ABSTRACT
Pharmaceutical industry-led access programs are growing in number globally and are increasingly adopting a hybrid approach intended to generate commercial and social value in parallel. We developed and applied a new conceptual framework in a descriptive analysis of observable indicators measuring commercial and social value for 91 programs registered in the Access Observatory. We found that most programs had features consistent with the generation of commercial value, directly through revenue generation (50.0%), or indirectly by creating competitive advantage (70.3%). We also found that most programs were implemented in countries where the company has commercial products registered (85.5%). While many programs had features consistent with the generation of social value, it was difficult to ascertain the level of that value because most did not share data (83.5%) and had not been evaluated (74.7%). Future efforts by the global health community and the pharmaceutical industry should focus on strengthening measurement and reporting on commercial and social indicators of industry-led access programs.
Introduction
Public-private partnerships are essential for improving access to medicinesCitation1 and achieving universal health coverage (UHC) by 2030 in low- and middle-income countries (LMICs).Citation2 Pharmaceutical companies have managerial expertise, technological capabilities, and investment capital that public sector counterparts do not have.Citation3 Particularly in countries where weak public infrastructure limits capacity to address health system needs, the private sector can complement public sector efforts to achieve UHC by 2030.Citation4 The pharmaceutical industry has a central role to play given the life-saving nature of their products.Citation5,Citation6
Many multinational pharmaceutical companies have independently responded to the call for industry contribution to UHC and access in lower-income settings, despite a lack of legal or regulatory requirements to do so. Indeed, pharmaceutical industry-led access programs have grown in number in recent years.Citation7 In this study, we adopt the definition of pharmaceutical industry-led access programs introduced in Rockers et al. (2018), “a subset of the full portfolio of access-related industry investments … [programs] designed and co-financed by companies and companies take responsibility and credit for them.” These programs utilize a diverse set of strategies, including health care worker trainings, community awareness campaigns, pricing schemes, and product donations.Citation8
Rather than being purely philanthropic, industry-led access programs are increasingly adopting a hybrid approach intended to generate commercial and social value in parallel.Citation8,Citation9 This hybrid approach mirrors social business and “shared value” models that are increasingly being adopted in other sectors, which have been argued to be a more sustainable and scalable way to pursue social development.Citation10 However, the features of industry-led access programs that might contribute to the generation of commercial and social value, respectively, are not well understood. While the potential commercial value generated by such programs has been observed,Citation6 there is no standard approach for characterizing this value or understanding how companies balance or trade-off the pursuit of commercial versus social value when designing and operating these programs. Given the growing importance of industry-led access programs using a hybrid approach, there is a need to better understand how commercial and social aims manifest in program strategies and whether they generate value. Assessing the commercial and social value of programs could enable governments, communities, citizens and shareholders to make informed decisions about investments in and support of these programs. For companies in the pharmaceutical industry, such assessments are critical to replicate, utilize and scale to identify the costs and value involved in access programs, and build partnerships to unlock shared opportunities with other companies at a pre-competitive level.
We developed a new conceptual framework that describes features of industry-led access programs that are consistent with the generation of commercial and social value, respectively. In this paper we present and apply the framework in a descriptive analysis of observable indicators for 91 programs registered in the Access Observatory, a public platform for measurement and reporting on access programs.Citation11
Conceptual Framework
We developed a new conceptual framework that aims to improve understanding of pharmaceutical industry-led access programs that adopt a hybrid approach (). The framework is grounded in Kramer and Porter’s notion of “shared value,” wherein an individual program could offset its costs by generating tangible commercial value for its company, including sales, profits, increased competitiveness, etc., while simultaneously advancing social conditions.Citation9
Figure 1. Conceptual framework for commercial and social value generated in industry-led access programs.
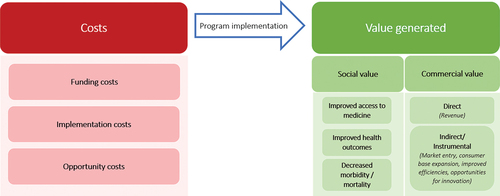
As displayed in , an industry-led access program can be thought of in terms of investment costs and value generated. Program costs can include financial costs attributed to the funding and implementation of programs, as well as the opportunity cost of allocating resources to the program rather than a purely commercial investment. Programs can generate commercial and/or social value. The social value of a particular program would be unique to that program, and tied to its key social objective, but could include outcomes such as improved access to medicine, improved health outcomes, or impact in the form of decreased morbidity or mortality. Improved health outcomes have utility that extends to broader society as they can contribute to progress in socioeconomic indicators, such as decreased healthcare expenditure, increased productivity, and GDP growth,Citation12 though these indirect effects have not been explicitly included in the framework. Commercial value generated from a program can be direct, where the program generates revenue, or indirect in the form of long-term competitive advantages. This notion of indirect commercial value was adapted from Droppert & Bennett (2015). The authors of that study found that pharmaceutical companies can be motivated by the competitive advantage presented by access programs, specifically in their potential to enable entering new markets, expanding consumer base, establishing long-term financial gain, improving efficiencies and creating opportunities for innovation.Citation9
It is important to note that access programs are not implemented within a vacuum; they are implemented within complex healthcare systems with a mix of public, private and donor-implemented health programs. While not included in the framework, we also acknowledge potential market effects of commercial value generation within health systems, including increased job creation within the health sector and decreased healthcare expenditure for health system actors (for example, through Astellas’ Action on Fistula program in Kenya, the company upgraded the Gynocare Women’s and Fistula Hospital, eliminating potential costs for the local government).Citation13 Alternatively, commercial value generation by multinational pharmaceutical access programs also poses a potential threat to local pharmaceutical companies that could face challenges entering a market already saturated by imported medicines.Citation14
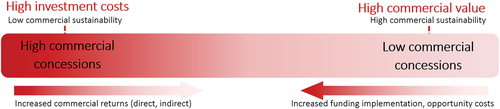
The elements of our framework are dynamic; programs can make varying degrees of commercial concessions or financial expenses, and these concessions can enable social benefit.Citation15 While this lens on concessions has primarily been applied to social impact investing, it applies well to our current analysis of access programs implemented by pharmaceutical companies. As these programs often offer products or services to individuals or institutions, they have the capacity to create, and therefore, concede commercial value for the company. The amount of commercial value that is conceded toward enhancing social value is critical to understand the nature of programs on the spectrum between fully philanthropic to fully commercial. Given the range of commercial value that access programs can generate for companies, individual programs can be further visualized to fall along a spectrum of the degree to which they make commercial concessions to generate social value (Appendix 1).
Materials and Methods
Applying the Framework
In order to apply the framework, we first reviewed three key data sources to understand what data were available. Next, we mapped relevant indicators onto the framework’s key concepts. Finally, we developed a 15-item instrument with these indicators and used it to review the sample of AO programs.
Three different data sources were reviewed: 1) the Access Observatory; 2) company websites and annual reports, and 3) national medicines regulatory authority databases.
The Access Observatory (AO) is a platform for public reporting of information on industry-led access programs focused mainly on non-communicable diseases (NCDs).Citation11 To our knowledge, AO offers the largest publicly available archive of information on industry-led access programs. The AO program reports are well-suited for this analysis as they contain information necessary to make assessments of the costs and value generated of individual industry-led access programs.
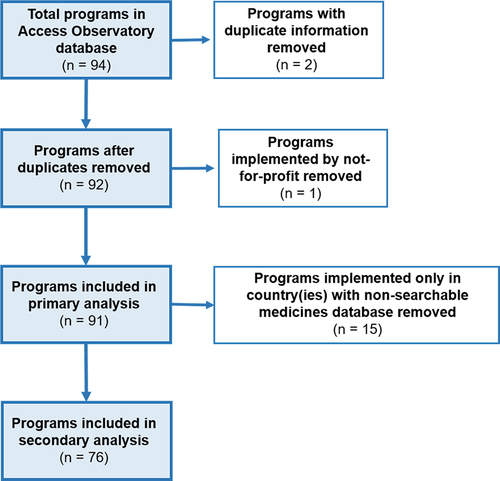
The AO includes 94 reports on individual access programs, however 3 reports were excluded from this analysis based on information duplication (n = 2) or the programs not being led by a for-profit company (n = 1).
To gain a better understanding of program features consistent with the generation of commercial value, publicly available company websites and annual reports were reviewed to assess whether a company was active within a program’s disease area. Moreover, medicine registration lists published by program countries’ health authority were reviewed to verify whether a company’s products are registered in program countries. Programs were excluded from a portion of the analysis if they were implemented only in countries where the national medicines regulatory authority did not have a functioning website or where no searchable database existed. See Appendix 2 for a flowchart describing the inclusion of programs for this analysis.
Data Extraction
The 15-item instrument was developed by study authors to extract data from the 91 program reports relating to the programs´ investment costs; their potential commercial value, both direct and indirect; and evidence of its social value. The data extraction instrument was developed based on the conceptual framework () and implemented using Kobo Toolbox, an online data collection software. Per the instrument, supporting justifications were recorded for each assessment made by reviewers, including the data source consulted. Data were extracted from program reports independently by two reviewers, discrepancies were discussed until consensus was reached. See Appendix 3 for the template data extraction instrument, Appendix 4 for detailed definitions of key variables in the instrument used by the reviewers during data extraction, and Appendix 5 for the program-level dataset (all are available as part of the online supplementary material).
A list of approved company products, noting both proprietary and nonproprietary drug names, was cross-referenced with online, searchable databases of medicines which were registered in the program country. Where a drug listed with a nonproprietary name could have multiple manufacturing companies, reviewers indicated they were “Unable to determine” whether a particular product was registered in a program country. Supporting justifications, including the specific data source consulted, were recorded for each determination made by the reviewer. Appendix 6 (see online supplementary material) contains the product registration verification dataset.
Data Processing and Analysis
Program review data were exported from Kobo Toolbox to Microsoft Excel, where data were stored and cleaned. Product registration data were also stored and cleaned in Microsoft Excel. Additional analytic variables around indirect commercial value variables were generated in RStudio by integrating the datasets. Descriptive analysis was performed using RStudio to describe frequencies and proportions of access programs according to selected cost, commercial value and social value variables.
Results
In this analysis, 91 access programs implemented by 18 companies across 115 countries were examined (). All of these programs were registered by their implementing companies into the Access Observatory. Many of these programs (67.0%) were implemented in multiple countries, though the majority were implemented in part in sub-Saharan Africa (63.7%). Similarly, programs were clustered in low- and middle-income countries, and only 7 programs (7.7%) were implemented in part in high-income countries.
Table 1. Implementation characteristics of AO programs
The most common program disease areas include cancer (56.0%), diabetes (15.4%), and mental & neurological disease (9.9%). The most common strategies that programs employed were those around health system strengthening (80.2%), community awareness and linkages (74.7%) health service delivery (53.8%), price schemes (18.7%) and donation (11.0%).
Nearly half of the program reports (47.3%) did not indicate whether a health technology, including medical devices, medical products and/or vaccines, had been provided as part of the access program. Of all programs, 23.1% indicated that they did provide health technologies through the program.
In-country registration was examined for a total of 423 approved products across all companies and countries. Of the 91 access programs, the vast majority (n = 76, 83.5%) were implemented in at least one country that had a searchable medicines registration database available on the national health authority website for that country.
Program Costs
No companies reported the total cost of their programs, and the vast majority of programs (87.9%) did not report the company’s financial contribution to the program (). For those 11 companies that did report, the average annual company contribution to AO programs in the past year (2019) reported was 1,024,863 USD. Of these 11 programs that did report their company’s contribution, 7 were implemented by a single company. Financial contribution was reported either in their description of the program or as an input indicator “Value of resources” contributed to the program.
Table 2. Company costs related to AO program implementation
The majority of programs were funded entirely by the reporting company (58.2%), meanwhile others were funded in part by the reporting company alongside other partners (37.4%), such as international NGOs, local universities or national ministries of health. While the reporting company’s costs for the vast majority of programs appeared to be in the form of a money transfer either directly to fund program activities or to an implementing partner (94.5%), for many programs costs also involved staff or in-country implementation (33.8%) or the provision of company products or health technologies (30.8%).
Commercial Value
The vast majority of programs appear to enable opportunities for commercial efficiencies (95.6%), by strengthening supply chains, addressing regulatory barriers, engaging government officials and other activities (). Additionally, the majority of programs (70.3%) employ a strategy such as access-based financing, or a technology, such as a digital health tool, that could enable innovation if it were transferred to another commercial area of the company.
Table 3. Commercial and social value across AO programs
Programs tended to be implemented in disease areas in which the company had products, and in countries where the company had registered products. Of the programs with product-level data (n = 76), the vast majority of programs (94.7%) focus on a disease area in which the company has at least one approved product. The majority of programs (85.5%) have at least one approved product registered in at least one of their program countries. Interestingly, the majority of programs (68.4%) also have at least one approved product within the program’s disease area registered in at least one of their program countries.
Of the programs with product-level data (n = 76), half (50.0%) had features consistent with the generation of revenue and half did not, either explicitly indicated in the AO program report or deduced by reviewers. Of the 38 programs that had features consistent with the generation of revenue, 19 (20.9% of all programs) explicitly presented these features in the program report, and 19 (20.9% of all programs) did not, but these features were deduced due to the specific program activities mentioned in the program report. Of the 19 programs where features consistent with generation of revenue were deduced, 17 (22.4% of all programs) were deduced due to their implementation of a health service delivery activity (such as diagnosis, screening or treatment) that were not explicitly donated in a program country where a treatment in the program’s disease area was registered. Of the 19 programs where revenue was assumed, 2 (2.6% of all programs) were deduced due to their implementation of other commercial activities where revenue was likely (such as clinical trials and a research summit). Of the 38 programs that do not appear to generate revenue, for 26 (34.2% of all programs) there was no explicit indication in the program report of any features consistent with the generation of revenue. For the remaining 12 programs (15.8% of all programs), reviewers deduced that features consistent with revenue generation were not present because there was no product in the program’s disease area registered in the program country, despite the program implementing some kind of health service delivery activity (such as diagnosis, screening or treatment).
Social Value
The majority of programs (73.6%) did report social indicators in the AO program report (). However, of the 67 programs that did report social indicators, the vast majority (77.6%) did not share any data. For those programs that reported social indicators, most reported output indicators (71.4%), some reported outcome indicators (44.0%), several reported input indicators (29.7%), and very few reported impact indicators (7.7%). Examples of output indicators reported for these programs included “Number of people trained” (Integrated Thyroid NCD Care in the Philippines); “Population exposed to community communication activities” (Mobile Healthcare Field Clinic Services); “Population screened” (Abundant Health); whereas impact indicators included “patients with complete cancer remission” (My Child Matters—Paraguay); disease-specific mortality rates (Celgene AMPATH Oncology Partnership); or “proportion of users satisfied with services received” (Health Camp against NCDs).
Published evidence of the social value of these programs was limited. A published evaluation was not found for the majority of programs (74.7%). Evaluations with an experimental or quasi-experimental design were only found for 5 programs (5.5%). Examples of such evaluations include Rockers et al. (2019), who conducted a cluster-randomized controlled trial to examine the effect of Novartis Access on the price and availability of medicines in Kenyan health facilities and households; or Patel et al. (2019)Citation16 who performed a quasi-experimental investigation of the effect of the SmartHEALTH mobile technology for cardiovascular health on the proportion of high-risk individuals using appropriate preventive medications.Citation17 A number of other programs had been evaluated with a non-experimental or descriptive design (19.8%).
Discussion
We presented a new conceptual framework that describes features of industry-led access programs that are consistent with the generation of commercial and social value. We applied the framework in an analysis of 91 programs registered in the Access Observatory, characterizing their costs, commercial value and social value. Our findings contribute importantly to the understanding of industry-led access programs, which are increasingly adopting a hybrid approach intended to generate commercial and social value in parallel.
Our analysis produced three key findings. First, half of the access programs reviewed had features consistent with the generation of revenue, a direct form of commercial value. Second, most programs had features consistent with the generation of indirect commercial value, whether by implementing within a disease area in which the company has approved products registered in the program country, or by implementing strategies that could lead to long-term commercial gains for the company. Third, data for this study were limited, particularly around the social value generated by programs, highlighting both a limitation in the applied methodology and the opportunity for companies to address the lack of information on and evaluation of industry-led access programs.
While specific financial data were limited, we found that access program funding was generally provided entirely by the company, and almost always involved money transfer to local implementing partners. This observation reinforces findings around the partnership typologies and the common industry-funded model adopted by programs registered with the AO.Citation18 Further study would be required to better understand if and how investment costs in industry-led access programs vary with the commercial and social value generated by these programs.
Many industry-led access programs have features consistent with the generation of commercial value, both direct and indirect. This finding is consistent with pharmaceutical industry trends, where access strategies have been associated with improved corporate financial performance,Citation19 and have even been recommended by business leader McKinsey & Company as important for success in emerging markets.Citation20,Citation21 However, given that conceding commercial value can be seen to enable social benefit,Citation12 the commercial value generated by access programs can be considered along a spectrum, with programs involving high commercial concessions on one end and those with low concessions on the other (). How companies balance these concessions with financial sustainability, and how commercial strategy is considered when implementing access programs, are areas for further research.
The majority of programs examined were implemented in the same disease area as their companies’ approved products, and in countries in which those products were registered. This finding demonstrates an alignment between the social aims of access programs and the commercial markets of companies. This is perhaps unsurprising, as companies are likely best suited to pursue access efforts that build on their established capabilities. Implementing access programs in settings where companies already work could involve lower opportunity cost and help expand consumer bases for established products or create consumer bases for new products.Citation9 Depending on the nature of a given access program, such expansions could generate social value in addition to commercial value, though this is not guaranteed. This observed alignment between access programs and companies’ commercial markets also raises the normative question of whether companies should be aiming for such an alignment (i.e., a company manufacturing antihypertensive drugs should aim to build access programs supporting antihypertensive service delivery), and whether access program activities can possibly distract from or compete with activities implemented by other stakeholders, including local governments. The interactions between industry-led access programs and local healthcare system activities is an area that warrants further specific academic attention, as they should operate synergistically in a holistic health system to generate the best outcomes for patients and society.
Identifying and measuring specific commercial value created from industry-led access programs has not yet been pursued. Our findings empirically demonstrate an observed trend of access programs exemplifying elements of shared value,Citation7 where commercial value and social value are being created simultaneously.Citation9
Evidence is limited around the social value generated by these programs, due to insufficient measurement and evaluation lacking scientific rigor.Citation7 Our findings reinforce these previous observations around limited reporting and the low-rigor evaluation of industry-led access programs.Citation7,Citation9 Rigorous evaluation of access programs is useful in understanding the social value these programs generate. In this analysis, we identified access programs that have undergone an experimental or quasi-experimental evaluation. Novartis Access was experimentally evaluated by Rockers et al. (2019), who found that the program did not affect the availability of portfolio medicines overall.Citation12 In contrast, Umeh et al. (2020) evaluated the Glivec International Patient Assistance Program (GIPAP), and found that the program did improve survival for patients with chronic myelogenous leukemia or gastrointestinal stromal tumor.Citation22 Evaluation and reporting of these programs presents an important opportunity for companies to understand the relationship between social and commercial objectives, identify and resolve tradeoffs, improve programs where outcomes are not being achieved, or increase investment and engagement in those programs where outcomes are being achieved.
Given that many of these access programs are hybrid,Citation7 wherein social and commercial objectives are intertwined, limited reporting and lack of evaluation raises the concern of mission drift. Mission drift away from social objectives and toward commercial objectives and revenue-generation is a documented phenomenon in nonprofitsCitation23 and social enterprises,Citation24 and can be applied to industry-led access programs that have explicit social aims but are embedded within larger, profit-driven corporations. While mission drift in access programs has not yet been studied, its possibility coupled with our findings around limited data emphasizes the need for companies to transparently report on social and commercial indicators of their access programs.
This analysis spotlights the limited data available on the costs, commercial value and social value generated by industry-led access programs. AO program reports held information pertinent to these fields but did not always provide clear and consistent observations. This finding highlights a noteworthy limitation of our framework and our descriptive analysis: the framework is perhaps most useful for stakeholders external to the pharmaceutical industry who want to understand the nature of industry-led access programs. The utility of the framework to these stakeholders as an analytic tool is therefore limited by the types of data that are publicly available. However, this limitation further highlights the significant opportunity for pharmaceutical companies to measure and publicly report these elements, to identify gains and losses in access program implementation, and to build innovative partnerships to enable programs to meet their desired outcomes.
As investigators external to the pharmaceutical industry, we mitigated this limitation by using additional data sources, including company websites and annual reports, as well as product registration databases from national health authorities. By supplementing AO program report data with product registration data, reviewers deduced those programs that were likely to have a revenue-generating component. Inconsistent data in AO program reports were addressed by employing systematic methodology for review through a structured questionnaire and having multiple reviewers. In addition to limited data availability, there were other limitations in our methodology. First, for the program review we relied on publicly available, company self-reported data found in AO program reports. Additionally, the AO dataset focuses primarily on programs targeting non-communicable diseases (NCDs), and so some findings, particularly those related to registration of products within a program disease area, might not be generalizable to industry-led access programs of other disease areas such as communicable diseases.
Conclusions
Pharmaceutical industry-led access programs are growing in number globally and are increasingly adopting a hybrid approach intended to generate commercial and social value in parallel. We developed and applied a new conceptual framework to systematically describe key features of these programs in order to better understand their hybrid nature. We found strong evidence that industry-led access programs generate direct and indirect commercial value for companies. However, comprehensive understanding of the commercial and social value from these programs is limited by insufficient publicly available data. Further collaborative work is needed to systematize the application of this framework; to improve standard reporting; and to consistently and more rigorously evaluate industry-led access programs within complex multi-stakeholder healthcare systems. Leveraging central institutions, like the Access Observatory, to facilitate this work is an effective means of creating an enabling environment and governance for these programs. This work can help build necessary transparency, accountability, and sustainability of pharmaceutical companies in ensuring access to medicines and universal health coverage globally.
Figure A1. Theoretical spectrum of commercial concessions in industry-led access programs. When commercial concessions are high, commercial sustainability is low because there is little commercial value generated by the program for the company. When commercial concessions are low, commercial sustainability is high because the commercial value generated by the program for the company is high.
Disclosure of Potential Conflicts of Interest
JSS, PK and MM report no potential conficts of interest. ACH reports grants from Access Accelerated, Gilead Sciences, Amgen and F. Hoffmann-La Roche outside the submitted work. VJW reports grants from Access Accelerated, Gilead Sciences, Amgen and F. Hoffmann-La Roche outside the submitted work. PCR reports grants from Access Accelerated, Gilead Sciences, Amgen and F. Hoffmann-La Roche outside the submitted work.
Supplemental Material (Appendices 3-6)
Download Zip (373.1 KB)Supplemental data
Supplemental material for this article can be accessed online at https://doi.org/10.1080/23288604.2022.2057831
Additional information
Funding
References
- Wirtz VJ, Hogerzeil HV, Gray AL, Bigdeli M, de Joncheere CP, Ewen MA, Gyansa-Lutterodt M, Jing S, Luiza VL, Mbindyo RM, et al. Essential medicines for universal health coverage. Lancet. 2017;389(10067):403–10. doi:10.1016/S0140-6736(16)31599-9.
- United Nations. Sustainable development goals. 2015 https://sustainabledevelopment.un.org/sdgs Accessed 20 February 2021.
- Clarke D, Doerr S, Hunter M, Schmets, Schmets G, Soucat A, Paviza A. The private sector and universal health coverage. Bull World Health Organ. 2019;97(6):434–35. doi:10.2471/BLT.18.225540.
- Hamidu H, Amran 8 October . Corporate Social Responsibility stages of transformation, drivers and dimensional models: a review. SSRN. 2016.
- Nations U. Report of the United Nations Secretary-General’s High-Level Panel on Access to Medicines: promoting innovation and access to health technologies [Internet]. New York (NY): UN; 2016 Sep [cited 2021 Apr 15]. Available from: http://www.unsgaccessmeds.org/final-report/
- International Federation of Pharmaceutical Manufacturers & Associations (2019). Achieving a healthier and sustainable future for all: policy perspectives on Universal Health Coverage from the innovative biopharmaceutical company. Available from: https://www.ifpma.org/wp-content/uploads/2019/09/IFPMA_Policy_Perspectives_on-_UHC_Full_Report.pdf Accessed 22 February 2021
- Rockers PC, Wirtz VJ, Umeh CA, Swamy PM, Laing RO. Industry-Led Access-To-Medicines Initiatives In Low- And Middle-Income Countries: strategies And Evidence. Health Aff. 2017; 36(4): 706–13. http://dx.doi.org.ezproxy.bu.edu/10.1377/hlthaff.2016.1213 Accessed 05 January 2021
- Rockers PC, Reich MR, Kettler H, Wirtz, VJ, et al. Commitment to Impact: strengthening Measurement of Industry-Led Access-to-Medicines Programs. Health Systems & Reform. 2018;4(3):188–93. doi:10.1080/23288604.2018.1483710.
- Droppert H, Bennet S. Corporate social responsibility in global health: an exploratory study of multinational pharmaceutical firms. Global Health. 2015:11. doi:10.1186/s12992-015-0100-5.
- Kramer MR. Creating shared value. Harv Bus Rev. 2011;Jan-Feb:62–77.
- Access Observatory. Programs. Available at: www.accessobservatory.org/programs/.
- McKinsey Global Institute (2020). Prioritizing health: a prescription for progress. Available from: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/prioritizing-health-a-prescription-for-prosperity Accessed 28 February 2022.
- Sruamsiri R, Wagner AK, Ross-Degnan D, Lu CY, Dhippayom T, Ngorsuraches S, Chaiyakunapruk N. Expanding access to high-cost medicines through the E2 access program in Thailand: effects on utilisation, health outcomes and cost using an interrupted time-series analysis. BMJ Open. 2016 Mar 17;6(3):e008671. doi:10.1136/bmjopen-2015-008671. PMID: 26988346.
- Conway M, Holt T, Sabow Holt T (2019). Should sub-Saharan Africa make its own drugs? What’s next for pharma in emerging markets? McKinsey & Company. https://www.mckinsey.com/industries/public-and-social-sector/our-insights/should-sub-saharan-africa-make-its-own-drugs Accessed 20 February 2022
- Brest P, Born K (2013). Unpacking the impact in impact investing. Stanford Social Innovation Review. Available at https://ssir.org/articles/entry/unpacking_the_impact_in_impact_investing.
- Rockers PC, Laing RO, Ashigbie PG, Onyango MA, Mukiira CK, Wirtz VJ. Effect of Novartis Access on availability and price of non-communicable disease medicines in Kenya: a cluster-randomised controlled trial. Lancet Global Health. 2019;7(4):e492–e502. doi:10.1016/S2214-109X(18)30563-1.
- Patel A, Praveen D, Maharani A, Oceandy D, Pilard Q, Kohli MPS, Sujarwoto S, Tampubolon G. Association of multifaceted mobile technology-enabled primary care intervention with cardiovascular disease risk management in Rural Indonesia. JAMA Cardiology. 2019;4(10):978–86. doi:10.1001/jamacardio.2019.2974.
- Umeh, Umeh CA, Rockers PC, Laing RO, Wagh O, Wirtz VJ. Pharmaceutical industry-led partnerships focused on addressing the global burden of non-communicable diseases: a review of Access Accelerated. Public Health. 2020;181:73–79. doi:10.1016/j.puhe.2019.12.008.
- Adamu SA (2017). Impact of corporate social responsibility on financial performance in the pharmaceutical industry. Theses and Dissertations. 903. https://digitalcommons.pepperdine.edu/etd/903 Accessed 13 February 2021
- Agarwal A, Dreszer J, Mina J (2017). What’s next for pharma in emerging markets? McKinsey & Company. Available at: https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/whats-next-for-pharma-in-emerging-markets Accessed 20 February 2021
- Ascher J, Bogdan B, Dreszer J, Zhao, G, et al. (2015). Pharma’s next challenge. McKinsey & Company. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/pharmas-next-challenge Accessed 21 February 2021
- Umeh CA, Garcia-Gonzalez P, Tremblay D, Laing R. The survival of patients enrolled in a global direct-to-patient cancer medicine donation program: the Glivec International Patient Assistance Program (GIPAP). EClinicalMedicine. 2020:19. doi:10.1016/j.eclinm.2020.100257.
- Jones MB. The multiple sources of mission drift. Nonprofit and Voluntary Sector Quarterly. 2007;36(2):299–307. doi:10.1177/0899764007300385.
- Ebrahim A, Battilana J, Mair J. The governance of social enterprises: mission drift and accountability challenges in hybrid organizations. Research in Organizational Behavior. 2014;34:81–100. doi:10.1016/j.riob.2014.09.001.