Abstract
A population of proliferating neural stem/progenitor cells located in the subgranular zone of the adult hippocampal dentate gyrus (DG) gives rise to new neurons continuously throughout life, and this process is referred to as adult hippocampal neurogenesis. To date, it has generally been accepted that impairments of adult hippocampal neurogenesis resulting from pathological conditions such as stress, ischemia and epilepsy lead to deficits in hippocampus-dependent learning and memory tasks. Recently, we have discovered that microglia, the major immune cells in the brain, attenuate seizure-induced aberrant hippocampal neurogenesis to withstand cognitive decline and recurrent seizure. In that study, we further showed that Toll-like receptor 9, known as a pathogen-sensing receptor for innate immune system activation, recognizes self-DNA derived from degenerating neurons to induce TNF-α production in the microglia after seizure, resulting in inhibition of seizure-induced aberrant neurogenesis. Our findings provide new evidence that interaction between the innate immune and nervous systems ensures homeostatic neurogenesis in the adult hippocampus and should pave the way for the development of new therapeutic strategies for neurological diseases including epilepsy.
In the adult mammalian brain, neural stem/progenitor cells (NS/PCs) reside in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and generate new neurons continuously.Citation1 This adult neurogenesis has been observed in all examined mammalian species including human.Citation2,3 Although many subsets of newborn neurons cannot survive as mature neurons at the end of a 4-week period, those cells that do survive functionally integrate into existing neuronal circuits and participate in the process of learning and memory formation.Citation4 Adult neurogenesis is responsive to both environmental and endogenous factors, and its levels are altered in pathological conditions such as ischemia, Alzheimer disease and epilepsy.Citation1,5 Among these pathological insults, for example, epileptic seizures induce aberrant hippocampal neurogenesis, including increased proliferation of NS/PCs, production of ectopic granule cells, and persistence of hilar basal dendrites on adult-generated granule neurons.Citation5-7 Therefore, regulating endogenous neurogenesis is a potential strategy for therapeutic treatment of neurological disorders such as epilepsy.
Regulation of Neurogenesis by Toll-Like Receptors
Toll-like receptors (TLRs) are well known as innate immune receptors that recognize conserved pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), flagellin, peptidoglycan and nucleic acid of bacteria, viruses, yeast, fungi and parasites.Citation8 The activation of TLR signaling in immune cells including macrophages and microglia induces production of pro-inflammatory factors and contributes to the clearance of pathogens. At least 13 TLR genes exist in mammals, and each TLR recognizes distinct ligands. TLRs 1–9 exist in both mice and humans, whereas TLRs 10–13 exist only in mice. The association of TLRs with their cognate ligands triggers the activation of the transcription factor nuclear factor κB (NF-κB), culminating in the production of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6).Citation8 Inflammation caused by LPS (a TLR4 ligand) reportedly inhibits neurogenesis in the adult rat hippocampus via the induction of pro-inflammatory cytokines.Citation9,10 Moreover, administration of Poly I:C (a TLR3 ligand) during pregnancy reduces cortical neurogenesis in fetal mice.Citation11 The finding that inflammation induced by TLR activation impairs neurogenesis represented a great contribution to research related to regulation of neurogenesis by innate immune receptors. Since recent studies have shown that activated TLR signaling induces pro-inflammatory cytokines in pathological conditions such as ischemia, Alzheimer disease and multiple sclerosis, even in the absence of any pathogen,Citation12,13 these studies have raised new questions: What are the endogenous ligands for the activation of TLR signaling and what types of cells produce these ligands?.
Under physiological conditions, TLR2 and TLR4, both of which are expressed in adult NS/PCs, have been implicated in differentiation and proliferation of NS/PCs, respectively.Citation14 TLR4 deficiency increases NS/PC proliferation in the hippocampus of adult mice.Citation14 () Although TLR2 deficiency does not alter proliferation of NS/PCs in the adult hippocampus, it reduces neuronal differentiation and promotes astrocytic differentiation of NS/PCs.Citation14 () TLR3 deficiency enhances the proliferative capacity of embryonic but not adult NS/PCs, probably because TLR3 expression gradually reduces during development.Citation15 () However, it remains unclear what the endogenous ligands are and how the activated TLR signaling regulate the behavior of NS/PCs in the absence of pathogen.
Figure 1. Schematic models showing the regulation of neurogenesis by TLR-related factors. Under physiological conditions (A), microglia engulf apoptotic cells in the course of neuronal differentiation/maturation of NS/PCs to maintain homeostatic neurogenesis.Citation4 TLR4 but not TLR2 and TLR3 deficiency in NS/PCs enhances their proliferation in the adult hippocampus.Citation14,15 While TLR2 deficiency reduces neuronal differentiation of NS/PCs, TLR4 deficiency increases it.Citation14 In the epileptic condition (B), self-DNAs derived from degenerating neurons activate microglia through TLR9, resulting in the release of TNF-α to inhibit aberrant NS/PC proliferation and neurogenesis as shown in our study.Citation16 TLR function in NS/PCs is still poorly understood in pathological conditions including epilepsy.
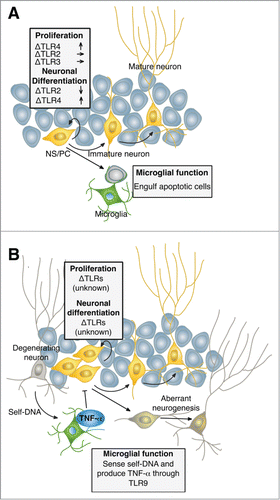
To elucidate the functions of other TLRs in the behavior of NS/PCs, we investigated whether TLR7 and TLR9 modulate neurogenesis under physiological conditions.Citation16 BrdU tracing of proliferating NS/PCs in the hippocampus revealed no significant difference among wild type (WT), TLR7-knockout (KO) and TLR9-KO mice,Citation16 probably due to the absence of endogenous ligands for TLR7 and TLR9 signaling under normal conditions. Although TLR7 and TLR9 were initially identified as receptors that recognize microbial RNA and DNA, respectively, recent studies have suggested that they can also sense self-RNA and -DNA derived from dying or degenerating cells as damage-associated molecular patterns (DAMPs).Citation17,18 We therefore asked whether TLR7 and TLR9 signaling activated by DAMPs affects neurogenesis in the hippocampus. Since we had previously shown that convulsive seizure causes neuronal degeneration in the DG,Citation6 we examined the effects of TLR7 and TLR9 deficiency on hippocampal neurogenesis after convulsive seizure induced by kainic acid (KA), a potent central nervous system excitant. Injection of KA into WT, TLR7-KO and TLR9-KO mice revealed that TLR9 but not TLR7 deficiency exacerbated aberrant neurogenesis compared to WT mice after seizure,Citation16 indicating that TLR9 attenuates seizure-induced aberrant neurogenesis in normal mice. Furthermore, in vitro analysis revealed that KA-treated degenerating neurons release self-DNA, thereby activating TLR9 signaling in microglia.Citation16 () These results are in accordance with previous studies that activated TLR signaling inhibits neurogenesis, and, more importantly, provide a new insight for the regulation of homeostatic neurogenesis by endogenous ligand-activated TLR9 signaling in the absence of pathogen.
TLR-Expressing Microglia and Neurogenesis
Microglia are the nerve tissue's resident immune cells and are widely distributed in the adult brain and spinal cord.Citation19 The primary function of microglia is to phagocytose dying cells and cellular debris without producing inflammation.Citation19 Phagocytosis of dying cells during brain development, neurodegenerative diseases and senescence is essential for the maintenance of tissue homeostasis throughout the organism's lifespan. A recent study has shown that microglia extend their processes to NS/PCs in the adult hippocampus and clear apoptotic newborn cells generated from NS/PCs by phagocytosis.Citation4 (). This finding highlights the importance of adequate and appropriate microglial function under physiological conditions for the maintenance of homeostatic neurogenesis. In contrast to physiological conditions, activated microglia produce a variety of pro-inflammatory factors in response to pathological insults such as traumatic brain injury, stroke, infection and seizure.Citation20-23 It has been shown that various inflammatory cytokines are released from microglia after seizure, and this is considered to exacerbate epileptic pathology.Citation24,25
In our study, we found that the expression of TLR9 is high in microglia but not in other cell types in the CNS, including NS/PCs.Citation16 Moreover, TLR9-expressing microglia are in close proximity to NS/PCs in the adult hippocampus under both KA-treated and -untreated conditions.Citation16 Therefore, we hypothesized that TLR9 mediates niche-resident microglial activation and reduces seizure-induced aberrant neurogenesis probably through the production of cytokines by activated microglia.
The first studies suggesting an acute detrimental role of activated microglia in neurogenesis were performed in rats following intraparenchymal LPS injections.Citation9 LPS-induced microglial activation occurred through TLR4 signaling and dramatically decreased the survival of new-born neurons in the adult hippocampus.Citation9 In addition, the reduced neurogenesis in response to LPS could be rescued by attenuation of microglial activation through the administration of minocycline, a well-known inhibitor of microglial activation.Citation26 In these pioneering studies, pro-inflammatory cytokines like IL-1β, IL-6 and TNF-α, produced acutely by activated microglia, were proposed to inhibit adult neurogenesis in the hippocampus. In this relation, TLR9-KO mice showed a reduction of sustained microglial activation and TNF-α production in the hippocampus after seizure compared to WT mice.Citation16 No significant change was observed in the mRNA expression levels of other TLRs, including TLR2, TLR3 and TLR4, in the DG between WT and TLR9-KO mice (unpublished data), suggesting that other TLRs do not contribute to this reduction of sustained microglial activation and TNF-α production in TLR9-KO mice after seizure. Thus, although all TLRs can regulate microglial activation, our study revealed that TLR9 is essential for the maintenance of sustained microglial activation through the recognition of self-DNA released from degenerating neurons. We also revealed that inhibiting microglial activation by minocycline or inhibiting Tnf-α expression by thalidomide in WT mice exacerbated seizure-induced aberrant 0neurogenesis, indicating that activated TLR9 signaling in microglia upregulates Tnf-α expression, resulting in an attenuation of aberrant neurogenesis induced by seizure.Citation16 (). However, we cannot exclude the possibility that TLR9 expressed in NS/PCs also affects the behavior of NS/PCs after seizure, although the expression level of TLR9 in NS/PCs is extremely low compared to that in microglia.Citation16 To address this issue will require further studies using NS/PC-specific KO mice.
While we and others have revealed that activated microglia have a negative effect on neurogenesis,Citation16,27,28 the opposite function for these cells has been also reported.Citation29,30 Indeed, activated microglia can be divided into 2 distinct subtypes:Citation19 the classical M1 state (characterized by the expression of pro-inflammatory factors such as TNF-α, IL-6 and iNOS) and the alternative M2 state (characterized by the expression of anti-inflammatory factors such as CD206, IL10 and arginase-1). Therefore, it is conceivable that each type of activated microglia contributes differently to regulate neurogenesis by producing different factors. In our study, seizure-induced aberrant neurogenesis in WT mice was exacerbated by minocycline, which selectively inhibits M1 microglia-related gene expression.Citation31 Thus, it is likely that M1 microglia play a role in the attenuation of seizure-induced aberrant neurogenesis. In the animal model of spinal cord injury (SCI),Citation32 increased numbers of iNOS-expressing M1 microglia and macrophages were observed in association with tissue damage.Citation33 Resident microglia and peripheral macrophages rapidly mobilize to the injured site and begin to release pro-inflammatory factors for the recruitment of other immune cells.Citation34 Although an increasing number of studies indicate that microglia and macrophages are highly plastic cells that can engage different functional programs in response to specific microenvironmental signals,Citation20,34 both of these cells express inflammatory cytokines such as TNF-α at injured sites.Citation33 This inflammation further induces neuronal cell death, thus exacerbating pathology in spinal cord injury.Citation35 In light of these observations, we might expect that M1 microglia (and possibly M1 macrophages) in the injured spinal cord would interfere with the behavior of transplanted NS/PCs by producing inflammatory cytokines. For more than a decade, NS/PCs have been an attractive and promising cell source for treatments of SCI, because they have the potential to differentiate into neurons that can replace lost neurons to regenerate disrupted neuronal circuits at lesion sites.Citation36-40 Since our work has suggested that neurogenesis is suppressed by M1 microglia,Citation16 it is tempting to speculate that over-activation of M1 microglia would also reduce neurogenesis in NS/PCs transplanted into the injured spinal cord. Therefore, a combination of NS/PC transplantation and pharmacological inhibition of M1 microglia by means of a drug such as minocycline would be worthwhile to examine whether such combinatorial treatment might enhance neuronal differentiation of transplanted NS/PCs at the injured spinal cord, resulting in improved functional recovery.
Aberrant Neurogenesis and Recurrent Seizure Activity
Approximately 50 million people worldwide suffer from different epilepsies, and 20–40% of epileptic patients still have poorly controlled seizures despite therapy.Citation41 Temporal lobe epilepsy (TLE) is the most common type of epilepsy in adults and is often intractable. The long-term morbidity of TLE patients includes an increased incidence of depression and memory deficits.Citation42 Therefore, progress in the study of TLE is essential for developing better therapies. We and others have used mouse or rat models to show that seizure induces augmentation of aberrant neurogenesis in the adult hippocampus.Citation5-7,43 This aberrant neurogenesis incurs cognitive decline, whereas pharmacological modulation of seizure-induced aberrant neurogenesis, by valproic acid or endoneuraminidase, restored hippocampal-dependent memory function.Citation6,44 However, it is unclear how animals withstand aberrant neurogenesis to maintain brain integrity. Moreover, it remains elusive whether neurons aberrantly generated in response to seizure indeed play a critical role in the development of epilepsy, although aberrant neurogenesis in the hilus after febrile seizure at postnatal stage has been suggested to contribute to epileptogenesis.Citation45 In our work, we revealed that the loss of TLR9 enhances seizure-induced aberrant neurogenesis, resulting in the aggravation of cognitive decline and of recurrent seizure severity, indicating that TLR9 signaling normally ameliorates cognitive decline and recurrent seizure severity.Citation16 Consistent with this finding, another group also showed that ablation of aberrant neurogenesis suppresses cognitive decline and chronic seizure frequency for nearly one year,Citation46 suggesting that controlling seizure-induced aberrant neurogenesis can effectively reduce recurrent seizure as well as memory deficits. We believe that these new revelations offer an excellent framework for the discovery of new drugs to inhibit epileptogenesis in patients for whom treatment with existing anti-epileptic drugs provides inadequate control of seizure.
Concluding Remarks
Our recent work has provided new evidence that activation of TLR9 signaling in microglia by self-DNA derived from degenerating neurons induces the production of TNF-α to suppress aberrant neurogenesis, reducing cognitive decline after seizure and recurrent seizure severity.Citation16 This indicates clearly the existence of bidirectional communication between the innate immune and nervous systems for homeostatic neurogenesis in the adult hippocampus. As well as in epileptic conditions, increasing evidence suggests that endogenous molecules activate microglia under pathological conditions such as ischemia, Alzheimer disease and aging.Citation47-50 In association with the behavioral change of microglia, abnormal neurogenesis is observed in these pathological conditions, implying that activated microglia have a powerful impact on abnormal neurogenesis.Citation1 As shown in the epileptic condition,Citation16 we believe that future challenges include elucidating the machinery by which TLR-expressing microglia regulate abnormal neurogenesis in other pathological conditions (e.g., ischemia, Alzheimer disease and aging), and we await with great interest the identification of endogenous ligands and subsequent development of therapeutic applications for neurological disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/10.1016/j.neuron.2011.05.001
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013; 153:1219-27; PMID:23746839; http://dx.doi.org/10.1016/j.cell.2013.05.002
- Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: Why the dentate gyrus? Learn Mem 2013; 20:710-29; PMID:24255101; http://dx.doi.org/10.1101/lm.026542.112
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010; 7:483-95; PMID:20887954; http://dx.doi.org/10.1016/j.stem.2010.08.014
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 1997; 17:3727-38; PMID:9133393
- Jessberger S, Nakashima K, Clemenson GD, Jr., Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 2007; 27:5967-75; PMID:17537967; http://dx.doi.org/10.1523/JNEUROSCI.0110-07.2007
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci 2010; 30:2051-9; PMID:20147533; http://dx.doi.org/10.1523/JNEUROSCI.5655-09.2010
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373-84; PMID:20404851; http://dx.doi.org/10.1038/ni.1863
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302:1760-5; PMID:14615545; http://dx.doi.org/10.1126/science.1088417
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 2008; 13:717-28; PMID:17700577; http://dx.doi.org/10.1038/sj.mp.4002055
- De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R, Lipkin WI. Induction of Toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio 2010; 1:e00176-10
- Marsh BJ, Stenzel-Poore MP. Toll-like receptors: novel pharmacological targets for the treatment of neurological diseases. Curr Opin Pharmacol 2008; 8:8-13; PMID:17974478; http://dx.doi.org/10.1016/j.coph.2007.09.009
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010; 58:253-63; PMID:19705460
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 2007; 9:1081-8; PMID:17704767; http://dx.doi.org/10.1038/ncb1629
- Lathia JD, Okun E, Tang SC, Griffioen K, Cheng AW, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci 2008; 28:13978-84; PMID:19091986; http://dx.doi.org/10.1523/JNEUROSCI.2140-08.2008
- Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun 2015; 6:6514; PMID:25751136; http://dx.doi.org/10.1038/ncomms7514
- Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007; 8:487-96; PMID:17417641; http://dx.doi.org/10.1038/ni1457
- Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 2012; 15:827-35; PMID:22610069; http://dx.doi.org/10.1038/nn.3113
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011; 11:775-87; PMID:22025055; http://dx.doi.org/10.1038/nri3086
- Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics 2010; 7:366-77; PMID:20880501; http://dx.doi.org/10.1016/j.nurt.2010.07.002
- Wood PL. Microglia as a unique cellular target in the treatment of stroke - potential neurotoxic mediators produced by activated microglia. Neurol Res 1995; 17:242-8; PMID:7477737
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev 2004; 17:942-64, table of contents; PMID:15489356; http://dx.doi.org/10.1128/CMR.17.4.942-964.2004
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci 1999; 19:5054-65; PMID:10366638
- Kosonowska E JK, Setkowicz Z. Inflammation induced at different developmental stages affects differently the range of microglial reactivity and the course of seizures evoked in the adult rat. Epilepsy Behav 2015; S15525-5050:242-5
- Zhang XM, Zhu J. Kainic Acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol 2011; 9:388-98; PMID:22131947; http://dx.doi.org/10.2174/157015911795596540
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 2003; 100:13632-7; PMID:14581618; http://dx.doi.org/10.1073/pnas.2234031100
- Sultan S, Gebara E, Toni N. Doxycycline increases neurogenesis and reduces microglia in the adult hippocampus. Front Neurosci 2013; 7:131; PMID:23898238
- Gebara E, Sultan S, Kocher-Braissant J, Toni N. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci 2013; 7:145; PMID:23970848; http://dx.doi.org/10.3389/fnins.2013.00145
- Nikolakopoulou AM, Dutta R, Chen Z, Miller RH, Trapp BD. Activated microglia enhance neurogenesis via trypsinogen secretion. Proc Natl Acad Sci U S A 2013; 110:8714-9; PMID:23650361; http://dx.doi.org/10.1073/pnas.1218856110
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 2014; 34:2231-43; PMID:24501362; http://dx.doi.org/10.1523/JNEUROSCI.1619-13.2014
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 2013; 4:e525; PMID:23470532; http://dx.doi.org/10.1038/cddis.2013.54
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 2011; 12:388-99; PMID:21673720; http://dx.doi.org/10.1038/nrn3053
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014; 83:1098-116; PMID:25132469; http://dx.doi.org/10.1016/j.neuron.2014.07.027
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab 2013; 33:1864-74; PMID:23942366; http://dx.doi.org/10.1038/jcbfm.2013.146
- Yune TY, Chang MJ, Kim SJ, Lee YB, Shin SW, Rhim H, Kim YC, Shin ML, Oh YJ, Han CT, et al. Increased production of tumor necrosis factor-α induces apoptosis after traumatic spinal cord injury in rats. J Neurotrauma 2003; 20:207-19; PMID:12675973; http://dx.doi.org/10.1089/08977150360547116
- Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells 2012; 30:1163-73; PMID:22419556; http://dx.doi.org/10.1002/stem.1083
- Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S, Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest 2010; 120:3255-66; PMID:20714104; http://dx.doi.org/10.1172/JCI42957
- Okada S, Ishii K, Yamane J, Iwanami A, Ikegami T, Katoh H, Iwamoto Y, Nakamura M, Miyoshi H, Okano HJ, et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J 2005; 19:1839-41; PMID:16141363
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 2005; 102:14069-74; PMID:16172374; http://dx.doi.org/10.1073/pnas.0507063102
- Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, Bregman BS, Koike M, Uchiyama Y, Toyama Y, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 2002; 69:925-33; PMID:12205685; http://dx.doi.org/10.1002/jnr.10341
- Schmidt D, Loscher W. Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia 2005; 46:858-77; PMID:15946327; http://dx.doi.org/10.1111/j.1528-1167.2005.54904.x
- Helmstaedter C, Sonntag-Dillender M, Hoppe C, Elger CE. Depressed mood and memory impairment in temporal lobe epilepsy as a function of focus lateralization and localization. Epilepsy Behav 2004; 5:696-701; PMID:15380121; http://dx.doi.org/10.1016/j.yebeh.2004.06.008
- Murao N, Matsuda T, Noguchi H, Koseki H, Namihira M, Nakashima K. Characterization of Np95 expression in mouse brain from embryo to adult: A novel marker for proliferating neural stem/precursor cells. Neurogenesis 2014; 1:1, e976026; http://dx.doi.org/10.4161/23262133.2014.976026
- Pekcec A, Fuest C, Muhlenhoff M, Gerardy-Schahn R, Potschka H. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem 2008; 105:389-400; PMID:18194217; http://dx.doi.org/10.1111/j.1471-4159.2007.05172.x
- Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, Matsuki N, Ikegaya Y. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med 2012; 18:1271-8; PMID:22797810; http://dx.doi.org/10.1038/nm.2850
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun 2015; 6:6606; PMID:25808087; http://dx.doi.org/10.1038/ncomms7606
- Rupalla K, Allegrini PR, Sauer D, Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol 1998; 96:172-8; PMID:9705133; http://dx.doi.org/10.1007/s004010050878
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr., Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and interferon-gamma. Nature 1995; 374:647-50; PMID:7715705; http://dx.doi.org/10.1038/374647a0
- Barger SW, Harmon AD. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997; 388:878-81; PMID:9278049; http://dx.doi.org/10.1038/42257
- Wong WT. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci 2013; 7:22; PMID:23493481
