Abstract
Persons with severe non-fluent aphasia would benefit from using gestures to substitute for their absent powers of speech. The use of gestures, however, is challenging for persons with aphasia and concomitant limb apraxia. Research on the long-term recovery of gestures is scant, and it is unclear whether gesture performance can show recovery over time. This study evaluated the recovery of emblems and tool use pantomimes of persons with severe non-fluent aphasia and limb apraxia after a left hemisphere stroke. The Florida Apraxia Screening Test-Revised (FAST-R) was used for measurements. The test includes 30 gestures to be performed (i) after an oral request, (ii) with the aid of a pictorial cue, or (iii) as an imitation. The gestures were rated on their degree of comprehensibility. The comprehensibility of gestures after an oral request improved significantly in five out of seven participants between the first (1–3 months after the stroke) and the last (3 years after) examination. Improvement continued for all five in the period between six months and three years. The imitation model did improve the comprehensibility of gestures for all participants, whereas the pictorial cue did so just slightly. The skill of producing gestures can improve even in the late phase post-stroke. Because of this potential, we suggest that gesture training should be systematically included in the rehabilitation of communication for persons with severe non-fluent aphasia.
Introduction
Aphasia, resulting from a stroke in the left Perisylvian region, is an acquired language disorder that causes linguistic problems in speech (word finding, phrasing, and sentence construction) and understanding speech, as well as difficulties in writing and reading (Hertrich et al., Citation2020; Papathanasiou & Coppens, Citation2022). Apraxia, resulting from a stroke with lesions in the parietotemporofrontal areas (Dressing et al., Citation2021; Leiguarda & Marsden, Citation2000; Rounis et al., Citation2021) is a motor planning and execution disorder where difficulties in producing voluntary, learned, and intentional movements cannot be explained by muscle paralyzes, aphasia, attention, or motivation (Goldenberg, Citation2013; Rothi & Heilman, Citation2015).
Gestures function naturally alongside speech but can also be used to replace speech (Auer & Bauer, Citation2011; Wagner et al., Citation2014). Persons with aphasia (PWA) use gestures as a compensatory means of communication (De Beer et al., Citation2019; Hogrefe et al., Citation2017; Sekine & Rose, Citation2013), however their gestures are known to be more unclear than the gestures of healthy speakers (Glosser et al., Citation1986; Herrmann et al., Citation1988; Hogrefe et al., Citation2017; Sekine & Rose, Citation2013). Glosser et al. (Citation1986) detected that, in face-to-face dyadic interactions, persons with moderate aphasia and less verbalization produced semantically less complicated and more unspecific gestures than persons with mild aphasia and better verbalization. Hogrefe et al. (Citation2017) had similar findings, noticing that the reduced verbal output and a likewise reduced ability to produce comprehensible gestures were associated for PWA. Herrmann et al. (Citation1988) observed that, in natural interactions, persons with severe non-fluent aphasia produced simple gestures that were restricted mainly to head nods and shakes and shoulder shrugs, instead of more complex gestures that could replace absent speech. As for persons with non-fluent aphasia (Broca or conduction aphasia), Sekine and Rose (Citation2013) found that they produced more linguistically meaningful gestures, than persons with fluent aphasia (Wernicke). In any case, a recent review by De Kleine et al. (Citation2023) emphasizes that gestures alongside speech enhance the overall level of communication success for PWA of any severity.
Persons with severe non-fluent aphasia, with absent or profoundly limited speech output but often better language comprehension, would especially benefit from the use of gestures as a communicative means. However, concomitant limb apraxia, which often co-occurs with severe non-fluent aphasia (Duffy, Citation2020; Hogrefe et al., Citation2017; Hybbinette et al., Citation2021; Roby-Brami et al., Citation2012; Weiss et al., Citation2016), hampers the use of gestures (De Kleine et al., Citation2023). It is known that gesture performance can be improved through systematic training (Rose et al., Citation2013). It is not known, however, whether gestures show overall long-term spontaneous recovery for persons with severe apraxia-aphasia. Thus, better knowledge of the potentiality for the recovery of gestures would help clinicians in planning the communication methods used in speech and language therapy for persons with severe apraxia-aphasia.
Limb apraxia and its influence on gestures
Before deliberate action can be produced expediently, the concept of the action needs to be comprehended and appropriately planned (Goldenberg, Citation2013; Rothi & Heilman, Citation2015). This process can be disturbed due to problems in a conceptual system (i.e., semantics), or problems in a production system (i.e., movement planning). Problems in both systems are often present in limb apraxia (Leiguarda & Marsden, Citation2000).
Limb apraxia influences the ability to use gestures and the comprehensibility of gestures (Borod et al., Citation1989; De Kleine et al., Citation2023; Hogrefe et al., Citation2012, Citation2013), whereas good comprehension in general, for those without limb apraxia, is connected to the effective use of gestures (Akhavan et al., Citation2018). Limb apraxia affects the manipulation of objects, making their use clumsy and prone to errors, and also affects the performance of gestures, making them difficult to identify (Goldenberg, Citation2013; Rothi & Heilman, Citation2015). Praxic errors in gestures are typically categorized into content, temporal, and spatial errors (Rothi & Heilman, Citation2015, pp. 61–66). Content errors are, for example, cases where the produced gesture is not related to the target gesture. Temporal errors are exemplified by cases where the timing or sequencing of movements is incorrect, or the gesture includes additional movements that make the gesture inaccurate. Spatial errors can be exemplified by cases where the positions of arms and/or fingers are not specific to the target gesture.
Evaluating the gestures of a person with aphasia
The ability of PWA’s to use gestures has been studied by observing gesture production in natural communicative situations (De Beer et al., Citation2019; Rose et al., Citation2017) as well as situations that resemble spontaneous communication: for example, gesture use in semi-structured interviews (Glosser et al., Citation1986; Sekine & Rose, Citation2013), and constructing narratives from a picture (De Beer et al., Citation2019) or a video (Hogrefe et al., Citation2013, Citation2017). Spontaneous communication in natural interactions is considered the best way to capture the real use of gestures among PWA (De Ruiter, Citation2006; Glosser et al., Citation1986; Rose et al., Citation2017). The gestures used in natural communication are supported by their context, which can be one reason why PWA’s are known to gesture spontaneously even though their gesture performances in a formal test situation are deficient. Nonetheless, gestures have also been studied using structured tests, where the subject is asked to perform gestures (typically tool use pantomimes) after an oral request or imitation (Mimura et al., Citation1996; Rothi & Heilman, Citation2015; Stamenova et al., Citation2011). These are often referred to as pantomime/gesture-to-command tasks. The benefits of using such structured tests are that they are easy to implement in therapy settings and can be repeated similarly in a follow-up of the performance. A structured test can also eliminate the conversational partner’s influence on the interaction and the subjects’ performance (e.g., Klippi, Citation2006). Another advantage is that there is a specific and defined target gesture, allowing accuracy and error types to be scored more systematically (as in Hogrefe et al., Citation2012, Citation2013, Citation2017).
A few studies have compared how gestures are produced in structured test versus spontaneous communication (Borod et al., Citation1989; Hogrefe et al., Citation2012, Citation2013, Citation2017). In studies by Hogrefe et al. (Citation2012, Citation2013, and 2017) PWA’s performance in gesture-to-command tests correlated positively to the comprehensibility of gestures in the retelling of a narrative by using only gestures as a communicative means. Gesture performance in these studies was examined by asking the participants (N 24, 16, and 30, of which most had global aphasia) to mime the use of everyday objects with the aid of seeing a picture of the object. In a study by Borod et al. (Citation1989) of 41 participants with a left hemisphere stroke, poor performance in a formal pantomime test correlated with ineffective gestural communication in everyday life. These studies provide support for the valid use of the test and suggest that poor performance in a gesture-to-command task, even though it is an artificial condition, correlates with inadequate spontaneous gesturing.
For gestures to function as a replacement for speech in severe non-fluent aphasia, the emphasis should be on gestures that can convey a comprehensible linguistic meaning without speech. Gesture types that can substitute for speech include deictics (pointing movements), iconic (body movements according to the content of the speech, e.g., moving one’s arms when referring to running), emblems (symbolic gestures that have a specific learned meaning, e.g., the “thumbs up” sign), and tool use pantomimes (gestures representing the use of an object) (Wagner et al., Citation2014).
Recovery of gesture use in aphasia
Apart from intervention studies of gesture use in aphasia (Alashram et al., Citation2022; Rose et al., Citation2013), long-term follow-up studies about gesture recovery are scarce. The most significant recovery from a stroke overall occurs within the first few months post-stroke (Papathanasiou & Coppens, Citation2022), although long-term follow-up studies on recovery from aphasia have suggested that improvement is possible even at the chronic stage post-stroke (Harvey et al., Citation2022; Holland et al., Citation2017; Johnson et al., Citation2019; Nakagawa et al., Citation2019). Nevertheless, while limb apraxia often co-occurs with severe non-fluent aphasia (Duffy, Citation2020), knowledge about long-term recovery from apraxia in PWA’s is mostly lacking (Lemmetyinen et al., Citation2020). Most of the studies have analyzed only imitated gestures, and the focus has been on the recovery of limb apraxia after the stroke, not gestures as a means of communication for persons with aphasia (Basso et al., Citation1987, Citation2000; Donkervoort et al., Citation2006; Dressing et al., Citation2021). Stamenova et al. (Citation2011) reported that participants who were impaired in gesture tasks (pantomime by verbal command, by picture, or imitation) did show some improvement in the acute and subacute stage (within three months) post-stroke, but no clear improvement was noticed in the chronic stage (after three months) in the two-year follow-up. The study included participants both with left and right hemisphere lesions but did not mention whether any had aphasia.
To our knowledge there are only two studies that have specifically addressed the recovery of gestures for persons with aphasia. A group study by Mimura et al. (Citation1996) noted a significant improvement in gestures after an oral request between examinations at 4,5 months and nearly seven years post-stroke. However, valid conclusions cannot be drawn regarding the gesture recovery of persons with severe non-fluent aphasia, as the study included persons with both fluent and non-fluent aphasia. Furthermore, no information was given on the exact timing of the recovery within the 4,5 months to 7 years time period. One more study (a dissertation by Braddock, Citation2007) noted slight, albeit not significant, improvement in tool use pantomimes and emblems for persons with Broca’s aphasia during the six months follow-up from early-stage post-stroke.
The aims of the study
This study aimed to evaluate the long-term recovery of gestures (emblems and tool use pantomimes) produced after an oral request in persons with initially severe non-fluent aphasia and limb apraxia after a left hemisphere stroke. The potential recovery of gestures with this challenging communication disorder is a significant and highly understudied clinical question. We expect that the knowledge our study adds to the field will help clinicians predict whether the use of gestures have an overall potential to improve over time for persons with severe apraxia-aphasia.
The main research question is as follows: (1) Does the comprehensibility of the gestures that participants produce after an oral request in the test situation show improvement over three-year post-stroke follow-ups? In addition, we were interested in studying: (2) Does the improvement differ for emblems and tool use pantomimes; (3) Does seeing a picture of the relevant object improve the comprehensibility of gestures; and (4) Does imitation improve the comprehensibility of gestures?
Methods
Participants
The study included seven right-handed participants with Finnish as their native language (see for the background information of each participant). The participants met the following inclusion criteria: severe non-fluent aphasia and limb apraxia, left hemisphere stroke no later than three months before the first examination; preferably aged no more than 75 years (considering the long-term follow-up and general health with aging); no previous neurological or psychiatric illnesses, as confirmed from medical records; and an adequate understanding of speech in everyday conversation, confirmed by an informant (next of kin or health care personnel with a close relationship to the participant). At the time of inclusion, the participants met the diagnostic criteria of severe initial aphasia according to the Western Aphasia Battery (WAB), specifically a standardized Finnish version (Kertesz et al., Citation2005). The aphasia quotient (AQ) measures the skills of spoken language (speech and auditive verbal comprehension) and reveals the overall severity of aphasia. The limit value for an aphasia diagnosis in WAB is 93.8, and scores below 50 indicate severe or very severe aphasia (Kertesz, Citation1982). See for the participants’ AQ scores. Participants had an absent or minimal spontaneous speech output (the scores in WAB: naming between 0–8/60, fluency 0–2/20, and speech answers 0–2/10). The limb apraxia scores in the WAB at the first examination (see ) varied between 7–46/60.
Table 1. Selected data on study participants (P). The table shows the scores of the examinations given at the first evaluation time post-stroke.
The participants’ mean age was 69 years (standard deviation, SD, 5.8) at the onset of the stroke. The age of participant 1 was over 75 years at the time of onset, but she was in very good condition in relation to her age. All of the participants had a left hemisphere infarct. Most of the participants had functionality only in the left arm, but some could still use the right arm for assistance. See for relevant details about the participants.
Before the stroke, the participants had been independent in their daily lives and either lived with a spouse or alone without outside help. Three of the participants with total right-sided hemiplegia (P4, P6, and P7) needed physical help with activities of daily living after the stroke. Two of the participants, P2 and P5 with right-sided weakness, needed occasional minor physical help. Two others, P1 with mild right arm weakness and P3 with right arm plegia, remained physically independent.
Outside of this study, all of the participants received speech and language therapy (SL therapy) in differing amounts during the follow-up period. The length of the therapy varied between the first-year post-stroke and continuing through the end of the follow-up. SL therapy was the most intensive, implemented from two to five times a week, in the first weeks or months post-stroke, which was the period that participants stayed at the rehabilitation center. After discharge from the center, the intensity varied between once a week to twice a week. This information on SL therapy was received either from the participant or their next of kin. The exact content of the SL therapy was not detailed for this study. It is known, however, that the therapy did not include systematic gesture training.
Examination and scoring of gestures
Besides the short apraxia section in the Finnish version of the WAB (Kertesz et al., Citation2005) other standardized comprehensive tests that could be used to evaluate the gesture performance of PWA’s have not been made available to speech and language therapists (SL therapists) in Finland. For this study, gestures were examined by the Florida Apraxia screening test, revised (FAST-R) (Rothi & Heilman, Citation2015, pp. 61–66), which includes 30 gesture tasks with 20 transitive (i.e., tool use pantomimes) and 10 intransitive gestures (i.e., emblems) (see Supplementary Material, Task S1). The tasks were translated from the English version to Finnish, except for two tasks that were modified to be more suitable to the Finnish culture. In this adaptation, task 29, which was originally a transitive gesture (“How to use an ice pick to chop ice in front of you”), was changed to “how to pound a fist on the table”. In our study this gesture was considered intransitive. A table was positioned in front of the participants during the examination, and it thus would not have been relevant to show them a picture of a table. Therefore, the Finnish version of FAST-R in our study included 11 intransitive gestures and 19 transitive gestures.
First, the instruction was given orally (e.g., “Show me how to drink from a glass”), and if the produced gesture was clearly comprehensible (clearly corresponded to the intended gesture) the examiner then moved on to the next gesture. However, if a transitive gesture was unclear (partially corresponded to the targeted gesture), not recognizable, or no action was produced, the examiner showed a picture of an associated tool (e.g., a glass). If the gesture was still deficient, the examiner showed a model of the gesture for imitation. Due to the nature of intransitive gestures, they did not include a pictorial cue. If an intransitive gesture was unclear, not recognizable, or no action was produced, the examiner gave a model of the gesture for imitation (see the example of scoring in the Supplementary Material, Table S2).
The healthy control group was not tested with FAST-R in this study. A similar type of test, “Florida Apraxia Battery–Extended and Revised Sydney”, was used for testing healthy elders in a study by Power et al. (Citation2010) where the healthy participants received almost full scores without any praxis errors even though they used their non-dominant left hand in order to be better comparable to persons with right-sided hemiplegia. Therefore, clear errors in FAST-R were determined to reflect impairment of praxis skills in the present study.
To our knowledge, a standardized scoring system for FAST-R has not been previously available. The scoring for this study was developed by the first author with the following criteria: the maximum score for the section on oral requests was 60 (transitive and intransitive gestures, 30 gestures in total), for the section on pictorial cues 38 (19 transitive gestures), and for the section on imitations 60 (transitive and intransitive gestures, 30 in total). The performance of each task (gesture produced after an oral request, after a pictorial cue, or after showing a model of a gesture) was given a score of two if the gesture was interpreted as comprehensible. The performance score was one if the gesture was unclear but resembled the targeted gesture. The performance score was zero if the gesture was not at all recognizable, the performance was not associated with the intended gesture, or the participant did not attempt to perform any gesture. To be more exact, the comprehensibility of the gesture was assessed according to the evaluator’s judgment of how well the gesture corresponded to the targeted gesture: was it clear and communicatively meaningful (i.e., was the gesture comprehensible itself without speech). Only gestures produced by a hand were accepted. For example, the request “show me, be quiet” got a score of zero if it was performed only by protruding lips while making a hissing sound. The advantage of this type of scoring is in its relevance for natural communicative situations: judging the meaning of gestures simply by how comprehensible they are, instead of focusing on the spatial or temporal errors. A similar type of scoring system was used in the studies by Borod et al. (Citation1989) and Mimura et al. (Citation1996): scoring gestures with a scale from unrecognizable to recognizable, or inadequate to adequate.
Altogether, five examinations of each participant were conducted within three years by the first author: the first examination was within one to three months post-stroke, the second at six months, then at one year, two years, and three years post-stroke. FAST-R was conducted once during each examination point for each participant. All tasks were conducted with the participant seated at a table and were video recorded for later scoring.
Examination of speech comprehension
To ensure the participants were able to understand the oral requests in FAST-R, their understanding of short, simple sentences was examined using Yes/No questions developed for this study. The Yes/No Questions were administered during the same examination times as the FAST-R follow-ups (see the individual scoring of Yes/No Questions in Supplementary Material, Table S1). The Yes/No questions include 50 simple, short questions requiring only “yes” or “no” answers (see Supplementary Material, Task S2), without any requirement for conducting actions with one’s hands. Taking into account the possibility that the participant understood the question but had difficulty giving the desired answer, participants were allowed to give yes or no answers by whatever means available: speech, gestures, voicing, or facial expressions. A participant’s responses were interpreted to be either unclear or clear. The answer was unclear if, for example, the speech response (yes or no) was inconsistent with the gesture (head nod or shake), or the participant produced only unclear voicing without a clear affirmative or negative prosody. The correctness of the answer (whether it was true or false) was also evaluated and, if necessary, verified by the next of kin. Correct and clear yes or no answers were given a score of two, incorrect but clear answers a score of one (the participant’s answer was clearly interpreted as affirmative or negative, but the given information was wrong), and unclear answers a score of zero.
In the Yes/No Questions, the second (6 months) examination of P7 was omitted due to a corruption of the recording. Other data were also missing in 13 items across the examinations: 1 for P3, 11 for P4, and 1 for P7. The Yes/No Questions were analyzed from recordings by the research assistant (a student of speech and language pathology). The reliability of the analyses was evaluated by peer review for 21% of the data; the first author of this study analyzed seven random examinations. The randomization was done by blind selection by a third person. The level of agreement for the scoring was evaluated with SPSS using intraclass correlation coefficient (ICC); the agreement was found to be very good: ICC(2,1) = 0.97.
Examination of visual perception
To ensure that the participants were able to recognize the pictorial cues in FAST-R, their visual recognition was examined using three Poppelreuter overlapping figures (Christensen, Citation1979). The test involves three cards with overlapping line drawings of 4 or 5 everyday objects in each, and a sheet with all 14 objects drawn separately. A subject is asked to point to the objects from the sheet that they recognize as corresponding to the overlapping line drawings. The maximum correct score is 14. In the Poppelreuter test, P1, P2, and P3 had scores indicating no clear problems in visual perception (see ). P7 scored 8/14, but according to the video recordings she correctly pointed to each item from the overlapping line drawings, only failing to point to some examples from the corresponding separate pictures. This implies the problem was not in visual perception but was rather a difficulty in following the test instructions. P4, P5, and P6 were unable to perform the test: P4, after a manual example, pointed out some incorrect items, while P5 did not even try to do the test. The pointing gestures of P6 were too unclear to be scored.
Data analysis of FAST-R
The data were processed in MS Excel. The total number of gestures evaluated in this study was 936. The total number of gesture tasks evaluated was 2101, as gestures were performed (i) after an oral request, (ii) a pictorial cue, and (iii) imitation (see Supplementary Material, Table S1). The pictorial cues and imitation were performed under specific conditions explained later in this section. All analyses were conducted by the first author. The analyses of each examination were carried out in random order (not in chronological order or on a case-by-case basis). The reliability of the analyses was evaluated by peer review for 20% of the data, which included a total of 406 gestures; the third author analyzed all examinations conducted on participants at one- and three-years post-stroke from gestures produced after an oral request. The agreement level of the scoring was evaluated on a group level with SPSS using Cohen’s kappa (Landis & Koch, Citation1977). The agreement level was found to be good (kappa 0.74 at one-year post-stroke) or very good (kappa 0.81 at three years post-stroke).
Gestures performed after an oral request was scored first. Transitive and intransitive gestures were analyzed also separately. All of the results are given as proportion percentages of the total score, as the maximum score differs both between the task types and individually. To analyze the influence of pictorial cues on gesture performances, only transitive gestures were used; the scores of gestures produced with pictorial cues were compared with scores of gestures produced with the oral request only. Similarly, to analyze the influence of imitation the scores of gestures (both transitive and intransitive) produced after imitation were compared with scores of gestures produced after an oral request only. During an examination, the participant was not given further cues if the gesture was judged as clearly comprehensible already after an oral request. However, when later analyzing the gestures through video recordings, some of the performances originally judged to be comprehensible after an oral request were interpreted to be unclear. In these cases, a participant was not able to achieve points for the gesture after a pictorial cue or imitation because further cues had not been offered. These gestures were not included in the total scores of analyses of a pictorial cue or imitation. In addition, the maximum score (2) was given also for the pictorial cue condition or imitation if the maximum score was obtained already after the oral request (see an example of the scoring in Supplementary Material, Table S2).
Part of the data was missing for three participants. P4 had four examinations instead of five during the follow-ups. For P5, the recording of the fourth examination (two years post-stroke) was corrupted and could not be used in this study. The first examination of P7 was discontinued after five gestures. Furthermore, altogether 27 gestures (from the total amount of 936) were excluded from the analysis, as the pictorial cue was erroneously given at the same time as an oral request (1 time for P1, 1 for P2, 6 for P4, 3 for P5, 2 for P6, and 14 for P7).
Statistical analyses were implemented on a group level to determine whether the difference between the tasks or the changes over time reached a level of statistical significance; the significance level was set at 0.05. The statistical analyses were performed using non-parametric statistics in SPSS version 28. The change in the repeated measurement of gestures after the oral request was analyzed with the Wilcoxon signed-rank test. The performance for transitive and intransitive gestures at all time points combined, as well as the performance with the aid of pictorial cues and imitation at all time points combined, were compared using the Mann-Whitney U-test.
Ethics
The study was performed in accordance with the principles in the Declaration of Helsinki. The research was approved and implemented in accordance with the policies of the local social and healthcare authorities in Southern Finland, the area where the participants lived. After receiving both oral and written information about the study, including information on any spouse or next of kin, the participants provided informed consent to participate in the study, and voluntary participation was ensured at every stage of the follow-up. All of the originally recruited participants remained in the study until the end of the follow-up process.
Results
Each research question is examined separately in the following subsections. The raw scores of the tasks for each participant in the FAST-R are summarized in the Supplementary Material, Table S1. The initial scores in FAST-R varied between 0–32/60 in gestures produced after an oral request, and between 4–41/60 after imitation.
Comprehensibility of gestures produced after an oral request
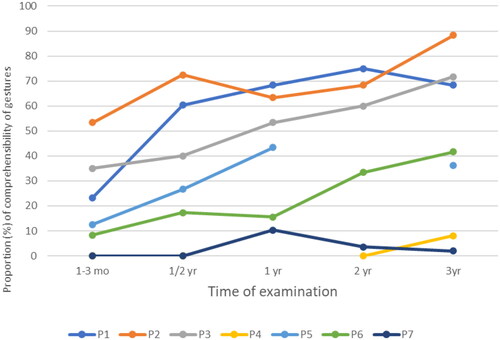
On a group level, the difference in mean performances of gestures produced after an oral request between the first (1–3 mo) and the last (3 yrs) examinations was statistically significant, according to the Wilcoxon signed-rank test. When examining the examination intervals, only the difference between 1–3 months and 6 months was statistically significant. displays the significance values and the scores on a group level.
Table 2. The mean percentages of comprehensibility of gestures produced after an oral request during five different examination times, and the difference in means between the examinations as measured by the Wilcoxon signed-rank test. The results are presented on a group level.
Table 3. Individual mean percentages of comprehensibility of transitive and intransitive gestures produced after an oral request, all time points from 1–3 months to 3 years combined.
On an individual level (see ), five (P1, 2, 3, 5, and 6) out of seven participants showed a clear improvement in the comprehensibility of gestures after an oral request in FAST-R during the three-year follow-up. All five improved within the first six months post-stroke. All five continued to improve between six months and three years post-stroke, but the pace varied. Three participants (P1, 3, and 5) showed improvement between 6 months and one-year post-stroke, four (P1, 2, 3, and 6) between one and two years post-stroke, and three (P2, 3, and 6) still between two and three years post-stroke. Two participants (P4 and 7) had very poor skills in gesture performance already at the early stages post-stroke, and they did not show noticeable improvement during the follow-ups.
Comparing performance in transitive (tool use pantomime) and intransitive (emblem) gestures
On a group level, the mean proportional percentage for all time points combined was 31.6 (SD 27.3) for transitive gestures and 31.4 (SD 30.1) for intransitive gestures. The difference between the performances, as analyzed with the Mann-Whitney U-test, was not significant (p = 0.710).
On an individual level, there was no noticeable difference between transitive and intransitive gestures produced after an oral request across the follow-up for P1, P3, P4, and P6. However, three participants (P2, P5, and P7) produced some more comprehensible intransitive gestures than transitive gestures (see for individual scores). The few gestures that P7 was able to perform with some comprehensibility were of the intransitive type.
Gestures produced after a pictorial cue or imitation
Pictorial cue
On a group level, the mean proportional percentage of all time points combined for comprehensibility of transitive gestures produced after an oral request was 33.5 (SD 31.3), and after a following pictorial cue 40.7 (SD 32.2). The difference, analyzed with the Mann-Whitney U-test, between these two performance types across the follow-ups was not statistically significant (p = 0.620). On an individual level, a pictorial cue seemed to improve the comprehensibility of gestures produced after an oral request for all participants during follow-ups, except for P3, although the improvement was subtle. P5 benefited from a pictorial cue the most, with a 22.3% difference favoring gestures performed after a pictorial cue over an oral request alone. See for individual scores for all time points combined and the Supplementary Material, Table S1, for the raw scores from each task.
Table 4. Individual scores for the comprehensibility of gestures produced after an oral request in relation to pictorial cue and imitation in FAST-R, with all time points combined.
Imitation
On a group level, the mean proportional percentage of all time points combined for comprehensibility of gestures (transitive and intransitive) produced after an oral request was 34.0 (SD 26.8), and after the imitation 62.3 (SD 18.1). The difference on a group level, as analyzed with the Mann-Whitney U-test, between these two performance types across the follow-ups was not statistically significant (p = 0.073). On an individual level, the comprehensibility of gestures seemed to improve with imitation for all participants during the follow-ups. The improvement varied between 17.4% (P2) and 43.8% (P4) favoring imitation over oral requests (see . for individual scores). In particular, P4 and P7 were able to produce some gestures after imitation, while otherwise their performances were extremely weak.
Discussion
This study aimed to determine whether seven participants with left hemisphere stroke and initially severe non-fluent aphasia and limb apraxia showed improvement in gesture performance in a formal gesture-to-command task over a series of three-year follow-ups. Knowledge about such potential recovery is needed, as persons with severe non-fluent aphasia may benefit from the effective use of gestures as a compensatory means to replace absent speech. Although the study comprises only a limited number of individual cases, due to the scarcity of published literature on the recovery of gestures our findings bring new knowledge to the field. The results indicate that most of the participants’ (5/7) gesture performance improved up to three years post-stroke. The progress was not limited to the first months post-stroke, although the greatest improvement occurred in the first six months, which is in line with earlier stroke-related studies of recovery. Both types of gestures studied, emblems (intransitive gestures) and tool use pantomimes (transitive gestures), showed improvement, without a noticeable difference in performances. When the gesture produced after an oral request was not comprehensible enough, seeing a model for imitation clearly improved the performance of gestures, whereas seeing a picture of the relevant object did not.
Improvement of gestures produced after an oral request
Five out of seven participants showed improvement in gesture performances produced after an oral request during the follow-up. At a group level, the progress between the first three months and three years post-stroke was statistically significant. The most noticeable, and statistically significant, improvement at the group level occurred within the first six months post-stroke. On a group level, the improvement was no longer statistically significant at later stages, however individual progress continued beyond six months for five out of seven participants, and for three participants the progress continued until the end of follow-ups.
Similar to our study, Mimura et al. (Citation1996) and Braddock (Citation2007) followed up on gesture performance after an oral request for persons with aphasia after a left hemisphere stroke. Mimura et al. (Citation1996) showed significant recovery between 4,5 months and nearly seven years post-stroke, but the study lacked specific information on gesture performance within this period. Therefore, it is not possible to form conclusions about the specific timing of the recovery. In the study by Braddock (Citation2007), gesture performances indicated significant improvement within six months post-stroke, which was the entire period of the follow-up. We extended our examination beyond the early phases, because the most evident recovery from the symptoms of cerebral vascular accidents is already known to occur within the first few months post-stroke (Papathanasiou & Coppens, Citation2022).
Comparing performance of emblems and tool use pantomimes
Three out of seven participants seemed to perform better in emblems (i.e., intransitive gestures) than in tool use pantomimes (transitive gestures). On a group level, the difference favoring emblems across the follow-up compared with tool use pantomimes was small and without statistical significance. However, the results are in line with those of Belanger et al. (Citation1996), who noted that emblems appeared easier to perform than tool use pantomimes for subjects with a left hemisphere stroke (Leiguarda & Marsden, Citation2000).
Emblems are used for commenting (e.g., a “thumbs up”), regulating the behavior of others (e.g., a sign to stop), or expressing one’s emotional state or opinion (e.g., shaking a fist) (McNeill, Citation2005). An emblem can be produced after deliberate thinking, but it can also be a spontaneous reaction in a communicative situation. A tool use pantomime, instead, always requires considered cognitive planning; before the action, a correct mental image of the object and its appropriate use must be formed (Goldenberg, Citation2013). This may explain why emblems were easier to perform than tool use pantomimes for some participants in this study.
According to the study by van Nispen et al. (Citation2017), the linguistically meaningful gestures that PWA’s are observed to use most often are emblems, concrete deictics, and iconic gestures (Sekine et al., Citation2013). Only persons with Broca’s aphasia (having relatively good comprehension, but laborious and scarce speech) were observed to produce tool use pantomimes to compensate for a difficulty in finding words. Healthy speakers typically use tool use pantomimes only in situations where speaking is not allowed or cannot be heard, whereas emblems are also frequently used by healthy speakers (Goldenberg, Citation2013; Goldin-Meadow, Citation2023; Wagner et al., Citation2014). Emblem gestures are used considerably more often in spontaneous speech than tool use pantomimes, which also might explain why emblems were easier to perform than tool use pantomimes for some participants in this study.
To conclude, the level of improvement in using emblems compared to tool use pantomimes was not noticeable for the participants in our study. Therefore, associated conclusions, for example regarding which one would have the more potential to progress, cannot be drawn.
Considering the aid of a pictorial cue in gesture performance
It can be difficult to distinguish whether the poor performance in a gesture-to-command task of a person with aphasia is due to limb apraxia, problems in understanding the requests, or problems in visual perception. Showing a picture of an object is assumed to help one to obey the request, as a picture can function as a concrete cue for a targeted tool use pantomime, facilitating the performance of the gesture (Goldenberg, Citation2013; Rothi & Heilman, Citation2015). In general, a pictorial cue seemed to improve the comprehensibility of gestures slightly for six out of seven participants in our study, but this change was not statistically significant on a group level.
The phenomenon could reflect some uncertainty in understanding oral requests in FAST-R. However, participants already performed noticeably better in the task of Yes/No Questions than in gestures produced after an oral request at the first examination in the early stages post-stroke, and this tendency continued until the end of the follow-ups. An exception was P2, whose performance between these two tests did not show a noticeable difference during the follow-ups. Better performance in Yes/No Questions in relation to FAST-R implies that the participant’s speech comprehension for simple and short sentences was good, and the problem in performing comprehensible gestures was caused by limb apraxia rather than problems in understanding oral requests.
A stroke can also cause problems in visual perception, as well as visual inattention toward the right hemispace, although such neglect tends to be more severe in right rather than left hemisphere lesions (Murray, Citation2012). The participants in our study did not display noteworthy right hemispace inattention during examinations. Also, the pictures of objects in FAST-R used in this study were simple, with a plain white background, and therefore easy to recognize. As was described in the methods section, at the beginning of the follow-up three participants (P4, P5, P6) were not able to perform the test of visual recognition. Based on the observations on video recordings during the first examinations, the problem in performing the task seemed to be due to difficulty in following or understanding the test instructions; these participants communicated confusion with facial expressions or gestures (head shakes, shoulder shrugs) and did not even try to perform the task. In this context, it should be kept in mind that deficient performance can also be due to other factors than deficient comprehension due to aphasia or deficient action performance due to paresis or apraxia (also Rounis et al., Citation2021). A stroke can cause difficulties in dividing and maintaining visual attention in a task, difficulties keeping the instructions in mind, or in keeping motivated if the task feels too complicated (e.g., Papathanasiou & Coppens, Citation2022). Overall, it is not surprising that adding more material to process, such as a pictorial cue to the oral request, does not improve the performance for all persons with apraxia-aphasia following a stroke.
Imitation of emblems and tool use pantomimes
Imitation seemed to improve the recognizability of the gestures overall for all participants, although the improvement did not reach a statistical significance on a group level. Imitated gestures for individuals with limb apraxia, as well as for healthy controls (see Power et al., Citation2010), are often easier to produce than gestures after an oral request or a pictorial cue (e.g., Goldenberg, Citation2013; Leiguarda & Marsden, Citation2000). This can be explained by a dual-route model in which the cognitive processes underlying praxis include conceptual and/or visuospatial systems (Baumard et al., Citation2014; Dresang et al., Citation2023; Etcharry-Bouyx et al., Citation2017; Power et al., Citation2010). A conceptual system, operating in a ventral stream, offers semantic information about a familiar object or symbolic gesture and sensorimotor knowledge about an appropriate movement relating to them. A visuospatial system, operating in a dorsal stream, offers visual input to hand movements. Imitated gestures can bypass conceptual knowledge of gestures and can be processed only via the visuospatial system, which makes their cognitive demand simpler.
Imitation can be used to differentiate limb apraxia from problems in speech comprehension in aphasia (i.e., understanding verbal commands in gesture tasks) (Goldenberg, Citation2013; Rothi & Heilman, Citation2015). To be exact, if gesture performance fails after verbal command only, but is correct without apraxic features after imitation, the problem is assumed to be in speech comprehension. All of the participants showed apraxic features, such as incorrect hand postures and movements (Rothi & Heilman, Citation2015), even in their imitated gestures.
Strengths and limitations of the study
Instead of using a structured test, a method that is based on the observation of natural spontaneous conversation could be seen to better capture the real use of gestures (Auer & Bauer, Citation2011; De Ruiter, Citation2006). The problem with this approach, however, is how to organize communicative situations across the participant population that are equivalent and can be compared to follow-up in time (Auer & Bauer, Citation2011; Klippi, Citation2006; van Nispen et al., Citation2017). Some studies support the use of a structured gesture task as a valid method for predicting the spontaneous use of gestures (Borod et al., Citation1989; Hogrefe et al., Citation2012, Citation2013). Based on that view, in our study the participants’ gesture performance in the tests may well have correlated with their gesture skills in everyday communication. Furthermore, improved gesture performance over time can mean improved use of gestures in everyday communication. Valid conclusions cannot be made with regard to this, however, as this study did not include data about the participants’ everyday gesture use. In future research, it would be beneficial to assess the participants’ use of gestures in natural everyday communication and make a comparison with their performance in formal gesture tasks.
It was possible to receive points in the gesture task only if the action was performed with one or both hands. The scoring for the comprehensibility of gestures in this study also did not take into account vocalizations, facial gestures or expressions, or head or shoulder movements that PWA’s might include in their gestures. It is important to remember that in everyday gesturing all of these factors have an essential role in multimodal communication (Doedens & Meteyard, Citation2020; Rautakoski, Citation2011), and may clarify the otherwise unclear gesturing of PWA’s in interaction with another person (Auer & Bauer, Citation2011).
We did not have a control group of healthy speakers. We considered it unnecessary, as this was a longitudinal follow-up of severely apraxic-aphasic participants who served as their own controls.
An obvious limitation in our study is the small sample size, which hampers the generalizability of the results and the ability to statistically detect significant differences. The use of non-parametric statistical methods may also suffer from decreased sensitivity. However, the results of this study nevertheless contribute a rare knowledge of the recovery of gestures in persons with apraxia-aphasia, which otherwise is still lacking in the research literature.
Additionally, the study has limitations concerning detailed information on the cognitive factors that could have been used to explain the results. However, a comprehensive cognitive evaluation of the participants was outside of the scope of this study, as the valid evaluation of the cognition of persons with severe apraxia-aphasia is very difficult, considering that many methods require a functional praxia or speech to provide answers.
In the end, the effect of therapy was not part of the current study, and exact information about the content of SL therapy is not available. The received amount of speech therapy has been established to be positively related to long-term recovery from aphasia (Harvey et al., Citation2022; Johnson et al., Citation2019). Nonetheless, it should be noted that in Finland aphasia therapy has not traditionally included systematic training in gestures. Instead, persons with aphasia are encouraged to engage in “total communication” (e.g., Rautakoski, Citation2011) and to use gestures among other communicative methods. Intervention studies have indicated improvement specifically in systematically trained gestures, with less effects on gestures that have not been trained (Alashram et al., Citation2022; Rose et al., Citation2013). Based on these issues, it is not to be expected that SL therapy would have had a substantial influence on the participants’ gesture performances.
Conclusions
The ability to communicate through gestures may be beneficial for individuals who have lost their ability to speak after a stroke. However, the effective use of gestures by a person with severe non-fluent aphasia and limb apraxia is limited. There is very little long-term research about aphasia and stroke-related recovery of gestures. This study addresses this need and contributes new knowledge to the issue. The study used a gesture after verbal command task to examine the performance of emblems and tool use pantomimes during a three years long follow-up period. As a result, it can be seen that skills in gesture performance can improve even one or two years post-stroke for persons with severe non-fluent aphasia and limb apraxia. The tendency toward improvement can be seen already during the first few months post-stroke.
We suggest that to support the progress of gesture communication for a person with severe non-fluent aphasia, gestures should be actively practiced among other means of communication.
Supplemental Material
Download MS Word (47.5 KB)Supplementary material.docx
Download MS Word (47.5 KB)Acknowledgements
We wish to thank Miamaria Hintikka, a student of speech and language pathology, for analyzing the task of Yes/No Questions. We also wish to thank Leena Tuomiranta, PhD., university lecturer, for her contribution to the master theses of Viivi Vehviläinen and Miamaria Hintikka. Many thanks to Kirsi Viilo, a speech and language pathology, for her good comments to the introduction at the earlier stage of writing. We also want to thank the anonymous reviewers for their valuable comments that helped to improve the manuscript. Finally, sincere gratitude for the participants in this study.
Disclosure statement
The authors have no declaration of interest to report.
Additional information
Funding
References
- Akhavan, N., Göksun, T., & Nozari, N. (2018). Integrity and function of gestures in aphasia. Aphasiology, 32(11), 1310–1335. https://doi.org/10.1080/02687038.2017.1396573
- Alashram, A. R., Annino, G., Aldajah, S., Raju, M., & Padua, E. (2022). Rehabilitation of limb apraxia in patients following stroke: A systematic review. Applied Neuropsychology. Adult, 29(6), 1658–1668. https://doi.org/10.1080/23279095.2021.1900188
- Auer, P., & Bauer, A. (2011). Multimodality in aphasic conversation: Why gestures sometimes do not help. Journal of Interactional Research in Communication Disorders, 2(2), 215–243. https://doi.org/10.1558/jircd.v2i2.215
- Basso, A., Burgio, F., Paulin, M., & Prandoni, P. (2000). Long-term follow-up of ideomotor apraxia. Neuropsychological Rehabilitation, 10(1), 1–13. https://doi.org/10.1080/096020100389264
- Basso, A., Capitani, E., Sala, S. D., Laiacona, M., & Spinnler, H. (1987). Ideomotor apraxia: A study of initial severity. Acta Neurologica Scandinavica, 76(2), 142–146. https://doi.org/10.1111/j.1600-0404.1987.tb03557.x
- Baumard, J., Osiurak, F., Lesourd, M., & Gall, D. L. (2014). Tool use disorders after left brain damage. Frontiers in Psychology, 5, 473–413. https://doi.org/10.3389/fpsyg.2014.00473
- Belanger, S. A., Duffy, R. J., & Coelho, C. A. (1996). The assessment of limb apraxia: An investigation of task effects and their cause. Brain and Cognition, 32(3), 384–404. https://doi.org/10.1006/brcg.1996.0072
- Borod, J. C., Fitzpatrick, P. M., Helm-Estabrooks, N., & Goodglass, H. (1989). The relationship between limb apraxia and the spontaneous use of communicative gesture in aphasia. Brain and Cognition, 10(1), 121–131. https://doi.org/10.1016/0278-2626(89)90079-1
- Braddock, B. (2007). Links between language, gesture, and motor skill: A longitudinal study of communication recovery in adults with Broca’s aphasia [Thesis]. University of Missouri–Columbia. https://doi.org/10.32469/10355/4656
- Christensen, A.-L. (1979). Luria’s neuropsychological investigation (2nd ed.). Munksgaard.
- De Beer, C., De Ruiter, J. P., Hielscher-Fastabend, M., & Hogrefe, K. (2019). The production of gesture and speech by people with aphasia: Influence of communicative constraints. Journal of Speech, Language, and Hearing Research: JSLHR, 62(12), 4417–4432. https://doi.org/10.1044/2019_JSLHR-L-19-0020
- De Kleine, N., Rose, M. L., Weinborn, M., Knox, R., & Fay, N. (2023). Does gesture improve the communication success of people with aphasia?: A systematic review. Aphasiology, 38(3), 462–486. https://doi.org/10.1080/02687038.2023.2207781
- De Ruiter, J. P. (2006). Can gesticulation help aphasic people speak, or rather, communicate? Advances in Speech Language Pathology, 8(2), 124–127. https://doi.org/10.1080/14417040600667285
- Doedens, W. J., & Meteyard, L. (2020). Measures of functional, real-world communication for aphasia: A critical review. Aphasiology, 34(4), 492–514. https://doi.org/10.1080/02687038.2019.1702848
- Donkervoort, M., Dekker, J., & Deelman, B. (2006). The course of apraxia and ADL functioning in left hemisphere stroke patients treated in rehabilitation centres and nursing homes. Clinical Rehabilitation, 20(12), 1085–1093. https://doi.org/10.1177/0269215506071257
- Dresang, H. C., Wong, A. L., & Buxbaum, L. J. (2023). Shared and distinct routes in speech and gesture imitation: Evidence from stroke. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 162, 81–95. https://doi.org/10.1016/j.cortex.2023.01.010
- Dressing, A., Kaller, C. P., Martin, M., Nitschke, K., Kuemmerer, D., Beume, L.-A., Schmidt, C. S. M., Musso, M., Urbach, H., Rijntjes, M., & Weiller, C. (2021). Anatomical correlates of recovery in apraxia: A longitudinal lesion-mapping study in stroke patients. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 142, 104–121. https://doi.org/10.1016/j.cortex.2021.06.001
- Duffy, J. (2020). Motor speech disorders: Substrates, differential diagnosis, and management (4th ed.). Elsevier.
- Etcharry-Bouyx, F., Gall, D. L., Jarry, C., & Osiurak, F. (2017). Gestural apraxia. Revue Neurologique, 173(7–8), 430–439. https://doi.org/10.1016/j.neurol.2017.07.005
- Glosser, G., Wiener, M., & Kaplan, E. (1986). Communicative gestures in aphasia*1. Brain and Language, 27(2), 345–359. https://doi.org/10.1016/0093-934X(86)90024-6
- Goldenberg, G. (2013). Apraxia. Wiley Interdisciplinary Reviews. Cognitive Science, 4(5), 453–462. https://doi.org/10.1002/wcs.1241
- Goldin-Meadow, S. (2023). Thinking with your hands: The surprising science behind how gestures shape our thoughts (1st ed.). Basic Books.
- Harvey, D. Y., Parchure, S., & Hamilton, R. H. (2022). Factors predicting long-term recovery from post-stroke aphasia. Aphasiology, 36(11), 1351–1372. https://doi.org/10.1080/02687038.2021.1966374
- Herrmann, M., Reichle, T., Lucius-Hoene, G., Wallesch, C.-W., & Johannsen-Horbach, H. (1988). Nonverbal communication as a compensative strategy for severely nonfluent aphasics?—A quantitative approach. Brain and Language, 33(1), 41–54. https://doi.org/10.1016/0093-934X(88)90053-3
- Hertrich, I., Dietrich, S., & Ackermann, H. (2020). The margins of the language network in the brain. Frontiers in Communication, 5. https://doi.org/10.3389/fcomm.2020.519955
- Hogrefe, K., Ziegler, W., Weidinger, N., & Goldenberg, G. (2012). Non-verbal communication in severe aphasia: Influence of aphasia, apraxia, or semantic processing? Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 48(8), 952–962. https://doi.org/10.1016/j.cortex.2011.02.022
- Hogrefe, K., Ziegler, W., Weidinger, N., & Goldenberg, G. (2017). Comprehensibility and neural substrate of communicative gestures in severe aphasia. Brain and Language, 171, 62–71. https://doi.org/10.1016/j.bandl.2017.04.007
- Hogrefe, K., Ziegler, W., Wiesmayer, S., Weidinger, N., & Goldenberg, G. (2013). The actual and potential use of gestures for communication in aphasia. Aphasiology, 27(9), 1070–1089. https://doi.org/10.1080/02687038.2013.803515
- Holland, A., Fromm, D., Forbes, M., & MacWhinney, B. (2017). Long-term recovery in stroke accompanied by aphasia: A reconsideration. Aphasiology, 31(2), 152–165. https://doi.org/10.1080/02687038.2016.1184221
- Hybbinette, H., Schalling, E., Plantin, J., Nygren-Deboussard, C., Schütz, M., Östberg, P., & Lindberg, P. G. (2021). Recovery of apraxia of speech and aphasia in patients with hand motor impairment after stroke. Frontiers in Neurology, 12, 634065. https://doi.org/10.3389/fneur.2021.634065
- Johnson, L., Basilakos, A., Yourganov, G., Cai, B., Bonilha, L., Rorden, C., & Fridriksson, J. (2019). Progression of aphasia severity in the chronic stages of stroke. American Journal of Speech-Language Pathology, 28(2), 2. https://doi.org/10.1044/2018_AJSLP-18-0123
- Kertesz, A. (1982). Western Aphasia Battery Test Manual. New York: Grune and Stratton.
- Kertesz, A., Pietilä, M-L., Lehtihalmes, M., Klippi, A., & Lempinen, M. (Eds.). (2005). Western aphasia battery: Käsikirja. Psykologien kustannus. Psykologien kustannus.
- Klippi, A. (2006). Nonverbal behavior as turn constructional units in aphasic conversation. In Texas Linguistic Forum 49: Proceedings of the Thirteenth Annual Symposium about Language and Society (pp. 158–169). Austin.
- Landis, J. R., & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 1. https://doi.org/10.2307/2529310
- Leiguarda, R. C., & Marsden, C. D. (2000). Limb apraxias. Brain: A Journal of Neurology, 123 (Pt 5)(5), 860–879. https://doi.org/10.1093/brain/123.5.860
- Lemmetyinen, S., Hokkanen, L., & Klippi, A. (2020). Long-term recovery from apraxia and its relation to severe apraxic-aphasic disorder in left hemisphere stroke – A systematic review. Aphasiology, 34(6), 6. https://doi.org/10.1080/02687038.2019.1636932
- McNeill, D. (2005). Gesture and thought. University of Chicago Press.
- Mimura, M., Fitzpatrick, P. M., & Albert, M. L. (1996). Long-term recovery from ideomotor apraxia. Neuropsychiatry. Neuropsychology and Behavioral Neurology, 9(2), 127–132.
- Murray, L. L. (2012). Attention and other cognitive deficits in aphasia: Presence and relation to language and communication measures. American Journal of Speech-Language Pathology, 21(2), S51–S64. https://doi.org/10.1044/1058-0360(2012/11-0067)
- Nakagawa, Y., Sano, Y., Funayama, M., & Kato, M. (2019). Prognostic factors for long-term improvement from stroke-related aphasia with adequate linguistic rehabilitation. Neurological Sciences, 40(10), 10. https://doi.org/10.1007/s10072-019-03956-7
- Papathanasiou, I., & Coppens, P. (2022). Aphasia and related neurogenic communication disorders (3rd ed.). Jones & Bartlett Learning.
- Power, E., Code, C., Croot, K., Sheard, C., & Gonzalez Rothi, L. J. (2010). Florida Apraxia Battery–Extended and Revised Sydney (FABERS): Design, description, and a healthy control sample. Journal of Clinical and Experimental Neuropsychology, 32(1), 1–18. https://doi.org/10.1080/13803390902791646
- Rautakoski, P. (2011). Training total communication. Aphasiology, 25(3), 3. https://doi.org/10.1080/02687038.2010.530671
- Roby-Brami, A., Hermsdörfer, J., Roy, A. C., & Jacobs, S. (2012). A neuropsychological perspective on the link between language and praxis in modern humans. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 367(1585), 144–160. https://doi.org/10.1098/rstb.2011.0122
- Rose, M. L., Mok, Z., & Sekine, K. (2017). Communicative effectiveness of pantomime gesture in people with aphasia: Communicative effectiveness of pantomime gesture in aphasia. International Journal of Language & Communication Disorders, 52(2), 227–237. https://doi.org/10.1111/1460-6984.12268
- Rose, M. L., Raymer, A. M., Lanyon, L. E., & Attard, M. C. (2013). A systematic review of gesture treatments for post-stroke aphasia. Aphasiology, 27(9), 1090–1127. https://doi.org/10.1080/02687038.2013.805726
- Rothi, L. J., & Heilman, K. M. (2015). Apraxia: The neuropsychology of action.
- Rounis, E., Halai, A., Pizzamiglio, G., & Lambon Ralph, M. A. (2021). Characterising factors underlying praxis deficits in chronic left hemisphere stroke patients. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 142, 154–168. https://doi.org/10.1016/j.cortex.2021.04.019
- Sekine, K., & Rose, M. L. (2013). The relationship of aphasia type and gesture production in people with aphasia. American Journal of Speech-Language Pathology, 22(4), 4. https://doi.org/10.1044/1058-0360(2013/12-0030)
- Sekine, K., Rose, M. L., Foster, A. M., Attard, M. C., & Lanyon, L. E. (2013). Gesture production patterns in aphasic discourse: In-depth description and preliminary predictions. Aphasiology, 27(9), 1031–1049. https://doi.org/10.1080/02687038.2013.803017
- Stamenova, V., Black, S. E., & Roy, E. A. (2011). A model-based approach to long-term recovery of limb apraxia after stroke. Journal of Clinical & Experimental Neuropsychology: Official Journal of the International Neuropsychological Society, 33(9), 954–971.
- van Nispen, K., van de Sandt-Koenderman, M., Sekine, K., Krahmer, E., & Rose, M. L. (2017). Part of the message comes in gesture: How people with aphasia convey information in different gesture types as compared with information in their speech. Aphasiology, 31(9), 9. https://doi.org/10.1080/02687038.2017.1301368
- Wagner, P., Malisz, Z., & Kopp, S. (2014). Gesture and speech in interaction: An overview. Speech Communication, 57, 209–232. https://doi.org/10.1016/j.specom.2013.09.008
- Weiss, P. H., Ubben, S. D., Kaesberg, S., Kalbe, E., Kessler, J., Liebig, T., & Fink, G. R. (2016). Where language meets meaningful action: A combined behavior and lesion analysis of aphasia and apraxia. Brain Structure & Function, 221(1), 563–576. https://doi.org/10.1007/s00429-014-0925-3