ABSTRACT
An effective atypical antipsychotic medication for treating psychosis is clozapine, especially for patients who have failed to respond to other treatments. However, clozapine-induced cardiotoxicity has generated safety concerns as it may result in sudden mortality. In addition, there is relatively little information on the safety of clozapine during pregnancy. According to several earlier studies, Withania somnifera (ashwagandha) extract has a cardioprotective effect. Therefore, this study aimed to investigate the therapeutic potential of ashwagandha root extract on clozapine-induced cardiotoxicity in rat fetuses. Pregnant rats were administered clozapine (40 mg/kg bw) and/or ashwagandha extract (300 mg/kg bw.) from the sixth to fifteenth day of gestation. Clozapine-induced oxidative stress in developing hearts by decreasing superoxide dismutase and catalase activities and increasing the concentration of cardiac malondialdehyde. Moreover, there was a significant increase in the percentage of apoptotic cells and a decrease in the proliferative capacity of cardiomyocytes. Evidently, clozapine triggered severe pathological changes in fetal cardiac tissue. In conclusion, ashwagandha root extract can be used to improve the heart oxidative stress, apoptotic and proliferation rates and histopathological changes induced by clozapine in rat fetuses during gestation.
Introduction
A typical antipsychotics are the primary treatments for psychotic disorders such as schizophrenia. On the other hand, atypical antipsychotics such as olanzapine, clozapine (Cloz), quetiapine, and risperidone have been related to a variety of side effects, such as dyslipidemia, diabetes mellitus, and weight gain. As a result, patients taking these medications face an increased risk of developing metabolic and cardiovascular diseases [Citation1,Citation2].
Cloz is a dibenzodiazepine antipsychotic drug of the second generation. Cloz is much more effective at controlling symptoms than first-generation and some second-generation antipsychotics, according to meta-analyses [Citation3,Citation4]. Numerous factors, including genetic polymorphisms in cytochrome oxidase enzymes, concurrent drug usage, inflammation, and intervening physical illnesses predispose to Cloz toxicity. Additionally, seizures, respiratory depression, disorientation, agitation, sleepiness, hypotension, and lethargy are signs of Cloz toxicity, as reported by Macfarlane et al. [Citation5–7]. The mechanisms underlying the effects and side effects of Cloz are unknown. However, Cloz has been reported to have extrapyramidal effects by inhibiting serotonin (5HT2), muscarinic, dopamine D2, and H1 histaminic receptors [Citation8]. In contrast to other antipsychotics, it does not significantly influence other hormone levels or create prolonged rises in blood prolactin levels, which can prevent conception [Citation9].
There is relatively little information on the risk of Cloz during pregnancy and breastfeeding [Citation10]. According to multiple case reports and previous research, Cloz appears to be linked to a higher risk of diabetes mellitus in pregnant cases [Citation11,Citation12] and congenital abnormalities in infants [Citation13]. Cardiotoxicity is a typical, severe side effect of Cloz that requires ongoing monitoring [Citation14]. International reports of Cloz-induced myocarditis cases have been made in a number of cases, with mortality occurring in 85% of those cases [Citation15]. According to clinical research, using Cloz might cause heart failure, myocarditis, pericarditis, and possibly death [Citation16]. Cloz may impair immunity, and its toxicity may result in severe heart inflammation [Citation17].
The use of natural therapies has been on the rise internationally [Citation18]. The safety of medicinal plants is one of the key benefits promoted for their therapeutic use in treating a variety of illnesses, in addition to their affordability, potency, and accessibility [Citation19,Citation20]. The medicinal herb Withania somnifera, often known as ashwagandha, or Indian ginseng, is a member of the Solanaceae family [Citation21]. It has broad energizing and regenerating properties and is used, among other agents, to treat nervous weariness, memory-related disorders, insomnia, tiredness-related problems with potency, skin problems, and coughing [Citation20–22]. Ashwagandha extracts exhibit anti-inflammatory, anticancer, antiviral, neuroprotective, cardioprotective, and antioxidant effects [Citation22,Citation23]. Alkaloids, glycowithanolides, steroidal lactones (withanolides), flavanol glycosides, sterols, and phenolics are among the more than 200 primary and secondary metabolites found in plant extracts from diverse sections [Citation24]. Withanolides, the main components of ashwagandha extracts, are the active chemicals that are responsible for most of the biological effects of Withania somnifera [Citation25].
Numerous epidemiological investigations have shown that phenolics and flavonoids are excellent anticancer, anti-inflammatory, and cardiovascular disease risk-reducing agents [Citation26]. However, compared to other plant components, ashwagandha has higher concentrations of catechin [Citation27]. Catechin appears to lower the risk of ischemic heart disease, according to several earlier studies [Citation28–30].
Furthermore, among other cardiovascular illnesses, myocardial ischemia and reperfusion are caused largely by oxidative stress, which is a significant stimulant of programmed cell death [Citation31]. According to some reports, antioxidants can prevent programmed cell death. However, few studies have examined how apoptosis inhibition affects the antioxidant state of the myocardium [Citation32,Citation33]. In addition, Ashwagandha extract inhibited H2O2-induced DNA damage in peripheral blood cells [Citation34]. As a result of the encouraging results in health promotion achieved through in vivo and in vitro studies, ashwagandha requires further investigation for use in the treatment of different chronic diseases and health complications to determine the potential mechanisms. Therefore, the present study attempted to assess the impact of ashwagandha supplementation on pregnant rats prenatally exposed to Cloz, and to determine whether it can reverse the cardiac degeneration caused by Cloz.
Materials and methods
Drugs and chemicals
Cloz tablets (100 mg) containing the active ingredients were manufactured by Copad Egypt for trade and pharmaceutical industries (Copad Pharma), Obour City, Cairo, Egypt. The tablets were crushed, diluted in purified water, and gavaged orally every day at a dose of 40 mg/kg body weight (bw.) according to Oltulu and Karadag [Citation35]. Biochemical kits for malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were purchased from Sigma Chemical Co., St. Louis, MO, USA.
Plant extraction and analysis
The dry roots of the plant Withania somnifera (L.) Dunal were obtained from a local herbal store and authenticated by the National Network of Egyptian Herbaria, Cairo University Herbarium. The voucher specimen (Prodr. 2002/453/1852). The roots are crushed into a fine powder by an electric grinder machine. Fifty grams of Withania somnifera have been extracted twice with 100% distilled water at room temperature for two days each. The filtered extracts were then concentrated by freeze drying to yield a dry powder. Samples were frozen using liquid nitrogen at −196 ◦C, allowed to acclimate to −80 ◦C for two hours, and then freeze-dried for 48 hours. By measuring little amounts of dried particles (about 0.5 g) both before and after drying in an oven at 105°C until constant weight, the moisture content of the freeze-dried particles was ascertained gravimetrically [Citation36,Citation37].
The material was analyzed with liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) utilizing an ExionLC AC system for isolation and a SCIEX Triple Quad 5500+ MS/MS system with electrospray ionization (ESI) for detection. An Ascentis® Express 90 C18 column (2.1×150 mm, 2.7 µl) was used for the separation. MS-DIAL and the Respect library were used to identify compounds.
Experimental animals
The study was conducted according to the guidelines of the ARRIVE and the institutional guideline and was approved by the Institutional Animal Care and Use Committee at the Faculty of Science, Menoufia University, Egypt (permit number MUFS/S/EM/2/22). Eighty mature virgin females and forty fertile male Wister albino rats (Rattus norvegicus) weighting 165 ± 10 g and aged 16 ± 1 weeks were kept in clean, well-ventilated cages in the animal facility. For acclimatization, the animals were kept for one week before the onset of the experiments and had a normal basal diet as well as complete access to water with an internal temperature of 22 ± 1°C.
Mating was performed by placing female rats with sexually mature males at a ratio of two females to one male overnight. The day on which a copulatory plug was observed in females and sperm was detected in the vaginal smear was day zero of gestation. The outcomes of this experiment were evaluated on day 20 of pregnancy.
Experimental design
The pregnant rats (n = 60) were randomly divided into four groups of 15 rats each, as follows:
Group (1): served as a control and was received 1 ml of distilled water.
Group (2): A single daily dose of 300 mg of ashwagandha/Kg/bw [Citation38] was given from the 6th to 15th day of gestation.
Group (3): A single daily dose of 40 mg of Cloz/Kg/bw [Citation35] was given from the 6th to 15th day of gestation.
Group (4): Pregnant rats were received a single daily dose of 40 mg of Cloz/kg/bw, followed by 300 mg of ashwagandha/kg/bw. One hour later, from the 6th to 15th day of gestation.
Halothane (Pharco, Egypt) was used for anesthesia, then the uterus was exposed through the lower midline abdominal incision. This study was based on 80 females (only 60 became pregnant) and 122 fetuses.
Biochemical analysis of heart tissue
The heart tissues were homogenized in 20 mL of 1X PBS, immediately followed by two ice-cold PBS washes, and stored at -20 °C overnight. After two freeze-thaw cycles, the samples were centrifuged at 1200×g for 20 min, and the supernatant was removed, analyzed immediately, or aliquoted and stored at −80°C [Citation39].
In tissue homogenates, malondialdehyde (MDA), was quantified calorimetrically (n mol/mg) according to the manufacturer’s instructions for the ELISA kit (MDA; Cat No: KA1206, Abnova, USA).
Superoxide dismutase (SOD) and catalase (CAT) enzyme activities (Unit/mg) as markers of oxidative stress were measured calorimetrically in tissue homogenates according to the manufacturer’s instructions for the ELISA kit (EnzyChromTM Superoxide Dismutase Assay Kit (ESOD-100), BioAssay System, USA) and (Rat Catalase (CAT) Kit; Cat No. MBS2600683; My BioSource, USA).
Flow cytometry analysis of the proliferative marker ki-67
Proliferating cells displayed the marker Ki-67, which measured by flow cytometry (BD AccuriTM C6) and its compatibility software (Becton Dickinson, Sunnyvale, CA, USA), according to the technical protocol of Immunostep Biotech. (Centro Investigación del Cáncer (C.I.C) Avda. Universidad de Coimbra, s/n Campus Miguel de Unamuno 37,007 Salamanca (Spain)) in a prepared cardiomyocyte suspension.
Annexin-V/PI double labeling for apoptosis
The cells were washed, incubated in PBS with 30% human AB serum, and then annexin V-FITC and PI added using a BD Pharmingen FITC Apoptosis Kit (Cat. No. 556547) in accordance with the manufacturer’s instructions. A BD AccuriTM C6 flow cytometer (Becton Dickinson, Sunnyvale, CA, USA) was used immediately to analyze the cells. The percentage of glowing cells in all quadrants was calculated after displaying the fluorescence pattern as a two-color dot.
Histopathological analysis
The cardiac tissues from all groups were fixed in 10% neutral-buffering formalin and prepared according to Suvarna et al. [Citation40]. Microscopical examination and imaging were performed using an Olympus microscope (BX41, Japan).
Transmission electron microscopy analysis
The heart tissue from fetuses of all groups was split into fine pieces, rapidly fixed in 2.5% glutaraldehyde, afterward fixed in a buffered 1% osmium tetraoxide, and processed according to Kuo [Citation41]. The grids were examined and photographed with a JEOL electron microscope (TEM-1400 plus, Japan).
Statistical evaluation
For analysis, Graph-Pad Prism 9 was utilized. All data was analyzed and interpreted as the mean ± standard deviation (SD) using computerized one-way ANOVA adhering to Tukey’s pos hoc test. The significance level was set at a probability of p < 0.05. The significance of the obtained data was classified as p < 0.001 or p < 0.05 according to the obtained p values.
Results
LC‒ESI‒MS/MS analysis
Eight compounds were defined from Withania somnifera extract, five compounds exhibited abundant [M+H]+ and three compounds exhibited abundant deprotonated [M−H]− therefore, both positive and negative ionization modes were selected for further studies. (, ).
Figure 1. LC-ESI-MS spectra of the withania somnifera L root extract with the proposed fragmentation of the compounds.
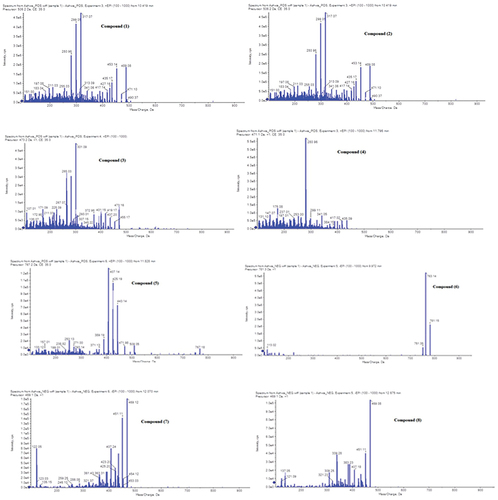
Table 1. Identification of phytoconstituents in 3 mg withania somnifera root extract. In 1 ml 80% methanol.
Changes in cardiac tissues antioxidants and MDA concentration
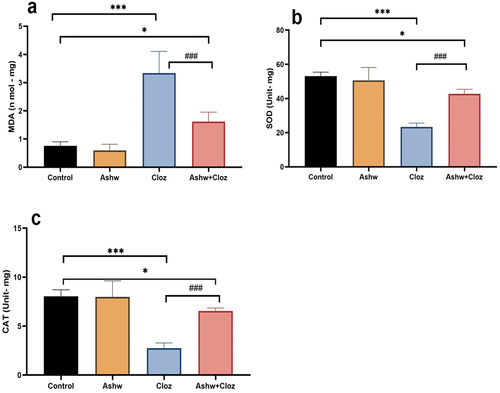
There was a highly significant (p < 0.001) increase in the MDA concentration in the fetal heart of the rats treated with Cloz during gestation compared to that in the control and ashwagandha groups. In contrast, the administration of ashwagandha after Cloz resulted in a highly significant (p < 0.001) decrease in activity compared with that in the Cloz group ().
Figure 2. Graph showing the effect of prenatal administration of cloz and/or ashwagandha in the different groups. (a) MDA concentrations, (b) SOD and (c) CAT enzymes activities. The data are presented as the means ± SDs (n = 6/group). *p < 0.05, ***p < 0.001 indicates a significant difference compared with the control group, and ###p < 0.001 indicates a significant difference compared with the cloz group.
SOD and CAT activities
The hearts of fetuses prenatally exposed to Cloz exhibited highly significant (p < 0.001) decreases in SOD and CAT activities compared to those in the control group. On the other hand, the combined treatment with ashwagandha after Cloz had a highly significant (p < 0.05) increase in the activities of SOD and CAT compared with that in the Cloz group and the change was significant (p < 0.05) compared with that in the control group ().
Changes in the proliferation rate
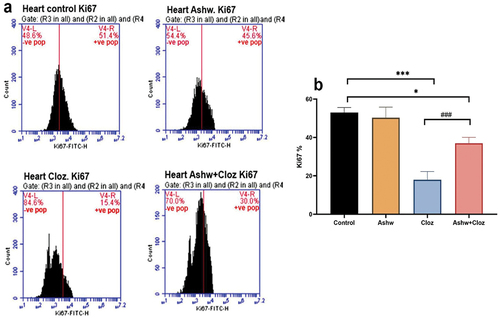
Flow cytometric analysis of Ki67 (a proliferation marker) expression in fetal cardiomyocytes is shown in . Similarly, the percentage of positively Ki67 cardiac cells was significantly (p < 0.001) lower in the Cloz group (15.4%) than in the control group (51.4%). Moreover, compared with those in the Cloz group, Ki67+ cardiac cells in the Cloz and ashwagandha group exhibited a highly significant (p < 0.001) increase in Ki67+ cardiac cells (30%).
Figure 3. Effect of cloz and/or ashwagandha on fetal cardiomyocyte proliferation. (a) the presented histogram is representative of one trial from a total of three trails conducted independently. (b) pooled data from the three experiments are presented as mean percentage of Ki67+ cells ± SD. ***p < 0.001 and *p < 0.05 indicate significant differences compared with the control group. ###p < 0.001 indicates a significant difference compared with the cloz group.
Apoptotic changes in cardiac cells
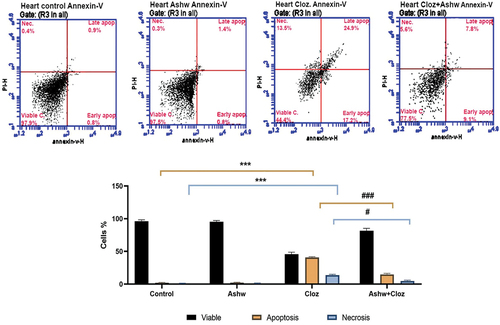
The influence of Cloz and/or ashwagandha on fetal cardiomyocytes is shown in . Most of the cells in the control and ashwagandha groups were viable (97.5 and 97.7% for the two groups, respectively), while the cardiomyocytes in the Cloz group showed a decrease in the percentage of viable cells (44.4%) and a highly significant (p < 0.001) increase in the rates of total apoptosis and necrosis (42.1 and 13.5%, respectively). Conversely, co-treatment with ashwagandha significantly decreased the rate of cardiomyocyte apoptosis (16.9%) and the rate of necrosis (5.6%) compared to those in the Cloz group.
Figure 4. Effect of cloz and/or ashwagandha on fetal cardiomyocytes. (a) The presented histogram is representative of one trial from a total of three trials conducted independently. Different cardiomyocyte populations were distinguished by flow cytometry based on their PI/Annexin-V staining patterns; viable cells (negative for both stains), early apoptotic cells (positive for annexin), late apoptotic cells (positive for both stains) and necrotic cells (positive for PI). (b) Pooled data from the three experiments are presented as the mean percentage of different cardiomyocyte populations ± SD. ***p < 0.001 indicates a significant difference compared with the control group. ###p < 0.001 and #p < 0.05 indicate significant differences compared with the cloz group.
Histopathological changes in fetal heart tissue
Analysis of the hearts of the control and ashwagandha rat fetuses stained with H and E revealed a normal appearance. The myocardial fibers were smooth, and most of the cells were elongated and rod-shaped with central, oval vesicular nuclei. The myofibrils consisted of repeating sections of sarcomeres and typical intercalated disks were observed between cells ().
Figure 5. Photomicrographs of sections of the cardiac muscle of 20-day-old fetuses. (a) Control, (b) Ashwagandha, (c-f) Cloz, and (g) Cloz + ashwagandha groups.
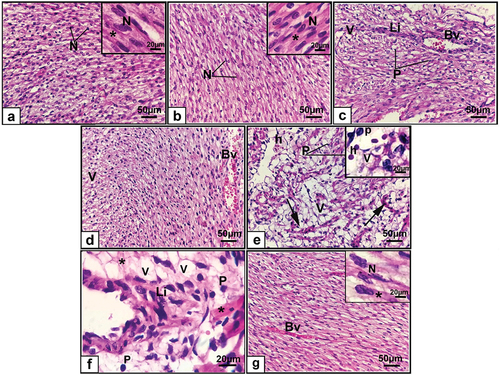
The hearts of the rats maternally administered Cloz had degenerated myocardium. The cardiac myofiber exhibited a disarray pattern and lost their branched structure compared with those of the control group. Moreover, inflammatory cell infiltration, blood vessel congestion, fragmented myocardial fibers, and intercellular hemorrhage were observed. Additionally, the myocardium had many vacuolated cells, which is an indication of cardiomyocyte hypertrophy and pyknotic nuclei (). The combined group exhibited fewer histological changes in the myocardium than did the Cloz group. The cardiac fibers retained their arranged pattern, and many nuclei regained their oval shape. The degree of inflammatory cell infiltration remained within the normal range, but some congested blood vessels were observed ().
Ultrastructural changes in fetal cardiomyocytes
In the control group, the cardiac myocytes of 20-day-old fetuses had oval euchromatic nuclei in a central position, a prominent nucleolus, and a regular nuclear envelope. The sarcomere distribution throughout the myofibrils was uniform and the myofibrils were arranged regularly between the Z lines. A large number of mitochondria were arranged in rows between the cardiac myofibrils. Additionally, highly coordinated intercalated discs linking adjacent cardiomyocytes were observed (). Cardiomyocytes from the ashwagandha group had typical arrangement like that of the control group ().
Figure 6. Transmission electron micrographs of cardiomyocytes from 20-day-old fetuses. (a , b) Control showed oval euchromatic nuclei, prominent nucleolus, regular nuclear envelope, will arrange myofibrils with Z lines, rows of mitochondria and highly coordinated intercalated discs (c) Ashwagandha, (d-g) Cloz showed degenerated myofibrils, degenerated intercalated discs, pyknotic and shrunken, nuclei with marginated chromatin, degenerated mitochondrial damage and accumulation of collagen. (h-i) Cloz + ashwagandha groups.
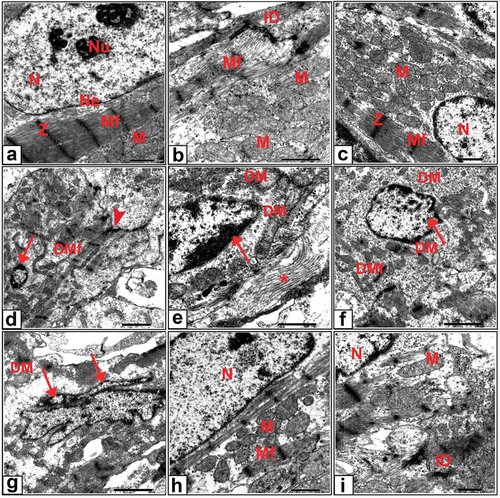
The cardiac myocytes in the Cloz group were disorganized and the myofibrils were fragmented. Additionally, disruption of the Z line and degeneration of the intercalated discs were evident. The nuclei of cardiac myocytes exhibited several abnormalities. These nuclei appeared pyknotic and shrunken with marginated chromatin, or irregular and fragmented as shown in . Mitochondrial damage with loss of cristae and accumulation of collagen fibers was also observed (). An examination of cardiac myocytes from rat fetuses in the combined group revealed marked improvement in most cardiac myocytes. The cardiomyocyte nuclei exhibited an oval euchromatic shape, and the mitochondria with normal cristae were arranged in rows. Moreover, the sarcomeres exhibited an ordinary organization, and the intercalated discs were partially organized ().
Discussion
Cloz is the best treatment for resistant schizophrenia. Nonetheless, there have been risks expressed about Cloz-induced cardiotoxicity, which can result in unexpected death, especially in young patients [Citation42–44].
The current investigation revealed a statistically significant increase in the mean heart MDA concentration with a significant decline in the heart SOD and CAT activities in fetuses from the Cloz group. Lipid peroxidation is known to be sensitively indicated by MDA which is used to assess the level of oxidative stress [Citation45]. SOD and CAT, on the other hand, are critical enzymes in cellular antioxidant defense mechanisms [Citation46,Citation47]. Because cardiac tissue has a highly oxidative metabolic process and less antioxidant defenses, it is particularly susceptible to damage from free radicals [Citation48], these findings might suggest that oxidative stress is the primary cause of Cloz-induced myocarditis, as suggested by Heiser et al. [Citation49], Abdel-Wahab and Metwally [Citation42], Nair et al. [Citation50] and Rabkin and Tang [Citation51].
Depending on the mechanism, Cloz either directly causes cellular damage, lipid peroxidation, and free radical formation through bioactivation to a chemically reactive nitrenium ion [Citation51] or indirectly through raising serum catecholamine levels, which raise myocardial oxygen demand through direct myocardial stimulation and increased cardiac loads. Furthermore, catecholamines reduce oxygen perfusion in the heart, which might result in ischemia in the myocardium and free radical production [Citation52]. The reactive oxygen species (ROS) and free radicals can target membrane phospholipids, enhancing lipid peroxidation and degenerate the cardiac cell membrane [Citation53]. The increased MDA level observed in this investigation is indicative of this mechanism. The decreased antioxidant enzyme activities shown in this study may be due to the excess production of ROS in cardiac cells, which consumes the stores of cellular enzymes and impairs antioxidant defenses [Citation54].
According to the results of the present study, Cloz induces apoptosis in fetal cardiomyocytes. In addition, it decreased proliferation capacity, whereas the percentage of Ki67+ cardiomyocytes was significantly decreased. This is due to the reported increase in oxidative stress, which is accompanied by a decrease in antioxidant defenses and the resulting cellular and DNA damage. These changes can result in myocarditis and cell death in the heart muscle, as well as severe cardiac damage and cardiomyopathy [Citation55]. A previous study showed increases in cardiac 8-OHdG (a biomarker of DNA damage), caspase-3, (a marker of apoptosis), and NF-κβ p65 (a nuclear factor that contributes to the inflammatory response and cell apoptosis) levels following exposure to Cloz [Citation56].
The present histological analysis of the myocardium from the Cloz group revealed several pathological consequences including disorganization, inflammatory cell infiltration, blood vessel congestion, fragmented myocardial fibers, hemorrhage, cardiomyocyte hypertrophy, and pyknotic nuclei. These findings coincide with those of Yuen et al. [Citation6], who reported that Cloz therapy is associated with orthostatic hypotension, fatal myocarditis, tachycardia and paradoxical hypertension. Additionally, Hägg [Citation57] reported that myocarditis has been associated with the use of Cloz in several case reports. These histological alterations were also observed by Wang et al. [Citation58], Kamel and Kamel [Citation59], Abdel-Wahab et al. [Citation60], and Abdel-Wahab and Metwally [Citation42]. Moreover, it has been shown that Cloz, ziprasidone, and sertindole have effects on rat hearts, which are characterized by toxic myocarditis. These three drugs cause degenerative changes in cardiomyocytes, which are accompanied by lymphocytic infiltration and redox imbalance and play a role in the development of histopathological alterations in cardiac tissue [Citation61].
Ultrastructurally, myofibrils and intercalated disc fragmentation, disruption in the Z lines, pyknotic nuclei, collagen fiber accumulation, and degenerated mitochondria were evident. Consequently, Kamel and Kamel [Citation59] showed that Cloz injection at a dose of 25 mg/kg to male rats caused fragmentation of myofibrils, disorganization of the Z lines, and mitochondrial rupture. Moreover, Saito et al. [Citation62] reported that the injection of the typical antipsychotic chlorpromazine at a dose of 5 mg/kg into rats caused ultrastructural alterations of the myocardium, such as contracted myofibers, collagen fibers accumulation, degenerated sarcoplasmic reticulum and mitochondria.
The deterioration of cardiac cells with pyknotic nuclei observed in this study could be attributed to apoptotic or proapoptotic characteristics. According to many studies, the key pathogenetic element of cardiotoxic effects is the detrimental effect of Cloz reactive metabolites [Citation63]. Cloz was discovered to be bioactivated in cardiac tissue to a chemically active nitrenium ion metabolite. The metabolite attaches to proteins in the myocardium, resulting in the creation of an antigenic complex that stimulates the immune response and macrophages, followed by the release of cytokines, which cause cellular infiltration and myocarditis [Citation64].
The oxidative stress caused by increased free radical formation in cardiomyocytes causes an energy imbalance, mitochondrial dysfunction, activation of stress-related signaling pathways, p53 accumulation, and cellular death [Citation65]. In addition, antioxidant supplementation has been linked to a reduction in ROS-induced cardiomyocyte damage and may have a role in decreasing drug-induced cardiotoxicity [Citation66,Citation67].
Ashwagandha appears to have a favorable effect on the endocrine, cardiovascular, and central nervous systems [Citation68]. It is also an effective anti-inflammatory drug. Its naturally occurring steroidal content is substantially greater than that of hydrocortisone, a routinely prescribed anti-inflammatory agent [Citation69]. The fact that ashwagandha significantly recovered SOD and CAT activities when compared to the Cloz group suggested that they may have a cardio-ameliorative impact. Treatment with ashwagandha improved the cardiac antioxidant status as well as membrane integrity as evidenced by a decrease in MDA levels. Furthermore, histological and ultrastructural analysis confirmed the cardio ameliorative effects of ashwagandha. Previous studies have reported that ashwagandha restores normal levels of cardiac oxidative stress markers and antioxidant enzymes induced by doxorubicin [Citation70], and by isoproterenol [Citation71] in animal models. Ashwagandha may scavenge free radicals produced by lead and gentamicin toxicity in the liver and kidney, as revealed by decreased lipid peroxidation and enhanced activity of antioxidant enzymes in tissues [Citation72,Citation73].
The fundamental therapeutic benefits of ashwagandha are derived from the activities of specific lactones and steroidal alkaloids, which constitute a group of components known as withanolides [Citation68,Citation71]. Together with numerous alkaloids, the root contains the steroid lactone (withaferin A) and related withanolides. Sitoindosides VII, VIII, IX, and X have been found to be adaptogenically active compounds in ashwagandha. The specific mechanism underlying this myocardial adaptation is unknown. However, it has been proposed that it functions by inducing a number of antioxidant enzymes such as CAT, SOD and GPx, as well as antioxidants such as glutathione and proteins such as heat shock proteins [Citation71].
Besides antioxidant capacity, the anti-apoptotic activity that contributes to cardio-protection was demonstrated by increased Ki67+ proliferation capacity and decreased apoptotic rates. The antiapoptotic properties of ashwagandha have previously been described in a stroke rat model [Citation74]. Moreover, Mohanty et al. [Citation33] demonstrated that ashwagandha protects against ischemia and reperfusion injury due to its antioxidant and antiapoptotic properties. It increased the expression of Bcl-2, an antiapoptotic protein, and decreased the expression of Bax, a proapoptotic protein, as well as attenuated terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) positivity, a hallmark of apoptosis. In addition, Preclinical studies have demonstrated that this plant can regulate apoptosis, modulate mitochondrial activity, and reduce inflammation in addition to inhibiting inflammatory indicators such as cytokines (TNF-a and IL-6), NO, and ROS.
The results of the present study confirmed the importance of ashwagandha in preventing the toxic effects of Cloz, and the administration of ashwagandha after Cloz treatment, ameliorated the myocardial histopathological and ultrastructural changes which further confirmed the biochemical findings. Similarly, Hamza et al. [Citation70] showed that ashwagandha protects against myofibril degeneration, focal necrosis, hemorrhage, and inflammatory cell infiltration induced by doxorubicin in the hearts of rats. Furthermore, Khalil et al. [Citation30] discovered that ashwagandha considerably decreases the damage to the heart induced by isoproterenol. Because of its antiapoptotic, anti-inflammatory qualities and ability to restore oxidative balance, ashwagandha has a cardioprotective impact against ischemia and reperfusion injury [Citation71]. Moreover, it has been shown that ashwagandha protects the cardiac muscle. Studies have demonstrated that ashwagandha can lessen the ischemia-induced damage to the cardiac muscle. This protective effect might aid in preventing cardiovascular events such as heart attacks.
Conclusions
Based on the obtained biochemical, flow cytometric, histopathological, and ultrastructural data, it was concluded that ashwagandha administration during gestation is an effective cardioprotective agent against Cloz-induced cardiotoxicity in fetal hearts. The antioxidant and antiapoptotic properties of ashwagandha’ phytoconstituents may attributed to the beneficial effects. Consequently, additional research is needed to assess the advantages of adopting ashwagandha as a cardioprotective remedy, particularly in clinical research, because we are currently unable to fully understand the precise mechanisms underlying the plant’s possible therapeutic effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bushe C, Paton C. The potential impact of antipsychotics on lipids in schizophrenia: is there enough evidence to confirm a link? J Psychopharmacol. 2005;19(6):76–83. doi: 10.1177/0269881105058719
- Haddad P. Weight change with atypical antipsychotics in the treatment of schizophrenia. J Psychopharmacol. 2005;19(6):16–27. doi: 10.1177/0269881105058378
- Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a metaanalysis. Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X
- Johal H, Barrera A. Clozapine-induced pericarditis: an ethical dilemma. BMJ Case Rep. 2019;12(6):e229872. doi: 10.1136/bcr-2019-229872
- Macfarlane M, Shahab J, Willis D, et al. Clozapine toxicity: a cautionary palliative care tale. BMJ Support Palliat Care. 2020;10(3):1–2. doi: 10.1136/bmjspcare-2019-001988
- Yuen JW, Kim DD, Procyshyn RM, et al. Clozapine-induced cardiovascular side effects and autonomic dysfunction: a systematic review. Front Neurosci. 2018;12:203. doi: 10.3389/fnins.2018.00203
- De Berardis D, Rapini G, Olivieri L, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther Adv Drug Saf. 2018;9(5):237–256. doi: 10.1177/2042098618756261
- Stahl S. “Clozapine,” in stahl essential psychopharmacology: prescriber’s Guide. 6th ed. New York, NY: Cambridge University Press; 2017. pp. 177–186.
- Haddad P, Wieck SA. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291–2314. doi: 10.2165/00003495-200464200-00003
- Mehta T, Van Lieshout R. A review of the safety of clozapine during pregnancy and lactation. Arch Womens Ment Health. 2017;20(1):1–9. doi: 10.1007/s00737-016-0670-0
- Beex-Oosterhuis M, Van Gool A, Heerdink E, et al. Clozapine treatment during pregnancy and the postpartum period: a systematic literature review. J Clin Psychiatry. 2021;83(1):21r13952. doi: 10.4088/JCP.21r13952
- Bodén R, Lundgren M, Brandt L, et al. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69(7):715–721. doi: 10.1001/archgenpsychiatry.2011.1870
- Dev V, Krupp P. Adverse event profile and safety of clozapine. Rev Contem Pharmacoth. 1995;6:197–208.
- Killian J, Kerr K, Lawrence C, et al. Myocarditis and cardiomyopathy associated with clozapine. Lancet. 1999;354(9193):1841–1845. doi: 10.1016/S0140-6736(99)10385-4
- Haas S, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993–2003. Drug Saf. 2007;30(1):47–57. doi: 10.2165/00002018-200730010-00005
- Markovic J, Momcilov-Popin T, Mitrovic D, et al. Clozapine-induced pericarditis. Afr J Psychiatry. 2011;14(3):236–238. doi: 10.4314/ajpsy.v14i3.7
- Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157(7):498–511. doi: 10.7326/0003-4819-157-7-201210020-00525
- Garcia-Cortes M, Robles-Diaz M, Ortega-Alonso A, et al. Hepatotoxicity by dietary supplements: a tabular listing and clinical characteristics. Int J Mol Sci. 2016;17(4):537. doi: 10.3390/ijms17040537
- El-Borm H, Gobara M, Badawy G. Modulatory effect of ginger on skeletal malformations, cell cycle, apoptosis and structural changes in the liver of rat fetuses prenatally exposed to labetalol. Beni-Suef Univ J Basic Appl Sci. 2023;12(1):4. doi: 10.1186/s43088-023-00345-0
- Verma S, Kumar A. Therapeutic uses of withania somnifera (ashwagandha) with a note on withanolides and its pharmacological actions. Asian J Pharm Clin Res. 2011;4:1–4.
- Mukherjee P, Harwansh R, Bahadur S, et al. Withania somnifera (L.) dunal-modern perspectives of an ancient rasayana from ayurveda. J Ethnopharmacol. 2021;26:113–157. doi: 10.1016/j.jep.2020.113157
- Widodo N, Priyandoko D, Shah N, et al. Selective killing of cancer cells by ashwagandha leaf extract and its component withanone involves ROS signaling. PLoS One. 2010;5(10):e13536. doi: 10.1371/journal.pone.0013536
- Palliyaguru D, Singh S, Kensler T. Withania somnifera: from prevention to treatment of cancer. Mol Nutr Food Res. 2016;60(6):1342–1353. doi: 10.1002/mnfr.201500756
- Trivedi M, Panda P, Sethi K, et al. Metabolite profiling in Withania somnifera roots hydroalcoholic extract using LC/MS, GC/MS and NMR spectroscopy. Chem Biodivers. 2017;14(3):3. doi: 10.1002/cbdv.201600280
- Kulkarni S, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol. Biol Psychiatry. 2008;32(5):1093–1105. doi: 10.1016/j.pnpbp.2007.09.011
- Saravanan G, Prakash J. Effect of garlic (allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol. 2004;94(1):155–158. doi: 10.1016/j.jep.2004.04.029
- Alam N, Hossain M, Khalil M, et al. High catechin concentrations detected in Withania somnifera (ashwagandha) by high performance liquid chromatography analysis. BMC Complement Altern Med. 2011;11(1):1–8. doi: 10.1186/1472-6882-11-65
- Arts I, Hollman P, Feskens E, et al. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen elderly study. Am J Clin Nutr. 2001;74(2):227–232. doi: 10.1093/ajcn/74.2.227
- Devika P, Prince P. Epigallocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats: a transmission electron microscopic and in vitro study. Pharmacol Res. 2008;57(5):351–7. doi: 10.1016/j.phrs.2008.03.008
- Khalil M, Ahmmed I, Ahmed R, et al. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by withania somnifera leaf extract. Biomed Res Int. 2015;2015:1–10. doi: 10.1155/2015/624159
- Narula J, Pandey P, Arbustini E, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci U S A. 1999;96(14):8144–8149. doi: 10.1073/pnas.96.14.8144
- Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71(1–2):23–43. doi: 10.1016/S0378-8741(00)00213-0
- Mohanty I, Arya D, Gupta S. Withania somnifera provides cardioprotection and attenuates ischemia–reperfusion induced apoptosis. Clin Nutr. 2008;27(4):635–642. doi: 10.1016/j.clnu.2008.05.006
- Kumar N, Yadav A, Gupta R, et al. Antigenotoxic effect of withania somnifera (ashwagandha) extract against DNA damage induced by hydrogen peroxide in cultured human peripheral blood lymphocytes. Int J Curr Microbiol Appl Sci. 2016;5(4):713–719. doi: 10.20546/ijcmas.2016.504.082
- Oltulu Ç, Karadağ ÇH. The effect of intrauterine antipsychotic drug exposure on learning and memory in adult rats. Klinik Psikofarmakoloji Bülteni-Bulletin of Clin Psychopharmacol. 2016;26(4):364–373. doi: 10.5455/bcp.20160627090254
- Khan MA, Ahmed RS, Chandra N, et al. In vivo, extract from Withania somnifera root ameliorates arthritis via regulation of key immune mediators of inflammation in experimental Model of arthritis. Antiinflamm Antiallergy Agents Med Chem. 2019;18(1):55–70. doi: 10.2174/1871523017666181116092934
- Kashaninejad M, Blanco B, Benito-Román O, et al. Maximizing the freeze-dried extract yield by considering the solvent retention index: extraction kinetics and characterization of moringa oleifera leaves extracts. Food Bioprod Process. 2021;130:132–142. doi: 10.1016/j.fbp.2021.09.008
- Khan MA, Subramaneyaan M, Arora VK, et al. Effect of withania somnifera (ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J Complementary Integr Med. 2015;12(2):117–125. doi: 10.1515/jcim-2014-0075
- Elsayed A, Elkomy A, Elkammar R, et al. Synergistic protective effects of lycopene and N-acetylcysteine against cisplatin-induced hepatorenal toxicity in rats. Sci Rep. 2021;11(1):13979. doi: 10.1038/s41598-021-93196-7
- Suvarna K, Layton C, Bancroft J. Bancroft’s theory and practice of histological techniques. 8th ed. Elsevier; 2018.
- Kuo J. Electron microscopy. Methods and protocols. 2 ed. New Jersey: Humana Press Inc. Totowa; 2007. p. 369.
- Abdel-Wahab BA, Metwally ME. Clozapine-induced cardiotoxicity in rats: involvement of tumor necrosis factor alpha, NF-κβ and caspase-3. Toxicol Rep. 2014;1:1213–1223. doi: 10.1016/j.toxrep.2014.11.012
- Patel RK, Moore AM, Piper S, et al. Clozapine and cardiotoxicity–a guide for psychiatrists written by cardiologists. Psychiatry Res. 2019;282:112491. doi: 10.1016/j.psychres.2019.112491
- Daniel P, Rajaree KM, Rudy L, et al. Myocarditis in patients on long-term antipsychotics–mechanism, management and recent updates. Heliyon. 2023;9(3):e13930. doi: 10.1016/j.heliyon.2023.e13930
- Pan HZ, Zhang H, Chang D, et al. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92(4):548–551. doi: 10.1136/bjo.2007.130542
- Muller FL, Lustgarten MS, Jang Y, et al. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034
- Li XR, Xiu MH, Guan XN, et al. Altered antioxidant defenses in drug-naive first episode patients with schizophrenia are associated with poor treatment response to risperidone: 12-week results from a prospective longitudinal study. Neurotherapeutics. 2021;18(2):1316–1324. doi: 10.1007/s13311-021-01036-3
- Yilmaz S, Atessahin A, Sahna E, et al. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology. 2006;218(2–3):164–171. doi: 10.1016/j.tox.2005.10.015
- Heiser P, Sommer O, Schmidt AJ, et al. Effects of antipsychotics and vitamin C on the formation of reactive oxygen species. J Psychopharmacol. 2010;24(10):1499–1504. doi: 10.1177/0269881109102538
- Nair GM, Skaria DS, James T, et al. Clozapine disrupts endothelial nitric oxide signaling and antioxidant system for its cardiovascular complications. Drug Res (Stuttg). 2019;69(12):695–698. doi: 10.1055/a-0991-7684
- Rabkin SW, Tang JKK. Clozapine-induced myocarditis: pathophysiologic mechanisms and implications for therapeutic approaches. Curr Mol Pharmacol. 2023;16(1):60–70. doi: 10.2174/1874467215666220211094910
- Panda PK, Mokta J, Mokta K, et al. Catecholamine induced cardiomyopathy in phaeochromocytoma. Indian J Endocr Metab. 2014;18(3):432–433. doi: 10.4103/2230-8210.131228
- Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111(10):5944–5972. doi: 10.1021/cr200084z
- Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 2005;390(2):377–394. doi: 10.1042/BJ20042006
- Mishra P, Samanta L. Oxidative stress and heart failure in altered thyroid states. Sci World J. 2012;2012:741861. doi: 10.1100/2012/741861
- Abdel-Wahab BA, Metwally ME. Clozapine-induced cardiotoxicity: role of oxidative stress, tumour necrosis factor alpha and NF-κβ. Cardiovasc Toxicol. 2015;15(4):355–365. doi: 10.1007/s12012-014-9304-9
- Hägg S, Spigset O, BAHons AB, et al. Myocarditis related to clozapine treatment. J Clin Psychopharmacol. 2001;21(4):382–388. doi: 10.1097/00004714-200108000-00005
- Wang JF, Min JY, Hampton TG, et al. Clozapine-induced myocarditis: role of catecholamines in a murine model. Eur J Pharmacol. 2008;592(1–3):123–127. doi: 10.1016/j.ejphar.2008.06.088
- Kamel A, Kamel E. Cardiotoxicity and hepatotoxicity induced by clozapine in adult male albino rats and possible protection by selenium: a histological study. Egypt J Anat. 2011;34(1):31–45. doi: 10.21608/ejana.2011.3651
- Abdel-Wahab BA, Metwally ME, El-Khawanki MM, et al. Protective effect of captopril against clozapine-induced myocarditis in rats: role of oxidative stress, proinflammatory cytokines and DNA damage. Chem Biol Interact. 2014;216:43–52. doi: 10.1016/j.cbi.2014.03.012
- Nikolić-Kokić A, Tatalović N, Nestorov J, et al. Clozapine, ziprasidone, and sertindole-induced morphological changes in the rat heart and their relationship to antioxidant enzymes function. J Toxicol Environ Health Part A. 2018;81(17):844–853. doi: 10.1080/15287394.2018.1495587
- Saito K, Daitoku K, Fukunaga H, et al. Chlorpromazine-induced cardiomyopathy in rats. Heart Vessels. 1985;1(Suppl. S1):283–285. doi: 10.1007/BF02072410
- Ishiyama S, Hiroe M, Nishikawa T, et al. Nitric oxide contributes to the progression of myocardial damage in experimental autoimmune myocarditis in rats. Circulation. 1997;95(2):489–496. doi: 10.1161/01.CIR.95.2.489
- Arzuk E, Karakuş F, Orhan H. Bioactivation of clozapine by mitochondria of the murine heart: possible cause of cardiotoxicity. Toxicology. 2021;447:152628. doi: 10.1016/j.tox.2020.152628
- Turillazzi E, Cerretani D, Cantatore S, et al. Myocardial oxidative damage is induced by cardiac fas-dependent and mitochondria-dependent apoptotic pathways in human cocaine-related overdose. Sci Rep. 2017;7(1):1–12. doi: 10.1038/srep44262
- Matsui H, Morishima I, Numaguchi Y, et al. Protective effects of carvedilol against doxorubicin-induced cardiomyopathy in rats. Life Sci. 1999;65(12):1265–1274. doi: 10.1016/S0024-3205(99)00362-8
- D’Errico S, La Russa R, Maiese A, et al. Atypical antipsychotics and oxidative cardiotoxicity: review of literature and future perspectives to prevent sudden cardiac death. JGC. 2021;18(8):663. doi: 10.11909/j.issn.1671-5411.2021.08.002
- Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of withania somnifera (ashwagandha): a review. Altern med review. 2000;5(4):334–346.
- Mikulska P, Malinowska M, Ignacyk M, et al. Ashwagandha (withania somnifera)—Current research on the health-promoting activities: a narrative review. Pharmaceutics. 2023;15(4):1057. doi: 10.3390/pharmaceutics15041057
- Hamza A, Amin A, Daoud S. The protective effect of a purified extract of withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 2008;24(1):63–73. doi: 10.1007/s10565-007-9016-z
- Mohanty I, Arya DS, Dinda A, et al. Mechanisms of cardioprotective effect of withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol. 2004;94(4):184–190. doi: 10.1111/j.1742-7843.2004.pto940405.x
- Chaurasia SS, Panda S, Kar A. Withania somnifera root extract in the regulation of lead-induced oxidative damage in male mouse. Pharmacol Res. 2000;41(6):663–666. doi: 10.1006/phrs.1999.0634
- Govindappa PK, Gautam V, Tripathi SM, et al. Effect of withania somnifera on gentamicin induced renal lesions in rats. Revista Brasileira de Farmacognosia. 2019;29(2):234–240. doi: 10.1016/j.bjp.2018.12.005
- Palojoki E, Saraste A, Eriksson A, et al. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280(6):H2726–31. doi: 10.1152/ajpheart.2001.280.6.H2726
