 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nephrotic syndrome (NS) is one of the most commonly diagnosed glomerular disorders, particularly in children and adolescents. The study aimed to explore the link between uteroglobin gene polymorphism rs41364547 (G38A) and serum uteroglobin level with the risk of nephrotic syndrome in Egyptian children. The current case-control research involved 100 children with NS and 100 healthy, age- and gender-matched controls. They were grouped into 45 steroid-sensitive (SSNS) and 55 steroid-resistant (SRNS) groups. The subjects were evaluated for uteroglobin (G38A) polymorphism using TaqMan genotyping and an ELISA to determine uteroglobin expression. There was a non-statistically relevant association between uteroglobin alleles, genotypes, and NS (P = 0.149, 0.288, respectively). Furthermore, there was no detectable variation in the genotypes and uteroglobin alleles between SSNS and SRNS (P = 0.803 and 0.8, respectively). In NS patients, uteroglobin levels were statistically lower than controls (p < 0.001). According to the ROC curve study, uteroglobin was an excellent discriminant between NS patients and control participants, with an AUC of 0.997. Lower serum uteroglobin is helpful in the diagnosis and prediction of NS. There is no statistically significant difference in the genotype distribution and allele frequencies between NS patients and the control subjects, as well as between SSNS and SRNS.
Introduction
Nephrotic syndrome (NS) is a major common pediatric glomerular illness, affecting between 1.15 and 16.9 per 100,000 children worldwide each year. It is distinguished by severe proteinuria, hypoalbuminemia, and/or edema [Citation1].
The exact cause of NS is unclear, but it is believed to be T lymphocyte dysfunction and abnormal glomerular permeability factor secretion, with recent studies suggesting B lymphocyte dysfunction [Citation2,Citation3]. In addition, several environmental, hereditary, and epigenetic factors influence the disease’s onset and progression [Citation4].
Corticosteroids serve as the cornerstone of treatment, with steroid response being the primary prognostic indicator. Most children respond to an oral steroid cycle and are diagnosed with steroid-sensitive nephrotic syndrome (SSNS). In contrast, others who do not respond are identified with steroid-resistant nephrotic syndrome (SRNS) [Citation5].
Uteroglobin (UG) protein is a steroid-inducible, multi-functional protein produced mainly in epithelial cells lining mucosal surfaces such as the lungs and trachea [Citation6]. It is found in blood and other body fluids, including urine. Mesangial cell receptors identify it even though the kidneys do not produce it [Citation7].
The human UG gene is situated on the long arm of chromosome 11, specifically at q12.3-q13.1, having three exons and two introns [Citation8]. Atopy and respiratory disorders are linked to this region. The guanine-to-adenine alteration at position 38 (G38A), which occurs downstream of the transcription start site inside the non-coding region of exon 1, is of special relevance among the many polymorphisms in the UG gene that have been found thus far [Citation6,Citation9].
Several clinical studies on UG gene polymorphism in IgA nephropathy (IgAN) patients have produced conflicting results. Some studies found the GG genotype more common in patients with progressive disease, while others found an increased risk of disease progression with increased ‘A’ allele numbers. Several inflammatory and autoimmune illnesses, including systemic lupus erythematosus, have been linked to the same variant in the UG gene [Citation10].
Furthermore, previous research revealed that recombinant uteroglobin is effective in treating human chronic glomerulonephritis and can prevent crescentic glomerulonephritis in an animal model [Citation11]. Also, a polymorphism in the 5’ UTR region of UG exon 1 is a key marker for IgAN development and may affect protein expression levels [Citation12]. These findings supported the suggestion that this UG SNP might be an additional genetic predisposition for developing NS.
The study aimed to explore the potential association of the uteroglobin gene single nucleotide polymorphism rs41364547 (G38A) and serum uteroglobin level with the risk of developing nephrotic syndrome in Egyptian children.
Material and methods
Study population
This case-control study included 100 Egyptian children with nephrotic syndrome fulfilling the diagnostic criteria of the International Study of Kidney Disease in Children (ISKDC) criteria [Citation13]. The patient group was initially treated with corticosteroids. Based on their responses, they were divided into 45 steroid-sensitive (SSNS) and 55 steroid-resistant (SRNS) groups. Some of them have received alternative agents such as MMF and/or calcineurin inhibitors, especially those with frequent relapse and SRNS. The patients involved in this research are hospitalized, either as recurrence or first-diagnosed.
The control group consisted of 70 children followed up in the Well-Child Outpatient Clinic with normal renal function and normotension, who had no other known illness (such as allergy or autoimmune disease).
The control group consisted of 70 children followed up in the Well-Child Outpatient Clinic with normal renal function and normotension, who had no other known illness (such as allergy or autoimmune disease).
The control group included 100 age- and sex-matched healthy children. They have normal kidney function, normal blood pressure, and no autoimmune or allergic disease.
The patients were recruited from Mansoura University Children’s Hospital from November 2020 to November 2021. All participants were of the same ethnic origin and declared to be of Egyptian ethnicity. Signed, informed written consent from all subjects was obtained. The protocol of the study was approved by the Institutional Research Board of the Faculty of Medicine, Mansoura University (approval number: MS.20.10.28).
The inclusion criteria consist of the following:
age ≤18 years
Meeting the diagnostic criteria of ISKDC
Exclusion criteria:
Age >18
Children with systemic diseases such as infectious diseases, liver diseases, or other kidney diseases
Children with inflammatory diseases such as IgA nephropathy and pulmonary active diseases
Children with autoimmune disease.
Demographic, clinical, and laboratory data
The demographic data about the patients was collected, including their sex as well as their age.
Diagnostic evaluation is dictated by the findings on history, general, physical (eg. Edema), clinical examination (eg. Blood pressure), and radiological investigation (eg. Ultrasonography). Among 100 patients, 50 subjects had a biopsy. The laboratory evaluation included urine analysis, serum albumin, serum creatinine, protein/creatinine ratio, lipid profile (Cholesterol and Triglyceride), and complement (C3).
Samples collection
Five milliliters of whole blood were drawn from each participant. For DNA extraction, 2 ml were put into EDTA-treated tubes and kept at −20°C. The remaining 3 milliliters were collected on a plain tube and allowed to stand for 15 to 30 minutes at room temperature and then centrifuged for 10 minutes to separate serum. The serum was kept at −20°C until used for uteroglobin assay using ELISA.
Estimation of serum uteroglobin level
A commercially available ELISA kit has been used for measuring Uteroglobin serum levels due to the manufacturer’s instructions (Inova, cats. No: In-Hu4111, China).
DNA extraction and genotyping of the uteroglobin gene (rs41364547)
DNA was extracted from whole blood using GeneJET Purification Mini Kit (Thermo Scientific, USA, Cat. no. #K0781), following the manufacturer’s instructions. NanoDrop™ 2000 (Thermo Scientific, Wilmington, DE) has been used to determine the quality and amount of the DNA. A score of 1.8–2 was judged high quality.
Real-time polymerase chain reaction with Taq Man allelic discrimination assay has been used. Predesigned primer and probe sets were utilized (Applied Biosystems, USA). Probes were created with a reporter dye FAM or VIC covalently bonded at the 5´end and a quencher dye MGB coupled to the 3´end of the probe. A mixture of 200 ng/μL genomic DNA, 10 μL of 1X Taq-Man Master Mix, 1 μL of 1X TaqMan Genotyping Assay, and nuclease-free water was used to start up the reaction. We applied the following thermal cycling parameters: 40 cycles of 95°C for 15 seconds, 60°C for 1 minute, and 95°C for 10 minutes. The last extension was programmed for 30 seconds at 60°C.
Hardy – Weinberg was calculated in each group to ensure that the study groups were in equilibrium using the following Hardy – Weinberg equilibrium equation:
P is defined as the frequency of the dominant allele
q is defined as the frequency of the recessive allele
Statistical analysis
IBM-SPSS software (IBM Corp., Released in 2017) was utilized to analyze the obtained data. The qualitative data, expressed as n (%), was examined by Pearson’s chi-square. To determine normality in quantitative data, Shapiro-Wilk’s test was performed. Results were presented as median and interquartile for non-parametric data and mean ± SD for parametric data. Tests used for comparing two sets of quantitative data were the Mann-Whitney U test for non-parametric data and the Student t-test for parametric one. The predictors for steroid resistance were identified using univariate and multivariate logistic regression analyses. The diagnostic power of every test was ascertained using the receiver operating characteristic (ROC) curve. A statistically relevant p value < 0.05 was considered.
Results
Characteristics of the study population
A total of 200 participants were enrolled in the study. The patient group included 100 INS patients, 53 female (53%) and 47 male (57%) with a mean age of 8.5 ± 3.8 years. The control group consisted of 100 young, healthy individuals, 45 females (45%) and 55 males (55%); their average age was 9.6 ± 4.9 years. The two groups had similar age and sex distributions (p = 0.258 and 0.166, respectively). Their median age at onset was 4 years (IQR = 2.4–7.1) and the median duration of the disease was 2.5 years (IQR = 1–5). As regards steroid treatment response, 45 (45%) were steroid sensitive (SSNS) and 55 (55%) were steroid resistant (SRNS). SRNS cases were significantly associated with older age, higher SBP, higher DBP, longer duration of disease onset, and renal failure as compared to SSNS (p = 0.001, 0.004, 0.002, 0.039, and 0.008, respectively). Most patients with a longer duration of disease onset are in a recurrency state. There was no statistically significant difference between SSNS and SRNS regarding the laboratory data. Among all the studied NS cases, 50 were subjected to renal biopsy. No significant association was found regarding steroid response with biopsy results ().
Table 1. Demographic, clinical, and laboratory data of the study NS patients (2).
Serum uteroglobin level
Serum uteroglobin levels were statistically significantly lower in NS patients (p < 0.001) than in controls. In addition, there was a significant decrease in serum uteroglobin levels in SSNS and SRNS cases compared to the control group (p < 0.001). However, no significant difference was found in serum uteroglobin level between SSNS and SRNS (p = 0.537) ().
Table 2. Comparison of serum uteroglobin level among studied groups.
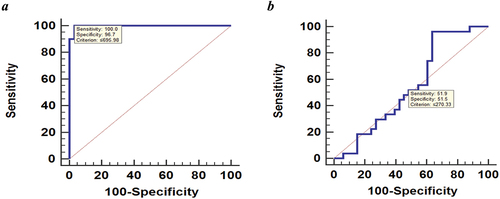
The ROC curve analysis was conducted for discrimination between the NS and control groups; high accuracy AUC (0.997), at the best cutoff value of ≤695.98 pg/ml, with sensitivity 100% and specificity 96.7%), and between SSNS and SRNS; Low accuracy AUC was found (0.547), at the best cutoff value of ≤270.33 pg/ml, with sensitivity 51.9% and specificity 51.5%) ().
Figure 1. ROC of serum uteroglobin level for discrimination between (a) NS and control groups; high accuracy AUC (0.997), at the best cutoff value of ≤695.98 pg/ml, with sensitivity 100% and specificity 96.7%, (b) SSNS and SRNS; Low accuracy AUC was found (0.547), at the best cutoff value of ≤270.33 pg/ml, with sensitivity 51.9% and specificity 51.5%.
Genotyping of the uteroglobin (G38A) polymorphism
The genotype distribution and allele frequencies did not differ significantly between the NS group and controls (p = 0.288 and 0.149, respectively). Both the NS and control groups achieve Hardy-Weinberg equilibrium (HWE). The actual test findings for HWE (p values = 0.757 and 0.256, respectively) reveal statistical insignificance. After classifying NS patients based on steroid responsiveness, uteroglobin genotypes, and allele frequencies did not differ significantly between SRNS compared to SSNS cases (p = 0.8 and 0.803, respectively) (). Moreover, no significant association was found regarding serum uteroglobin level with uteroglobin gene (G38A) genotypes in the dominant model among INS cases (p = 0.837) as well as in controls (p = 0.522) ().
Table 3. Distribution of uteroglobin alleles and genotypes in all studied groups.
Table 4. Association of serum uteroglobin level with uteroglobin gene (G38A) genotypes in dominant model among NS cases and controls.
Predictors of steroid resistance among INS cases
Logistic regression analysis was conducted for the prediction of INS susceptibility. Lower serum uteroglobin was found to be a risk predictor of NS susceptibility (p = 0.013). Regression analysis was directed for the prediction of steroid resistance among NS cases. Participants with older age, higher SBP, higher DBP, and longer disease duration were significantly associated with the risk of steroid resistance among NS cases. However, on conducting multivariable analysis, only longer disease duration was considered a risk predictor of steroid resistance ().
Table 5. Regression analysis for prediction of steroid resistance among NS cases.
Discussion
One of the most frequent kidney illnesses in children is nephrotic syndrome (NS). It is distinguished by significant proteinuria, pitting edema, hypoalbuminemia, and hyperlipidemia [Citation14]. This study explored the potential association of the uteroglobin gene single nucleotide polymorphism rs41364547 (G38A) and serum uteroglobin level with the risk of developing nephrotic syndrome in Egyptian children.
Uteroglobin protein is an immunomodulatory agent that inhibits neutrophil and monocyte function, prevents immune recognition, and has anti-inflammatory and anti-chemotactic properties. It inhibits phospholipase A2 activity, limits arachidonic acid metabolism, and reduces prostaglandin and leukotriene mediators, which are linked to glomerular disease pathogenesis [Citation15]. Uteroglobin protein also has a high affinity for fibronectin (Fn) and forms Fn-uteroglobin heterodimers, which counteract the Fn-collagen interactions required for abnormal deposition in some diseases [Citation16].
Our results showed no statistically significant difference in UG gene (G38A) polymorphism between NS cases and the control group. Also, UG gene G38A genotypes and allelic frequencies did not differ significantly between SRNS compared to SSNS cases.
A study by Demircioglu Kilic et al. [Citation17] in Turkish children reported a significantly increased risk of INS and UG G38A polymorphisms. Children with the AA genotype had a nearly 4-fold greater chance of developing INS, whereas SRNS had a 4.8-fold increased risk. They also showed no statistically significant difference between the SRNS and SSNS groups as regards the UG G38A polymorphism.
In our study, the ‘G’ allele was predominant in both the NS and control groups. The ‘G’ allele is the wild allele. This difference in allele frequencies between our study and Demircioglu Kilic et al. [Citation17] study may be attributed to the different ethnicity and environmental factors in combination with the genetic background toward the risk of the disease. Over the past few decades, there has been minimal evidence of a relationship between the uteroglobin G38A gene polymorphism and NS, due to the paucity of research linking the uteroglobin G38A gene polymorphism and the risk of NS.
IgA nephropathy (IgAN) risk and UG G38A gene polymorphism have gained a lot of interest, and several studies investigating the association of UG G38A gene polymorphism with IgAN risk revealed inconsistent findings. Studies by Lü et al. [Citation18] and Lim et al. [Citation19] concluded no association between UG G38A gene polymorphism and IgAN risk. A meta-analysis study by Lin et al. [Citation20] that included 7 case-control studies, containing 1197 patients with IgAN and 1083 controls, also came to the same conclusion of no association between UG G38A gene polymorphism and IgAN risk. All these studies share the same findings as ours: there is no association between UG G38A gene polymorphism and the risk of renal disorder.
Numerous studies have investigated the link between uteroglobin G38A polymorphism and pulmonary disorders, particularly bronchial asthma. Nie et al. [Citation21] found a significant increase in asthma risk, while Cheng et al. [Citation22] and Gribben et al. [Citation23] found no association between UG G38A polymorphism and asthma susceptibility, based on 15 case-control studies and 1,623 asthma cases and 3,294 controls.
In this study, the serum level of UG protein in NS cases was significantly lower than in the control group. No significant association was also found regarding serum uteroglobin level and steroid treatment response (SRNS and SSNS). In serum, UG protein concentration is determined by both the production mainly from the lung and the clearance by the kidney due to its small size [Citation24], so the lower level of serum UG protein in NS patients may be attributed to the protein-losing nature of the disease (mainly albuminuria), especially with UG protein’s small size (16 kDa) compared to albumin (66.4 kDa) [Citation25].
In a study by Zhu et al. [Citation26], IL-4 and IL-13 were found to repress the expression of the UG protein; these cytokines are associated with the pathophysiology of NS [Citation27,Citation28]. So, the low level of serum UG protein may be a result of the combined effect of cytokines lowering UG expression as well as the excretion of UG protein in the urine; this needs more investigation.
Many studies have investigated the relationship between uteroglobin protein serum levels and pulmonary disorders. Lower serum levels of UG protein were reported in COPD [Citation29,Citation30] and bronchial asthma [Citation31], while higher serum levels were reported in ARDS [Citation32,Citation33]. Studies have demonstrated that UG levels were affected by disease duration, also Clara cells are sensitive to direct lung injury (as they are non-ciliated and non-mucus secretary cells), which may explain the high levels in ARDS (due to initial cell irritation) and lower levels in chronic disorders such as asthma and COPD [Citation34]. A limited sample size, a single-center study design, and consideration of a single SNP were limitations of this study.
Conclusion
To the best of our knowledge, our study is the first to investigate the relationship between uteroglobin serum levels and INS risk. Lastly, lower serum uteroglobin is a risk predictor of NS susceptibility. Furthermore, our findings indicate a poor association between uteroglobin gene variants and steroid resistance risk in the Egyptian population.
Recommendation
Considering the research findings, we recommend more studies based on larger sample sizes and more ethnic groups, assessing urine and serum UG protein levels to eliminate the protein-losing effect of INS on UG protein levels, and studying the correlation between inflammatory cytokines and UG gene expression.
Abbreviations
| IgAN | = | IgA nephropathy |
| INS | = | Idiopathic nephrotic syndrome |
| SSNS | = | Steroid Sensitive Nephrotic Syndrome |
| SRNS | = | Steroid Resistance Nephrotic Syndrome |
| UG | = | Uteroglobin |
Author contributions
SA and HM designed the research. MS and ME performed the experiments. The final version of this paper was reviewed and approved by all authors, who also collaborated in data analysis and text writing.
Availability of data and materials
The data that support the findings of this study are accessible from the corresponding author upon request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Mansoura Faculty of Medicine with approval number [MS.20.10.28].
Informed written consent was obtained from all participants included in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Trautmann A, Boyer O, Hodson E, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2023;38(3):877–919. doi: 10.1007/s00467-022-05739-3
- Chen J, Qiao XH, Mao JH. Immunopathogenesis of idiopathic nephrotic syndrome in children: two sides of the coin. World J Pediatr. 2021;17(2):115–122. doi: 10.1007/s12519-020-00400-1
- Liu J, Guan F. B cell phenotype, activity, and function in idiopathic nephrotic syndrome. Pediat Res. 2022;31:1–9.
- Yang F, Lai X, Deng L, et al. Association of endothelin-1 gene polymorphisms with the clinical phenotype in primary nephrotic syndrome of children. Life Sci. 2014;118(2):446–450. doi: 10.1016/j.lfs.2014.04.010
- Vivarelli M, Gibson K, Sinha A, et al. Childhood nephrotic syndrome. Lancet. 2023;402(10404):809–882. doi: 10.1016/S0140-6736(23)01051-6
- Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocrine Rev. 2007;28(7):707–725. doi: 10.1210/er.2007-0018
- Menegatti E, Nardacchione A, Alpa M, et al. Polymorphism of the uteroglobin gene in systemic lupus erythematosus and IgA nephropathy. Lab Invest. 2002 May;82(5):543–546. doi: 10.1038/labinvest.3780448
- Chen LC, Tseng HM, Wu CJ, et al. Evaluation of a common variant of the gene encoding Clara cell 10 kd protein (CC10) as a candidate determinant for asthma severity and steroid responsiveness among Chinese children. J Asthma. 2012;49(7):665–672. doi: 10.3109/02770903.2012.697954
- Candelaria PV, Backer V, Laing IA, et al. Association between asthma-related phenotypes and the CC16 A38G polymorphism in an unselected population of young adult Danes. Immunogenetics. 2005;57(1–2):25–32. doi: 10.1007/s00251-005-0778-2
- Menegatti E, Davit A, Francica S, et al. Genetic factors associated with rheumatoid arthritis and systemic vasculitis: evaluation of a panel of polymorphisms. Dis Markers. 2009;27:217–223. doi: 10.1155/2009/435108
- Lee DS, Yang SH, Kim HL, et al. Recombinant uteroglobin prevents experimental crescentic glomerulonephritis. Kidney Int. 2004;66(3):1061–1067. doi: 10.1111/j.1523-1755.2004.00855.x
- Kim YS, Kang D, Kwon DY, et al. Uteroglobin gene polymorphisms affect the progression of immunoglobulin a nephropathy by modulating the level of uteroglobin expression. Pharmacogenetics. 2001 Jun;11(4):299–305. doi: 10.1097/00008571-200106000-00004
- International Study of Kidney Disease in Children (ISKID). The primary nephrotic syndrome in children identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the international study of kidney disease in children. J Pediatr. 1981;98(4):561–564. doi: 10.1016/S0022-3476(81)80760-3
- Prince S, Naresh K, Tulasi R. Case Report on Paediatric Nephrotic Syndrome. EJIFCC. 2020 Jun 2;31(2):164–168.
- Dadar M, Shahali Y, Chakraborty S, et al. Antiinflammatory peptides: current knowledge and promising prospects. Inflammation Res. 2019;68(2):125–145. doi: 10.1007/s00011-018-1208-x
- Zhang Z, Kundu GC, Zheng F, et al. Insight into the physiological function(s) of uteroglobin by gene-knockout and anti-sense transgenic approaches. Ann NYA Cad Sci. 2000;923(1):210–233. doi: 10.1111/j.1749-6632.2000.tb05532.x
- Demircioglu Kilic B, Buyukcelik M, Balcı S, et al. Uteroglobin gene polymorphism (G38A) may be a risk factor in childhood idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33(2):295–303. doi: 10.1007/s00467-017-3800-7
- Lü JC, Zhang H, Chen YQ, et al. Uteroglobin G38A polymorphism is associated with the progression of IgA nephropathy in Chinese patients. Zhonghua Nei Ke Za Zhi. 2004;43(1):37–40.
- Lim CS, Kim SM, Oh YK, et al. Association between the Clara cell secretory protein (CC16) G38A polymorphism and the progression of IgA nephropathy. Clin Nephrol. 2007;67(2):73–80. doi: 10.5414/CNP67073
- Lin D, Li S, Xu H, et al. Association of uteroglobin G38A gene polymorphism with IgA nephropathy risk: an updated meta-analysis. J Recept Signal Transduction Res. 2015;35(2):115–121. doi: 10.3109/10799893.2014.936460
- Nie W, Xue C, Chen J, et al. Secretoglobin 1A member 1 (SCGB1A1)+ 38A/G polymorphism is associated with asthma risk: A meta- analysis. Gene. 2013 Oct 10;528(2):304–308. doi: 10.1016/j.gene.2013.06.049
- Cheng D, Di H, Xue Z, et al. CC16 gene A38G polymorphism and susceptibility to asthma: an updated meta-analysis. Intern Med. 2015;54(2):155–162. doi: 10.2169/internalmedicine.54.2979
- Gribben KC, Wyss AB, Poole JA, et al. CC16 polymorphisms in asthma, asthma subtypes, and asthma control in adults from the agricultural lung health study. Respir Res. 2022;23(1):1–1. doi: 10.1186/s12931-022-02211-6
- Zhu L, Di PY, Wu R, et al. Repression of CC16 by cigarette smoke (CS) exposure. PLOS ONE. 2015;10(1):e0116159. doi: 10.1371/journal.pone.0116159
- Hermans C, Petrek M, Kolek V, et al. Serum Clara cell protein (CC16), a marker of the integrity of the air-blood barrier in sarcoidosis. Eur Respir J. 2001 Sep 1;18(3):507–514. doi: 10.1183/09031936.01.99102601
- Zhu L, An L, Ran D, et al. The club cell marker SCGB1A1 downstream of FOXA2 is reduced in asthma. Am J Respir Cell Mol Biol. 2019 Jun;60(6):695–704. doi: 10.1165/rcmb.2018-0199OC
- Kim AH, Chung JJ, Akilesh S, et al. B cell-derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight. 2017 11;2(21). doi: 10.1172/jci.insight.81836
- Stangou M, Spartalis Μ, Daikidou DV, et al. Impact of Τh1 and Τh2 cytokines in the progression of idiopathic nephrotic syndrome due to focal segmental glomerulosclerosis and minimal change disease. J Nephropathol. 2017;6(3):187. doi: 10.15171/jnp.2017.32
- Park HY, Churg A, Wright JL, et al. Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188(12):1413–1419. doi: 10.1164/rccm.201305-0892OC
- Guerra S, Halonen M, Vasquez MM, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015 Aug 1;3(8):613–620. doi: 10.1016/S2213-2600(15)00196-4
- Guerra S, Vasquez MM, Spangenberg A, et al. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol. 2016 Sep 1;138(3):932–934. doi: 10.1016/j.jaci.2016.03.047
- Lesur O, Langevin S, Berthiaume Y, et al. Critical care research group of the Québec respiratory health network. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive care Med. 2006;32(8):1167–1174. doi: 10.1007/s00134-006-0235-1
- Lin J, Zhang W, Wang L, et al. Diagnostic and prognostic values of Club cell protein 16 (CC 16) in critical care patients with acute respiratory distress syndrome. J Clin Lab Analysis. 2018;32(2):e22262. doi: 10.1002/jcla.22262
- Almuntashiri S, Han Y, Zhu Y, et al. CC16 Regulates Inflammation, ROS generation and apoptosis in bronchial epithelial cells during Klebsiella pneumoniae infection. Int J Mol Sci. 2021;22(21):11459. doi: 10.3390/ijms222111459
