ABSTRACT
Accumulating findings have begun to unveil the important role of the endosomal machinery in the nervous system development. Endosomes have been linked to the differential segregation of cell fate determining molecules in asymmetrically dividing progenitors during neurogenesis. Additionally, the precise removal and reinsertion of membrane components through endocytic trafficking regulates the spatial and temporal distribution of signaling receptors and adhesion molecules, which determine the morphology and motility of migrating neurons. Emerging evidence suggests that the role of the endosomal sorting adaptors is dependent upon cell type and developmental stage. The repertoire of the signaling receptors and/or adhesion molecules sorted by the endosome during these processes remains to be explored. In this commentary, we will briefly address the progress in this research field.
Introduction
Early during neocortical development, apical radial glia (RG) divide symmetrically generating 2 identical daughter cells, thereby increasing the pool of progenitor stem cells. Later, RG divide asymmetrically producing another RG and an intermediate progenitor. The later will ultimately generate 2 post-mitotic multipolar neurons that migrate away from the ventricle border. These multipolar neurons later retract all their undifferentiated neurites and acquire a bipolar morphology, composed of a pia-directed leading process and a prospective axon that grows toward the ventricle. Once neurons have arrived at their final position within the cortical plate, the leading process branches and matures into the apical dendrite, and the axon elongates through the white matter until reaching its target cell.
There is an extensive literature concerning neurogenesis and neuronal migration during cortical development. Both processes are regulated under the control of cytoskeletal dynamics, internal and external signaling, and membrane trafficking (Reviewed in refs. Citation1 and Citation2). The endosomal system has emerged as a new multifactorial regulator in different nervous system development models, including brain cortex, cerebellum, spinal cord, and sensory organ precursor.Citation3,4 Its machinery comprises a mosaic of interconnected membrane compartments in which both the endocytic and exocytic pathways intersect. Through the addition and removal of membrane signaling components at precise subcellular sites, the endosomal trafficking modules cell behavior and morphology of both progenitor cells and neurons.
In this commentary, we will briefly summarize the state of our knowledge of the mechanisms by which the endosomal system coordinates different aspects of nervous system development while focusing on discussing the recent advances in our understanding of the emerging role of Smad Anchor for Receptor Activation (SARA) in both neurogenesis and neuronal migration.
Cell fate determinant distribution during asymmetric mitosis
Several non-mutually exclusive models — centrosome dynamics, cell cycle length, asymmetric inheritance of cell fate determinants during mitosis — have been proposed to explain the asymmetric division of cortical progenitors and other dividing progenitors.Citation5,6,7 Emerging evidence shows that endosome-associated proteins can regulate the differential sorting of cell fate instructing molecules between daughter cells during asymmetric division (). A notable example is the endosome-bound adaptor protein Numb. Its overexpression enhances the proliferation of avian neuroepithelial progenitors.Citation8 In line with this, a Numb knockout mouse line exhibits precocious neuronal differentiation in the mouse neocortex.Citation9 However, in examining a different Numb knockout mouse model, Zilian et al., did not observe any premature neurogenesis in the forebrain. Instead, arrested neuronal differentiation was discernable in the hindbrain.Citation10 Dissimilar results were also found in cultured cortical progenitors by Shen et al.Citation11 While Numb is evenly distributed into the 2 daughters of a symmetric division, Numb accumulates into the daughter cell that acquires a neurogenic cell fate in asymmetric mitosis.Citation11 These authors proposed that, in the Numb-predominant cell, the Notch signaling pathway is inhibited to promote neuronal differentiation. Recent studies suggested that the Numb-accumulating cell sequesters Notch into late endosomes, while the Numb-devoid cell recycles Notch back to the plasma membrane for signaling activation.Citation12 These different findings raise the possibility that Numb's endosomal trafficking role in asymmetric cell fate choice is context-dependent. Perhaps distinct cell determinant factor(s) besides Notch is sorted by Numb under different contexts.
Table 1. Participation of endocytic components in asymmetric mitosis during nervous system development.
SARA is also an endosome-bound adaptor protein. SARA specifically binds to the early endosome through its FYVE (Fab1p, YOTB, Vac1p, and EEA1) domain, and acts as a downstream effector of Rab5-mediated early endosomal fusion.Citation13 Similar to Numb, the function of SARA during asymmetric divisions is also cell model dependent.
SARA, labeled by ectopic expression of fluorescently-tagged SARA, was first linked to asymmetric division in the Drosophila sensory organ precursor (SOP). Gonzalez-Gaitan and his colleagues noticed that during SOP mitosis internalized Notch receptor and its ligand Delta traffic through Sara+ endosomes.Citation14 They showed that 15 times more SARA is segregated to the pIIa daughter than to the pIIb daughter cell. However, SARA mutant flies show normal internalized Notch and Delta asymmetric distribution into pIIa cells, as well as normal overall sensory organ development (), indicating that SARA is dispensable in SOP asymmetric mitosis and binary cell fate choice.Citation14 The same group later showed that the Notch receptor itself is required for the asymmetric segregation of SARA+ endosomes.Citation15
Figure 1. Schematic diagrams depicting SARA's distribution and roles in asymmetric mitosis of 3 different nervous systems. (A) SARA--positive endosomes (depicted in red compartments) accumulate in the pIIa cell during Drosophila SOP mitosis, although SARA mutant flies exhibit normal cell fate choice and SOP development. (B) In the zebrafish spinal cord, SARA endosomes predominantly accumulate to one of the daughter cells irrespectively if mitosis is symmetric or asymmetric. Only in the asymmetric lineage, SARA accumulation correlates with the progenitor type (P) fate. Either SARA KD or KO increases the proportion of neurons (N) at the expense of the progenitor type cell number. (C) In the mouse cortex, SARA endosomes distribute equally between daughter cells of apical progenitors. The proportion of progenitor (P) and neuron (N) daughter cells is not altered after SARA KD.
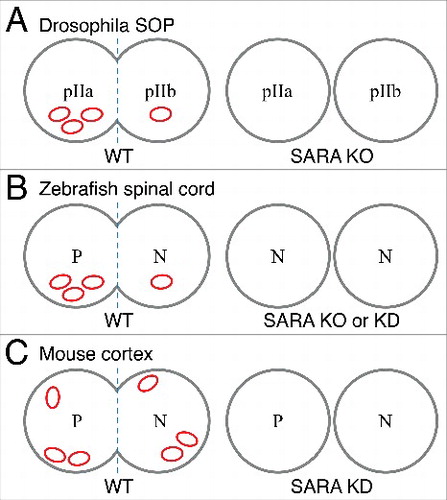
In the zebrafish spinal cord system, SARA has been shown to be functionally critical to maintain the progenitor population number. Either knockout or knockdown of SARA leads to the generation of 2 post-mitotic neurons at the expense of self-renewing mitosisCitation16 (). In this model, SARA segregates differentially into the 2 daughter cells of either symmetric or asymmetric divisions. Interestingly, only in the asymmetric lineages, the daughter cell with accumulated SARA+ endosomes remains proliferative, while the other differentiates into a neuron. Nevertheless, it is not clear why SARA+ endosomes are also unevenly segregated during symmetric mitosis.
In mouse embryonic cortex, we recently showed that SARA labeled endosomes are distributed roughly equally into dividing apical progenitor cells, independently of the mitotic orientation angle (). In these experiments we examined endogenous SARA since ectopically overexpressed SARA may affect endosomal dynamics.Citation13,18-20 Acute knockdown of SARA expression in the developing neocortex does not crucially affect the progenitor asymmetric division nor cell fate choice of daughter cells.Citation17 Accordingly, SARA knockout mouse brains develop to normal size (unpublished).
Surface receptor treadmill is necessary for neuronal migration and morphogenesis
To reach their final destinations, the trajectories of migrating neurons are guided by chemotactic cue-transmitted signaling axes. Neuronal movement requires concerted cytoskeletal dynamics and membrane trafficking, which are coupled to attachment formation between the leading process and the extracellular matrix and/or neighboring cells, as well as detachment at the cell rear.Citation21–23 It is generally thought that chemotactic surface receptors and adhesion molecules have to be sequestered from the cell surface, and later transcytosed and/or reinserted on different locations to ensure a vectorial movement.Citation4 Compromised coordination of the trafficking events leads to migration defects that culminate in neuronal malpositioning within the cortex (), and perhaps aberrant synaptic contacts as well.Citation24
Table 2. Post mitotic events regulated by endocytic components through surface receptors during neurodevelopment.
Cerebellar granule cell precursors (GCPs) migrate along a gradient of brain-derived neurotrophic factor (BDNF). The chemoattraction toward BDNF orients and stimulates neuronal migration in a process that relies on endosomal trafficking and signaling. Upon activation, the BDNF receptor TrkB in the leading process is endocytosed through a Numb-dependent pathway, which in turn stimulates local BDNF secretion. This feedback generates an asymmetric accumulation of signaling endosomes at the subcellular site with the highest level of BDNF. Thus, regulated TrkB endocytosis and resulting BDNF release coordinately determine the migratory direction of GCPs.Citation25,26
By using the strategy to perturb known components of the endosomal trafficking regulation (i.e., Rab5, Rab11) or endocytosis (i.e., clathrin, dynamin), several investigators have highlighted the role of the endosomal trafficking in cortical neuron migration.Citation27,23 In migrating neurons in which Rab5 or Rab11 GTPase are suppressed, N-cadherin which is normally found at the distal and tip region of the leading process, is delocalized to the soma and the proximal region of the leading process.Citation27 In turn, the mislocalization of N-cadherin leads to migration arrest of postmitotic neurons in the intermediate zone (IZ) of the brain cortex. Similarly, interference with dynamin increases the accumulation of β1-integrin and focal adhesion kinase at the cell rear, and hence inhibits cell detachment and movement.Citation23 Moreover, Rab7-dependent lysosomal degradation (of N-cadherin and perhaps other adhesion components) is important for the final phase of somal translocation and dendrite morphogenesis.Citation27 ADP ribosylation factor 6 (Arf6) also regulates N-cadherin trafficking through Rab11 family-interacting protein 3 (FIP3). Knockdown of either Arf6 or FIP3 leads to cytoplasmic accumulation of N-cadherin and neuronal migration arrest at the IZ.Citation28 These results highlight that unbalanced temporal-spatial distribution of adhesion molecules impairs both the location and morphogenesis of cortical neurons.
L1CAM (L1) is also a cell adhesion molecule best known for its importance in axon growth, guidance and fasciculation.Citation29 We and others showed that either up- or down-regulating the expression level of L1 in cortical neurons disrupts their radial migration.Citation17,30 Mutations in the L1 gene have been linked to hydrocephalus in several human congenital brain disorders.Citation29,31 While in vitro studies have shown that the axonal plasma membrane distribution of L1 is regulated by a transcytotic pathway,Citation32,33 how the surface expression of L1 is regulated in migrating neurons was unknown until recently. In the developing neocortex, our work showed that SARA knockdown led to an overall ∼3-fold increase in surface L1 distribution. SARA knockdown, phenocopying L1 overexpression, delayed neuronal migration. This phenotype is concomitant with compromised multipolar-to-bipolar transition of migrating neurons; which get “stuck” at the IZ, and with soma and leading processes misalignment. We also found that SARA-silenced neurons with increased surface L1 preferably bind to their neighboring neurons instead of radial glial cells, as control neurons do.Citation17
In all, a handful of papers have provided the first pieces of evidence about how the endosomal system coordinates different aspects of nervous system development. Even though differential sorting has been implicated in asymmetric mitosis, it seems highly context-dependent. To better decipher the mechanisms underlying endosome-mediated cell fate specification, we will need to delineate the cargoes sorted out, while tracking endogenous endosomal components. This may also clarify contradicting findings coming from different systems. Finally, aside from neurons, other types of cells, such as astroglia, oligodendrocytes, and microglia, also migrate within the developing brain. Whether or not the endosome-mediated turnover of surface receptors also modulates the movement of these cells remains to be explored.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH NEI (EY11307 and EY016805), Starr Foundation, and Research to Prevent Blindness to C-H S. C-H S is a recipient of Research To Prevent Blindness Stein Innovation Award. Travel grants from Journal of Cell Science and International Society for Neurochemistry were awarded to IM. We want to thank M. Lorenzatti and M. Otsu for assistance with .
References
- Cooper JA. Mechanisms of cell migration in the nervous system. J Cell Bio 2013; 202(5):725-34; PMID:23999166; https://doi.org/10.1083/jcb.201305021
- Taverna E, Götz M, Huttner W. The Cell Biology of Neurogenesis: Toward an Understanding of the Development and Evolution of the Neocortex. Annu Rev Cell Dev Biol 2014; 30:465-502; PMID:25000993; https://doi.org/10.1146/annurev-cellbio-101011-155801
- Yap CC, Winckler B. Adapting for endocytosis: roles for endocytic sorting adaptors in directing neural development. Front Cell Neurosci 2015; 9:119; PMID:25904845; https://doi.org/10.3389/fncel.2015.00119
- Kawauchi T. Cellullar insights into cerebral cortical development: focusing on the locomotion mode of neuronal migration. Front Cell Neurosci 2015; 9:394; PMID:26500496; https://doi.org/10.3389/fncel.2015.00394
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nat 2009; 461:947-55; PMID:19829375; https://doi.org/10.1038/nature08435
- Calegari F, Huttner W. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci 2003; 116:4947-55; PMID:14625388; https://doi.org/10.1242/jcs.00825
- Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 1995; 82(4):631-41; PMID:7664342; https://doi.org/10.1016/0092-8674(95)90035-7
- Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron 1999; 23:71-81; PMID:10402194; https://doi.org/10.1016/S0896-6273(00)80754-0
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric Localization of a Mammalian Numb Homolog during Mouse Cortical Neurogenesis. Neuron 2000; 17:43-53; PMID:8755477; https://doi.org/10.1016/S0896-6273(00)80279-2
- Zilian O, Saner C, Hagedorn L, Lee HY, Säuberli E, Suter U, Sommer L, Aguet M. Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol 2001; 11(7):494-501; PMID:11412999; https://doi.org/10.1016/S0960-9822(01)00149-X
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development 2002; 129:4843-53; PMID:12361975
- Johnson SA, Zitserman D, Roegiers F. Numb regulates the balance between Notch recycling and late-endosome targeting in Drosophila neural progenitor cells. Mol Biol Cell 2016; 27(18):2857-66; PMID:27466320; https://doi.org/10.1091/mbc.E15-11-0751
- Hu Y, Chuang JZ, Xu K, Mcgraw TG, Sung CH. SARA, a FYVE domain protein, affects Rab5-mediated endocytosis. J Cell Sci 2002; 115:4755-63; PMID:12432064; https://doi.org/10.1242/jcs.00177
- Coumailleau F, Fürthauer M, Knoblich JA, González-Gaitán M. Directional Delta and Notch trafficking in Sara endosomes during asymmetric cell division. Nat 2009; 458:1051-55; PMID:19295516; https://doi.org/10.1038/nature07854
- Loubéry S, Seum C, Moraleda A, Daeden A, Fürthauer M, González-Gaitán M. Uninflatable and Notch Control the Targeting of Sara Endosomes during Asymmetric Division. Curr Bio 2014; 24(18):2142-8; PMID:25155514; https://doi.org/10.1016/j.cub.2014.07.054
- Kressmann S, Campos C, Castanon I, Fürthauer M, González-Gaitán M. Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat Cell Biol 2015; 17(3):333-9; PMID:25706234; https://doi.org/10.1038/ncb3119
- Mestres I, Chuang JZ, Calegari F, Conde C, Sung CH. SARA regulates neuronal migration during neocortical development through L1 trafficking. Development 2016; 143:3143-53; PMID:27471254; https://doi.org/10.1242/dev.129338
- Itoh F, Divecha N, Brocks L, Oomen L, Janssen H, Calafat J, Itoh S, Dijke PT. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes to Cells 2002; 7(3):321-31; PMID:11918675; https://doi.org/10.1046/j.1365-2443.2002.00519.x
- Seet LF, Hong W. Endofin, an Endosomal FYVE Domain Protein. J Biol Chem 2001; 276:42445-54; PMID:11546807; https://doi.org/10.1074/jbc.M105917200
- Arias CI, Siri SO, Conde C. Involvement of SARA in Axon and Dendrite Growth. PLoS ONE 2015; 10(9):e0138792; PMID:26405814; https://doi.org/10.1371/journal.pone.0138792
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature 2007; 448:901-7; PMID:17713529; https://doi.org/10.1038/nature06063
- Jossin Y and Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 2011; 14:697-703; PMID:21516100; https://doi.org/10.1038/nn.2816
- Shieh J, Schaar B, Srinivasan K, Brodsky F, McConnell S. Endocytosis regulates cell soma translocation and the distribution of adhesion proteins in migrating neurons. PLoS ONE 2011; 6:e17802; PMID:21445347; https://doi.org/10.1371/journal.pone.0017802
- Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, Takeuchi A, Miyazaki T, Watanabe M, Yuasa S, Manabe T, Wakabayashi K, Kaneko S, Saito T, Ohno H. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Bio 2004; 167(2):293-302; PMID:15492041; https://doi.org/10.1083/jcb.200405032
- Zhou P, Alfaro J, Chang EH, Zhao X, Porcionatto M, Segal R. Numb Links Extracellular Cues to Intracellular Polarity Machinery to Promote Chemotaxis. Dev Cell 2007; 20(5):610-22; PMID:21571219; https://doi.org/10.1016/j.devcel.2011.04.006
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal R. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron 2011; 55:53-68; PMID:17610817; https://doi.org/10.1016/j.neuron.2007.05.030
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo KI, Nakajima K, Nabeshima YI, Hoshino M. Rab GTPases-Dependent Endocytic Pathways Regulate Neuronal Migration and Maturation through N-Cadherin Trafficking. Neuron 2010; 67(4):588-602; PMID:20797536; https://doi.org/10.1016/j.neuron.2010.07.007
- Hara Y, Fukaya M, Sakagami H, Hayashi K, Kawauchi T. ADP Ribosylation Factor 6 Regulates Neuronal Migration in the Developing Cerebral Cortex through FIP3 / Arfophilin-1-dependent Endosomal Trafficking of N-cadherin. eNeuro 2016; 6:e0148-16; PMID:27622210; https://doi.org/10.1523/ENEURO.0148-16.2016
- Kamiguchi H, Hlavin ML, Lemmon V. Role of L1 in neural development: what the knockouts tell us. Mol Cell Neurosci 1998; 12:48-55; PMID:9770339; https://doi.org/10.1006/mcne.1998.0702
- Kishimoto T, Itoh K, Umekage M, Tonosaki M, Yaoi T, Fukui K, Lemmon VP, Fushiki S. Downregulation of L1 perturbs neuronal migration and alters the expression of transcription factors in murine neocortex. J Neu Res 2013; 91:42-50; PMID:23073969; https://doi.org/10.1002/jnr.23141
- Weller S and Gärtner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): Mutations in the L1CAM gene. Hum Mutat 2001; 18(1):1-12; PMID:11438988; https://doi.org/10.1002/humu.1144
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Fölsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Bio 2003; 162(7):1317-28; PMID:14517209; https://doi.org/10.1083/jcb.200307069
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Hirling H, Klumperman J, Winckler B. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J Cell Bio 2008; 180(4):827-42; PMID:18299352; https://doi.org/10.1083/jcb.200707143
