ABSTRACT
Cadmium is a hazardous metal swiftly generated through industrialization and persists in the environment due to its non-biodegradability. Cadmium induces liver and kidney damage primarily by triggering free radical production causing oxidative stress. This study explored the potential of methanolic extract of Vernonia amygdalina in ameliorating cadmium-induced oxidative stress. Twenty adult male Wistar rats were used for this experiment, with each group consisting of five rats. The first group was the unexposed control group given standard rat chow and distilled water ad libitum while the test groups (II-IV) were orally administered with cadmium at standard doses of 12 mg/kg/BW. Vernonia amygdalina at standard doses of 100 mg/kg/BW and 200 mg/kg/BW was administered to rats in groups III and IV respectively. After 28 days, the rats were euthanized using cervical dislocation and the livers and kidneys were analyzed for histological and mRNA analysis of tumor necrosis factor-alpha (TNF-α) and interleukin-10 (IL-10). The results showed significant pathological changes in the histoarchitecture of the livers and kidneys, indicating oxidative stress and the effectiveness of Vernonia amygdalina, which mitigated cadmium-induced oxidative damage by modulating TNF-α and IL-10 expression and ameliorating the histological alteration seen in the studied organs.
Introduction
Cadmium is a silvery-white soft metal that belongs to Periodic Group II B and has a density of 8.64 g/cm3, an atomic weight of 112.41 u, and an atomic number of 48. Cadmium is not found in nature in its purest form until needed. It is produced as a byproduct of zinc ore and is found in the ground at a rate of 0.15–0.2 mg/kg [Citation1]. Cadmium being an environmental pollutant and a hazardous heavy metal poses substantial health concerns to humans with the respiratory and digestive tracts being the principal entry points into the human body, causing damage to many tissues and organs, including the liver, kidney, lung, bone, and brain [Citation2,Citation3]. Additionally, human activities such as mining, smelting, manufacturing nickel-cadmium batteries, plastic stabilizers, and the use of phosphate fertilizers and manures lead to cadmium being released into the environment [Citation4].
The liver is the largest gland in the body and has numerous physiological functions, making it more susceptible to pathogenic agents and damage [Citation2]. Recent studies by Wang et al. [Citation5], Ren et al. [Citation6] and Fang et al. [Citation7] earlier demonstrated the liver as one of the primary target organs that accumulates the most cadmium following exposure. Cadmium is delivered to liver tissue by adhesion to the cellular membrane and through membrane transporters. Following translocation, it suppresses the activity of hepatic antioxidant enzymes and releases reactive oxygen species (ROS), which induce lipid peroxidation, cause damage to proteins, deoxyribonucleic acid (DNA), and lipids, and result in hepatic injury, accompanied by oxidative damage, inflammation, and apoptosis [Citation3]. Humans initially accumulate high levels of cadmium in the liver due to the presence of metallothioneins, which gradually decrease over time, however, redistributes to the kidney via transportation in the bloodstream and uptake into the proximal tubular cells, and eventually becomes the organ with the highest cadmium concentration [Citation8].
Oxidative stress is generally defined as an imbalance between oxidants and antioxidants, which alters redox signaling and control and/or molecular damage [Citation9–12]. TNF-α is extensively studied for its pro-inflammatory properties, central to mammalian immunity and cellular balance [Citation13]. During acute inflammation, macrophages/monocytes produce TNF-α, crucially coordinating the cytokine cascade in various inflammatory diseases, by activating immune cells and promoting the production of other pro-inflammatory cytokines such as IL-1 and IL-6 [Citation14–16]. IL-10 is an anti-inflammatory cytokine that prevents the formation of free radicals and the secretion of pro-inflammatory cytokines by monocytes and/or macrophages [Citation17,Citation18]. Macrophage plays an important role in protecting the host from infection, repairing damaged tissue and secreting inflammatory cytokines such as IL-10 to regulate inflammation [Citation19]. Vernonia amygdalina, also known as ‘bitter leaf’ in English, ‘Ewuro’ in Yoruba, ‘Shuwaka/Chusadoki’ in Hausa, and ‘Olubu/Onugbu’ in Igbo, is a tropical shrub that grows up to 3 meters tall and has a dark green color, distinctive odor and bitter taste [Citation20,Citation21]. Vernonia amygdalina is a perennial herb of the Asteraceae family, whose aqueous leaf extract has antibacterial, anticancer, antioxidant, antidiabetic, hepatoprotective, hypolipidemic, and antifertility activities and a long history of use in anti-coagulant, anti-cancer, anti-thrombic, antipyretic, and anti-inflammatory medications [Citation22,Citation23]. Vernonia amygdalina Delile has been extensively studied for its chemical constituents. It is enriched with various compounds such as flavonoids, sesquiterpene lactones, fatty acids, steroidal saponins, terpenoids, alkaloids, anthraquinone, phenols, and tannins, contributing to its bitter taste and various health benefits [Citation24–26]. Vernonia amygdalina has also been established to possess anti-inflammatory activity, as the administration of its extract can increase malondialdehyde levels and decrease superoxide dismutase levels, hence making it useful for treating inflammatory disease conditions [Citation27]. This study evaluates the ameliorative potential of methanolic extract of Vernonia amygdalina in cadmium-induced oxidative stress.
Materials and methods
Chemical
Chemically pure cadmium chloride hydrated (CdCl2.2.5H20 = 228.34) with batch number 6802/3 and product number C0262 obtained from Surechem Products Limited United Kingdom was used for this study.
Plant collection, extraction and preparation
Fresh leaves of Vernonia amygdalina were harvested from a farm in Akure, Ondo State, Nigeria. An expert from the Plant Biology and Biotechnology Unit of the Department of Biological Sciences, University of Medical Sciences, Ondo, identified and authenticated the plant with herbarium number, 032. The leaves were washed with tap water and then sterilized with 70% ethyl alcohol to get rid of the impurities. The leaves were then rinsed thoroughly in sterile distilled water, shade-dried, and then properly dried in an oven at 60°C before being ground into powder with an electric blender [Citation26]. The powder was dissolved in methanol, and the mixture was allowed to stand for 24 hours while being shaken intermittently. The mixture was first filtered with cheesecloth and the filtrate was filtered again using Whatman filter paper No. 1. After filtering, the filtrate was evaporated to dryness using a rotary evaporator under reduced pressure at 40°C. Appropriate concentrations of the extract were then diluted in distilled water before being administrated throughout the experiment [Citation28].
Experimental animals
Twenty adult male Wistar rats weighing 150–200 g were purchased from the animal house at the University of Medical Sciences, Ondo. The rats were fed with New Hope pelleted feed manufactured by New Hope Agriculture and Technology, Nigeria, purchased from Jopoka feed depot in Ondo town, Ondo state. The rats were housed in plastic cages measuring 101 cm long, 54.6 cm wide and 45.4 cm high and fed appropriately in a hygienic environment with good ventilation, and they had access to standard rat chow and water ad libitum. The rats were subjected to an acclimatization period of two weeks before commencing the experiment, which spanned four weeks.
Experimental design
The rats were then randomly assigned to four groups, with each group including five rats (n = 5), as follows:
Group I (unexposed negative control group): Distilled water was administered only.
Group II (Cadmium exposed untreated group): Orally administered with cadmium at a standard dosage of 12 mg/kg/BW.
Group III: Cadmium exposed group (12 mg/kg/BW) administered with Vernonia amygdalina at a standard dosage of 100 mg/kg/BW.
Group IV: Cadmium exposed group (12 mg/kg/BW) administered with Vernonia amygdalina at a standard dosage of 200 mg/kg/BW.
Oral administration via oral cannula was employed during this study and the duration of this administration spanned 28 days. At the end of the experiment, the rats were fasted for 12 hours and euthanized using cervical dislocation. The livers and kidneys of the rats were excised and immediately transferred into 10% neutral buffered formalin for adequate fixation for further histological processing for microscopic studies. Additionally, liver and kidney samples for mRNA expression were washed and homogenized in TRIzol for mRNA extraction as described by Ruart et al. [Citation29].
Histological examination
Upon completion of the experiment, the livers and kidneys were excised and fixed in 10% neutral buffered formalin and prepared for paraffin sectioning through a process of dehydration using various concentrations of alcohol, followed by clearing with xylene and embedding in paraffin blocks. Sections approximately 4 mm thick were obtained using Leica RM2125 RTS microtome and stained with hematoxylin and eosin [Citation30]. The tissues were then examined under a light microscope to assess any pathological changes [Citation31].
mRNA analysis
RNA was extracted from the tissues, and the gene expression level was determined using PCR according to the method outlined by Ruart et al. [Citation29]. Briefly, RNA purification was carried out from 200 mg of tissue using TRIZOL reagent (Inqaba Biotech West Africa Ltd), following the manufacturer’s instructions (InvitrogenTM, Denmark). Then, following the manufacturer’s instructions, the complementary DNA (cDNA) was synthesized using the ProtoScript II First Strand cDNA Synthesis kit (Biolabs, New England), following a 3-step reaction. Polymerase Chain Reaction (PCR) for gene expression was conducted using the Luna Mastermix kit (Biolabs, New England) and Taqman kit probes obtained from TibM-01bio (Berlin, Germany), within a thermocycler. Gel imaging was performed utilizing an electrophoresis gel imager, with β-actin serving as the reference gene. Standard gel electrophoresis was employed as the technique for analyzing the quality and yield of DNA products from the reaction.
The cDNA primers utilized were procured from Inqaba Biotech (Hatfield, South Africa). The specific primers employed for PCR are as follows:
TNF-α: forward (5’-TGAACTTCGGGGTGATCG-3′)
reverse (5’-GGGCTTGTCACTCGAGTTTT-3′);
IL-10: forward (5’-TTGAACCACCCGGCATCTAC-3′)
reverse (5’CCAAGGAGTTGCTCCCGTTA-3′);
β-actin: forward (5’-CCCGCGAGTACAACCTTCT-3′)
reverse (5’-CGTCATCCATGGCGAACT-3′).
Statistical analysis
The results were pooled and expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) was employed to analyze the results, followed by Duncan’s multiple tests for post hoc analysis (DMRT). Statistical Package for Social Science (SPSS) version 17.0 for Windows was utilized for the statistical analysis. The significance level was established at p < 0.05.
Result
Histopathological findings
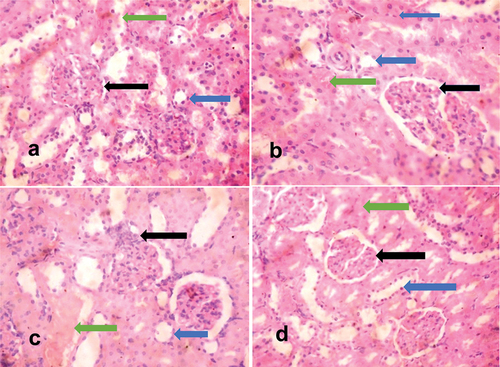
The histopathological findings demonstrated the efficacy of methanolic extracts of Vernonia amygdalina, as there was a significant ameliorative effect in the organs of cadmium-exposed rats treated with the extracts of Vernonia amygdalina. The liver tissues of unexposed rats exhibited normal histological features, characterized by well-preserved hepatocytes, central veins, portal triads, and sinusoids. The liver morphology appeared normal, devoid of any pathological lesions (). The kidney tissues of unexposed rats showed normal histological features composed of glomeruli, renal tubules and interstitial spaces, and no pathological lesion was observed (). In contrast, the liver tissues of exposed, untreated rats displayed moderately poor morphology evidenced by parenchyma inflammation coupled with dilated and congested sinusoidal spaces (). The kidney tissues of exposed, untreated rats had tubular degeneration and congested interstitial space (). Liver tissues of exposed rats, which were administered Vernonia amygdalina at a dosage of 100 mg/kg/BW, showed evident repair, with the restoration of normal histological features evidenced by the absence of non-necrotic hepatocytes with non-congested and un-infiltrated sinusoids (). Kidney tissues of exposed rats, which were administered Vernonia amygdalina at a dosage of 100 mg/kg/BW, showed normal glomeruli and normal renal tubules (). Similarly, liver tissues of exposed rats given Vernonia amygdalina at a dosage of 200 mg/kg/BW displayed substantial repair and maintained normal histological features. The liver parenchyma exhibited a normal profile without congestion, the morphology of the hepatocytes remained normal, and the sinusoids exhibited a normal appearance without any signs of inflammatory cell infiltration (). Kidney tissues of exposed rats given Vernonia amygdalina at a dosage of 200 mg/kg/BW showed normal glomeruli and normal proximal convoluted tubules devoid of inflammation and congestion ().
Figure 1. H and E-stained liver sections of rats.
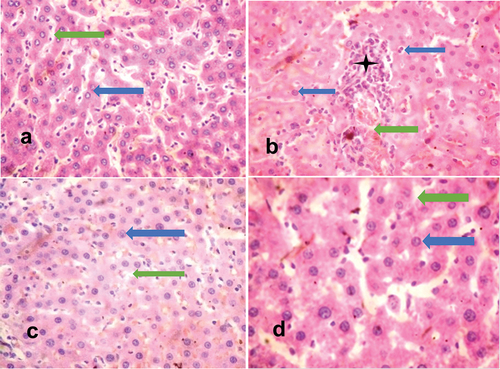
Figure 2. H and E-stained kidney sections of rats.
mRNA findings
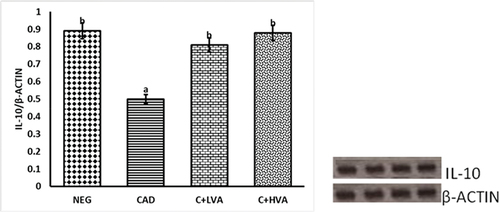
portrays the relative TNF-α gene expression levels in the livers of exposed and non-exposed groups relative to the control groups. Notably, the exposed untreated rats exhibited a marked elevated TNF-α level, indicating cadmium-induced oxidative stress. Conversely, in the treatment groups administered varying doses of Vernonia amygdalina, a significant reduction in TNF-α activity was observed compared to the exposed untreated group. This reduction suggests that the treatment effectively suppressed the TNF-α-mediated inflammatory response, ultimately leading to a substantial decrease in oxidative stress and associated damage when contrasted with the positive control group. The suppression of TNF-α activity in the treatment groups further implies the successful mitigation of the inflammatory response by Vernonia amygdalina, due to its anti-oxidant and anti-inflammatory properties. shows the immunomodulatory effect of Vernonia amygdalina and cadmium on IL-10 gene expression levels in the kidneys of cadmium-exposed untreated and cadmium-exposed Vernonia amygdalina-treated groups relative to the unexposed control groups. IL-10 expression was significantly reduced in the cadmium-exposed rats when compared to other test groups (p < 0.05). However, IL-10 was better expressed in the Vernonia amygdalina-treated rats administered with 100 mg/kg/bw and 200 mg/kg/bw relative to the cadmium-exposed untreated rats (p < 0.05).
Figure 3. The graph illustrates the relative TNF-α gene expression in the livers of adult male Wistar rats across all groups.
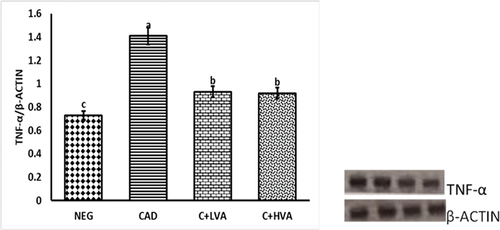
Figure 4. The graph illustrates the relative IL-10 gene expression in the kidneys of adult male Wistar rats across all groups.
Discussion
Our investigations revealed that cadmium exposure at a dosage of 12 mg/kg/BW exerted oxidative stress in the livers and kidneys of the exposed, untreated rats which is evidenced by the substantial elevation of TNF-α and reduced IL-10 levels in the organ studied, which signifies disturbance in hepatic and renal tissues, including consequent impairment of organ function. These processes contributed to the lesions observed in the liver parenchyma, ultimately leading to the disruption of the overall architecture of the liver, as observed in the cadmium-exposed untreated rats. In addition, histoarchitectural studies of the kidneys of the cadmium-exposed untreated rats showed epithelial tubular degeneration coupled with congested interstitial spaces. These findings align with studies by Kousar et al. [Citation3] and Ojo et al. [Citation10] who highlighted that cadmium exposure disrupts cellular redox balance and induces oxidative stress. Our present findings demonstrate that rats exclusively exposed to cadmium, had elevated expression levels of liver TNF-α proteins when compared to the unexposed control group which agrees with the findings of Sanjeev et al. [Citation32]. Also, the kidney tissues exclusively exposed to cadmium had markedly reduced IL-10 levels while histopathological findings revealed a mildly congested interstitial space further indicating cytotoxicity induced by cadmium. This agrees with Wang et al. [Citation18] and Zhu et al. [Citation33], which showed pronounced histological changes in the cadmium-exposed group. Intriguingly, treatment with Vernonia amygdalina demonstrated a significant reduction in the expression of TNF-α protein in the liver and improvement in IL-10 expression in the kidney of the treated rats at standard doses of 100 mg/kg/bw and 200 mg/kg/bw respectively. In contrast to the untreated cadmium-exposed rats, recovery of the liver as well as kidneys of the treated rats was observed as no pathological lesion was observed in the liver and kidneys of the treated rats indicating the mitigating potential of Vernonia amygdalina in cadmium-induced oxidative stress via the regulation of inflammatory stress proteins. Hence, it can be logically deduced from the study that the administration of Vernonia amygdalina effectively alleviated the acute liver damage prompted by cadmium exposure. These findings are consistent with previous investigations by Ogbuagu et al. [Citation20] and Orororo et al. [Citation34], highlighting the hepatoprotective and nephroprotective properties of Vernonia amygdalina and its potential as a therapeutic agent for liver and kidney disorders caused by heavy metal toxicity. Our study further suggests that Vernonia amygdalina possesses strong antioxidant and anti-inflammatory attributes, mainly due to the presence of various phytochemicals, as previously documented by Ossai et al. [Citation26].
In summary, the improvement in liver and kidney architecture, and the significant alteration in oxidative stress, antioxidant, and anti-inflammatory markers align with the existing body of knowledge by Wang et al. [Citation18] and Precious et al. [Citation31]. These findings highlight the potential of Vernonia amygdalina in alleviating cadmium-induced hepato-renal damage and dysfunction, hence reinforcing the potential of Vernonia amygdalina leaf extracts as a natural remedy and a promising option for the management of heavy metal toxicity.
Conclusion
Vernonia amygdalina has potent antioxidative and anti-inflammatory properties that could mitigate cadmium-induced hepatorenal toxicity. This study establishes that Vernonia amygdalina at standard doses of 100 mg/kg/bw and 200 mg/kg/bw ameliorates cadmium-induced oxidative stress by exerting a negative regulatory influence on TNF-α expression which is a key pro-inflammatory marker, in the livers and a marked increase in the level of IL-10 in the kidneys of the treated rats. Conclusively, the beneficial therapeutic supplement potential of Vernonia amygdalina is recommended for the development of new pharmacophores aimed at addressing and mitigating cadmium-induced hepatorenal toxicity in living organisms.
Ethical approval
Before initiating the research project, ethical approval was sought from the Ministry of Agriculture, Akure, ensuring alignment with established guidelines for the use of experimental animals. The study received the necessary approval, indicated by the ethical clearance registration reference number: V.384/33. The research adhered rigorously to the guidelines governing the care and utilization of research animals, as outlined by the research committee, which aligns with the standards outlined by the World Health Organization.
Acknowledgments
The authors are grateful to Mr Otegbade, University College Hospital, Ibadan for the processing of the histological samples and Dr Gideon Oladipo of the Biochemistry Department of Achievers University, Owo for the mRNA studies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Unsal V, Dalkıran T, Çiçek M, et al. The role of natural antioxidants against reactive oxygen species produced by cadmium toxicity: a review. Tabriz Univ Med Sci. 2020;10(2):184–202. doi: 10.34172/apb.2020.023
- Liu C, Zhu Y, Lu Z, et al. Cadmium induces acute liver injury by inhibiting Nrf2 and the role of NF- κ B, NLRP3, and MAPKs signaling pathway. Int J Environ Res Public Health. 2019;17(1):138. doi: 10.3390/ijerph17010138
- Kousar K, Ijaz MU, Ehsan N, et al. Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: a biochemical, inflammatory, apoptotic and histopathological investigation. Biomed Pharmacother. 2022;150:112934. doi: 10.1016/j.biopha.2022.112934
- Schaefer HR, Dennis S, Fitzpatrick S. Cadmium: mitigation strategies to reduce dietary exposure. J Food Sci. 2020;85(2):260–267. doi: 10.1111/1750-3841.14997
- Wang J, Fang Z, Li Y, et al. Ameliorative effects of oyster protein hydrolysates on cadmium-induced hepatic injury in mice. Mar Drugs. 2022;20(12):758. doi: 10.3390/md20120758
- Ren L, Qi K, Zhang L, et al. Glutathione might attenuate cadmium-induced liver oxidative stress and hepatic stellate cell activation. Biol Trace Elem Res. 2019;191(2):443–452. doi: 10.1007/s12011-019-1641-x
- Fang J, Yin H, Yang Z, et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol Environ Saf. 2021;208:111610. doi: 10.1016/j.ecoenv.2020.111610
- Nordberg GF. Metallothionein and cadmium toxicology—historical review and commentary. Biomolecules. 2022;12(3):1–15. doi: 10.3390/biom12030360
- Sies H. Oxidative stress: Concept and some practical aspects. Antioxidants. 2020;9(9):1–6. doi: 10.3390/antiox9090852
- Ojo OA, Rotimi DE, Ojo AB, et al. Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats. Sci Rep. 2023;13(1):1–11. doi: 10.1038/s41598-023-28893-6
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38(2):167–197. doi: 10.1016/j.ccell.2020.06.001
- Pisoschi AM, Pop A, Iordache F, et al. Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891
- Yamashita M, Passegué E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration. Cell Stem Cell. 2019;25(3):357–372.e7. doi: 10.1016/j.stem.2019.05.019
- Mahmoud AH, Taha NM, Zakhary M, et al. PTEN gene & TNF-alpha in acute myocardial infarction. IJC Hear Vasc. 2019;23:100366. doi: 10.1016/j.ijcha.2019.100366
- Robinson PC, Liew DFL, Liew JW, et al. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med. 2020;1(1):90–102. doi: 10.1016/j.medj.2020.11.005
- Wu L, Zhang G, Guo C, et al. MiR-128-3p mediates TNF-α-induced inflammatory responses by regulating Sirt1 expression in bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2020;521(1):98–105. doi: 10.1016/j.bbrc.2019.10.083
- Tan W, Luo X, Li W, et al. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2019;40:446–456. doi: 10.1016/j.ebiom.2018.12.047
- Te Wang W, Liao SF, Wu ZL, et al. Simultaneous study of antioxidant activity, DNA protection and anti-inflammatory effect of Vernonia amygdalina leaves extracts. PLOS ONE. 2020;15(7):1–17. doi: 10.1371/journal.pone.0235717
- Xuan T, Nguyen T, Dang DL, et al. Anti-inflammatory activity of a new compound from Vernonia amygdalina. Nat Prod Res. 2020;35(23):5160–5165. doi: 10.1080/14786419.2020.1788556
- Ogbuagu EO, Airaodion AI, Ogbuagu U, et al. Prophylactic propensity of methanolic extract of Vernonia amygdalina leaves against acute ethanol-induced oxidative stress in Wistar rats. Int J Bio-Sci Bio-Technol. 2019;11(7):37–46. doi: 10.9734/ajrimps/2019/v7i330122
- Oyeyemi IT, Akinlabi AA, Adewumi A, et al. Vernonia amygdalina: A folkloric herb with anthelminthic properties. Beni-Suef Univ J Basic Appl Sci. 2018;7(1):43–49. doi: 10.1016/j.bjbas.2017.07.007
- Asante D, Henneh IT, Acheampong DO, et al. Anti-inflammatory, anti-nociceptive and antipyretic activity of young and old leaves of Vernonia amygdalina. Biomed Pharmacother. 2019;111:1187–1203. doi: 10.1016/j.biopha.2018.12.147
- Innih SO, Ubhenin AE. The protective effect of Vernonia amygdalina in lead acetate‑induced nephrotoxicity in Wistar rats. Niger J Exp Clin Biosci. 2022;9(4):227–233. doi: 10.4103/njecp.njecp_29_21
- Hasibuan PAZ, Harahap U, Sitorus P, et al. The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon. 2020;6(7):1–5. doi: 10.1016/j.heliyon.2020.e04449
- Yusoff SF, Haron FF, Tengku Muda Mohamed M, et al. Antifungal activity and phytochemical screening of Vernonia amygdalina extract against botrytis cinerea causing gray mold disease on tomato fruits. Biology. 2020;9(9):1–14. doi: 10.3390/biology9090286
- Ossai NR, Ojieh AE, Wilson JI, et al. Vernonia amygdalina ethanol leaf extract protects against tramadol-induced organ damages through inhibition of oxidative stress. Int Conf Sci Innov Stud. 2023;1(1):424–445. doi: 10.59287/icsis.637
- Ugbogu EA, Emmanuel O, Dike ED, et al. The phytochemistry, ethnobotanical, and pharmacological potentials of the medicinal Plant-Vernonia amygdalina L. (bitter Leaf). Clin Complement Med Pharmacol. 2021;1(1):100006. doi: 10.1016/j.ccmp.2021.100006
- Tunasamy K, Suryadevara N, Athimoolam T. Screening of Vernonia amygdalina leaf extracts for antioxidant and antimicrobial activity. In: Ismail H, Mohamed NM, Muhamad N, editors. Materials Today: Proceedings. Malaysia: Elsevier Ltd; 2019. p. 1809–1818. doi: 10.1016/j.matpr.2019.06.055
- Ruart M, Chavarria L, Campreciós G, et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70(3):458–469. doi: 10.1016/j.jhep.2018.10.015
- Moronkeji A, Eze IG, Bejide RA, et al. Evaluation of herbal cocktail used in the treatment of malaria on liver tissue of adult Wistar rats. J Med Plants Res. 2018;12(28):508–521. doi: 10.5897/JMPR2018.6661
- Barnes P, Yeboah JK, Gbedema W, et al. Ameliorative effect of Vernonia amygdalina plant extract on heavy metal-induced liver and kidney dysfunction in rats. Adv Pharmacol Pharm Sci. 2020;2020:1–7. doi: 10.1155/2020/2976905
- Sanjeev S, Bidanchi RM, Murthy MK, et al. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ Sci Pollut Res. 2019;26(20):20631–20653. doi: 10.1007/s11356-019-05420-7
- Zhu MK, Li HY, Bai LH, et al. Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult Sci. 2019;99(6):3215–3228. doi: 10.1016/j.psj.2019.12.073
- Orororo OC, Efekemo O, Osain BP, et al.Combined leaf extracts of Vernonia amygdalina and Occimum gratissimum ameliorates cadmium-induced nephro-toxicity in Wistar rats. Asia-Pacific J Sci Technol. 2022;28(3):1–8.
