ABSTRACT
Synthetic biology predominantly follows a market-driven approach, both within the private sector and academia. We present a research journey undertaken by a synthetic biologist who received guidance from responsible innovation scholars, reflecting on the wider effects of synthetic biology technologies. The outcome is a re-evaluation of synthetic biology through the lens of ‘conviviality’, a concept introduced by Ivan Illich to designate a modern society of responsibly limited tools, where individual freedom is realised through personal interdependence. We find that in its current form, synthetic biology is not convivial since it relies on centralisation, monopolies and technologies which have the capacity to negatively affect the biosphere and its inhabitants. We argue that a broader conception of biotechnology, beyond genetics, is needed to conceive convivial biotechnologies. In our research journey we explore a range of approaches for responsible biotechnology innovation, which includes open-source, commons-based, decentralised organisations, and post-growth models.
Conviviality and synthetic biology
In the book Tools for Conviviality, the eclectic intellectual Ivan Illich stated ‘I have chosen “convivial” as a technical term to designate a modern society of responsibly limited tools ’ Illich (Citation2018). According to Illich, ‘convivial tools’ are those that empower individuals and communities to pursue their own goals and meet their own needs, fostering autonomy and self-reliance. Illich argued that modern industrial societies had become overly dependent on complex technologies that served to centralise power and control, often at the expense of individual freedom and human dignity. He believed that convivial tools should be simple, accessible, and under the control of the users themselves, rather than being monopolised by large institutions or corporations. Convivial tools, in Illich's view, would promote a more equitable and participatory society by enabling people to actively engage with their environment and each other. They facilitate collaboration, creativity, and cooperation, rather than fostering dependence on expert knowledge or hierarchical structures.
The first author of this perspective came across Illich's notion of convivial tools during a responsible innovation training course for synthetic biologists (Pansera et al. Citation2020), where the concept of ‘conviviality’ was suggested as a guide for the development and use of new technologies. The idea of conviviality resonated with the sentiment of some of the young researchers in the room that synthetic biology might aid a transition from an economy based upon finite resources to a more sustainable society based on renewable plant-based materials as inputs. The idea of conviviality complicates the optimistic hypes of synthetic biology by considering who controls access to such technologies and who benefits (local enterprises, global companies or the people using them). The social system that constructs and is constructed by the technology is important (Vetter Citation2018): people are seen as inherently interwoven in social networks and driven by complex motivations (Godbout and Caille Citation1998; Vetter Citation2018). In 1973 Illich argued
I consider conviviality to be individual freedom realised in personal interdependence and, as such, an intrinsic ethical value. I believe that, in any society, as conviviality is reduced below a certain level, no amount of industrial productivity can effectively satisfy the needs it creates among society's members. (Illich Citation2018)
The concept of convivial technology considers the interdependence between people and between technology and humans, Vetter, 2016 – ‘reflecting the social construction of technology as well as the technological construction of human behaviour. This makes it possible to talk about “convivial technologies”, a term that Ivan Illich did not use ’ Vetter (Citation2018). The concept of convivial technology focuses on the ideas that Illich raised – the need for creativity and autonomy for convivial tools (Illich Citation2018; Vetter Citation2018).
What implications do these ideas have on the development and application of hyper-complex technological regimes such as synthetic biology? Somebody could argue that Illich might turn in his grave at the thought of the term ‘convivial synthetic biology’. Some would consider this an oxymoron, since creativity in synthetic biology is kept in the hands of a minority of experts with access to prohibitively expensive equipment most often in start-ups and universities, leaving little room for a conviviality. Furthermore, synthetic biology technologies often use genetic modification to engineer DNA, or the genome of living organisms (Baldwin et al. Citation2012), often microorganisms. The impact of such genetic technologies goes further than the newly modified DNA or microorganism, given the propensity of DNA to be shared between microbes (Von Wintersdorff et al. Citation2016) and for microbes themselves to travel (Qin et al. Citation2022). Thus, genetic tools developed by synthetic biology can have lasting consequences outside of the lab (Chemla et al. Citation2022; Paracchini et al. Citation2017). The concept of ‘one health’ can help to understand the implications: van Bruggen et al. argue that ‘the health of all organisms in an ecosystem are interconnected and mediated through the cycling of subsets of microbial communities from the environment (in particular the soil) to plants, animals and humans, and back into the environment’ (van Bruggen et al. Citation2019). From the perspective of convivial technology, the flaws of synthetic biology tools lie in its potential impact on people, other organisms and the environment in ways which cannot be easily changed in the absence of experts and expensive equipment. That said, adaptation of the sequences by microorganisms would likely follow, in ways that would not always be predictable. Synthetic biology tools which do not have such impacts on living organisms may exist, but the dependence on experts and centralised equipment will likely remain, ruling out conviviality. To find convivial biological tools, we argue that one must take a step outside of the frame of synthetic biology, to biotechnology and more specifically, a broader conception of biotechnology.
In the past 30 years, the term ‘biotechnology’ has often been used to refer exclusively to technologies that operate on a genetic level (Crowe Citation2021). This specific understanding of biotechnology (that it must be implemented genetically) has emerged from the groundbreaking work of scientists who revolutionised our comprehension of cellular functioning (Morange Citation2020). These efforts have culminated in synthetic biology, a thriving field of research, endeavouring to leverage genetic engineering for the creation of organisms and molecules possessing specific functionalities (Baldwin et al. Citation2012), which scientists are applying to challenges in medicine, agriculture, and manufacturing (Greco, Tarnowski, and Gorochowski Citation2019; Voigt Citation2020). However, this is just one understanding of what biotechnology, or biological technology, is and can be. More broadly, biotechnology can be defined as all interactions within this living world. By redefining biotechnology in this way, convivial biotechnologies such as fermentation and agroecology might be included.
But what makes a biotechnology convivial, or not? In tandem with the advancements in genetic biotechnology, the field of science and technology studies (STS) has emerged, resulting in tools which can be used to evaluate new technologies and scientific research (Martin, Nightingale, and Yegros-Yegros Citation2012). STS scholars have effectively demonstrated that the development of technologies is not a neutral and apolitical process, but rather reflects the values, ideologies, and worldviews of the society in which it originates (Pansera and Fressoli Citation2020; Toynbee Citation1953; Winner Citation1980). The pathways of technological change are shaped by socio-economic conditions, interests, and historical contexts (Pansera and Fressoli Citation2020). It is crucial to recognise that multiple trajectories of technological change are possible, and they often coexist (Leach et al. Citation2012). However, over time, a dominant path may emerge and become ‘naturalized’, creating the illusion that it is the sole approach despite being the product of converging interests, asymmetrical power dynamics, domination, and violence (Pansera and Fressoli Citation2020).
The emergence and development of synthetic biology seems to have followed a very similar path. The advent of synthetic biology as a scientific discipline has been accompanied by lofty expectations of its potential achievements. The field has promised remarkable advancements in agriculture, revolutionary applications in medicine and the environment, among many others. These promises have often been framed to align with corporate interests, thereby attracting significant attention from investors. Consequently, Synthetic Biology has swiftly become entrenched as just another domain of scientific inquiry that is moulded to generate economic value. What becomes evident through studies in the field of responsible innovation (RI) within STS is that synthetic biology does not solely emerge from the curiosity and inventiveness of disinterested scientists (Ribeiro and Shapira Citation2020). Rather, it is the outcome of increasingly intricate socio-technical assemblages that serve the interests of global capitalism, aiming to extract and accumulate value. These mechanisms seek to subordinate even the most intimate and microscopic aspects of life to the service of capital valorisation. Consequently, it is not surprising that technology development in synthetic biology often aligns with an ideology of productivism that exalts economic growth for its own sake and a determinism that views technological progress as an inevitable force (Pansera and Fressoli Citation2020).
This framing of innovation is problematic as it imposes limitations on the potential actions that researchers and innovators can pursue. It restricts the discourse on what can be said and done, creating boundaries and defining what is permissible and what is not. By perpetuating this restrictive perspective, opportunities for alternative approaches and critical engagement are stifled, hindering the exploration of diverse possibilities and transformative pathways. It is essential to challenge and transcend these limitations to foster a more inclusive and ethically grounded innovation landscape.
Our responsible research journey
Considerations of responsible research can remain irrelevant to the vast majority of scientists and engineers working in the field of synthetic biology, as they are immersed in the demanding pursuit of securing grants and conducting their research (Glerup, Davies, and Horst Citation2017). However, the field of Responsible Innovation (RI) has demonstrated its capacity to create spaces for reflection on alternative modes of innovation that challenge this prevailing notion within synthetic biology (Pansera et al. Citation2020). Achieving this has been made possible through efforts to institutionalise RI (Pansera et al. Citation2020), as well as through personal interactions between synthetic biologists and social scientists. A pertinent example is the collaboration between the two authors: as we said above, the first author encountered the concept of RI during their time as a PhD student in synthetic biology at BrisSynBio, a multi-disciplinary research centre that focuses on the biomolecular design and engineering aspects of synthetic biology, while the second author was a post-doctoral fellow in social science at the University of Bristol. We met in the context of a EU funded project designed to promote responsible practices within research funding and conducting institutions. Through a number of workshops, seminars and retreats, we became mutually aware of the complicated relation between personal values and aspirations, our individual agency and the broader institutional settings in which we both operated at the time.
For the first author, this research journey took place in the context of doctoral studies in synthetic biology. Whilst we are often taught that scientific research is objective, his PhD showed him that the experiences of researchers (Batty et al. Citation2020) and the cultures they participate in influence their studies (Saini Citation2020), funding and research priorities, methods and outputs (Baggini Citation2018). Human endeavours are born of and influenced by the world around them; a PhD journey is influenced by the experiences of the researcher. Science does not happen in a laboratory isolated from the world, but exists as part of society and culture. He came to the perspective that the broader focuses of scientific research is influenced and mediated by culture, just as science offers ideas and concepts – as well as technologies – that get incorporated into and influence the direction and workings of society. At the outset of the PhD he was both enthusiastic and wary of the promise of designing genetically engineered microorganisms to produce materials. Modifying the genetic code of life sounded like something to do cautiously and with care. Whilst unsure of what to make of synthetic biology, the degree was an exciting opportunity to understand it in greater detail. Initially he was guided by engineering principles commonly utilised in synthetic biology, such as abstraction, whereby genetics is simplified to a set of separate parts (Baldwin et al. Citation2012). This led him to focus his doctoral studies on developing methods to create novel genetic parts (Tarnowski and Gorochowski Citation2022). Along the way, training in responsible research and innovation (Pansera et al. Citation2020) led to consideration of the place of scientific research and innovation in society. Undertaking research responsibly (Owen et al. Citation2013) became a priority and he attempted to apply reflection and reflexivity, the capacity to adapt research direction in response to new knowledge, to his own research.
Learning of reflections and practices from RI scholars, and in particular his personal interaction with the second author, were pivotal. Realising that the tools developed in his PhD research within synthetic biology could contribute to inequality by virtue of their inaccessibility (both economically and technologically), the author experienced a profound internal conflict, sensing a misalignment between his work and personal values. Motivated by the exploration of alternative approaches, he contemplated shifting his research focus towards a more accessible biotechnology that would leverage his skills in microbiology. However, mentors cautioned against such a change, deeming it untimely and deviating too far from the original research project. This raises significant questions regarding the role and responsibility of social scientists – in this case the second author – engaging with young natural scientists in the early stages of their careers. It is important to acknowledge that exposing scholars, who often possess limited agency, to the intricate web of power dynamics and corporate interests that sustain their research field can disrupt their motivation and, most importantly, their career prospects. In our particular case, although the journey was challenging, persisting with the initial research project presented an opportunity for profound reflection on how synthetic biology can be approached and practiced in a responsible manner. This introspection enabled a deeper understanding of the complexities at play and led to an exploration of possibilities for responsible practices within the field.
Our reflection began when we examined the prevailing norms relating to the desired outcomes of synthetic biology research and innovation. One norm that stands out prominently is the singular focus on a specific innovation strategy presented to young scholars in the field: to bring their research output to market by founding start-up companies backed by venture capitalist funding, enabling rapid economic growth. While this approach undoubtedly generates wealth, it also contributes to the concentration of wealth (Brynjolfsson, McAfee, and Spence Citation2014). The consequences of increased wealth inequality extend beyond purely economic concerns and encompass environmental degradation both directly (Rip and Kemp Citation1998) and indirectly (Shahbaz Citation2013). This ‘exponential’ growth model heavily relies on the creation of intellectual property, which is another norm within the synthetic biology community. This norm often hinges on the development of unique genetic sequences (Meyer et al. Citation2021) and genetically engineered organisms as the basis for proprietary rights and commercial advantage (Van Doren, Koenigstein, and Reiss Citation2013).
Another norm that emerges from this growth-focussed and innovation-driven approach is the emphasis on private ownership, leading to governance by investor shareholders who often prioritise profit over social and environmental well-being. The pursuit of rapid growth and substantial profits in synthetic biology typically revolves around centralised production at scale, which can result in the displacement of livelihoods (Peplow Citation2016; Thomas Citation2013). To facilitate such large-scale production, bulk inputs are required, necessitating the use of crop monocultures which carry significant environmental consequences (Azadi et al. Citation2012; Higgins, Short, and South Citation2013; Luo, van der Voet, and Huppes Citation2009). This perpetuates extractive economies, where low-value materials are acquired and value is added within centralised production facilities before being sold, further concentrating wealth (Haraway Citation2015; Murphy and Schroering Citation2020). All of these norms appear to stem from a paradigm that separates humans from nature and from one another. The consequence is the creation of technologies and systems that are fragile and susceptible to being stuck in patterns of power concentration, inaccessibility (Illich Citation2018), and technological lock-in Foxon (Citation2014). This hinders the adaptability of the resultant technologies, which could otherwise offer resilience. In the face of ongoing climate change (Bendell and Read Citation2021), resilient technologies offer the capacity to adapt. However, it is important to recognise that the norms governing synthetic biology innovation are just one of many possible approaches to innovating in the field of biotechnology.
Convivial biotechnology
Probably the most interesting outcome of our intellectual collaboration was the opening up of a new imaginative space to reframe what synthetic biology could be and what it should or might deliver. Having questioned some basic tenets and established assumption about the political economy and normativity of synthetic biology, our research journey led us to imagine potential alternatives. Our analysis, as illustrated in , is not intended to offer a definitive prescription for labelling synthetic biology practices as ‘bad’ or ‘good’. Rather, our aim is to start reflection regarding political aspects often overlooked by researchers in this domain. By questioning these dimensions and juxtaposing them with potential alternatives, we hope to initiate the journey toward envisioning more convivial biotechnological practices. When it comes to the overall approach to innovation, post-growth thinking presents alternative business philosophies that challenge the relentless pursuit of economic growth as the ultimate measure of progress. Instead, it prioritises sustainability, well-being, and equitable resource distribution (Pansera and Fressoli Citation2020). This perspective has significant implications for the innovation process itself (Robra et al. Citation2023).
Figure 1. Synthetic biology research and innovation norms and alternatives. Image credit: Matthew Tarnowski.
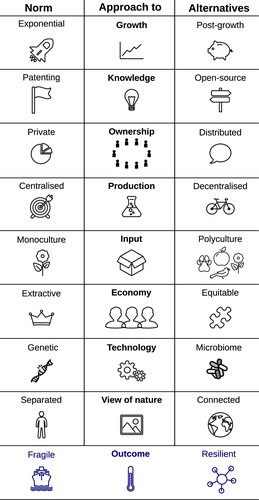
In terms of knowledge sharing, embracing open-source (Vavitsas Citation2018; Williamson et al. Citation2016), copyleft (Mustonen Citation2003), and commoning (Hess and Ostrom Citation2008) approaches can facilitate the development of more accessible and adaptable technologies. Within the synthetic biology community, there are many open-source methodologies, such as the use of biobricks (Shetty, Endy, and Knight Citation2008) in the iGEM competition and repository, and the synthetic biology open language for representing genetic designs (McLaughlin et al. Citation2020). Another recent advance in the field is the introduction of open material transfer agreements (Kahl et al. Citation2018), which could enable wider sharing of biological materials with less strings attached. However, wider use of such agreements may only come about with wider changes in the organisational structures that bind researchers, who are sometimes constrained by grant and institutional conditions. Such changes may only be possible with broader social, political and economic changes. Addgene, a library of DNA sequences stored and distributed as plasmids (Kahl et al. Citation2018), serves as an excellent example of a commons utilised in synthetic biology. Microbial culture collections around the world provide access to microbial strains. Genetic sequences of interest to the synthetic biology community and beyond, are regularly annotated on a voluntary basis which is coordinated online (Terlouw et al. Citation2023). The Nagoya protocol governs access to biological materials and sharing of benefits from its use, however the relevance of this protocol to digital sequence information has been questioned (Bond and Scott Citation2020). A change in the approach to developing genetic parts for synthetic biology, from ‘bioprospecting’ to ‘biorespecting’, has been proposed too (Tarnowski et al. Citation2023). However, the precedent for corporate capitalism profiting from commons-based production, or ‘commonswashing’, is worth bearing in mind (de Rosnay Citation2020). This may be especially the case for synthetic biology since despite these open source efforts, the utility of such genetic technologies remains inaccessible outside of academia and industrial biotechnology.
In terms of ownership and governance, various models that distribute decision-making power exist, while still allowing for the creation of highly valued enterprises (Dawson, Paeglis, and Basu Citation2017). One such model is steward ownership, wherein the business is owned by a trust whose board comprises individuals with a vested interest in the company's values rather than solely its economic prospects (Purpose Economy Citation2022). Other potential models are cooperatives or common-based-peer production collectives (Robra et al. Citation2023). We are not aware of any synthetic biology organisations set up on this basis. The scale of production embedded within technologies is a choice that determines how people and resources can be organised (Winner Citation1980). Synthetic biology innovation often focuses on large-scale production of a single molecule or product, using bioreactors in the region of 10,000s of litres. An alternative choice is to incorporate diverse inputs from the local bioregion, fostering collaboration with landworkers in the area to cultivate biodiverse ‘agroecologies’ that support biodiversity and the production of raw materials, or processed products. The report ‘An agroecological Europe in 2050’ found that sustainable food could be provided for 530 million Europeans by generalising agroecology, abandoning imports of plant proteins and adopting healthier diets (Poux and Aubert Citation2018). Re-purposing existing breweries as community bioreactors, using fermentation for production of food, materials, medicine and more, could be an important part of this potential future agroecological economy. The synthetic biology approach to biotechnology generally necessitates genetic engineering, which relies upon experts and technical facilities, making it inaccessible. Alternative approaches to biotechnology, such as fermentation and selective breeding, have been used to select microbiomes and plants respectively, for over 13,000 years (Liu et al. Citation2018), in the absence of DNA sequencing or synthesis, the main tools of synthetic biology. Fermentation offers is a biotechnology that remains both accessible and adaptable. Convivial biotechnology may also currently be limited by broader constraints: opportunities to learn relevant skills, access land and an economic framework which values conviviality.
The principles outlined above are a direct inspiration from Ivan Illich's concept of ‘convivial’ (Illich Citation2018), responsibly limited, tools. This approach can be used to design technologies that consider the interdependence between people, technology and the environment (Vetter Citation2018). The ideas of Illich have been transformed in to a matrix that can be used to assess the conviviality of a technology (Vetter Citation2018). The matrix of convivial technology (MCT) assesses four life-cycle levels of a technology (materials, production, use, infrastructure) in terms of five dimensions: relatedness, access, adaptability, bio-interaction and appropriateness. This results in a matrix comprising 20 fields. This is summarised in and the full table which can be used for technology assessment is provided in the Supplementary Information. The dimensions of conviviality were selected based upon qualitative research of innovators developing low impact technologies. Assessing each field requires the assessor to select the most appropriate descriptors from a list of options, enabling them to answer the overarching question related to each dimension of conviviality. Whilst it is qualitative and subjective, the MCT provides a tool that researchers and innovators can use to reflect upon the technologies they are developing.
Figure 2. The matrix of convivial technology The matrix of convivial technology can be used to assess four life-cycle levels of a technology in terms of five dimensions of conviviality. Figure adapted from Vetter (Citation2018).
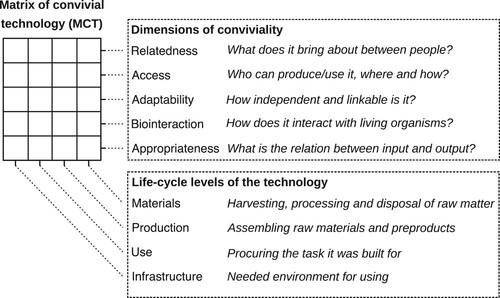
Broadly, the approaches that we focus upon in our analysis () map to some of the dimensions of the matrix of convivial technology . ‘Production’, ‘Ownership’ and ‘Knowledge’ in our analysis all relate to the ‘Access’ dimension of conviviality since it considers who can produce / use the technology, where and how. ‘Growth’ and ‘Economy’ relate to the ‘Relatedness’ dimension: they consider what is brought about between people. ‘Technology’ and ‘View of Nature’ both relate to the ‘Bio-interaction’ dimension, they consider how the technology interacts with living organisms. Finally ‘Input’ maps to the ‘Appropriateness’ dimension, which focuses on the relation between input and output. Thus, our analysis identifies some alternatives to norms within synthetic biology that, if addressed, could modify the conviviality of the resulting technologies.
In conclusion, we would like to draw attention to the potential risks associated with the interaction between social scientists and early-stage researchers in synthetic biology. While such engagements may seem innocuous, there are instances where they can inadvertently undermine personal motivations and career objectives. However, these dialogues also have the potential to foster radically different perspectives and practices for research and innovation process. This has been the case for the first author whose academic work now focuses primarily on microbiology and agroecology and now enjoys seed-swaps, community-supported agriculture, brewing and fermentation. Meanwhile for the social scientist, there has been a realisation that in such interactions, there is a degree of responsibility for young researchers, where the impact can disrupt their career. As a result of our research journey, we propose ‘conviviality’ as a framework to reframe the discourse surrounding synthetic biology and its promises. Whilst synthetic biology may never be convivial, recalling that it is one of many approaches to biotechnology and innovation opens possibilities that can lead to its transformation. By embracing conviviality, we can foster a more collaborative and inclusive approach to biotechnology innovation.
gpmap-synbio-seq.bib
Download Bibliographical Database File (119.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Azadi, H., S. A. de Jong, B. Derudder, P. De Maeyer, and F. Witlox. 2012. “Bitter Sweet – How Sustainable is Bio-Ethanol Production in Brazil?” Renewable and Sustainable Energy Reviews 16 (6): 3599–3603. https://doi.org/10.1016/j.rser.2012.03.015.
- Baggini, J. 2018. How the World Thinks – a Global History of Philosophy. London, UK: Granta Books.
- Baldwin, G., T. Bayer, R. Dickinson, T. Ellis, K. M. Polizzi, and G. B. Stan. 2012. “Synthetic Biology: A Primer.” Imperial College Press, https://doi.org/10.1142/p837.
- Batty, C., E. Ellison, A. Owens, and D. Brien. 2020. “Mapping the Emotional Journey of the Doctoral ‘Hero’: Challenges Faced and Breakthroughs Made by Creative Arts and Humanities Candidates.” Arts and Humanities in Higher Education 19 (4): 354–376. https://doi.org/10.1177/1474022219844986.
- Bendell, J., and R. Read. 2021. Deep Adaptation: Navigating the Realities of Climate Chaos. Cambridge, UK: Polity.
- Bond, M. R., and D. Scott. 2020. “Digital Biopiracy and the (Dis)assembling of the Nagoya Protocol.” Geoforum 117:24–32. https://doi.org/10.1016/j.geoforum.2020.09.001.
- Brynjolfsson, E., A. McAfee, and M. Spence. 2014. “New World Order: Labor, Capital, and Ideas in the Power Law Economy.” Foreign Affairs 93 (4): 44–53.
- Chemla, Y., Y. Dorfan, A. Yannai, D. Meng, P. Cao, S. Glaven, D. B. Gordon, J. Elbaz, and C. A. Voigt. 2022, December. “Parallel Engineering of Environmental Bacteria and Performance Over Years Under Jungle-Simulated Conditions.” PLoS One 17 (12): 1–24. https://doi.org/10.1371/journal.pone.0278471.
- Crowe, N. 2021. “The Historiography of Biotechnology.” In Handbook of the Historiography of Biology, 217–241. Cham, UK: Springer Cham Springer Cham Springer Cham.
- Dawson, A., I. Paeglis, and N. Basu. 2017. “Founder as Steward or Agent? A Study of Founder Ownership and Firm Value.” Entrepreneurship Theory and Practice 42 (6): 886–910. https://doi.org/10.1177/1042258717725522.
- de Rosnay, M. D. 2020. “Commonswashing – a Political Communication Struggle.” In Global Cooperation Research – a Quarterly Magazine, 2 (3):11–13.
- Foxon, T. J. 2014. “Technological Lock-In and The Role of Innovation.” In Handbook of Sustainable Development, edited by G. Atkinson, S. Dietz, E. Neumayer, and M. Agarwala, 304–316. Cheltenham, UK: Edward Elgar Publishing. https://ideas.repec.org/h/elg/eechap/15312_20.html.
- Glerup, C., S. R. Davies, and M. Horst. 2017. “‘Nothing Really Responsible Goes on Here’: Scientists' Experience and Practice of Responsibility.” Journal of Responsible Innovation 4 (3): 319–336. https://doi.org/10.1080/23299460.2017.1378462.
- Godbout, J. T., and A. C. Caille. 1998. World of the Gift. McGill-Queen's Press-MQUP.
- Greco, F. V., M. J. Tarnowski, and T. E. Gorochowski. 2019. “Living Computers Powered by Biochemistry.” The Biochemist 41 (3): 14–18. https://doi.org/10.1042/BIO04103014.
- Haraway, D. 2015. “Anthropocene, Capitalocene, Plantationocene, Chthulucene: Making Kin.” Environmental Humanities 6 (1): 159–165. https://doi.org/10.1215/22011919-3615934.
- Hess, C., and E. Ostrom. 2008. Understanding Knowledge as a Commons: From Theory to Practice. Cambridge, MA, USA: Harvard University Press.
- Higgins, P., D. Short, and D. South. 2013. “Protecting the Planet: A Proposal for a Law of Ecocide.” Crime, Law and Social Change 59:419–426. https://doi.org/10.1007/s10611-013-9413-6.
- Illich, I. 2018. Tools for Conviviality. New York, USA: Harper and Row.
- Kahl, L., J. Molloy, N. Patron, C. Matthewman, J. Haseloff, D. Grewal, R. Johnson, and D. Endy. 2018. “Opening Options for Material Transfer.” Nature Biotechnology 36 (10): 923–927. https://doi.org/10.1038/nbt.4263.
- Leach, M., J. Rockström, P. Raskin, I. Scoones, A. C. Stirling, A. Smith, J. Thompson, E. Millstone, A. Ely, E. Arond, C. Folke, and P. Olsson. 2012. Transforming innovation for sustainability. Ecology and Society 17 (2): 11. https://doi.org/10.5751/ES-04933-170211.
- Liu, L., J. Wang, D. Rosenberg, H. Zhao, G. Lengyel, and D. Nadel. 2018. “Fermented Beverage and Food Storage in 13,000 Y-old Stone Mortars at Raqefet Cave, Israel: Investigating Natufian Ritual Feasting.” Journal of Archaeological Science: Reports 21:783–793.
- Luo, L., E. van der Voet, and G. Huppes. 2009. “Life Cycle Assessment and Life Cycle Costing of Bioethanol from Sugarcane in Brazil.” Renewable and Sustainable Energy Reviews 13 (6): 1613–1619. https://doi.org/10.1016/j.rser.2008.09.024.
- Martin, B. R., P. Nightingale, and A. Yegros-Yegros. 2012. “Science and Technology Studies: Exploring the Knowledge Base.” Research Policy 41 (7): 1182–1204. https://doi.org/10.1016/j.respol.2012.03.010.
- McLaughlin, J. A., J. Beal, G. Mısırlı, R. Grünberg, B. A. Bartley, J. Scott-Brown, P. Vaidyanathan, et al. 2020. “The Synthetic Biology Open Language (SBOL) Version 3: Simplified Data Exchange for Bioengineering.” Frontiers in Bioengineering and Biotechnology 8:1009. https://doi.org/10.3389/fbioe.2020.01009.
- Meyer, C., Y. Nakamura, B. J. Rasor, A. S. Karim, M. C. Jewett, and C. Tan. 2021. “Analysis of the Innovation Trend in Cell-Free Synthetic Biology.” Life 11 (6): 551. https://doi.org/10.3390/life11060551.
- Morange, M. 2020. The Black Box of Biology. Cambridge, MA, USA: Harvard University Press.
- Murphy, M. W., and C. Schroering. 2020. “Refiguring the Plantationocene: Racial Capitalism, World-Systems Analysis, and Global Socioecological Transformation.” Journal of World-Systems Research 26(2): 400–415.
- Mustonen, M. 2003. “Copyleft–the Economics of Linux and Other Open Source Software.” Information Economics and Policy 15 (1): 99–121. https://www.sciencedirect.com/science/article/pii/S0167624502000902. https://doi.org/10.1016/S0167-6245(02)00090-2.
- Owen, R., J. Stilgoe, P. Macnaghten, M. Gorman, E. Fisher, and D. Guston. 2013. “A Framework for Responsible Innovation.” In Responsible Innovation: Managing the Responsible Emergence of Science and Innovation in Society. Hoboken, NJ, USA: John Wiley and Sons, Ltd.
- Pansera, M., and M. Fressoli. 2020. “Innovation Without Growth – Frameworks for Understanding Technological Change in a Post-Growth Era.” Organization 28 (3): 380–404. https://doi.org/10.1177/1350508420973631.
- Pansera, M., R. Owen, D. Meacham, and V. Kuh. 2020. “Embedding Responsible Innovation Within Synthetic Biology Research and Innovation: Insights from a UK Multi-Disciplinary Research Centre.” Journal of Responsible Innovation 7 (3): 384–409. https://doi.org/10.1080/23299460.2020.1785678.
- Paracchini, V., M. Petrillo, R. Reiting, A. Angers-Loustau, D. Wahler, A. Stolz, and B. SchÖnig. 2017. “Molecular Characterization of an Unauthorized Genetically Modified Bacillus Subtilis Production Strain Identified in a Vitamin B2 Feed Additive.” Food Chemistry 230:681–689. https://doi.org/10.1016/j.foodchem.2017.03.042.
- Peplow, M. 2016. “Synthetic Biology's First Malaria Drug Meets Market Resistance.” Nature 530:389–390. https://doi.org/10.1038/530390a.
- Poux, X., and P. M. Aubert. 2018. “An Agroecological Europe in 2050: Multifunctional Agriculture for Healthy Eating.” Findings from the Ten Years For Agroecology (TYFA) Modelling Exercise, Iddri-AScA, Study 9:18.
- Purpose Economy. 2022. “Steward Ownership”. https://purpose-economy.org/en/
- Qin, Q. L., Z. B. Wang, Q. Q. Cha, S. S. Liu, X. B. Ren, H. H. Fu, and M. L. Sun. 2022. “Biogeography of Culturable Marine Bacteria from Both Poles Reveals that ‘Everything is Not Everywhere’ at the Genomic Level.” Environmental Microbiology 24 (1): 98–109. https://doi.org/10.1111/emi.v24.1.
- Ribeiro, B., and P. Shapira. 2020. “Private and Public Values of Innovation: A Patent Analysis of Synthetic Biology.” Research Policy 49 (1): 103875. https://doi.org/10.1016/j.respol.2019.103875.
- Rip, A., and R. Kemp. 1998. “Technological Change.” In Human Choice and Climate Change, 327–399. Washington, DC: Battelle Press.
- Robra, B., A. Pazaitis, C. Giotitsas, and M. Pansera. 2023. “From Creative Destruction to Convivial Innovation-A Post-Growth Perspective.” Technovation125:102760. https://doi.org/10.1016/j.technovation.2023.102760.
- Saini, A. 2020. “Want to Do Better Science? Admit You're Not Objective.” Nature 579:175. https://doi.org/10.1038/d41586-020-00669-2.
- Shahbaz, M. 2013. “Does Financial Instability Increase Environmental Degradation? Fresh Evidence from Pakistan.” Economic Modelling 33:537–544. https://doi.org/10.1016/j.econmod.2013.04.035.
- Shetty, R. P., D. Endy, and T. F. Knight. 2008. “Engineering BioBrick Vectors from BioBrick Parts.” Journal of Biological Engineering 2 (1): 1–12. https://doi.org/10.1186/1754-1611-2-5.
- Tarnowski, M. J., and T. E. Gorochowski. 2022. “Massively Parallel Characterization of Engineered Transcript Isoforms Using Direct RNA Sequencing.” Nature Communications 13 (1): 434. https://doi.org/10.1038/s41467-022-28074-5.
- Tarnowski, M. J., G. Varliero, J. Scown, E. Phelps, and T. E. Gorochowski. 2023. “Soil as a Transdisciplinary Research Catalyst: From Bioprospecting to Biorespecting.” Royal Society Open Science 10 (11): 230963. https://doi.org/10.1098/rsos.230963.
- Terlouw, B. R., K. Blin, J. C. Navarro-Muñoz, N. E. Avalon, M. G. Chevrette, S. Egbert, and S. Lee. 2023. “MIBiG 3.0: A Community-Driven Effort to Annotate Experimentally Validated Biosynthetic Gene Clusters.” Nucleic Acids Research 51 (D1): D603–D610. https://doi.org/10.1093/nar/gkac1049.
- Thomas, J. 2013. “Synthetic Anti-Malarial Compound is Bad News for Artemisia Farmers”.
- Toynbee, A. 1953. The World and the West. Oxford, UK: Oxford University Press.
- van Bruggen, A. H., E. M. Goss, A. Havelaar, A. D. van Diepeningen, M. R. Finckh, and J. G. Morris. 2019. “One Health-Cycling of Diverse Microbial Communities as a Connecting Force for Soil, Plant, Animal, Human and Ecosystem Health.” Science of the Total Environment 664:927–937. https://doi.org/10.1016/j.scitotenv.2019.02.091.
- Van Doren, D., S. Koenigstein, and T. Reiss. 2013. “The Development of Synthetic Biology: A Patent Analysis.” Systems and Synthetic Biology 7:209–220. https://doi.org/10.1007/s11693-013-9121-7.
- Vavitsas, K. 2018. “OpenMTA, a Paradigm Shift in Exchanging Biological Material.” Synthetic Biology 3 (1): ysy021. https://doi.org/10.1093/synbio/ysy021.
- Vetter, A. 2018. “The Matrix of Convivial Technology – Assessing Technologies for Degrowth.” Journal of Cleaner Production 197:1778–1786. https://doi.org/10.1016/j.jclepro.2017.02.195.
- Voigt, C. A. 2020. “Synthetic Biology 2020–2030: Six Commercially-Available Products that are Changing Our World.” Nature Communications 11 (1): 6379. https://doi.org/10.1038/s41467-020-20122-2.
- Von Wintersdorff, C. J., J. Penders, J. M. Van Niekerk, N. D. Mills, S. Majumder, L. B. Van Alphen, P. H. Savelkoul, and P. F. Wolffs. 2016. “Dissemination of Antimicrobial Resistance in Microbial Ecosystems Through Horizontal Gene Transfer.” Frontiers in Microbiology 173:1–10.
- Williamson, A. E., P. M. Ylioja, M. N. Robertson, Y. Antonova-Koch, V. Avery, J. B. Baell, and H. Batchu. 2016. “Open Source Drug Discovery: Highly Potent Antimalarial Compounds Derived from the Tres Cantos Arylpyrroles.” ACS Central Science 2 (10): 687–701. https://doi.org/10.1021/acscentsci.6b00086.
- Winner, L. 1980. “Do Artifacts Have Politics?” Daedalus 121–136.
