 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Breast cancer (BC) is one of the most common malignant tumors among women worldwide that contributes to the high mortality rate among other malignancies. The resistance developed by cancer cells against different treatment strategies such as radiotherapy, inspired the researchers to use other products such as phytochemicals to sensitize cancer cells to therapy. This study is designed to evaluate the anti-cancer and/or radio-sensitizing effect of Indole-3-carbinol (I3C) on the triple negative breast cancer (TNBC) cell line MDA-MB-231. The concentration of I3C that is used as a radio-sensitizing dose was assessed using an MTT assay. Treatment of MDA-MB-231 cells with I3C and/or ionizing radiation led to significant downregulation at cell proliferation which was indicated by the decrease at colony formation ratio and reduction in Ki67 expression. The combined treatment showed upregulation of Bax and downregulation of Bcl-2 gene expression. Wound healing assay indicated the significant effect of combined treatment in reducing MDA-MB-231 cell motility, which was molecularly validated by significant downregulation at CD44, MMP-9 and VEGF transcripts levels. The combined therapy led to a significant reduction at the antioxidant enzyme levels as well as the Epithelial-mesenchymal transition indicated by downregulation of IR-dependent vimentin upregulation. The stemness markers Nanog and Sox2 were significantly downregulated in combined therapy compared to IR-only treatment. In conclusion, synchronized therapy showed a potential role in enhancing the cell responsiveness to IR therapy by ceasing cell proliferation, survival, metastasis and reversing IR-dependent induction of EMT, and stemness in TNBC cell line MDA-MB-231.
Introduction
Breast cancer (BC) is one of the most common malignant tumors among women worldwide, which is one of the leading causes of cancer-related death in women [Citation1]. Approximately 2.3 million new cases per year worldwide and 520,000 new cases per year are detected in Europe. Over 90% of cases are diagnosed at early stages of breast cancer [Citation2]. Breast cancer varies in grades and stages and its aggressiveness is correlated to the expression of estrogen, progesterone and human epidermal growth factor receptor 2 (Her2) receptors on the cell membrane [Citation3]. However, cancer treatment with surgery, chemotherapy, and radiotherapy were still limited to a high rate of reverse side effects [Citation4]. Several studies clarified the major role of adjuvant radiotherapy as a standard clinical protocol due to its ability to improve locoregional recurrence rates and lower cancer-related mortality by care for women with early stage invasive breast cancer [Citation5,Citation6].
Radiotherapy (RT) is one of the most common non-surgical approaches that are used for treatment of some cancers. RT plays a main role in the therapeutic management of cancer disease in patients at both early-stage and local cases [Citation7]. RT effectiveness is due to the high energy of ionizing radiation that impacts biological molecules, living cells, and tissues [Citation8]. X-rays and Gamma rays are the most common forms of ionizing radiation that can strike and kill cancer cells through two mechanisms; direct action, by inducing a DNA single- or double-strand breakage, thus, halts the cancer cells repair mechanisms, and induces cell transition to death, and indirect effect, by initiating highly reactive hydroxyl radicals that increases the density and number of free radicals which leads to cell injury and death [Citation9,Citation10]. A high level of oxidative stress is considered a novel target for anti-cancer cure due to increasing exogenous reactive oxygen species (ROS) and/or antioxidant system inhibition [Citation11]. Excessive amounts of ROS may also act as cellular toxicants, which can lead to cancer-cell growth arrest, apoptosis, and necrosis. It is speculated that malignant cells under increased levels of oxidative stress are more vulnerable to further ROS attacks [Citation12]. In spite of the role of IR in upregulating the level of oxidative stress of kill cancer cells, accumulating evidences supported its profound role in inducing epithelial mesenchymal transition of non-stem cancer cells to acquire the phenotype of stemness and radioresistancy [Citation13,Citation14].
One of the mechanisms by which cancer cells can resist radiotherapy was due to its ability to downregulate pro-apoptotic P53 and Bax, and upregulate anti-apoptotic Bcl-2, TRAF2, and NF-kB protein expression [Citation15]. Moreover, Cancer cells have the ability to resist radiotherapy via activating DNA damage repair pathways, undergoing EMT and upregulating the expression of stemness markers such as Sox2 and Nanog [Citation16]. Therefore, it is important to find a strategy to elevate the radiosensitivity of cancer cells while lowering their systematic reverse effect.
In the past two decades, numerous literatures clarified the anti-cancer activity of several phytochemicals that are naturally extracted from plants; highlighting them owing to their high potential as a possible alternative therapy that needs more consideration from oncologists [Citation17]. Several literatures elucidated those phytochemicals to play an important role in cancer treatment due to their anti-inflammatory, antioxidants, anti-bacterial, and anti-fungal characteristics [Citation18]. Recent report indicated the main role of phytochemicals in cancer treatment via targeting different pathways involved in cancer cell proliferation, survival, invasiveness and metastasis by downregulating Ki67, SVV, CD44, MMP9 expressions, respectively [Citation19].
Indole-3-carbinol (I3C) is one of the phytochemicals that are naturally extracted from several cruciferous vegetables such as broccoli, kale, cabbage, Brussels sprouts, cauliflower, turnip, and collard greens [Citation20,Citation21]. I3C is produced by degradation of glucobrassicin A and is considered as one of the Indole-3-methyl isothiocyanate hydrolysis products [Citation20,Citation22]. Several studies referred to the major role of I3C in inhibiting breast cancer cell proliferation by targeting the NF-kB pathway [Citation22]. Some studies showed that I3C plays an important role in blocking estrogen receptors as an implicant of tamoxifen that used in treating estrogen-dependent tumors and preventing breast cancer cell proliferation [Citation23]. A study by Odongo et al., proved that the combined treatment of I3C with actein, withaferin A, and CKI revealed its capacity to simultaneously regulate multiple processes of carcinogenesis in breast cancer [Citation24]. Several reports indicated in vitro and in vivo effects of I3C in reducing cell proliferation, migration, and metastasis of MDA-MB-231 and MCF7 cells-derived xenograft mice [Citation21,Citation25].
The present study was designated to determine the potential therapeutic effect of I3C as a radiosensitizer on diminishing the progression of the triple negative breast cancer cell line MDA-MB-231 by targeting cell proliferation, invasion, survival and halting the IR-dependent increment at treated cells stemness and EMT.
Materials and methods
Chemicals and antibodies
- MDA-MB-231 cell line was provided by Tissue culture lab, faculty of science/El-Mansoura university, Egypt., was cultured in DMEM (Dulbecco’s Modified Eagle Medium, biowest, Canada), supplemented with 10% FBS (Fetal Bovine Serum), and 1% pen/strep (penicillin/streptomycin, biowest, Canada, trypsin-EDTA 1X in solution w/o calcium, w/o magnesium, and w/Phenol Red). MDA-MB-231 cells were incubated with 5% Co2 and 37°C. Harvesting was conducted by washing cells twice with PBS 1× (Phosphate Buffer Saline, biowest, Canada) followed by trypsinization using trypsin 0.25% at 37°C and 5% Co2 for 2 minutes.
- Natural plant extraction phytochemical (Indole-3-carbinol) was purchased from MedChemExpress (MCE), USA.
- The anti-bodies included (Bax, Bcl-2, Ki67, Survivin (SVV), nuclear factor erythroid 2-related factor 2 (Nrf2), e-cadherin (e-cad), Vimentin (vim), vascular endothelial growth factor (VEGF), CD44, Nanog (a member of the homeobox family of DNA binding transcription factors, and the name derives from Tir Na Nog, the mythical Celtic land of youth), Sox2 (sex-determining region homeobox2) and GAPDH) were purchased from (Santa Cruz, CA, USA).
Cell line and cell culture
Proliferation assay (MTT assay)
MDA-MB-231 cells were seeded in a 96-well plate with 5 × 105 cell/well in 100 µl of complete media (DMEM supplemented with 10% FBS and 1% pen/strep) in duplicates for each concentration and incubated at 37ºC and Co2 5% for 24 hr. different concentrations of I3C were prepared by serial dilution. Cells were incubated with descending concentrations of I3C (200 µg/ml, 100 µg/ml, 50 µg/ml, 25 µg/ml, 12.5 µg/ml, and 6.25 µg/ml) for 48 hr. untreated cells were considered as a negative control. The IC50 of I3C and the inhibition of cell proliferation was evaluated by using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide, 5 mg/ml), a 100 µl per well of MTT was added and incubated at 37°C and 5% Co2 for 4 hr. Following the incubation, a 100 µl Sodium Lauryl Sulfate (SDS) was added to each well and incubated for 14 hr, the absorbance was measured at a wavelength of 570 nm using (Bio Tek, Elx800, US) [Citation26]. The inhibition of cell proliferation was calculated from (Eq.):
Where (O.D) is the optical density.
Data analysis was carried out using the Prism software program (GraphPad Prism 6).
Group designation
To evaluate the therapeutic potential of I3C with/without IR on MDA-MB-231 cells, cells were divided into four groups as follows: (a) The Control group (MDA-MB-231 cells without any treatment), (b) I3C group (cells treated with 58.82 µg/ml of I3C for 24 hr), (c) IR group (cells treated with single dose of 2 Gy of x-ray), (d) I3C + IR group (cells treated with 58.82 µg/ml of I3C for 24 hr, then exposed to single dose of 2 Gy of x-ray). The groups were designed to study the effect of I3C as radio-sensitizer by colony developed ratio, pro-apoptotic protein Bax, anti-apoptotic protein Bcl-2, the ability of cells migration by wound healing assay, CD44, MMP-9, Vegf Nrf2, Nanog and Sox2 genes expression, and the activity of oxidative enzymes (CAT, and GSH). The untreated group of MDA-MB-231 was considered a control group.
Clonogenic assay
MDA-MB-231 cells were seeded in T-75 flasks and incubated until 80% confluency, Afterwards, cells were pretreated with I3C for 24 hours, then exposed to a single dose of 2 Gy of x-ray irradiation, using a SHINVA® XHA600D medical linear accelerator (linac) and Prowess® panther 3D planning system as a radiation source. After irradiation, cells of each group were washed twice with 1× Phosphate Buffer Saline (PBS), and dissociated using trypsin. Cells were sub-cultured in triplicates in 6-well plate at seeding density of 1000 cells/well. After incubation for an additional 7 days, the surviving cells developed colonies countable by the naked eye. Cells were then fixed with 70% ice-cold ethanol and then stained with crystal violet. Colonies of at least 50 cells were counted. Survival factor and colony formation ratio were estimated for each group [Citation27].
Migration assay (wound healing assay)
MDA-MB-231 cells were seeded in T-75 flasks and incubated till 80% confluency then pre-treated with I3C for 24 hr and irradiated. Cells were washed more than once with 1× PBS and trypsinization, the MDA-MB-231 treated cells were cultured in a 6-well plate with 25,000 cells/well and then incubated even completely confluence, a scratch with 1000 μL micro-pipette tip was induced diagonally in the middle of each plate. Afterward, the culture was washed twice with PBS to remove the undetached cells and then incubated for 24 hours. Microscopic photographs were taken using an inverted microscope at 0 and 20 hrs [Citation28].
Quantitative reverse transcription polymerase chain reaction (RT-PCR) for evaluation of Ki67, Bax, Bcl2, CD44, SVV, MMP-9, VEGF, Nrf2, vim, e-cad, Nanog, and Sox2 transcriptional levels
Total RNA was extracted from MDA-MB-231 cells using the Trizol method according to the manufacturer’s instructions. The concentration and quality of extracted RNA were measured by the NanoDrop (ND-2000, USA). Complementary DNA was synthesized from 1 μg of total RNA using SensiFAST™ cDNA Synthesis Kit (Bioline, Australia) according to the manufacturer’s protocol. Determination of the expression levels was assessed using Maxima SYBR Green Kit according to the manufacturer’s protocol (Thermo Scientific, CA, USA) using the following primers: B-cell lymphoma protein 2-associated X (Bax) (Forward)5′-GGTTGTCGCCCTTTTCTA-3′, (Reverse) 5′-CGGAGGAAGTCCAATGTC-3′, B-cell lymphoma 2 (Bcl2): (Forward)5′-GATGTGATGCCTCTGCGAAG-3′, (Reverse) 5′-CATGCTGATGTCTCTGGAATCT-3′, cluster of differentiation 44 (CD44): (Forward)5′-TTTGCATTGCAGTCAACAGTC-3′, (Reverse) 5′-TTACACCCCAATCTTCATGTCCAC-3′, Matrix metallopeptidase 9 (MMP9): (Forward)5′-ATCCAGTTTGGTGTCGCGGAGC-3′, (Reverse) 5′-GAAGGGGAAGACGCACAGCT-3′, vascular endothelial growth factor (Vegf): (Forward)5′- TGCAGATTATGCGGATCAAACC-3′, (Reverse) 5′- TGCATTCACATTTGTTGTGCTGTAG-3′, Nuclear factor erythroid 2-related factor 2 (Nrf2): (Forward)5′- GCTATGGAGACACACTACTTGG-3′, (Reverse) 5′- CCAGGACTTACAGGCAATTCT-3′, Nanog: (Forward)5′- 5 -ATGCCTGTGATTTGT GGGCC-3′, (Reverse) 5′-GCCAGTTGTTTTTCTGCCAC-3′, sex-determining region homeobox2 (Sox2): (Forward)5′- GGATGGTTGTCTATTAACTT-3′, (Reverse) 5′- TCAAACTTCTCTCCCTTT-3′, Ki67: (Forward)5′- TCCTTTGGTGGGCACCTAAGACCTG-3′, (Reverse) 5′- TGATGGTTGAGGTCGTTCCTTGATG-3′, Survivin (SVV): (Forward)5′- TTTTGTGGCTTTGCTCTATTGT-3′, (Reverse) 5′- GGTAGGAGGACTCATCAGAAGGA-3′, Vimentin (Vim): (Forward)5′- AAAGTGTGGCTGCCAAGAAC-3′, (Reverse) 5′- AGCCTCAGAGAGGTCAGCAA-3′, E- cadherin (e-cad): (Forward)5′- TACGGGGGGCGGTGCTCCGG −3′, (Reverse) 5′- CTGGGGCGCGGAGCTTGCGG −3′, and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): (Forward) 5′-TGGCACCCCAGCACAATGAA-3′, (Reverse) 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Thermal cycling conditions were conducted by initial heating step at 95 ◦C for 10 min. followed by 40 cycles at 95 ◦C for 30 s, annealing at 49 ◦C, 64 ◦C, and 51 ◦C for Bax, Bcl-2, KI67, SVV, MMP9, Vegf, Nrf2,Vim, e-cad, Nanog, Sox2 and GAPDH, respectively, for 30 s each and 72 ◦C for 1 min and final extension at 72 ◦C for 10 s. Relative expression of the targeted genes were normalized against GAPDH transcript as an internal reference control and then calculated according to the mathematical model introduced by M Pfaffl 2−ΔΔCt [Citation29].
Assessment of oxidative stress biomarkers
MDA-MB-231 cells were treated with I3C for 24 hours and irradiated with 2 Gy x-ray, cells were washed with 1× PBS and trypsinization then kept with 1 ml of 1× PBS in −20°C till use. ELISA technique was used to determine the Catalase (CAT) and Glutathione Synthesis (GSH) antioxidants according to the kit instructions (Biodiagnostic and research reagents, Giza, Egypt).
Statistical analyses
The data was statistically analyzed using the statistical software GraphPad Prism program (V.6). mean value and standard deviation were calculated. The mean differences were acquired by One-way ANOVA analysis. The results were expressed as mean ± standard error of the mean value (Mean ± SEM) and used at least 3 values for the samples (n = 3).
Results
Determination of I3C IC50
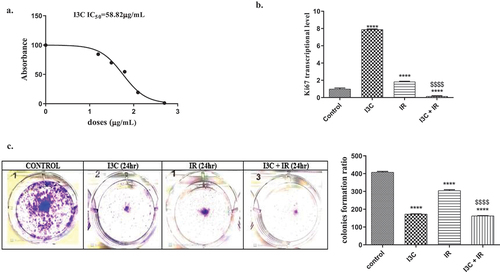
The sensitivity of MDA-MB-231 to I3C was first evaluated by the MTT colorimetric method which is a useful tool for assessing cell proliferation, cytotoxicity, and cellular metabolic activity against the log of [I3C doses individually] as illustrated in (). The data analysis indicated that the IC50 of I3C was 58.82 µg/mL. So, the dose of 58.82 µg/mL, and 2 Gy x-ray for I3C, and ionizing radiation (IR), respectively, were chosen for radio-sensitizing studies.
Figure 1. Anti proliferative effect of IR and/or I3C on MDA-MD-231 cell line. (a) MTT assay indicated the cytoxicity of I3C and the IC50 was calculated (58.82 μg/ml). (b) The combined therapy significantly downregulated the expression of the tumor proliferation marker Ki67 compared to I3C and IR, where the cells were pretreated with I3C for 24 hr before exposure to IR. (c) Colony formation assay revealed the significant effect of combined therapy in diminishing cell proliferation compare to control group and IR. Significance was denoted *as p < 0.05 as compared to control and significance was denoted $ as p < 0.05 as compared to IR. Significance was denoted *as p < 0.05 as compared to control and significance was denoted $ as p < 0.05 as compared to IR.
Effect of I3C with/without IR on MDA-MB-231 cells proliferation and survival
Effect of I3C with/without IR on MDA-MB-231 cells colony formation ratio
To evaluate the radio-sensitizing effect of I3C, and/or IR, MDA-MB-231 cells were irradiated with a single dose of 2 Gy of X-rays, due to its relevance to the cumulative dose used in human conventional radiotherapy as a routine protocol, and it also showed a significant effect on clonogenic assay, in the presence and absence of 58.82 µg/mL of I3C. The number of developed colonies was assessed after 7 days of incubation. The data reached a significant reduction in colony formation number by 24-hour sensitization of the cells with I3C (58.82 µg/mL) (). The significant reduction in the ratio of developed colonies for I3C treatment compared to the control group highlighted the anti-growth and anti-survival effect of the combination on MDA-MB-231 cells (). The present data showed a significant reduction in the number of colony formation in all treated groups I3C, IR, and I3C + IR by (172.66 ± 1.45, 304.66 ± 5.17, 162.66 ± 4.63), respectively, as compared to the control group (408.33 ± 4.41) p < 0.05, respectively, (). Remarkably, the treatment with I3C and I3C + IR viewed a significant reduction in the developed colonies ratio as compared with IR treatment ().
Impact of I3C, and/or IR on cell proliferation
To elucidate the impact of I3C, with/without IR by using the RT-PCR technique to evaluate Ki67 expression as a proliferation marker, MDA-MB-231 cells treated with I3C, IR, and I3C + IR. The data revealed a significant increase in Ki67 level expression when cells are treated with I3C, and IR (7.896 ± 0.029, 1.847 ± 0.023, p < 0.05, respectively), One the other hand, the combined therapy achieved a significant reduction in Ki67 expression (0.158 ± 0.026), compared to the control group (1 ± 0.058). On the other hand, data expressed a significant elevation in Ki67 level when cells were treated with I3C, and a significant decrease in Ki67 expression when cells were treated with combined therapy as a comparison to IR treatment ().
I3C, and/or IR treatment induce cell apoptosis on MDA-MB-231 cells
Apoptosis was evaluated by RT-PCR technique that was designed to assess the expression levels of pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 in MDA-MB-231 cells. The analyzed data referred to a significant increase in the level of Bax gene expression when MDA-MB-231 cells treated with I3C, IR, and I3C + IR for 24hrs (1.74 ± 0.029, 1.947 ± 0.048, 4.05 ± 0.104, p < 0.05, respectively) 24hrs when compared to the control group (1 ± 0.058) (). On the other hand, combined treatment showed a significant increase in the level of Bax expression as compared with IR treatment (). The data illustrated a significant decrease of Bcl-2 expression levels in MDA-MB-231 cells treated with I3C, and I3C + IR (0.42 ± 0.012, 0.349 ± 0.023, p < 0.05, respectively), while groups treated with IR, revealed significant increase of Bcl-2 expression levels (6.409 ± 0.115) after 24 hr of treatment as compared to the control group (1 ± 0.058), p < 0.05, (). I3C, and combined treatment on MDA-MB-231 cells indicated a significant decrease on Bcl-2 levels as compared to IR treatment ().
Figure 2. Anti apoptotic effect of IR and/or I3C on MDA-MD-231 cell line. (a) the cell treatment with I3C and/or IR significantly upregulated the expression of Bax compared to the control group. (b) The cell treatment with I3C and combined significantly downregulated the expression of Bcl2. Meanwhile IR significantly upregulated Bcl2 expression compared to the control. (c)The statistical analysis of Bax/Bcl2 ratio pointed out the significant proapoptotic effect of combined therapy, IR and I3C compared to control group, respectively. The combined therapy significantly upregulated Bax/Bcl2 ratio compared to IR group. (d) The I3C, combined therapy and IR significantly downregulated the expression of the anti apoptotic SVV compared to the control group, respectively. Significance was denoted *as p < 0.05 as compared to control and significance was denoted $ as p < 0.05 as compared to IR.
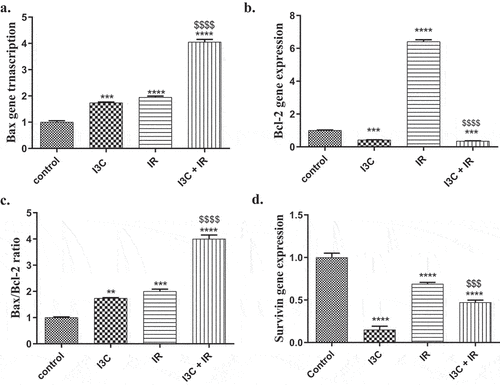
Interestingly, the index of Bax/Bcl-2 ratio data confirmed the apoptotic therapeutic effect of I3C, and/or IR on MDA-MB-231 cells, the data showed a significant elevation at the levels of expression of Bax against Bcl-2 with a synergistic effect on MDA-MB-231 cells treated with I3C, IR, and I3C + IR for 24 hr of treatment with expression levels of (1.741 ± 0.17, 2 ± 0.006, 4 ± 0.153) compared to untreated cells group (1 ± 0.058), p < 0.05 (). Combined therapy revealed a significant increase in the Bax expression level versus Bcl-2 when compared with IR treatment.
The data elucidated a significant decrease in SVV (anti apoptotic marker) expression levels when cells treated with I3C, IR, and combined treatment (152 ± 0.023, 0.678 ± 0.021, 0.473 ± 0.015, p < 0.05, respectively), as compared to the control group (1 ± 0.058). Besides, the data expressed a significant decrease in SVV levels of MDA-MB-231 cells treated with I3C and combined therapy in comparison with IR-treated cells ().
Effect of I3C, and/or IR on MDA-MB-231 cell motility
Functional validation of I3C and/or IR effect on cell migration
The effect of I3C, with/without IR on MDA-MB-231 cell motility was tested by measuring the migration ability of treated cells to migrate following a mechanically induced wound in the culture plate, whereas the increase at wound size clarifies an inhibition at cell migration capability. Evaluation of the size of the space after 24hrs of treatment showed a significant effect of IR, and I3C + IR by (270.66 ± 12.979, 526.66 ± 49.008, p < 0.05) as compared to the control group (0 ± 0.0), respectively, in lessening the MDA-MB-231 cells motility compared with control cells which migrated into the space and healed the induced wound after 20 hr of starting the experiment (.
Figure 3. Anti metastatic effect of IR and/or I3C on MDA-MD-231 cell line. (a–b) Wound healing assay indicated the significant effect of IR and combined therapy in increasing the wound size compared to control group. (c–d) Molecular validation of antimetastatic effect of IR and/or I3C showed a significant upregulation at CD44 and Vegf and downregulation at MMP9 in IR group compared to control. The combined therapy significantly downregulated CD44, MMP9 and Vegf expressions in comparison with IR and control groups. Significance was denoted *as p < 0.05 as compared to control and significance was denoted $ as p < 0.05 as compared to IR.
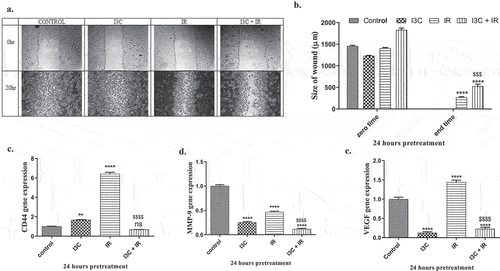
Interestingly, the data pointed out a significant effect of combined therapy in lowering the cell’s motility compared with IR treatment ().
Molecular validation of the inhibitory effect of combined therapy on MDA-MB-231 cells metastasis by targeting CD44, MMP-9 and Vegf genes expression
The impact of I3C, with/without IR was evaluated by RT-PCR technique, and the collected data suggested that MDA-MB-231 cells exposure to I3C, and IR significantly increased the expression of CD44 (1.678 ± 0.26, 6.409 ± 0.173, p < 0.05, respectively), while the combined treatment showed no significant decreased on CD44 expression by (0.677 ± 0.012) as compared to control group (1 ± 0.058). I3C and combined therapy observed a significant decrease in CD44 expression when compared to IR treatment ().
In addition, the effect of I3C, IR, and I3C + IR on MDA-MB-231 cells induced a significant decrease in MMP-9 gene expression when treated with I3C, IR, and dual treatment (0.25 ± 0.02, 0.466 ± 0.18, 0.116 ± 0.15, p < 0.05, respectively), as compared to the control group (1 ± 0.058). Moreover, I3C, and I3C plus IR treatment revealed a significant decrease in MMP-9 expression level compared to IR treatment ().
RT-PCR technique was used to evaluate the expression of vim, VEGF, and e-cad as tumor markers for metastasis in cancer cells. MDA-MB-231 cells were treated with I3C, IR, and I3C + IR. The data reported a significant decrease in VEGF expression in cells treated with I3C, and I3C plus IR by (0.128 ± 0.015, 0.23 ± 0.023), p < 0.05, respectively, while IR treatment revealed a significant increase in VEGF expression recorded (1.44 ± 0.029), p < 0.05, as compared to the control group (1 ± 0.058). Besides, the treatment with I3C and combined therapy showed a significant decrease in VEGF expression in comparison to IR treatment ().
Impact of I3C, and/or IR on the antioxidant machinery of MDA-MB-231
MDA-MB-231 cells were exposed to I3C, and/or IR for 24hrs, the data revealed a significant decrease of Nrf2 levels when cells treated with I3C by (0.428 ± 0.24), p < 0.05, while treatment with IR revealed a significant increase in Nrf2 expression recorded (7.963 ± 0.39), p < 0.05, besides, the combined treatment showed a non-significant effect on Nrf2 gene levels recorded (0.99 ± 0.42), p < 0.05, as compared to the control group (1 ± 0.058). Remarkably, the data showed a significant reduction in Nrf2 gene levels on MDA-MB-231 cells when treated with I3C, and combined therapy in comparison with IR treatment ().
Figure 4. (a–c) The role of IR and/or I3C therapy in cellular antioxidant capacity. The IR therapy significantly upregulated the expression of Nrf2 with non significant effect on CAT and GSH expression. The combined therapy significantly downregulated Nrf2 expression. (d–f) Effect of IR and/or I3C on EMT. The combined therapy significantly upregulated E-cadherin expression and downregulated vimentin expression compared to IR group. (g–h) Effect of IR and/or I3C on cell stemness. The IR treatment significantly upregulated the expression of Sox2 and Nanog compared to control group. The combined therapy significantly over countered this effect. Significance was denoted * as p < 0.05 as compared to control and significance was denoted $ as p < 0.05 as compared to IR.
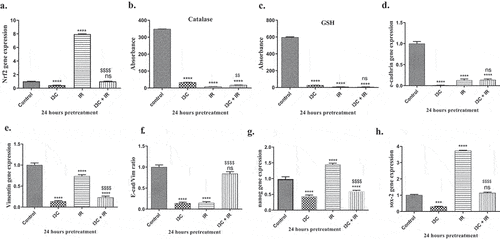
Furthermore, the data expressed a significant decrease in the antioxidant enzyme CAT when cells treated with I3C, IR, and I3C + IR by recorded (33.095 ± 0.85, 7.545 ± 1, 16.913 ± 1), p < 0.05, respectively, when compared with untreated control group (348.286 ± 0.3). On the other hand, I3C, and dual therapy indicated a significant elevation in CAT activity compared to IR treatment (). By the way, the activity of GSH enzyme reached a significant decrease when MDA-MB-231 cells treated with I3C, IR, and I3C + IR which recorded (26.894 ± 0.85, 7.608 ± 0.7, 6.57 ± 0.9), p < 0.05, respectively, as compared to control group (596.946 ± 5.1), p < 0.05, while the data observed a significant increased on GSH activity on cells treated with I3C compared to IR treatment ().
Evaluation of the effect of I3C, IR, and combined treatment on MDA-MB-231 cells EMT gene expression
The effect of I3C, and/or IR on EMT markers of MDA-MB-231 cells was assessed using the RT-PCR method. The data observed a significant decrease in e-cad gene expression on cells treated with I3C, IR, and combined therapy recorded (0.11 ± 0.0006, 0.129 ± 0.018, 0.138 ± 0.012), p < 0.05, respectively, as compared to control group (1 ± 0.058). The data showed a significant reduction in e-cad expression in cells treated with I3C, with no significant effect on cells treated with combined therapy in comparison with IR-treated cells (). On the other hand, the data marked a significant decrease in vim gene expression when cells treated with I3C, IR, and I3C plus IR recorded (0.136 ± 0.012, 0.737 ± 0.021, 0.232 ± 0.017), p < 0.05, respectively, as compared to control group (1 ± 0.58). The treatment with I3C and combined therapy showed a significant decrease in the level of vim expression as compared to the IR-treated group ().
The index of the e-cad/Vim ratio revealed a significant decrease at e-cad levels versus vim levels as compared to the control group, ().
Effect of I3C, and/or IR on MDA-MB-231 cell stemness
To estimate the therapeutic potential effect on pluripotent Nanog, and Sox2 as stemness biomarkers, MDA-MB-231 cells treated with I3C, IR, and I3C plus IR. The data reached a significant decrease in the Nanog expression when cells treated with I3C, and I3C + IR recorded (0.426 ± 0.019, 0.614 ± 0.006), p < 0.05, respectively, while treatment with IR alone revealed a significant increase in the level of Nanog expression by (1.417 ± 0.017), p < 0.05, as compared to the control group (1 ± 0.058), p < 0.05 () observable, I3C and combined treatment pointed to a significant decrease in Nanog expression level compared to IR treatment ().
On the other hand, the data expressed a significant decrease in Sox2 level expression when cells treated with I3C recorded (0.265 ± 0.015), p < 0.05, while data proved a significant increase in the Sox2 expression with IR treatment by (3.804 ± 0.046), p < 0.05, the combined treatment on MDA-MB-231 cells revealed no significant elevation on Sox2 gene expression by (1.75 ± 0.015), p < 0.05, as compared to control group (1 ± 0.058), p < 0.05 (). Remarkable, the data reached the cells treated with I3C, and dual treatment indicated a significant decrease in Sox2 expression levels compared to IR treatment ().
Discussion
Radio-resistance developed by highly invasive cancers is still a major delinquent that tackles the radiotherapists worldwide [Citation19]. Despite the numerous reports that illustrated the high therapeutic potential of phytochemicals in cancer treatment, their implication in clinical protocols is still limited because of their low bioavailable levels [Citation30]. For this reason, the implication of phytochemicals in adjuvant and neoadjuvant therapy to overcome its low bioavailability was developed to target cancer cell proliferation, survival, and metastasis [Citation31].
In this article, we aimed at estimating the proposition of using I3C as a radiosensitizer, to enhance MDA-MB-231 cells’ responsiveness to radiotherapy by reducing their proliferation, survival, metastasis and IR dependent EMT and stemness. In addition, the anti-cancer characteristics of I3C were tested as well.
Several literatures clarified the potentiality of I3C in targeting different cancer cells such as liver and breast cancers either solitary or in combined therapy [Citation21,Citation22]. However, its impact as radiosensitizer in breast cancer is still unclear and not fully understood. To investigate the anti-cancer and the possible radio-sensitizing effects of I3C, the IC50 of I3C was first calculated and a dose of (58.82 µg/mL) was selected for further radio-sensitizing studies as it was the dose that induced a significant inhibition at cell proliferation with enough number of cells remains for clonogenic assay after irradiation (). The significant decrease in the developed colony ratio in I3C + IR treatment compared to its representable dose of IR (2 Gy) treated MDA-MB-231 cells as well as untreated cells (control group) elucidated the adjuvant inhibitory effect of I3C plus IR on breast cancer cell growth (). Moreover, the anti-proliferative effect of I3C combined with IR revealed a significant downregulation at proliferation marker Ki67 ().
The role of Ki67 as a cell cycle marker and a prognostic factor for breast cancer progression was illustrated by several literatures [Citation32]. Several literatures clarified the positive correlation of CD44 and Ki67 with tumor grades [Citation33]. Besides, Nemours reports clarified the correlation between Nrf2 and Ki67, whereas Nrf2 downregulation or elevation led to decrease or increase at Ki67 expression, respectively [Citation34].
The role of phytochemical such as I3C or its diverts [3,3’-diindolylmethane (DIM)] as an adjuvant approach to conventional chemo- and radiotherapies for murine breast adenocarcinoma and prostate cancer was stated by several studies [Citation35,Citation36]. A study by Zamanian et al., proved that combined therapy of glioblastoma (U87MG cell line) with conventional IR (2 Gy) dose and genistein (50 µM) gave the highest significant effect on inhibiting cells proliferation and colony formation ratio compared to 2 Gy and fractionated doses [Citation37].
In order to investigate the proapoptotic effect of I3C on MDA-MB-231 cells, the treated cells showed significant upregulation at Bax gene expression and downregulation of Bcl-2 gene expression in combined treatment compared with either I3C and IR which indicated the potent proapoptotic effect of adjuvant therapy ().
I3C significantly stimulated P53-dependent apoptosis pathway in treated ovarian, Lung and adeno gastro carcinoma cancer cells [Citation38,Citation39]. The proapoptotic effect of adjuvant therapy was further validated by the significant diminishment at SVV expression compared to IR and control groups () The antiapoptotic effect of SVV, as IAP family member, is an established fact and its expression in controllable by several upstream proteins such as CD44/E2F1/SVV pathway [Citation40].
Furthermore, the anti-metastatic effect of IR and I3C plus IR treatment was structurally validated by the significant increase at the wound size compared to control group in wound healing assay (). Furthermore, these results were molecularly confirmed by the significant decrease at the expression of CD44 and its downstream targets MMP9 and Vegf expressions in combined therapy compared to control and IR-treated groups. Interestingly in IR group, the transcription of CD44 and its downstream target Vegf were significantly upregulated compared to control; whereas MMP9 transcription was elevated compared to combined therapy ().
Recent publication illustrated the role of IR in uprising the expression of CD44 by activating-RAS/ERK signaling as one of the underlying mechanisms of radioresistance [Citation41]. A study on the expression patterns of genes that were highly expressed in radioresistant colorectal cancer RKO cells; indicated CD44 as one of the top five targeted genes [Citation42].
The role of CD44 in signaling for neo-angiogenesis in breast cancer was illustrated by its novel role in upregulating MMP9 expression which in turn sheds the CD146 ectodomain from cell membrane. The upraise at soluble CD146 signals for VEGF overexpression and new blood vessels formation in CD44/sCD146/VEGF pathway [Citation43].
A study by Abd Elhakeem et al., clarified the role of MMP-9 in tumor invasiveness, malignancy, or recurrence for brain anaplastic meningiomas [Citation44]. In prostate cancer, a decrease at the surface expression of CD44 was recorded in MMP-9 knocked-down PC3 cells. Meanwhile, a decrease at the activation of MMP-9 was indicated as well in cells null for CD44 [Citation45]. In addition, the downregulation of MMP-9 protein expression in PC3 cells switched CD44 isoform expression from CD44s to CD44v6 which is more glycosylated [Citation46]. A study by Ludwig and colleagues elucidated that the activation of the HA-CD44 pathway led to the elevation of VEGF and MMP-9 by endothelial cells (ECs) to induced EMT and activate invasion during cancer progression [Citation47].
Furthermore, CD44 was found to play a possible role in signaling for epithelial-mesenchymal transition (EMT) and stemness in MDA-MB-231 cells. The downregulation at CD44 transcription upon treatment may lead to Nrf2 downregulation, which in turn led to significant elevation of e-cad and reduction at Vim genes transcription. On the other hand, the role of adjuvant therapy in downregulating the treated cells antioxidant capacity was indicated by the significant downregulation at Nrf2 transcription and antioxidant enzymes CAT and GSH expression levels compared to IR and control groups, respectively.
Overstimulation of cellular antioxidant activity via upregulating NrF2 expression is a vital approach that cancer cells developed to resist cell apoptosis [Citation11]. Interestingly, the adjuvant therapy significantly succeeded in opposing the IR dependent upraise at Nrf2 expression and its downstream targets antioxidant enzymes CAT and GSH, indicated its profound effect in targeting the antioxidant defense mechanism that cancer cells developed to resist radiotherapy (). Overexpression of Nrf2 is one of the cytoprotective mechanisms that cancer cells developed to evade cell death [Citation48]. [Citation49], illustrated the role of IR in elevating Nrf2 expression in breast cancer treated cells which in turn increased their resistance to radiotherapy in a negative feedback mechanism [Citation49].
The activation of P53 dependent apoptosis requires an accumulation of Reactive oxygen species which seems to prevent the transactivation of Nrf2 [Citation50]. The overexpression of Nrf2 and Svv was significantly associated with lymph node and distant metastasis in esophageal squamous cell carcinoma which was negatively correlated with the presence of immune-infiltrating cells in sectioned tumors [Citation51].
The expression of Nrf2 was found to be regulated by the expression of CD44. The high level of the CD44 (Hyaluronic acid receptor) mediated the chemoresistance of doxorubicin resistant breast cancer cell line MCF7-DR by elevation of Nrf2 expression; whereas, silencing of Hyaluronic acid synthase-2 or CD44 led to inhibition of Nrf2 signaling with significant enhancement in cell responsiveness to chemotherapy [Citation52]. Double targeting of autophagic and Nrf2-signaling pathways with selective chemical inhibitors, increases the response of oral CD44+ cancer cells to cisplatin cytotoxicity [Citation53].
Interestingly, the combined therapy significantly over countered the IR effect in provoking the EMT by downregulating e-cad/Vim ratio compared to control cells which may be mediated by CD44 and Nrf2 overexpression (). The role of IR in promoting EMT was illustrated by several literatures which indicated that cancer cells survive the radiotherapy by enters EMT [Citation14,Citation54]. The CD44 expression significantly signals for EMT by activating TGF-β/Smad signaling, which enhanced EMT [Citation55]. In colon cancer, the expression of the variant CD44v6 positively correlated with vimentin expression and inversely correlated with E-cadherin expression [Citation56]. Recent publications illustrated the role of ionizing radiation in upregulating the expression of CD44 and Nrf2 in breast cancer [Citation57].
Finally, we were interested in testing the impact of combined therapy on cancer cell stemness and the data revealed its effect on tackling on of the major disadvantage at cancer therapy. The adjuvant therapy significantly downregulated the expression of the stem cell markers Sox2 and Nanog compared to IR group which significantly upregulated their expression (). Recent literatures elucidated the implication of IR in upregulating the cancer cell stemness markers Oct4, Sox2, and Nanog mediated by the upregulation of CD44 [Citation58].
Several literatures proved the phytochemicals’ role in downregulating the stemness markers Nanog and Sox2 expression and reduced cancer cell proliferation, invasion, and survival [Citation59,Citation60]. In breast cancer and Pancreatic cancer, several literatures highlighted the high accumulation of ROS due to the reduction at antioxidant enzymes inhibits cell proliferation, colony development, metastasis, stemness, and EMT [Citation61,Citation62].
In summary, the present study demonstrated, to-the-best of our knowledge, for the first time I3C as a radiosensitizer that enhances the MDA-MB-231 cell responsiveness to IR to diminish cell proliferation, migration, and survival as well as I3C significantly over countered the IR dependent increment at MDA-MB-231 EMT and stemness. Thus, suggesting this adjuvant therapy as a potential therapeutic strategy for breast cancer, to be considered for application in future clinical trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Jayachandran P, Battaglin F, Strelez C, et al. Breast cancer and neurotransmitters: emerging insights on mechanisms and therapeutic directions. Oncogene. 2023;42(9):627–637. doi: 10.1038/s41388-022-02584-4
- Pruneri G, Lorenzini D, Mastropasqua MG, et al. The central role of pathology labs in breast cancer precision oncology: a call for action. NPJ Breast Cancer. 2023;9(1):3. doi: 10.1038/s41523-023-00506-5
- To NH, Nguyen HQ, Thiolat A, et al. On behalf of the TransAtlantic Radiation Oncology Network (TRONE) & Association of Radiotherapy, and Oncology of the Mediterranean Area (AROME), radiation therapy for triple-negative breast cancer: emerging role of microRnas as biomarkers and radiosensitivity modifiers. A systematic review. Breast Cancer Res Treat. 2022;193(2):265–279. doi: 10.1007/s10549-022-06533-3
- Wang Y, Liu F, Sun L, et al. Association between human blood metabolome and the risk of breast cancer. Breast Cancer Res. 2023;25(1):9. doi: 10.1186/s13058-023-01609-4
- He Y, Chen S, Gao X, et al. Robustness of VMAT to setup errors in postmastectomy radiotherapy of left-sided breast cancer: Impact of bolus thickness. PLOS ONE. 2023;18(1):e0280456. doi: 10.1371/journal.pone.0280456
- Sjostrom M, Fyles A, Liu F-F, et al. Development and validation of a genomic profile for the omission of local adjuvant radiation in breast cancer. J Clin Oncol. 2023;41(8):1533–1540. doi: 10.1200/JCO.22.00655
- Bronzatti E, Siqueira LO. Radiotherapy for breast cancer. Springer Nature Switzerland AG 2019 467, G. Novita et al. (eds.). Breast Dis. 2019. doi: 10.1007/978-3-030-13636-9_57
- Bensadoun R-J, Nair R. Principles and practice of radiation oncology and modern radiation therapy techniques. In: Nair R, editor. Orofacial supportive care in cancer. Springer Nature Switzerland AG; 2022. doi: 10.1007/978-3-030-86510-8_4
- Lee V-F, Lee A-M. Principle of cancer radiotherapy. In: Seong J, editor. Radiotherapy of liver cancer. Springer Nature Singapore Pte Ltd; 2021. doi: 10.1007/978-981-16-1815-4_1
- Nosrati H, Salehiabar M, Charmi J, et al. Enhanced in vivo radiotherapy of breast cancer using gadolinium oxide and gold hybrid nanoparticles. Appl Biomater. 2023;6(2):784–792. doi: 10.1021/acsabm.2c00965
- Loenhout VJ, Peeters M, Bogaerts A, et al. Oxidative stress-inducing anticancer therapies: taking a closer look at their immunomodulating effects. Antioxidants. 2020;9(12):1188. doi: 10.3390/antiox9121188
- Mileo AM, Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies, oxid. Med Cell Longev. 2016;2016:1–17. doi: 10.1155/2016/6475624
- Li F, Zhou K, Gao L, et al. Radiation induces the generation of cancer stem cells: a novel mechanism for cancer radioresistance. Oncol Lett. 2016;12(5):3059–3065. doi: 10.3892/ol.2016.5124. PMCID: PMC5103903.
- Zhou S, Zhang M, Zhou C, et al. The role of epithelial-mesenchymal transition in regulating radioresistance. Crit Rev Oncol Hematol. 2020;150:102961. doi: 10.1016/j.critrevonc.2020.102961
- Yang HJ, Youn H, Seong KM, et al. Phosphorylation of ribosomal protein S3 and antiapoptotic TRAF2 protein mediates radioresistance in non-small cell lung cancer cells. J Biol Chem. 2013;288(5):2965–2975. doi: 10.1074/jbc.M112.385989
- Wu Y, Song Y, Wang R, et al. Molecular mechanisms of tumor resistance to radiotherapy. Mol Cancer. 2023 15;22(1):96. doi: 10.1186/s12943-023-01801-2. PMID: 37322433; PMCID: PMC10268375.
- Choudhari AS, Mandave PC, Deshpande M, et al. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2019;10:1614. doi: 10.3389/fphar.2019.01614
- Prakash D, Gupta C. Therapeutic implications of phytochemicals in ROS induced cancer. In: Chakraborti S, editor. Handbook of oxidative stress in cancer: therapeutic aspects. Springer Nature Singapore Pte Ltd; 2022. doi: 10.1007/978-981-16-1247-3_14-1
- Abdraboh ME, Essa ZS, Abdelrazzak AB, et al. Radio-sensitizing effect of a cocktail of phytochemicals on HepG2 cell proliferation, motility and survival. Biomed Pharmacother. 2020;131:110620. doi: 10.1016/j.biopha.2020.110620
- Wang T, Zhang D, Yang B, et al. Salicylic acid regulates indole-3-carbinol biosynthesis under blue light in broccoli sprouts (Brassica oleracea L.). Front Plant Sci. 2022;13:848454. doi: 10.3389/fpls.2022.848454
- Wang X, Zhang L, Dai Q, et al. Combined luteolin and indole-3-carbinol synergistically constrains ERα-positive breast cancer by dual inhibiting estrogen receptor alpha and cyclin-dependent kinase 4/6 pathway in cultured cells and xenograft mice. Cancers (Basel). 2021;13(9):2116. doi: 10.3390/cancers13092116
- Qi Y, Zhang C, Wu D, et al. Indole-3-carbinol stabilizes p53 to induce miR-34a, which targets LDHA to block aerobic glycolysis in liver cancer cells. Pharmaceuticals. 2022;15(10):1257. doi: 10.3390/ph15101257
- Martín-Ruiz A, Peña L, González-Gil A, et al. Effects of indole-3-carbinol on steroid hormone profile and tumor progression in a mice model of canine inflammatory mammary cancer. BMC Cancer. 2018;18(1). doi: 10.1186/s12885-018-4518-z
- Odongo R, Demiroglu-Zergeroglu A, Çakır T. A systems pharmacology approach based on oncogenic signalling pathways to determine the mechanisms of action of natural products in breast cancer from transcriptome data. BMC Complement Med Ther. 2021;21(1):181. doi: 10.1186/s12906-021-03340-z
- Poloznikova AA, Muyzhnekb EL, Nikulinc SV, et al. Antitumor activity of indole-3-carbinol in breast cancer cells: phenotype, genetic pattern, and DNA methylation inversion. Appl Biochem Microbiol. 2020;56(9):909–919. ISSN 0003-6838. doi: 10.1134/S0003683820090070
- Wilsher NE, Arroo RR, Matsoukas MT, et al. Cytochrome p450 cyp1 metabolism of hydroxylated flavones and flavonols: selective bioactivation of luteolin in breast cancer cells. Food And Chemical Toxicology. 2017;110:383–394. doi: 10.1016/j.fct.2017.10.051
- Abdraboh ME, Essa ZS, Abdelrazzak AB. Radio-sensitizing effect of a cocktail of phytochemicals on HepG2 cell proliferation, motility and survival. Biomed Pharmacother. 2020;131:110620. doi: 10.1016/j.biopha.2020.110620
- Pandya V, Githaka JM, Patel N, et al. BIK drives an aggressive breast cancer phenotype through sublethal apoptosis and predicts poor prognosis of ER-positive breast cancer. Cell Death Dis. 2020;11(6):448. doi: 10.1038/s41419-020-2654-2
- Golestani BE, Sanati MH, Houshmand M, et al. Expression and prognostic significance of bcl-2 and bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014;15(4):356–363.
- Kotecha R, Takami A, Espinoza JL. Dietary phytochemicals and cancer chemoprevention: a review of the clinical evidence. Oncotarget. 2016;7(32):52517. doi: 10.18632/oncotarget.9593
- Nisar S, Masoodi T, Prabhu KS, et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomedicine & Pharmacotherapy. 2022;154:113610. doi: 10.1016/j.biopha.2022.113610
- Davey GM, Hynes SO, Kerin MJ, et al. Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers (Basel). 2021 3;13(17):4455. doi: 10.3390/cancers13174455
- Ma B, Huang X-T, Zou G-J, et al. Relationship between Ki-67 and CD44 expression and microvascular formation in gastric stromal tumor tissues. World J Clin Cases. 2022 14;10(2):469–476. doi: 10.12998/wjcc.v10.i2.469
- Huang P, He Y, Cao J, et al. Up-regulated Nrf2 in colorectal carcinoma and predicts poor prognosis. Int J Clin Exp Med. 2017;10(1):1034–1042. Available from: www.ijcem.com/ISSN:1940-5901/IJCEM0039190
- Hajra S, Patra AR, Basu A, et al. Indole-3-carbinol (I3C) enhances the sensitivity of murine breast adenocarcinoma cells to doxorubicin (DOX) through inhibition of NF-κβ, blocking angiogenesis and regulation of mitochondrial apoptotic pathway. Chem Biol Inter. 2018;290:19–36. doi: 10.1016/j.cbi.2018.05.005
- Singh-Gupta V, Banerjee S, Yunker CK, et al. B-DIM impairs radiation-induced survival pathways independently of androgen receptor expression and augments radiation efficacy in prostate cancer. Cancer Lett. 2012;318(1):86–92. Elsevier Ireland Ltd. doi: 10.1016/j.canlet.2011.12.006
- Zamanian A, Changizi V, Nedaie H, et al. Combination treatment of glioblastoma by low-dose radiation and genistein. Curr Radiopharm. 2016;9(3):258–263. doi: 10.2174/1874471009666160813232031
- Singh AA, Jo S-H, Kiddane AT, et al. Indole-3-carbinol induces apoptosis in AGS cancer cells via mitochondrial pathway. Chem Biol Drug Des. 2023;101(6):1367–1381. doi: 10.1111/cbdd.14219
- Xu X-L, Deng S-L, Lian Z-X, et al. Resveratrol targets a variety of oncogenic and oncosuppressive signaling for ovarian cancer prevention and treatment. Antioxidants. 2021;10(11):1718. doi: 10.3390/antiox10111718
- Abdraboh ME, Gaur RL, Hollenbach AD, et al. Survivin is a novel target of CD44-promoted breast tumor invasion. Am J Pathol. 2011 Aug;179(2):555–563. doi: 10.1016/j.ajpath.2011.04.042
- Zhao Y, Kang J-H, Yoo K-C, et al. K-RAS acts as a critical regulator of CD44 to promote the invasiveness and stemness of GBM in response to ionizing radiation. Int J Mol Sci. 2021;22(20):10923. doi: 10.3390/ijms222010923
- Lin S, Shen Z, Yang Y, et al. Expression profiles of radio-resistant genes in colorectal cancer cells. Radiat Med Prot. 2021;2(2):48–54. doi: 10.1016/j.radmp.2021.04.006
- Ouhtit A, Abdraboh ME, Hollenbach AD, et al. CD146, a novel target of CD44-signaling, suppresses breast tumor cell invasion. Cell Commun Signaling. 2017;15(1):45. doi: 10.1186/s12964-017-0200-3
- Abd Elhakeem AA, Essa AA, Soliman RK, et al. Novel evaluation of the expression patterns CD44 and MMP9 proteins in intracranial meningiomas and their relationship to the overall survival. Egypt J Neurosurg. 2022;37(1):33. doi: 10.1186/s41984-022-00173-x
- Desai B, Ma T, Zhu J, et al. Characterization of the expression of variant and standard CD44 in prostate cancer cells: identification of the possible molecular mechanism of CD44/MMP9 complex formation on the cell surface. J Cell Biochem. 2009;108(1):272–284. doi: 10.1002/jcb.22248
- Gupta A, Cao W, Sadashivaiah K, et al. Promising noninvasive cellular phenotype in prostate cancer cells knockdown of matrix metalloproteinase 9. Sci World J. 2013;2013:1–13. doi: 10.1155/2013/493689
- Ludwig N, Szczepanski MJ, Gluszko A, et al. CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019;28(467):85–95. doi: 10.1016/j.canlet.2019.10.010
- Kumar H, Kumar RM, Bhattacharjee D, et al. Role of Nrf2 signaling cascade in breast cancer: strategies and treatment. Front Pharmacol. 2022;29(13):720076. doi: 10.3389/fphar.2022.720076
- McDonald JT, Kim K, Norris AJ, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010 1;70(2):8886–8895. doi: 10.1158/0008-5472.CAN-10-0171
- Faraonio R, Vergar P, Di Marzo D, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem. 2006;281(52):39776–39784. doi: 10.1074/jbc.M605707200
- Gao Y, Wan L, Li M, et al. NRF2/HO-1 axis, BIRC5, and TP53 expression in ESCC and its correlation with clinical pathological characteristics and prognosis. Int J Biol Markers. 2023;38(3–4):174–184. doi: 10.1177/03936155231176571
- Choi B-H, Ryoo I, Sim KH, Ahn H-J, Lee YJ, and Kwak M-K. High levels of hyaluronic acid synthase-2 mediate NRF2-driven chemoresistance in breast cancer cells. Biomol Ther. 2022;30(4):368–379. doi: 10.4062/biomolther.2022.074
- Praharaj PP, Singh A, Patra S, et al. Co-targeting autophagy and NRF2 signaling triggers mitochondrial superoxide to sensitize oral cancer stem cells for cisplatin-induced apoptosis. Free Radic Biol Med. 2023;207:72–88. doi: 10.1016/j.freeradbiomed.2023.07.008
- Qiao L, Chen Y, Liang N, et al. Targeting epithelial-to-mesenchymal transition in radioresistance: crosslinked mechanisms and strategies. Front Oncol. 2022;16(12):775238. doi: 10.3389/fonc.2022.775238
- Xu H, Tian Y, Yuan X, et al. The role of CD44 in epithelial–mesenchymal transition and cancer development. Onco Targets Ther. 2015;16(8):3783–3792. doi: 10.2147/OTT.S95470
- Saito S, Okabe H, Watanabe M, et al. CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer. Oncol Rep. 2013;29(4):1570–1578. doi: 10.3892/or.2013.2273
- Frascogna C, Mottareale R, La Verde G, et al. Role of the mechanical microenvironment on CD-44 expression of breast adenocarcinoma in response to radiotherapy. Sci Rep. 2024 3;14(1):391. doi: 10.1038/s41598-023-50473-x
- Mesrati MH, Syafruddin SE, Mohtar MA, et al. CD44: a multifunctional mediator of cancer progression. Biomolecules. 2021 9;11(12):1850. doi: 10.3390/biom11121850
- Hu C, Li M, Guo T, et al. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine. 2019;58:152740. doi: 10.1016/j.phymed.2018.11.001
- Loh C-Y, Chai JY, Tang TF, et al. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10):1118. doi: 10.3390/cells8101118
- Das U, Kundu J, Shaw P, et al. Self-transfecting GMO-PMO chimera targeting Nanog enable gene silencing in vitro and suppresses tumor growth in 4T1 allografts in mouse. Mol Ther Nucl Acids. 2023;32. doi: 10.1016/j.omtn.2023.03.011
- Peng G, Tang Z, Xiang Y, et al. Glutathione peroxidase 4 maintains a stemness phenotype, oxidative homeostasis and regulates biological processes in Panc‑1 cancer stem‑like cells. Oncol Rep. 2019;41:1264–1274. doi: 10.3892/or.2018.6905

