabetic rats
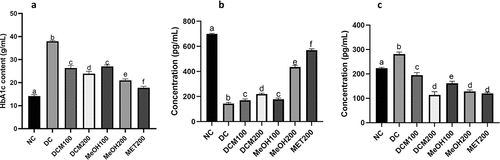
HbA1c was significantly elevated in diabetic rats compared to normal rats (NC), as shown in . Treatment with extracts and metformin significantly reduced HbA1c levels in diabetic rats compared to the DC group (p < 0.05), with no significant difference between DCM100 and MeOH100 (p < 0.05). The most effective regimen for lowering HbA1c levels was MET200, followed by MeOH200 and DCM200.
Figure 4. Effects of Euclea natalensis leaf extracts on plasma levels of glycated hemoglobin (a), adiponectin (a), and leptin (c) in diabetic rats. The results are the mean (n = 5) ± standard deviation (SD). Bars with different letters (a, b, c, d) are significantly (p < 0.05) different. NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
Euclea natalensis improved plasma adiponectin and leptin levels in diabetic rats
The effect of Euclea natalensis extract treatment on adipokines was studied on plasma leptin and adiponectin, as shown in . There was a significant (p < 0.05) decrease in adiponectin levels in diabetic rats (DC) compared to all other groups. All extracts and metformin significantly increased the adiponectin levels, with the most effective extract being the MeOH200 extract. On the other hand, the plasma levels of leptin were significantly (p < 0.05) increased in the diabetic rats (DC) when compared to normal rats (NC) and other groups, as shown in . The treatments with extracts resulted in a significant (p < 0.05) decrease in leptin levels in diabetic rats, with the most effective extract being DCM200.
Euclea natalensis increased plasma insulin and alleviated insulin resistance in diabetic rats
According to , plasma insulin levels were significantly (p < 0.05) decreased in diabetic rats (DC) compared to normal rats (NC) and the other treatment groups. After treatment with the extracts and metformin, a significant (p < 0.05) increase in plasma insulin levels was observed, as shown in . Using insulin levels and plasma glucose levels, levels of markers of insulin sensitivity and resistance were measured. There was a significant (p < 0.05) increase in HOMA-IR in the diabetic control (DC) and DCM100 groups in comparison to the normal rats (NC) and other groups, as indicated in . The insulin sensitivity index QUICKI was significantly (p < 0.05) decreased in the DC and DCM100 groups. Conversely, a significant (p < 0.05) increase in the QUICKI index was observed in the other diabetic groups treated with extracts and metformin. The pancreatic β-cell function index, HOMA-β, was significantly (p < 0.05) reduced in diabetic rats, as shown in . While DC recorded the lowest HOMA-β score, treatment with extracts significantly elevated the HOMA-β index but failed to come close to that of NC.
Table 2. Effects of Euclea natalensis leaf extracts on plasma insulin concentration, insulin resistance (HOMA-IR), sensitivity (QUICKI), and pancreatic function index (HOMA-β) in diabetic rats.
Euclea natalensis alleviated oxidative stress in diabetic rats
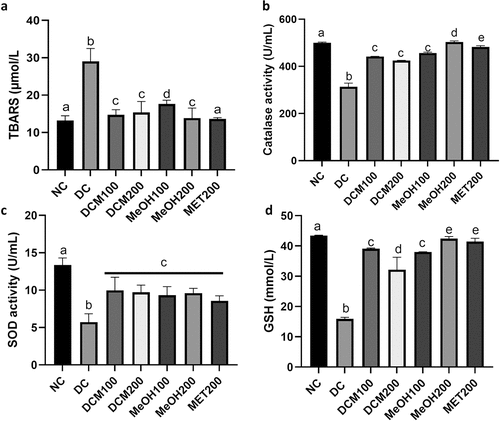
The level of lipid peroxidation, as shown for TBARS (), was significantly (p < 0.05) elevated in diabetic rats (DC) when compared to NC. Treatment with the extracts and metformin significantly (p < 0.05) decreased the plasma TBARS levels. shows that superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) were significantly (p < 0.05) decreased in diabetic rats (DC) compared to normal rats (NC). Administration of DCM and MeOH extracts and metformin to diabetic rats significantly (p < 0.05) elevated the activities of CAT (), GSH () and SOD ().
Figure 5. Effects of Euclea natalensis leaf extracts on plasma TBARS (a), CAT (b), SOD (c) activity, and GSH (d) of diabetic rats. The results are the mean (n = 5) ± standard error (SE). Bars with different letters (a, b, c, d) are significantly (p < 0.05) different. NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
Euclea natalensis improved plasma lipid profiles in diabetic rats
shows that triglycerides and total cholesterol were significantly (p < 0.05) elevated in the diabetic rats (DC) group when compared to normal rats (NC). Treatment with the DCM and MeOH extracts, and metformin significantly (p < 0.05) decreased them, with MeOH200 being the most effective in reducing triglycerides and total cholesterol. On the other hand, a significant (p < 0.05) decline in HDL levels was observed in diabetic rats (DC) when compared to the normal control (NC) and other groups. The extracts and metformin significantly (p < 0.05) increased HDL levels. Regarding LDL and VLDL plasma levels, a significant (p < 0.05) increase in plasma levels of the two lipids was notable in diabetic rats (DC) when compared to the normal control (NC). Metformin extract treatment ameliorated this lipidemic spike by significantly (p < 0.05) decreasing both LDL and VLDL cholesterol levels. There was also an increased risk of atherosclerosis in diabetic animals (DC) compared to normal rats (NC) and treated animals as measured by the atherogenic index.
Table 3. Plasma lipid levels (mg/mL) and atherogenic index of different treatment groups.
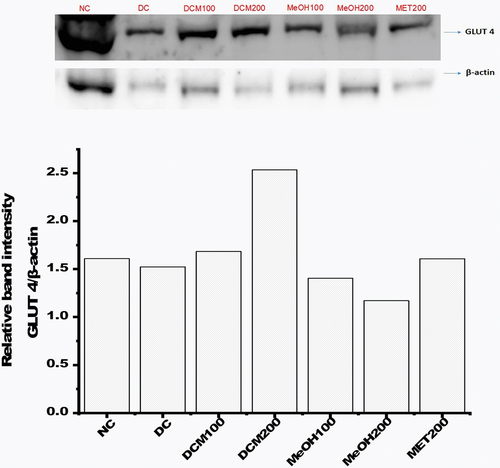
Euclea natalensis improved GLUT4 protein expression in diabetic rats
The expression GLUT 4 in skeletal muscle lysates from normal, diabetic, and diabetic rats treated with extracts was determined by western blotting. The bands were explicitly obtained for GLUT 4 and β-actin on the blots (). The intensity of GLUT 4 bands was normalized to that of the housekeeping gene β-actin to determine the protein expression level, as shown in . The results indicated a slight reduction in the expression of GLUT4 in diabetic rats (DC) compared to normal rats (NC). Treatment with DCM100, DCM200, and metformin modulated the expression of GLUT 4, with DCM200 increasing the relative expression of GLUT 4 compared with all extracts and metformin.
Figure 6. The expression of skeletal muscle GLUT 4 relative to β-actin by western blot. Data are represented as relative band intensity. NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
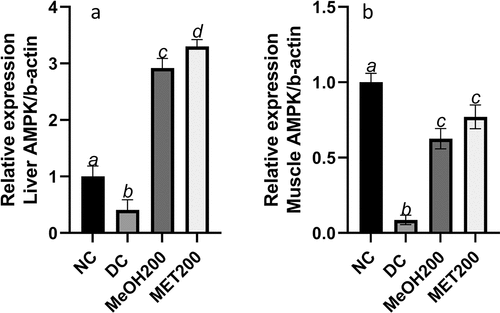
Euclea natalensis upregulated AMPK gene expression in diabetic rats
The expression of AMPK was significantly (p < 0.05) downregulated in both the muscle () and liver () of diabetic rats (DC) when compared to the normal rats (NC). Administration of MeOH200 and MET200 resulted in a significant (p < 0.05) upregulation of AMPK expression in diabetic rats. Relative to NC, there was a 0.63- and 0.77-fold increase in the expression of muscle AMPK for MeOH200 and MET200, respectively, as well as a 2.9- and 3.3-fold increase in the expression of hepatic AMPK for MeOH200 and MET200, respectively. Additionally, the enhancement of AMPK expression by MeOH200 and MET200 was much higher in the liver than in the muscle tissue.
Figure 7. Skeletal muscle (a) and hepatic (b) AMPK expression in different treatment groups. The results are the mean (n = 5) ± standard deviation (SD). Bars with different letters (a, b, c, d) are significantly (p < 0.05) different. NC: negative control; DC: diabetic control; MeOH200: 70% methanol extract at 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
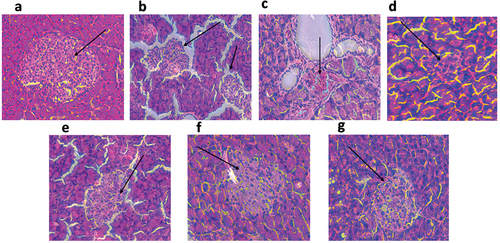
Euclea natalensis improved the pathological morphology of the pancreas in diabetic rats
shows that while the normal rats had normal and healthy islets of Langerhans in their pancreas, the diabetic control (DC) had ruptured and degenerated islets with decreased functionality. Treatment with Euclea natalensis extracts and metformin resulted in the regeneration of healthy islets in the pancreas. The degree of damage observed in the DC pancreas was ameliorated by treatment with the extracts.
Figure 8. Hematoxylin and eosin-stained histopathological sections of the pancreas at 200X magnification after 21 days of treatment. Black arrows show the islets of Langerhans. (a) Normal rats (NC) maintained a healthy islet; (b) untreated diabetic rats (DC) showed ruptured and dying islets; (c) regenerating islets after 21 days of DCM100 treatment; (d) developing islets with normal round shape after treatment of diabetic rats with DCM200; (e) ruptured islets surviving after treatment with MeOH100; (f) healthy islets reinstated by MeOH200 treatment; (g) healthy islets reinstated by MET200 treatment. NC: negative control; DC: diabetic control; DCM: dichloromethane-methanol (1:1) extract at 100 and 200 mg/kg bw; MeOH: 70% methanol extract at 100 and 200 mg/kg bw; MET200: metformin at 200 mg/kg bw.
Discussion
The study of plant extracts with antidiabetic effects offers a promising alternative to the development of new antidiabetic drugs, and the present study was aimed at evaluating how the leaf extracts Euclea natalensis affect the biochemical activity of HFD-STZ induced diabetic rats. The extracts showed no toxicity in normal rats as their median lethal dose (LD50) was more than 2 g/kg bw, a level set by the OECD [Citation19]. The antihyperglycemic potential of the extracts was evaluated using the oral glucose tolerance test (OGTT). OGTT is a standard method for assessing the glucose tolerance status of an individual and provides information about insulin secretion and resistance [Citation32]. All the extracts positively affected the glucose tolerance curves, and blood glucose levels returned to near-normal levels after 3 hours of the experiment. In the area under the curve (AUC) of the glucose tolerance curves, the DCM and MeOH extracts at 100 and 200 mg/kg bw effectively reduced blood glucose levels. Hence, they were chosen as potent extracts and doses for further studies.
The antihyperglycemic potential of DCM and MeOH extracts was assessed in type 2 diabetic Sprague Dawley rats induced by feeding them a high-fat diet (HFD) followed by a single low-dose intraperitoneal injection of streptozotocin (STZ) to induce hyperlipidemia, hyperglycemia, and insulin resistance [Citation33]. STZ is an N-nitroso-containing antibiotic, alkylating, and antineoplastic agent used to cause experimental diabetes by selective destruction of the pancreatic β-cells that produce insulin [Citation34]. Partial destruction of pancreatic β-cells causes insulin deficiency and is coupled with insulin resistance, resulting in hyperglycemia and mimicking the pathophysiology of human T2DM [Citation35]. In the current study, the DCM200, MeOH100, and MeOH200 extracts reduced fasting blood glucose (FBG) when comparing the day 1 FBG to day 21 results. MeOH at 200 mg/kg bw was the most effective extract in reducing FBG.
The reduction in FBG was also coupled with modulation of weight gain, food, and water intake in diabetic-treated rats. Untreated diabetic rats (DC) experienced decreased body weight and increased food and water intake compared to normal rats, which is consistent with some published studies [Citation36]. It has been documented that in an uncontrolled diabetic state, a subject experiences exaggerated weight loss due to muscle degeneration [Citation37]. Fasting blood glucose levels are influenced by glucose production via glycogenolysis and gluconeogenesis [Citation38], which account for more than 90% of endogenous glucose production. However, under diabetic conditions, the rate of glycogenolysis is highly increased, resulting in increased hepatic glucose output and diminished glycogen levels. In this study, glycogen content in DC rats was significantly reduced compared to that in normal rats. Treatment with DCM and MeOH extracts, and metformin significantly ameliorated the hepatic glycogen content compared to DC. MeOH200 caused the highest increase in glycogen content. These results are consistent with other published results that reported decreased glycogen levels in untreated diabetic subjects and increased glycogen levels in diabetic rats treated with extracts of different plants [Citation26,Citation39]. The extracts also ameliorated hepatic glucose output based on the oral pyruvate tolerance test. The pyruvate tolerance test (PTT) assesses the appearance of glucose following a pyruvate bolus administered by oral gavage (O-PTT) in conscious mice and helps estimate hepatic gluconeogenesis [Citation40]. The reduced hepatic glucose output in diabetic rats administered the DCM200 and MeOH200 extracts implies that the extracts might inhibit gluconeogenesis in the liver, and the results agree with published findings [Citation24,Citation41].
Hepatic glucose output during fasting conditions occurring through gluconeogenesis is a well-known phenomenon, and the key enzymes involved in the gluconeogenic pathway are pyruvate carboxylase, phosphoenolpyruvate carboxykinase, fructose 1,6-bisphosphatase, and glucose 6 phosphatase [Citation42]. When evaluating the effects of Euclea natalensis leaf extracts on fructose 1,6-bisphosphatase (F16P) and glucose 6 phosphatase (G6P) from liver microsomes, the activities of both enzymes were significantly increased in DC rats compared to NC rats consistent with existing literature [Citation24]. Treatment with the extracts and metformin reduced the activities of the enzymes. The DCM200 extract was the most effective in ameliorating F16P activity, while the MeOH200 extract was the most effective for G6P activity.
The antihyperglycemic effect of Euclea natalensis extracts was further cemented by the significant reduction in glycated hemoglobin (HbA1c) in diabetic rats administered DCM and MeOH extracts. A significant increase in blood HbA1c was observed in diabetic rats compared to normal rats (NC), consistent with the literature [Citation43,Citation44]. Prolonged hyperglycemia exacerbates the glycosylation of hemoglobin and other proteins, resulting in their structural and functional impairment [Citation45,Citation46]. In addition to ameliorating HbA1c, diabetic rats treated with plant extracts had significantly higher insulin levels than the diabetic untreated group (DC). Diabetic control (DC) rats experienced a significant decrease in plasma insulin levels, which may be due to β-cell dysfunction and reduced β-cell mass [Citation32], consistent with previous findings [Citation47]. In the assessment of insulin resistance using the homeostatic model assessment of insulin resistance (HOMA-IR), the diabetic control group showed significantly higher HOMA-IR than normal rats. However, treatment with the extracts and metformin significantly reduced the score except for the DCM100 extract. Insulin sensitivity was increased after treatment of diabetic animals with extracts and metformin for 21 days, except for the DCM100 extract, based on the quantitative insulin sensitivity check index (QUICKI). In addition, diabetic animals showed drastically reduced beta function based on the homeostasis model assessment of β-cell function (HOMA-β) compared to normal rats. Increases in insulin secretion and sensitivity, decreases in insulin resistance, and improvements in beta cell functionality after treatment with plant extracts or drugs have been documented by previous studies [Citation48,Citation49].
The leaf extracts of Euclea natalensis also ameliorated plasma levels of adiponectin and leptin. While adiponectin was significantly decreased in diabetic controls compared to normal controls, leptin levels were significantly elevated. Surprisingly, administration of the DCM and MeOH extracts increased plasma adiponectin levels and decreased leptin levels. The MeOH200 extract significantly increased adiponectin and reduced leptin levels compared with the DCM extract. Adiponectin is known to improve insulin resistance in peripheral tissues [Citation50] and inhibit the expression of genes essential for glucose production, suppressing hepatic gluconeogenesis [Citation51]. At the same time, leptin regulates appetite [Citation52], thus affecting body weight and energy expenditure [Citation44].
The leaf extracts of Euclea natalensis also showed antihyperlipidemic potential. LDL cholesterol, triglycerides, total cholesterol, and very low-density lipoprotein (VLDL) were significantly elevated in diabetic rats compared to normal rats. However, they were reduced differently by the extracts and metformin after treatment. MeOH200 extract was the most effective in decreasing triglycerides, cholesterol, LDL, and VLDL cholesterols, while the DCM100 was the most efficient in increasing HDL cholesterol. This observed amelioration of lipids in diabetic animals treated with extracts is supported by previous studies [Citation36,Citation53]. The amelioration of plasma hyperlipidemia is critical in reducing the rate of development or progression of cardiovascular diseases in diabetic individuals [Citation54]. This was observed by the reduction in the atherogenic index in diabetic rats treated with extracts and metformin [Citation55].
HFD – STZ – induced DM is associated with elevated lipid peroxidation and drastically decreased activities of SOD, CAT, and GSH [Citation56,Citation57]. In the present study, the level of lipid peroxidation measured in terms of thiobarbituric acid reactive substances (TBARS) was grossly elevated in diabetic rats and significantly reduced in rats treated with DCM and MeOH extracts consistent with previous findings [Citation58,Citation59]. The activities of the enzymatic antioxidants SOD and CAT were significantly reduced in diabetic rats and were significantly enhanced upon treatment with DCM and MeOH extracts. SOD scavenges superoxide radicals by converting them to hydrogen peroxides and molecular oxygen, while catalase breaks down hydrogen peroxide into molecular oxygen and water [Citation60]. Reduced GSH, on the other hand, was significantly decreased in diabetic rats but also interestingly elevated in treated animals. Reduced GSH is a sulfhydryl antioxidant that uses its thiol group to scavenge and neutralize reactive oxygen species [Citation61]. Therefore, the enhancement of SOD, CAT, and GSH may have contributed to the protection of beta cells from oxidative damage, hence the increase in insulin secretion and HOMA-β index indicating beta cell functionality.
One mechanism by which plant extracts exert antihyperglycemic effects is by enhancing the expression and translocation of GLUT 4 to the plasma membrane. In a diabetic state, GLUT4 is downregulated by lack of insulin [Citation62], and its translocation is impaired due to defects in signaling pathways such as the AMPK pathway [Citation63]. The impairment of GLUT 4 translocation and its downregulation culminates in insulin resistance [Citation64], leading to hyperglycemia. In the current study, the relative expression of GLUT4 was downregulated in diabetic rats. Treatment with the DCM and MeOH extracts and metformin upregulated the relative expression of GLUT 4, consistent with previous findings of other plant extracts [Citation30,Citation65]. The increased expression of total GLUT 4 May have significantly contributed to the reduced blood glucose levels observed in diabetic rats treated with the extracts.
Recognizing the importance of AMP – activated protein kinase (AMPK) in regulating the translocation of GLUT 4 from its cytoplasmic vesicles to the plasma membrane, the present study investigated the effect of DCM and MeOH extracts on the AMPK pathway. The AMPK gene was significantly downregulated in both the muscle and liver tissues of the diabetic group. Surprisingly, the MeOH200 extract and MET200 corrected this by significantly upregulating the expression of the AMPK gene in both liver and muscle tissues. The upregulation of AMPK may have caused the upregulation of GLUT 4 expression and subsequently increased glucose uptake by peripheral tissues. The upregulation of AMPK may have contributed to the antihyperglycemic and antihyperlipidemic effects of the MeOH200 extract. AMPK also inhibits glycogen phosphorylase [Citation66], leading to preserved glycogen content in the liver, limiting hepatic glucose output, and reducing FBG, which were observed in this study. The implication of AMPK in the acetyl-CoA (ACC)-malonyl-CoA pathway in the hypothalamus to regulate food intake and body weight [Citation8,Citation67] may also have contributed to the modulation of food intake and body weight in diabetic rats treated with extracts in this study. Therefore, the increased gene expression of AMPK by the MeOH200 extract makes this extract a promising antidiabetic agent that may increase glucose uptake by muscle tissues and alleviate hyperlipidemia and hepatic steatosis, which are prevalent in diabetic conditions.
Finally, the histopathological investigation of the pancreas showed a severely damaged pancreas with reduced sizes of islets and a reduced number of beta-cells in diabetic rats. The islets of Langerhans were ruptured in diabetic rats, which could be linked to the destructive nature of streptozotocin on the pancreas [Citation68,Citation69]. Treatment with the DCM and MeOH extracts resulted in the regeneration of islets and an increased number of β-cells consistent with previous studies that reported the regeneration of islets in response to other plant extracts [Citation69,Citation70]. Protecting the pancreas against hyperglycemia-induced oxidative stress and regeneration against cell damage due to STZ may have resulted in increased plasma insulin, improved beta-cell function (HOMA-β), and general control of glycemia observed in rats treated with extracts. These ameliorative effects of extracts on the biochemical and cellular abnormalities of diabetic rats may be due to phytochemicals such as flavonoids, tannins, and polyphenols, which have been reported in plant extracts [Citation11,Citation14], hence warranting the isolation of bioactive compounds with antidiabetic potential.
Conclusion
The present study has demonstrated that leaf extracts of Euclea natalensis have profound ameliorative effects on the biochemical and cellular abnormalities of type 2 diabetes, partly via the modulation of the AMPK – GLUT 4 pathway. These were shown by the ability of the extracts to reduce insulin resistance and increase insulin sensitivity, reduce hepatic glucose output, and the activities of gluconeogenic enzymes modulating glycogen storage, adiponectin, and leptin levels and ameliorating lipid peroxidation. The extracts increased the expression of GLUT 4 and the relative expression of AMPK in skeletal muscles and liver tissues. Therefore, further studies are needed to isolate and identify the bioactive compounds in the extracts.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047
- Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255 doi: 10.2337/db16-0806
- ElSayed NA, Aleppo G, Aroda VR, et al. (2023) 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care 46:S19–S40. doi: 10.2337/dc23-S002
- Li M, Chi X, Wang Y, et al. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Sig Transduct Target Ther. 2022;7(1). doi: 10.1038/s41392-022-01073-0
- Batista TM, Haider N, Kahn CR (2021) Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia.64(5):994–1006. doi: 10.1007/s00125-021-05415-5
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50(5):567–575. doi: 10.1016/j.freeradbiomed.2010.12.006
- Kjøbsted R, Hingst JR, Fentz J, et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018;32(4):1741–1777. doi: 10.1096/fj.201700442R
- Vázquez MJ, Novelle MG, Rodríguez-Pacheco F, et al. (2020) AMPK-Dependent mechanisms but not hypothalamic lipid signaling mediates GH-Secretory responses to GHRH and ghrelin. 10.3390/cells9091940
- Xu J, Rajaratnam R. Cardiovascular safety of non-insulin pharmacotherapy for type 2 diabetes. Cardiovasc Diabetol. 2017;16(1). doi: 10.1186/s12933-017-0499-5
- Sim R, Chong CW, Loganadan NK, et al. Comparative effectiveness of cardiovascular, renal and safety outcomes of second-line antidiabetic drugs use in people with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetic Med. 2022;39(3). doi: 10.1111/dme.14780
- Maroyi A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of euclea natalensis A.DC. Molecules. 2017;22(12):2128. doi: 10.3390/molecules22122128
- Sales-Peres SHDC, Brianezzi LFDF, Marsicano JA, et al. Evaluation of an experimental gel containing euclea natalensis: An in vitro study. Evid Based Complement Alternat Med. 2012;2012. doi: 10.1155/2012/184346
- Lall N, Kumar V, Meyer D, et al. In vitro and in vivo antimycobacterial, hepatoprotective and immunomodulatory activity of Euclea natalensis and its mode of action. J Ethnopharmacol. 2016;194:740–748. doi: 10.1016/j.jep.2016.10.060
- Lall N, Meyer JJM. Antibacterial activity of water and acetone extracts of the roots of euclea natalensis. J Ethnopharmacol. 2000;72(1–2):313–316. doi: 10.1016/S0378-8741(00)00231-2
- Lall N, Weiganand O, Hussein AA, et al. Antifungal activity of naphthoquinones and triterpenes isolated from the root bark of Euclea natalensis. South Afri J Bot. 2006;72(4):579–583. doi: 10.1016/j.sajb.2006.03.005
- Nkobole N, Houghton PJ, Hussein A, et al. Antidiabetic activity of Terminalia sericea constituents. Nat Prod Commun. 2011;6(11):1585–1588. doi: 10.1177/1934578x1100601106
- Deutschländer MS, Lall N, Van de Venter M, et al. Hypoglycemic evaluation of a new triterpene and other compounds isolated from Euclea undulata Thunb. var. myrtina (ebenaceae) root bark. J Ethnopharmacol. 2011;133(3):1091–1095. doi: 10.1016/J.JEP.2010.11.038
- Brkljača N, Đurović S, Milošević S, et al. Sequential extraction approach for sustainable recovery of various hemp (cannabis sativa L.) bioactive compounds. Sustain Chem Pharm. 2023;35:101213. doi: 10.1016/j.scp.2023.101213
- OECD. Test guideline 453: combined chronic Toxicity\Carcinogenicity studies. 1981;1–17. https://www.oecd.org/chemicalsafety/testing/41362977.pdf
- Benedé-Ubieto R, Estévez-Vázquez O, Ramadori P, et al. guidelines and considerations for metabolic tolerance tests in mice. Diabetes Metab Syndr Obesity. 2020;13:439–450. doi: 10.2147/DMSO.S234665
- Charan J, Kantharia N. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726
- Horakova O, Kroupova P, Bardova K, et al. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep. 2019;9(1). doi: 10.1038/s41598-019-42531-0
- Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi: 10.1016/j.phrs.2005.05.004
- Mata-Torres G, Andrade-Cetto A, Espinoza-Hernández FA, et al. Hepatic glucose output inhibition by Mexican plants used in the treatment of type 2 diabetes. Front Pharmacol. 2020;11:1–9. doi: 10.3389/fphar.2020.00215
- Liu S, Liu Q, Sun S, et al. The application of 2-NBDG as a fluorescent tracer for assessing hepatic glucose production in mice during hyperinsulinemic euglycemic clamp. Acta Pharm Sin B. 2012;2(4):403–410. doi: 10.1016/j.apsb.2012.06.009
- Aba PE; Aba PE. Evaluation of hepatic glycogen content, some haematological and biochemical parameters of alloxan-induced diabetic rats treated with combinations of glibenclamide and G. latifolium extract. J Complement Integr Med. 2017;14(4):1–8. doi: 10.1515/jcim-2016-0078
- Erukainure OL, Matsabisa MG, Salau VF, et al. Tetrahydrocannabinol-rich extracts from cannabis sativa L. Improve glucose consumption and modulate metabolic complications linked to neurodegenerative diseases in isolated rat brains. Front Pharmacol. 2020;11:1–10. doi: 10.3389/fphar.2020.592981
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499
- Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic Index of Plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29. doi: 10.1186/1476-511X-6-1
- Haddad PS, Benhaddou-Andaloussi A, Martineau L, et al. The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evid Based Complement Alternat Med. 2011;2011:1–9. doi: 10.1155/2011/538671
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262
- Wang Y, Wen L, Zhou S, et al. Effects of four weeks intermittent hypoxia intervention on glucose homeostasis, insulin sensitivity, GLUT4 translocation, insulin receptor phosphorylation, and akt activity in skeletal muscle of obese mice with type 2 diabetes. PloS One. 2018;13(9):1–22. doi: 10.1371/journal.pone.0203551
- Arika WM, Kibiti CM, Njagi JM, et al. Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats. Heliyon. 2019;5(11):e02800. doi: 10.1016/j.heliyon.2019.e02800
- Ng TL, Rohac R, Mitchell AJ, et al. An N-nitrosating metalloenzyme constructs the pharmacophore of streptozotocin. Nature. 2019;566(7742):94–99. doi: 10.1038/s41586-019-0894-z
- Vatandoust N, Rami F, Salehi A, et al. Novel high-fat diet formulation and streptozotocin treatment for induction of prediabetes and type 2 diabetes in rats. Adv Biomed Res. 2018;7(1):107. doi: 10.4103/abr.abr_8_17
- Mollica A, Zengin G, Locatelli M, et al. An assessment of the nutraceutical potential of Juglans regia L. leaf powder in diabetic rats. Food Chem Toxicol. 2017;107:554–564. doi: 10.1016/j.fct.2017.03.056
- Yadav VK, Mishra A. In vitro & in silico study of hypoglycemic potential of Pterocarpus marsupium heartwood extract. Nat Prod Res. 2019;33(22):3298–3302. doi: 10.1080/14786419.2018.1471078
- Liu X, Wang K, Zhou J, et al. Metformin and Berberine suppress glycogenolysis by inhibiting glycogen phosphorylase and stabilizing the molecular structure of glycogen in db/db mice. Carbohydr Polym. 2020;243:116435. doi: 10.1016/j.carbpol.2020.116435
- Tella T, Masola B, Mukaratirwa S. The effect of Psidium guajava aqueous leaf extract on liver glycogen enzymes, hormone sensitive lipase and serum lipid profile in diabetic rats. Biomed Pharmacother. 2019;109:2441–2446. doi: 10.1016/j.biopha.2018.11.137
- Hughey CC, Wasserman DH, Lee-Young RS, et al. Approach to assessing determinants of glucose homeostasis in the conscious mouse. Mammalian Genome. 2014;25(9–10):522–538. doi: 10.1007/s00335-014-9533-z
- Mata-Torres G, Andrade-Cetto A, Espinoza-Hernández F. Approaches to decrease hyperglycemia by targeting impaired hepatic glucose homeostasis using medicinal plants. Front Pharmacol. 2021;12. doi: 10.3389/fphar.2021.809994
- Wang Z, Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5(1):30–45. doi: 10.1016/J.TRECAN.2018.11.003
- Domínguez Avila JA, Rodrigo García J, González Aguilar GA, et al. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules. 2017;22:903–916. doi: 10.3390/molecules22060903
- Veeramani C, Alsaif MA, Al-Numair KS. Lavatera critica controls systemic insulin resistance by ameliorating adipose tissue inflammation and oxidative stress using bioactive compounds identified by GC–MS. Biomed Pharmacother. 2018;106:183–191. doi: 10.1016/j.biopha.2018.06.121
- Alamri BN, Bahabri A, Aldereihim AA, et al. Hyperglycemia effect on red blood cells indices. Eur Rev Med Pharmacol Sci. 2019;23:2139–2150. doi: 10.26355/eurrev_201903_17259
- Karimabad MN, Niknia S, Golnabadi MB, et al. Effect of Citrullus colocynthis extract on glycated hemoglobin formation (in vitro). Eurasian J Med. 2020;52(1):47–51. doi: 10.5152/eurasianjmed.2020.19223
- Yaribeygi H, Sathyapalan T, Atkin SL, et al. Review article molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longevity. 2020;2020:1–13. doi: 10.1155/2020/8609213
- Ahmed OM, Hassan MA, Abdel-Twab SM, et al. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother. 2017;94:197–205. doi: 10.1016/j.biopha.2017.07.094
- de Souza Cardoso J, Oliveira PS, Bona NP, et al. Antioxidant, antihyperglycemic, and antidyslipidemic effects of Brazilian-native fruit extracts in an animal model of insulin resistance. Redox Rep. 2018;23(1):41–46. doi: 10.1080/13510002.2017.1375709
- Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8(2):93–100. doi: 10.1093/jmcb/mjw011
- Kim B, Kim MS, Hyun CK. Syringin attenuates insulin resistance via adiponectin-mediated suppression of low-grade chronic inflammation and ER stress in high-fat diet-fed mice. Biochem Biophys Res Commun. 2017;488(1):40–45. doi: 10.1016/j.bbrc.2017.05.003
- Gruzdeva O, Borodkina D, Uchasova E, et al. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes. 2019;12:191–198. doi: 10.2147/DMSO.S182406
- Bartelt A, John C, Schaltenberg N, et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat Commun. 2017;8(1). doi: 10.1038/ncomms15010
- Silva JC, César FA, de Oliveira EM, et al. New PPARγ partial agonist improves obesity-induced metabolic alterations and atherosclerosis in LDLr−/− mice. Pharmacol Res. 2016;104:49–60. doi: 10.1016/j.phrs.2015.12.010
- Emini Veseli B, Perrotta P, De Meyer GRA, et al. Animal models of atherosclerosis. Eur J Pharmacol. 2017;816:3–13. doi: 10.1016/j.ejphar.2017.05.010
- Dordević M, Grdović N, Mihailović M, et al. Centaurium erythraea extract reduces redox imbalance and improves insulin expression and secretion in pancreatic β-cells exposed to oxidative and nitrosative stress. Arch Biol Sci. 2020;72:117–128. doi: 10.2298/ABS200127005D
- Phull AR, Majid M, Haq IU, et al. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. International Journal Of Biological Macromolecules. 2017;97:468–480. doi: 10.1016/j.ijbiomac.2017.01.051
- Kuo Y, Lin C, Shih C, et al. Antrodia camphorata , displays antidiabetic and antihyperlipidemic effects via glucose transporter 4 and AMP-Activated Protein Kinase Phosphorylation in muscles. Evid Based Complement Alternat Med. 2016;2016:1–16. doi: 10.1155/2016/4867092
- Okoh SO, Asekun OT, Familoni OB, et al. Antioxidant and free radical scavenging capacity of seed and shell essential oils extracted from abrus precatorius (L). Antioxidants. 2014;3(2):278–287. doi: 10.3390/antiox3020278
- Rehman K, Akash MSH. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J Cell Biochem. 2017;118(11):3577–3585. doi: 10.1002/jcb.26097
- Farina M, Aschner M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: an intriguing interplay. Biochim Biophys Acta Gen Subj. 2019;1863(12):129285. doi: 10.1016/j.bbagen.2019.01.007
- Naowaboot J, Pannangpetch P, Kukongviriyapan V, et al. Mulberry leaf extract stimulates glucose uptake and GLUT4 Translocation in rat adipocytes. Am J Chin Med (Gard City N Y). 2012;40:163–175. doi: 10.1142/S0192415X12500139
- Hu R, Yan H, Fei X, et al. Modulation of glucose metabolism by a natural compound from chloranthus japonicus via activation of AMP-activated protein kinase. Sci Rep. 2017;7(1):778. doi: 10.1038/s41598-017-00925-y
- Hardie DG, Grahame HD. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62:2164–2172 7 doi: 10.2337/db13-0368
- Kamga-Simo FDY, Kamatou GP, Ssemakalu C, et al. Cassia abbreviata enhances glucose uptake and glucose transporter 4 translocation in c2c12 mouse skeletal muscle cells. J Evid Based Integr Med. 2021;26:1–11. doi: 10.1177/2515690X211006333
- Zhang P, Li T, Wu X, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583–600. doi: 10.1007/s11684-019-0729-1
- Galic S, Loh K, Murray-Segal L, et al. AMPK signaling to acetyl-CoA carboxylase is required for fasting- and cold-induced appetite but not thermogenesis. Elife. 2018;7:1–22. doi: 10.7554/eLife.32656
- Dos Santos JM, Tewari S, Mendes RH. The role of oxidative stress in the development of diabetes mellitus and its complications. J Diabetes Res. 2019;2019:10–13. doi: 10.1155/2019/4189813
- Oyebode OA, Erukainure OL, Sanni O, et al. Crassocephalum rubens (Juss. Ex Jacq.) S. Moore improves pancreatic histology, insulin secretion, liver and kidney functions and ameliorates oxidative stress in fructose-streptozotocin induced type 2 diabetic rats. Drug Chem Toxicol. 2020;45:481–490. doi: 10.1080/01480545.2020.1716783 2
- Majd NE, Tabandeh MR, Shahriari A, et al. Okra (Abelmoscus esculentus) Improved Islets Structure, and Down-Regulated PPARs Gene Expression in Pancreas of High-Fat Diet and Streptozotocin-Induced Diabetic Rats. Cell J. 2018;20(1):31–40. doi: 10.22074/cellj.2018.4819

