ABSTRACT
The prenatal and postnatal growth of offspring may be adversely impacted by maternal stress experienced during pregnancy. The objective of this study is to investigate the adverse effects of immobilization stress (IS) on pregnant rats and the possible complications on their offspring. Thirty female dams were randomly divided into two groups (15/group), the control group and the IS group. At gestation day 20, and post natal day7 (PND -7) the levels of serum serotonin, cortisol, and insulin and plasma glucose were assessed in dams. The congenital anomalies of offspring were also investigated. At PND-21, the brain and heart were excised from both mothers and their pups for histological and ultrastructural investigations. The results revealed significant decrease in serotonin and insulin levels, while an increase in cortisol and glucose levels in dams exposed to IS. Also, there was a remarkable reduction in the body weight of mother’s rats and their pups compared to the control. Moreover, exposure of pregnant rats to IS caused relative abortion (26%), and congenital anomalies (9.1%) along with histopathological signs in brain and cardiac tissues in mothers and their pups. Stress during pregnancy resulted in adverse effects on brain and heart in mothers and their pups.
Introduction
Embryonic development is very sensitive to action of environmental maternal factors (drugs, diet, oxidative stress and many others), maternal status and others stressors, which can increase the likelihood of the newborn in developing diseases during pre- and post-natal life [Citation1]. Also, the viability of developing fetuses relies on the preservation of homeostasis [Citation2]. As reported, stress during gestation is considered as an epigenetics factor which is capable of inducing alterations in brain structure and function [Citation3]. Additionally, it is significantly contributing to the onset of cardiovascular and psychiatric disorders [Citation4].
Stress is defined as the ‘unspecific reaction of the body to any challenge requiring adaptation or modification’ [Citation5]. Different forms of stress like anger, anxiety, depression, malnutrition, sudden changes of life and socio-economic factors that can induce adverse effects on women had been reported [Citation6,Citation7]. The stress response proceeds by activating the hypothalamic-pituitary-adrenal (HPA) axis, which causes a release of glucocorticoids especially cortisol hormone in some animals and human [Citation8]. Excess liberation of cortisol hormone during gestation was found to induce a range of adverse effects on the development of fetuses [Citation9] and may be implicated in promoting spontaneous abortion [Citation10]. Bennett et al. revealed that the prevalence of depression during pregnancy has been reported between 10% and 16%, and it is increasingly becoming a serious health concern [Citation11]. Furthermore, reports on humans and experimental animals have investigated the hazard effects of stress on mothers, fetus and birth outcomes [Citation11,Citation12].
Laboratory studies have shown that the immobilization stress (IS) model of depression is widely acknowledged for conducting psychological stress studies, the IS paradigm involves subjecting the mice to repeated daily episodes of movement restrictions that directly enhance the neuroendocrine system [Citation13,Citation14].
Frequently, the maternal stress during gestation is accompanied by cardiovascular diseases (CVD). In the same context, maternal CVD impacts the uteroplacental circulation, which is vital for the survival and development of the fetus, leading to placental insufficiency that influences offspring development [Citation15]. Additionally, maternal stress can cause alteration of placental functions and perfusion [Citation16]. Furthermore, the endocrine alterations linked to prenatal stress influence the synthesis of placental serotonin, which plays a crucial role in fetal brain development [Citation17].
Serotonin is known as 5-hydroxytryptamine (5-HT), serves as a monoamine neurotransmitter. Its biological role is intricate and diverse, influencing mood, cognition, reward, learning, memory, as well as various physiological processes [Citation18]. 5-HT plays an effective role in the regulation and function of the adult human brain. It also helps in regulating central nervous system development of fetuses [Citation19,Citation20]. Schlienger and Meier reported that, rat model of serotonin deficiency recapitulates many several features of effects of prenatal maternal stress on serotonin and fetal development [Citation21]. Serotonin deficiency in early embryogenesis can cause neurogenesis disorders and defects in neurotransmitter system development. These effects induce many neuro-functional disorders [Citation22].
Herein, we aimed to investigate the impact of immobilization stress on pregnant rats and their offspring’s, and how that affect the developing brain and heart.
Material and methods
Animal experiment
For this experiment, a total of thirty adult female rats (weighing 150–160 g), were obtained from MRC, Deanship of Scientific Research, Jazan University, Saudi Arabia. After acclimatization for one-week female rats were mated with males. Conception was confirmed by observation of vaginal plug at the morning (8 am) and examination of vaginal smear. At fourth day of gestation, the pregnant rats were randomly divided into two groups (n = 15); control and IS group. All procedures were performed in accordance with the guidelines of Standing Committee for Scientific Research – Jazan University (HAPO-10-Z-006) (HAPO-10-Z-001).
Induction of Immobilization stress
A standardized rodent-sized immobilization bags (Braintree Scientific, Inc. Cat# DC200, Braintree, MA, USA) were used to immobilize the pregnant rats. Briefly, at the fourth day of gestation, 15 rats were subjected to three 60-min episodes of immobilization stress at 9 a.m., 1 p.m., and 5 p.m daily till the end of gestation period [Citation23,Citation24].
Samples collection
At 20th day of gestation, five pregnant rats from each group were dissected to check the characteristic morphology of uterus as well as to investigate the gross morphology and anatomy of embryos. To estimate the biochemical parameters, the blood samples were collected from the dissected mother’s rats at 20th day of gestation and from the lived rats through the tail vein at postnatal day 7 (PND7). The blood samples were taken in sodium fluoride tube and serum was separated from the other part on serum separation tube (ss tube) and kept frozen at (−20°C) until use. Furthermore, the fetuses were dissected at PND7, 14 and 21 to obtain the brain and heart for histological investigation. Other samples were processed for immunohistochemical and ultrastructural investigations at PND21.
Investigated parameters
Monitoring food intake of the pregnant dams
To determine the food consumption during the gestation and lactation periods, the amount of food placed in the cages at the beginning of a 24-hour cycle was weighed. The weight of the remaining food at the end of each 24-hour cycle was subtracted, and this process was repeated daily. The average weekly food consumption was then calculated. Additionally, the cage floors were examined for any food pellets that may have been spilled, which were collected and measured [Citation23].
Recording of aborted mothers and congenital anomalies of offspring’s
The total number of aborted (manifested by heavy white secretions and the embryos live for few minutes, these mostly recorded in day 12 and 14) and pregnant mother’s rats was recorded along the gestation period. The offspring of the two groups were examined for morphological abnormalities, also for skeletal abnormalities by staining with Alizarin red stain according to the protocol reported by Weesner and Parry [Citation25].
Estimation of body weight changes
The body weight changes of the experimental groups of mother’s rats and their fetuses were recorded once every week at PND7, 14 and 21.
Biochemical assays
The levels of serum insulin, cortisol, serotonin, and blood glucose were estimated at 20th day of gestation and PND7 for mother’s rats. The level of serum serotonin was measured using a commercial enzyme linked immunoassay (ELISA) kit that was purchased from USA Immuno-Biological Laboratories, Inc. - IBL – Minneapolis. The level of serum cortisol was measured using chemiluminescent enzyme immunoassay (IMMULITE 2000 ACTH and IMMULITE2000 Cortisol, Siemens Healthcare Diagnostics Inc., CA, USA) [Citation26]. The level of serum insulin was estimated by ELISA Kit purchased from Boehringer Mannheim, Germany, using Boehringer analyzer ES300. The level of plasma glucose was estimated using SPINREACT diagnostics kit, Spain, where the intensity of the color formed was proportional to the glucose concentration in each sample [Citation27].
Histological and transmission electron microscopic investigations
The brain and heart of both mothers and their offspring were processed and stained by hematoxylin and eosin for histological investigations. This was focused for offspring at PND7, 14 and 21 while for mothers at PND21 (at the end of lactation period) [Citation28]. For ultrastructural investigations, pieces from the cerebellum and ventricular muscles were processed at PND21 for both mothers and their offspring using TEM (Joel 100CXl, Musashino 3- chome; Akishima, Tokyo, Japan, TEM unit, Mansoura University).
Immunohistochemical labeling of synaptophysin
Immunohistochemical labeling of synaptophysin was focused only on the cerebellar cortex of mother’s rats and their pups at PND21. Briefly, the de-paraffinized 5–6 μm sections of cerebellar cortex were cut with LeicaRM2125 (RTS microtome Germany). Immunohistochemical detection of Synaptophysin was done using a rabbit polyclonal primary antibody (PA5–27286, Thermo Scientific, USA), diluted 1:400 at 4°C, exposure for 20 h, in a moist chamber. A Thermo Scientific Super PictureTM Polymer Detection Kit was used to detect primary antibody binding [Citation29]. So, the expression of synaptophysin in the cerebellar cortex was assessed. Prepared slides of cerebellar tissue were examined using an Axioscop 2 plus microscope (Zeiss, Germany) and microphotographed using a Leica DFC 320 digital camera (Leica, Germany) to evaluate the immunohistochemical intensity of synaptophysin.
Statistical analysis
The statistical analysis tool SPSS (version 19), one-way ANOVA used to describe the data as means ±standard error. Different superscript letters in the same row of means indicate substantially different values (p < 0.05). When p < 0.05, *** Significant at P-value ≤0.001 ** Significant at P-value ≤0.01 and *Significant at P-value ≤0.05.
Results
Changes in food intake during gestation and lactation periods
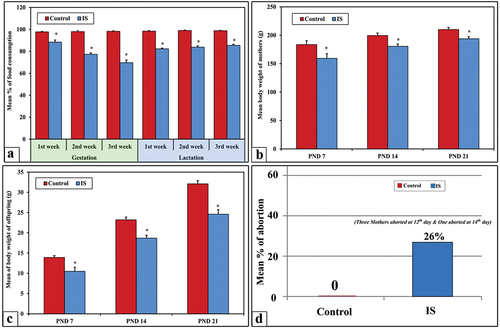
During the gestation and lactation period, control pregnant rats exhibited a consistent mean percentage of food consumption which was approximately 98% ±0.59 throughout the entire period. On the other hand, the immobilized stressed (IS) pregnant rats revealed gradual significant decrease in the mean % value of food intake as shown in .
Figure 1. The mean % value of food consumption among the control and the IS group of mothers rats (panel a), changes in body weight (g) of both mother’s rats (panel b), and their offspring (panel c), and percentage of abortion (panel d). Note a highly significant decrease (*) in food consumaption and body weight among IS rats comparatively with control.
Body weight gain of pregnant mothers
The obtained results showed high significant decrease (p < 0.001) in the mean body weight of IS mother’s rats at PND7, 14 and 21 () while their pups () showed moderate significant decrease (p < 0.05) if compared with their corresponding control.
The ratio of aborted pregnant rats
No abortion was recorded among the control, however, the abortion incidence among IS pregnant was represented by 26% (4:15). Such abortion appeared at 12th day of gestation for 3 mother’s rats while the fourth abortion occurred at 14thh days ().
Morphological and anatomical changes of fetuses
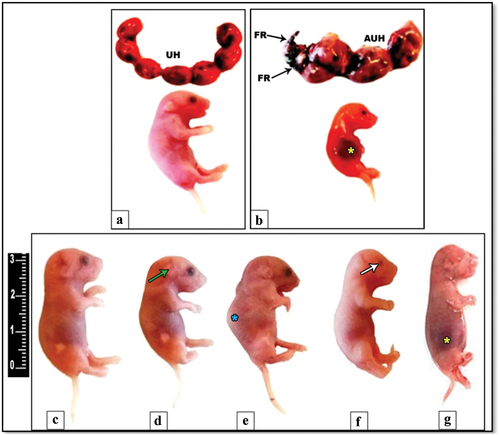
The obtained result revealed that one fetus from the aborted IS rats at 14th day of gestation showed one sign of congenital anomaly represented by subcutaneous hemorrhage. On the other side, the dissected five IS female rats at gestation day 20 revealed a total of 25 embryos, four of which (16%) showed pronounced morphological anomalies. Such anomalies included microtia, subcutaneous hemorrhage, microphthalmia and Kyphosis. Additionally, partial fetal resorption was recorded in the uterine horns of only in two IS pregnant rats ().
Figure 2. Photograph illustrating the uterine horns and congenital anomalies among the fetuses of control rat (a) and aborted IS (b) at 20th day of gestation. Note that the uterine horns (UH) and fetuses (image a) of control rats with normal anatomical pattern. On the other hand, a pronounced atrophied uterine horn (AUH) with partial fetus resorption (FR) appears in the IS rat (image b). Also, some fetuses from dissected IS rats at 20th day showing different morphological anomalies (images d–g) like microtia (green arrow), kyphosis (blue star), microphthalmia (white arrow) and subcutaneous hemorrhage in abdominal are (yellow star) with obvious hind limb paralysis. Image C representing the normal gross morphological pattern of fetuses taken from control.
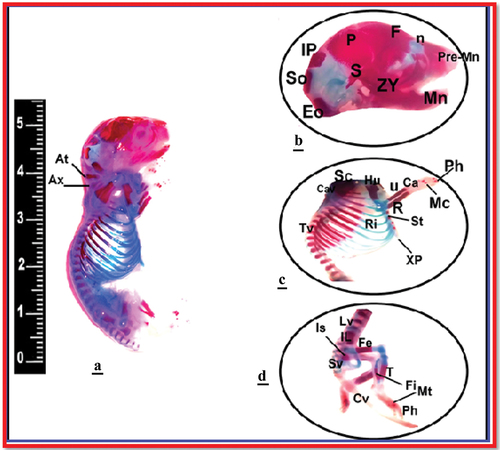
Anatomically, the long bones of only three fetuses maternally induced by immobilization stress revealed bone anomalies especially in skull and long bones. The skull appeared with atrophied nasal and occipital bones. Additionally, pronounced deformity was recorded in the skull, ribs, clavicle, and vertebrae (lumbar and sacral) as well as in the bones of lower limb (femur and tibia) ().
Figure 3. Photograph illustrating the congenital anomalies of bones among the fetuses of IS induced pregnant rats at 20th day of gestation. The skeleton of fetuses from control rats (image a), for IS group, skull occipital bones (image b), upper limbs, thoracic vertebrae, and ribs (image c), and lumber vertebrae and lower limbs (image d). (Alizarin-stained bony skeleton).
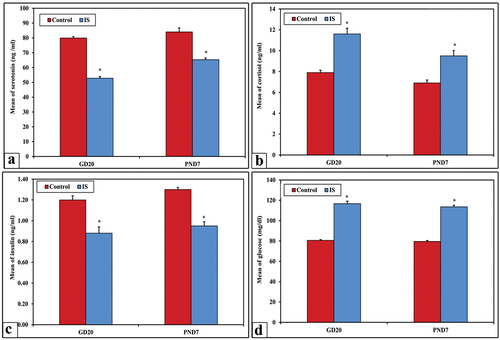
Biochemical assays of blood glucose, insulin, cortisol, and serotonin
The obtained data of biochemical blood analysis revealed high significant increase (p < 0.001) in the mean levels of blood glucose and cortisol among IS group of mother’s rats if compared with control. In contrast, a high significant decreased levels (p < 0.001) of serum insulin and serotonin were found among the IS group of mother’s rats unlike the control group ().
Histopathological and ultrastructural changes in the brain and heart
Brain
In control mother’s rats and their offspring at PND21, the cerebral cortex displayed normal histological distribution of pyramidal neurons, glial cells, and interstitial neuropil (). In IS group of mother’s rats, the cerebral cortex displayed hypertrophied pyramidal neurons infiltrated glial cells as well as pronounced thick-walled capillaries (), however the cerebral cortex of their offspring at PND21 revealed obvious giant hypertrophied pyramidal neurons and dilated cerebral capillaries ().
Figure 5. Images from histological sections of cerebral cortex of control and IS mother’s rats (panels a&b) and their offspring at PND21 (b&b1) respectively. The cerebral cortex of IS mothers rats and their offspring display obvious hypertrophied neurons (HN), dilated blood capillaries and infiltrated cells (star). (H&E stain, scale bar: 25µm). Abbreviations: Glial cells (GC), Pyramidal neurons (N) and Interstitial neuropils (NI), Blood capillaries (BC), Hypertrophied neurons (HN), Dilated blood capillaries (DBC), and Infiltrated neurons (asterisk).
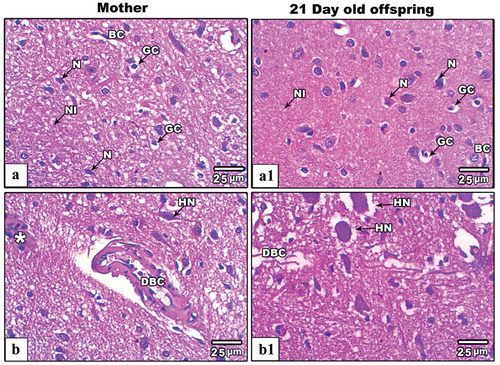
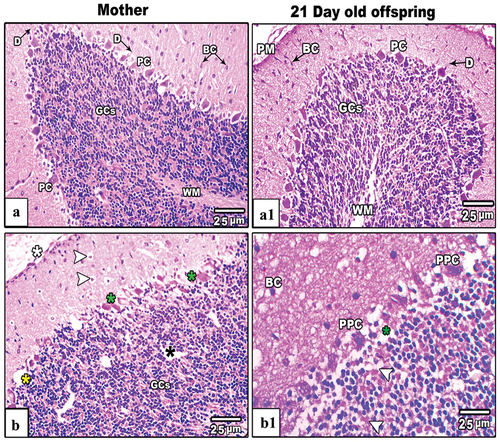
The cerebellum of control mother’s rats and their offspring at PND21, showed two distinctive well-organized layers; outer cortex (gray matter) and inner medulla (white matter). The cerebellar cortex, including the inner granular layer, the intermediate Purkinje layer, and the outer molecular layer, were observed in their typical arrangement. The granular layer, characterized by densely packed rounded and oval cells of varying sizes, exhibited a dark staining. The Purkinje cell layer, situated between the molecular and granular layers, consisted of a single layer of flask-shaped cells that were uniformly arranged, with basophilic nuclei deeply stained. Molecular layer cells were distributed sparsely. Axons, dendrites and capillaries penetrated deep into the layer as well (). Severe histopathological signs were recorded in the cerebellar cortex of IS mother rats these included fragmented pia matter, dislocated granular cell layer with little vacuolated cells, pyknotic Purkinje and basket cells (). Likewise, the cerebellar cortex of maternally IS induced offspring showed mild histopathological signs included little pyknotic Purkinje cells as well as mild dislocation of cerebellar layers ().
Figure 6. Images from histological sections of cerebellar cortex of control and IS mother’s rats (panels a&b) and their offspring at PND21 (b&b1) respectively. The cerebellar cortex of IS mothers rats and their pups showing fragmented pia matter (white asterisk), vacuolated basket and granular cells (white arrow heads), nonadjacent granular cels (black asterisk). Also, some Purkinje cells appear lysed (yellow asterisk), hypertrophied (green asterisks) and pyknotic (PPC). (H&E stain, scale bar: 25µm).
In control mothers’ rats () and their offspring at 21 days old (), the TEM results revealed that, the cerebellar cortex cells appeared ultra-structurally normal. In further details, the basket cells appeared with dark stained condensed nucleus, oval mitochondria with well-organized cristae, scattered rough endoplasmic reticulum (RER) and little lysosomes. Purkinje cell appeared huge with large centrally located nucleus and high density of rounded and elongated mitochondria as well as obvious scattered RER and smooth endoplasmic reticulum (SER). The granular cells appeared with darkly stained nuclei and granulated cytoplasm with tight junction among the cell membranes of adjacent cells. Additionally, the mitochondria and RER appeared rarely scattered in the cytoplasm in comparing with those found in Purkinje cells ().
Figure 7. Transmission electron micrograph (TEM) through the basket (panel a&a1), Purkinje (panel b&b1) and granular (panel c&c1) cells of the cerebellar cortex of control (c) and IS mother rats respectively. Cellular images from control cerebellar cortex (a–c) appear with normal nucleus (N) and nucleolus (Nu), mitochodria (M), RER (arrow heads), SER (arrow), lysosomes (L). The cellular images from IS cerebellar cortex (a1–c1) there are pyknotic nuclei (PN), vacuoalted cytoplasm (white asterisks), dilated and de-granulated RER (double arrow heads), dialted Golgi bodies (black asterisk) and atrophied and vacuolated mitochondria (zigzag arrows). (Scale bar is indicated in the lower left handed side for each image).
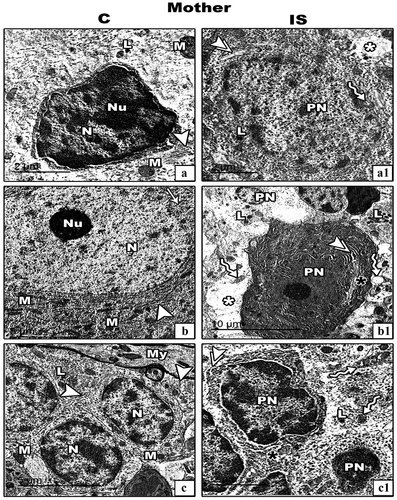
Figure 8. Transmission electron micrograph (TEM) through the basket (panel a&a1), Purkinje (panel b&b1) and granular (panel c&c1) cells of the cerebellar cortex of control and IS maternally induced rats offspring respectively. The cellular images from IS cerebellar cortex (a1-c1) there are pyknotic nuclei (PN), vacuoalted cytoplasm (white asterisks), dilated and de-granulated RER (double arrow heads), condensed Golgi bodies (black asterisk) and atrophied and vacuolated mitochondria (zigzag arrows). (Scale bar is indicated in the lower left handed side for each image).
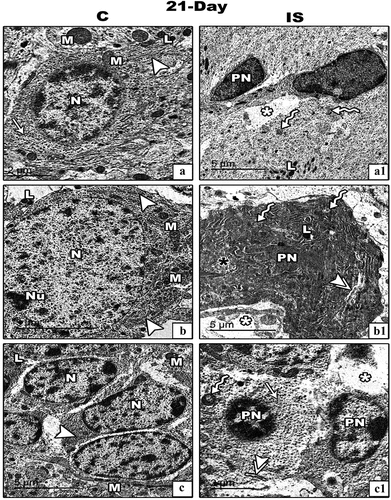
In immobilized stressed mothers’ rats () and their offspring (), all categories of cerebellar cortex cells appeared with remarkable deleterious ultrastructural changes. Such changes were represented by pyknotic nuclei and cytoplasm vacuolation as well as dilated and de-granulated RER. Also, most of mitochondria appeared with lost crista.
Changes in ventricular tissues of the heart
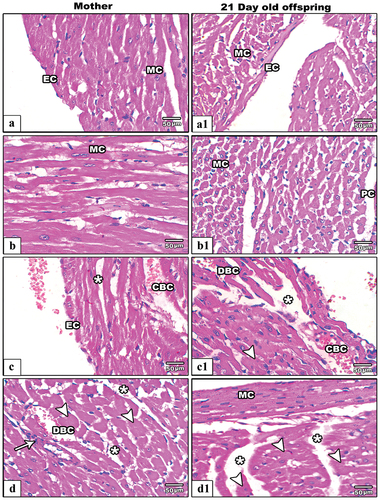
In control mother rats () and their pups at PND21 (), the ventricular sections appeared with normal histological architecture including intact layers of pericardium, endocardium, well striated myocardial muscles with normal striation and centrally located nuclei. On the other hand, several histological lesions were recorded among the ventricular histological sections of IS female rats. In IS pregnant rats, the ventricular cardiac sections showed severe hypertrophied muscle cells with multiple scattered necrotic nuclei and dilated coronary capillaries as well as the cardiac fibers appeared non-tight (). In 21-day old offspring from IS rats, the ventricular histological sections revealed deleterious changes included aggregated and hypertrophied myocardial cells with obvious loss of striation pattern. Additionally, the coronary capillaries appeared dilated and congested ().
Figure 9. Images from histological sections of heart ventricles of control (a&b) and IS mother rats (c&d) (left hand panels) and their offspring at PND21(control:a1&b1and IS:c1&d1)(right hand panels). In control group the pericardium (PC) endocardium (EC) and mocardium (MC) appear intact. In IS female rats and their pups the ventricular sections showing hypertrophied (arrow heads), necrotic myocardial cells (arrows), congested (CBC) and dilated blood capillaries (DBC), and less tight junction among myocardial fibers (star).
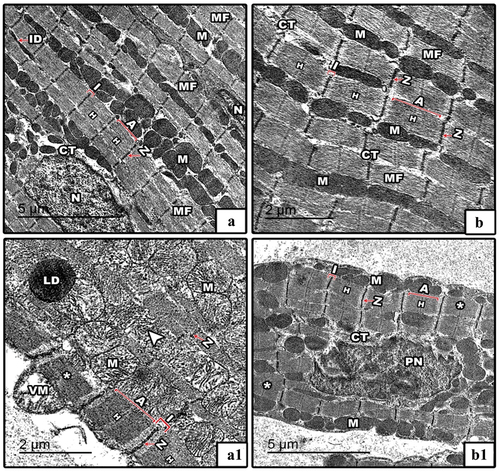
In control mothers’ rats () and their offspring at PND21 (), the TEM results revealed well developed striated bundles of interconnected myocardial muscle fibers. Each myocardial fiber consists of longitudinally arranged myofibrils and high density of adjacent rows of mitochondria with centrally located nuclei. The myofibrils displayed regular sarcomeres which determined by the distance between two successive Z- lines and showed distinctive alternating dark and light bands. In IS pregnant rats, the myocardial fibers appeared hypertrophied and compressed with pronounced loss of normal architecture of myofibrils and sarcomeres. Furthermore, most of mitochondria appeared lysed and vacuolated. Additionally, a lipid droplet appeared accumulated on the myocardial fibers (). In offspring from IS rats, the myocardial fibers appeared hypertrophied with markedly pyknotic nucleus while the mitochondria appeared unchanged ().
Figure 10. TEM through the myocardial fibers of control and IS mother’s rats (panels a&a1) and their offspring at PND21 (b&b1) respectively. In control mothers’ rats and their offspring, the TEM of myocardial fibers appear with well striated myofibrils which had regular architectures of sarcomeres (distance between two successive Z-lines; red line in images). In IS rats and their pups, the myocardial fibers appear compressed and hypertrophied (*) with lysed (arrowhead) and vacuolated moitochondria (VM) as well as pyknotic nucleus (PN). Abbreviations: MF; myofibrils, M; mitochondria, ID; intercalated disc, N; nucleus, I; isotropic band (light band), A; anisotropic band (dark band), Z; zigzag-line, CT; connective tissue, H; H-zone, PN; pyknotic nucleus and LD; lipid droplet. Sarcomere is indicated by the distance between two successive Z-lines.
Immunohistochemical localization of synaptophysin in cerebellar cortex
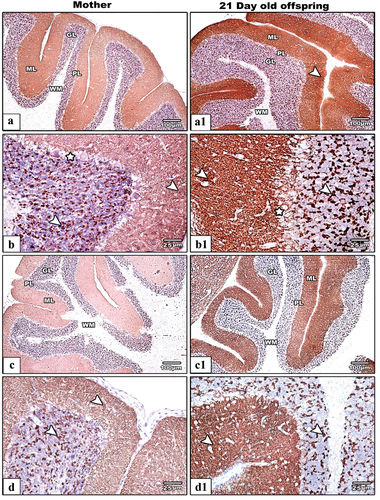
The cerebellar cortex sections from control mothers rats dispalyed moderate synaptophysin immunoreactiveity () while their offspring revealed strong expression (). In IS mothers rats () and their pups at PND21(), the cerebellar cortex showed remarkable decrease in the immune reaction for synaptophysin protein if comparred with control. Generally, SYP immunoreactivity was shown to be more concentrated in the cerebellar cortex’s granular and molecular layers.
Figure 11. Photomicrograph of SYN antibody-stained embedded paraffin slices of cerebellar cortex from control (Images a&b) and IS (Images c&d) mother rats and their offspring (control: (Images A1&B1 IS: (Images c1&d1). In IS mothers rats and their pups the SYP immunoreactivity appear less expressed comparatively with their control. The arrows heads point to the localization of SYP immune expression.) Synaptophysin antibody satin, images a,a1,c&c1 the scale bar = 100 µm, images b,b1d&d1 the scale bar = 25µm).
Discussion
Maternal depression during pregnancy may affects about 20% of women [Citation30]. It is a growing public health challenge and is a high-risk factor for both mothers and their offspring’s [Citation31,Citation32]. The goal of this study was to develop a rat model of human maternal depression during pregnancy in rats.
In the current work, a remarkable significant decrease in the body weight was recorded in IS rats. This finding is in line with the previous study that found significant decrease in the body weight of rats induced with immobilization stress [Citation33]. The decreased body weight in IS rats is mainly attributed to hypercortisolism which implicated in induction of lipolysis and protein breakdown in tissues [Citation34]. Also, our results indicated significant decline in the mean percentage of food consumption which agree with previous study noticed by Amugongo and Hlusko [Citation23]. All these postulated that immobilization stress is one of the fundamental factors promoting appetite loss.
The obtained results revealed that exposure of rats to immobilization stress during gestation results in significant elevation in the levels of cortisol and glucose while significant decline in the levels of serotonin and insulin. It had been reported that pregnant rats that subjected to immobilization stress had a higher cortisol level more than normal that may result in disturbance in HPA system [Citation35,Citation36]. Additionally, the researchers find an association of maternal symptoms of immobilization stress during pregnancy and the elevated levels of cortisol which affected directly and cause lowering of serotonin level that help in formation of some developmental anomalies in brain tissues of offspring and their mothers. So, there is a reverse co-relation between the level blood serotonin and cortisol. Serotonin plays a very important role in development and maturation of the mammalian brain before its action as a neurotransmitter [Citation37,Citation38]. Furthermore, it was reported that acute psychological stress may play a role in the glycemic instability, whereas emotional stress was found to induce a delay in the disposal of a carbohydrate load [Citation39]. This delay in the disposal of the carbohydrate load results in an undue elevation of the blood glucose [Citation40]. Other studies explained that because hypercortisolism is a major causative factor for insulin resistance which consequently results in high peripheral glucose levels and hyperinsulinemia because of the reduced ability of insulin to reduce blood glucose levels by taking to muscle tissue [Citation41]. Furthermore, exposure to depression during gestation was found to decline the plasma insulin level [Citation42,Citation43].
Studies in rats have shown that maternal exposure to prenatal stress is associated with induction of intrauterine growth restriction (IUGR) [Citation44] and glucose intolerance [Citation45], this retardation of fetal growth may result due to crossing of cortisol to the placenta [Citation46]. Furthermore, serotonin plays a role in development before it acts as a neurotransmitterc [Citation38,Citation47]. This provides novel evidence that serotonin affects craniofacial, neurological, and cardiovascular morphogenesis in developing rat. This result is also proved by previous refreshers [Citation48,Citation49]. Our results declared obvious congenital morphological and anatomical anomalies in rats fetuses maternally exposed to immobilization stress, these in line with above mentioned reports
Immobilization stress limits the movements of the body, which can affect the body systems and can produce various pathological states. A number of diseases are related to immobilization under conditions of chronic stress that can cause degenerative diseases such as aging and many other brain dysfunctions [Citation50]. Our results showed remarkable histopathological signs in the brain and cardiac muscles tissues in IS rats and their pups. The cerebral cortex of IS mothers rats and their offspring displayed obvious hypertrophied neurons, dilated blood capillaries and infiltrated cells. The cerebellar cortex of IS mothers rats and their pups indicated fragmented pia matter, vacuolated basket and granular cells nonadjacent granular cells. Also, some Purkinje cells appear lysed (yellow asterisk), hypertrophied (green asterisks) and pyknotic. On the level of cardiac tissues, the ventricular tissues dispalyed hypertrophied and necrotic myocardial cells, congested and dilated blood capillaries, and less tight junction among myocardial fibers. Similar histopathological observations were recorded in the glutamate induced stress in cerebral cortex [Citation51] and restraint stress cerebellar cortex tissues of rats [Citation52]. It has been declared that psychological and immobilization stress can inhibit the levels of brain antioxidants especially reduced glutathione (GSH) resulting in pronounced cell damage and apoptosis [Citation53]. Other study proved that, the pathological changes in the cerebral and cerebellar cells under the influence of IS can be attributed to the excessive liberation cortisol, which is involved in stress reactions. High levels of serum cortisol have previously been found to lead to multiple structural and physiological changes in the nervous system [Citation53].
The obtained results concerned with the ventricular histopathological observations in this study go in accordance with study reported by Esrefoglu et al. who found ventricular muscles damage in rats exposed to immobilization and starvation stress [Citation54]. The authors attributed myocardial cell damage to oxidative stress induced by the excessive liberations of cellular free radicals. Other reports found that depression during gestation in human is often associated with CVD and consequent developmental abnormalities in the offspring [Citation55,Citation56].
Conclusion
Based on our finding, immobilization stress during gestation is implicated in decrement of food consumption, body weight loss, pronounced increase in the levels of cortisol and glucose as well as significant decrease in the levels of serotonin. Also, deleterious histological and ultrastructural alterations in the myocardial and brain tissues of mother’s rats and their pups were obviously appeared. Additionally, various morphological and anatomical anomalies were recorded in some examined fetuses during gestation time.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lamberto F, Peral-Sanchez I, Muenthaisong S, et al. Environmental Alterations during Embryonic Development: Studying the Impact of Stressors on Pluripotent Stem Cell-Derived Cardiomyocytes. Genes (Basel). 2021;12(10):1564. doi: 10.3390/genes12101564
- Giordana LN, Bozzo AA, Cots DS, et al. The effect of chronic stress on prenatal development of the central nervous system. Biotechnic Histochemist. 2015;90(2):146–151. doi: 10.3109/10520295.2014.976269
- Lemaire V, Koehl M, Le Moal M, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA; 2000. 97(20). p. 11032–11037.
- Esler M. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev. 2017;74:269–276. doi: 10.1016/j.neubiorev.2016.10.011
- Lee HY, Rhee Y, Choi KS. Urinary incontinence and the association with depression, stress, and self-esteem in older Korean Women. Sci Rep. 2021;11(1):9054. doi: 10.1038/s41598-021-88740-4
- Lazinski MJ, Shea AK, Steiner M. Effects of maternal prenatal stress on offspring development: a commentary. Arch Womens Ment Health. 2008;11(5–6):363–375. doi: 10.1007/s00737-008-0035-4
- Strekalova T, Liu Y, Kiselev D, et al. Chronic mild stress paradigm as a rat model of depression: facts, artifacts, and future perspectives. Psychopharmacology. 2022;239(3):663–693. doi: 10.1007/s00213-021-05982-w
- Juruena MF, Eror F, Cleare AJ, et al. The role of early life stress in HPA axis and anxiety, advances in experimental medicine and biology. Springer Singapore; 2020. p. 141–153.
- Obel C, Hedegaard M, Henriksen TB, et al. Stress and salivary cortisol during pregnancy. Psychoneuroendocrinology. 2005;30(7):647–656. doi: 10.1016/j.psyneuen.2004.11.006
- Nepomnaschy PA, Welch KB, McConnell DS, et al. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci USA; 2006. 103(10). p. 3938–3942.
- Bennett HA, Einarson A, Taddio A, et al. Prevalence of depression during pregnancy: systematic review. Obstet & Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f
- Ryan D, Milis L, Misri N. Depression during pregnancy. Can Fam Physician. 2005;51(8):1087–1093.
- Ramirez-Franco J, Oros-Pantoja R, Torres-Garcia E, et al. Effects of chronic immobilization stress on biokinetics and dosimetry of (67)Ga in a murine model. Radiat Environ Biophys. 2020;59(2):257–263. doi: 10.1007/s00411-020-00839-w
- Son H, Yang JH, Kim HJ, et al. A chronic immobilization stress protocol for inducing depression-like behavior in mice. J Vis Exp. 2019;(147). doi: 10.3791/59546-v
- Palinski W. Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129(20):2066–2077. doi: 10.1161/CIRCULATIONAHA.113.001805
- Sohlberg S, Mulic-Lutvica A, Lindgren P, et al. Placental perfusion in normal pregnancy and early and late preeclampsia: a magnetic resonance imaging study. Placenta. 2014;35(3):202–206. doi: 10.1016/j.placenta.2014.01.008
- St-Pierre J, Laurent L, King S, et al. Effects of prenatal maternal stress on serotonin and fetal development. Placenta. 2016;48(Suppl 1):S66–S71. doi: 10.1016/j.placenta.2015.11.013
- Young SN. How to increase serotonin in the human brain without drugs. J Psychiatry Neurosci Jpn. 2007;32:394.
- Oberlander TF. Fetal serotonin signaling: setting pathways for early childhood development and behavior. J Adolesc Health. 2012;51(2):S9–16. doi: 10.1016/j.jadohealth.2012.04.009
- Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267(2):87–93. doi: 10.1002/ar.10089
- Schlienger RG, Meier CR. Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction? Am J Cardiovasc Drugs. 2003;3(3):149–162. doi: 10.2165/00129784-200303030-00001
- Volkova OV. V.A. Otellin, L.I. Khozhai, N.E. Ordyan, prenatal stress actions on the developing brain. Adaptive mechanisms, direct and delayed effects, St. Petersburg, “Desyatka”, 2007, 237 pp. J Evol Biochem Physiol. 2011;44(1):119–121. doi: 10.1134/S0022093008010155
- Amugongo SK, Hlusko LJ. Impact of maternal prenatal stress on growth of the offspring. Aging Dis. 2014;5:1–16. doi: 10.14336/AD.2014.05001
- Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18(2):158–168. doi: 10.1016/j.numecd.2007.06.004
- Weesner S, Parry JM. Mutagenicity testing: a practical approach. (NY): Oxford, IRL Press; 1984.
- Tormo MA, Gomez-Zubeldia MA, Ropero F, et al. Effect of insulin and gliclazide on glucose utilization by a perfused intestine-pancreas preparation isolated from diabetic and non-diabetic rats. Acta Diabetol. 1994;31(3):151–155. doi: 10.1007/BF00570370
- Kemmer FW, Bisping R, Steingruber HJ, et al. Psychological stress and metabolic control in patients with type I diabetes mellitus. N Engl J Med. 1986;314(17):1078–1084. doi: 10.1056/NEJM198604243141704
- Bancroft JD, Gamble M. General acknowledgments, theory and practice of histological techniques. Elsevier; 2008. p. xiii.
- Grillo CA, Piroli GG, Wood GE, et al. Reagan LP.Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136(2):477–486. doi: 10.1016/j.neuroscience.2005.08.019
- Czarzasta K, Makowska-Zubrycka M, Kasarello K, et al. A rat model to study maternal depression during pregnancy and postpartum periods, its comorbidity with cardiovascular diseases and neurodevelopmental impact in the offspring. Physiol Behav. 2019;199:258–264. doi: 10.1016/j.physbeh.2018.11.024
- Ding XX, Wu YL, Xu SJ, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103–110. doi: 10.1016/j.jad.2014.02.027
- Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003
- Radahmadi M, Shadan F, Karimian SM, et al. Effects of stress on exacerbation of diabetes mellitus, serum glucose and cortisol levels and body weight in rats. Pathophysiology. 2006;13(1):51–55. doi: 10.1016/j.pathophys.2005.07.001
- Sentari M, Harahap U, Sapiie TWA, et al. Blood cortisol level and blood serotonin level in depression mice with basil leaf essential oil treatment. Open Access Maced J Med Sci. 2019;7(16):2652–2655. doi: 10.3889/oamjms.2019.819
- Gifford RM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans. Early Hum Dev. 2017;114:7–10. doi: 10.1016/j.earlhumdev.2017.09.011
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009
- Cote F, Fligny C, Bayard E, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA; 2007. 104. p. 329–334.
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28(3):171–185. doi: 10.1159/000091915
- Wiesli P, Schmid C, Kerwer O, et al. Acute psychological stress affects glucose concentrations in patients with type 1 diabetes following food intake but not in the fasting state. Diabetes Care. 2005;28(8):1910–1915. doi: 10.2337/diacare.28.8.1910
- Pamidi N, Nayak BS, Mohandas KG, et al. Environmental enrichment exposure restrains the neuronal damage induced by diabetes and stress in the motor cortex of rat brain. Bratisl Lek Listy. 2014;115(4):197–202. doi: 10.4149/BLL_2014_042
- van Donkelaar EL, Vaessen KR, Pawluski JL, et al. Long-term corticosterone exposure decreases insulin sensitivity and induces depressive-like behaviour in the C57BL/6NCrl mouse. PLOS ONE. 2014;9(10):e106960. doi: 10.1371/journal.pone.0106960
- Entringer S, Wust S, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199(5):498 e491–497. doi: 10.1016/j.ajog.2008.03.006
- Valsamakis G, Papatheodorou DC, Chalarakis N, et al. In pregnancy increased maternal STAI trait stress score shows decreased insulin sensitivity and increased stress hormones. Psychoneuroendocrinology. 2017;84:11–16. doi: 10.1016/j.psyneuen.2017.06.008
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch gen psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111
- Lesage J, Del-Favero F, Leonhardt M, et al. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol. 2004;181(2):291–296. doi: 10.1677/joe.0.1810291
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19(4):296–308. doi: 10.1016/j.bbi.2004.09.006
- Nguyen L, Rigo JM, Rocher V, et al. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305(2):187–202. doi: 10.1007/s004410000343
- Buznikov GA, Lambert HW, Lauder JM. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305(2):177–186. doi: 10.1007/s004410100408
- Fiorica-Howells E, Maroteaux L, Gershon MD. Serotonin and the 5-HT 2B receptor in the development of enteric neurons. J Neurosci. 2000;20(1):294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000
- Kahn CR, Weir GC. Joslinʼs diabetes mellitus. Endocrinologist. 1995;5(2):157–158. doi: 10.1097/00019616-199503000-00013
- Borisova T, Krisanova N, Himmelreich N. Artificial gravity and functional plasticity of nerve system. L-[14C]-glutamate uptake by nerve terminals from rat cerebellum and cerebral hemispheres under hypergravity stress, Life in space for life on earth. 2002. p. 135–136.
- Amin SN, Hassan SS, Khashaba AS, et al. Hippocampal and cerebellar changes in acute restraint stress and the impact of pretreatment with ceftriaxone. Brain Sci. 2020;10(4):193. doi: 10.3390/brainsci10040193
- Samarghandian S, Farkhondeh T, Samini F, et al. Protective effects of carvacrol against oxidative stress induced by chronic stress in rat’s brain, liver, and kidney. Biochem Res Int. 2016;2016:2645237. doi: 10.1155/2016/2645237
- Esrefoglu M, Akinci A, Taslidere E, et al. Ascorbic acid and beta-carotene reduce stress-induced oxidative organ damage in rats. Biotechnic Histochemist. 2016;91(7):455–464. doi: 10.1080/10520295.2016.1220019
- Kallen B. Fluoxetine use in early pregnancy. Birth Defects Res B Dev Reprod Toxicol. 2004;71:395–396. doi: 10.1002/bdrb.20025
- Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407