Abstract
Memory impairment imposes a great burden on stroke patients and can be divided into Objective Memory Problems (OMPs) and Subjective Memory Complaints (SMCs). Studies have shown that these do not always co-occur. Possibly, the gap between SMCs and OMPs can be bridged when using a more ecologically valid memory test and considering the impact of other common stroke symptoms such as sensory hypersensitivity (SHS) and fatigue. In the present study, we applied Virtual Reality (VR) to create a sensory-rich environment with real-life stimuli. Memory performance was tested with the 15-Verbal Word Learning Test (VLT). Furthermore, we assessed SMCs (Everyday Memory Questionnaire), and the levels of SHS (Multi-Modal Evaluation of Sensory Sensitivity) and fatigue in the previous month. 31 chronic stroke patients and 32 healthy controls participated. The results showed that participants’ memory performance decreased in a sensory-rich compared to a neutral environment. This decrease did not significantly differ between the groups. Interestingly, fatigue and SHS are related to the level of SMC in stroke patients but no such evidence was found in healthy controls. Last, for stroke patients, we found a significant negative correlation between SMCs and memory performance in a sensory-rich environment, but not in a neutral environment. In conclusion, our study implicates that in stroke patients, fatigue and SHS are related to SMCs and that using a sensory-rich VR environment might be a more ecologically valid way to objectify SMCs. However, interpretative caution is warranted due to the absence of sex and age-matched controls and potential selection bias.
Introduction
Approximately 60% of stroke survivors experience Post Stroke Cognitive Impairment (PSCI) in the first year after their stroke (El Husseini et al., Citation2023; Huang et al., Citation2022). PSCI is defined as the occurrence of any type of cognitive decline after a stroke (Lim et al., Citation2021; Salvadori et al., Citation2013). Cognitive impairment following a stroke imposes a great burden on patients since it affects their quality of life and requires them to have ongoing support; this in turn has profound effects on caregivers and society (Huang et al., Citation2022). An important aspect of PSCI is memory impairment. A survey by the Stroke Association found that 77% of patients experience post-stroke memory problems (Tang et al., Citation2020). These problems have a great impact on patients’ recovery and long-term well-being. For instance, stroke patients with low scores on a verbal memory task have been reported to show higher scores on depression questionnaires (Salis et al., Citation2021).
Memory impairments can be divided into subjective and objective impairments. For the scope of the current study, these will be referred to as Subjective Memory Complaints (SMCs) and Objective Memory Problems (OMPs). SMCs reflect the perception of one’s memory and everyday memory performance and state whether this is irritating and/or worrying one (Cho et al., Citation2020; Hun Sung et al., Citation2022; van Rijsbergen et al., Citation2014). SMCs are reported based on the patient’s experience during daily life activities, are often assessed with questionnaires, and are particularly suited to assess the extent of stroke consequences (Salis et al., Citation2021). OMPs are measurable with standardized neuropsychological tests and are usually assessed in a quiet testing room. However, these standard tests provide little information about how stroke patients experience memory problems when they perform activities in their daily lives (Salis et al., Citation2021).
Studies have shown that SMCs and OMPs do not always co-occur. For example, patients who have had a stroke and score within the average range on neuropsychological memory tests can still report memory complaints in daily life (Dubreuil et al., Citation2007; van Rijsbergen et al., Citation2014). This could be related to the fact that neuropsychological tests often show poor ecological validity (Matheis et al., Citation2007). Since the tests are administered in a controlled environment, which retains little resemblance to real-life cognitive challenges, the performance on neuropsychological tests only accounts for 5–21% of the variance in patients’ daily functioning (Miskowiak et al., Citation2022). Therefore, it is of great importance to create a more ecologically valid way of assessing OMPs, to bridge the gap between OMPs and SMCs, by increasing the way the tests explain the variance in the patients’ daily functioning. This could for example, be done with the use of virtual reality (VR).
VR enables to create a virtual environment that better resembles a more realistic real-life situation than a standardized neuropsychological testing session (Neguț et al., Citation2016; Parsons & Rizzo, Citation2008; Parsons et al., Citation2008). Unlike the real world, VR allows more control over the stimulus environment, aiding the standardization of behavioral measurements (Huygelier et al., Citation2022). VR has often been used to assess neuropsychological functions (Neguț et al., Citation2016; Parsons & Rizzo, Citation2008; Parsons et al., Citation2008). More specifically it has been used to assess memory performance in individuals with Traumatic Brain Injury (TBI) in a VR office space, where it was able to distinguish between patients and controls and had good construct validity (Matheis et al., Citation2007). Head-mounted VR was shown to be a safe tool and older adults had a positive attitude toward using it (Huygelier et al., Citation2019). A study showed that it was promising and feasible to use VR for neglect rehabilitation in stroke patients (Huygelier et al., Citation2022). VR tools have also been proposed as a possible method to assess cognitive functions in stroke patients (Kang et al., Citation2008). VR can benefit neuropsychological assessment, by balancing the demands of high ecological validity along with the requirements of sensitivity and specificity (Hogan et al., Citation2023). By using VR technology, it may be possible to measure SMCs with an objective tool. With VR, factors that possibly contribute to SMCs in daily life can be manipulated.
Previous research shows that several factors might influence SMCs, such as sleep, stress, depression, cognitive reserve, personality, coping style, and living conditions (Aguiar et al., Citation2010; Miley-Akerstedt et al., Citation2018; Steinberg et al., Citation2013). In the current study, we aimed to focus on two other factors that are separate but related complaints after a stroke, namely sensory hypersensitivity (SHS) and fatigue, and investigate whether they contribute to the gap between SMCs and OMPs.
According to research by Marzolla, Thielen, et al. (Citation2023), individuals with Acquired Brain Injury (ABI) report SMCs as a result of SHS. Post-injury SHS or atypical sensory sensitivity is reported by 75% of stroke survivors (Thielen, Tuts, et al., Citation2023). It is defined as a self-reported increase in sensitivity to sensory stimuli post-injury, which may manifest in an altered response to these stimuli (Marzolla, Thielen, et al., Citation2023; Marzolla, Wijenberg, et al., Citation2023; Thielen, Huenges Wajer, et al., Citation2023a; Thielen, Tuts, et al., Citation2023). They may experience changes in various sensory modalities including auditory, visual, touch, proprioception, and taste (Thielen, Huenges Wajer, et al., Citation2023a). Furthermore, their tolerance level to environmental stimuli can affect their participation in different aspects of life, and potentially lead to social isolation, reduced quality of life, and reduced physical and mental health (Callahan & Lim, Citation2018; Shepherd et al., Citation2020). Studies have shown that sensory sensitivity to noise correlates with cognitive flexibility in female participants with acute TBI (Shepherd et al., Citation2019). Although Marzolla, Thielen, et al. (Citation2023) have reported that individuals with ABI report worsened memory in situations of SHS, this association has not yet been investigated in an experimental setting.
Another common complaint after stroke is post-stroke fatigue, which is prevalent in 30–71% of stroke survivors (Hubacher et al., Citation2012). This is characterized by mental and physical exhaustion and lack of energy that is unrelated to a preceding level of exertion and does not ameliorate with rest (De Groot et al., Citation2003; Lagogianni et al., Citation2018). Subjective fatigue is defined as a feeling of early exhaustion developing during or after mental activity or usual activities, with wariness, lack of energy, and aversion to effort (Staub & Bogousslavsky, Citation2001). Fatigue is a widespread issue for stroke survivors (Cumming et al., Citation2016). It has been associated with cognitive impairment, especially sustained attentional performance (Dillon et al., Citation2022, 2023). In addition, post-stroke fatigue has been associated with memory deficits even after having controlled for depression (Pihlaja et al., Citation2014).
In the present study, we applied VR to create a sensory-rich environment with real-life stimuli, in order to mimic daily life situations where stroke patients report SMCs. The present study aimed to investigate the difference in memory performance of stroke patients and healthy controls in a sensory-rich compared to a sensory-neutral testing condition using VR. The stroke patients were previously tested in a different project on a paper Verbal Word Learning Test (VLT), and all participants that signed up for this study had SMC (inclusion criterion). Although they scored above the norm score (absent OMP), they did complain about their memory performance (present SMC). In an evaluation, several of these participants reported that they experienced these problems mostly in a busy environment (e.g., with a radio on, or at their homes with family) and that the complaints were strongest at the end of the day. These conversations made us come to the current study design. It raised the question whether factors such as SHS and being fatigued might contribute to these differences in OMPs and SMCs. In the current study, it was expected that participants would show a decrease in objective memory performance in a sensory-rich VR environment as compared to a neutral VR environment. In addition, it was hypothesized that the decrease in memory performance would be larger in the stroke group as compared to the control group. Furthermore, we tested if SMC and OMP (in the neutral and sensory-rich VR condition) were related and whether SMCs and OMPs were related to experienced levels of SHS and fatigue.
Methods
All procedures were approved by the Ethical Review Committee Psychology and Neuroscience of Maastricht University (ERCPN-264_28_02_2023). All participants provided written informed consent.
Participants
Recruitment
Participants were recruited between March and May 2023. The study population consisted of a post-stroke group and a control group. The post-stroke group (>1 year after stroke) had participated in the ROSTMEMA study (Clinicaltrials.gov ID: NCT04854811). All participants had SMCs, but did not have OMP (which made them fail the ROSTMEMA screening). They showed a normal memory performance (i.e., above the norm score) on a paper VLT. The people who gave consent to be contacted for future research on their Informed Consent Form, were asked to participate in this study. The participation rate was 86%. Individuals for the control group were recruited via advertisement on social media, such as Facebook and LinkedIn, word-of-mouth, and via the database of the Limburg Brain Injury Center.
Inclusion/exclusion criteria
Individuals were eligible for the post-stroke group if they met the following inclusion criteria: they were between 41 and 72 years of age; suffered a stroke at least one year before recruitment and at the age of 40 or older, and were willing to give informed consent. Individuals were eligible for the control group if they were between 41 and 72 years of age; did not suffer a stroke; and were willing to give informed consent. Participants in both groups were excluded from the present study if they met any of the following criteria: visual disabilities or neglect; presence of any (other) disorder that can affect memory (such as a brain tumor, epilepsy, Normal Pressure Hydrocephalus (NPH), Huntington’s disease, Parkinson’s disease); a recent Transient Ischemic Attack (TIA; less than 1 year ago); history of schizophrenia, bipolar disorder or psychotic symptoms not otherwise specified or previous treatment for these diseases; current affective disorder (i.e., anxiety or major depression); history of or present alcohol abuse or use of illicit drugs.
Sample size
Based on the primary research question the sample size of the present study was calculated with GPower 3.1 (Faul et al., Citation2009). Based on a GLM using within- and between-subjects interaction and using a power of β = 0.85, a significance level of α = .05, and a medium effect size of f = 0.20, a total number of 60 participants were needed, leading to 30 participants in each group.
Procedure
All participants were tested individually in a VR laboratory at the Faculty of Psychology and Neuroscience of Maastricht University, equipped with a head-mounted VIVE Pro Series™. Participants were instructed to refrain from caffeinated drinks the morning of the experiment. Test sessions took about 1.5–2 h. Participants were rewarded with a €10 voucher and a reimbursement for their travel costs. All participants underwent two memory tests in a VR environment. The memory task was displayed first on a neutral and then on a sensory-rich background or vice versa. This order was randomized and equally distributed between the groups. In the neutral condition, the words were displayed on a grey background. In the sensory-rich condition, the words were presented with a video of a village and background noise consisting of someone speaking about the living conditions in the Stone Age. In this 360° video, participants could look around and see the surroundings of a village, which enhanced the immersiveness of the situation.
The questionnaires were administered once and included a demographics questionnaire, the Multi-Modal Evaluation of Sensory Sensitivity (MESSY) (Thielen, Huenges Wajer, et al., Citation2023a), a fatigue questionnaire (Thielen, Huenges Wajer, et al., Citation2023b) and the Everyday Memory Questionnaire – revised (EMQ-revised) (Royle & Lincoln, Citation2008). The MESSY and fatigue questionnaire were completed on a computer; the demographics questionnaire and EMQ-revised questionnaire were filled out on paper.
Materials
Memory: Verbal word Learning test (VLT) in VR environment
The memory task participants completed was the VLT. The VLT is an adapted version of the original 15-word Rey Auditory Verbal Learning Test (Rey, Citation1958). This original test assesses short- and long-term memory function for verbal information. The VLT has been proven a reliable and valid tool (Van der Elst et al., Citation2005). In the VLT, 15 monosyllabic words are presented in five consecutive trials. Words were shown for 2 seconds, followed by a 1-second interval. The words were presented in the lower part of the screen, and independent of where the participants were looking in the video condition. The participants had to recall the words immediately after each trial (immediate recall) and this was repeated five times. After 30 min, during which the participants filled out the questionnaires, there was an additional free recall trial (delayed recall). Outcome scores were defined as the number of recalled items in the immediate trials, calculated by the sum of the five trials (ranging from 0 to 75), and the score on the delayed recall test (ranging from 0 to 15). In order to correct for age, sex, and education differences between groups, z-scores were calculated based on the normative data of a large sample (Van der Elst et al., Citation2005). In each VR environment (sensory rich vs neutral), previously validated parallel word-lists of the VLT were used (unpublished data).
Demographics
A demographic questionnaire was used to assess age, sex, education level, and for the post-stroke group also type of and time since injury.
Sensory hypersensitivity: multi-modal evaluation of sensory sensitivity (MESSY)
The MESSY was developed by Thielen, Huenges Wajer, et al. (Citation2023a) to assess sensory sensitivity across a variety of sensory modalities. 30 items are divided over 7 domains: i.e., visual, auditory, tactile, olfactory, gustatory, motion sensitivity, and environmental temperature sensitivity, and are answered on a scale from 1 (never/not at all) to 5 (very often/extremely) based on experiences in the previous month. The MESSY includes questions, such as “I suffer or feel overwhelmed when there are a lot of people around me”, “I get annoyed by sounds that are not bothersome for other people”, and “When I turn my body or when I stretch or bend, I feel dizzy.” Furthermore, an open-ended question was administered to assesses whether patients with acquired brain injury experience an increased sensitivity from pre- to post-injury for each modality. As an outcome measure, the total score on the multiple-choice items of the MESSY was used (ranging from 30 to 150), with higher scores indicating higher sensitivity. The MESSY shows high convergent validity and test-retest reliability. Internal consistency ranged from α = 0.94 in healthy adults to α = 0.96 in an acquired brain injury group (Thielen, Huenges Wajer, et al., Citation2023a).
Fatigue: fatigue questionnaire
The fatigue questionnaire consists of 13 items that can be scored from 1 (never/not at all) to 5 (very often/extremely) based on the subject’s experiences in the previous month (Thielen, Huenges Wajer, et al., Citation2023b). This questionnaire included questions such as “Fatigue influences my daily life”, “I need to rest during the day because I am tired”, and “When I am tired, I have a headache.” The outcome measure was the sum score of all items (ranging from 13 to 65), with a higher score indicating more fatigue in daily life. The Cronbach’s Alpha of the questionnaire in the current study showed good internal consistency (α = 0.89). Other data on the psychometric properties of the test are not yet available.
Everyday memory questionnaire – revised (EMQ-R)
Finally, the EMQ-R was used to assess SMCs. This 13-item questionnaire aims to measure failure in everyday memory. Each item can be scored from 0 (once or less in the last month) to 4 (once or more in a day) (Royle & Lincoln, Citation2008). Some examples of items included in the EMQ-revised include “Having to check whether you have done something”, “Completely forgetting to do things you said you would”, and “Getting the details mixed up.” As an outcome measure, the sum score of all items was used (ranging from 0 to 52), with higher scores indicating more memory complaints.
According to Royle and Lincoln (Citation2008), the 13-item questionnaire is a valid and reliable tool that has good face validity for use with neurological patients, however further research on the validity and reliability is needed. The reliability of the full EMQ is good (r = 0.85) (Efklides et al., Citation2002).
Data processing and analysis
Data processing and analysis were performed in SPSS 27.0. Descriptive statistics were used to analyze the characteristics of the population, which included sample size, sex, educational level, and mean and standard deviation (SD) for age. Additionally, for the stroke group, these included type of injury, and mean and SD for time since injury. Furthermore, the mean and SD of the z-scores of the primary outcome (memory performance), and the mean and SD of questionnaire scores were presented. The age distribution of the stroke and control group was tested with an independent samples t-test. The sex distribution of the stroke and control group was tested with Fisher’s exact test. The educational level distribution was tested with a Mann-Whitney U test. Data were checked on assumptions of normality, homoscedasticity, multicollinearity, and outliers. The assumptions were met for all data, except for the regression analysis for the effects of SHS and fatigue on memory performance (see below). Differences between the groups on the predictor variables were tested with an independent samples t-test; homogeneity of variances was assumed. In case equal variances could not be assumed, we used adjusted t-values.
A mixed within- (neutral vs sensory rich) and between-subject (stroke vs control) repeated-measures ANOVA was used to compare the memory performance between the conditions. We checked whether the order of presentation of the VR conditions influenced results. Furthermore, we aimed to investigate the relationship between the difference in memory performance between the two conditions (difference score on VLT, for both immediate recall and delayed recall) and subjective memory (EMQ sum score), and the level of SHS (MESSY sum score) and level of fatigue (fatigue sum score). A regression analysis in which SHS and fatigue were used to predict memory performance was not possible since the assumption of multicollinearity was violated: the correlation between the two independent variables (fatigue and MESSY) was highly significant (r = 0.852, p ≤ .001). Due to this fact, it was decided to calculate the correlations between the fatigue and the MESSY scores and the difference scores of the VLT. Finally, we aimed to check the relationship between the SMC and OMP, and whether the sensory-rich VR condition could better predict SMC and OMP as compared to the neutral condition. To check this we calculated the correlations between the EMQ scores (SMC) and VLT scores (OMP) in the sensory-rich and neutral VR condition. A significance level of α = .05 was used and corrections for multiple comparisons were applied.
Results
Participants
The stroke group initially consisted of 31 participants and the control group of 33 healthy controls. One healthy control was excluded due to technical issues during the experiment. The characteristics of the participants are described in . Participants in the stroke group were significantly older than participants in the control group (t(61) = 2.361, p = .021). Additionally, Fisher’s exact test showed that sex distribution was significantly different in the groups (p ≤ .001). The stroke group contained fewer females compared to the control group. A Mann-Whitney U test did not reveal a significant difference in educational level between the two groups (Z = −1.857, p = .066).
Table 1. Demographics.
Difference in memory performance between conditions
To assess the difference in memory performance between the sensory-rich and sensory-neutral VR condition, and the effect of group (stroke vs. control) on this difference, a GLM analysis was performed on the data. In , the mean and SD of total scores of the immediate recall z-score and delayed recall z-score of the VLT are shown per group. In , these mean scores are visualized per group. The VLT immediate recall z-score was significantly lower in the sensory-rich VR condition compared to the neutral VR condition (F(1,59)=33.409, p ≤ .001), (M = −0.06 ± 1.16 (SD) and M = 0.65 ± 0.98 (SD), respectively). There was no evidence for a significant difference between groups (F(1,59)=0.652, p = .423) or order (F(1,59)=3.942, p = .052). Additionally, the interaction between order and group was not significant (F(1,59)=0.919, p = .342).
Figure 1. Mean performance of the Stroke and Control group on the immediate recall (left panel) and delayed recall (right panel) in the two VR conditions on the VLT. Data represent mean z-scores (and SE), with a higher score indicating better performance.
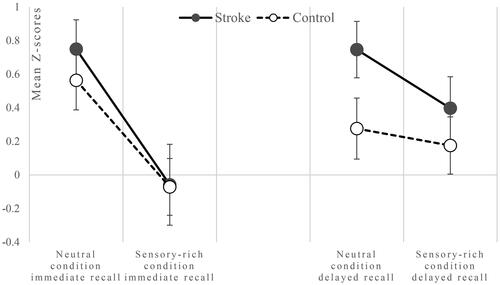
Table 2. Performance of the Stroke and Control group on the immediate and delayed VLT recall in the two different VR conditions and EMQ sum scores.
The VLT delayed recall z-score also significantly differed between conditions (F(1,59)=4.374, p = .041), indicating a lower performance in the sensory-rich VR condition compared to the neutral (M = 0.28 ± 1.00 (SD), M = 0.51 ± 1.00 (SD), respectively). This did not significantly differ between groups (F(1,59)=1.745, p = .192) or order (F(1,59)=2.364, p = .130). Additionally, the interaction between order and group was not significant (F(1,59)=0.004, p = .953).
Correlations between outcome variables and predictor variables
shows the mean and SD for EMQ, MESSY, and fatigue total scores for both groups. According to an independent samples t-test, the stroke group had significantly higher scores on the total score of the MESSY, fatigue, and EMQ compared to the control group.
Table 3. Scores of the Stroke and Control group on the MESSY and Fatigue questionnaires.
Correlations were calculated between the MESSY and fatigue score, and the difference scores of the VLT (difference in VLT scores between the sensory-neutral and the sensory-rich VR condition). These results are shown in . No evidence was found for a significant correlation between the MESSY and fatigue scores and the VLT difference scores in both groups. Correlations were also calculated for EMQ scores, and MESSY and fatigue scores (). The results show a significant positive correlation between the EMQ and the MESSY score, as well as between the EMQ and the fatigue score for the stroke group; for the healthy controls, no evidence for a significant correlation was found.
Table 4. Correlations between study variables (EMQ, difference scores VLT, MESSY, and fatigue).
To check the relation between the subjective and objective memory measurements, correlations were calculated between the EMQ sum score and the VLT z-scores (sensory-rich and neutral). Interestingly, significant negative correlations were found between the EMQ sum score and VLT z-score, immediate as well as delayed, in the sensory-rich VR condition only, and only for the stroke group; stroke patients with more SMCs score worse on the objective VLT (see ).
Table 5. Correlations between VLT z-scores per VR condition and EMQ sum score.
Discussion
The present study aimed to investigate the effect of a sensory-rich VR environment on the memory performance of stroke patients and healthy controls. Additionally, it aimed to explain whether this possible effect could be related to SHS and fatigue in daily life. The stroke patients that were selected for this study all scored above the normative score on a standard VLT but reported subjective complaints about their memory performance in daily life. Analysis showed that the stroke patients reported more SMC, more SHS, and were more fatigued in daily life as compared to the control group. As expected, the results from the current study showed that the participants recalled fewer words in the sensory-rich VR condition as compared to the neutral VR condition; however contrary to our expectations, the memory performance of the stroke patients was not more affected in the sensory-rich condition when compared to the healthy control group.
The observation that memory performance was decreased in the sensory-rich environment was as expected based on earlier research. For example, previous research showed that the presence of visually irrelevant environmental stimuli diminishes long-term memory performance (Wais et al., Citation2010). Additionally, studies consistently show that the presence of auditory distractions, such as background speech, either at encoding or at retrieval, disrupts memory performance (Beaman et al., Citation2014; Liebl et al., Citation2012; Salamé & Baddeley, Citation1982; Wais & Gazzaley, Citation2011). A possible explanation could be higher demands placed upon general attentional resources (e.g., attentional filtering) when we attempt to block out unwanted stimulation, which consequently lead to memory deficits. In addition, distractive stimulation often is qualitatively similar to task-relevant information (Craik, Citation2014). This may lead to falsely remembering closely related but irrelevant information.
In contrast to what would be expected based on earlier research, the decrease in memory performance in the sensory-rich environment did not differ between the stroke and control group. This deviates from earlier findings that the learning and memory of patients with moderate to severe TBI are impaired compared to healthy controls when performing a memory task in a VR office setting (Matheis et al., Citation2007). During this memory task, participants were immersed in a VR office, programmed to resemble a common work office. Participants had to remember items visually presented to them in the office (typical and atypical office items). TBI patients performed significantly worse than healthy controls. In addition, Kang et al. (Citation2008) previously investigated cognitive performance in a VR shopping simulation in stroke patients and healthy controls. In this shopping simulation, participants were tasked with recalling items within the simulated store. The results of that study showed that stroke patients performed worse on this task compared to healthy controls. A possible explanation for the fact that we did not replicate these results could be that in the previously mentioned studies, they used visual items instead of verbal words, testing visual memory instead of verbal memory. Another explanation might also pertain to the selection of stroke patients. In our study, stroke patients were people who had already scored above the normative score on the VLT under standard testing conditions. Conversely, our control participants were not subjected to such preselection. This could explain why in , mean z-scores on the VLT are higher for the stroke group than for the control group. In addition, it is noteworthy that this preselection process resulted in stroke patients having prior experience and practice with the VLT, enabling them to have acquired strategies and better anticipate the current situation. In contrast, for many control participants, the neuropsychological test session during this research was their first time being tested.
In the secondary analysis, an investigation of the differences in the scores between the stroke group and healthy controls confirmed that the stroke group was significantly more fatigued in daily life, experienced more problems with SHS, and had more SMC. It was then examined whether an individual’s level of self-reported SHS and fatigue was related to the decrease in memory performance. In contrast to our hypothesis, no evidence for a significant correlation was found for the difference in VLT scores between the two conditions (neutral vs. sensory-rich) and the level of SHS and fatigue. However, the analysis of SMCs revealed that for the stroke group, the EMQ score was significantly correlated with the MESSY and fatigue scores. This indicates that in stroke patients, fatigue and SHS in daily life are associated with subjective everyday memory problems. This could be expected based on earlier research (Dillon et al., Citation2022; Marzolla, Thielen, et al., Citation2023; Pihlaja et al., Citation2014).
Last, earlier studies investigating the relationship between SMCs and OMPs found that SMCs did not necessarily result in OMPs (Hun Sung et al., Citation2022; van Rijsbergen et al., Citation2014). Interestingly, in the current study, significant correlations have been found between SMC (EMQ score) and OMP (VLT z-scores delayed and immediate) but only in the sensory-rich condition, and only for the stroke group. No significant correlations were found in the sensory neutral condition, and no significant correlations were found for the control group. The finding that the memory performance in the sensory-rich condition significantly correlated with the EMQ scores of the stroke group underscores the notion that measuring objective memory performance in a sensory-rich VR situation is predictive for SMCs in stroke patients. This implies that in future studies, this method should be further explored for use in research and clinical settings.
Some limitations of the current study need to be discussed. First, our participants were not required to use the content of the narrative in the sensory-rich VR condition. It is possible that the participants actively ignored the narrative context and focused solely on the words they needed to remember. Distraction could be enhanced by giving the instruction that participants have to remember what was said during the video. This would enhance the attention the participants have to pay to the content. If this had been done, this might have resulted in a bigger difference in memory performance between the stroke patients and healthy controls. Alternatively, a more distracting video could have been selected or the loudness could have been increased. An advantage of the VR method is that it offers many ways in which the distraction can be modulated; this can be standardized, thereby offering a variety of simulated everyday situations mimicking distractions in real life. These advantages can be investigated in further studies.
Secondly, we did not measure other factors that might have influenced memory, such as other cognitive functions, sleep, stress, depression, cognitive reserve, personality, coping style, and living conditions (Aguiar et al., Citation2010; Miley-Akerstedt et al., Citation2018; Steinberg et al., Citation2013). These could also have influenced the memory performance. Third, it has to be noted that in the present study, we chose to use the z-scores on the VLT as the primary outcome measure (Van der Elst et al., Citation2005). The normative scores we used are based on the standard VLT as measured with words presented on paper; normative scores for VR (with or without distractions) are not available. Since the stroke and control groups differed concerning age and sex, we preferred using z-scores as they allowed us to directly compare the performance of both groups. A final limitation is, as mentioned above, the fact that the stroke patient group previously scored above the normative score on the VLT under standard testing conditions. The control group did not have such preselection, possibly creating a baseline difference between the groups. In future studies, it is recommended to use age- and sex-matched controls, as well as controls with similar performance levels on the memory tests.
In conclusion, the current study showed that adding distraction via VR decreases word-list learning performance. This is the case for healthy controls and stroke patients. Objective memory performance was not influenced by SHS or fatigue. For stroke patients, however, subjective memory complaints were influenced by SHS and fatigue. In addition, subjective memory complaints in stroke patients were related to memory performance in a sensory-rich VR condition. This implies that measuring memory performance in a sensory-rich VR environment is possibly a better way to objectify SMCs within this patient group. However, caution should be applied when drawing these conclusions due to the absence of age and sex-matched controls and the potential selection bias as described above. Further studies on patients with brain injury, with age- and sex-matched controls, and using VR technologies, are indicated. They could result in creating a better understanding of the relationship between subjective complaints and cognitive problems, and in clinically relevant measures that are a more ecological way of objectifying these subjective complaints with neuropsychological tests.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aguiar, A. C. P. D O., Ribeiro, M. I., & Jacinto, A. F. (2010). Subjective memory complaints in the elderly may be related to factors other than cognitive deficit. Dementia & Neuropsychologia, 4(1), 54–57. https://doi.org/10.1590/S1980-57642010DN40100009
- Beaman, C. P., Hanczakowski, M., & Jones, D. M. (2014). The effects of distraction on metacognition and metacognition on distraction: evidence from recognition memory. Frontiers in Psychology, 5, 439. https://doi.org/10.3389/fpsyg.2014.00439
- Callahan, M. L., & Lim, M. M. (2018). Sensory sensitivity in TBI: Implications for chronic disability. Current Neurology and Neuroscience Reports, 18(9), 56. https://doi.org/10.1007/s11910-018-0867-x
- Cho, I., Kim, S., Choi, J. G., & Shin, J.-H. (2020). Subjective memory complaints and sensitivity of the subjective memory complaint questionnaire in post-stroke dementia patients. Dementia and Geriatric Cognitive Disorders, 49(3), 279–285. https://doi.org/10.1159/000509083
- Craik, F. I. (2014). Effects of distraction on memory and cognition: a commentary. Frontiers in Psychology, 5, 841. https://doi.org/10.3389/fpsyg.2014.00841
- Cumming, T. B., Packer, M., Kramer, S. F., & English, C. (2016). The prevalence of fatigue after stroke: a systematic review and meta-analysis. International Journal of Stroke, 11(9), 968–977. https://doi.org/10.1177/1747493016669861
- De Groot, M. H., Phillips, S. J., & Eskes, G. A. (2003). Fatigue associated with stroke and other neurologic conditions: implications for stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 84(11), 1714–1720. https://doi.org/10.1053/s0003-9993(03)00346-0
- Dillon, A., Casey, J., Gaskell, H., Drummond, A., Demeyere, N., & Dawes, H. (2022). Is there evidence for a relationship between cognitive impairment and fatigue after acquired brain injury: a systematic review and meta-analysis. Disability and Rehabilitation, 45(26), 4359–4372. https://doi.org/10.1080/09638288.2022.2152503
- Dubreuil, P., Adam, S., Bier, N., & Gagnon, L. (2007). The ecological validity of traditional memory evaluation in relation with controlled memory processes and routinization. Archives of Clinical Neuropsychology, 22(8), 979–989. https://doi.org/10.1016/j.acn.2007.08.002
- Efklides, A., Yiultsi, E., Kangellidou, T., Kounti, F., Dina, F., & Tsolaki, M. (2002). Wechsler Memory Scale, Rivermead Behavioral Memory Test, and Everyday Memory Questionnaire in healthy adults and Alzheimer’s patients. European Journal of Psychological Assessment, 18(1), 63–77. https://doi.org/10.1027/1015-5759.18.1.63
- El Husseini, N., Katzan, I. L., Rost, N. S., Blake, M. L., Byun, E., Pendlebury, S. T., Aparicio, H. J., Marquine, M. J., Gottesman, R. F., & Smith, E. E. (2023). Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke, 54(6), e272–e291. https://doi.org/10.1161/STR.0000000000000430
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
- Hogan, C., Cornwell, P., Fleming, J., Man, D. W. K., & Shum, D. H. K. (2023). Assessment of prospective memory after stroke utilizing virtual reality. Virtual Reality, 27(1), 333–346. https://doi.org/10.1007/s10055-021-00576-5
- Huang, Y.-Y., Chen, S.-D., Leng, X.-Y., Kuo, K., Wang, Z.-T., Cui, M., Tan, L., Wang, K., Dong, Q., & Yu, J.-T. (2022). Post-stroke cognitive impairment: epidemiology, risk factors, and management. Journal of Alzheimer’s Disease, 86(3), 983–999. https://doi.org/10.3233/JAD-215644
- Hubacher, M., Calabrese, P., Bassetti, C., Carota, A., Stöcklin, M., & Penner, I.-K. (2012). Assessment of post-stroke fatigue: the fatigue scale for motor and cognitive functions. European Neurology, 67(6), 377–384. https://doi.org/10.1159/000336736
- Hun Sung, J., Kim, S., & Shin, J.-H. (2022). Subjective memory complaints and their relationship with the objective cognitive performance of stroke patients. Dementia and Geriatric Cognitive Disorders, 51(6), 475–484. https://doi.org/10.1159/000527685
- Huygelier, H., Schraepen, B., Lafosse, C., Vaes, N., Schillebeeckx, F., Michiels, K., Note, E., Vanden Abeele, V., van Ee, R., & Gillebert, C. R. (2022). An immersive virtual reality game to train spatial attention orientation after stroke: A feasibility study. Applied Neuropsychology. Adult, 29(5), 915–935. https://doi.org/10.1080/23279095.2020.1821030
- Huygelier, H., Schraepen, B., van Ee, R., Vanden Abeele, V., & Gillebert, C. R. (2019). Acceptance of immersive head-mounted virtual reality in older adults. Scientific Reports, 9(1), 4519. https://doi.org/10.1038/s41598-019-41200-6
- Kang, Y. J., Ku, J., Han, K., Kim, S. I., Yu, T. W., Lee, J. H., & Park, C. I. (2008). Development and clinical trial of virtual reality-based cognitive assessment in people with stroke: preliminary study. CyberPsychology & Behavior, 11(3), 329–339. https://doi.org/10.1089/cpb.2007.0116
- Lagogianni, C., Thomas, S., & Lincoln, N. (2018). Examining the relationship between fatigue and cognition after stroke: A systematic review. Neuropsychological Rehabilitation, 28(1), 57–116. https://doi.org/10.1080/09602011.2015.1127820
- Liebl, A., Haller, J., Jödicke, B., Baumgartner, H., Schlittmeier, S., & Hellbrück, J. (2012). Combined effects of acoustic and visual distraction on cognitive performance and well-being. Applied Ergonomics, 43(2), 424–434. https://doi.org/10.1016/j.apergo.2011.06.017
- Lim, J.-S., Lee, J.-J., & Woo, C.-W. (2021). Post-stroke cognitive impairment: pathophysiological insights into brain disconnectome from advanced neuroimaging analysis techniques. Journal of Stroke, 23(3), 297–311. https://doi.org/10.5853/jos.2021.02376
- Marzolla, M. C., Thielen, H., Hurks, P., Borghans, L., & van Heugten, C. (2023). Qualitative data on triggers and coping of sensory hypersensitivity in acquired brain injury patients: A proposed model. Neuropsychological Rehabilitation, 1–21. https://doi.org/10.1080/09602011.2023.2242616
- Marzolla, M. C., Wijenberg, M., Stapert, S., Hurks, P., Schepers, J., & van Heugten, C. (2023). Hypersensitivity to noise and light over 1 year after mild traumatic brain injury: A longitudinal study on self-reported hypersensitivity and its influence on long-term anxiety, depression, and quality of life. The Journal of Head Trauma Rehabilitation, 38(3), 259–267. https://doi.org/10.1097/htr.0000000000000813
- Matheis, R. J., Schultheis, M. T., Tiersky, L. A., DeLuca, J., Millis, S. R., & Rizzo, A. (2007). Is learning and memory different in a virtual environment? The Clinical Neuropsychologist, 21(1), 146–161. https://doi.org/10.1080/13854040601100668
- Miley-Akerstedt, A., Jelic, V., Marklund, K., Walles, H., Åkerstedt, T., Hagman, G., & Andersson, C. (2018). Lifestyle factors are important contributors to subjective memory complaints among patients without objective memory impairment or positive neurochemical biomarkers for Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders Extra, 8(3), 439–452. https://doi.org/10.1159/000493749
- Miskowiak, K. W., Jespersen, A. E., Kessing, L. V., Aggestrup, A. S., Glenthøj, L. B., Nordentoft, M., Ott, C. V., & Lumbye, A. (2022). Cognition Assessment in Virtual Reality: Validity and feasibility of a novel virtual reality test for real-life cognitive functions in mood disorders and psychosis spectrum disorders. Journal of Psychiatric Research, 145, 182–189. https://doi.org/10.1016/j.jpsychires.2021.12.002
- Neguț, A., Matu, S.-A., Sava, F. A., & David, D. (2016). Virtual reality measures in neuropsychological assessment: a meta-analytic review. The Clinical Neuropsychologist, 30(2), 165–184. https://doi.org/10.1080/13854046.2016.1144793
- Parsons, T. D., & Rizzo, A. A. (2008). Initial validation of a virtual environment for assessment of memory functioning: virtual reality cognitive performance assessment test. CyberPsychology & Behavior, 11(1), 17–25. https://doi.org/10.1089/cpb.2007.9934
- Parsons, T. D., Silva, T. M., Pair, J., & Rizzo, A. A. (2008). Virtual environment for assessment of neurocognitive functioning: virtual reality cognitive performance assessment test. Studies in Health Technology and Informatics, 132, 351–356.
- Pihlaja, R., Uimonen, J., Mustanoja, S., Tatlisumak, T., & Poutiainen, E. (2014). Post-stroke fatigue is associated with impaired processing speed and memory functions in first-ever stroke patients. Journal of Psychosomatic Research, 77(5), 380–384. https://doi.org/10.1016/j.jpsychores.2014.08.011
- Rey, A. (1958). L’examen clinique en psychologie. [The clinical examination in psychology]. Presses Universitaries De France.
- Royle, J., & Lincoln, N. B. (2008). The everyday memory questionnaire–revised: Development of a 13-item scale. Disability and Rehabilitation, 30(2), 114–121. https://doi.org/10.1080/09638280701223876
- Salamé, P., & Baddeley, A. (1982). Disruption of short-term memory by unattended speech: Implications for the structure of working memory. Journal of Verbal Learning and Verbal Behavior, 21(2), 150–164. https://doi.org/10.1016/S0022-5371(82)90521-7
- Salis, C., Murray, L., & Vonk, J. M. (2021). Systematic review of subjective memory measures to inform assessing memory limitations after stroke and stroke-related aphasia. Disability and Rehabilitation, 43(11), 1488–1506. https://doi.org/10.1080/09638288.2019.1668485
- Salvadori, E., Pasi, M., Poggesi, A., Chiti, G., Inzitari, D., & Pantoni, L. (2013). Predictive value of MoCA in the acute phase of stroke on the diagnosis of mid-term cognitive impairment. Journal of Neurology, 260(9), 2220–2227. https://doi.org/10.1007/s00415-013-6962-7
- Shepherd, D., Landon, J., Kalloor, M., & Theadom, A. (2019). Clinical correlates of noise sensitivity in patients with acute TBI. Brain Injury, 33(8), 1050–1058. https://doi.org/10.1080/02699052.2019.1606443
- Shepherd, D., Landon, J., Kalloor, M., Barker-Collo, S., Starkey, N., Jones, K., Ameratunga, S., Theadom, A., & Group, B. R. (2020). The association between health-related quality of life and noise or light sensitivity in survivors of a mild traumatic brain injury. Quality of Life Research, 29(3), 665–672. https://doi.org/10.1007/s11136-019-02346-y
- Staub, F., & Bogousslavsky, J. (2001). Fatigue after stroke: a major but neglected issue. Cerebrovascular Diseases, 12(2), 75–81. https://doi.org/10.1159/000047685
- Steinberg, S. I., Negash, S., Sammel, M. D., Bogner, H., Harel, B. T., Livney, M. G., McCoubrey, H., Wolk, D. A., Kling, M. A., & Arnold, S. E. (2013). Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. American Journal of Alzheimer’s Disease & Other Dementias, 28(8), 776–783. https://doi.org/10.1177/1533317513504817
- Tang, E. Y. H., Price, C., Stephan, B. C. M., Robinson, L., & Exley, C. (2020). Impact of memory problems post-stroke on patients and their family carers: A qualitative study. Frontiers in Medicine, 7, 267. https://doi.org/10.3389/fmed.2020.00267
- Thielen, H., Huenges Wajer, I. M., Tuts, N., Welkenhuyzen, L., Lafosse, C., & Gillebert, C. R. (2023a). The multi-modal evaluation of sensory sensitivity (MESSY): Assessing a commonly missed symptom of acquired brain injury. The Clinical Neuropsychologist, 38(2), 377–411. https://doi.org/10.1080/13854046.2023.2219024
- Thielen, H., Huenges Wajer, I. M., Tuts, N., Welkenhuyzen, L., Lafosse, C., & Gillebert, C. R. (2023b). The relationship between fatigue and sensory hypersensitivity after acquired brain injury [Unpublished manuscript]. Department of Brain & Cognition.
- Thielen, H., Tuts, N., Welkenhuyzen, L., Huenges Wajer, I. M., Lafosse, C., & Gillebert, C. R. (2023). Sensory sensitivity after acquired brain injury: A systematic review. Journal of Neuropsychology, 17(1), 1–31. https://doi.org/10.1111/jnp.12284
- Van der Elst, W., van Boxtel, M. P., van Breukelen, G. J., & Jolles, J. (2005). May) Rey’s verbal learning test: normative data for 1855 healthy participants aged 24-81 years and the influence of age, sex, education, and mode of presentation. Journal of the International Neuropsychological Society, 11(3), 290–302. https://doi.org/10.1017/s1355617705050344
- van Rijsbergen, M. W., Mark, R. E., de Kort, P. L., & Sitskoorn, M. M. (2014). Subjective cognitive complaints after stroke: a systematic review. Journal of Stroke and Cerebrovascular Diseases, 23(3), 408–420. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.05.003
- Wais, P. E., & Gazzaley, A. (2011). The impact of auditory distraction on retrieval of visual memories. Psychonomic Bulletin & Review, 18(6), 1090–1097. https://doi.org/10.3758/s13423-011-0169-7
- Wais, P. E., Rubens, M. T., Boccanfuso, J., & Gazzaley, A. (2010). Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. Journal of Neuroscience, 30(25), 8541–8550. https://doi.org/10.1523/JNEUROSCI.1478-10.2010
