ABSTRACT
Lung cancer is the most lethal malignancy and is often associated with a poor prognosis because of a lack of prognostic markers. Thus, this study aimed to determine whether irisin, resistin, and leptin could be useful biomarkers for lung cancer prognosis. The study is designed on 100 lung cancer patients, (66) patients with non-small cell lung cancer (NSCLC) and (34) patients with small cell lung cancer (SCLC). For the purpose of comparison, (66) samples as control group. Using the ELISA technique to estimate leptin, resistin, and irisin levels in patients and controls. The results indicate a significant increase in mean leptin in NSCLC and SCLC groups (10.71 ± 0.30 and 10.13 ± 0.51) ng/ml, respectively, in contrast to the control (8.26 ± 0.47) ng/ml. The mean of irisin and resistin had been significantly increased in SCLC group (5.86 ± 0.13 pg/ml and 7.25 ± 0.38 ng/ml, respectively) and NSCLC group (5.08 ± 0.09 pg/ml and 6.35 ± 0.13 ng/ml, respectively) in contrast to the control (4.13 ± 0.09 pg/ml and 3.96 ± 0.17 ng/ml, respectively). The higher levels of leptin in NSCLC patients could serve as a prognostic marker for NSCLC. The variations in Resistin and Irisin levels across different stages of lung cancer suggest that they might be useful in predicting the prognosis of lung cancer.
Introduction
Lung cancer is one of the most pathogenic and fatal types of cancer in the world, affecting both men and women [Citation1]. Lung cancer that starts in the lungs, or the cells that line the air passages, is referred to as primary lung cancer [Citation2]. Every year, approximately 2 million people are diagnosed with lung cancer, annually and majority of cases diagnosed at late stages [Citation1]. The total 5-year survival rate is still only 16%, despite improvements in outcomes and advancements in treatment [Citation3].
The most significant fator in enhancing patient outcomes and survival rates is the discovery of accurate biomarkers to support early detection, diagnosis, and treatment monitoring [Citation4]. Researchers have recently been examining the relationship between different physiological markers and the onset and spread of lung cancer, which can offer important insights into a person’s physiological condition [Citation5,Citation6]. Leptin, Resistin, and Irisin are a few examples of genetic, molecular, cellular, or biochemical components that may be included in these indicators and their levels may change as cancer progresses [Citation7]. Elucidating the correlation between these markers and lung cancer may improve our comprehension of the fundamental mechanisms of the illness and pave the way for the development of tailored treatments [Citation8].
Leptin is non-glycosylated hormone [Citation9] mostly secreted by adipose tissue [Citation10]. Leptin regulates several physiological processes, including energy metabolism, appetite control, immunity, effect on tumor development, and the stimulation of the oxidation of fatty acids, so as to prevent the accumulation of fat [Citation9]. Elevated blood leptin levels have been found to be an independent risk factor for non-small cell lung cancer (NSCLC) [Citation11], although one study suggested a significant correlation between lower serum leptin levels and lung cancer prognosis [Citation12]. Even after adjusting for BMI and recent weight loss, the study by Bereda [Citation9] found elevated leptin levels in lung cancer patients and identified leptin as a cancer risk factor.
Resistin pro-inflammatory cytokine, that is secreted mainly from monocytes, dendritic cells, and macrophages in humans [Citation13]. Resistin may be involved in many inflammatory and autoimmune processes, including cancer, inflammatory bowel disorders, and atherosclerosis, according to Increasing recent research [Citation14]. There is still a dearth of information on resistin’s relationship to various cancer types, despite prior research’ demonstration of this relationship [Citation13,Citation15]. According to one study, resistin and leptin might play a role in the development and spread of lung cancer [Citation14]. Although resistin is less well studied and contentious in lung cancer, the amount of expression of this protein in cancer tissues has not been assessed in many papers [Citation16]. One study found that higher circulating levels of resistin were associated with an increased risk of lung cancer [Citation17]. Additionally, antiresistin antibody-treated mice have a lower incidence of lung tumors, indicating that resistin accelerates the progression of lung cancer. As a result, resistin may be a desirable target for the development of therapeutic approaches that target immune cells in the cancer microenvironment [Citation18]. Irisin is an exercise-inducible myokine that plays a significant role in the regulation of body energy homeostasis [Citation19]. Recent studies have shown that irisin is involved in the occurrence and development of a variety of tumors [Citation20–22]. Additionally, it was discovered that irisin treatment was able to lessen the chemoresistance of NSCLC cells that had developed resistance to a chemotherapeutic drug used in the treatment of lung cancer [Citation19]. This study aimed to investigate whether leptin, resistin, and irisin could be potential as a prognostic factors in lung cancer patients.
Materials and methods
Study design
Lung cancer samples were collected from Anbar Cancer Center in AL-Ramadi city and the tumor Center in Baghdad Teaching Hospital-Medical City. Specimens collection started in December, 2022 and continued until the end of November, 2023. The local Research Ethics Committee of the university of Anbar/College of Medicine accepted the study protocol (Certificate No: 123 on 23 November 2023) in compliance with the Helsinki Declaration for Human Studies.
A cross-sectional study was designed with 100 lung cancer patients at age range (40–75) years, where these patients included (67) smoking patients and (33) nonsmoking patients. Lung cancer patients divided into (66) patients with non-small cell lung cancer (NSCLC) (41 males and 25 females) and (34) patients with small cell lung cancer (SCLC) (21 male and 13 females). The diagnosis was performed by a multidisciplinary team, including a pulmonologist, a pathologist, and an oncologist. This study divided lung cancer patients into four stages: stage I, stage II (limited), stage III, and stage IV (extensive), which are based on the results of physical exams, radiology, biopsy, and bronchoscopic staging. This is important to determine the disease extent in the lungs. For the purpose of comparison, (66) samples as control group (34 males and 32 females) with an age range (40–70) years included in the study, control group included (25 smoking individual and 41 nonsmoking individuals).
Sample selection
Patient inclusion criteria
Patients were newly diagnosed with lung cancer, and they were not initiating any therapeutic approach or receiving (1, 2, and 3) cycles of therapy.
Patient exclusion criteria
They had a history of other types of cancer or a family history of cancer, or received more than three cycles of therapy. Lung cancer patients with co-morbidities like Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), Hepatitis B surface antigen-A (HBsAg) positivity. Patients who had a disorder that causes an increase in hormones as leptin.
Controls inclusion criteria
Controls were chosen from among participants who were in good health and were free of acute illness or infection at the time of sampling.
Control exclusion criteria
They have any endocrine abnormalities, are diabetic or hypertensive, and are comparatively similar in age and sex to the patient participants.
Sample collection and laboratory measurements
Before the blood samples were collected, a careful history was taken from each patient and control via interview: age, sex, duration of lung cancer, height/weight, and smoking status, and written informed consent was obtained from each participant before to their involvement in the study. Each patient and control had 5 ml of blood taken by disposable syringe after 12- hours of fasting. The blood samples were placed in plain tubes and left 30 minutes to clot and centrifuged at 5000 round per minute (r.p.m) to obtained sera, which were placed in 1.5 mL Eppendorf tubes and kept cold at -20° C until the analysis. In the lab, samples were examined using the enzyme-linked immunosorbent test (ELISA) using the Human Leptin (LEP) ELISA Kit (Cat. No: ELK1160), the Human Resistin ELISA Kit (Cat. No: ELK1225), and the Human Irisin ELISA Kit (Cat. No: ELK6625). All ELISA kits were provided by ELK Biotechnology Company in China. The biomarkers were analyzed following the methods recommended by the kit manufacturer, and the color of the samples was measured by ELISA reader. The optical density (OD) of the samples was compared with standard curve, the quantities of these markers in the samples were ascertained. The information gathered was documented.
Statistical analysis
The SAS (2018) software was utilized to ascertain the impact of variance variables on the study parameters. The T-test and the least significant difference (LSD) test (ANOVA) were used to compare means statistically. The chi-square test was utilized to assess the different forms of lung cancer statistically (0.05 and 0.01 likelihood). Using ROC (Operating characteristic) curve to determinate the diagnostic specificity and sensitivity of study hormones. The area under the curve was calculated to assess the capability of serum hormones to accurately differentiate between lung cancer patients and control. The optimal diagnostic cutoff value for the highest clinical specificity and sensitivity was determined using the ROC curve analysis.
Results
Clinical characteristics of the study groups
The results have shown significant difference between patients and control groups according to sex, BMI, and smoking, except age, where the mean age in lung cancer patients was (66.15 ± 0.82) year and that in control was (63.27 ± 1.60) year. Both the lung cancer patient group and control group showed higher percentage of males (64% and 51.5%) than females (36% and 48.5%), respectively. The mean of BMI in lung cancer patients (26.30 ± 0.33) kg/m2 is higher than the mean of BMI in control (24.52 ± 0.68) kg/m2, as shown in .
Table 1. Show clinical characteristics of patients and control.
shows a larger percentage of lung cancer patients (67%) were smokers compared to nonsmokers (33%). In contrast, the control group showed a high percentage of nonsmokers (62.12%) compared to smokers (37.87%).
Table 2. Distribution of patients according to smoking status.
Types of lung cancer
A total of 100 sera from lung cancer were included in this study. Among them, there are (30) patients with small cell lung cancer (SCLC) and (70) patients with non- small cell lung cancer (NSCLC). The present study found that significant higher percentage (70%) in patients with NSCLC compared with SCLC patients (30)% at level (p ≤ 0.01), as shown in .
Table 3. Distribution of patients according to lung cancer types.
Stages of lung cancer
The patients in this study are divided into four stages according to the staging or TNM system, which is based on the results of physical exams, biopsies, and other tests. The results show a significant difference at a level of p ≤ 0.05 in lung cancer stages among patients, with a high percentage (35%) of patients in stage IV, followed by stage III show a percentage (27%), while stage I and II shows a lower percentage (17 and 21%) respectively, as show in .
Table 4. Distribution of patients according to stages in patients group.
Determination of leptin, irisin, and resistin hormone level between the different groups
indicates that there was a significant difference in leptin, irisin, and resistin hormone levels between the study groups (p ≤ 0.01). There was significant increase in the mean leptin level in NSCLC and SCLC groups (10.51 ± 0.30 and 9.83 ± 0.51) ng/ml respectively, in contrast to that in the control group (8.26 ± 0.47) ng/ml. Resistin had been significant increase in SCLC group (7.25 ± 0.38) ng/ml compared with other groups. Also, resistin had been significant increase in NSCLC group (6.35 ± 0.13) ng/ml in contrast to the control group (3.96 ± 0.17) ng/ml at (p ≤ 0.01). However, the mean irisin level was significant increase in SCLC group (5.86 ± 0.13) pg/ml compared with other groups. Additionally, the mean irisin level was significantly greater in the NSCLC group (5.08 ± 0.09) pg/ml than in the control group (4.13 ± 0.09) pg/ml.
Table 5. Comparisons leptin, resistin, and irisin levels among the different groups.
Effect of stage on hormones level in lung cancer patients
shows the levels of several hormones parameters according to lung cancer stage. The mean leptin-level show was not significantly different among the lung cancer stage. The mean level of resistin was significantly greater in stage I (7.88 ± 0.56) ng/ml than in the other stages, while there was no significant difference in the mean of resistin between Stages II (7.05 ± 0.29) and III (6.79 ± 0.34), significant decreased at Stage IV (5.67 ± 0.13). There was, a significant increase was observed in irsin level at Stage I (6.42 ± 0.13) pg/ml compared to the other stages, followed by stage II (5.86 ± 0.15). There were no significant differences in the irsin level between the III, and IV stages (4.84 ± 0.15, and 4.57 ± 0.12 pg/ml, respectively).
Table 6. Effect of stage on hormone level in patients group.
Correlation coefficient between study hormones and BMI in study groups
As shown in the there are significant and positive correlations between BMI with resistin (r = 0.55, p = 0.0001; ), leptin with BMI (0.21, p = 0.038; ), irsin with BMI (0.31, p = 0.0001; ) in lung cancer patients. In the control group, all correlations were no significant except irsin with BMI show significant and positive correlations coefficient (r = 0.53, p = 0.0001).
Figure 1. (a) Relationship between leptin and BMI in control. (b) relationship between resistin and BMI in lung cancer patients.
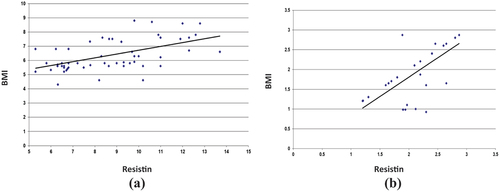
Figure 2. (a) Relationship between irisin and BMI in control. (b) relationship between irisin and BMI in lung cancer patients.
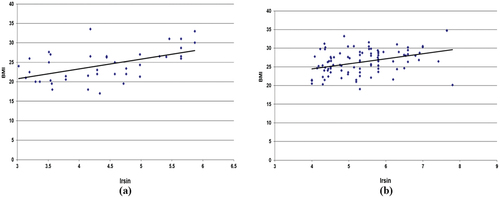
Figure 3. (a) Relationship between leptin and BMI in control. (b) relationship between irisin and BMI in lung cancer patients.
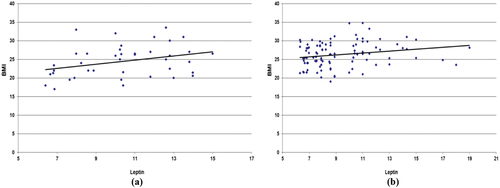
Table 7. Correlation coefficient between parameters study in patients and control groups.
Assessment of the diagnostic specificity and sensitivity of study hormones in study groups
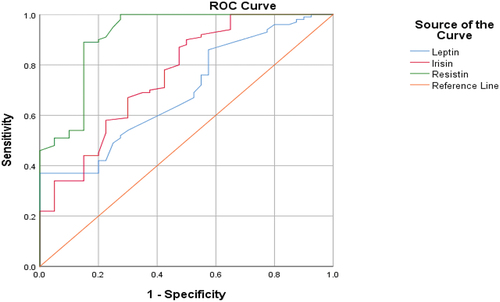
The ROC analysis of leptin, irisin, and resisitin levels in lung cancer patients and control are given in . The pest cutoff value of leptin was 1.37 ng/ml. Leptin levels greater than 1.37 demonstrated a specificity 86% and sensitivity 92% for lung cancer diagnosis. The pest cutoff value of irisin was 1.40 ng/ml. Irisin levels greater than 1.40 demonstrated a specificity 90.1% and sensitivity 94.7% for lung cancer diagnosis. The pest cutoff value of resistin was 1.74 ng/ml. Resistin levels greater than 1.74 demonstrated a specificity 80% and sensitivity 96% for lung cancer diagnosis.
Figure 4. ROC curve analysis for leptin, irisin, and resistin levels (lung cancer patients versus controls). Leptin ROC AUC = 0.693; 95% CI, 0.602–0.785. Irisin ROC AUC = 0.759; 95% CI, 0.669–0.849. Resistin ROC AUC = 0.916; 95% CI, 0.858–0.973. The sensitivity and specificity of lung cancer diagnoses were more than 80%.
Discussion
The results show a higher percentage of males (64%) compared to females (36%) in the lung cancer group. The larger percentage in men may suggest that there are sex-specific risk factors for lung cancer development, as more men than women engage in risky behaviors like smoking or occupational exposures that are associated with the disease [Citation23,Citation24].
The variations in mean BMI between the control group and lung cancer patients could point to an association between a higher risk of developing lung cancer and an elevated BMI. While obesity is an established risk factor for several malignancies, it’s vital to remember this. There are several factors that could contribute to the observed variation in BMI, including age, sex, and lifestyle choices [Citation25,Citation26].
Our results reported that the mean age of patients with lung cancer is 65.15 years. This suggests that the study’s lung cancer patients are older than those in the control group. The variation in mean age suggests that being older might be a risk factor for lung cancer. This discovery is consistent with the well-accepted notion that the risk of cancer rises with age. Understanding the age distribution in lung cancer patients is crucial for screening strategies, treatment plans, and diagnostic approaches [Citation24]. The correlation between smoking and lung cancer is well established, and this is supported by the higher percentage of smokers among patients with lung cancer. According to Hecht [Citation27], tobacco smoke contains carcinogens that can harm lung cells and raise the risk of developing cancer. It is not always the case that lung cancer patients who smoke have a larger percentage of smoking among them. Although smoking is a significant risk factor for lung cancer, other factors might also be involved [Citation15]. A significant majority of individuals may be diagnosed with lung cancer at an advanced stage, as indicated by the increased percentage of patients in stage IV. This is problematic because advanced phases frequently present more difficult treatment and prognostic situations [Citation28]. There are significant clinical ramifications for the distribution of phases. Prognosis and treatment outcomes are typically better with early discovery (stages I and II) [Citation29].
Large-scale research is needed to validate our findings, as serum leptin and resistin levels play a crucial role as adipokines in lung cancer. Nevertheless, their application as diagnostic or prognostic indicators is still viable. Leptin was detected high in our patients, particularly in patients with NSCLC. This might explain that leptin hormone is secreted from many adenomatous structures in the body, particularly from adipose tissue. Therefore, leptin secretion may be induced by paraneoplastic events or cytokine effects in lung carcinoma [Citation30]. Some authors reported that leptin expression was significantly higher in NSCLC patients than in SCLC and healthy control, these suggesting leptin as an independent prognostic factor for NSCLC and shorter survival in NSCLC [Citation14]. Our results agree with [Citation31,Citation32] who indicated that mean of leptin increase in lung cancer group contrast with control. The results of the present study disagree with results by Tas [Citation33]; Tong [Citation34], who they recorded that the mean of leptin decrease in lung cancer compared with control. In Chine, according to the meta-analysis, leptin levels in serum and tissue may have a role in the etiology of lung cancer and tumor metastasis [Citation35]. An earlier investigation revealed that leptin stimulation increases the growth of lung cancer by stimulating the production of immunoinflammatory cytokines and causing resistance to apoptosis [Citation34].
In one study, increased leptin in lung tissue was found to be associated with impairment in lung tissue, along with an increased risk of NSCLC [Citation12]. Many studies have concluded that elevated blood leptin levels may be predictive of lung cancer diagnosis and progression, suggesting that leptin plays a significant role in the pathophysiology of lung cancer [Citation35,Citation36]. Additionally, the current study’s findings along with those of earlier research may enable us to pinpoint a novel marker for the diagnosis of lung cancer as well as a therapeutic target. A large number of studies have suggested that leptin has the potential as an anticancer drug target [Citation37,Citation38].
While, resistin hormone had been significant increase in SCLC and NSCLC group compared with control group. These results agree with results by Demiray et al. [Citation15], who indicated that lung cancer patients’ serum resistin levels were noticeably elevated (6.3 ± 1.9) ng/ml at (p = .025) than the control group (4.4 ± 0.5) ng/ml. It was stated that tissue inflammation and organ failure were linked to this increase [Citation18]. A study by Nigro et al. [Citation39], indicated that NSCLC patients showed significantly higher serum resistin levels than healthy volunteers and they found that resistin might be involved in the pathophysiology of weight reduction in NSCLC patients. Resistin, according to one study, was substantially expressed in lung cancer tissues and, in a dose-dependent manner, facilitated the migration and invasion of lung adenocarcinoma cells [Citation17]. Our results disagree with [Citation18] when who indicated that no significant correlation between the resistin level in the lung cancer and control groups. There aren’t many studies looking into the connection between resistin and cancer. Peripheral blood monocytes, macrophages and adipose tissue produce resistin in tumor tissues [Citation17]. Lung cancer patients may have elevated resistin levels due to both adipose tissue involvement and monocyte activation as a component of the greater inflammatory process [Citation40,Citation41]. The results by Gong et al. [Citation17], implied that resistin could increase the migration and invasion of lung adenocarcinoma cells. A previous report found that, in comparison to controls, NSCLC patients had higher blood levels of resistin [Citation40]. In the borderline regions of human lung cancer tissue, there is more resistin than in the non-cancerous portions reported by Kuo et al. [Citation42]. Clinical study has reported that the prognosis, development, and propensity for tumor formation are all substantially correlated with resistin, which was strongly correlated with increasing tumor size, clinical stage, and lymph node metastasis [Citation18,Citation41].
Our results show mean of Irisin significant increase in SCLC and NSCLC groups compared with control group. These results agree with results by Nowinska et al. [Citation43], who indicated that the irisin level increase in NSCLC patients (2.25 ± 0.09) ng/ml compared with in control (1 ± 0.97) ng/ml. The results by Tsiani et al. [Citation44], consistent with our results increase Irisin levels in NSCLC tissue and in stromal fibroblasts, and they suggest that Irisin expression in stromal fibroblasts may be a separate predictor of survival for NSCLC patients as well as a possible link to enhanced cancer cell proliferation. In China, a study by Shao et al. [Citation45] found that Irisin dramatically reduced NSCLC cell proliferation at concentrations of 20–50 nM, and decreased the migration and invasiveness of NSCLC cells at values greater than 20 nM [Citation19,Citation46]. Irisin is expressed in the greatest energy-demanding cells in normal bodily tissues, such as hepatocytes, cardiac muscle cells, and skeletal muscle fibers [Citation44]. Furthermore, irisin enhances glucose absorption and is linked to glucose homeostasis. This could account for the increased production of irisin in lung cancer cells, which also have a high glucose absorption need as a result of tumor growth [Citation46]. Many researches have observed that increased irisin expression in fibroblasts may function as an independent prognostic factor and is linked to a worse patient prognosis [Citation44,Citation45]. Perhaps only lung cancer exhibits irisin expression in the malignant stroma. No other form of cancer has been shown to express irisin in the stromal cells. This may just be a feature of lung tumors [Citation46]. Using an in vitro model, Shao et al. [Citation45] showed in a previous study that irisin inhibits the proliferation, migration, and invasion of lung cancer cells. Pinkowska et al. [Citation47] found that Irisin was elevated in the cancer cells and tumor stromal fibroblasts in patients with NSCLC. In tumor cells, Irisin expression levels were decreased with higher grades of malignancy and in larger tumors (T) [Citation46,Citation47]. The correlation coefficient indicates a moderate to strong positive correlation of BMI with resistin and BMI with irisin and a weak positive correlation between leptin and BMI in lung cancer patients. The positive correlations suggest that as BMI increases, the levels of resistin and irsin also tend to increase in lung cancer patients. However, the correlation between Leptin and BMI is weaker [Citation11,Citation13,Citation19].
Conclusion
Based on our results, the higher levels of leptin in NSCLC patients could serve as screening and independent prognostic factor for NSCLC. The significant variations in resistin and Irisin levels across different stages of lung cancer suggest their potential utility in predicting the prognosis or progression of lung cancer. However, further large-scale studies are required to confirm our results.
Abbreviations
| SCLC | = | Small cell lung cancer |
| NSCLC | = | Non-small cell lung cancer |
| HCV | = | Hepatitis C virus |
| HIV | = | Human immunodeficiency virus |
| HBsAg | = | Hepatitis B surface antigen – A |
Disclosure statement
No conflict of interest was reported by the author(s).
References
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol (Pozn). 2021;25(1):45–52. doi: 10.5114/wo.2021.103829
- Parma B. Identification of the mitochondrial HSPD1 as metabolic vulnerability in non-small cell lung cancer [ dissertation]. Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU); 2023.
- Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623
- Seijo LM, Peled N, Ajona D, et al. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14(3):343–357. doi: 10.1016/j.jtho.2018.11.023
- Ganster DC, Crain TL, Brossoit RM. Physiological measurement in the organizational sciences: a review and recommendations for future use. Annu Rev Organ Psychol Organ Behav. 2018;5(1):267–293. doi: 10.1146/annurev-orgpsych-032117-104613
- Ismaeel GL, Abdul-Hussein AH, Qasim HM, et al. Therapeutic targeting of dormant cancer stem cells in solid tumors. Gene Rep. 2023;30:101717. doi: 10.1016/j.genrep.2022.101717
- Saleem HM, Ramaiah P, Gupta J, et al. Nanotechnology-empowered lung cancer therapy: from EMT role in cancer metastasis to application of nanoengineered structures for modulating growth and metastasis. Environ Res. 2023;232:115942. doi: 10.1016/j.envres.2023.115942
- Zhu C, Ma H, He A, et al. Exercise in cancer prevention and anticancer therapy: efficacy, molecular mechanisms and clinical information. Cancer Lett. 2022;544:215814. doi: 10.1016/j.canlet.2022.215814
- Bereda G. Application of leptin in enhancing immunity and maintaining obesity. J Clin Stud Rev Rep. 2023;5(1):1–2. doi: 10.47363/JCCSR/2023(5)239
- Jiménez-Cortegana C, López-Saavedra A, Sánchez-Jiménez F, et al. Leptin, both bad and good actor in cancer. Biomolecul. 2021;11(6):913. doi: 10.3390/biom11060913
- Caruso A, Gelsomino L, Panza S, et al. Leptin: a heavyweight player in obesity-related cancers. Biomolecul. 2023;13(7):1084. doi: 10.3390/biom13071084
- Karatas F, Yalcin B, Sahin S, et al. The significance of serum leptin level in patients with early stage nonsmall cell lung cancer. J Cancer Res Ther. 2017;13(2):204–207. doi: 10.4103/0973-1482.196859
- Abdalla MMI. Serum resistin and the risk for hepatocellular carcinoma in diabetic patients. World J Gastroenterol. 2023;29(27):4271. doi: 10.3748/wjg.v29.i27.4271
- Ashour MA, Wadea FM, Hussein NMM, et al. Insulin resistance, resistin hormone and hepatocellular carcinoma interplay: a review article. Egypt J Hosp Med. 2023;90(2):2041–2044. doi: 10.21608/ejhm.2023.285028
- Demiray G, Değirmencioğlu S, Uğurlu E, et al. Effects of serum leptin and resistin levels on cancer cachexia in patients with advanced-stage non–small cell lung cancer. Clin Med Insights Oncol. 2017;11:1179554917690144. doi: 10.1177/1179554917690144
- Zhao CC, Chen J, Niu R, et al. Increased resistin suggests poor prognosis and promotes development of lung adenocarcinoma. Oncol Rep. 2018;40(6):3392–3404. doi: 10.3892/or.2018.6736
- Gong WJ, Liu J-Y, Yin J-Y, et al. Resistin facilitates metastasis of lung adenocarcinoma through the TLR 4/Src/ EGFR / PI 3K/ NF -κB pathway. Cancer Sci. 2018;109(8):2391–2400. doi: 10.1111/cas.13704
- Göktepe M, Korkmaz C, Zamani A, et al. Evaluation of serum resistin, visfatin and chemerin levels in patients with lung cancer and chronic obstructive pulmonary disease. Turk Thorac J. 2019;21(3):169. doi: 10.5152/TurkThoracJ.2019.19001
- Temur AA, Rashid FA. The relationship between circulating irisin and oxidative stress in gastric and colorectal cancer patients. Asian Pac J Cancer Prev. 2022;23(8):2649. doi: 10.31557/APJCP.2022.23.8.2649
- Aslan R, Alp HH, Eryılmaz R, et al. Can the irisin be a biomarker for prostate cancer? A case control study. Asian Pac J Cancer Prev. 2020;21(2):505–509. doi: 10.31557/APJCP.2020.21.2.505
- Temur AA, Rashid FA. Irisin and carcinoembryonic antigen (CEA) as potential diagnostic biomarkers in gastric and colorectal cancers. Rep Biochem Mol Biol. 2021;10(3):488–494. doi: 10.52547/rbmb.10.3.488
- Liu S, Cui F, Ning K, et al. Role of irisin in physiology and pathology. Front Endocrinol. 2022;13:962–968. doi: 10.3389/fendo.2022.962968
- Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer. 2020;146(9):2376–2382. doi: 10.1002/ijc.32716
- Mederos N, Friedlaender A, Peters S, et al. Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. 2020;5:e000796. doi: 10.1136/esmoopen-2020-000796
- Ardesch FH, Ruiter R, Mulder M, et al. The obesity paradox in lung cancer: associations with body size versus body shape. Front Oncol. 2020;10:591110. doi: 10.3389/fonc.2020.591110
- Li M, Cao SM, Dimou N, et al. Association of metabolic syndrome with risk of lung cancer: a population-based prospective cohort study. Chest. 2024;165(1):213–223. doi: 10.1016/j.chest.2023.08.003
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–2732. doi: 10.1002/ijc.27816
- Alemán MR, Santolaria F, Batista N, et al. Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine. 2002;19(1):21–26. doi: 10.1006/cyto.2002.1051
- Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7(9):170070. doi: 10.1098/rsob.170070
- Vita E, Stefani A, Piro G, et al. Leptin-mediated meta-inflammation may provide survival benefit in patients receiving maintenance immunotherapy for extensive-stage small cell lung cancer (ES-SCLC). Cancer Immunol Immun. 2023;72(11):3803–3812. doi: 10.1007/s00262-023-03533-0
- Mou W, Xue H, Tong H, et al. Prognostic value of serum leptin in advanced lung adenocarcinoma patients with cisplatin/pemetrexed chemotherapy. Oncol Lett. 2014;7(6):2073–2078. doi: 10.3892/ol.2014.1988
- Song CH, Liao J, Deng Z-H, et al. Is leptin a predictive factor in patients with lung cancer? Clin Biochem. 2014;47(3):230–232. doi: 10.1016/j.clinbiochem.2013.12.003
- Tas F, Duranyildiz D, Argon A, et al. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol. 2005;22(4):353–358. doi: 10.1385/MO:22:4:353
- Tong H, Mou W, Xue H, et al. Prognostic value of serum leptin in advanced lung adenocarcinoma patients with cisplatin/pemetrexed chemotherapy. Oncol Lett. 2014;7(6):2073–2078.
- Tong X, Ma Y, Zhou Q, et al. Serum and tissue leptin in lung cancer: a meta-analysis. Oncotarget. 2017;8(12):19699. doi: 10.18632/oncotarget.14963
- Bocian-Jastrzębska A, Malczewska-Herman A, Kos-Kudła B. Role of leptin and adiponectin in carcinogenesis. Cancers (Basel). 2023;15(17):4250. doi: 10.3390/cancers15174250
- Greco M, De Santo M, Comandè A, et al. Leptin-activity modulators and their potential pharmaceutical applications. Biomolecules. 2021;11(7):1045. doi: 10.3390/biom11071045
- Wang J, Zhou F, Li F, et al. Autocrined leptin promotes proliferation of non-small cell lung cancer (NSCLC) via PI3K/AKT and p53 pathways. Ann Transl Med. 2021;9(7):568–568. doi: 10.21037/atm-20-7482
- Nigro E, Perrotta F, Monaco ML, et al. Implications of the adiponectin system in non-small cell lung cancer patients: A case-control study. Biomolecules. 2020;10(6):926. doi: 10.3390/biom10060926
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2(8):1014–1022. doi: 10.1001/jamaoncol.2016.0173
- Sudan SK, Deshmukh SK, Poosarla T, et al. Resistin: an inflammatory cytokine with multi-faceted roles in cancer. Biochimica Et Biophysica Acta (BBA) - Rev Cancer. 2020;1874(2):188419. doi: 10.1016/j.bbcan.2020.188419
- Kuo CH, Chen K-F, Chou S-H, et al. Lung tumor‐associated dendritic cell‐derived resistin promoted cancer progression by increasing Wolf–Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis. 2013;34(11):2600–2609. doi: 10.1093/carcin/bgt281
- Nowinska N, Jablonska J, Pawelczyk P, et al. Expression of Irisin/FNDC5 in cancer cells and stromal fibroblasts of non-small cell lung cancer. Cancers (Basel). 2019;11(10):1538. doi: 10.3390/cancers11101538
- Tsiani E, Tsakiridis N, Kouvelioti R, et al. Current evidence of the role of the myokine irisin in cancer. Cancers (Basel). 2021;13(11):2628. doi: 10.3390/cancers13112628
- Shao L, Li H, Chen J, et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem Biophys Res Commun. 2017;485(3):598–605. doi: 10.1016/j.bbrc.2016.12.084
- Zhang D, Tan X, Tang N, et al. Review of research on the role of Irisin in tumors. OTT. 2020;Volume 13:4423–4430. doi: 10.2147/OTT.S245178
- Pinkowska A, Podhorska-Okołów M, Dzięgiel P, et al. The role of irisin in cancer disease. Cells. 2021;10(6):1479. doi: 10.3390/cells10061479
