ABSTRACT
This review summarizes what is known about the influence of water temperature and velocity on the migration and spawning success of an inland population of Chinook salmon Oncorhynchus tshawytscha. Models are then developed and used to illustrate how migration and spawning success might change if temperatures and velocities increase under a future climate. The illustration shows the potential for moderate increases in temperature and velocity to reduce homing and increase energy expenditure. Those two outcomes would reduce the abundance, productivity, and diversity of the population studied. Under the future scenario illustrated, it would become difficult for fish management actions alone to recover conservation-reliant populations of inland Chinook salmon.
Introduction
Self-sustaining native populations of Chinook salmon Oncorhynchus tshawytscha once existed from southern California to northern Alaska, and from northern Japan to northern Russia (Scott and Crossman, Citation1973). Human development of river systems eliminated access to many historical production areas, and altered the seasonal and spatial patterns of velocities and temperatures of presently occupied habitat. Such changes contributed to a decline in the abundance and spatial distribution of many Chinook salmon populations (e.g., Nehlsen et al., Citation1991). Nine Evolutionarily Significant Units of Chinook salmon in the Pacific Northwest United States were listed under the United States Endangered Species Act (NOAA, Citation2017). Those Evolutionarily Significant Units essentially have become “conservation reliant” in that their persistence relies heavily on human intervention (terminology attributed to Dr. J.M. Scott, retired U.S. Geological Survey Cooperative Fish and Wildlife Research Unit, University of Idaho).
Adult Chinook salmon from inland populations are highly susceptible to the effects of human development because upon freshwater return they have a lengthy migration and a narrow window of time to reach the spawning grounds and spawn. Being semelparous, the adults do not feed in freshwater, and have a fixed amount of stored somatic energy to swim to their natal waters and spawn before dying. The rate of energy expenditure as the fish complete their final life stage is directly dependent on swimming speed (fish speed plus water velocity) and temperature (e.g., Geist et al., Citation2000a). Chinook salmon generally behave and function normally at water temperatures less than 20°C (Brett, Citation1952), but there are disruptions in behavior and physiological functions as temperature rises above 20°C to the point of death between ≈25 and 28°C (Brett et al., Citation1982; Stuehrenberg et al., Citation1978; Marine and Cech, Citation2004; Richter and Kolmes, Citation2005; Yanke, Citation2006; Geist et al., Citation2010; Perry et al., Citation2015).
In addition to the habitat changes caused in the past by humans, adult Chinook salmon will be challenged with any alterations to river flow and temperature affected by future climate conditions. Bilby et al. (Citation2007) wrote a review on future climates with emphasis on fish and wildlife resources of the Columbia River basin within the Pacific Northwest United States. Most of the 20 modeled climate scenarios reviewed by Bilby et al. (Citation2007) predicted an increase in annual precipitation that was partitioned into increases in precipitation in the winter, a general shift in winter precipitation from snow to rain, and a decrease in precipitation during the summer. The lowest level of change in predicted annual precipitation was –2%, the average was +6%, and the highest was +18%. All of the models reviewed predicted an increase in annual average air temperature within a range of +1.6°C to +4.9°C. Future increases in precipitation and air temperature during the migration and spawning of inland populations of Chinook salmon could increase the water velocity and temperature exposures of the fish and affect changes in behavior, energy use, and survival.
The goal of this review is to “illustrate” how fish behavior, temperature, and velocity under a future climate might influence the migration and spawning success of inland populations of Chinook salmon in highly developed river systems. “Illustrate” is defined here as “to explain logically by use of (a) the literature, (b) empirical data that were collected under the technical and logistical constraints common to all large river studies, and (c) models that provide empirically-based, biologically intuitive results that come with error that is likely large.” After a general background on the fish and river system is presented, five topics are covered each in a separate section: Migration Timing, Behavior, and Success; Spawning Behavior, Timing, and Success; Illustrating the Effects of Velocity and Temperature on Migration and Spawning Success Measured through Spawning Site Selection and the Initiation of Redd Construction; Illustrating the Effect of Temperature on Embryo Loss; and Illustrating the Effects of a Future Climate on Migration and Spawning Success. The review is concluded with a discussion of what the review illustrated and at its relevance to research and management.
The fish and the river system
The fish
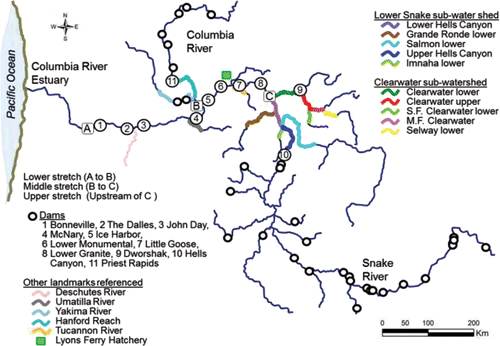
Chinook salmon in the Columbia River basin are divided into management units as spring, summer, and fall runs based on the timing of upstream passage at dams. Fall Chinook salmon are further divided into tules and upriver brights. Tule fall Chinook are native to the lower Columbia River and its tributaries. Upon freshwater entry, tules exhibit advanced maturation and the quality of their flesh degrades rapidly, turning almost white. Upriver brights, so named for their silvery skin color, mature 1–3 months after freshwater entry, and migrate further upriver. Today, the largest population of upriver bright fall Chinook salmon referenced periodically in this review is found in the Hanford Reach of the Columbia River (; Huntington et al., Citation1996). This review focuses on upriver bright fall Chinook salmon population from the Snake River basin ().
Figure 1. The river system including the lower, middle, and upper stretches where the studies reviewed were conducted and the new data analyzed were collected. The spawning areas located within the Lower Snake and Clearwater sub-water sheds covered in are also shown.
Fish origin is dependent on the spawning location. Natural-origin fish are the offspring of parents that spawned in the wild, whereas hatchery-origin fish are the offspring of parents spawned in captivity. Fish of both origins spend from a few months to 4 years, and sometimes but rarely 5 years, maturing in the Pacific Ocean (Connor et al., Citation2005). The number of years between fry emergence in freshwater and return to the estuary after maturation in saltwater is referred to here as “apparent ocean age” (hereafter Age-I, Age-II, Age-III, Age-IV, and Age-V). Females have apparent ocean-age distributions composed of higher proportions of older fish compared to males regardless of origin primarily because Age-I females are rare, and it is common for the proportion of Age-III females in the run to be large. Natural-origin fish (both sexes combined) have apparent ocean-age distributions composed of higher proportions of older fish compared to hatchery-origin fish primarily because Age-I hatchery males can be numerically abundant. This review focuses largely on fish that are apparent Age-II or older that are classified as “adults.” Emphasis is placed on females because they return almost exclusively as adults, and they construct countable spawning nests (hereafter, redds) that provide an indicator of geographic distribution and abundance.
Connor et al. (Citation2016) estimated that the Snake River basin once supported a minimum of ≈ 408,500 to 536,200 wild fall Chinook salmon adults (i.e., no hatchery influence), but by 1990 only a portion of the historical habitat remained free-flowing and accessible. Presently, every natural-origin fish produced in the wild has some level of hatchery parentage. In 1990, only 78 natural-origin adults were estimated to have arrived at Lower Granite Dam () that had the potential to spawn in riverine habitat upstream of the dam (Cooney, Citation1991). The Snake River fall Chinook salmon Evolutionarily Significant Unit, composed of a single population (hereafter, the population), was listed as threatened under the U.S. Endangered Species Act in 1992 (NMFS, Citation1992). The population is strongly conservation-reliant as its persistence is dependent on protective harvest regulations, a hatchery program, and the adaptive management of dams and reservoirs.
Newcomers to the Columbia River basin began to overharvest Chinook salmon both in saltwater and freshwater in the late 1800s (e.g., USCFF, Citation1894). Of the fish that entered the Columbia River, up to 88% were harvested (Chapman, Citation1986). Freshwater harvest rates fluctuated at levels below 88% as the population declined up until listing under the U.S. Endangered Species Act (NOAA, Citation2014). Since listing, a harvest schedule has been implemented to protect the fish. Tribal members have treaty rights (i.e., the Nez Perce, Umatilla, Warm Springs, and Yakima Treaties of 1855) to harvest Chinook salmon throughout the Columbia River basin. The rightful treaty harvest of fall Chinook salmon is predominantly focused between Bonneville and McNary dams (). Scheduled treaty harvest increases from 20% to 30% of the run depending on the estimated abundance of the fish entering the mouth of the Columbia River (TAC, Citation2008). There is additional non-treaty harvest that cannot exceed a rate of 15%, a significant portion of which takes place downstream of Bonneville Dam (TAC, Citation2008).
A hatchery program was initiated in 1975 after the threat of extinction had been fully recognized. The program started as a genetic conservation effort that included the construction and operation of Lyons Ferry Hatchery () in concert with a network of existing hatcheries (e.g., Bugert et al., Citation1995). The contemporary hatchery program releases a portion of the juveniles produced into riverine spawning areas with the intent of increasing the number of adults that return, spawn in the wild, and produce natural-origin juveniles. Over the past 10 years the number of adult spawners estimated to have passed Lower Granite Dam has been as high as ≈53,000 (see Young et al., Citation2012 for methods). During those years, the majority of the spawners have been first generation, hatchery-origin fish (mean 68%; Ford et al., Citation2015).
Dam construction in the Pacific Northwest United States was the primary form of development that eliminated habitat connectivity, and completely altered the hydrology of the Columbia and Snake rivers. Numerous modifications have been made to reservoir operations and fish passage structures at dams in an effort to reverse the declines of anadromous fish populations. The modifications that involve adult passage and temperature management are addressed to various extents later in this review. The states of Oregon and Washington established a maximum water temperature standard of 20°C for the Columbia and Lower Snake rivers to protect salmonids pursuant to the U.S. Clean Water Act (Washington Administrative Code Citation2011; Oregon Administrative Rule Citation2011). Similarly, the state of Idaho established a maximum temperature limit of 13°C in Hells Canyon to protect Snake River fall Chinook salmon during spawning (Idaho Administrative Procedures Act Citation2014).
To describe the spatial and temporal aspects of the velocity and thermal exposure of the fish as they swim upstream, the study area was demarcated into lower, middle, and upper stretches (). The upper stretch was further divided into the Lower Snake and Clearwater River sub-watersheds (). Before the fish enter the lower stretch of the study area, they are subjected to environmental conditions, non-treaty harvest, and predation over a distance of roughly 235 km between the Columbia River estuary and the tailrace of Bonneville Dam (). The energy used by the fish to swim from the estuary to Bonneville Dam is accounted for in subsequent analyses of migration and spawning success, whereas fish loss between those two points is not evaluated. Hereafter, the study area is referred to as “the river system.”
The lower stretch of the river system
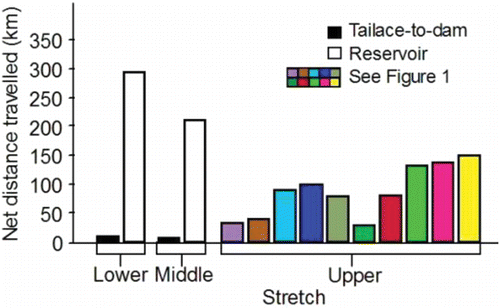
Returning adult salmon first enter the lower stretch of the river system when they arrive in the tailrace of Bonneville Dam (). The lower stretch of the river system is impounded and extends from the tailrace of Bonneville Dam upstream along the Columbia River to the Lower Snake River mouth. The fish pass through four dam tailraces (measured to the upstream ladder exit) and reservoirs before arriving at the Lower Snake River mouth. The tailrace-to-dam distances are much shorter compared to the reservoir distances (Bonneville, The Dalles, John Day, and McNary in ; Lower stretch in and ).
Table 1. Tailrace, ladder, and reservoir distances (km) calculated from the tailrace of Bonneville Dam to the upper end of the south arm of Lower Granite Reservoir.
Figure 2. Net distances fall Chinook salmon adults must travel to complete tailrace-to-dam and reservoir passage events in the lower and middle stretches of the river system, and to the most consistently and heavily used spawning sites within the spawning areas located within the upper stretch.
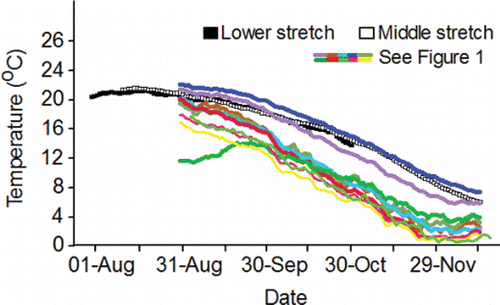
The fish are counted in both of the ladders (one on each side of the river) at each of the four lower Columbia River dams and are assigned to a run. The run schedules for fall Chinook salmon range from 01-Aug–15-Aug at Bonneville Dam to 09-Aug–31-Oct at McNary Dam (CBR, Citation2017). Longitudinal temperature and flow patterns do not vary substantially throughout the lower stretch of the river system over the 01-Aug–31-Oct date span of the fall Chinook salmon run schedules. As such, the run schedule at every dam in the lower stretch overlaps with the warmest, low-flow days of summer ( and Citation4). For example, daily mean temperature measured at the dam tailraces exceeded 20°C 30 times in 2010, 25 times in 2011, 18 times in 2012, 52 times in 2013, 38 times in 2014, and 33 times in 2015.
Figure 3. Inter-annual daily mean temperature (°C) measured in the lower stretch of the river system in the tailrace of McNary Dam, the middle stretch in the tailrace of Ice Harbor Dam, and the upper stretch within the spawning areas within the Lower Snake River sub-watershed, and within the Clearwater River lower and upper reaches within the Clearwater River sub-watershed, 2010–2015. Data for the dams are from CBR (Citation2017). Data for the Lower Snake River watershed, S.F. Clearwater lower reach, M.F. Clearwater, and Selway River lower reach were collected by the authors of this review and their staff. Data for the remaining spawning areas within the Clearwater River watershed were collected by the U.S. Geological Survey at Spalding (Station 13342500) and Orofino (Station 13340000), Idaho.
The tailrace velocities at the four dams along the lower stretch of the river system are hydraulically complex and dynamic as the velocities vary considerably across the channel depending on how the flow is proportioned between and among the powerhouse and spillways (see Rakowski et al., Citation2010). In this review, tailrace velocities (as well as reservoir velocities) are illustrated simply as mean cross-sectional velocities that were coarsely simulated as described in . Mean cross-sectional tailrace velocities in the dam tailraces along the lower stretch of the river system decline throughout the fall Chinook salmon run schedule ().
Reservoir velocities in the lower stretch of the river system are far less hydraulically complex and dynamic compared to tailrace velocities, and they are affected primarily by flow volume, channel width, and channel depth. Wind and thermal gradients can also influence anomalies in reservoir velocities (Cook et al., Citation2006). Mean cross-sectional reservoir velocities in the lower stretch decline over the period fall Chinook salmon are passing upstream and are much slower compared to tailrace velocities ().
The middle stretch of the river system
After exiting the lower stretch, fall Chinook salmon destined for the Snake River basin spawning grounds enter the middle stretch of the river system that is also impounded. The middle stretch extends from the mouth of the Lower Snake River to the upper ends of the south and east arms of Lower Granite Reservoir formed within the Lower Snake and Clearwater rivers, respectively (). Fall Chinook salmon encounter four dams along the middle stretch each of which is equipped with one or two fish ladders in which fish are counted. The run schedules for fall Chinook salmon at the four Lower Snake River dams range from 12-Aug–15-Dec at Ice Harbor Dam to 18-Aug–15-Dec at Lower Granite Dam (CBR, Citation2017).
Unlike the lower stretch of the river system, the temperature regime in the middle stretch can be modified by releasing stored, cold water from Dworshak Reservoir formed by Dworshak Dam on the North Fork Clearwater River (). Dworshak Dam () is equipped with multi-level selector gates that are operated to control outflow temperatures (USACE, Citation1986). Dworshak Reservoir releases are used to meet a temperature standard of 20°C and a flow standard of 50 to 55 thousand cubic feet per second (KCFS), measured in the tailraces of the Lower Snake River dams from 21-Jun to 31-Aug (NMFS, Citation1995). The primary intent was initially to enhance migratory conditions for juvenile fall Chinook salmon, but the standards are in effect during the early portion of the adult fall Chinook salmon run schedule. The Snake River Basin Water Rights Adjudication among the Nez Perce Tribe, the State of Idaho, and the U.S. Government, mandated that a portion of the stored water in Dworshak Reservoir be put under the jurisdiction of the Tribe. Since 2002, the Tribe has used that adjudicated water to cool temperatures in the middle stretch of the river system through about 20-Sep depending on year.
Cook et al. (Citation2006, Citation2007) used 2- and 3-dimensional temperature modeling to describe the thermal environment along the middle stretch of the river system. The most thermally diverse area is located near the confluence where relatively warm water from the Lower Snake River sub-watershed meets the relatively cold water from the Clearwater River sub-watershed. As the water moves downstream from the confluence it stratifies in Lower Granite Reservoir. On average at Lower Granite Dam, water temperatures 1.5 m below the surface were 5°C warmer than water temperatures 20–40 m below the surface. Cook et al. (Citation2006) called that difference between the two depth-specific means “stratification intensity.” Stratification intensity diminished as thermally heterogeneous water mixed as it passed downstream through each Lower Snake River reservoir and dam. Mean stratification intensity was 2.5°C in the forebay of Little Goose Dam, 2.1°C in the forebay of Lower Monumental Dam, and 1.8°C in the forebay of Ice Harbor Dam.
Acknowledging the complex thermal environment in Lower Granite Reservoir, but noting that the flow pattern does not vary considerably from Lower Granite Reservoir to Ice Harbor Dam tailrace, temperature and flow measured in the tailrace of Ice Harbor Dam are used here to illustrate the temperature and flow regimes of the middle stretch of the river system. Even when cool water is released from Dworshak Reservoir, the date span of the fall Chinook salmon run schedules in the middle stretch (12-Aug–15-Dec) includes the warmest, low-flow days of summer ( and ). For example, daily mean temperature exceeded 20°C 21 times in 2010, 23 times in 2011, 29 times in 2012, 41 times in 2013, 33 times in 2014, and 28 times in 2015. Mean cross-sectional reservoir velocities decline over the run schedule, noting that reservoir velocities are much slower compared to the tailrace velocities ().
Figure 4. Inter-annual daily mean flow (KCFS) measured in the lower and middle stretches of the river system in the tailraces of McNary and Ice Harbor dams, respectively, 2010–2015 (CBR, Citation2017).
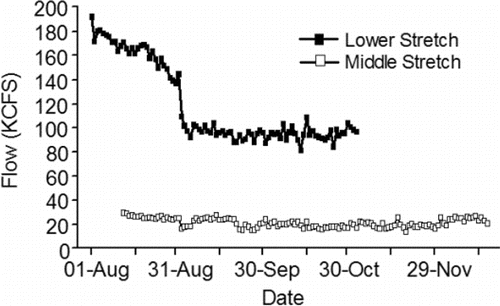
Figure 5. Inter-annual daily mean simulated velocities (cm/s) for the dam tailraces and reservoirs in the lower stretch of the river system (see Eqs. 3 and 4 in ), for the dam tailraces and reservoirs in the middle stretch of the river system (see Eqs. 9 and 10A–10O in ), and for the Upper and Lower Hells Canyon spawning areas (distance weighted 1-D modeling; Borden and Manning, Citation2011).
The upper stretch of the river system
The upper stretch of the river system is partly regulated by dams, but it is composed entirely of riverine habitat (). Fall Chinook salmon arrive in the upper stretch of the river system and enter the spawning areas as early as August, but most enter starting in September (e.g., Connor and Garcia, Citation2006). Fall Chinook salmon redds are counted in the Lower Snake and Clearwater sub-watersheds to monitor the status and trends in the spatial distribution of the population (see Groves and Chandler, Citation1999; Groves et al., Citation2013, Citation2016 for methods). Those data were used here to identify and rank the reaches of rivers that support spawning (hereafter, spawning areas) according to production potential. Spawning areas that could support at least 500 females based on observed redd counts (assuming each female constructed only one redd; e.g., Connor et al., Citation2001; Groves et al., Citation2013) were classified as primary production areas. Spawning areas that could support at least 200 females were classified as secondary production areas. If the annual redd counts were above 0 but less than 200 with no consecutive lapses in occupancy during years when redd surveys were conducted, a spawning area was classified as a tertiary spawning area.
The primary spawning areas in the Lower Snake sub-watershed include the Upper and Lower Hells Canyon spawning areas (; ). Within the Lower Snake sub-watershed, the Grande Ronde lower reach is a secondary spawning area, and the Imnaha and Salmon lower reaches are tertiary spawning areas (; ). The Clearwater lower reach is the primary spawning area in the Clearwater sub-watershed (; ). There are four tertiary spawning areas in the Clearwater sub-watershed including the Clearwater upper reach, South Fork Clearwater lower reach, contiguous Middle Fork Clearwater, and Selway lower reach (; ).
Table 2. Snake River basin fall Chinook salmon spawning areas located upstream of Lower Granite Dam. Accessible channel length is given in kms. Channel length (kms) was determined from river charts or redd locations documented by the authors except for the Lower Snake upper and lower reaches that are from Dauble et al. (Citation2003). Abbreviations: rkm, river km (river mouth = rkm 0); D., Dam; Res., Reservoir; S. F., South Fork; M.F., Middle Fork.
After entering the upper stretch of the river system, the fish have to traverse considerable net channel distances to spawning sites (). For example, fish destined to spawn at the most frequently used sites in the Lower Snake sub-watershed travel net distances of 32.8 km to 100.7 km. Fish destined to spawn in the most frequently used sites in Clearwater sub-watershed travel net distances of 29.4 km to 151.1 km.
Exposure of adults to warm water is not a phenomenon restricted to the lower and middle stretches of the river system (). Every fish destined for spawning areas in the Lower Snake sub-watershed has to enter the Lower Hells Canyon spawning area (). Of the spawning areas, the Lower Hells Canyon spawning is the second warmest from 01-Sep through 13-Dec when fall Chinook salmon are making pre-spawning movements and spawning in the upper stretch of the river system (). Daily mean temperature recorded after 01-Sep along the Lower Hells Canyon spawning area exceeded 20°C 1 time in 2010, 27 times in 2011, 14 times, in 2012, 23 times in 2013, 17 times in 2014, and 11 times in 2015. Fish entering secondary or tertiary spawning areas can sometimes be exposed to temperatures above 20°C after tributary entry within the Lower Snake River watershed (). For example in 2013, daily mean temperature exceeded 20°C in the Grande Ronde, Imnaha, and Salmon lower reaches 17, 16, and 18 times, respectively. The Upper Hells Canyon spawning area is the warmest spawning area from 01-Sep through 13-Dec (). After 01-Sep, daily mean temperature along the Upper Hells Canyon spawning area exceeded 20°C 17 times in 2010, 33 times in 2011, 30 times in 2012, 28 times in 2013, 29 times in 2014, and 28 times in 2015.
The release of cold water from Dworshak Reservoir creates a benign temperature environment after 01-Sep in the Clearwater River lower reach (). Temperatures increase as fish swim upstream from the Clearwater River lower reach into the Clearwater River upper reach (). Temperatures above 20°C after 01-Sep are sometimes observed in the Clearwater upper reach. For example in 2013, daily mean temperature exceeded that standard 17 times. Fish destined to spawn in the South Fork Clearwater lower reach, the Middle Fork Clearwater, and the Selway lower reach must pass through the Clearwater upper reach and can be exposed to temperatures above 20°C while doing so. Once the fish enter those three spawning areas they are rarely exposed to temperatures above 20°C ().
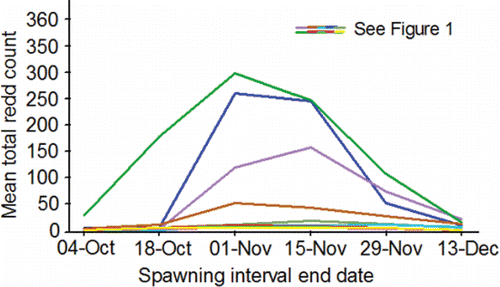
Fish from the population spawn in the fall with some temporal variation between sub-watersheds and among spawning areas (). Spawn timing and temperature of a given spawning area affect the amount of time the embryos spend developing in the gravel. After fertilization and egg deposition, the embryos develop in the gravel from five to nine months before absorbing their yolk sac and emerging as fry (Connor et al., Citation2002, Citation2003).
Figure 6. Inter-annual mean total redd counts estimated on the end dates of six spawning intervals (21-Sep‒04-Oct, 05-Oct‒18-Oct, 19-Oct‒01-Nov, 02-Nov‒15-Nov, 16-Nov‒29-Nov, 30-Nov‒13-Dec) from data collected during aerial surveys conducted by the authors and their staff during 1991‒2015 (methods, Groves and Chandler, Citation1999; Groves et al., Citation2013, Citation2016) within the primary, secondary, and tertiary spawning areas used by Snake River basin fall Chinook salmon upstream of Lower Granite Reservoir (; ).
Flow is important to consider in the upper stretch of the river system because large decreases in flow can dewater redds (e.g., Harnish et al., Citation2014). Redd dewatering can kill developing embryos. Flow in the Upper and Lower Hells Canyon spawning areas, and in the Clearwater lower reach, is regulated by Hells Canyon () and Dworshak dams, respectively. Minimum stable flows from Hells Canyon Dam were established in 1991 to prevent redd dewatering in the Upper Hells Canyon spawning area. That practice also minimized the potential for redd dewatering in the Lower Hells Canyon spawning area that receives unregulated inflow from the Imnaha, Salmon, and Grande Ronde rivers (IPC, Citation1990). In the case of the Clearwater River lower reach, use of Dworshak Reservoir water to supplement flow and decrease temperatures in the lower four Snake River reservoirs draws the reservoir down to near-minimum operating elevations, greatly reduces power peaking operations in the fall and winter that can dewater redds, and results in stable or naturally increasing flows from Dworshak Dam during and after spawning. In short, the dams capable of causing redd dewatering are generally operated to avoid it, and redd dewatering is not presently considered to be a large factor for spawning success.
Velocities in the upper stretch of the river system are complex and vary widely both laterally and longitudinally within a spawning area, as well as among spawning areas. With regard to describing velocity, this review focuses on mean cross-sectional velocities in the Upper and Lower Hells Canyon spawning areas because those areas have been most intensively modeled (see Borden and Manning, Citation2011 for methods). Mean cross-sectional velocities are higher in the Upper Hells Canyon spawning area compared to velocities in the Lower Hells Canyon spawning area (). Mean cross-sectional velocities are higher in both of those spawning areas compared to velocities in the dam tailraces and reservoirs in the lower and middle stretches of the river system ().
Migration timing, behavior, and success
This section covers four objectives that describe:
| (1) | the use of radiotelemetry and PIT-tag data to help understand migration timing, behavior, and success; | ||||
| (2) | migration timing; | ||||
| (3) | migration behavior; and | ||||
| (4) | migration success. | ||||
The radiotelemetry and PIT-tag data
Much of the understanding of fall Chinook salmon migration timing, behavior, and success is derived from studies of individual fish using radiotelemetry and PIT tags. Bjornn and Peery (Citation1992) provided a comprehensive review of the numerous early (1956–1991) telemetry studies of adult salmon and steelhead (O. mykiss) that were initiated to better understand fish migration rates, dam passage behavior, and loss between dams in the Columbia and Snake rivers. Many of the studies reviewed in this section were brought to the attention of the authors by the Bjornn and Peery (Citation1992) paper. Bjornn and Peery initiated a series of intertwined radiotelemetry studies on upriver bright fall Chinook salmon in 1998.
The radiotelemetry studies started by Bjornn and Peery provided key components of many of the analyses in this review. In general, adult fish were diverted as they passed upstream in a fish ladder at Bonneville Dam where they were identified to species, sexed, measured, and checked for fin clips, tags, and injuries. Based on run schedule and morphological features, the majority of the fish tagged were probably from the healthy, abundant Hanford Reach population of upriver brights. Temperature-related tagging restrictions prevented tagging fish during the warmest portions of most years, but after close inspection by the researchers the data proved to be generally representative of the upriver bright populations. Adults were gastrically tagged with uniquely coded radio transmitters and released either downstream of Bonneville Dam or in the dam forebay. Fixed radio-telemetry stations were established at various locations from Bonneville Dam to Priest Rapids Dam () and the upper end of Lower Granite Reservoir to provide data on passage behaviors.
The introduction of PIT tags overcame many of the technological and biological limitations of radiotelemetry. Fish could be tagged as juveniles with few negative effects, the tags had an unlimited life and could be detected in the adults as they ascended the ladders at dams, and were uniquely coded to allow the retrieval of fish-specific data (Prentice et al., Citation1990a, Citation1990b, Citation1990c; Tiffan et al., Citation2015). As such, known-origin upriver bright fall Chinook salmon adults from the Snake River basin population that had been implanted with a tag as juveniles could be detected passing dams as they moved upstream from Bonneville Dam to Lower Granite Dam without being handled or stressed. The number of dams with adult ladders or other passage structures fitted with PIT-tag monitoring devices (e.g., Prentice et al., Citation1990c) increased over time as did the number of juvenile fall Chinook salmon that were PIT tagged.
From this point on in the review, several original analyses are conducted with PIT-tag data to compliment or fill in gaps left by the radiotelemetry studies. The PIT-tag data were collected on hatchery-origin fish that were released as juveniles upstream of Lower Granite Dam during 2005–2012, which then returned to spawn during 2010–2015. The data were downloaded from the central database (PTAGIS, Citation2017). The analyses focuses on hatchery-origin adults (Age-II and older) because the numbers of natural-origin adults that had been PIT tagged as juveniles was too small for meaningful, stand-alone analyses. The return years analyzed include 2010 to 2015 because the proportions of the returning adults that had been tagged as juveniles were the highest on record and provided large sample sizes of detected fish. Detection data collected at Bonneville, McNary, Ice Harbor, and Lower Granite dams were analyzed because all four of those dams had PIT-tag monitoring systems in the ladders during 2010–2015 and the detection efficiencies of the systems exceeded 96% in all years (e.g., Tenney et al., Citation2011, Citation2014, Citation2015; PSMFC, Citation2013). Based on PIT-tag detection histories, the prevalent age class of the PIT-tagged adults was Age-III in 2011(61.8%), 2014 (49.8%), and 2015 (75.4%), whereas Age-II fish were most prevalent in 2010 (97.9%), 2012 (83.4%), and 2013 (69.7%). The preponderance of Age-II PIT-tagged adults in the age class distributions of those three years probably resulted from the sex composition of the population. Males can be numerically dominant in the run in some years and can return at younger ages compared to females (Connor et al., Citation2005).
Migration timing
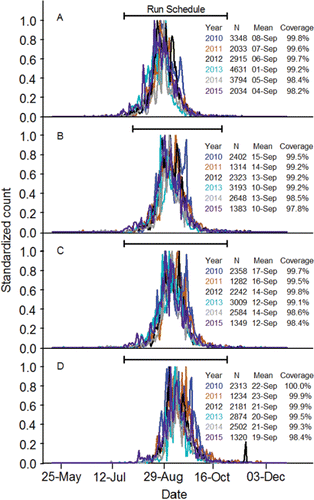
Sample sizes of known Snake River fish in the radiotelemetry studies were too small to describe migration timing accurately. The passage date distributions calculated using the last date a PIT-tagged adult was detected at Bonneville, McNary, Ice Harbor, and Lower Granite dams were compressed and symmetrical (). The mean passage dates at each dam, and the corresponding passage date distributions, varied little among years at a particular dam. Annual mean passage dates increased as the fish moved upstream. On average, the fish passed Bonneville Dam during the first week of September, McNary and Ice Harbor dams during the second week of September, and Lower Granite Dam during the third week of September. At least 95% of the fish detected at each dam during each year was detected within the run schedules. The run schedules tended to miss very early and very late migrants.
Figure 7. Standardized passage date distributions (2010‒2015) at Bonneville (panel A), McNary (panel B), Ice Harbor (panel C), and Lower Granite (panel D) dams for PIT-tagged, hatchery-origin fall Chinook salmon adults compared to the run schedules at those dams (CBR, Citation2017).
Migration behavior
General dam passage behaviors
Radiotelemetry studies conducted in the 1980s showed that the total time required for adult fall Chinook salmon to pass individual lower Columbia River dams ranged from 14 h (McNary Dam) to 31 h (John Day Dam; Liscom and Stuehrenberg, Citation1983; Ross, Citation1983; Turner et al., Citation1984). Most adult fall Chinook salmon used the fishway ladders to pass dams, and they passed primarily during daylight hours (Calvin, Citation1975); but the occasional adult passed the dams via the navigation locks, and a few fish passed at night (Boggs et al., Citation2004; Keefer et al., Citation2004). Once fish entered the fish ways, times to ascend the fish ladders only ranged from 3 h to 7 h (Duncan et al., Citation1978; Johnson et al., Citation1982; Ross, Citation1983; Shew et al., Citation1985), and passage rates were faster at reduced powerhouse discharges (Monan and Liscom, Citation1974).
One behavior that can adversely affect adult salmon behavior and energetics is dam fallback (i.e., passing back downstream of the dam after initial, successful passage). Early telemetry studies showed that fallback rates were relatively low (range, 0–2%) and were likely influenced by low flows in late summer and fall (Young et al., Citation1974; Liscom and Stuehrenberg, Citation1983; Ross, Citation1983). Later studies confirmed that fallback was observed at every dam, but was sometimes higher than reported in earlier studies especially at Little Goose Dam (). “Permanent” fallbacks are those fish that never re-ascend fish ladders after falling back. If a fish fell back and remained healthy but did not re-ascend the ladder, it was likely that the ladder and dam were located upstream of, or adjacent to, the direct route to the natal tributary or hatchery of the fish. Boggs et al. (Citation2004) termed that particular behavior “overshoot fallback.” For example, upriver brights destined for the Hanford Reach might wander into the Lower Snake River and pass Ice Harbor Dam before falling back and continuing on the correct course to their natal spawning location (e.g., see Ice Harbor Dam fallback; ; also see Mendel et al., Citation1992, Citation1993; Mendel and Milks, Citation1997). Likewise, a strong return to Lyons Ferry Hatchery could increase fallback rates at Little Goose and Lower Granite dams (e.g., see Little Goose and Lower Granite Dam fallback; ). Consequently, subsequent analyses of energetics omitted adults returning from Lyons Ferry Hatchery releases, and only included adults returning from releases of juveniles made upstream of Lower Granite Dam.
Table 3. Information on fallback of presumed, upriver bright fall Chinook salmon adults at the lower dams within the lower and middle stretches of the river system. The data are from Boggs et al. (Citation2004; years 1998, 2000, and 2001) and was pooled across years for this table. The denominator for gross and net fallback percentages was the number of unique fish that passed. The numerator for net fallback was the number of unique fish that re-ascended the ladder (n under Reascension). The denominator for all other percentages was the number of unique fallbacks.
Upstream movement, temperature, and flow
Initial studies of upstream movement rate focused on travel times (elapsed days) and rates (distance traveled divided by elapsed days) within reservoirs between dams. Fall Chinook salmon migrated between Bonneville and McNary dams at 20.0–27.2 km/d (Oregon Fish Commission, Citation1960; Young et al., Citation1974). Trefethen and Sutherlin (Citation1968) measured migration rates of fall Chinook salmon in Brownlee Reservoir on the Snake River and showed that fish travelled from 16.1 to 16.8 km/d. Fallback behavior was shown to increase upstream travel times, which is potentially detrimental to adults because it can lengthen migration thereby increasing energy expenditure during the warmest, low-flow days of summer. Using radiotelemetry, Keefer et al. (Citation2004) found that the annual median Bonneville-to-McNary and Bonneville-to-Lower Granite travel times were longer (10–15 d and 9–26 d, respectively) for fish that fell back compared to fish that did not fall back (8–9 d, Bonneville-to-McNary; 15–17 d, Bonneville-to-Lower Granite).
Because adult fall Chinook salmon migrate during the warmest, low-flow days of summer, managers have been interested in the effect of temperature, flow, and passage date on upstream movement. Keefer et al. (Citation2004) analyzed radiotelemetry data collected in 2000 and 2001 to explore those effects. Univariate and quadratic regression models were fitted to data aggregated from individuals into semi-monthly blocks with movement rates being expressed as the median observed in each block. The results were inconsistent and unconvincing. It was not possible to judge the explanatory power of the models because the coefficients of determination were not reported.
Goniea et al. (Citation2006) expanded on the work of Keefer et al. (Citation2004) by analyzing data collected during 1998 and 2000‒2004 that were aggregated into 52 weekly bins for plotting against mean weekly temperature intervals (range, 14.0‒14.9°C to 22.0‒22.9°C) at Bonneville Dam. Upstream movement rate between Bonneville and John Day dams was slower in warmer years and at higher temperatures, particularly when temperatures exceeded 21°C. Analysis of variance identified temperature interval as the most influential predictor of rate of upstream movement, followed by year, and then the interaction between year and temperature.
Goniea et al. (Citation2006) also provided the first evidence that adult upriver brights temporarily used cool tributaries for behavioral thermoregulation. Of the 2,121 fish studied, 9.1% (n = 194) resided in those tributaries for an average (±SD) of 5.1±5.8 d (range, 12 h to 34 d). The relation between the proportions of fish in each bin that used tributaries and mean weekly Columbia River temperatures was exponential (). Less than about 5% of fish used tributaries when Columbia River temperatures were below 20.0°C, but tributary use increased to about 40% when temperatures exceeded 21°C.
Figure 8. Relation between the percent of presumed upriver bright fall Chinook salmon adults that used cool water tributaries for more than 12 h along the lower stretch of the river system and mean weekly water temperatures at Bonneville Dam. Circles represent 52 weekly bins (mean 41 fish/bin; range 4‒122 fish/bin). The curve is the exponential regression line that best fits the data (r2 = 0.80; P ≤ 0.0001; percent = 6.558‒7e0.802xtemperature). The figure originally published in Goniea et al. (Citation2006) was provided for use by the editorial staff of Transactions of American Fisheries Society. The font was modified and color added.
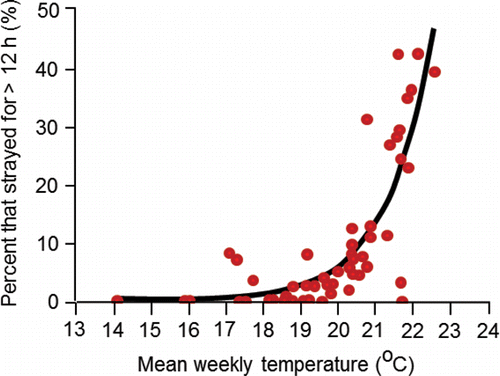
Goniea et al. (Citation2006), consistent with the results of Keefer et al. (Citation2004), concluded that many fall Chinook salmon exhibited thermoregulatory behaviors, but the response to warm temperature varied among fish, and whether or not a given fish would halt their migration upon exposure to temperatures above 20°C was unpredictable. A salient point made by Goniea et al. (Citation2006) is that inland fall Chinook salmon continue migration under sub-lethal, warm temperatures because they have a long way to migrate and a narrow window of time to reach the spawning grounds and spawn.
Caudill et al. (Citation2013) was another publication produced by the series of radiotelemetry studies started in 1998. That study was conducted during 2000‒2003 on 227 fish in the middle stretch of the river system and focused on the behavioral response of fish to temperature gradients in the fish ladders. At the time of the study, the top of the ladders drew warm surface water from the forebay of each dam, whereas water in the tailrace at the bottom of the ladders was relatively cool as it exited the powerhouse turbines having been drawn from deeper in the forebay. Caudill et al. (Citation2013) referred to the difference between top-of-ladder and base-of-ladder temperatures as ΔT, and characterized the temporal and spatial trends in ΔT observed in the ladders of the lower Snake River dams. Not unexpectedly, the frequency ΔT exceeded 0°C was highest during summer, and values of ≥ 2.0°C were most frequently observed at Lower Monumental, Ice Harbor, and Lower Granite dams. At Lower Granite Dam where the temperature gradient in the forebay was greatest, ΔT values above 3.0°C were common and some values ≥ 4.0°C were observed. Fish fitted with data-storage radio transmitters had body temperatures directly proportional to ladder temperatures (inter-annual range of r2; 0.97–0.98), and as such, body temperatures increased during ladder passage when ΔT exceed 0.0°C.
Caudill et al. (Citation2013) analyzed the effect of ΔT on passage time through the ladders, and after accounting for the variation in ladder passage time affected by other covariates, showed that passage time increased significantly as ΔT increased in the ladders at Ice Harbor and Lower Granite dams. Consequently, increases in ΔT could increase travel time from Ice Harbor Dam tailrace to Lower Granite Dam forebay, which highlights the potential for excess energy expenditure and unsuccessful migration. As per the suggestion of Caudill et al. (Citation2013) and efforts of managers, cooler water was directed into the top-of-ladder exit pools to remedy the problem with high ΔT.
Before moving on to the analyses of the PIT-tag data intended to compliment the radiotelemetry research, two additional telemetry studies are briefly reviewed here. Mann (Citation2007) established that external and internal temperatures measured on the fish were nearly identical and highly correlated (e.g., r2 = 0.97), and Mann (Citation2007) and Keefer and Caudill (Citation2015) showed that temperatures recorded by temperature loggers on a given fish were highly correlated with temperature exposure indices calculated from temperature data collected at the dams during fish passage. These findings were important because they showed that the mean temperature calculated over the elapsed time between passage at a downstream dam and the next upstream dam could be used to generally characterize the thermal experience of a given PIT-tagged fish.
Upstream movement behavior of PIT-tagged adults returning during 2010–2015 showed similar results to those of radiotelemetry studies. Travel time and upstream movement rates of PIT-tagged adults were calculated by subtracting the first detection date at a given downstream dam (e.g., Bonneville) from the last detection date at a given upstream dam (e.g., McNary Dam), with the exception of Lower Granite Dam in some instances. If a fish was trapped at Lower Granite Dam and hauled to Lyons Ferry Hatchery for brood stock, then the first detection date, not the last, of that fish at Lower Granite Dam was used to calculate passage date distributions, travel time, and upstream movement rate.
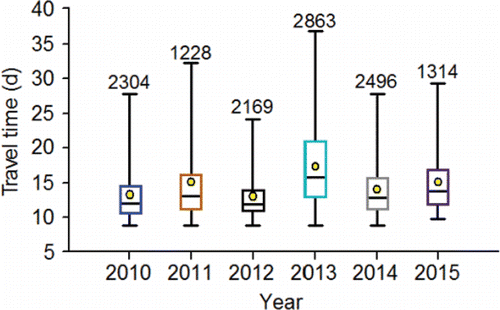
Despite high water temperature (e.g., ), the PIT-tagged adults typically traversed the 460-km channel distance between Bonneville and Lower Granite dams in about three weeks or less (). For example, the 75th percentiles in the travel time distributions for PIT-tagged adults were 14.2 d in 2010, 16.1 d in 2011, 14.0 d in 2012, 21.0 d in 2013, 13.0 d in 2014, and 16.7 d in 2015. Some fish took upwards of a month or more to make the 460-km journey.
Figure 9. Travel time distributions (2010‒2015) for PIT-tagged, hatchery-origin fall Chinook salmon adults that swam from Bonneville Dam to Lower Granite Dam. The lower whisker is the 2.5th percentile, the bottom of the box is the 25th percentile, the horizontal line in the box is the median, the circle in the box is the mean, the upper end of the box is the 75th percentile and the top whisker is the 97.5th percentile. The numbers above the 97th percentile are the sample sizes.
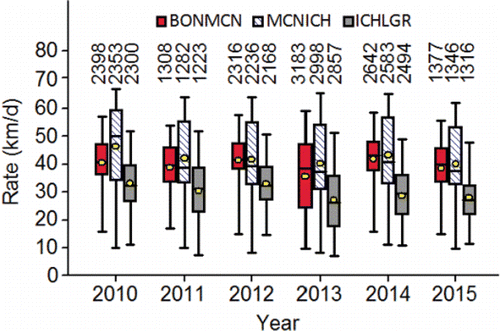
Within a year, the PIT-tagged adults consistently slowed their rate of upstream movement after they entered the middle stretch of the river system (). Behavioral thermoregulation near Lyons Ferry Hatchery and the Palouse River mouth, combined with slow passage due to ΔT that intensified as the fish passed from Ice Harbor to Lower Granite Dam are plausible explanations for that decrease in upstream movement rate.
Figure 10. Upstream movement rate (km/d) distributions (2010‒2015) calculated for PIT-tagged, hatchery-origin fall Chinook salmon adults that swam from Bonneville Dam to McNary Dam (BONMCN), McNary Dam to Ice Harbor Dam (MCNICH), and Ice Harbor Dam to Lower Granite Dam (ICHLGR). The lower whisker is the 2.5th percentile, the bottom of the box is the 25th percentile, the horizontal line in the box is the median, the circle in the box is the mean, the upper end of the box is the 75th percentile and the top whisker is the 97.5th percentile. The numbers above the 97th percentile are the sample sizes.
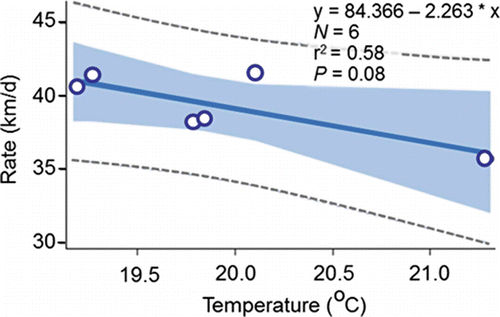
Other analyses of the PIT-tag data tended to support the findings from the radiotelemetry studies described above. Keefer et al. (Citation2004) and Goniea et al. (Citation2006) brought out the difficulty of understanding the factors for the variation in upstream movement rates of individual fish, which was also the case with the PIT-tag data analyzed for this review. For example, the r2 values for annual regression models fitted from temperature data collected on individual fish were all small because there was no clear relation between temperature and upstream movement rate (; 2010‒2015 r2 range 0.002 to 0.04). An inverse relation between temperature and inter-annual variation in upstream movement rate was observed after aggregating the data (). Notably, the analysis on the aggregated data oversimplified the cause-and-effect relation and exaggerated the amount of variation that is actually explained at the level of the individual fish.
Figure 11. Upstream movement rates (km/d) of PIT-tagged, hatchery-origin fall Chinook salmon adults that swam from Bonneville Dam to McNary Dam plotted against the mean temperature measured in the tailrace of McNary Dam for each fish between detection at the two dams during 2010‒2015. Annual sample sizes are given in .
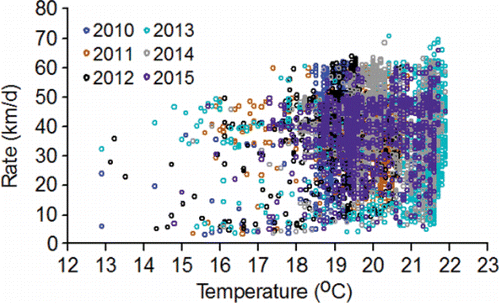
Figure 12. The relation between the annual mean upstream movement rate (km/d) and annual mean temperature calculated from the data in .
One recent analysis is mentioned here to close this subsection of the review. Bond et al. (Citation2016) used quantile regression to evaluate the factors for variation in travel time of PIT-tagged, hatchery-origin fall Chinook salmon from Bonneville Dam to Lower Granite Dam during 2002‒2011. Quantile regression analyzes data that are arguably aggregated, but the aggregates are broad and include observations of travel times within a selected range of percentiles opposed to single observations of annual means or medians. Specifically, the quantile regression model fitted by Bond et al. (Citation2016) focused on the relation between the groups of fish surrounding the 10th (i.e., the fastest), 50th (i.e., the median), and 90th (i.e. the slowest) percentile travel times observed at each temperature the fish were exposed to during migration. Bond et al. (Citation2016) reported that temperature did not have a significant effect on travel time for the 10th and 50th percentile groups of fish, whereas increasing temperature was associated with a decrease in travel time of the 90th percentile group that included the slowest migrants that made up 10% of the fish in each temperature grouping. It is pointed out here that the majority of the PIT-tagged fish were not slow migrants, and there was considerable variability in travel time at a given temperature among individual fish that formed the percentile groupings (e.g., ).
Migration success
Based on radiotelemetry studies
Estimating survival of Chinook salmon adults as they pass upstream along the Columbia and Snake rivers is a complicated task and researchers continue to contend with the inherent problems and search for solutions (see review by Dauble and Mueller, Citation2000). The detection rate of a group of tagged fish between two points is an established proxy for a minimum survival rate and it is referred to as migration success. The primary goal of migration success analyses is to understand the behavioral and environmental factors that determine whether or not a given fish will be a successful migrant. Radiotelemetry data sets for evaluating migration success are typically made up of fish that were tagged at Bonneville Dam, escaped harvest, and were then detected passing McNary Dam before they proceeded to swim to points upstream.
Analyzing the same radiotelemetry data set as Goniea et al. (Citation2006), Caudill et al. (Citation2007) explored the effect of travel times from Bonneville Dam to McNary Dam on migration success from McNary Dam to points upstream. Fish that swam from McNary Dam to a potential spawning location (e.g., the Hanford Reach or hatcheries) were classified as successful migrants. Adults for which natal location and likely upstream spawning location were determined, were considered to be successful migrants even if the fish was last tracked to non-natal spawning locations (i.e., the fish strayed). Fish classified as unsuccessful migrants were not tracked to a spawning location and their final fate was not known. Fish harvested in sport fisheries upstream of McNary Reservoir downstream of the Lower Snake River mouth, and along the Hanford Reach, were not included in the analyses.
Caudill et al. (Citation2007) reported that unsuccessful migrants had longer travel times from Bonneville Dam to McNary Dam compared to successful migrants. The differences in travel times were generally small in most years (<1 d in 1998, P > 0.05; ≈ 2 d in 2000, P = 0.06; ≈ 4 d in 2001, P < 0.05; < 1 d in 2002 and 2003, both P values > 0.05). Pooled across years, mean travel time was significantly (P = 0.02) longer for unsuccessful migrants compared to successful migrants. Caudill et al. (Citation2007) concluded that the majority of adults passed the dams rapidly and successfully reached spawning locations, and generally, fish that did not reach spawning locations were the slower migrants.
Mann (Citation2007) completed another of the series of studies that began in 1998 and contributed to the understanding of migration success. One of the objectives of Mann (Citation2007) was to analyze the effects of the temperature exposures on migration success in the middle stretch of the river system. Adult fall Chinook were fitted with a combination of radio tags, externally-mounted temperature recorders, and internal temperature tags at Ice Harbor Dam. The daily mean temperature each fish was exposed to between Ice Harbor and Lower Granite dams was determined. If a daily mean temperature was greater than 20°C, a value of 20 was subtracted from that mean to obtain a daily difference. The daily differences were summed to calculate degree days above 20°C (hereafter, DD>20) for a given fish.
The results of Mann (Citation2007) comported with those of Goniea et al. (Citation2006). The fish with comparatively lower temperature exposures typically exhibited behavioral thermoregulation by holding in ≈ 11°C water discharged from Lyons Ferry Hatchery. Fish that did not escape to a spawning tributary tended to experience warmer temperatures than fish that did escape to a spawning tributary. Those findings prompted Mann (Citation2007) to conclude: “warm but sub-lethal water temperatures experienced by anadromous salmonids during migration in the mainstem Snake River may have an indirect or delayed effect on survival.”
Based on analysis of PIT-tag data
Two presently unanswered questions that resonate in the migration success analyses conducted with radiotelemetry data are “What are the primary factors for migration success, and what are the final fates of unsuccessful migrants?” Those questions are explored in this section of the review by analyzing the data collected on the PIT-tagged adults during 2010‒2015. In contrast to Caudill et al. (Citation2007), the PIT-tagged adults that strayed were classified as unsuccessful migrants in the following analyses.
Migration success from Bonneville Dam to McNary Dam
Migration success of the PIT-tagged adults in the lower stretch of the river system measured between Bonneville and McNary dams averaged (± SE) 70.3 ± 2.1% during 2010‒2015. The majority of the PIT-tagged adults that did not migrate successfully from Bonneville Dam to McNary Dam were rightfully harvested by Columbia River tribes as part of treaty fisheries implemented under the aforementioned voluntary abundance-based harvest schedule. It can also be surmised that some portion of the unsuccessful migrants died as the result of injury attributable to marine mammal bites (e.g., Fryer, Citation1998) or predation, gill net or angling wounds (e.g., Chopin and Arimoto, Citation1995), or injury from dam passage (e.g., Wagner and Hillson, Citation1993). Additionally, some of the unsuccessful migrants probably strayed permanently to non-natal locations downstream of McNary Dam.
Bond et al. (Citation2016) evaluated straying of PIT-tagged, hatchery-origin fall Chinook salmon from the Snake River basin that returned during 2002–2011. Of the 24,533 PIT-tagged adults detected at Bonneville Dam, 18,463 had adult detection histories that were adequate for determining if any of the PIT-tagged adults strayed downstream of the Lower Snake River mouth. A total of 152 (0.8%) of the fish with adult detection histories permanently strayed in the lower stretch of the river system, of which 139 strayed into the Deschutes River (). Bond et al. (Citation2016) applied logistic regression to the data and found that the probability of a fish straying increased as temperature increased.
Migration success from McNary Dam to Lower Granite Dam
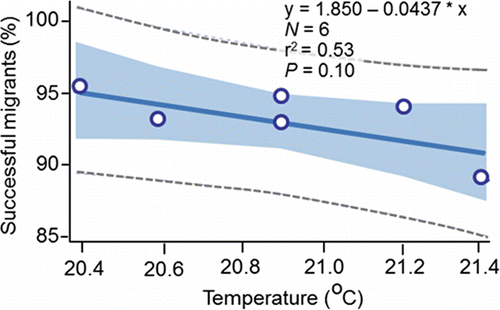
Across the years 2010‒2015, 93.4% of the PIT-tagged adults that were detected at both Bonneville and McNary dams then successfully migrated to Lower Granite Dam, whereas the remaining 6.6% were considered to be unsuccessful migrants (). Temperature measured at Ice Harbor Dam explained 53% of the inter-annual variation in migration success rate, which declined as temperature increased (). Successful migrants to Lower Granite Dam traveled between Bonneville and McNary dams in an average (±SD) of 6.7 ± 0.9 d while unsuccessful migrants took almost twice as long (11.2 ± 2.0 d; ). Within-year comparisons showed that every annual mean travel time of successful migrants was shorter than the corresponding annual mean travel time of unsuccessful migrants ().
Table 4. Mean travel times of PIT-tagged, hatchery-origin fall Chinook salmon adults from Bonneville Dam to McNary Dam for fish that were and were not detected at Lower Granite Dam (i.e., successful [S] and unsuccessful [U] migrants). Fisher's test for honestly significant differences showed that every annual mean travel time (loge transformed) of successful migrants was significantly (P < 0.05) shorter than the corresponding annual mean travel time of unsuccessful migrants. Results of logistic regression models fitted separately by year and jointly across years (All) to predict g(x) and calculate the probability of success Pi (set at 0.5; Pi = eg(x) / 1 + eg(x)) of individual fish from their travel times are given. The percentage of the known unsuccessful and successful migrants that were predicted to be successful by those models is also given.
Figure 13. The relation between the annual percentages of the PIT-tagged, hatchery-origin fall Chinook salmon adults that migrated successfully between McNary and Lower Granite dams, and the annual mean temperature measured in the tailrace of Ice Harbor Dam from 12-Aug to 14-Sep, 2010‒2015.
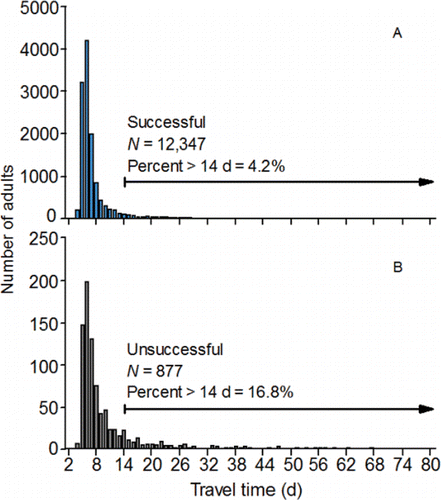
Travel time between Bonneville and McNary dams was also related to the probability of a PIT-tagged adult successfully migrating from McNary to Lower Granite Dam. The probability of successful migration to Lower Granite Dam was inversely related to travel time, but each annual model predicted that over 90% of the unsuccessful migrants should have been successful migrants (). Classification inaccuracy could not be reduced substantially by varying the cutoff probability for migrants classified as being successful, and was caused by the nearly complete overlap in the right-skewed, travel time frequency distributions of successful and unsuccessful migrants. The primary difference between the distributions was the length of the right tail ().
Figure 14. Travel time distributions (across 2010‒2015) calculated for PIT-tagged, hatchery-origin fall Chinook salmon adults that swam upstream from Bonneville Dam to McNary Dam, and were subsequently detected or not detected at Lower Granite Dam (i.e., successful panel A and unsuccessful migrants panel B).
The final fate of PIT-tagged adults that did not successfully migrate to Lower Granite Dam during 2010‒2015 was categorized as follows. Fish that were last detected downstream of McNary Dam were categorized as permanent fallbacks. Fish that were last detected along the Lower Snake River from Ice Harbor Dam to Little Goose Dam including tributaries and Lyons Ferry Hatchery (not including fish trapped at Lower Granite Dam) but failed to home to their riverine release points upstream of Lower Granite Reservoir were categorized as “undershoots.” Fish that presumably passed McNary Dam, but were never detected at Snake River dams, were categorized as strays.
Permanent fallbacks made up very small percentages of the PIT-tagged adults that passed McNary Dam, but were not successful migrants to Lower Granite Dam (). Across the 6 years, the majority (46.2%) of the permanent fallbacks were last detected in the Umatilla River (). The Umatilla River once supported spawning of upriver bright fall Chinook salmon, is the present location of a fall Chinook salmon hatchery (ODFW, Citation2005), and some fish likely spawn in the river rather than in the hatchery as is commonly observed in the vicinity of hatcheries. The remainder was last detected as fallbacks in the juvenile fish passage facility at McNary Dam (30.8%), in the Deschutes River (7.7%; that supports spawning of upriver bright fall Chinook salmon; Myers et al. Citation1998), and as fallbacks in the juvenile fish facilities at John Day Dam (7.7%) and Bonneville Dam (7.7%).
Figure 15. Pie charts showing the annual percentages (2010‒2015; charts A‒F, respectively) of the PIT-tagged, hatchery-origin fall Chinook salmon adults that did not migrate successfully from McNary to Lower Granite Dam (N = 877), but were last detected as permanent fallbacks downstream of McNary Dam, as mid-Columbia strays between McNary and Priest Rapids dams, as undershoots between Ice Harbor and Little Goose dams, or as upper Columbia strays at dams, within tributaries, or at hatcheries upstream of the Lower Snake River mouth.
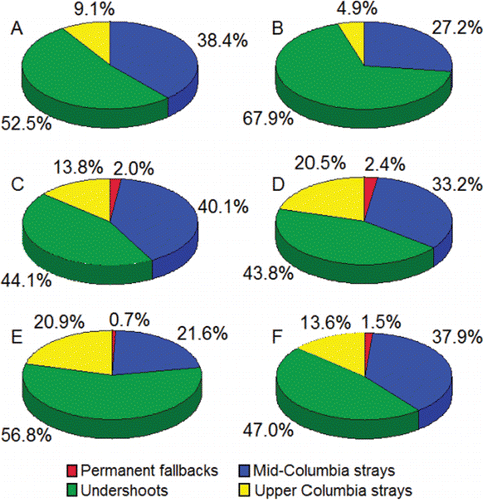
In all 6 years, the majority of the fish that did not migrate successfully from McNary Dam to Lower Granite Dam were undershoots (). Of the undershoots, 74.7% was last detected in the fish ladders of Ice Harbor Dam. The remaining 25.3% of the fish was detected in the Lower Monumental Dam fish ladder (6.0%), Little Goose Dam fish ladder (11.5%), as fallbacks in the juvenile fish passage facilities at Lower Monumental (2.5%) and Little Goose (1.8%) dams, the Tucannon River (2.3%) where fall Chinook salmon spawn (Schoning Citation1947), and Lyons Ferry Hatchery (1.2%) where fall Chinook salmon are spawned.
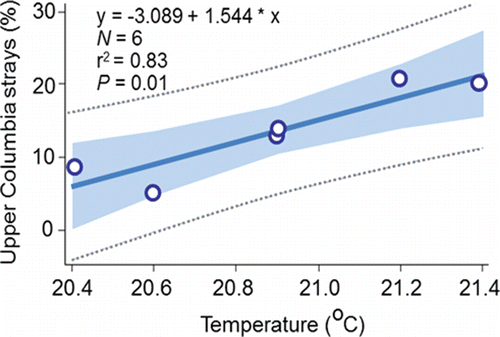
Straying into the mid-Columbia River and Upper Columbia River was the second most prevalent final fate of the PIT-tagged adults that did not migrate successfully from McNary Dam to Lower Granite Dam (). The PIT-tag monitoring systems where the strays were last detected were in the vicinity of known spawning areas or at hatcheries. It is probable that many of the strays, both PIT-tagged and non-tagged from the population of strays as a whole, spawned in the Hanford Reach or entered Priest Rapids Hatchery. In support of that conclusion it is noted that: (1) adults that pass the Snake River mouth have unimpeded access to those sites; and (2) the spawning population in the Hanford Reach is large (Huntington et al., Citation1996), and spawning fall Chinook salmon attract other fish that have yet to spawn (Groves et al., Citation2013). Thus, it is likely that an unknown—but large—number of the unsuccessful migrants during 2010–2015 halted their migration in the Hanford Reach.
The results presented in this section suggest that a large percentage of the 877 unsuccessful PIT-tagged migrants were strays that successfully swam to a final destination where they had the opportunity to spawn. That proposal is consistent with the conclusions of Mendel et al. (Citation1992, Citation1993) and Mendel and Milks (Citation1997) who conducted the earliest radiotelemetry studies on fall Chinook salmon passing upstream through the Lower Snake River reservoirs. Moreover, the 6.6% figure for the unsuccessful PIT-tagged migrants () aligns closely with the overall stray rate of 4.2% reported by Caudill et al. (Citation2007) who was able to radio track fish to spawning locations upstream of McNary Dam. Large percentages of unsuccessful migrants were classified as strays and the percentage of those fish known with certainty to have strayed into the upper Columbia River were directly proportional to temperature measured at Ice Harbor Dam (). Consistent with Bond et al. (Citation2016), it can be concluded that homing increases as temperature at the Lower Snake River mouth decreases, or conversely, that straying increases as temperature at the Snake River mouth increases.
Spawning behavior, timing, and success
This section covers four objectives including the description of:
| (1) | pre-spawning movement; | ||||
| (2) | spawning behavior; | ||||
| (3) | spawning timing and | ||||
| (4) | spawning success. | ||||
Pre-spawning movement
Connor and Garcia (Citation2006) provided the most detailed published information on movement up to the time of spawning initiation by fall Chinook salmon adults in the upper stretch of the river system. Natural-origin (females, n = 4; males n = 5) and hatchery-origin (females, n = 27) adults were captured in the Lower Granite Dam ladder during 1998, 1999, and 2001 all of which had been PIT tagged as juveniles as they reared, or were released, in the Upper or Lower Hells Canyon spawning areas. The above 36 adults were radio tagged, and then tracked an average of 15 observations per fish from Lower Granite Dam to spawning sites using a combination of fixed stations and mobile tracking. Of the 36 radio-tagged fish, 29 spawned in the Upper Hells Canyon spawning area, and 7 spawned in the Lower Hells Canyon spawning area upstream of the Grande Ronde River.
The fish did not swim directly to spawning sites and initiate spawning. A migratory phase was observed that consisted of consecutive detections made in an upstream direction from Lower Granite Dam (). The fish typically made a transition from the migratory phase to a search phase after natural-origin adults passed their known natal rearing areas, and hatchery-origin adults passed the known locations where they were released as Age-0 juveniles (). The search phase was commonly characterized by a series of downstream and upstream excursions ending with spawning initiation ().
Figure 17. Examples of detection plots for natural-origin male (panel A), natural-origin female (panel B), and hatchery-origin female (panel C) fall Chinook salmon adults including the migratory phase, transition date and location, search phase, spawning site selection, and detections made after spawning site selection. The arrows next to y axis show the river km where the adults were released as Age-0 juveniles. The figure originally published in Connor and Garcia (Citation2006) was provided for use by the editorial staff of Transactions of American Fisheries Society. The font was modified and color added.
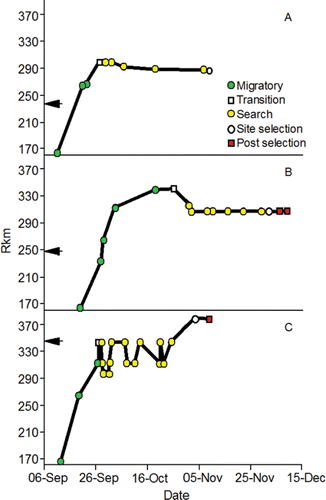
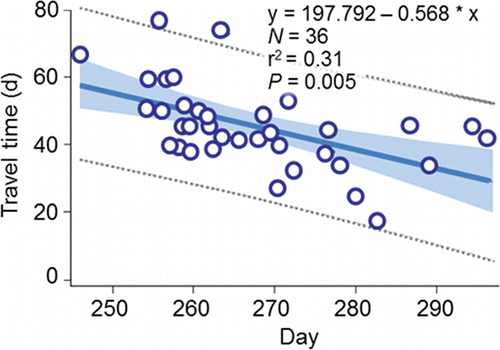
The fish spent considerable time, and covered relatively long swimming distances, prior to spawning initiation. The median travel time between release at Lower Granite Dam and spawning initiation was 45 d for fish that spawned along the Upper Hells Canyon spawning area, and 39 d for fish that spawned along the Lower Hells Canyon spawning area. Travel time from Lower Granite Dam to spawning initiation was inversely proportional to passage day of year at the dam (). Therefore, spawning date generally became later as passage date became later. The net channel distances to the most frequently and heavily used spawning sites along the Upper and Lower Hells Canyon spawning areas after entering the upper section are 100.7 km and 32.8 km, respectively (). The median total distances fish swam in the upper section were 330.2 km and 94.7 km for the radio-tagged fish that spawned in Upper or Lower Hells Canyon, respectively. The extra swimming distance (i.e., > 100.7 km or 32.8 km) was accrued during the downstream and upstream excursions of the search phase.
Figure 18. The relation between travel time (d) from Lower Granite Dam to spawning site selection and day of the initiation of redd construction along the Upper and Lower Hells Canyon spawning areas combined and passage day of year (01/01 = 1) at the dam for radio-tagged fall Chinook salmon adults, 1998, 2000, and 2001. The data for the regression were collected by Connor and Garcia (Citation2006).
Spawning behavior
The Master Fish Warden of Oregon at the turn of the 20th century (Reed, Citation1901) made some of the earliest observations on spawning behavior, timing, and habitat of fall Chinook salmon.
“Those [fish] that have been so fortunate as to run the gauntlet and escape the many devices used to capture them, and have arrived in the vicinity of their spawning grounds, seem to enjoy the rest after their long and perilous journey from the sea by lying in the deep, shady pools along the streams for the greater part of the day, and as night approaches, if they have not yet reached the place where they first saw the light of day, they will move on up the stream, passing over rapids where the current is very strong, with apparently little exertion till they have reached the next pool, and so on from day to day until they find the desired spot and are ready to deposit their eggs. When this time comes they may be seen in pairs, male and female, going from the pools into shallow water, say from one to two feet deep, where the current runs from six to eight miles an hour over a good, gravelly bottom, where each pair chooses their beds, so termed, and begin spawning. In this process the female turns on its side, and while making several vigorous strokes with her tail, deposits from twenty-five to fifty eggs. The strokes with the tail are made for the purpose of loosening the gravel so that when the eggs reach the bottom the greater part of them may be covered up with the moving gravel. When the female has performed this operation, the male, if he is not chasing trout away, goes over the bed and deposits the milt which, coming in contact with the eggs, fertilizes them.
This performance is repeated at intervals of from ten to twenty minutes, until the female has deposited all her eggs (the average number being about five thousand), the time required to deposit them being from three to four days.
The holes in the gravel (or nests as some call them) are formed by the movement of the salmon tail, and are not dug or rooted out with the nose as some writers have claimed.”
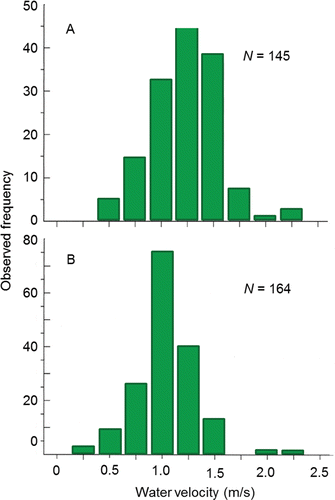
Spawning behavior is entwined with habitat selection as redd construction is essentially a behavioral response to the habitat into which the eggs are laid and fertilized. Redds are constructed in distinct patches of substrate that are partially or wholly submerged under water (e.g., ). Groves and Chandler (Citation1999) visually assessed substrate composition and measured depth, mean water column velocity, and substrate-level velocity at 112, 205, 145, and 164 redds, respectively, located within the Upper and Lower Hells Canyon spawning areas. The majority of redds (77%) were excavated in medium-to large-sized gravel (). Water depth over the redds ranged from 0.5 m to 6.5 m with mode of 2.0 m (). The range of mean water column and substrate-level velocities overlapped, whereas the modal mean water column velocity was higher than the modal substrate level velocity that was likely experienced by the fish ().
Figure 19. An overhead example of a spawning site located along the Upper Hells Canyon spawning area including spawning substrate distribution (e.g., ), channel bathymetry, redd locations, and a sampling transect where depth, velocity, and substrate were measured to quantify spawning habitat. The figure originally published in Connor et al. (Citation2001) was provided for use by the editorial staff of Northwest Science. The font was modified and color added.
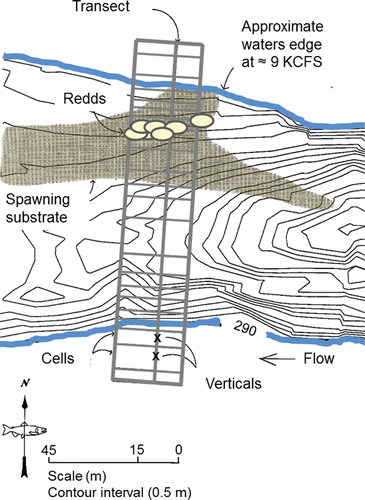
Figure 20. Frequency distribution of combined dominant (left) and subdominant (right) substrate types observed at fall Chinook salmon redds in the Upper and Lower Hells Canyon spawning areas, 1993‒1995. The figure originally published in Groves and Chandler (Citation1999) was provided for use by the editorial staff of Transactions of the American Fisheries Society. The font was modified and color added.
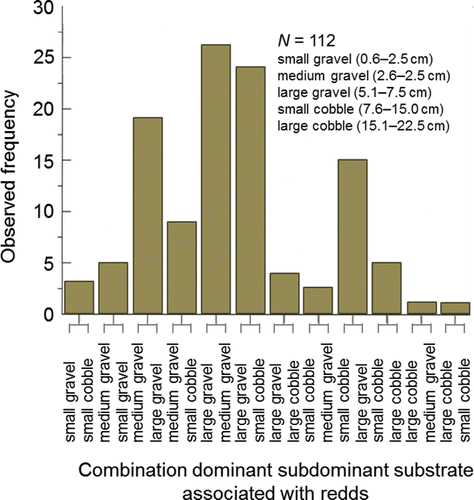
Figure 21. Frequency distribution of water depths measured at fall Chinook salmon redds in the Upper and Lower Hells Canyon spawning areas, 1993‒1995. Reproduced from Groves and Chandler (Citation1999) with permission from the American Fisheries Society. The font was changed and color added.
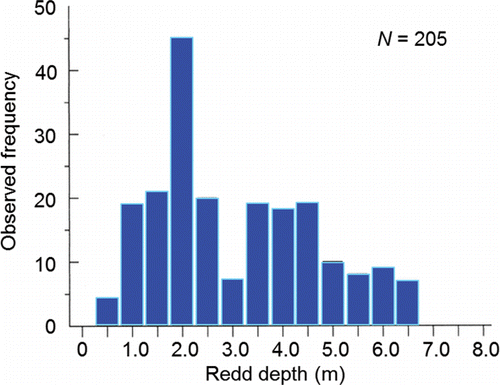
Figure 22. Frequency distribution of mean water column (panel A) and substrate-level (panel B) velocities measured at fall Chinook salmon redds in the Upper and Lower Hells Canyon spawning areas, 1993‒1995. Reproduced from Groves and Chandler (Citation1999) with permission from the American Fisheries Society. The font was changed and color added.
Geist and Dauble (Citation1998) moved the understanding of spawning habitat use beyond the fundamental, substrate-depth-velocity paradigm by developing a conceptual spawning habitat model for upriver bright fall Chinook salmon in the Hanford Reach of the Columbia River. The model described how geomorphic features of river channels affect hydraulic processes, and how those processes influence where the fish spawn in large rivers. Some of the key points brought out by Geist and Dauble (Citation1998) are summarized hereafter.
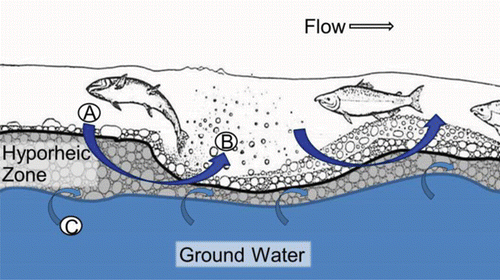
The hyporheic zone is the subsurface portion of the substrate within habitats such as the upstream end of riffles and gravels bars where groundwater and surface water mix (). Downwelling occurs when surface water enters the hyporheic zone of the substrate, whereas upwelling occurs when water reemerges from the hyporheic zone. The proportion of surface and groundwater in the hyporheic zone is dependent on discharge, groundwater quantity, and the time of year. Runoff and velocity are high during the spring and surface water forced into the hyporheic zone makes up the largest portion of subsurface flow, whereas during periods of low flow groundwater dominates within the hyporheic zone (Geist and Dauble, Citation1998). In short, field data and literature published after the conceptual model was developed, supported the spawning habitat model and showed how the geomorphic features of the riverbed could help prevent the percentage of fine substrates (hereafter, percent fines) from becoming high enough to completely embed the incubation environment, while insuring that incubating embryos were supplied with oxygenated water (Geist and Dauble, Citation1998; Geist, Citation2000; Geist et al., Citation2000b, Citation2002).
Figure 23. The post-spawn incubation environment according to the conceptual model of Geist and Dauble (Citation1998) including downwelling (A) and upwelling (B) of surface water (i.e., river water), and ground water inflow (C). Not drawn to scale. The figure of the fish digging the redd was adapted from Burner (Citation1951) with permission of the National Oceanic and Atmospheric Administration. The font was changed and color added.
Spawning timing
Because the eggs are laid and fertilized in the last stages of redd digging, and the redd can be distinguished from the surrounding substrates due to the disturbance caused by the digging, spawn timing in the wild is determined based on frequency distributions of redd counts. Fish from the population spawn in the fall with some temporal variation between sub-watersheds and among spawning areas (). With regard to the primary spawning areas where the majority of production occurs, fish begin to spawn earlier in the Clearwater River lower reach compared to fish in the Upper and Lower Hells Canyon spawning areas ().
Spawning success
Spawning success is measured in this review as survival between the time the adults pass Lower Granite Dam and the fry produced by spawning emerge from the gravel. Spawning success is directly determined by the level of pre-spawning mortality in the adults combined with embryo loss caused by the thermal history of the female parent, initial incubation temperature, and substrate composition.
Pre-spawning mortality
Bowerman et al. (Citation2016) reviewed the literature on pre-spawning mortality of the anadromous species from the genus Oncorhynchus and defined pre-spawning mortality as the mortality observed after the adults reach the spawning grounds but before the eggs and milt are successfully released. The review covered methods used to estimate pre-spawning mortality including escapement-based estimates (1 – number of redds counted/female escapement x 100), and examining egg retention (number of eggs/expected fecundity) in female carcasses. Bowerman et al. (Citation2016) suggested that pre-spawning mortality increased as the time in freshwater and distance migrated to the spawning grounds increased supporting a link between energy expenditure over time and space and death. More in-depth material on this topic is presented later in the review.
The thermal history of the female parent
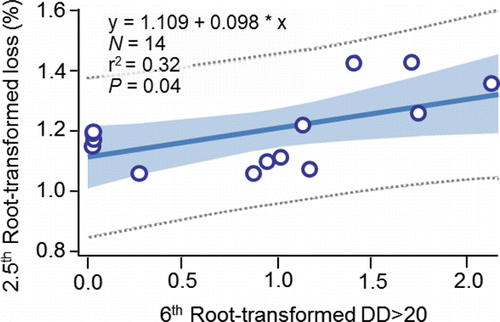
Mann (Citation2007) conducted an innovative but limited study on embryo loss affected by the thermal histories of the females he had radio tagged at Ice Harbor Dam. A subsample of those radio-tagged females was subsequently trapped at Lower Granite Dam, taken to hatcheries, held at ≈ 11 to 12°C, and spawned. Embryo loss (%) was monitored through the button-up stage (equivalent to fry emergence in the wild). Of the females studied, 15 had been fitted with temperature loggers. Their thermal exposures from Ice Harbor Dam to Lower Granite Dam were measured in terms of DD>20 (). Mann (Citation2007) removed one outlier (tag 25162; ), 2.5th root transformed DD>20, and 6th root transformed embryo loss (%). He then fitted a regression equation to test the relation between DD>20 and embryo loss (%). Transformed DD>20 was directly proportional to embryo loss and explained 32% of the variation in embryo loss ().
Table 5. Embryo loss (%) from fertilization to button up (equivalent to fry emergence in the wild) and degree days above 20°C (DD>20) measured on female fall Chinook salmon fitted with temperature loggers at Ice Harbor Dam, trapped at Lower Granite Dam, and spawned at either Lyons Ferry or Nez Perce Tribal hatcheries during 2004 (from Mann, Citation2007). To calculate DD>20 in the middle stretch of the river system (i.e., observed), the daily mean temperature each fish was exposed to between Ice Harbor and Lower Granite dams was determined. If a daily mean temperature was greater than 20°C, 20 was subtracted from that mean to obtain a daily difference. The daily differences were summed to calculate DD>20 in the middle stretch. Bonneville Dam passage dates and DD>20 in the lower stretch of the river system were simulated (Eq. 14; ) to provide data for fitting Eq. 15 () that predicted embryo mortality from a spatially-extended value of DD>20.
Figure 24. The relation between degree days above 20°C (DD>20) calculated for the female parent and embryo loss measured from spawning to the button-up stage (equivalent to fry emergence in the wild). The equation was fitted by Mann (Citation2007). The untransformed data are given in (x = DD>20 in the middle stretch of the river system; y = observed embryo loss).
Initial incubation temperature
The ability to duplicate exposure to the warmest temperatures observed during redd construction and egg laying has eluded researchers in the laboratory. Although fall Chinook salmon adults can survive at temperatures above 20°C in the wild, they are extremely sensitive to handling and holding in captivity at such temperatures. Trapping and hauling incoming adults at temperatures close to 20°C, and then holding them in captivity at warm temperatures as they matured, would most certainly kill an unacceptable number of the fish (e.g., see the historical review by Connor et al. (Citation2016) and the laboratory study by Geist et al. (Citation2000a).
Geist et al. (Citation2006) avoided high levels of temperature-related handling mortality by obtaining eggs and milt at Lyons Ferry Hatchery, fertilizing the eggs at ≈ 12°C, and then assigning the gametes to replicated, initial incubation temperature treatments (13.0°C, 15.0°C, 16.0°C, 16.5°C, and 17.0°C). Dissolved oxygen in the 13.0°C and 17.0°C treatment replicates was held at saturation, whereas the remaining three treatment replicates were subdivided and held at oxygen levels of 4 mg/L, 6 mg/L, 8 mg/L, and saturation. The apparatus that produced each temperature treatment was programmed to drop the temperature by approximately 0.2°C /d for 40 d, while increasing the dissolved oxygen level by 2 mg/L/d starting 16 d post fertilization. The 40-d temperatures were selected to span the range of the 1991–2003 inter-annual mean temperatures in the Upper Hells Canyon spawning area, and the 4 mg/L oxygen treatment represented the lowest level observed at a spawning site located within that spawning area. After 40 days, the temperatures were equilibrated among the treatments to match the 2001 drought year temperatures.
Mean (± SD) survival from fertilization to emergence calculated across the three coolest temperature treatments and the corresponding oxygen treatments was 92.8 ± 4.4% compared to 93.1 ± 1.4% for fish in the 16.5°C treatment and 1.7 ± 1.6% for fish in the 17.0°C treatment. Geist et al. (Citation2006) concluded that exposure to water temperatures up to 16.5°C would not have deleterious effects on survival or growth from egg to emergence if temperatures decline at a rate of 0.2°C /d or more after spawning and dissolved oxygen levels remain above 4 mg/L.
There are two noteworthy aspects of the Geist et al. (Citation2006) results. The first is that the high level of mortality (i.e., 98.3%) observed in the 17.0°C treatment, which had a 17.0°C starting temperature and then declined by 0.2°C per day, resulted from the embryos being exposed to only 0.9 degree days above 16.5°C (i.e., 17.0-16.5 + 16.8-16.5+ 16.6-16.5; hereafter DD>16.5). In other words, embryo survival decreased by 10.9 percentage points for every 0.1 °C increase in DD>16.5. Secondly, it was possible that embryo loss would have been higher in the warmer temperature treatments had the fish been spawned and the eggs fertilized at the treatment temperatures.
Substrate composition
Substrate composition expressed as percent fines is not generally considered a determinant of temperature-related spawning success. Substrate composition is important to cover in this review because embryo loss under typical levels of percent fines is high (≈ 70% from Healey, Citation1991; but see McMichael et al., Citation2005 for lower values). To measure percent fines, Arnsberg et al. (Citation1992) built an enlarged tri-tube freeze core sampler (Everest et al., Citation1980) that was capable of extracting large substrate cores from the river bed. Between 1990 and 2001, those researchers extracted cores from spawning sites within the Clearwater lower reach, Imnaha lower reach, Salmon lower reach, Clearwater upper reach, South Fork Clearwater lower reach, Middle Fork Clearwater, and Selway lower reach. The particle sizes of the core samples were determined, but substrate composition was not statistically related to embryo loss.
Bennett et al. (Citation2003) equipped SCUBA divers with a dome suction sampler (Gale et al., Citation1975) to collect substrate samples at four known spawning sites along the Upper Hells Canyon spawning area and three spawning sites along the Lower Hells Canyon spawning area. In the laboratory, eight substrate mixtures were created each of which was placed in six replicate troughs (N = 48) to represent substrate mixtures observed in the field. Eggs from Lyons Ferry Hatchery were fertilized and planted in the troughs. The regression equation: emergence success (%) = 46.643 + 1.194*S0.85 – 0.0111*S6.4S0.85 was fitted from the laboratory results; where emergence success was the mean percent survival to emergence, S0.85 was the mean percentage of fines less than 0.85 mm in diameter in each of the eight substrate mixtures, and S6.4S0.85 was a quadratic term calculated from S0.085 and the mean percentage of the eight substrate mixtures composed of fines less than 6.4 mm in diameter (i.e., S6.4). That regression equation explained 97% of the variability observed in mean emergence success (N = 8; P < 0.0001). Bennett et al. (Citation2003) then predicted emergence success at the seven spawning sites based on the average values of S0.085 and S6.4 observed at each site in their field samples. Predicted mean emergence success ranged from 29% to 48% (N = 7 sites). The equation could not be used to predict emergence success for the spawning areas sampled by Arnsberg et al. (Citation1992) because they did not measure S6.4.
To put what is presently known about substrate composition and embryo loss in the contemporary spawning areas in the context of the 70% embryo loss rate reported by Healey (Citation1991), the laboratory data from Bennett et al. (Citation2003) were used here to fit the regression equation: embryo loss (%) = -52.689 – 0.160*S0.85 + 0.186*S0.85S0.85 (N = 8; r2 = 0.89; P = 0.004). That equation was then used to predict embryo loss from the value of S0.85 measured in each individual substrate sample collected in the contemporary spawning areas. The average (± 95% CI) of the predictions was 53.5 ± 0.3% for the Upper Hells Canyon substrate samples (N = 40), 64.1 ± 4.6% for the Lower Hells Canyon substrate samples (N = 29), and 64.3 ± 8.2% for the Clearwater River lower reach substrate samples (N = 6). Only two samples collected in the primary, secondary, and tertiary spawning areas differed significantly from the 70% benchmark based on the coverage of the 95% prediction intervals of the individual predictions used to calculate the above averages (both from the Lower Hells Canyon; ). To restate the primary point of this section, it is important to recognize that embryo mortality is high even when temperatures are favorable for survival.
Figure 25. Embryo loss (%) predicted (± 95% CI) for the primary, secondary, and tertiary fall Chinook salmon spawning areas in the sub-watersheds that make up the upper stretch of the river system. The equation was: embryo loss (%) = -52.689 – 0.160*S0.85 + 0.186*S0.85S0.85 (N = 8; r2 = 0.89; P = 0.004); S0.85 was the mean percentage of fines less than 0.85 mm in diameter in each of the substrate samples collected by either Arnsberg et al. (Citation1992) or Bennett et al. (Citation2003). The prediction marked by an asterisk in panel was 105.1 ± 27.8%.
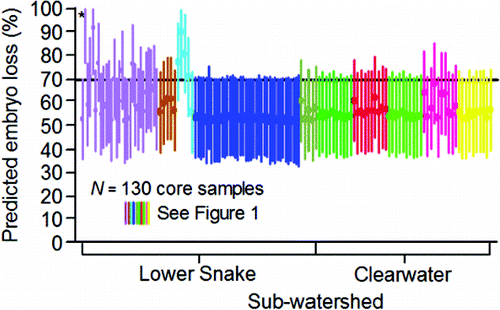
Illustrating the effects of behavior, velocity, and temperature on migration and spawning success measured through spawning site selection and the initiation of redd construction
A three-stage somatic energy use model was developed in this review (). The model is used to illustrate how behavior, and temperature and velocity exposure of adults from Bonneville Dam to the Upper Hells Canyon spawning area, act as joint factors for migration and spawning success measured through spawning site selection and redd construction. For the sakes of brevity and readability, the detailed methods are presented separately in the Appendix unless noted otherwise.
Figure 26. Stages of the model developed to simulate somatic energy use, migration success, and spawning success of PIT-tagged, hatchery-origin fall Chinook salmon adults during 2010‒2015.
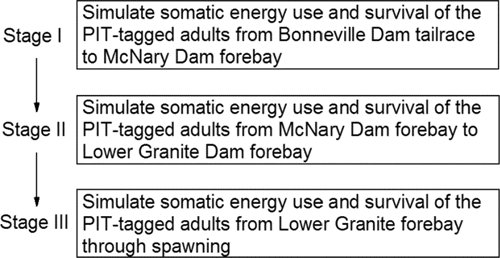
The individual PIT-tagged adults in were the source of the data for modeling. Each of those fish survived from Bonneville Dam tailrace to Lower Granite Dam forebay. Those fish were selected with the understanding that the large majority of the fish detected at Bonneville Dam that were not subsequently detected at McNary Dam had been rightfully harvested in the Treaty fisheries. Thus, it was assumed that death due to exhaustion was not a factor for migration success in that portion of river; but information is subsequently provided that supports that assumption. Similarly, this review has established that straying after the fish reach the mouth of the Lower Snake River is the primary source of loss between the forebays of McNary and Lower Granite Dam. As such, the primary goal of this section of the review was to illustrate how mortality incurred by the fish after passage at Lower Granite Dam might be affected by fish behavior and environmental exposure after arrival in the Bonneville Dam tailrace.
Each fish was treated as if it was a female destined to spawn in the Upper Hells Canyon spawning area. Spawners destined for the Upper Hells Canyon spawning area must swim some of the longest distances to reach the spawning sites, through the highest velocity habitats, and are the most susceptible to warm temperatures upstream of Lower Granite Dam as there is little thermal refuge available after passing the mouth of the Clearwater River ().
This section covers five objectives including:
| (1) | evaluating the realism of the model simulations; | ||||
| (2) | comparing the simulated cumulative temperature exposures (1°C above 0°C for 1 day = 1 cumulative temperature unit) between Bonneville Dam and McNary Dam, and the simulated expended useable energy (cumulative %) upon arrival in the forebay of McNary Dam, between fish that successfully migrated from Bonneville Dam tailrace to Lower Granite Dam forebay, and fish that that were unsuccessful migrants between those two dams; | ||||
| (3) | comparing the Lower Granite Dam passage dates, simulated cumulative temperature exposures, and simulated expended useable energy content (cumulative %) upon arrival in the forebay of Lower Granite Dam between fish that were classified as successful or unsuccessful spawners by the model; | ||||
| (4) | depicting the role of cumulative temperature exposure, as the fish swam from the lower to upper stretch of the river system, on spawning success classification (i.e., successful versus unsuccessful spawners according to the model; see Appendix for detailed methods); and | ||||
| (5) | comparing simulated somatic energy use among tailrace-to-dam passage, reservoir passage, and pre-spawning movement events for fish classified as successful spawners. | ||||
Evaluating the realism of model simulations
The realism of the model simulations was evaluated based on: (1) the estimated lengths and weights that were input to the model; (2) the observed and simulated migration success rates between Bonneville Dam forebay and McNary Dam forebay, and between McNary Dam forebay and Lower Granite Dam forebay; (3) the typical age-class distribution of natural-origin females and the observed age-class distribution of the PIT-tagged fish; (4) levels of pre-spawning mortality reported in the literature and simulated levels of pre-spawning mortality; (5) the temporal pattern in pre-spawning mortality reported in the literature and the simulated temporal pattern in pre-spawning mortality; (6) the spawning date distributions estimated for fish in the wild and the simulated spawning date distributions; and (7) the fraction of pre-spawning, somatic energy content used to spawn according to the literature and that same fraction simulated by the model.
Weights and fork lengths were estimated at Bonneville Dam, and in turn, used to simulate the amount of energy that was used or was available at various times and locations. In all cases, estimated weights and lengths were well within the bounds of expectations, and estimated weight declined as the fish moved upstream and spawned as observed in the river. Jointly across the 6 years modeled (N = 18,755 fish), the overall average (±SD) estimated weight of the PIT-tagged adults at Bonneville Dam was 5.6 ± 1.3 kg. The overall average (±SD) estimated fork length of the PIT-tagged adults at Bonneville Dam was 75 ± 5 cm. The overall average estimated weights of the fish at McNary Dam fell to 5.0 ± 1.2 kg (N = 13,224), 4.5 ± 1.3 kg at Lower Granite Dam (N = 12,347), and then to 3.7 ± 1.3 kg (N = 11,.364) at the simulated time of spawning initiation.
Every PIT-tagged adult used in the model survived from Bonneville Dam tailrace to McNary Dam forebay (i.e., migration success during Stage I modeling). Likewise, every PIT-tagged adult survived from McNary Dam forebay to Lower Granite Dam forebay (i.e., migration success during State II modeling). Simulated migration success for the fish in Stages I and II of the model was expected to be 100% if the model was not markedly underestimating useable somatic energy, or overestimating somatic energy use. That expectation was met.
The apparent ocean-age distributions of the PIT-tagged adults were not perfectly aligned with the apparent ocean-age distribution typically observed for natural-origin females. Age-II fish predominated in the returns of PIT-tagged fish in 2010, 2011, and 2012. It is important to keep that age discrepancy in mind because spawning success classification was dependent on apparent ocean age during all 6 years modeled. The annual percentages of the fish classified as successful spawners were lower for Age-II fish compared to Age-III, Age-IV, and Age–V fish ().
Figure 27. The percentage of the apparent ocean age II, III, IV, and V PIT-tagged, hatchery-origin fall Chinook salmon adults that migrated successfully during Stage II modeling (i.e., McNary Dam forebay to Lower Granite Dam forebay) that were classified as successful spawners, 2010–2015. The numbers above the bars are the sample sizes of fish classified as successful spawners.
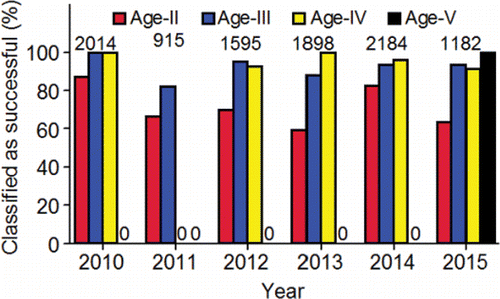
The model classified unsuccessful spawners into two groups including prespawning mortalities that died before spawning site selection and the initiation of redd construction, and premature spawning mortalities that began to construct redds and lay eggs but died before egg laying was complete. Prespawning mortality for the PIT-tagged fish simulated between passage at Lower Granite Dam and spawning site selection was 2.7% in 2010, 0.8% in 2011, 0.6% in 2012, 10.5% in 2013, 3.6% in 2014, and 2.6% in 2015. The distribution observed in the simulated pre-spawning mortality rates for the Upper Hells Canyon spawning area did not stand out as an anomaly when compared to pre-spawning mortality distributions for other fall Chinook salmon in rivers impacted by human development ().
Figure 28. Pre-spawning mortality (%) distributions (5th, 25th, 50th, 75th, and 95th percentiles) for basin-level groupings of fall Chinook salmon calculated with empirical data from Bowerman et al. (Citation2016) compared to the distributions calculated from simulations made with the somatic energy use model for the PIT-tagged, hatchery-origin fall Chinook salmon adults in the Upper Hells Canyon spawning area (UHC). The numbers above the 95th percentile are the sample sizes. Other abbreviations: CLR, Clearwater River lower reach, ID; KAL, Klamath Falls, OR; MCOL, Mid-Columbia, OR; PUG, Puget Sound, WA; SAC, Sacramento, CA; SJO, San Joaquin, CA. The data were provided by the corresponding author of Bowerman et al. (Citation2016), except for the Clearwater River lower reach that were taken from Arnsberg et al. (Citation2007, Citation2010) and Arnsberg and Kellar (Citation2007a, Citation2007b, Citation2007c).
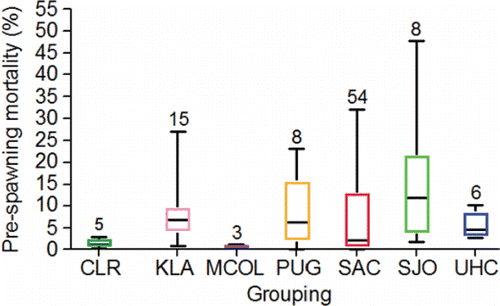
Within and across years, the majority of the PIT-tagged fish classified as unsuccessful spawners fell into the premature spawning mortality category (). To provide a temporal perspective on mortality, Eq. 8 in was used to simulate a spawning initiation date for each premature spawning mortality, acknowledging that those fish were assumed to have died without beginning redd construction. To simulate a daily mortality rate, the sum of the simulated pre-spawning and premature spawning mortalities on each spawning initiation date was divided by the total number of fish that were simulated to have initiated spawning on that date. The resulting simulated daily mortality rates declined throughout the spawning period () consistent with the pattern reported by Bowerman et al. (Citation2016).
Figure 29. The percentages of the PIT-tagged, hatchery-origin fall Chinook salmon adults that were classified as unsuccessful spawners and fell into the pre-spawning or premature spawning mortality categories, 2010–2015. The numbers above the bar are the sample sizes of fish classified as unsuccessful spawners.
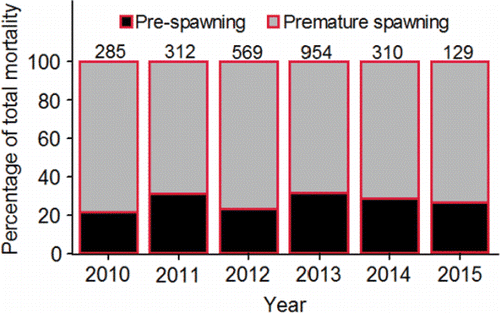
Figure 30. Simulated daily pre-spawning mortality rates (%) of PIT-tagged, hatchery-origin fall Chinook salmon adults along the Upper Hells Canyon spawning area, 2010–2015.
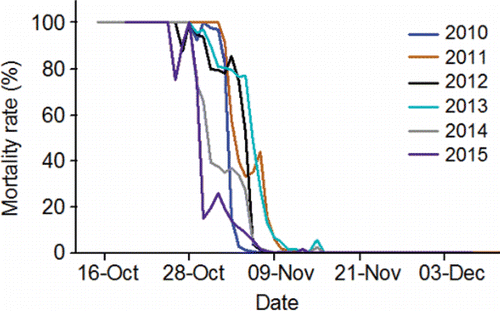
If the model simulations were realistic, then spawning date distributions estimated from observed redd count data would be similar to simulated spawning date distributions, and depict a temporal trend associated with temperature-related spawning success. The simulated spawning date distributions were constructed jointly from the simulated spawning dates of both premature spawning mortalities and successful spawners. The observed and simulated distributions were similar (). If the pre-spawning mortalities had survived to spawn, the simulations indicated that they would have been the earliest to construct redds, followed by the premature spawning mortalities, and then the successful spawners ().
Figure 31. Standardized, simulated spawning initiation date distributions for PIT-tagged, hatchery-origin fall Chinook salmon adults classified as premature spawning mortalities and successful spawners (combined) compared to the actual distributions observed during the four annual redd survey intervals along the Upper Hells Canyon spawning area, 2010–2015 (data collected by the authors and their staff; e.g., Groves et al., Citation2013).
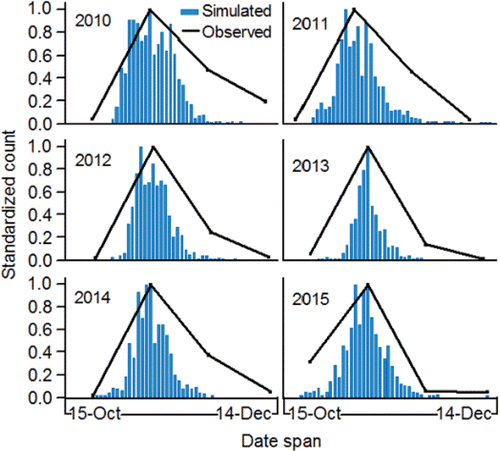
Figure 32. Standardized, simulated spawning initiation date distributions for PIT-tagged, hatchery-origin fall Chinook salmon adults that fell into the pre-spawning mortality category (had they survived to initiate spawning), into the category of premature spawning mortalities, or were classified as successful spawners, 2010–2015.
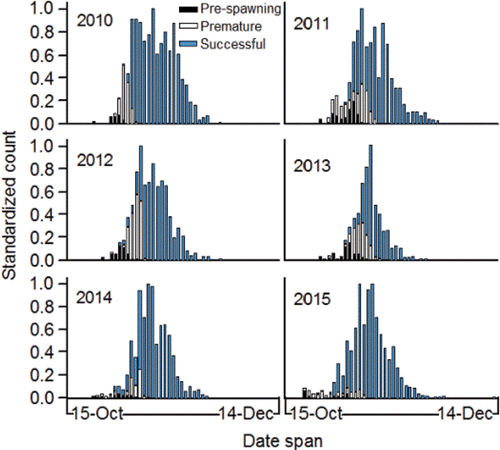
Successful spawners only use a fraction of their pre-spawning, somatic energy to spawn. For example, Jonsson et al. (Citation1991) measured somatic energy content immediately before and after spawning. On average females lost 25% of their pre-spawning, somatic energy reserves by the time they were spent. Thus, the simulated somatic energy loss calculated between spawning site selection and the completion of spawning for the PIT-tagged adults classified as successful spawners was expected to be well below 100% of the simulated somatic energy content at the time of spawning site selection. If mean simulated somatic energy loss between spawning initiation and completion approached 100%, evidence would be provided for the underestimation of somatic energy content, or the overestimation of somatic energy use (and vice versa). Inter-annual mean (± 95% C.L.) annual somatic energy loss (%) between the time of spawning site selection and the completion of spawning was 25.1 ± 0.1%.
Having provided the reader with some information to evaluate the realism of the simulations made by the somatic energy use model, the following simulations of spawning success are reported. It is important to note that these values are the annual means calculated from simulations for individual PIT-tagged fish. Those individual values were intended as a basis for evaluating behavior, velocity, and temperature as determinants of variation in spawning success. The means resulted from numerous estimation procedures, and they do not include measures of precision or uncertainty. Of the PIT-tagged adults that successfully migrated to Lower Granite Dam forebay (), the somatic energy use model classified 87.6% as successful spawners in 2010, 74.6% % in 2011, 73.7% in 2012, 66.6% in 2013, 87.6% in 2014, and 90.2% in 2015.
Comparing thermal exposure and energy content between successful and unsuccessful migrants
There was a difference in simulated cumulative temperature exposures, and useable energy expenditure (cumulative %) during Stage I modeling (i.e., Bonneville Dam tailrace to McNary Dam forebay) between fish of the two migration success classes during State II modeling (i.e., McNary Dam forebay to Lower Granite Dam forebay). The successful migrants to Lower Granite Dam forebay had lower mean cumulative temperature exposures, and expended less usable energy on average, when swimming from Bonneville Dam tailrace to McNary Dam forebay compared to unsuccessful migrants according to the model (). That difference was not influential in terms of migration success between Bonneville and McNary dams because simulated expended useable energy (cumulative %) was a relatively small fraction of the simulated somatic energy available to the fish at Bonneville Dam even in the case of unsuccessful migrants (range of annual means [±SE]; 10.7 ± 0.1% to 13.8 ± 0.1%).
Figure 33. Annual mean (95% C.I.) cumulative temperature exposures (panel A) and expended useable energy (cumulative %; panel B) simulated during Stage I modeling (i.e., Bonneville Dam tailrace to McNary Dam forebay) for PIT-tagged, hatchery-origin fall Chinook salmon adults that were either successful or unsuccessful migrants from McNary Dam forebay to Lower Granite Dam forebay, 2010–2015. Sample sizes are given in Table 4.
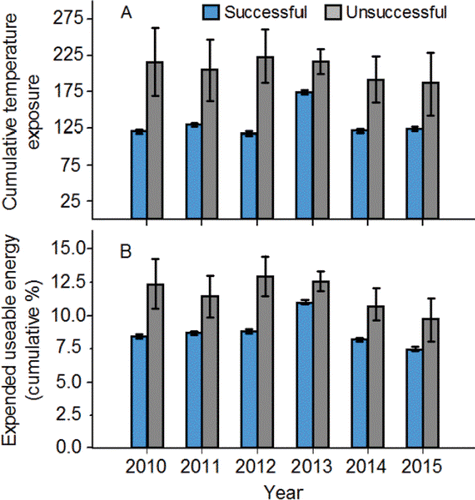
Comparing Lower Granite Dam passage dates, simulated cumulative temperature exposures, and simulated expended useable energy content (cumulative %) between successful and unsuccessful spawners
The PIT-tagged adults classified as successful spawners passed Lower Granite Dam later compared to fish classified as unsuccessful spawners during all 6 years modeled (; panel A). The annual mean (± 95% C.L.) simulated cumulative temperature exposures from Bonneville Dam tailrace to Lower Granite Dam forebay were consistently lower for fish classified as successful spawners compared to fish classified as unsuccessful spawners (; panel B). Fish classified as successful spawners also expended smaller fractions of their simulated useable energy content between Bonneville Dam tailrace and Lower Granite Dam forebay compared to fish classified as unsuccessful spawners (; panel C). The simulated amount of the useable energy expended (cumulative %) between Bonneville Dam tailrace to Lower Granite Dam forebay was far below the simulated energy thresholds for death due to exhaustion, even in the case of the fish classified as unsuccessful spawners (range of annual means [±SE]; 17.1 ± 0.1% to 25.4 ± 0.1%). Therefore, the majority of the useable energy content simulated at Bonneville Dam for the average fish remained available for making pre-spawning movements and spawning.
Figure 34. Annual mean (95% C.I) day of passage at Lower Granite Dam (panel a; numbers in bars = n), simulated cumulative temperature exposures between Bonneville and Lower Granite dams (panel b), and simulated expended useable energy (cumulative %; panel c) during Stages I and II for PIT-tagged, hatchery-origin fall Chinook salmon adults that were classified as either successful or unsuccessful spawners, 2010–2015.
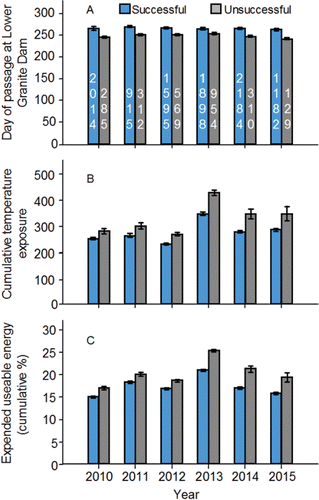
Depicting the role of cumulative temperature exposure on spawning success
The annual cumulative temperature exposure indices were lower within and across years for the PIT-tagged adults classified as successful spawners compared to the fish classified as unsuccessful spawners (). On average within each year, the simulations indicated that the fish accumulated more cumulative temperature units after passing Lower Granite Dam than they did downstream of the dam ().
Figure 35. Annual cumulative temperature exposure indices for the PIT-tagged, hatchery-origin fall Chinook salmon adults that successfully migrated to Lower Granite Dam forebay, and were subsequently classified as either successful (S) or unsuccessful (U) spawners during 2010–2015. Sample sizes are given in .
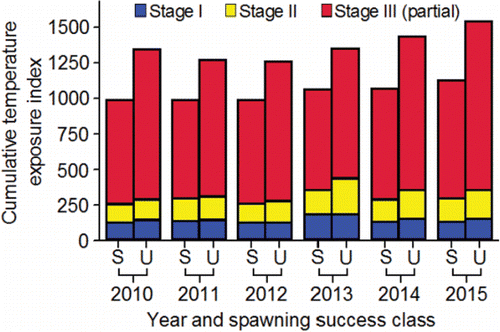
To evaluate the relative influence of cumulative temperature among the three modeling stages, logistic regression models were fitted for each of the six modeled years. The models predicted the probability of an individual PIT-tagged adult being classified as a successful spawner based on its simulated weight at Bonneville Dam, and the cumulative temperature exposures simulated for the PIT-tagged adults during the modeling stages I and II, and modeling stage III through spawning initiation.
The models with an intercept and covariates (i.e., the full models) provided more information on the probability of a fish being classified as a successful spawner compared to intercept-only models (i.e., the null models; ). Classification accuracy of the full models was above 90% for fish that were classified successful spawners, and 70% for fish that were classified as being unsuccessful spawners (). Each of the annual models predicted that the probability of being classified as a successful spawner decreased as cumulative temperature exposure increased (see the β values; ). Based on the odds ratios, cumulative temperature exposure upstream of Lower Granite Dam had the most relative influence on the probability of a fish being classified as a successful spawner ().
Table 6. Logistic regression diagnostics for models fitted to predict the probability of a PIT-tagged, hatchery-origin fall Chinook salmon adult being a successful spawner based on fish weight at Bonneville Dam, and cumulative temperature exposure during Stages I, II, and II. Abbreviations: CUMDEGR, cumulative temperature exposure; S, successful spawner; U, unsuccessful spawner.
Comparing simulated somatic energy use by successful spawners among tailrace-to-dam passage, reservoir passage, and pre-spawning movement events in kcal and kcal/km/d
In all modeled years, the simulated amount of energy expressed in kcal used by adults for swimming (i.e., without consideration for ovarian development) during pre-spawning movements and initiating spawning upstream of Lower Granite Dam far exceeded simulated energy use during swimming through the tail-race-to-dam and reservoir reaches downstream (; panel A).
Figure 36. The mean (95% C.I.) total amount of simulated somatic energy used for swimming during tailrace-to-dam passage, reservoir passage, and pre-spawning movement events by PIT-tagged, hatchery-origin fall Chinook salmon adults classified as successful spawners expressed in kcal (panel A) and kcal/km/d (panel B), 2010–2015. Sample sizes are given in .
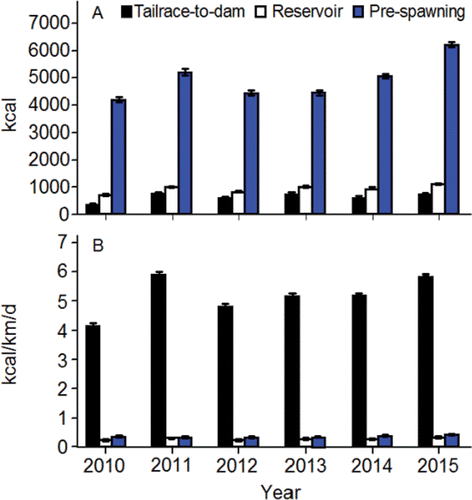
To express the simulated amount of energy used in kcal/km/d, it was necessary to know the net upstream distance travelled from Lower Granite Dam to spawning initiation. That distance (161.7 km) was calculated by subtracting the location of Lower Granite Dam (rkm 173.0) from the most consistently and heavily used spawning site along the Upper Hells Canyon spawning area (rkm 334.7). Irrespective of the short distances and times inherent to tailrace-to-dam passage (; and ), simulated somatic energy use expressed in kcal/km/d during tailrace-to-dam passage events, by far exceeded simulated energy use for reservoir passage and pre-spawning movement and spawning initiation (; panel B).
Figure 37. Median tailrace-to-dam passage time (d) of presumed upriver bright fall Chinook salmon adults at dams in the lower and middle stretches of the river system during 1998, 2000, and 2001. The inter-annual mean (±95%CI) are given above the bars. The data are from Keefer et al. (Citation2004). Abbreviations: Bonneville, BON; The Dalles, DAL; John Day, JD; McNary, MCN; Ice Harbor, ICH;, Lower Monumental, LMN; Little Goose, LGS; and Lower Granite, LGR.
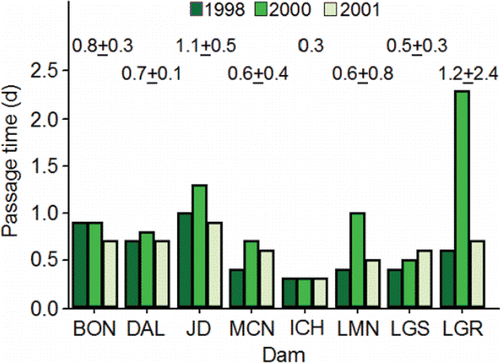
Illustrating the effects of temperature on embryo loss
As demonstrated earlier in this review, the effect of temperature on spawning success is not restricted to death of the adult. The cumulative exposure of the female parent to temperatures above 20°C (a.k.a., DD>20), and the exposure of the fertilized gametes to temperatures above 16.5°C (a.k.a., DD>16.5) might reduce egg to fry survival. The analyses presented in this section were conducted on the PIT-tagged adults that were classified as successful spawners in the previous section. This section accomplishes three objectives:
| (1) | explore the temporal and spatial characteristics of system-wide exposure to temperatures above 20°C; | ||||
| (2) | simulate embryo loss due to DD>20 with an emphasis on the temporal nature of the loss (see Appendix for methods); | ||||
| (3) | simulate embryo loss due to DD>16.5 with an emphasis on the temporal nature of the loss (see Appendix for methods). | ||||
Temporal and spatial characteristics of system-wide exposure to temperatures above 20°C
The PIT-tagged adults that passed Bonneville Dam early in the run schedule in a given simulation year generally accumulated more system-wide DD>20 units compared to fish that passed the dam late in the run schedule (). During four of the five annual simulations, the average PIT-tagged adult accumulated more DD>20 units in the Upper Hells Canyon spawning area than in any other stretch of river (). In the 2013 simulation, the average PIT-tagged adult accumulated more DD>20 units in the lower stretch of the river system than in any other stretch (). In all simulation years, the average PIT-tagged adult did not accumulate any DD>20 units while swimming through Lower Granite Reservoir ().
Figure 38. System-wide values of degree days above 20°C (DD>20) for PIT-tagged, hatchery-origin fall Chinook salmon adults that were classified as successful spawners by the somatic energy use model plotted against the observed dates of passage at Bonneville Dam of those adults, 2010‒2015.
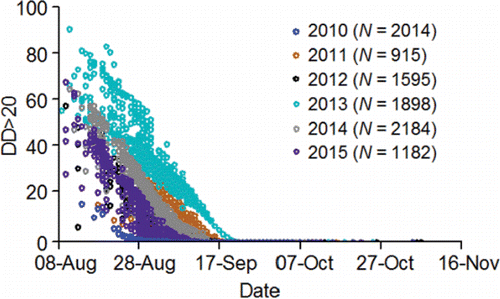
Figure 39. The annual mean (±95% C.L.) number of degree day units above 20°C (DD>20) accumulated by PIT-tagged, hatchery-origin fall Chinook salmon adults that were classified as successful spawners by the somatic energy use model as they swam through the lower, middle, and upper stretches of the river system, 2010‒2015. The upper stretch was divided into Lower Granite Reservoir, Lower Hells Canyon, and Upper Hells Canyon.
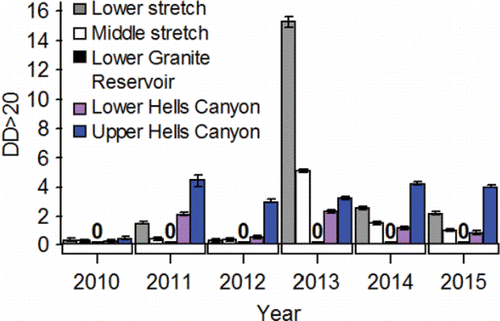
Simulating embryo loss resulting from DD>20
Simulated embryo loss averaged (± SE) 0.05 ± 0.003% in 2010, 1.76 ± 0.066% in 2011, 0.77 ± 0.033% in 2012, 5.63 ± 0.079% in 2013, 1.82 ± 0.055% in 2014, and 1.62 ± 0.074% in 2015. Simulated embryo loss was highest for the PIT-tagged adults with October simulated spawning initiation dates, declined for fish with simulated spawning initiation dates that fell within the first two weeks of November, and then stabilized around annual values ranging from 0% to ≈ 6.0% depending on year ().
Figure 40. Simulated embryo loss (%) due to exposure to degree days above 20°C for PIT-tagged, hatchery-origin fall Chinook salmon adults that were classified as successful spawners by the somatic energy use model plotted against the simulated spawning initiation dates of those adults along the Upper Hells Canyon spawning area, 2010‒2015.
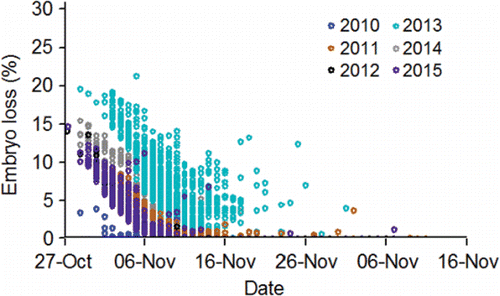
Simulating embryo loss resulting from DD>16.5
Mean (± SE) simulated embryo loss was 0 ± 0% each year from 2010 to 2014, and 2.5 ± 0.4% in 2015. Simulated embryo loss was low because the fish that would have begun to spawn at temperatures above 16.5°C had already been classified as premature and prespawning mortalities during somatic energy use modeling. In 2015, a warm low-flow year, mean simulated embryo loss was 45.8 ± 4.1% for the PIT-tagged adults with October simulated spawning initiation dates, declined to 0.4 ± 0.1% for fish with simulated spawning initiation dates in November, and then was 0 ± 0% thereafter.
Illustrating the effects of a future climate scenario on migration and spawning sucess
This section uses the somatic energy use model to accomplish two goals. First, the model is used to illustrate how changes in velocity and temperature under a future climate scenario might influence the migration success of the population of Snake River basin fall Chinook salmon. The second goal is to illustrate how changes in velocity and temperature may further affect spawning success measured through fry emergence of fall Chinook salmon that migrate back to the Upper Hells spawning area. As pointed out earlier in the review, the Upper Hells Canyon spawning area has the warmest prespawn and spawning thermal regime, and as such, it would be the most affected by future changes in precipitation and water temperature.
The objectives of this section are to:
| (1) | develop baseline and 2080 climate scenarios to provide approximated flows and temperatures for simulating migration and spawning success; | ||||
| (2) | use the 2010–2015 data collected on the PIT-tagged adults to create a data set with a representative age-class distribution for the simulations; | ||||
| (3) | simulate migration and spawning success through fry emergence under the two scenarios and | ||||
| (4) | simulate spawner demographics under the two scenarios including age and size class distributions, fecundity, migrant-to-fry productivity, and spawn timing. | ||||
The baseline and 2080 climate scenarios
The baseline scenario was created by averaging the mean daily flows and water temperatures across the years 1991‒2006 to capture changes in dam operations that were made in association with recovery efforts. The 2006 end point was selected to correspond with the last year in the 1915‒2006 study period assessed by the Climate Impacts Group (CIG; Hamlet et al., Citation2010). The second scenario used flow and water temperatures derived from model simulations to represent the climate in the Columbia River basin by the year 2080 (hereafter, the 2080 scenario). The 2080 scenario modeled assumed significant greenhouse gas mitigation in the early 21st century would begin to stabilize gas concentrations and warming by the end of the century. The methods used to create the 2080 scenario flows and temperatures are given in the Appendix.
Compared to the baseline scenario, daily mean flows in the 2080 scenario were higher from about November through mid-April, and lower from about mid-April through October (e.g., ), consistent in pattern with the output of most of the models reviewed by Bilby et al. (Citation2007). There was a 15.7% increase in annual flow, which falls just under the upper range of the modeling results reviewed by Bilby et al. (Citation2007).
Figure 41. Baseline (1991‒2006 average) and adjusted 2080 flows for Upper Hells Canyon from Hells Canyon Dam to the Imnaha River mouth. Refer to the Appendix to see how flows were simulated starting with information provided by Hamlet et al. (Citation2010). The figure style is also attributed to Hamlet et al. (Citation2010).
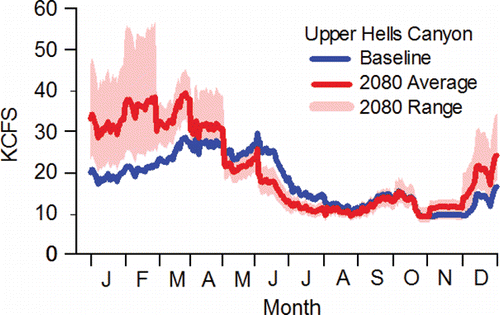
Much effort has been expended to shape the pattern of the hydrograph to protect fish within portions of the river system that are regulated by dams. It was assumed here that managers would shape the higher flow volumes observed under the 2080 scenario in the same manner they had shaped the baseline flow patterns (see Appendix). The adjusted 2080 flows used in the simulations to approximate flow shaping exhibited two general patterns that were largely affected by how much potential there was for flow shaping at a given location. At locations where flow volume was largely shaped by dam operations, the adjusted 2080 flows were higher than the baseline flows throughout the year (; panels A, B, and G). At locations where flow was largely affected by the inflow from large, unregulated tributaries located upstream, the pattern observed in persisted (; panels C, D, E, and F).
Figure 42. The baseline scenarios (1991‒2006 average), and 2080 average flow scenarios adjusted to match the pattern in the hydrograph in the baseline scenario, for Upper Hells Canyon from Hells Canyon Dam to the Imnaha River mouth (panel A), Upper Hells Canyon from the Imnaha River mouth to the Salmon River mouth (panel B), Lower Hells Canyon from the Salmon River mouth to Grande Ronde River mouth (panel C), Lower Hells Canyon from the Grande Ronde River mouth to the Clearwater River mouth (panel D), Lower Granite Dam (panel E), Ice Harbor Dam (panel F), and McNary Dam (panel G). Refer to the Appendix to see how flows were simulated starting with information provided by Hamlet et al. (Citation2010).
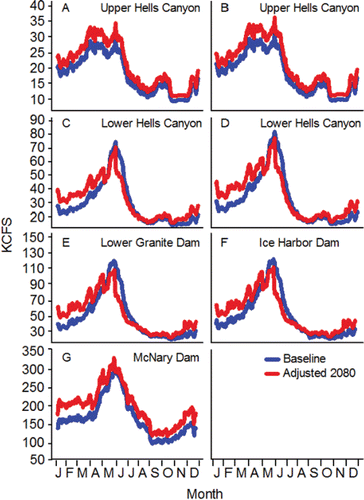
Comparisons of the baseline and adjusted 2080 temperatures at primary locations in the river system for which temperature data were needed for subsequent analyses are shown in . Approximated daily mean temperatures were higher than baseline daily mean temperatures at all locations every day of the year. The mean daily difference between baseline and approximated 2080 daily mean water temperatures was 2.0°C for the Snake River locations, and 1.9°C at McNary Dam. Concerning Dworshak Dam and Reservoir operations, it was assumed that the temperature control efforts would continue under the 2080 scenario, but under the operational restrictions observed during the baseline period (see Appendix for additional detail).
Figure 43. Baseline (1991‒2006 average) and adjusted 2080 daily mean temperatures for Upper Hells Canyon (panel A), Lower Hells Canyon (panel B), Lower Granite Dam (panel C), Ice Harbor Dam (panel D), and McNary Dam (panel E). Refer to the Appendix to see how temperatures were simulated starting with information provided by Hamlet et al. (Citation2010).
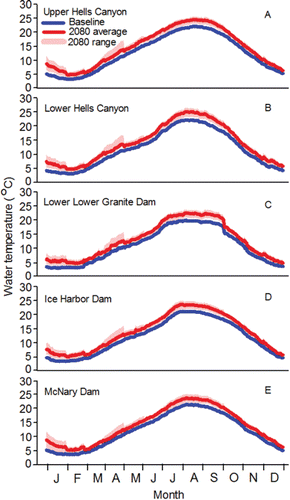
The PIT-tag data set
Expansion methods were applied to estimates of adult passage at Lower Granite Dam to estimate the age-class distribution of natural-origin females arriving at Lower Granite Dam during 2004–2015 (for methods Young et al. (Citation2012); data provided by B. Young). Overall, 23.7% of the natural-origin females were Age-II, 58.9% Age-III, 17.4% Age-IV and 0.2% Age-V. In this review, the PIT-tag data collected on the hatchery–origin adults that were successful migrants from Bonneville Dam to Lower Granite Dam (N = 12,347; ) were pooled across the period of years 2010‒2015, and fish from each age-class were randomly selected to create a data set (N = 1,997) composed of Age-II (23.5%), Age-III (59.1%), Age-IV (17.3%) and Age-V (0.1%) adults that were assigned the sex of female.
The 1,997 adults in the randomly drawn PIT-tag data set had been successful migrants from Bonneville Dam to Lower Granite Dam. The approach to making the data set did not account for fish that migrated successfully from Bonneville Dam to McNary Dam, but then failed to migrate to Lower Granite Dam (e.g., and ). To simulate migration and spawning success rates for comparison between PIT-tagged fish under the baseline and 2080 scenarios, the denominator used to calculate the rates had to include those unsuccessful migrants. The denominators were obtained by dividing 1,997 by the probability of a fish being a successful migrant taken from the equation in . Clarification follows.
The mean temperatures measured in the tailrace of Ice Harbor Dam from 12-Aug to 14-Sep under the baseline (20.5°C) and 2080 (23.0°C) were input into the regression equation in Figure 13 to predict (P.I.) migration success rates that were then expressed as proportions (baseline, 0.955 [0.899,1.000]; 2080, 0.846 [0.715, 0.976]). To produce starting numbers of fish at Bonneville Dam for the baseline and 2080 scenarios (i.e., the denominators of the success rates), 1997 was divided by the proportion reported above that corresponded to each scenario. The starting numbers were 2,091 and 2,316 for the baseline and 2080 scenarios, respectively.
Migration and spawning success through fry emergence under the two scenarios
Simulated migration success rate from Bonneville Dam tailrace to Lower Granite Dam forebay was 95.5% for fish under the baseline scenario and 84.6% for fish under the 2080 scenario (). The success rates simulated for the fish from Bonneville Dam tailrace to the completion of spawning were 84.3% and 60.1% under the baseline and 2080 scenarios, respectively ().
Figure 44. Simulated success rates (%) migration from Bonneville Dam to the Upper Hells Canyon spawning area, from Bonneville Dam to spawning completion, and from Bonneville Dam to fry emergence simulated for natural-origin fall Chinook salmon females under the baseline and 2080 scenarios, where N is the starting number of adults that arrived at Bonneville Dam and n is the number of adults that successfully completed each event given above the bars (except in the case of emergence; see text for description).
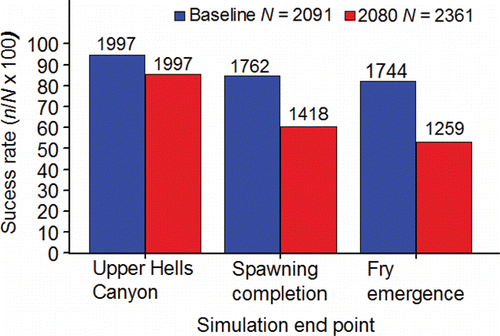
In the case of success rate from the completion of spawning to fry emergence, embryo survival was expressed in adult equivalents, where the adult equivalent for a given successful spawner was equal to 1 minus the sum of the simulated proportions of embryos lost due to the DD>20 and DD>16.5 exposures of that fish. The total number of adult equivalents produced by the successful spawners was 1,744 fish under the baseline scenario, and 1,259 fish under the 2080 scenario. As such, the success rate simulated from arrival in the tailrace of Bonneville Dam to fry emergence was 30.1 percentage points higher for fish under the baseline scenario (83.4%) compared to fish under the 2080 scenario (53.3%; ).
The numbers reported in can be used to calculate success rates separately for each of the three events just covered. Success rates calculated in that manner listed respectively for the baseline and 2080 scenarios were: (a) 95.5% and 84.6% from Bonneville Dam tailrace to Lower Granite Dam forebay, (b) 88.2% and 71.0% from Lower Granite Dam forebay to spawning completion, and (c) 99.0% and 88.8% from spawning completion to fry emergence (does not include mortality attributable to percent fines).
Changes in spawner demographics under the two scenarios
The adult equivalent value for each successful spawner was used to calculate proportions and means in this section of the review. For example, fecundity for a given successful spawner was simulated with Eq. 16 () and multiplied by the adult equivalent for that fish. The number of fry produced by that fish was then multiplied by 0.535 (i.e., the mean embryo survival rate for the Upper Hells Canyon spawning area from ) to account for embryo loss due to fines in the substrate.
Table A1. The regression equations used in the somatic energy model and other models developed in this review. Abbreviation: LGR, Lower Granite.
The simulated age class distributions, fork length distributions, and mean fecundities of the spawners under the two scenarios were similar, whereas there were differences between the scenarios in migrant-to-fry productivity and spawn timing. Simulated migrant-to-fry productivity was 2,513 under the baseline scenario and 1,808 under the 2080 scenario. The primary difference between the spawn timing distributions of fish between the two scenarios was the near absence of effective spawning during the third spawning interval (19-Oct‒01-Nov) under the 2080 scenario (), which in the case of the Hells Canyon spawning area, is presently the first interval when spawning is observed ().
Figure 45. Effective spawn timing distributions simulated for natural-origin fall Chinook salmon females along the Upper Hells Canyon spawning area under the baseline and 2080 scenarios, where N is the total number of fish classified as successful spawners expressed in adult equivalents (see text), and n is the total number of adult equivalents in each spawning interval given above the bars.
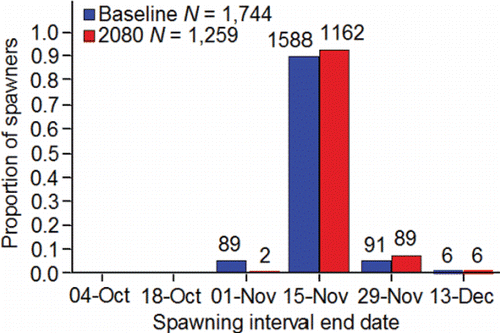
A conceptual model for research and management
Assumptions and limitations
The word “illustrate” was defined in the introduction of this review for good reason. Fish are difficult to study especially in large river systems because they live and move underwater out of sight of human beings almost all of the time. Consequently, the results of large river studies on fish are founded on data collected under extreme limitations that are then used in analyses that are always fraught with assumptions. Those limitations and assumptions need to be diligently considered and balanced against the biological realism of the results.
In terms of biology and hydrology, limitations associated with resources and available technology made it necessary to make numerous assumptions when analyzing and interpreting data in this review. The assumptions made in somatic energy use modeling serve as good examples for discussion. The accuracy and bias of the simulations of migration and spawning success cannot be assessed because there is no known baseline on Snake River basin fall Chinook salmon for comparison. In the case of hydrology, the temperature and velocity simulations were clearly gross oversimplifications of the temperatures and velocities observed along the river system studied. Details on the most influential biologic and hydrologic assumptions and limitations of the somatic energy use model are discussed hereafter.
It was necessary to extrapolate when using Eq. 1 () to simulate somatic energy use at temperatures above 20.0°C because handling the fish in the laboratory at temperatures above 20°C in the Geist et al. (Citation2000a) study would likely have killed the fish. It is possible, that the relation between somatic energy use and temperature becomes nonlinear at warmer temperatures meaning the fish either start using energy at a faster rate or the rate of energy use tapers off at some temperature threshold. The model simulated energy use based on mean tailrace temperatures. The actual temperatures encountered by the fish were undoubtedly more complex compared to those used to simulate somatic energy use, but again, the radiotelemetry study by Mann (Citation2007) reviewed earlier provided support for the approach used here. It was assumed that the fish selected velocities equivalent to mean velocities in the tailrace areas and mean cross-sectional velocities in the reservoirs and riverine habitat. It is known that migrating adults orient themselves along the shorelines (Bjornn and Peery Citation1992), and it is probable that fish do not consistently select velocities that are equivalent to the mean. Anyone who has ever watched a salmon knows that the fish sometimes orient themselves in current to maximize movement efficiency, while minimizing energy use. That innate characteristic could not be captured in the model without adopting some arbitrary correction factor. Furthermore, the prespawning movement templates used to develop Eqs. 12A–12F were largely based on the observed movements of hatchery-origin fish, and the limited evidence available indicates that hatchery-origin fish swim farther and longer after reaching the spawning grounds compared to natural-origin fish (Connor and Garcia Citation2006).
Concerning the hydrological aspects of the model, it was assumed that the mean tailrace temperatures at McNary and Ice Harbor dams provided an adequate representation of the temperatures in reservoirs, ladders, and tailraces. For a time at Lower Granite Dam, Caudill et al. (Citation2013) showed that that assumption was not met with regard to temperature. Velocity models fitted to data collected in John Day and Lower Granite reservoirs were used to predict velocities in the other Columbia and Lower Snake River reservoirs in the lower and middle stretches of the river system, respectively. As clearly indicated in , the equations used to simulate tailrace velocities at the eight dams were visually approximated from figures in Rakowski et al. (Citation2010). It was also necessary to assume that velocities in the ladders were similar to the visually approximated, mean velocities in the tailrace area, even though it is known that ladders are designed to shelter fish from high velocities and facilitate upstream movement and elevation gain.
As with the somatic energy use model, there were limitations and assumptions associated with the embryo loss simulations. In particular, DD>20 of the females that were radio tagged by Mann (Citation2007) explained only 31% of the variation in embryo loss measured at hatcheries for those fish. It was also assumed that embryo loss was linear over the range of DD>20 values measured at the system-wide level. Embryo loss due to the thermal history of the parent could increase in a non-linear fashion in association with the rigors of swimming upstream and spawning the riverine environment upstream of Lower Granite Reservoir. Nevertheless, the embryo loss simulations were useful for illustrating how thermal exposure of the female parent and initial incubation temperature might influence spawning success measured in terms of the production of offspring.
Lastly, it is acknowledged that measures of uncertainty were not reported for the simulations. This review was not written to provide exact predictions of future events. Rather than undertaking the statistical gyrations that are part of many modeling endeavors, it is simply stated here that any honest estimate of uncertainty would be large especially if random process was considered. The standard caveat also applies: research, monitoring, and evaluation should be undertaken to evaluate the veracity of the illustration developed in this review. In practice, such research will be difficult to conduct until technology advances.
Acknowledging the above limitations and assumptions, the assessments of model performance reported earlier supported the use of the simulation results for accomplishing the stated goal of this review. That is, to illustrate how fish behavior, temperature, and velocity under a future climate might influence the migration and spawning success of inland populations of Chinook salmon that inhabit highly developed river systems.
The illustration
This subsection summarizes what the review illustrated in the form of a conceptual model. The model is intended to challenge researchers while providing managers with one alternative future to consider. It is organized around the following topics: (1) migration timing; (2) factors affecting migration and spawning success; (3) the final fate of unsuccessful migrants; (4) the final fate of unsuccessful spawners; (5) the future of water temperature management; and (6) the influence of future climate patterns on abundance, diversity, and spatial distribution of inland populations of Chinook salmon in warm, highly developed river systems.
The conceptual model
Inland fall Chinook salmon from southerly latitudes of the northern hemisphere begin to enter freshwater during the warmest time of the year. Through the process of natural selection, inland anadromous fishes evolved to have an inherently strong migratory disposition. It is quite possible that some inland populations of Chinook salmon do not greatly alter their time of freshwater entry in response to environmental conditions because of the spatial and temporal constraints imposed on them in terms of migration distance and maturation schedule. Such rigidity in migration timing makes it probable that a portion of the population will always be exposed to temperatures that are warm enough to reduce migration and spawning success.
Within the constraints of available energy, the migration and spawning success of inland populations of Chinook salmon in highly developed river systems are jointly determined by inherent migratory disposition, temperature, and velocity. Seasonally warm, sub-lethal temperatures and localized temperature gradients can elicit short-term disruptions in upstream movement by some of the fish that actively avoid warm water, and then incidentally encounter or actually seek thermal refuge. Extreme temperature gradients can temporarily halt movement altogether as has been shown in fish ladders. Despite such temperature-related disruptions in movement, temperature accounts for little variation in the upstream movement rate of individual fish measured over the entire length of the migration corridor. Migratory disposition overrides exposure to sub-lethal temperatures. Concerning velocity, impounding rivers exposes fish to lower velocities compared to those of riverine habitat. The velocities in the reservoirs are less energetically demanding compared to the historical riverine velocities, thus in that respect the reservoirs formed by the dams have made it is easier for the fish to migrate. Tailrace-to-dam passage, on the other hand, presents the fish with an energetic challenge that is analogous to ascending rapids and water falls that were engineered to reduce energy expenditure. Although the technology for facilitating passage at dams has evolved, tailrace-to-dam passage remains an energetically demanding process that influences migration success.
Under contemporary temperature conditions, a relatively small percentage of the fish stray after migrating to their natal river mouth. Unless the fish had been previously injured, there is little reason to believe that large numbers of the strays die from exhaustion before spawning. Most spawn “out-of-basin.” Straying and spawning in the Hanford Reach by fish from the Snake River basin fall Chinook salmon Evolutionarily Significant Unit serve as an example of out-of-basin spawning. The level of out-of-basin spawning increases as the temperature at the mouth of the natal river basin increases. Straying and out-of-basin spawning represents a modest loss in production to the natal or “source” population under present climate conditions.
Death due to exhaustion after natal river entry, but before the completion of spawning, is the final fate of a moderate proportion of inland Chinook salmon, especially those fish destined for the warmest spawning areas. Death due to exhaustion is a cumulative, temporal, and spatial process. Comparing simulated somatic energy use expressed as kcal/km/d illustrated the importance of providing the fish with the least stressful, and most efficient, tailrace and ladder passage conditions at dams along the migration corridor when the relatively short distances and times that are inherent to tailrace-to-dam passage are considered. Comparing simulated somatic energy use during tailrace-to-dam passage, reservoir passage, and pre-spawning movement and spawning events in kcal illustrated the importance of the environment in the upper stretch of river systems to migration and spawning success.
With regard to managing water temperature in the future, any increase in air temperature would make it more difficult to keep water temperatures from exceeding existing management standards. To the extent that the heat-retention properties of some reservoirs contribute to warming, reducing reservoir volumes during certain times might contribute to cooling. For the most part, if it is only possible to release warm water from a particular reservoir, few management options are available short of dam removal or breeching. The fate of dams and reservoirs that warm downstream temperatures will depend on the availability of new energy sources, alternative means for transporting goods and facilitating commerce, the decision process with respect to how to remedy the aging infrastructure of many dams, and perhaps most importantly, whether or not there are changes in societal norms.
Some large storage reservoirs that hold relatively large volumes of cold water might continue to be important to temperature management, or be newly identified for future use in system-wide cooling efforts. Some cool-water reservoirs might have a diminished potential for temperature management as a consequence of past development. Two reservoirs within the river system studied in this review exemplify the preceding statements. Dworshak Reservoir has been managed to maintain maximum temperatures along the middle stretch of the river system close to the 20oC standard for over two decades. Brownlee Reservoir () water is released along with Dworshak Reservoir water to enhance flow conditions in the middle stretch of the river system. Cool water is seasonally available for release from Brownlee Reservoir, but altering current operations to release that cold water would be problematic. It is anoxic, laden with toxins, and carries a heavy nutrient and organic loading (Myers et al., Citation2003; Fosness et al., Citation2013). The reality is that even if the number of reservoirs operated for temperature management at the system-wide level could be increased, the efficacy of the system-wide effort would be largely dependent on future climate patterns.
This review illustrated how the velocity and temperature conditions under an optimistic 2080 climate scenario could contribute to further reductions in the abundance, spatial distribution, and diversity of inland populations of Chinook salmon. Those reductions would come about as follows. The proportion of the fish that seek and use thermal refuge would increase as temperature increased. Eventually the availability of thermal refuge would become a limiting factor to production as the demand for cold water needed for management exceeded the supply. The elevation in temperature would increase out-of-basin straying, increase the amount of energy that the salmon use to migrate and spawn, and ultimately increase pre-spawning and premature spawning mortality in the earliest migrating fish that homed successfully. The warmer temperatures would kill the embryos of earliest surviving spawners either indirectly through the thermal exposure of the female parent, or directly from exposure to lethal temperatures. Embryo loss due to fines in the substrate would continue to be high regardless of time of spawning. There would be a spatial redistribution of spawning among spawning areas with a shift to the coolest of rivers. In the river system studied, a shift might be observed from the Upper Hells Canyon spawning area to Clearwater River lower reach. Such a spatial redistribution would not offset the numerical loss in the source population as a whole because a moderate portion of the loss will come from out-of-basin straying. The end result being that it would become more difficult for fish management actions alone to recover conservation-reliant, inland populations of Chinook salmon.
Closing remarks and segue
This review is the first of a trilogy of reviews written to provide a comprehensive history of Snake River basin fall Chinook salmon to inform future research and management efforts. Completing it provided a background illustration of adult behavior and survival to form a foundation for the second and third reviews. The second review will provide a background illustration on the topics of juvenile life history, behavior, and survival. The final review will chronicle the recorded history of Snake River fall Chinook salmon from the 1880s to present. It will detail the changes in abundance, spatial distribution, and management of the population from its pristine state, through a period of decline, and lastly to its current conservation-reliant status. When completed, the trilogy will provide insight to the following questions. Does fish management repeat itself in the same manner as other aspects of human history—and if so—to what end?
Acknowlegments
The authors express our sincere respect and gratitude to everyone that was involved with this review. David Geist, Matt Keefer, and Tracy Bowerman provided data from their published works. In particular, the effort and quality of research conducted by staff of the University of Idaho and Pacific Northwest National Laboratory cannot be over admired. Countless professionals contributed to the studies reviewed. The editorial staff of the journal and peer review improved the manuscript prior to publication. The editor of the journal unfailingly provided the first author with words of encouragement. The Bonneville Power Administration project 199102900 and Idaho Power Company provided funding. Special thanks to Debbie Docherty for administering the BPA contract for over two decades. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the Idaho Power Company, Nez Perce Tribe Department of Fisheries Resources Management, U. S. Fish and Wildlife, U. S. Geological Survey, or the U.S. Government. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the Idaho Power Company, Nez Perce Tribe Department of Fisheries Resources Management, or U. S. Fish and Wildlife, but do represent the views of the U.S. Geological Survey.
Additional information
Funding
References
- Arnsberg, B. D., and D. S. Kellar. Nez Perce tribal hatchery monitoring and evaluation of fall Chinook Salmon supplementation in the Clearwater River Subbasin. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project 1983-350-003 2004 Annual Report to the Bonneville Power Administration, Portland, OR. Available from http://www.nptfisheries.org/PublicationLibrary.aspx (2007a).
- Arnsberg, B. D., and D. S. Kellar. Nez Perce tribal hatchery monitoring and evaluation of fall Chinook Salmon supplementation in the Clearwater River Subbasin. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project 1983-350-003 2005 Annual Report to the Bonneville Power Administration, Portland, OR. Available from http://www.nptfisheries.org/PublicationLibrary.aspx (2007b).
- Arnsberg, B. D., and D. S. Kellar. Nez Perce tribal hatchery monitoring and evaluation of fall Chinook Salmon supplementation in the Clearwater River Subbasin. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project 1983-350-003 2006 Annual Report to the Bonneville Power Administration, Portland, OR. Available from http://www.nptfisheries.org/PublicationLibrary.aspx (2007c).
- Arnsberg, B. D., D. S. Kellar, and M. A. Tuell. Nez Perce tribal hatchery monitoring and evaluation of fall Chinook Salmon supplementation in the Clearwater River Subbasin. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project 1983-350-003 2003 Annual Report to the Bonneville Power Administration, Portland, OR. Available from https://www.cbfish.org/Document.mvc/Viewer/00004414-5 (2007).
- Arnsberg, B. D., D. S. Kellar, and M. A. Tuell. Nez Perce tribal hatchery monitoring and evaluation of fall Chinook Salmon supplementation in the Clearwater River Subbasin. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project 1983-350-003 2007 Annual Report to the Bonneville Power Administration, Portland, OR. Available from https://www.cbfish.org/Document.mvc/Viewer/P118425 (2010).
- Arnsberg, B. D., W. P. Connor, and E. Connor. Mainstem Clearwater River study: assessment for salmonid spawning, incubation, and rearing. Nez Perce Tribe Department of Fisheries Resources Management, Research Division. Project number 88-15 Final report to the Bonneville Power Administration, Portland, OR. Available from https://www.cbfish.org/Document.mvc/Viewer/37474-3 (1992).
- Bennett, D. H., W. P. Connor, and C. A. Eaton. Substrate composition and emergence success of fall Chinook salmon in the Snake River. Northwest Sci., 77: 93–99 (2003).
- Bilby, R., S. Hanna, M. Healey, N. Huntley, S. Hurlbert, R. Lamberson, C. Levings, D. Montgomery, W. Pearcy, T. P. Poe, P. Smouse, N. Mantua, and E. Merill. Climate change impacts on Columbia River basin fish and wildlife. ISAB 2007-2. Available from https://www.nwcouncil.org/media/31247/isab2007_2.pdf (2007).
- Bjornn, T.C., and C.A. Peery. A review of literature related to movements of adult salmon and steelhead passed dams and through reservoirs in the lower Snake River. Technical Report 92-1 by the U.S. Fish and Wildlife Service, Idaho Cooperative Fish and Wildlife Research Unit to the U.S. Army Corps of Engineers, Walla Walla District. Available from http://www.dtic.mil/dtic/tr/fulltext/u2/a323311.pdf (1992).
- Boggs, C. T., M. L. Keefer, C. A. Peery, T. C. Bjornn, and L. C. Stuehrenberg. Fallback, reascension, and adjusted fishway escapement estimates for adult Chinook salmon and steelhead at Columbia and Snake River dams. Trans. Am. Fish. Soc., 133: 932‒949 (2004). doi:10.1577/T03-133.1.
- Bond, M. H., P. A. H. Westley, A. H. Dittman, D. Holecek, T. Marsh, and T. P. Quinn. Combined effects of barge transportation, river environment, and rearing location on straying and migration of adult Snake River fall-run Chinook salmon. Trans. Am. Fish. Soc., 146: 60–73 (2016). doi:10.1080/00028487.2016.1235614.
- Borden, C. and P. Manning. Hells Canyon MIKE 11 Model 2011 Model Calibration Update Final Report. Prepared for the Idaho Power Company by the Danish Hydraulic Institute. Portland, OR. (2011).
- Bowerman, T. E., A. Pinson-Dumm, C. A. Peery, and C. C. Caudill. Reproductive energy expenditure and changes in body morphology for a population of Chinook salmon Oncorhynchus tshawytscha with a long-distance migration. J. Fish Biol., doi:10.1111/jfb.13274 (2017).
- Bowerman, T. E., M. L. Keefer, and C. C. Caudill. Pacific salmon prespawn mortality: Patterns, methods, and study design considerations. Fisheries, 41: 739–749 (2016). doi:10.1080/03632415.2016.1245993.
- Brafield, A. E., and D. J. Solomon. Oxy-calorific coefficients for animals respiring nitrogenous substrates. Comp. Biochem. Physiol., 837–841 (1972). doi:10.1016/0300-9629(72)90155-7.
- Brett, J. R. Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J. Fish. Res. Board Can., 9: 265–323 (1952). doi:10.1139/f52-016.
- Brett, J. R., W. C. Clarke, and J. E. Shelbourn. Experiments on thermal requirements for growth and food conversion efficiency of juvenile Chinook salmon Oncorhynchus tshawytscha. Can. Tech. Rep. Fish. Aquatic Sci., 1127: 1–35 (1982).
- Bugert, R. M., C. W. Hopley, C. A. Busack, and G. W. Mendel. Maintenance of stock integrity in Snake River fall Chinook salmon. Am. Fish. Soc. Symp., 15: 267–276 (1995).
- Burner, C. J. Characteristics of spawning nests of Columbia River salmon. U.S. Fish and Wildlife Service Fishery Bulletin 52: 96‒110 (1951).
- Calvin, L. Estimating night fish passing over Bonneville, The Dalles, and John Day dams. Portland, OR: U.S. Army Corps of Engineers (1975).
- Caudill, C. C., M. L. Keefer, T. S. Clabough, G. P. Naughton, B. J. Burke, and C. A. Peery. Indirect Effects of Impoundment on Migrating Fish: Temperature gradients in fish ladders slow dam passage by adult Chinook salmon and Steelhead. PLoS One, 8(12): e85586. doi:10.1371/journal.pone.0085586 (2013).
- Caudill, C. C., W. R. Daigle, M. L. Keefer, C. T. Boggs, M. A. Jepson, B. J. Burke, R. W. Zabel, T. C. Bjornn, and C. A. Peery. Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: delayed negative effects of passage obstacles or condition-dependent mortality. Can. J. Fish. Aquatic Sci., 64: 979‒995 (2007). doi:10.1139/f07-065.
- CBR (Columbia Basin Research). Columbia River DART (data access in real time). Seattle: CBR, University of Washington. Available from www.cbr.washington.edu/dart/dart.html (2017).
- Chapman, D. W. Salmon and steelhead abundance in the Columbia River in the nineteeth century. Trans. Am. Fish. Soc., 115: 662‒670 (1986). doi:10.1577/1548-8659(1986)115%3c662:SASAIT%3e2.0.CO;2.
- Chopin, F. S., and T. Arimoto. The condition of fish escaping from fishing gears—a review. Fish. Res., 21: 315–327 (1995). doi:10.1016/0165-7836(94)00301-C.
- Connor, W. P., A. P. Garcia, A. H. Connor, E. O. Garton, P. A. Groves, and J. A. Chandler. Estimating the carrying capacity of the Snake River for fall Chinook salmon redds. Northwest Sci., 75: 363–370 (2001).
- Connor, W. P., and A. P. Garcia. Pre-spawning movement of wild and hatchery fall Chinook salmon in the Snake River. Trans. Am. Fish. Soc., 135: 131–139 (2006). doi:10.1577/T05-097.1.
- Connor, W. P., B. D. Arnsberg, J. A. Chandler, T. D. Cooney, P. A. Groves, J. A. Hesse, G. W. Mendel, D. J. Milks, D. W. Rondorf, S. J. Rosenberger, M. L. Schuck, K. F. Tiffan, R. S. Waples, and W. Young. A Retrospective (circa 1800–2015) on abundance, spatial distribution, and management of Snake River Basin fall Chinook salmon. Draft 2 Parts I, II, and III. Available from http://www.streamnetlibrary.org/?page_id=1357 (2016).
- Connor, W. P., C. E. Piston, and A. P. Garcia. Temperature during incubation as one factor affecting the distribution of Snake River fall Chinook salmon spawning areas. Trans. Am. Fish. Soc., 132: 1236–1243 (2003). doi:10.1577/T02-159.
- Connor, W. P., H. L. Burge, R. Waitt, and T. C. Bjornn. Juvenile life history of wild fall Chinook salmon in the Snake and Clearwater rivers. North Am. J. Fish., 22: 703–712 (2002). doi:10.1577/1548-8675(2002)022%3c0703:JLHOWF%3e2.0.CO;2.
- Connor, W. P., J. G. Sneva, K. F. Tiffan, R. K. Steinhorst, and D. Ross. Two alternative juvenile life histories for fall Chinook salmon in the Snake River basin. Trans. Am. Fish., 134: 291–304 (2005). doi:10.1577/T03-131.1.
- Cook, C. B., G. A. McMichael, J. A. Vucelick, B. Dibrani, E. E. Hockersmith, C. A. Duberstein, I. D. Welch, B. J. Bellgraph, C. A. McKinstry, P. S. Titzler, D. A. Ogden, B. P. Sandford, R. K. Kirkham, and M. D. Bleich. Lower Monumental Reservoir Juvenile Fall Chinook Salmon Behavior Studies, PNWD-3800. Richland, WA: Battelle – Pacific Northwest Division. Available from https://waterpower.pnnl.gov/jsats/pdf/LoMo_FinalReport_2006.pdf (2007).
- Cook, C. B., M. C. Richmond, S. P. Titzler, B. Dibrani, M. D. Bleich, and T. Fu. Hydraulic characteristics of the lower Snake River during periods of juvenile fall Chinook salmon migration. Contract DE-AC05-76RL01830 Final Report to the Bonneville Power Administration, Portland, OR. Available from https://www.cbfish.org/Document.mvc/Viewer/00000652-29 (2006).
- Cooney, T. D. Estimation of Snake River fall Chinook salmon returns to Ice Harbor, LF Hatchery, and over Lower Granite. Olympia WA: Washington Department of Fisheries Resource Planning and Research Division, memorandum May 7, 1991 (1991).
- Dauble, D. D., and R. P. Mueller. Upstream passage monitoring: Difficulties in estimating survival for adult Chinook salmon in the Columbia and Snake rivers. Fisheries 25(8): 24–34 (2000). doi:10.1577/1548-8446(2000)025%3c0024:UPMDIE%3e2.0.CO;2.
- Dauble, D. D., T. P. Hanrahan, D. R. Geist, and M. J. Parsley. Impacts of the Columbia River hydroelectric system on main-stem habitats of fall Chinook salmon. North Am. J. Fish. Manage., 23: 641–59 (2003). doi:10.1577/M02-013.
- Duncan, R. N., D. P. Arndt, J. R. Kuskie Jr., and G. A. Johnson. The Dalles Dam powerhouse fish collection system studies, 1974‒75. Portland, OR: U.S. Army Corps of Engineers (1978).
- Everest, F. H., C. E. McLemore, and J. F. Ward. An improved cryogenic gravel sampler. Portland, OR: U. S. Forest Service research note PNW-350, Pacific Northwest Forest and Range Experiment Station (1980).
- Ford, M. J., K. Barnas, T. Cooney, L. G. Crozier, M. Diaz, J. J. Hard, E. E. Holmes, D. M. Holzer, R. G. Kope, P. W. Lawson, M. Liermann, J. M. Myers, M. Rowse, D. J. Teel, D. M. Van Doornik, T. C. Wainwright, L. A. Weitkamp, and M. Williams. Status Review Update for Pacific Salmon and Steelhead Listed under the Endangered Species Act: Pacific Northwest. National Marine Fisheries Service, Northwest Fisheries Science Center. Available from https://www.nwfsc.noaa.gov/publications/scipubs/display_doctrack_allinfo.cfm?doctrackmetadataid=8623 (2015)
- Fosness, R. L., J. Naymik, C. B. Hopkins, and J. F. DeWild. Water column and bed-sediment core samples collected from Brownlee Reservoir near Oxbow, Oregon, 2012. U.S. Geological Survey Data Series 809. Available from https://pubs.er.usgs.gov/publication/ds809 (2013).
- Fryer, J. K. Frequency of pinniped-caused scars and wounds on adult spring-summer Chinook and Sockeye salmon returning to the Columbia River. North Am. J. Fish. Manage., 18: 46–51 (1998). doi:10.1577/1548-8675(1998)018%3c0046:FOPCSA%3e2.0.CO;2.
- Gale, W. F., J. D. Thompson, and J. Douglas. A suction sampler for quantitatively sampling benthos on rocky substrates on rivers. Trans. Am. Fish. Soc., 104: 398–405 (1975). doi:10.1577/1548-8659(1975)104%3c398:ASSFQS%3e2.0.CO;2.
- Geist, D. R. Hyporheic discharge of river water into fall Chinook salmon spawning areas in the Hanford Reach, Columbia River. Can. J. Fish. Aquat. Sci., 57: 1647–1656 (2000). doi:10.1139/f00-102.
- Geist, D. R., and D. D. Dauble. Redd site selection and spawning habitat use by fall Chinook salmon: the importance of geomorphic features in large river. Environ. Manage., 22(5): 655–669 (1998). doi:10.1007/s002679900137.
- Geist, D. R., C. S. Abernathy, K. D. Hand, V. I. Cullinan, J. A. Chandler, and P. A. Groves. Survival, development, and growth of fall Chinook salmon embryos, alevins, and fry exposed to variable thermal and dissolved oxygen regimes. Trans. Am. Fish. Soc., 135: 1462–1477 (2006). doi:10.1577/T05-294.1.
- Geist, D. R., C. S. Abernethy, S. L. Blanton, and V. I. Cullinan. The use of electromyogram telemetry to estimate energy expenditure of adult fall Chinook salmon. Trans. Am. Fish. Soc., 129: 126–135 (2000a). doi:10.1577/1548-8659(2000)129%3c0126:TUOETT%3e2.0.CO;2.
- Geist, D. R., J. Jones, C. J. Murray, and D. D. Dauble. Suitability criteria analyzed at the spatial scale of redd clusters improved estimates of fall Chinook salmon (Oncorhynchus tshawytscha) spawning habitat use in the Hanford Reach, Columbia River. Can. J. Fish. Aquat. Sci., 57: 1636–1646 (2000b). doi:10.1139/f00-101.
- Geist, D. R., T. P. Hanrahan, E. V. Arntzen, G. A. McMichael, C. J. Murray, and Yi-Ju Chien. Physiochemical characteristics of the hyporheic zone affect redd site selection by Chum and fall Chinook salmon in the Columbia River. North Am. J. Fish. Manage., 22: 1077–1085 (2002). doi:10.1577/1548-8675(2002)022%3c1077:PCOTHZ%3e2.0.CO;2.
- Geist, D. R., Z. Deng, R.P. Mueller, S.R. Brink, and J.A. Chandler. Survival and growth of juvenile Snake River fall Chinook salmon exposed to constant and fluctuating temperatures. Trans. Am. Fish. Soc., 139: 92–107 (2010). doi:10.1577/T09-003.1.
- Goniea, T. M., M. L. Keefer, T. C. Bjornn, C. A. Peery, D. H. Bennett, and L. C. Stuehrenberg. Behavioral thermoregulation and slowed migration by adult fall Chinook salmon in response to high Columbia River water temperatures. Trans. Am. Fish. Soc., 135: 408–419 (2006). doi:10.1577/T04-113.1.
- Groves, P. A., and J. A. Chandler. Spawning habitat used by fall Chinook salmon in the Snake River. North Am. J. Fish. Manage., 19: 912–922 (1999). doi:10.1577/1548-8675(1999)019%3c0912:SHUBFC%3e2.0.CO;2.
- Groves, P. A., B. Alcorn, B., M. M. Wiest, J. M. Maselko, and W. P. Connor. Testing unmanned aircraft systems (UASs) for salmon spawning surveys. FACETS. doi:10.1139/facets-2016-0019 (2016).
- Groves, P. A., J. A. Chandler, B. Alcorn, T. J. Richter, W. P. Connor, A. P. Garcia, and S. M. Bradbury. Evaluating salmon spawning habitat capacity using redd survey data. North Am. J. Fish. Manage., 33: 707–716 (2013). doi:10.1080/02755947.2013.793628.
- Hamlet, A. F., P. Carrasco, J. Deems, M. M. Elsner, T. Kamstra, C. Lee, S. Lee, G. S. Maugher, E. P. Salathe, I. Tohver, and L. W. Binder. Final report for the Columbia basin climate change scenarios project. http://www.hydro.washington.edu/2860/ (2010).
- Harnish, R. A., R. Sharma, G. A. McMichael, R. B. Langshaw, and T. N. Pearsons. Effect of hydroelectric dam operations on the freshwater productivity of a Columbia River fall Chinook salmon population. Can. J. Fish. Aquatic Sci., 71: 602–615 (2014). doi:10.1139/cjfas-2013-0276.
- Healey, M. C. Life history of chinook salmon (Oncorhynchus tshawytscha), pp. 312–393. In: Pacific salmon life histories. (Groot, C., and L. Margolis, Eds.) Vancouver, BC: UBC press (1991).
- Huntington, C., W. Nehlsen, and J. Bowers. A survey of healthy native stocks of anadromous salmonids in the Pacific Northwest and California. Fisheries, 21: 6–14 (1996). doi:10.1577/1548-8446(1996)021%3c0006:ASOHNS%3e2.0.CO;2.
- Idaho Administrative Procedures Act. Idaho Water Quality Standards. IDAPA 58.01.02, Available from https://adminrules.idaho.gov/rules/current/58/0102.pdf (2014).
- IPC (Idaho Power Company). Fall chinook interim recovery plan and study. Boise, ID: Idaho Power Company (1990).
- Johnson, G. A., J. R. Kuskie Jr., W.T. Nagy, K. L. Liscom, and L. Stuehrenberg. The John Day Dam powerhouse adult fish collection system evaluations, 1979–1980. Portland, OR: U.S. Army Corps of Engineers, Portland District (1982).
- Jonsson, N., B. Jonsson, and L. P. Hansen. Energetic cost of spawning in male and female Atlantic salmon (Salmo salar L.). J. Fish Biol., 39: 739–744 (1991). doi:10.1111/j.1095-8649.1991.tb04403.x.
- Keefer, M. L., and C. C. Caudill. Estimating thermal exposure of adult summer Steelhead and fall Chinook salmon migrating in a warm impounded river. Ecol. Freshwater Fish, doi:10.1111/eff.12238 (2015).
- Keefer, M. L., C. A. Peery, T. C. Bjornn, M. A. Jepson, and L. C. Stuehrenberg. Hydrosystem, dam, and reservoir passage rates of adult Chinook salmon and Steelhead in the Columbia and Snake Rivers. Trans. Am. Fish. Soc., 133: 1413–1439 (2004). doi:10.1577/T03-223.1.
- Liscom, K. L., and L. C. Stuehrenberg. Radio tracking studies of ‘upriver bright’ fall Chinook salmon between Bonneville and McNary dams, 1982. Seattle, WA: National Marine Fisheries Service, Northwest and Alaska Fisherie Center (1983).
- Mann, R. D. The effects of high temperature exposures on migration success and embryo quality of Snake River adult Chinook salmon and Steelhead. Master's Thesis. Moscow: University of Idaho (2007).
- Mann, R. D., C. A. Peery, A. M. Pinson, and C. R. Anderson. Energy use, migration times, and spawning success of adult spring-summer Chinook salmon returning to spawning areas in the South Fork Salmon River in central Idaho: 2002‒2007. Technical report 2009-4. Moscow: Idaho Cooperative Fish and Wildlife Research Unit, University of Idaho (2009).
- Marine, K. R., and J. J. Cech. Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook salmon. North Am. J. Fish. Manage., 24: 198–201 (2004). doi:10.1577/M02-142.
- McMichael, G. A., C. L. Rakowski, B. B. James, and J. A. Lukas. Estimated fall Chinook salmon survival to emergence in dewatered redds in a shallow side channel of the Columbia River. North Am. J. Fish. Manage., 25: 876–884 (2005). doi:10.1577/M04-168.1.
- Mendel, G., and D. Milks. Upstream passage and spawning of fall Chinook salmon in the Snake River. Olympia: Washington Department of Fish and Wildlife. Contract report #DOE/BP 60415-2 submitted to Bonneville Power Administration, Portland, OR (1997).
- Mendel, G., D. Milks, and R. Bugert. Upstream passage and spawning of fall Chinook salmon in the Snake River 1992. Olympia: Washington Department of Fisheries. Contract report DE-BI 79–92 BP60415 to Bonneville Power Administration, Portland, OR (1993).
- Mendel, G., D. Milks, R. Bugert, and K. Petersen. Upstream passage and spawning of fall chinook salmon in the Snake River 1991. Olympia: Washington Department of Fisheries. Submitted to USFWS, LSRCP Office. Report # AFF1/LSR/92-11 (1992).
- Monan, G. E., and K. L. Liscom. Radio tracking studies of fall Chinook salmon to determine effect of peaking on the passage at Bonneville Dam, 1973. Seattle, WA: National Marine Fisheries Service, Northwest Fisheries Center (1974).
- Myers, J. M., R. G. Kope, G. J. Bryant, D. Teel, L. J. Lierheimer, T. C. Wainwright, W. S. Grant, F. W. Waknitz, K. Neely, S. T. Lindley, and R. S. Waples. Status review of Chinook salmon from Washington, Idaho, Oregon, and California. U.S. Department of Commerce, NOAA Technincal Memo NMFS-NWFSC-35. Available from https://www.nwfsc.noaa.gov/assets/25/7190_07042012_124647_Myers.et.al.1998-rev.pdf (1998).
- Myers, R., J. Harrison, S. K. Parkinson, B. Hoelscher, J. Naymik, and S. E. Parkinson. Pollutant transport and processing in the Hells Canyon Complex. Boise, ID: Idaho Power Company. Technical Report E.2.2-2 (2003).
- Nehlsen, W., J. E. Williams, and J. A. Lichatowich. Pacific salmon at the crossroads: stocks at risk from California, Oregon, Idaho, and Washington. Fisheries, 16: 4–21 (1991). doi:10.1577/1548-8446(1991)016%3c0004:PSATCS%3e2.0.CO;2.
- NMFS (National Marine Fisheries Service). Proposed recovery plan for Snake River salmon. U. S. Department of Commerce National Oceanic and Atmospheric Administration (1995).
- NMFS (National Marine Fisheries Service). Threatened status for Snake River spring/summer chinook salmon, threatened status for Snake River fall chinook salmon. Fed. Regist., 57: 78 (22 April 1992):14,653–14,663 (1992).
- NOAA (National Oceanographic and Atmospheric Administration). List of Endangered and Threatened Marine Species under NMFS' Jurisdiction. Available from http://www.nmfs.noaa.gov/pr/species/esa/listed.htm#fish (2017).
- NOAA (National Oceanographic and Atmospheric Administration). Snake River harvest module. Portland, OR: National Marine Fisheries Service West Coast Region. Available from http://www.westcoast.fisheries.noaa.gov/publications/recovery_planning/salmon_steelhead/domains/interior_columbia/snake/harvest_module_062514.pdf (2014).
- ODFW (Oregon Department of Fish and Wildlife). Oregon native fish status report. Volume II: Assessment methods and population results. Salem, OR. Available from http://www.dfw.state.or.us/fish/ONFSR/docs/final/02-fall-chinook/fc-methods-mid-col.pdf (2005).
- Oregon Administrative Rule. Oregon Water Quality Standards. OAR 340–041. Available from https://www.epa.gov/sites/production/files/2014–12/documents/orwqs-chapter340.pdf (2011).
- Oregon Fish Commission. An investigation of the effect of The Dalles Dam upon migration rates of adult salmonids, 1956 and 1957. Portland: Oregon Fish Commission, Fisheries Engineering Research Project (1960).
- Perry, R. W., J. M. Plumb, and C. W. Huntington. Using a laboratory-based growth model to estimate mass- and temperature-dependent growth parameters across populations of juvenile Chinook salmon. Trans. Am. Fish. Soc., 144: 331–336 (2015). doi:10.1080/00028487.2014.996667.
- Prentice, E. F., T. A. Flagg, and C. S. McCutcheon. Feasibility of using passive integrated transponder (PIT) tags in salmonids. Am. Fish. Soc. Symp., 7: 317–322 (1990a).
- Prentice, E. F., T. A. Flagg, C. S. McCutcheon, and D. F. Brastow. PIT-tag monitoring systems for hydroelectric dams and fish hatcheries. Am. Fish. Soc. Symp., 7: 323–334 (1990b).
- Prentice, E. F., T. A. Flagg, C. S. McCutcheon, D. F. Brastow, and D. C. Cross. Equipment, methods, and an automated data-entry station for PIT tagging. Am. Fish. Soc. Symp., 7: 335–340 (1990c).
- PSMFC (Pacific States Marine Fisheries Commission). Administration and system operation of the Columbia basin PIT tag information system. Project 1990-080-00 2011 and 2012 annual reports to the Bonneville Power Administration, Portland, OR. Available from http://www.ptagis.org/docs/default-source/ptagis-program-documents/2011-2012-annual-report-project-1990-080-00.pdf?sfvrsn=4 (2013).
- PTAGIS (PIT Tag Information System). Available from http://www.ptagis.org (2017).
- Quinn, T. P., and S. Bloomberg. Fecundity of Chinook salmon (Oncorhynchus tshawytscha) from the Waitaki and Rakaia Rivers, New Zealand. New Zealand J. Mar. Freshwater Res., 26: 429–434 (1992). doi:10.1080/00288330.1992.9516536.
- Rakowski, C. L., M. C. Richmond, J. A. Serkowski, and W. A. Perkins. Determining Columbia and Snake River project tailrace and forebay zones of hydraulic influence using MASS2 modeling. Richland, WA: PNNL-20030, Pacific Northwest National Laboratory. Available from https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-20030.pdf (2010).
- Raleigh, R. F., W. J. Miller, and P. C. Nelson. Habitat suitability index models and instream flow suitability curves: Chinook salmon. Instream flow and aquatic systems group. Biological report 82: 10.122 (1986).
- Reed, F. C. Annual reports of the Department of Fisheries of the State of Oregon to the legislative assembly, twenty–first regular session. Salem Oregon. W. H. Leeds State printer (1901).
- Richter, A., and S. A. Kolmes. Maximum temperature limits for Chinook, Coho, and Chum salmon, and Steelhead trout in the Pacific Northwest. Rev. Fisheries Sci., 13: 23–49 (2005). doi:10.1080/10641260590885861.
- Ross, C. V. Evaluation of adult fish passage at Bonneville Dam, 1982. Portland, OR: U.S. Army Corps of Engineers (1983).
- Schoning, R. W. Snake River Fall Report. Portland, OR: Oregon Fish Commission (1947).
- Scott, W. B. and E. J. Crossman. Freshwater fishes of Canada. Bulletin 184. Ottawa: Fisheries Research Board of Canada (1973).
- Shew, D. M., R. D. Peters, R. J. Stansell, and L. M. Beck. Evaluation of adult fish passage at McNary Dam and John Day Dam, 1985. Cascade Locks, OR: U.S. Army Corps of Engineers, Portland, District Fisheries Office Bonneville Lock and Dam (1985).
- Stuehrenberg, L. C., K. L. Liscom, and G. E. Monan. A study of apparent losses of Chinook salmon and steelhead based on count discrepancies between dams on the Columbia and Snake rivers, 1967‒1968. U.S. Army Corps of Engineers, Final Report, Portland, OR (1978).
- TAC (U.S. v. Oregon Technical Advisory Committee). 2008 Biological assessment of incidental impacts on salmon species listed under the Endangered Species Act in the 2008–2017 non-Indian and treaty Indian fisheries in the Columbia River basin, 4/21/2008 (2008).
- Tenney, J., D. Warf, D. Marvin, D. Clough, A. Brower, D. Chase, and J. Nighbor. Administration and system operation of the Columbia basin PIT tag information system. 2010 annual report to the Bonneville Power Administration Project 1990-080-00. Available from http://www.ptagis.org/docs/default-source/ptagis-program-documents/2010-annual-report-project-1990-080-00.pdf?sfvrsn=10 (2011).
- Tenney, J., D. Warf, and N. Tancreto. Columbia Bsin PIT tag information system Project 1990-080-00 2014 annual report to the Bonneville Power Administration. Available from http://www.ptagis.org/docs/default-source/ptagis-program-documents/project_1990-080-001_annualreport_2014.pdf?sfvrsn=4 (2015).
- Tenney, J., D. Warf, and N. Tancreto. Columbia Bsin PIT tag information system Project 1990-080-00 2013 annual report to the Bonneville Power Administration. Available from http://www.ptagis.org/docs/default-source/ptagis-program-documents/2013-annual-report-project-1990-080-00.pdf?sfvrsn=4 (2014).
- Tiffan, K. F., R. D. Garland, and D. W. Rondorf. Predicted changes in subyearling fall Chinook salmon rearing and migratory habitat under two drawdown scenarios for John Day Reservoir, Columbia River. North Am. J. Fish. Manage., 26: 894–907 (2006). doi:10.1577/M06-018.1.
- Tiffan, K. F., R. W. Perry, W. P. Connor, F. L. Mullins, C. D. Rabe, and D. D. Nelson. Survival, growth, and tag retention in age-0 Chinook salmon implanted with 8-, 9-, and 12-mm PIT tags. North Am. J. Fish. Manage., 35: 845–852 (2015). doi:10.1080/02755947.2015.1052163.
- Tiffan, K. F., T. J. Kock, C. A. Haskell, W. P. Connor, and R. K. Steinhorst. Water velocity, turbulence, and migration rate of subyearling fall Chinook salmon in the free-flowing and impounded Snake River. Trans. Am. Fish. Soc., 138: 373–384 (2009). doi:10.1577/T08-051.1.
- Trefethen, P. S., and D. F. Sutherland. Passage of adult Chinook salmon through Brownlee Reservoir, 1960–62. U.S. Fish Wildlife Serv. Fishery Bull., 67: 35–45 (1968).
- Turner, A. R., Jr., D. M. Shew, L. M. Beck, R. J. Stansell, and R. D. Peters. Evaluation of adult fish passage at Bonneville Lock and Dam in 1983. Portland, OR: U.S. Army Corps of Engineers (1984).
- USACE (U. S. Army Corps of Engineers). Water control manual for Dworshak Dam and Reservoir: North Fork, Clearwater River, Idaho. Walla Walla District, WA (1986).
- USCFF (United States Commission of Fish and Fisheries). Part XX Report of the commissioner for the year ending June 30, 1894. 54th Congress 1st session of the House of Representatives. Document No. 424. Washington D.C. Government Printing Office (1894).
- Wagner, P., and T. Hillson. 1991 Evaluation of adult fallback through the McNary Dam juvenile bypass system. Final report of the Washington Department of Fisheries to the U. S. Army Corps of Engineers (1993).
- Washington Administrative Code. Water quality standards for surface waters of the state of Washington. Chapter 173–201A WAC. Olympia, Washington. Available from https://www.epa.gov/sites/production/files/2014–12/documents/wawqs.pdf (2011).
- Yanke, J. A. Effects of passive integrated transponder (PIT) tags and elevated water temperatures on survival, growth, and physiology of Snake River fall Chinook salmon (Oncorhynchus tshawytscha). Master's thesis. Moscow: University of Idaho (2006).
- Young, F. R., R. T. Michimoto, G. G. Gibson, R. T. Westfall, H. E. Jensen, and D. W. Nichols. An investigation of the effects of peaking upon adult salmon in the Columbia River. Fish Commission of Oregon, Annual Progress Report, Portland (1974).
- Young, W. P., S. Rosenberger and D. Milks. Snake River Fall Chinook Salmon Run Reconstruction at Lower Granite Dam; Methods for Retrospective Analysis. Nez Perce Tribe, Department of Fisheries Resources Management. Available from http://www.nptfisheries.org/PublicationLibrary.aspx (2012).
Appendix:
Detailed methods not provided in main body of text
Somatic energy use stage I: Bonneville Dam tailrace to McNary Dam forebay
In Stage I of the model, somatic energy use was simulated for the PIT-tagged adults that successfully swam from Bonneville Dam tailrace to McNary Dam forebay during 2010–2015 (). The key equation in the model (Eq. 1; ) was fitted from raw data provided by the corresponding author of Geist et al. (Citation2000a) to simulate oxygen consumption (mg/kg/h) that was in turn converted to somatic energy use. The input data for the individual fish for Eq. 1 included their simulated weight (kg) at Bonneville Dam, swimming speed (cm/s) from Bonneville Dam tailrace to McNary Dam forebay, and the mean water temperature between passage at Bonneville Dam tailrace and McNary Dam forebay. Equation 2 () was used to predict the weight of each fish at Bonneville Dam. The water temperature for a given fish used for input to Eq. 1 was the mean temperature measured in the tailrace of McNary Dam between the detection dates of that fish at Bonneville and McNary dams (CBR, Citation2017).
Swimming speed was simulated separately for tailrace-to-dam and reservoir passage events as fish speed (cm/s) plus water velocity (cm/s). Keefer et al. (Citation2004) found that an average of 38% of the total time radio-tagged adults spent traveling between the Bonneville Dam and McNary Dam ladder exits was spent passing from the downstream end of the tailraces, to the ladder exits of The Dalles, John Day, and McNary dams. The total travel time expressed in seconds measured on each PIT-tagged adult between dates of detection in the Bonneville and McNary dams ladders was multiplied by 0.38 to simulate a tailrace-to-dam passage travel time, which was then increased by 72,000 seconds to account the time spent passing from the tailrace of Bonneville Dam to the Bonneville Dam ladder exit (after Keefer et al., Citation2004). Fish speed during tailrace to dam passage was then calculated as the sum of the total tailrace and ladder distances at Bonneville, John Day, The Dalles, and McNary dams (; but expressed in cm) divided by the tailrace-to-dam travel time of each fish. Equation 3 () was used to simulate the tailrace-to-dam passage velocities from the mean flow measured in the tailrace of McNary Dam between the detection dates of a given fish at Bonneville and McNary dams (e.g., ).
The remaining 62% of the travel time measured between the detections dates of a PIT-tagged adult at Bonneville and McNary dams represented the reservoir travel time for that fish (after Keefer et al., Citation2004). Fish speed during reservoir passage for a given fish was calculated as the combined lengths of Bonneville, The Dalles, and John Day reservoirs (; but expressed in cm) divided by the reservoir travel time of that fish. Eq. 4 () was used to simulate the reservoir passage velocities from the mean flow measured in the tailrace of McNary Dam between the detection dates of a given fish at Bonneville and McNary dams (e.g., ).
The weights, swimming speeds, and water temperatures were then input to Eq. 1 to simulate oxygen consumption for each PIT-tagged adult from Bonneville Dam tailrace to McNary Dam forebay separately for the tailrace-to-dam and reservoir passage events. Simulated oxygen consumption for the two events was summed and converted to energy use in kcal/kg/d and kcal (after Geist et al. Citation2000a; see footnotes 1 and 2; ).
To place each PIT-tagged adult into a migration success class it was necessary to simulate somatic energy content at Bonneville Dam and a somatic energy threshold for death. Somatic energy content at Bonneville Dam was simulated for each fish (Eqs. 5 and 6; ). Then the somatic energy threshold for death was simulated for each fish (Eq. 7; ). Subtracting simulated somatic energy threshold for death from simulated somatic energy content at Bonneville Dam provided a measure of the energy available for successful migration, spawning site selection, and spawning for each fish and is referred to as “useable somatic energy.”
It was also necessary to account for the somatic energy used for gonadal development. Bowerman et al. (Citation2017) found that summer Chinook salmon females destined for a spawning area located 920 km upstream of Bonneville Dam used 14% of their somatic energy content measured at Bonneville Dam for ovarian development. Summer Chinook salmon migrate longer distances and spawn later after freshwater entry compared to fall Chinook salmon, thus it was assumed that summer Chinook salmon have less developed ovaries at the time of passage at Bonneville Dam. As such, fall Chinook salmon likely would use less than 14% of their somatic energy content simulated at the time of passage at the dam for ovarian development. The percentage of the somatic energy content at the dam that was eventually used by each PIT-tagged adult was simulated as follows.
Bowerman et al. (Citation2017) found that the average summer Chinook salmon had a travel time of 37 d from Bonneville Dam to the spawning grounds. The value of 0.14 (i.e., 14.0%) was divided by 37 d to determine the proportion of somatic energy used per day (i.e., 0.003784) for ovarian development. The mean number of days it took the radio-tagged fish in the Connor and Garcia (Citation2006) study to reach the lower end of the spawning grounds after passing Lower Granite Dam (4.2 d) was added to the annual mean travel times of the PIT-tagged adults that successfully migrated from Bonneville Dam to Lower Granite Dam. That calculation provided an estimate of the annual mean travel times to the spawning grounds for the PIT-tagged adults (range, 17.6 d to 22.0 d). Multiplying each of those annual means by 0.003784 provided annual estimates of the percentage of the somatic energy content simulated at Bonneville Dam that was used for ovarian development as the PIT-tagged adults swam upstream from Bonneville Dam to the spawning grounds (range, 6.6% to 8.3%).
The percentage of the simulated somatic energy content used for ovarian development for a given PIT-tagged adult was then multiplied by the somatic energy content that was simulated at Bonneville Dam for that fish. The resulting value for the simulated total amount of energy used for ovarian development was apportioned among modeling stages based on the detection history of the fish. For fish that were detected at Bonneville, McNary, and Lower Granite dams, travel time to spawning initiation was simulated by use of Eq. 8 (). Total travel time from Bonneville Dam to spawning initiation was calculated for each fish as the sum of the observed travel times from Bonneville Dam to McNary Dam, McNary Dam to Lower Granite Dam, and the simulated travel time from Lower Granite Dam to spawning site selection. Then for each fish, the proportion of that total travel time spent during modeling Stages I, II, and III (up to the point of spawning site selection) was determined. Those proportions were used multiplicatively to apportion simulated somatic energy used for ovarian development among the three modeling stages. In the cases of the fish that were detected at Bonneville and McNary dams, but not at Lower Granite Dam, the annual mean percentages of the simulated somatic energy used by the fish that were detected at all three dams were used to apportion the energy use for Stage I modeling.
The total amount of energy used by each PIT-tagged adult as it swam from Bonneville to McNary Dam was equal to the sum of the simulated amounts of somatic energy that were used for ovarian development and for swimming (i.e., from Eq. 1; ). That total was then subtracted from the simulated amount of useable somatic energy at Bonneville Dam to calculate somatic energy content at McNary Dam. If the somatic useable energy simulated for a fish at Bonneville Dam was not exceeded upon arrival in McNary Dam forebay, the fish was classified as a successful migrant that survived during Stage I of the model, otherwise it was classified as an unsuccessful migrant that died due to exhaustion.
Somatic energy use stage II: McNary Dam forebay to Lower Granite Dam forebay
In Stage II of the somatic energy use model (), the calculations described in the first stage were repeated with data collected on each PIT-tagged adult that successfully passed from McNary Dam forebay to Lower Granite Dam forebay. The first step was to calculate the weight of every fish that successfully migrated to the forebay of McNary Dam by subtracting ≈ 0.5 kg (0.002279 kg/km; Mann et al., Citation2009) from their simulated weight at Bonneville Dam.
To simulate somatic energy use during Stage II, average percentages of the travel time spent in tailrace-to-dam (39%) and reservoir reaches (61%) were used to apportion the observed travel times of the PIT-tagged fish between Ice Harbor Dam tailrace and Lower Granite Dam forebay (from Keefer et al., Citation2004). The total tailrace, ladder, and reservoir distances are given in of the main body of the review. The daily mean flows and temperatures were measured in the tailrace of Ice Harbor Dam (CBR, Citation2017). Snake River-specific regression equations were fitted to simulate tailrace and reservoir velocities (Eqs. 9 and 10A–10O; ). The sum of simulated somatic energy use values from Stage I and II modeling for a given fish was then subtracted from the simulated amount of useable somatic energy for that fish at Bonneville Dam to classify the fish as a successful or unsuccessful migrant to Lower Granite Dam forebay.
Somatic energy use stage III: Spawning initiation and the completion of spawning
In Stage III of the model, somatic energy use from Lower Granite Dam forebay through the completion of spawning was simulated for the PIT-tagged adults that successfully migrated from McNary Dam forebay to Lower Granite Dam forebay (). The temporal and spatial movements of every PIT-tagged, successful migrant that passed Lower Granite Dam during 2010–2015 were not monitored. To fill that gap, the data collected by Connor and Garcia (Citation2006) were used to fit annual regression equations for simulating energy use of each PIT-tagged adult from Lower Granite Dam forebay to spawning initiation (hereafter, pre-spawning energy use). In brief, the energy expenditure of each of the 36 fish studied by Connor and Garcia (Citation2006) was determined from estimates of swimming speeds, water velocities, and temperatures that would have been experienced under year-specific flows and temperatures during 2010–2015. These are referred to annual pre-spawning movement templates, which were then used to fit regression equations to predict pre-spawning energy use of PIT-tagged adults based on their date of passage and estimated weight at Lower Granite Dam. This assumes that the pre-spawning movement patterns and energy expenditure of the Connor and Garcia (Citation2006) fish are representative and applicable to the PIT-tagged adults. Details follow.
The first step in the development of the pre-spawning energy use regression equations was to create annual (i.e., 2010–2015) pre-spawning movement templates representing the velocity conditions a fish would experience within those years for each of the 36 fish that were radio-tagged and tracked by Connor and Garcia (Citation2006; e.g., for 2010, ). The template approach assumed that the 36 fish would have the same movement behavior under those years, as they did during the years they were tagged and tracked by Connor and Garcia (Citation2006). Annual distance-weighted water velocities and temperatures between tracking events were entered into the templates. Velocities for Lower Granite Reservoir were simulated with Eqs. 10A–10O fitted for 16 velocity transects (), for the zone where the reservoir transitions into riverine habitat with Eqs. 11A‒11F fitted for 5 velocity transects (), for the lower reaches of the Clearwater, Grande Ronde, and Salmon rivers with Eq. 11A (), and for the stretch of the lower Snake River extending from the upper end of the transition zone to Hells Canyon Dam with a 1-D model (Borden and Manning, Citation2011) that simulated mean cross-sectional velocities at 1,124 transects.
Table A2. A movement template for a hatchery-origin female adult that had been PIT-tagged and released upstream of Lower Granite Dam as a subyearling in 1999, radio tagged at Lower Granite Dam (rkm 173) as a 68-cm FL (measured), 3.3 kg (predicted1) adult female in 2001, tracked to the lower end of the spawning grounds 0.4 d after being released at the dam, and then tracked to a spawning site (rkm 379.2) along the Upper Hells Canyon spawning area where it was assumed to have initiated redd construction. Distance and time weighted velocities and temperatures for 2010 were entered into the template. Abbreviation: DOY.h/24, day of year.hour divided by 24.
The radio-tagged fish used to create the templates often moved downstream after making upstream movements (e.g., 9-Oct to 11-Oct; ). In such cases swimming speed was set at fish speed. The radio-tagged fish also commonly held at a tracking location (e.g., 05-Oct to 09-Oct; ). The velocities at the holding location were sometimes high (e.g., > 3 m/s) because the last velocity transect of the 1-D model that the fish passed prior to holding was located across or near a rapid. When a fish held position, a velocity of 80 cm/s was assigned to the movement template (e.g., 09-Oct; ) after Raleigh et al. (Citation1986). The information in each pre-spawning movement template along with the simulated weight at Lower Granite Dam passage (see footnote 1 in ) of each radio-tagged fish was used to predict O2 consumption (Eq. 1; Table A1), and energy use (e.g., ).
Once the 36 pre-spawning movement templates were created for each of the 6 years, annual pre-spawning energy use regression models were fitted from day of passage and simulated weight of the radio-tagged fish at Lower Granite Dam (Eqs. 12A–12F, ). Those predictor variables were selected a priori because they were obtainable for the PIT-tagged adults at Lower Granite Dam. The weight of a given PIT-tagged adult at Lower Granite Dam for input was calculated by subtracting ≈ 0.5 kg (0.002279 kg/km) from the weight of that fish at McNary Dam. Fish were classified as unsuccessful spawners if the simulations indicated that they expended all of their useable somatic energy while making pre-spawning movements. Those unsuccessful spawners are referred to here as “pre-spawning mortalities,” as it was assumed that they did not survive to select a spawning site, begin redd construction, or expel any eggs.
To simulate the date of spawning initiation of each PIT-tagged adult that was not classified as a pre-spawning mortality, the travel time that was previously simulated to apportion the somatic energy used for ovarian development (i.e., Eq. 8; ) was added to the day of passage at Lower Granite Dam. The next two steps in Stage III of the model were simulating somatic energy use during the completion of redd construction and spawning for a given fish, and then adding that amount of energy to the simulated amount of pre-spawning energy used by that fish. Jonsson et al. (Citation1991) measured energy use during spawning of Atlantic salmon (Salmo salar) and reported the average (±SD) female used 299 ± 58 kcal/kg of somatic energy to spawn. To simulate the weight of each fish at the time of spawning site selection, the energy use rate of 0.002279 kg/km was multiplied by 332 km (the average distance the radio-tagged fish swam) and the product (≈ 0.8 kg) was subtracted from the simulated weight at Lower Granite Dam of each fish. That weight was then multiplied by 299 kcal to calculate energy use from spawning initiation to the completion of spawning.
To classify a particular fish that was simulated to have initiated spawning as a successful or unsuccessful spawner, the sum of the simulated somatic energy use values from Stages I, II, and III of the model was subtracted from simulated useable somatic energy content at Bonneville Dam. If the difference was positive the fish was then classified as a successful spawner, otherwise it was classified as an unsuccessful spawner.
It was assumed that successful spawners had expelled all of their eggs (i.e., were 100% spent), completed redd construction, and had the remaining energy needed to guard their redds. At this point in the simulations, it was also assumed that the fish classified as unsuccessful spawners had initiated redd construction, but expelled only a portion of their eggs before dying. Unsuccessful spawners that fell into that category are referred to here as “premature spawning mortalities.”
Depicting the role of cumulative temperature exposure on spawning success
To depict the role of cumulative temperature exposure, as the PIT-tagged adults swam from the lower to upper stretch of the river system, on spawning success classification, it was necessary to simulate cumulative temperature exposure of the individual fish between passage at Lower Granite Dam to spawning initiation or death. The data in the pre-spawning movement templates were used to fit regression Eqs. 13A–13F (). Under the 2010–2015 temperature regimes, simulated cumulative temperature exposure upstream of Lower Granite Dam decreased as day of passage at the dam became later (see main body).
Inputting the passage day of year observed for each PIT-tagged adult that successfully migrated to Lower Granite Dam into Eqs. 13A–13F provided annual, fish-specific simulations of cumulative temperature exposure between passage at Lower Granite Dam and spawning initiation or death. The annual averages calculated from those individual simulations were added to the annual averages calculated for the PIT-tagged adults between Bonneville and McNary dams, and McNary and Lower Granite dams, to calculate the total of the mean temperature exposures (hereafter, annual cumulative temperature exposure indices).
DD>20 simulations
One limitation of the Mann (Citation2007) analysis was that it was spatially restricted. No information was available on dam passage timing or thermal exposure of the radio-tagged females downstream of Ice Harbor Dam. Additionally, the radio-tagged females were maintained in artificially cool (≈ 12.0°C) well water at the hatchery, and were never exposed to the ambient temperatures within the warmer spawning areas upstream of Lower Granite Reservoir. These methods were developed to build on the Mann (Citation2007) analysis and illustrate how system-wide temperature exposure from Bonneville Dam to the Upper Hells Canyon spawning area measured in terms of DD>20 might influence spawning success as measured by embryo survival. It was necessary to (1) increase the spatial representation of the regression equation developed by Mann (Citation2007), and (2) explore the temporal and spatial characteristics of system-wide exposure to temperatures above 20°C and the potential effect on embryo mortality.
To extend the spatial representation of the Mann (Citation2007) analysis, a regression model was fitted to predict day of passage at Bonneville Dam from day of passage at Ice Harbor Dam (Eq. 14; ). Eq. 14 was then used to predict day of passage at Bonneville Dam for each of the radio-tagged females Mann (Citation2007) used in his embryo loss analysis (). Next, temperature data collected in the tailrace of McNary Dam were used to calculate DD>20 for each radio-tagged female between its predicted date of passage at Bonneville Dam and observed day of passage at Ice Harbor Dam (). The sum of the DD>20 values for the radio-tagged fish in the lower and middle stretches of river () were then used to fit Eq. 15 () for simulating embryo loss.
Next, the cumulative DD>20 for each PIT-tagged adult classified as a successful spawner had to be determined for use in Eq. 15. Detection dates at Bonneville, Ice Harbor, and Lower Granite dams and temperature data collected in the tailraces of McNary and Ice Harbor dams were used to calculate DD>20 for these fish as they swam through the lower and middle stretches of the river system. Values of DD>20 for each PIT-tagged adult after passage at Lower Granite Dam had to be simulated because the movement of the fish was not monitored in the upper stretch of the river system.
Each PIT-tagged adult had a known date of passage at Lower Granite Dam that marked entry into Lower Granite Reservoir. That date was used earlier in this review to simulate the number of days each PIT-tagged adult was at large between passage at the dam and the initiation of spawning. Thus, each PIT-tagged adult had a Lower Granite Reservoir entry date and a simulated spawning date in Upper Hells Canyon. The exit date from the reservoir, the entry date into and exit date from Lower Hells Canyon, and the entry date into Upper Hells Canyon were not known. That information was obtained through analysis conducted on a subsample of the radio-tagged fish used to create the pre-spawning movement templates. Of those fish, 18 swam directly upstream to the Upper Hells Canyon spawning area. On average (± SD), those 18 fish spent 4.1 ± 4.0%, 10.0 ± 4.9%, and 85.8% ± 8.3% of their total time at large between passage at Lower Granite Dam and the initiation of spawning, in Lower Granite Reservoir, Lower Hells Canyon, and Upper Hells Canyon, respectively. Those location-specific percentages were used to simulate the entry and exit dates needed to calculate DD>20 for each PIT-tagged adult as described in the following example.
Fish tagged with PIT tag 3D9.1C2C4125A5 passed Lower Granite Dam and entered Lower Granite Reservoir on 21-Sep-2013. The simulated number of days at large between dam passage and spawning initiation was 47 for that fish. Thus, its simulated date of spawning was 08-Nov-2013. Partitioning the 47d by the percentages of time spent in each location (described above) resulted in ≈ 2 d being spent in Lower Granite Reservoir, ≈ 5 d being spent in Lower Hells Canyon, and ≈ 40 d being spent in Upper Hells Canyon. Location-specific, daily mean temperature data collected with thermographs were then used to calculate DD>20 for the time the fish was in each reach. System-wide DD>20 was calculated by summing the lower stretch, middle stretch, Lower Granite Reservoir, Lower Hells Canyon, and Upper Hells Canyon DD>20 values.
To simulate embryo loss at the system-wide level, the system-wide DD>20 values for the PIT-tagged adults were input to Eq. 15. Some extrapolation was required because the system-wide DD>20 values included temperature exposure in the upper stretch of the river system. The intercept of Eq. 15 (1.86%) was subtracted from each simulated value of embryo loss to account for embryo loss that was not attributable to DD>20. To evaluate the potential association between time of spawning and embryo loss due to the thermal history of the female parent, the simulated values of embryo loss of the PIT-tagged adults were plotted against the simulated dates of spawning initiation of those adults.
DD>16.5 simulations
To simulate embryo loss affected by DD>16.5, daily mean temperature in the Upper Hells Canyon spawning area was determined for 4 days (after Reed Citation1901) starting on the simulated date of spawning initiation of each PIT-tagged adult classified as a successful spawner by the somatic energy use model. Embryo loss (%) was then simulated for each of those PIT-tagged adults by decreasing embryo survival by 10.9 percentage points for every 0.1°C increase in DD>16.5 (after Geist et al., Citation2006).
2080 climate scenario simulation flows
Hamlet et al. (Citation2010) used 9 Global Climate Models (GCM) to simulate precipitation and air temperature as the predictors for Northwest precipitation and air temperature. In this review, precipitation and air temperatures downscaled from Hamlet's GCM simulations with the hybrid delta downscaling method were used to simulate runoff and air temperature in 2080. Two of the historical data sets from Hamlet et al. (Citation2010) were used: (1) combined monthly average total runoff and base flow for selected watersheds within the Columbia River basin expressed as an average depth (inches); and (2) monthly average air temperatures for those basins (°F converted to °C). The historical and 2080 data sets were downloaded from the CIG web portal (http://warm.atmos.washington.edu/2860/products/sites/).
The combined monthly average total runoff and base flows were used to simulate daily mean flows under the 2080 climate scenario for the following locations:
Hells Canyon Dam to the Imnaha River mouth
Imnaha River
Salmon River
Grande Ronde River
Clearwater River upper reach
Dworshak Dam
Palouse River
Tucannon River
Priest Rapids Dam
Yakima River.
The daily mean flows for the above locations were simulated as described in the following example for the section of Upper Hells Canyon between Hells Canyon Dam and the Imnaha River mouth. First, each historical combined monthly average total runoff and base flow was divided by the average, minimum, and maximum combined monthly average totals taken from the GCM B1 simulations for 2080 to calculate three adjustment statistics per month (). The mean daily flow in the baseline data set was then divided by the average, minimum, and maximum adjustment statistics that corresponded to the month during which the daily mean flow was observed. That step was applied to every day of the year to produce the 2080 flow scenario for Upper Hells Canyon from Hells Canyon Dam to the Imnaha River mouth.
Table A3. The historical combined monthly average total runoff and base flows for the Snake River basin upstream of Hells Canyon Dam expressed as an average depth (inches); the average, minimum, maximum value of that combined monthly average from the GCM for the B1 greenhouse gas scenario; and the adjustment statistics (historical divided by the average, minimum, or maximum values) for each month used to adjust the 2080 daily simulation flows for expected hydropower operations for Upper Hells Canyon from Hells Canyon Dam to the Imnaha River mouth. Details are given in Hamlet et al. (Citation2010). The data were downloaded from http://warm.atmos.washington.edu/2860/products/sites/?site = 4010 (Available May 2017).
The 2080 simulation flows were shaped, as described by example here, for Upper Hells Canyon. The simulated daily mean 2080 simulated flows were divided by 0.864, where 0.864 was calculated by dividing the mean annual baseline flow of 17,937 CFS by the mean annual 2080 simulated flow of 20,758 CFS. This provided a new set of daily mean 2080 simulated flows referred to as Qsimadj.
The simulations flows under the 2080 climate scenario had to be calculated by using a combination of the simulation flows from the 10 locations described above. Such locations included:
Upper Hells Canyon from the Imnaha River mouth to the Salmon River mouth
Lower Hells Canyon from Salmon River mouth to the Grande Ronde River mouth
Lower Hells Canyon from Grande Ronde River mouth to Clearwater River mouth
Priest Rapids Dam to Yakima River mouth
Yakima River at Kiona
McNary Dam.
The equations and calculations are given in .
Table A4. The series of equations that were used to calculate simulation flows under the 2080 climate change scenario. Abbrevations: Q^, location-specific equation; 2080 Qsimadj, daily flow data adjusted for 2080 conditions assuming the dam could be operated to match the baseline hydrograph pattern; 2080 Qsim, daily flow data adjusted for 2080 conditions in an unregulated tributary. The locations are shown in .
2080 climate scenario simulation temperatures
The historical monthly average air temperatures predicted from historical meteorological data and the GCM models by Hamlet et al. (Citation2010) were used to approximate daily mean water temperatures for the 2080 scenario as described in the following example for Upper Hells Canyon. This was necessary because historical data were only available on a monthly time-step but data on a daily time-step were required for this analysis. First, historical monthly average air temperatures reported for the Snake River basin upstream of Hells Canyon Dam were regressed against baseline monthly average water temperatures measured in Upper Hells Canyon during 1991‒2006. Regression models were fitted separately for the months January‒July and August‒December to best capture the general increase in temperatures during the former period, and the general decrease in temperatures during the latter period (). Next, the regression models were used to predict baseline monthly average water temperatures from the historical monthly average air temperatures (). The average, minimum, and maximum 2080 monthly average air temperatures were then taken from the output of GCMs downscaled with the hybrid delta method (). Those three values of 2080 monthly air temperatures were input to the corresponding January‒July and August‒December regression equations to predict 2080 monthly average water temperatures (). Three sets of adjustment statistics were calculated for each month as shown in . Average daily mean and a range of water temperatures for the 2080 scenarios were then estimated as follows. The daily mean water temperatures in the baseline data set were divided by the adjustment statistics that corresponded to the months during which the daily mean temperatures were observed.
Table A5. Information on regression equations fitted to predict baseline monthly average water temperatures at the locations required for simulating migration and spawning success of Snake River fall Chinook salmon within the Upper Hells Canyon spawning area from historical monthly average air temperatures predicted from meteorological data and the GCM models by Hamlet et al. (Citation2010). The historical air temperatures were downloaded from http://warm.atmos.washington.edu/2860/products/sites/?site = 4010 (Available May 2017).
Table A6. The historical monthly average air temperatures (°C) predicted from meteorological data and the GCM models by Hamlet et al. (Citation2010) for the Snake River basin upstream of Hells Canyon Dam, baseline monthly average water temperatures (°C) measured in Upper Hells Canyon during 1991‒2006, and the baseline monthly average water temperatures in Upper Hells Canyon predicted with the January‒July and August‒December regression equations in . The average, minimum, maximum values of the 2080 monthly average air temperatures were taken from the GCMs using the B1 greenhouse gas scenario and used to predict 2080 monthly average water temperatures with those regression equations. Adjustment statistics (predicted baseline monthly average water temperatures divided by the predicted 2080 monthly average water temperatures) are also given. Details are reported in Hamlet et al. (Citation2010). The data were downloaded from http://warm.atmos.washington.edu/2860/products/sites/?site = 4010 (Available May 2017).
Pattern matching, as described for the 2080 daily flow adjustment, was deemed unnecessary for the 2080 water temperature. Presently, the only confirmed and tested source for cooling temperature in the river system is the release of Dworshak Reservoir water from Dworshak Dam. Temperature control operations at Dworshak Dam are shaped around a variety of restrictions, and as such, decreasing the release temperature below the 1991‒2006 baseline period would not be a simple matter. Moreover, it is quite possible that the thermal stratification observed in the reservoir would change if precipitation and air temperature patterns change. Modeling such patterns was beyond the scope of this review.