Abstract
Climate change is the defining environmental problem for our generation. The effects of climate change are increasingly evident and are anticipated to profoundly affect our ability to conserve fish habitats and fish assemblages. Reservoirs are important structures for coping with projected shifts in water supply, but they also provide refuge for riverine fishes and retain distinct fish assemblages that support diverse fisheries. The effects of climate change on reservoirs are unique among aquatic systems because reservoirs have distinctive habitat characteristics due to their terrestrial origin and strong linkage to catchments. This article reviews (1) the projected effects of rising temperature and shifting precipitation on reservoir fish habitats, and (2) adaptation strategies to cope with the anticipated effects. Climate warming impacts to reservoirs may include higher water temperatures and shifts in hydrology that can result in reduced water levels in summer and fall, altered water residence cycles, disconnection from upstream riverine habitats and backwaters, increased stratification, eutrophication, anoxia, and a general shift in biotic assemblages including plants, invertebrates, and fishes. What is needed to adapt to these changes is a perspective that focuses on maintaining ecosystem functionality rather than on retaining a certain species composition. To that end, various strategies organized into planning, monitoring, and managing compartments are identified.
1. Introduction
Climate change is the defining environmental issue of this generation. The effects of climate change are increasingly evident, from melting glaciers and coastal flooding to drying lakes, torrential downpours, and expansions and contractions of species’ distributions (Melillo et al. Citation2014). These and other changes are bellwethers for what climate scientists anticipate will be dramatic impacts in decades to come (Maclean and Wilson Citation2011). The projected climate could profoundly affect our ability to conserve fish habitats and fish assemblages.
Reservoirs are important artificial structures for coping with anticipated temporal shifts in water supply (Christensen et al. Citation2004). Next to water storage, reservoirs provide seasonal refuge for selected riverine fishes and support distinct fish assemblages that provide diverse fisheries. The effects of climate change on reservoirs are peculiar among aquatic systems because reservoirs have unique habitat characteristics due to their terrestrial origin and strong linkage to catchments (Knoll et al. Citation2003). Because reservoirs were engineered to capture large volumes of water they tend to have large catchments that receive large inputs of inorganic and organic loads. Depositional filling effectively results in relatively rapid surface area and volume reductions, habitat fragmentation, loss of depth, and associated changes in water quality (Patton and Lyday Citation2008; Miranda and Krogman Citation2015). Unnatural water‐level fluctuations degrade shorelines that were once uplands and are maladapted to regular flooding, promoting erosion and ultimately homogenization of once diverse nearshore habitats (Miranda Citation2017). Well‐established riparian zones and floodplain wetlands that provide key ecological services to natural lakes and the original river are mostly missing in upland reservoirs. Lack of woody debris deposition, limited access to backwaters, and lack of seed banks and stable water levels that discourage native aquatic vegetation often produce barren littoral habitats. Because of their artificial origin, reservoirs reveal unique fish habitat problems that stand to be compounded by anticipated shifts in climate.
This article reviews (1) the projected effects of climate change on reservoir fish habitats, and (2) adaptation strategies to cope with the anticipated effects of climate change on reservoir fish habitats. The strategies listed are broad and general and represent a starting line applicable at the agency or regional level for developing creative alternatives relevant to local reservoirs and climate conditions.
2. The shifting climate
Global mean surface temperatures have been rising over the last two centuries (Hartmann et al. Citation2013). Temperatures are predicted to continue to rise in the 21st century, although by 2020 the climate change signal may not be clearly distinguishable from the effect of natural long-term climatic variability. Representative Concentration Pathway (RCP) assessment models of climate change adopted by the IPCC (Intergovernmental Panel on Climate Change) (Citation2019) anticipate global temperatures to increase by an average 1.1-2.6 °C by late-century under the RCP4.5 model, and by 2.6-4.8 °C under the RCP8.5 model. A warmer climate increases evaporation of water from land and sea and allows more moisture in the atmosphere, which shifts the timing and quantity of global precipitation. Climate change is predicted to increase global precipitation, but precipitation is likely to be concentrated over shorter periods (Collins et al. Citation2013). The outcome of these shifts may be an intensified hydrologic cycle of high-flow events and flooding interspersed with drought (Kirtman et al. Citation2013; Gaeta et al. Citation2014). Nevertheless, climate predictions have large uncertainties and generally projected changes in precipitation are less certain than those for temperature (Kirtman et al. Citation2013).
Shifts in temperature and precipitation are projected to show regional differences. Increases in temperature are likely to be strongest inland and at higher latitudes, with lesser warming near the coasts. Warming is likely to be especially pronounced at higher latitudes in winter. Changes from snow to rain are expected primarily at the southern extent of current snow lines (Knowles et al. Citation2006). More precipitation is projected to come in heavy downpours rather than soaking events (Furniss et al. Citation2010). Changes in atmospheric circulation tend to move storm tracks possibly causing dry regions to become drier and wet regions to become wetter (Jain et al. Citation2005; Pagano and Garen Citation2005; Hamlet and Lettenmaier Citation2007). Some regions may be subject to all these conditions during different times of the year. Despite increased precipitation in some regions, annual water availability in reservoirs may decline under most climate scenarios due to the increase in evapotranspiration (Ehsani et al. Citation2017).
Extreme weather events may pose stronger threats to ecosystem functioning than changes in average conditions. Under extreme conditions reservoir habitats and biota can face novel events. Large floods or prolonged droughts can drastically change the normal conditions of reservoir habitats, perhaps permanently. Population recruitment and mortality events can be bound to extreme conditions, and thus community composition. Missing extreme cold events can lead to species outbreaks of an unprecedented magnitude
3. Effects on integrity of reservoir environments
Climate warming impacts to reservoirs may include higher water temperatures, more evapotranspiration, and shifts in hydrology (Ehsani et al. Citation2017) that can result in lower dissolved oxygen and less water, thus shifting biological assemblages and causing fish kills (). Impacts also could include reduced reservoir levels during dry seasons, loss of habitat, disconnectedness from upstream riverine habitats and adjacent backwaters, and loss of recreational access. Reductions in the frequency and intensity of cold winter temperatures can allow tropical and subtropical fishes to move and replace some temperate species. Where climatic thresholds are crossed, certain ecosystems and landscapes may be transformed by warming winter temperatures. For convenience, the projected transformations are categorized as changes to the integrity of physical, geochemical, and biological environments (), although these categories are often intertwined.
Figure 1. Climate warming impacts to reservoirs may include higher water temperatures and shifts in precipitation and hydrology that can result in altered physicochemical characteristics leading to changes in biological assemblages.
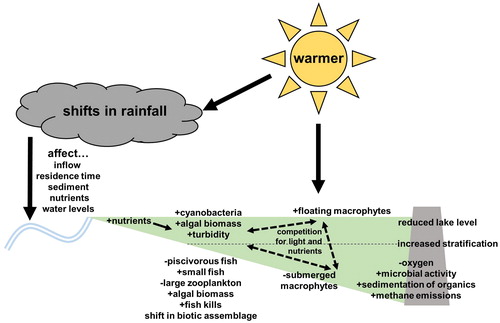
Table 1. Projected consequences of climate-induced changes in temperature and precipitation on physical, geochemical, and biological properties of reservoir environments.
3.1. Physical integrity
3.1.1. Catchment
Suspended sediment, sedimentation, and eutrophication are major problems in many reservoirs and are often linked to sediment and nutrient availability in the catchment and to transport capacity provided by runoff. Increased seasonal high flows anticipated in some regions are likely to lead to increased transport of sediment and nutrients into reservoirs. There is considerable uncertainty regarding the effects of climate change on sediment erosion and nutrient delivery, but it can be projected that in many catchments an increase in rainfall and runoff may be associated with an increase in reservoir seasonal turbidity and sedimentation (Arnell and Hulme Citation2000). Even where average rainfall decreases, an increasing frequency of intense rainfall separated by drought could increase suspended sediment and sedimentation in reservoirs (Furniss et al. Citation2010; DEFRA (Department for Environment and Food and Rural Affairs), Citation2013). These sediment increases may reduce reservoir lifespan (Palmer et al. Citation2008), impair trophic status, and have major repercussions on fish habitat degradation, with lopsided effects on shallow littoral areas of reservoirs (Patton and Lyday Citation2008; Miranda and Krogman Citation2015).
Changes in precipitation brought by climate warming could also alter the distribution of vegetation, agriculture, and forestry as land use within a basin may be converted to or from cropland depending on the occurrence of drought (Boehlert et al. Citation2015). In some cases, climate change may facilitate expanding agricultural areas into higher latitudes or changing the types of trees grown in managed forests, altering patterns of runoff. These changes in land use would have direct and indirect influences on reservoir ecosystems. If agricultural land uses increase in importance, the likely consequence is increased loads of sediment and nutrients, and associated water quality changes related to such inputs (Miranda Citation2017). The conversion of forest vegetation in riparian zones to agricultural land would also exacerbate the effects of warming on reservoirs because riparian shade creates unique water quality microhabitats that help promote fish assemblage diversity in nearshore contours of reservoirs (Raines and Miranda Citation2016). Adoption of catchment and riparian zone best-management practices for controlling nutrient inputs into reservoirs may improve hypolimnetic dissolved oxygen levels, thereby potentially offsetting rising water temperatures and preventing increased oxythermal stress (more below).
3.1.2. Horizontal habitat connectivity
Reductions in water levels due to drought, and excessive sedimentation due to torrential rains, can alter connectivity to lateral aquatic habitats, particularly in shallow lowland reservoirs (Patton and Lyday Citation2008). Water bodies adjacent to reservoirs, including tributaries, are used by many reservoir species for spawning and nursery sites, by permanent reservoir residents, and by species that live in the reservoir or tributaries seasonally. Drought can isolate backwaters from the main reservoir and from each other. With the projected increases in the intensity and frequency of drought, lateral disconnection from neighboring water bodies and riparian habitats could become more common for many reservoirs. Torrential rains move sediment that generally settles near the mouth of tributaries as water enters the reservoir. Loss of connectivity develops in shallow embayments and major tributaries through fragmentation created by the combination of sediment deposition and accretion (Patton and Lyday Citation2008). As embayments of the reservoir become filled with sediment, water that flows into the reservoir forms channels by depositing sediment on both sides of the flow channel. As discharges exceed the banks, water spills out of the channel, losing much of its energy and allowing sediment to fall out of the water column and deposit adjacent to the channel. Over time, this process tends to separate and isolate the channel from the backwaters and isolation can change the makeup of reservoir fish assemblages (Slipke et al. Citation2005; Patton and Lyday Citation2008). Under a changing climate that is projected to have more concentrated and intensified wet seasons, habitat fragmentation and isolation may be accelerated via alterations to sediment transport, deposition, and accretion pathways.
3.1.3. Storage
The projected changes in distribution of precipitation are expected to affect the current water supply-demand balance and challenge current water management strategies (Ehsani et al. Citation2017; Rahmani et al. Citation2018). The original storage and release designs may need adjustments to accommodate higher or lower inputs, shifts in seasonality, torrential short-term events, and earlier snowmelt. In many regions runoff is projected to occur earlier in the year reducing late-summer runoff. In these cases, existing reservoirs may be incapable of storing the added early water resulting from rain and snowmelt to later counter lower inflows in dry months, leading to overall lower water levels in late-summer and fall (Ehsani et al. Citation2017). Thus, earlier runoff leads to lower late-summer inflows, which stresses reservoir habitats through less water availability later in the year and overall higher water temperatures.
The vulnerability of reservoirs to floods and droughts will likely increase, and the tradeoffs between reservoir releases to maintain flood control storage, drought resilience, energy production, and suitable depth for navigation and fish requirements may need to be reconsidered. The need to release otherwise valuable water may require reassessing the tradeoffs between having a flood and drought resilient system and meeting in-lake and downstream water demands as these goals compete to utilize the same limited reservoir storage. Ehsani et al. (Citation2017) suggested that in addition to modifying reservoir operations, it may be necessary to increase the size and number of reservoirs. Many reservoir storage challenges may be exacerbated by the shrinkage of storage space caused by the accelerated sedimentation expected in some regions (Wisser et al. Citation2013).
3.1.4. Water residence time
Increased drought frequency and duration coupled with heavier precipitation pushed back from spring to winter may require longer residence time to store water from wet to dry seasons. In agricultural regions precipitation may arrive in reservoirs too early for use in irrigation and must be stored longer (USBR (U.S. Bureau of Reclamation)), Citation2008). Residence time affects water chemistry by controlling the time available for biogeochemical and photochemical processes to operate, and the extent of accumulation and loss of dissolved and particulate materials (Vincent Citation2009). In reservoirs that stratify, a prolonged residence time can result in accelerated eutrophication (more below). Conversely, phytoplankton production may be compromised in regions that experience reduced residence time due to increased precipitation, which is often associated with increased turbidity and flushing of nutrients (Ambrosetti et al. Citation2003; George and Hurley Citation2003).
3.1.5. Water level fluctuations
Increased drought frequencies, intensities, and durations particularly in summer are expected to intensify annual water level fluctuations. Large reservoirs with extensive shallow littoral zones, as well as most small reservoirs, may be impacted by long-term drought conditions that lower water levels and dewater key littoral fish habitats. When water levels drop, reservoirs with shallow and gently-sloping littoral slopes as well as small shallow reservoirs experience a greater magnitude of change than those with steep slopes (Stamou et al. Citation2007). These periodic drawdowns typically create barren shorelines with low habitat diversity and depressed species richness (Jeppesen et al. Citation2015; Hatcher et al. Citation2019) and may limit development of littoral species in favor of pelagic fish species.
Reservoir water levels are often regulated by guide curves that require a certain amount of storage space to capture potential large floods. Guide curves are engineered based on historical records of stream inflows, water levels, precipitation, and water demand (Yeh Citation1985; Mower and Miranda Citation2013a). Because climate change is anticipated to modify many aspects of the water cycle, past assumptions derived from the historical record about supply and demand may need to be revisited. The flexibility to modify guide curves is critical for the protection of infrastructure, for public safety, to ensure reliability of water delivery, and to protect fish assemblages and fisheries. There are, however, many institutional and legal barriers to such changes. Guide curves have not been easily changed as oftentimes modifications require congressional approval (Mower and Miranda Citation2013b).
3.2. Geochemical integrity
3.2.1. Stratification
In reservoirs with limited flows, higher surface temperatures lead to earlier onset and longer periods of stratification (i.e., when surface and bottom waters do not mix). During these periods, the hypolimnion isolated from the atmosphere and a vertical density gradient prevents mixing. Without mixing to replace dissolved oxygen, the hypolimnion, lacking enough light for photosynthesis, tends to have no or limited oxygen. Biotic respiration further depletes dissolved oxygen in the hypolimnion (Peeters et al. Citation2002). The circulation created by seasonal mixing moves oxygen from the surface to the deeper layers and resuspends nutrients trapped in the hypolimnion. The onset of stratification is postponed by inflows but exacerbated by warmer temperatures. The mixing regime strongly affects nutrient loadings, phytoplankton abundance, and reservoir water chemistry. The effects of climate warming on reservoir thermal structure and nutrient circulation are expected to be equivalent to considerable increases in the external nutrient loading and upgrade eutrophication status of many reservoirs (Trolle et al. Citation2011).
Seasonal hypolimnetic changes in dissolved oxygen could be more conspicuous in oligotrophic reservoirs because eutrophic reservoirs already have low dissolved oxygen. With longer stratification, bottom hypoxia could develop earlier in the year, last longer, and cover a greater spatial extent. Simulations have shown that because of warming dissolved oxygen concentrations in the epilimnion may decrease by <2 mg/L, but hypolimnion concentrations may decrease by much more (Stefan et al. Citation1993; Stefan et al. Citation2001), resulting in increased frequency of anoxia in bottom waters during mid- and late summer. Some climate scenarios predict warming epilimnetic temperatures that could possibly cause a cooling hypolimnion (De Stasio et al. Citation1996). By trapping heat at the surface layer, less heat is available for warming the lower column, and deep waters can become cooler, at least in some reservoirs.
The suitability of the hypolimnion as a thermal refuge for some reservoir fishes can be reduced by prolonged and strengthened stratification. Coolwater and coldwater stenotherms use the hypolimnion as refuge from high water temperatures (Coutant Citation1990; Jacobson et al. Citation2008). Fishes that seasonally depend on the hypolimnion for suitable temperatures can be faced with oxythermal stress created by a temperature-oxygen squeeze where they are vertically confined by the warm temperatures in the epilimnion and the low dissolved oxygen levels in the hypolimnion (Coutant Citation1985). This severely limits their habitat during warm months. When thermal refugia are reduced in volume, the fish are crowded into a smaller amount of water with higher likelihood of rapid oxygen depletion, low prey availability, stress, and disease transmission (Chang et al. Citation1992). Increased frequencies of warm summers may increase the likelihood of oxythermal stress in some reservoirs and the probability of local extinctions from successive mortality events.
3.2.2. Water quality
Climate change is likely to have far-reaching effects on overall water quality in reservoirs due to increases in water temperature, reduced vertical mixing, increased biotic respiration, and decreases in dissolved oxygen that influence a multiplicity of geochemical reactions. The effects of pollutants including sediment, nitrogen, phosphorus, and pesticides could be amplified by projected seasonal changes in precipitation. Heavy downpours earlier in the year when sun angle is low (climate change will not affect sun angle and photoperiod) and thus vegetative cover is light can lead to increased delivery of sediment and nutrients (Arnell and Hulme Citation2000). Loadings will likely increase seasonally when precipitation is higher, owing to agricultural fields being fallow or the crops not yet mature. Increased delivery of pollutants into reservoirs, coupled with reduced flow, water level, and increased temperature later in the year can result in blooms of algae and bacteria, some harmful. Predictions are uncertain because pollution can potentially be diluted in those reservoirs that experience heavy seasonal inflows. In the U.S. it is expected that the number of reservoirs listed as impaired by water pollution will increase (USEPA (US Environmental Protection Agency), Citation2017).
3.2.3. Anoxia
Next to temperature, dissolved oxygen is one of the principal water quality variables influencing fish assemblages. Increases in temperature lead to reductions in dissolved oxygen (DEFRA 2013), through reduced solubility in warmer water as well as higher respiration rates by all biota (Karl et al. Citation2009). Further reductions in dissolved oxygen are likely to be caused by nutrient increases and associated algal blooms that (1) tend to increase dissolved oxygen highs, and when they collapse, increase the lows, and (2) intensify diel cycles. Rising temperatures not only reduce dissolved oxygen in the hypolimnion but also in the epilimnion. Dissolved oxygen in the epilimnion is strongly dependent on trophic status – oligotrophic reservoirs are less affected. With mounting anoxia incidents, reservoirs may expect more summer and fall fish kills (Fang and Stefan Citation1997, Citation1999). Changing weather patterns are expected to produce stronger wind gusts, contributing to a mixed and deeper epilimnion, but increasing the risk of mixing epilimnetic and hypolimnetic waters to resuspend anoxic layers and exacerbate anoxia episodes. Alternatively, in shallow stratified reservoirs wind-induced mixing could preclude a hypolimnion and anoxic episodes. Thus, shifts in wind speed and timing may alter reservoir stratification and mixing patterns, and could bump some reservoirs into a different mixing regime (Woolway and Merchant Citation2019).
3.3. Biological integrity
3.3.1. Aquatic macrophytes
Many aquatic macrophyte species are limited in range by low winter temperatures, and therefore warming temperatures may facilitate their expansion locally and geographically. Higher temperatures can alleviate the latitudinal and altitudinal limitations placed by cold temperatures (e.g., frost kill, dormancy) as well as extend the number of days that plants experience temperatures warm enough to permit growth (Walther Citation2003). Warmer temperatures earlier in the year can foster earlier colonization by aquatic macrophytes (Haag and Gorham Citation1977) and allow some plants to more successfully compete in new habitats, and in higher altitudes and latitudes (Walther Citation2003). This increased advantage is an important factor when considering the future expansion of exotic plant species such as giant salvinia Salvinia molesta, hydrilla Hydrilla verticillata, and Eurasian watermilfoil Myriophyllum spicatum (Madsen and Owens Citation2000).
The eutrophic conditions fostered by warmer temperature may stimulate explosive macrophyte growth in some reservoirs. One study reported that a 2-3 °C temperature increase could cause a 300–500% increase in biomass of Elodea canadensis (Kankaala et al. Citation2002). Such a large increase in macrophytes would affect a reservoir in various ways. First, because plants take up the nutrients sequestered in the sediment, after the plants die and decompose there would be a large release of nitrogen and phosphorus into the water column (Cooper Citation1996; Kankaala et al. Citation2002). Second, this influx of nutrients can stimulate algal and macrophyte blooms and help perpetuate high plant production. Third, the elevated oxygen demand during the bacterial and fungal decomposition of these plants depresses levels of dissolved oxygen in the system, raising the likelihood of fish kills (Klapper Citation1991), and of stressful oxygen fluctuations that ultimately can shift the composition of fish assemblages. Lastly, in some eutrophic systems increased macrophyte growth could capture nutrients and potentially decrease algal blooms (Scheffer and van Nes Citation2007).
In contrast to factors that may boost plant growth, water level fluctuations are a primary limiting factor in the abundance of aquatic macrophytes in reservoirs (Poff et al. Citation1997; Lacoul and Freedman Citation2006). Increasingly variable water levels like those forecasted to occur in some regions could counteract aquatic plant growth, or favor generalist and fast-growing plants over species which thrive in late-succession, stable-stages of aquatic systems (Hudon Citation1997; Lacoul and Freedman Citation2006). Fluctuating water levels also create unfavorable light conditions via increased turbidity. The projected increase of water levels in reservoirs, combined with the erosive capacity of water level fluctuations, have the potential to seasonally increase turbidity and reduce aquatic plant growth.
3.3.2. Cyanobacteria
Changes in light, temperature, and access to nutrients can affect cyanobacteria species composition and diversity, and in turn impact higher trophic levels (Vincent Citation2009). A major climate change concern in temperate latitudes is the prospect of a shift in phytoplankton toward dominance by cyanobacteria that form noxious blooms (Moe et al. Citation2013). Cyanobacteria can create various water quality problems, including the release of unpleasant taste and odor compounds, the production of various toxins, and the overproduction of biomass composed of larger cells that disrupt feeding by zooplankton and causes oxygen depletions. Bloom-forming cyanobacteria are likely to be favored in a warming climate by at least two mechanisms. First, their temperature for optimum growth tends to be higher (≥25° C), and thus warmer conditions favor their more rapid accumulation and dominance. Second, a warmer climate can lead to increased phosphorus loadings under increased anoxic stratified conditions, and bloom-forming cyanobacteria tend to become increasingly abundant with increasing levels of phosphorus enrichment. The potential effects of climate change on cyanobacteria concentrations have been modeled in large reservoirs of the contiguous U.S. by Chapra et al. (Citation2017) who concluded that the mean number of days with harmful blooms is expected to increase by 16 − 23 d by 2050 and 18 − 39 d by 2090. Cyanobacteria are inedible to most zooplankton taxa that sustain planktivorous fishes (George et al. Citation1990; Kangur et al. Citation2002), so a shift toward cyanobacteria can negatively affect the fish assemblage and fisheries productivity.
3.3.3. Macroinvertebrates
Benthic macroinvertebrates play an important role in the food webs in reservoirs, including the conversion of organic material into food for fish, nutrient cycling, and the aeration of sediment (Covich et al. Citation1999). Climate-driven changes in temperature influence the productive capacity of benthic macroinvertebrates in at least two ways. First, extended bottom hypoxia facilitated by warmer temperatures and stratification could negatively influence benthic macroinvertebrate distribution and production. Second, benthic invertebrates are discouraged by fluctuating water levels, which cause a reduction in species diversity and abundance in nearshore invertebrate communities (Brauns et al. Citation2008). Most species have little ability to retreat with lowering water levels and become stranded in substrates. Species able to migrate with the changing water level (e.g., chironomids) or independent of the substrate (e.g., mobile benthos) may increase in abundance and representation in fluctuating reservoirs. The effects on macroinvertebrates are likely to be more severe in shallow, gently sloping reservoirs than in deep, steep‐sided reservoirs.
Sedimentation associated with shifting precipitation patterns is likely to also have impacts. Overall, sediment loadings tend to reduce the abundance of benthic invertebrates (Donohue and Garcia-Molinos Citation2009). Sedimentation has several direct and indirect effects including reduced feeding and growth rates and increased mortality (Donohue and Garcia-Molinos Citation2009). Moreover, alterations to taxonomic composition can occur, including reductions in species richness resulting from the decreases in substrate heterogeneity caused by sedimentation (Carew et al. Citation2007).
3.3.4. Fish assemblages
Habitat changes prompted by climate change are predicted to have substantial impacts on reservoir fish assemblages and associated recreational fisheries (Hunt et al. Citation2016; Lynch et al. Citation2016). Fishes often have broad temperature tolerances, but their population dynamics and community associations are sensitive to temperature changes in their environment. Because biochemical reaction rates vary as a function of temperature, and fish body temperature tracks environmental temperature, all aspects of fish physiology, including activity, growth, and reproduction are directly influenced by changes in temperature. Fish populations faced with changing thermal regimes may increase or decrease in abundance and experience range expansions or contractions, leading to changes in fish community makeups.
In many parts of the world the distribution of freshwater fishes is largely determined by temperature isolines that are principally latitudinal but also altitudinal (Baltz et al. Citation1987; Moyle and Cech Citation1988). Freshwater fishes within these clines are often divided into warmwater, coolwater, and coldwater guilds (Magnuson et al. Citation1997). As latitude and altitude increases, fish species are limited by low temperatures that delay the onset of spawning seasons and shorten growing seasons for juveniles, preventing juveniles from attaining enough size in their first year of life to stave off starvation or predation in long winters (Shuter and Post Citation1990; McCauley and Beitinger Citation1992). In the northern hemisphere climate warming is projected to produce a northward expansion of warmwater and coolwater fishes toward higher latitudes (Shuter and Post Citation1990; Jackson and Mandrack Citation2002). This expansion is likely to provide new opportunities for warmwater and coolwater fisheries, but at the expense of coldwater fisheries with substantial uncertainty about fish assemblage structure. Conversely, in lower latitudes the warm water can also restrict species physiology, for example by forcing spawning seasons that begin too early and last too long (Neal and Noble Citation2006; Rogers and Allen Citation2009). There may also be within-drainage latitudinal and altitudinal expansions where fish from reservoirs in lower elevations may now be able to occupy reservoirs further upstream that previously did not have adequate temperature. These invaders, whether indigenous or non-indigenous would vie for space and compete with local species that share similar thermal requirements, habitats, and diets.
Coldwater fish have the best chance of survival in deep, stratified reservoirs in higher latitudes of the contiguous U.S. but are projected to be lost in many regions especially in the mountains of the Northwest, Southwest, and the Northeast through Appalachia. Warming is also projected to have a negative impact on coolwater fish in southern reservoirs where suitable habitat exists under present conditions. The number of locations in the contiguous U.S. where reservoirs have suitable coldwater and coolwater fish habitat reportedly may decline by up to 45% and 30%, respectively (Stefan et al. Citation2001). Coolwater fish currently find habitat in seasonally-stratified reservoirs in most of the U.S., but climate warming could introduce summer kills of coolwater species in the southeastern U.S. in reservoirs where suitable habitat existed previously. According to Stefan et al. (Citation2001) the largest negative impact of warming on coldwater fish habitat is projected to occur in medium-depth lakes (13 m maximum depth); the largest negative impact on coolwater fish habitat is projected to occur in shallow lakes (4 m maximum depth).
3.3.5. Fish invasions
Climate warming can facilitate invasions by non-native species and diminish the ability of native fish assemblages to fend off invasions (Thuiller et al. Citation2007; Rahel and Olden Citation2008). Increases in temperature can raise the probability of invasion and establishment of species originating from southerly latitudes. Because the majority of exotic species in the northern hemisphere are from the tropics, increasing temperatures greatly favor their ability to expand northwards. The northern range limits are typically determined by minimum winter temperatures. Thus, as climate warms, some reservoirs may become suitable for the breeding populations of various non-native species, with often minor but occasionally major influences on native species and fish assemblages (Rahel and Olden Citation2008). Negative influences may originate from predation, competition, and spread of parasites and diseases (Wrona et al. Citation2006). The harmful impacts of non-native species can be especially large if they are directed at keystone species or if they substantially change trophic relationships. Altered precipitation and flow regimes resulting from climate warming may influence the pathways by which non-native species are introduced (Olden et al. Citation2006). Overflow of rearing ponds during flood events could promote escapes from aquaculture and tropical fish farm facilities (Padilla and Williams Citation2004).
4. Coping with shifting climate
Most reservoirs in the U.S. were constructed in the 20th century. Reservoir fish habitats and their fish assemblages have been changing quickly as reservoirs experience rapid aging, albeit at diverse rates (Miranda and Krogman Citation2015). Simultaneously, since the mid-20th century reservoir fisheries have been shifting as commercial fishers have mostly vanished and recreational fishers have become dominant (Klein et al. Citation2018). Recreational fisheries have also evolved over time as generations of fishers have switched techniques, target species, and leisure versus consumptive behaviors (Sass and Shaw Citation2020). Over their short history, reservoirs and management have aged rapidly and change is likely to continue with or without shifting climate. Hence, reservoir managers have already been coping with change. Yet, the extent of changes anticipated to be produced by climate warming are of a larger scale and may require that managers adopt a different operational framework.
Acclimation is the most tenable approach to cope with forthcoming climate change (Stein et al. Citation2013; Paukert et al. Citation2016; Magee et al. Citation2019). The word “acclimation” has been used by ecologists to describe the process by which populations adapt over time in response to their environment. In the context of climate change, acclimation refers to preparing for, coping with, and responding to the impacts of climate change with the goal of minimizing adverse effects (Paukert et al. Citation2016; Magee et al. Citation2019). Acclimation involves developing strategies to increase the capacity of reservoir ecosystems to absorb disturbances and retain the ability for self-regulation despite alterations (i.e., resilience). A resilient reservoir may be better positioned to serve all or at least some of its purposes under an unpredictable set of conditions generated by climate change (Folke et al. Citation2010). Resilient reservoirs can continue to function in an altered climate, albeit potentially with a different fish assemblage. If climate changes are large even a resilient reservoir may be unable to absorb all changes. Thus, managers may also have to allow for, and potentially initiate shifts to preserve at least some desired qualities, rather than waiting for irreparable damage to fish habitat that causes completely unwanted conditions. Promoting resilience or change may involve tradeoffs that sacrifice historical goals in favor of long-term functionality of the system (Holling Citation1996; West et al. Citation2009; Rist and Moen Citation2013). Management activities for resilience are varied but can be classified into three main classes: planning, monitoring, and managing of habitats, fish, and anglers ().
Table 2. Adaptation tools potentially applicable to cope with the consequences of climate-induced changes on reservoir fish habitats.
4.1. Planning
4.1.1. Clarify goals
Effective management relies on articulating clear goals, which in turn facilitates the development of relevant management objectives. Goals represent a vision of reservoir habitat conditions that largely reflect ecological, societal, and agency values. Goals can be diverse and may include aims such as preserving clean water, maintaining diverse habitats, and controlling erosion of shorelines. Reservoirs are artificial environments and are ephemeral over a large temporal scale given that they were constructed to last no more than about a century, although clearly some reservoirs will last longer. Considering their artificial nature, the goals for managing aquatic habitats are different than those applied to natural aquatic systems. Although occasionally protection of biodiversity may be a concern if the reservoir serves as temporary refuge for riverine species, the goals of habitat management in reservoirs are more likely to focus on habitat that can provide recreational opportunities, including fisheries. It is important to be clear about why habitat management is important (Barber and Taylor Citation1990; Slocombe Citation1998; Sass et al. Citation2017), and what aspects of habitats require management attention.
4.1.2. Assess vulnerabilities
Change in climate may result in irrecoverable loss of fish habitat in some reservoirs. Depending on various reservoir attributes (e.g., depth, area, catchment composition), reservoirs may differ in their sensitivity to change (Tingley et al. Citation2019). Because time, funding, and personnel are limited, it is critical to direct resources to reservoirs where the investment has the greatest likelihood of maintaining desired outputs at the least cost. Determining which reservoirs are most vulnerable enables managers to set priorities (Schneider et al. Citation2007). Distinctions between reservoirs can be made based on a variety of reservoir attributes sensitive to climate as well as the extent of exposure (e.g., magnitude of predicted temperature and precipitation shift). Vulnerability assessments are a tool that can provide managers answers to questions such as what reservoir characteristics are most likely to be affected by climate change, which fish species may be at risk of population decline or habitat loss, which areas or habitats in the reservoir are most likely to be affected by changes in water temperature and supply, which reservoirs are most at risk, which habitats and reservoirs are high priorities for management to sustain desired fish assemblages, and which habitats may serve as climate change refugia because they are expected to experience the least impact (Tingley et al. Citation2019). Moreover, understanding why certain reservoirs are more vulnerable than others provides a basis for developing appropriate management action. Nevertheless, methods for assessing vulnerability of reservoir habitats to climate change don’t exist, although some basic models have been developed (MDFW (Massachusetts Division of Fisheries and Wildlife), Citation2010).
4.1.3. Update management goals
As climate shifts, the characteristics of reservoirs may also shift, including changes in habitat properties and biotic community composition. Such long-term realignments can make protecting habitats and species in their original distribution increasingly difficult and in some cases unlikely. As a result, there may be a need to update goals from preserving existing conditions to managing for systems that may differ in habitat or species composition and structure yet continue to function (Cole and Yung Citation2010). Some goals may have to be abandoned and new goals established if climate change effects are severe. Even with substantial management efforts, some reservoir fisheries may not be able to maintain the desirability they currently have. For other reservoirs the cost of maintaining a resilient system may far outweigh the returns their fisheries would provide. In such cases, management resources may be better invested elsewhere. If the habitat for a certain species is becoming unsuitable, it might be best to actively manage a different habitat or for a different species, or for the same habitat and species elsewhere in another reservoir.
4.1.4. Prepare for change
Management goals may need to consider an unceasingly changing future, rather than be grounded exclusively on the current situation. Strategies to achieve goals while accounting for near-term conditions, may also need to take a long-term view of challenges in the horizon in decades ahead. Thus, managers need to be vigilant for opportunities to adjust goals as blurry forecasts about upcoming climatic, ecological, and societal changes come to focus. Most management plans have relatively short 5- to 10-year horizons. Forward-looking plans may require that managers begin incorporating some longer, but softer, 2- to 3-decade goals into current management plans. Moreover, climate change may be used as an opportunity to learn about reservoir systems. Reservoir management under a changing climate can be considered as a deliberate learning experiment (i.e., adaptive management, Walters and Holling Citation1990) in which management options may change depending on uncertain outcomes due to an uncertain climate.
4.1.5. Set priorities
Reservoir ecosystems and their fisheries are not equally valuable, nor are they equally sensitive to adverse impacts from climate change. Setting management priorities can help ensure that the management investment provides the greatest possible benefits (Tingley et al. Citation2019). Priorities may best be set in consultation with a diversity of stakeholders and considering a vulnerabilities assessment. Setting priorities can also involve identifying specific areas within a reservoir that warrant special protection or changes in management owing to their importance to maintaining the fish assemblage in the entire reservoir.
4.1.6. Ensure the needs of fish are represented
Major shifts in government policy can have major impacts on fish habitats. Catchment mismanagement has been identified as a key factor influencing various mechanisms by which reservoir habitats are impacted (Miranda Citation2017). Policies and initiatives that promote reduced runoff (e.g., conservation tillage, maintenance of buffer strips) are vital to control erosion and sedimentation. Water conservation can be key to maintaining fish habitats. Policies that establish caps on consumption, freeze urban water footprints, reduce agricultural consumption without reducing yield, encourage more efficient irrigation systems, and switch production toward less “thirsty” crops can ultimately determine availability of fish habitat in reservoirs (Postel Citation2001). Water level is an important characteristic of reservoir environments. Guide curves were designed with the assumption of a stationary climate to handle historical conditions, which may no longer be applicable under climate change. There is a need for agencies that control water levels to proactively evaluate existing guide curves to adjust to changing conditions and to develop the flexibility needed to adapt to projected changes (Zhang et al. Citation2017). The American Society of Civil Engineers estimated that at least US$64 billion is needed to rehabilitate nonfederal and federal dams across the nation, but only about $6 billion is provided through the Water Infrastructure Improvements for the Nation Act 2016 (ASCE (American Society of Civil Engineers) Citation2017). The lapse in appropriate rehabilitation of impoundments is reportedly driven largely by a lack of political will (ASCE Citation2017). Advocacy for policies that promote viable land use, water conservation, updated guide curves, and maintenance of our aging reservoir infrastructure may need to be an essential element of adaptation to climate change. Policy action can occur at various levels but is likely to be most beneficial at top levels of government. Organizations that advocate for fishery policy, such as the American Fisheries Society, are likely to be more effectual in shaping widespread change in water management of national scope.
4.1.7. Prevent arrival of new invasive species
Biological invasions are often irreversible (Rahel and Olden Citation2008). Given the expansion of invasive exotic species expected with climate warming, adaptation to climate change may need to consider improved policies for management of invasive species. Preventing further introductions is the most effective step toward managing invasive exotic species (Hulme Citation2006). Many invasion routes have substantial industries supporting them. For example, the aquarium, aquaculture, bait, and commercial shipping industries have in the past been responsible for the spread of exotic species (e.g., fish, mussels, aquatic plants) in North America (Kerr et al. Citation2005). These routes of introduction imply that more or new policies, or adequate enforcement of existing policies, are needed at the national and state levels to regulate these industries (Peters and Lodge Citation2009; Barbier et al. Citation2013).
4.1.8. Incorporate uncertainty
Planning for climate change is extremely difficult because some parts of the picture can be predicted with reasonable certainty, many may not, making prediction of the big picture challenging. To deal with this uncertainty managers may consider four general strategies (Hallegatte Citation2009). First, apply a no-regrets approach, where the management action can yield benefits even if the projected effect of climate change does not materialize. For example, habitat management activities that help maintain clean water or foster habitat diversity are nearly always a good investment even in the absence of climate change. Second, it is prudent to choose reversible management actions over irreversible options. For example, equipment installed to aerate reservoir hypolimnion or reduce stratification can be removed or turned off at any time if stratification is not as severe as predicted. Similarly, harvest regulations applied to selected piscivores to control expansion of awaited invaders could be rescinded without major impacts. Other decisions may not be reversible. Third, safety margins to account for uncertainty can often be introduced into habitat management structures during the installation period at small costs relative to making improvements after the project has been finalized. For example, constructed wetlands or pre-dams to control sediment inflows could be oversized, or shoreline riprap to control erosion at unpredicted water levels could be extended up or down cheaper during early planning. Fourth, reduce the time span of management choices or investments. The uncertainty regarding climate conditions increases with time and avoiding long-term commitments could be an option to lower uncertainty and corresponding costs. This strategy may be implemented, for example, when establishing regulatory practices such as relicensing of dams or acquiring conservation easements in riparian zones. In addition to these four general strategies, structured decision models provide alternative quantitative approaches to dealing with uncertainty in climate change. These models are examined by Littell et al. (Citation2011) and Nichols et al. (Citation2011).
4.2. Monitoring tools
4.2.1. Document trends
Monitoring is the backbone of management. Reservoir managers routinely rely on monitoring programs to assess spatial and temporal changes in resource status. In the face of climate change, monitoring may be even more essential given there is substantial uncertainty about possible effects. Also, regular monitoring allows detection of changes early and guides management response. Long-term monitoring of impacts of climate change is critical for our ability to adapt (Brekke et al. Citation2009). Extensive monitoring programs may not be feasible for all attributes of potential interest. A handful of selected attributes such as temperature, dissolved oxygen, water regime, and fish assemblage composition can be priorities in all reservoirs, or in a select group of indicator reservoirs. The attributes requiring monitoring are likely to vary among reservoirs or regions depending on the main sources of impacts.
4.2.2. Early detection
Management is often most effective if change is recognized early so that actions can be implemented at initial stages of change. Aspects such as sedimentation, shore erosion, population declines, and invasions are best addressed before they become extensive. Therefore, early detection and rapid response is an important management tool that provides another option when management to prevent negative influences has failed (Davidson et al. Citation2015). Early detection and rapid response may allow for a more flexible and measured response based on the potential extent of impact and the speed of impact development (e.g., the projected pace of colonization by an invader). Indeed, the thresholds for management action (e.g., control, restoration, eradication, no response) can be identified in advance through monitoring programs designed to detect early stages of change.
4.2.3. Hypothesis-driven monitoring
Monitoring programs are most effective if designed with a hypotheses (e.g., nutrient levels are stable; species abundance is above a threshold) and with a trigger point that will initiate a policy or a management reevaluation (Gregory et al. Citation2006). For instance, using a combination of baseline and historical data, a monitoring program could be set up with pre-defined thresholds for a species’ catch rate, growth rate, size structure, or nutrient levels that once transcended, would prompt a reexamination of management goals and objectives. Sampling site selection, although for most purposes is random, could instead focus on those sites that may best expose biotic and abiotic changes driven by climate change (Brekke at al., 2009), such as the shallow littoral zone of a deep reservoir or the habitat of a key bellwether species.
4.2.4. Increase flexibility and adaptability
Standardized monitoring programs need to have some flexibility so they can adapt to new unpredicted events and be informed with new knowledge that might develop as habitats and fishes respond to climate change. Monitoring programs may also need to track fisher attitudes and actions because the often-fickle responses of users to changing conditions may dictate how to best implement management actions (Hunt et al. Citation2016; Paukert et al. Citation2016). An effective monitoring program can help agencies adapt management programs to address unpredicted challenges.
4.2.5. Coordinate monitoring efforts
Climate change represents a major challenge to reservoir biologists and managers. Managers of other natural resources associated with the reservoir face similar challenges. Monitoring of some waters may need to be intensified to keep up with rapid development prompted by climate change. Sometimes multiple agencies, or branches of the same agency, collect overlapping data on the same reservoir. Coordination across agencies to collect and share complementary data facilitates expanded monitoring (Quevauviller et al. Citation2007; Peters et al. Citation2008).
4.2.6. Enlist volunteers and new technology
Fishers and citizens are often concerned about the quality of reservoir resources and many are willing to participate in monitoring activities. Volunteers and amateurs have added to scientific knowledge for centuries. For example, fields such as astronomy and ornithology encourage volunteers to collect data on stars and bird migrations. In the U.S., many monitoring programs have relied on water chemistry (Kerr et al. Citation1994; Hoyer et al. Citation2014), macroinvertebrates (Penrose and Call Citation1995; Fore et al. Citation2001), and fish (Cooke et al. Citation2000; Crandall et al. Citation2018) data collected by volunteers. Angler data programs can take a variety of forms, from paper‐based catch cards, logbooks, and diaries to more technologically advanced online databases and mobile phone applications. The advent of new reporting technologies (e.g., smartphones, text‐message‐based reporting, angler apps), provides the capacity for real-time data collection and reduces recall bias (Venturelli et al. Citation2017). Using data collected by volunteers presents various challenges, but if glitches are resolved, volunteers can greatly expand the reach of agency monitoring programs.
4.3. Managing tools
4.3.1. Partner with outside organizations
Climate change can bring changes too complex and extensive for agencies to address independently. To deal with this complexity, agencies may need to consider partnering across geographic and sociopolitical structures to efficiently use staff and limited fiscal resources. Partnering with catchment, water management, water quality, or non-government conservation organizations can provide the structure needed to plan, fund, and complete required habitat work. Partnerships can provide agencies the capacity to implement activities that may be beyond the exclusive purview of traditional reservoir fish management. As partners, reservoir fish managers can contribute skills, experience, and technical expertise about reservoir management. Fish managers are equipped to show the linkages between alterations in the catchment or tributaries and outcomes in the reservoir, and to advocate for practices outside the reservoirs that will benefit fish habitat in the reservoir. These efforts can help partners understand ecological processes within the reservoir and thereby fine-tune plans for management outside the reservoir.
4.3.2. Reduce anthropogenic stressors
Reinforcing the resilience of a reservoir system often involves reducing existing pressures on habitats (e.g., sedimentation, eutrophication) and fishes (e.g., overfishing, invasive species) that hinder the ability of reservoir habitats or fish assemblages to withstand stressful climatic events. Thus, this strategy seeks to reduce or remove non-climate stressors to give fishes the maximum flexibility to respond to climate change (Mawdsley et al. Citation2009). For example, agricultural catchments may require a strong emphasis on managing land use to improve or restore the natural water retention of the catchment, reduce high flows, and retain sediment and nutrients (Furniss et al. Citation2010). Less intensive land use of riparian zones and protection of filtering zones such as floodplains and wetlands along reservoir edges can also counter the input of sediment and nutrients (Jeppesen et al. Citation2009). The resilience of fish populations and assemblages could be enhanced by maintaining diverse age structures, modest mortality rates, and steady recruitment by strategically adapting a variety of harvest regulations including a combination of length limits, bag limits, closed seasons, and closed refuge zones, as well as occasional stocking to supplement recruitment as needed (Hansen et al. Citation2015; Paukert et al. Citation2016). Conversely, urging harvest of selected species may in some cases, although not always (Sass and Shaw Citation2020), help adjust assemblages or help control undesirable invaders. These tradeoffs are encapsulated by the safe operating space concept that suggests local management actions can be adjusted in response to environmental change to maintain ecosystem services and increase resilience (Carpenter et al. Citation2017).
4.3.3. Protect or restore key reservoir features
Reservoirs have distinctive attributes that are essential to promoting resilience of the overall system. Such key attributes could be important focal points for special management protections or actions. For example, some fish species require dispersal in and out of tributaries to complete their life history, while others may thrive when there is access to backwaters. In the worst-case scenario, if dispersal is prevented, species may be extirpated locally if they are not able to escape unsuitable thermal, geochemical, or hydrological conditions. Thus, if passage can go dry under shifting precipitation patterns, or changing water level patterns, or increased sedimentation, then preemptively maintaining and restoring connectivity to allow fish access to these adjacent habitats is key. If suitable connectivity is hampered, then the responses of fish to climate change may not be realized.
As another example, nearshore zones are key habitats for reservoir fishes, with most of the fish assemblage and fishing activity occurring in this zone. Targeting improvement of nearshore and riparian zones to maintain a healthy ecotone with diverse substrates, depths, cover, and shade can foster resilience (Furniss et al. Citation2010). Bank stabilization that prevents erosion caused by water level fluctuation can prevent homogenization of diverse nearshore environments. These effects could be mitigated by land-use planning to retain mostly intact riparian zones and by planting trees or other vegetation in the riparian areas currently lacking them. Setting aside segments of the riparian zone to protect from extensive development or requesting partners to apply strict guidelines for shoreline zoning can help maintain suitable fish habitat in nearshore areas.
4.3.4. Preserve representative habitats
Another management approach to improve resilience involves protecting an assortment of habitat types to maintain representation of all available environments. Representing the diversity of environmental conditions across a locale is a common objective in conservation planning; it has been considered a proxy and hedge for maintaining diverse species assemblages (Faith Citation2003). A diversity of habitats increases the chances that, regardless of the climatic change that occurs, somewhere in the reservoir system there may be areas that provide refuge or a renewal zone.
4.3.5. Provide refugia
Refugia are habitats less affected by climate change due to factors such as depth, shade, or other key local conditions. Refugia can provide protection from extreme temperature or the effects of extreme low or high precipitation on reservoir fishes. These sites could receive special attention for protection, access, maintenance, restoration, and fish harvesting control. For example, it may be possible to use restoration techniques to reforest steep riparian zones to create shaded thermal refugia that tend to promote species richness (Raines and Miranda Citation2016), particularly in north-facing shores that are likely to receive reduced radiation during daylight hours. As another example, excavated pockets along the floor of the littoral zone can provide deeper and cooler water in addition to providing habitat diversity. If deep enough, a pocket may have continuous access to groundwater and provide cooler water as well as refuge during droughts (Miranda Citation2017).
4.3.6. Manage for change
Agencies often refine management practices based on decades of experience. Fast-tracked climate change may render some long-established management practices ineffective. Reservoir systems, particularly the small and shallow with limited buffering capacity, may experience changes big enough that strategies suitable to increase ecosystem resilience may no longer be effective, drastically altering habitats and potentially eliminating some or all fisheries in some reservoirs. At that stage, major shifts in reservoir processes and components could be unavoidable, triggering a need to manage for change. Managing for change could mean actively guiding a reservoir through a transformation into a new state. This may involve, for example, nurturing fisheries suited to the new conditions because the original fisheries can no longer be supported by the reservoir. In a shallow reservoir with increased water level fluctuations due to shifts in seasonal precipitation, managing for change may mean that pelagic species may be favored over littoral species. Thus, habitat management programs targeting the nearshore, or stocking programs that support recruitment of littoral species, may be shifted to programs that manage reservoir stratification problems and stock pelagic species instead.
Managing for change may also mean applying actions that provide benefits across a range of likely future scenarios. When this is possible, such a tactic helps cope with uncertainties in forthcoming climate and in subsequent environmental and human responses. Focusing on management strategies that are pertinent across multiple future scenarios can provide managers and stakeholders confidence about management strategies and increase the likelihood that strategies will be implemented (Stein et al. Citation2013).
Managing for change may be complicated by societal expectations. The public in general may expect that agencies will maintain reservoir habitats and fisheries in their current state. They may not recognize the potential impossibility of this goal. Since management may not be able to prevent most changes, but only adjust for change, it could become critical to manage expectations as an integral part of managing for change (Tingley et al. Citation2019).
4.3.7. Adjust expectations
Maintaining resilience in reservoir fisheries also requires adjusting the expectations of users. Some angler groups are focused on single species and demand that management agencies optimize production of their focus species. These attitudes may require reshaping if managers expect to provide gratifying fisheries in the 21st century. In some cases, certain fisheries may be too costly to maintain (DEFRA Citation2013). Such a change in emphasis on species, or from fisheries that emphasize a single species to fisheries that reflect the ecological capacity of habitats, is no small challenge for reservoir managers and may take a decade or longer. Management agencies can foster more realistic attitudes through outreach and education designed to promote participation in resource management (e.g., Biggs et al. Citation2012) and a broader species preference.
4.3.8. Keep vigil on invasive species
Management of spreading invasive species that are established will reportedly continue to be essential (Vander Zanden and Olden Citation2008). There are species that have already been introduced but have not become problematic, although as climate changes they could (Crooks Citation2005). Government policy (national and state) and regulations are needed to support risk-reduction measures, including education. Voluntary codes and education alone may not be effective, but in combination with effective enforcement could lead to risk reduction.
The development of new technology and adaptation of old technology may assist with limiting impacts of invasive species. Barriers of several types are being adopted to exclude invasive fish, both for short-term and long-term protection (e.g., Clarkson Citation2004; Noatch and Suski Citation2012; Ruebush et al. Citation2012). Ongoing development of biological controls (Hoddle Citation2004), and habitat manipulation (Buckley Citation2008) may improve the efficiency of existing control methods, and enable a broader range of invasive species to be managed. Furthermore, development and adoption of new technologies such as fertility control treatments for feral fish (Jewgenow et al. Citation2006) or use of population genetics for planning control strategies (Hansen et al. Citation2007) may improve our ability to reduce the impacts and spread of invasive species.
5. Summary
Climate change has the potential to change fish habitat, fish assemblages, and fisheries in reservoirs through influences on habitat quantity and quality, and by altering species interactions and composition. With the rising temperatures and the shifts in timing and amount of precipitation forecasted in the 21st century, the habitats available to fish may change in many reservoirs. Changes in habitat are expected to alter biological components of reservoir ecosystems that are important to fisheries. The effects, however, may differ greatly across regions given the differences in climate patterns prognosticated among geographical regions, and even within regions given the high diversity of reservoirs relative to altitude, surface area, depth, inflow volume, water residence time, sediment and nutrient influx, and biotic assemblage composition.
Temperature rises could have a variety of effects on reservoirs. First, increased water temperatures are expected to enhance the symptoms of eutrophication through increases in primary production, respiration, and declines in oxygen storage capacity. Warming may increase the potential to produce nuisance algae through nutrient releases from the hypolimnion. Reservoirs may experience a longer stratification period in summer and fall and a single recirculation period. This could enhance eutrophication and lead to oxygen depletion in deep water, eliminating refuges for coolwater or coldwater fish species. Water quality may decline further if anticipated changes in precipitation increase sediment and nutrients entering the reservoir. Seasonal reservoir hydrology and water levels may change as a result of shifts in precipitation. In areas that become drier, lake levels are expected to drop, while in wetter areas levels may rise or become more erratic. Declining reservoir levels could expose littoral habitats, reduce connectivity to surrounding habitats, and reduce habitat availability for fish. In reservoirs where summer inflows decline, dissolved nutrients may linger, increasing productivity and likely contributing to nuisance algal blooms. Fish assemblage composition may shift as a result of climate change. Warmwater fish may expand into northern reservoirs, littoral species may decline in reservoirs with large water level fluctuations, and invasive species may infiltrate native assemblages.
Reservoir managers have already been dealing with shifting conditions as reservoirs have aged rapidly since intense construction in the 20th century. Moreover, managers already have many of the tools needed to address the impacts of climate change in reservoirs. What is most needed is a new perspective on management. The new perspective requires focusing on maintaining ecosystem functionality rather than on retaining composition of desired species. To help reservoir managers adapt to upcoming changes this paper has identified various strategies organized into three compartments, including planning, monitoring, and managing compartments. The coping strategies listed are broad and general and represent a starting line applicable for developing creative alternatives relevant to local conditions.
Acknowledgments
This review was funded by the Reservoir Fisheries Habitat Partnership. We thank G. Sass for a very helpful review.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ambrosetti W, Barbanti L, Sala N. 2003. Residence time and physical processes in lakes. J Limnol. 62(1s):1–15. doi:10.4081/jlimnol.2003.s1.1
- Arnell N, Hulme M. 2000. Implications of climate change for large dams and their management. Working Paper for the World Commission on Dams, Cape Town, South Africa.
- ASCE (American Society of Civil Engineers) 2017. 2017 infrastructure report card - dams. Baltimore, Maryland: American Society of Civil Engineers. Available online: https://www.infrastructurereportcard.org/cat-item/dams/
- Baltz DM, Vondracek B, Brown LR, Moyle PB. 1987. Influence of temperature on microhabitat choice by fishes in a California stream. Trans Am Fish Soc. 116(1):12–20. doi:10.1577/1548-8659(1987)116<12:IOTOMC>2.0.CO;2
- Barber WE, Taylor JN. 1990. The importance of goals, objectives, and values in the fisheries management process and organization: a review. North Am J Fish Manag. 10(4):365–373. doi:10.1577/1548-8675(1990)010<0365:TIOGOA>2.3.CO;2
- Barbier EB, Knowler D, Gwatipedza J, Reichard SH, Hodges AR. 2013. Implementing policies to control invasive plant species. Bioscience. 63(2):132–138. doi:10.1525/bio.2013.63.2.9
- Biggs R, Schlüter M, Biggs D, Bohensky EL, BurnSilver S, Cundill G, Dakos V, Daw TM, Evans LS, Kotschy K, et al. 2012. Toward principles for enhancing the resilience of ecosystem services. Annu Rev Environ Resour. 37(1):421–448. doi:10.1146/annurev-environ-051211-123836
- Boehlert B, Solomon S, Strzepek KM. 2015. Water under a changing and uncertain climate: lessons from climate model ensembles. J Climate. 28(24):9561–9582. doi:10.1175/JCLI-D-14-00793.1
- Brauns M, Garcia XF, Pusch MT. 2008. Potential effects of water-level fluctuations on littoral invertebrates in lowland lakes. Hydrobiologia. 613(1):5–12. doi:10.1007/s10750-008-9467-0
- Brekke LD, Kiang JE, Olsen JR, Pulwarty RS, Raff DA, Turnipseed DP, Webb RS, White KD. 2009. Climate change and water resources management: A federal perspective. Circular 1331, U.S. Geological Survey, Reston, Virginia.
- Buckley YM. 2008. The role of research for integrated management of invasive species, invaded landscapes and communities. J Appl Ecol. 45(2):397–402. doi:10.1111/j.1365-2664.2008.01471.x
- Carew ME, Pettigrove V, Cox RL, Hoffmann AA. 2007. The response of Chironomidae to sediment pollution and other environmental characteristics in urban wetlands. Freshwater Biol. 52(12):2444–2462. doi:10.1111/j.1365-2427.2007.01840.x
- Carpenter SR, Brock WA, Hansen GJA, Hansen JF, Hennessy JM, Isermann DA, Pedersen EJ, Perales KM, Rypel A, Sass GG, et al. 2017. Defining a safe operating space for recreational fisheries. Fish Fish. 18(6):1150–1160. doi:10.1111/faf.12230
- Chang LH, Railsback SF, Brown RT. 1992. Use of a reservoir water quality model to simulate global climate change effects on fish habitat. Clim Change. 20(4):277–296. doi:10.1007/BF00142423
- Chapra SC, Boehlert B, Fant C, Bierman VJ, Henderson J, Mills D, Mas DML, Rennels L, Jantarasami L, Martinich J, et al. 2017. Climate change impacts on harmful algae blooms in US freshwaters: a screening-level assessment. Environ Sci Technol. 51(16):8933–8943. doi:10.1021/acs.est.7b01498
- Christensen NS, Wood AW, Voisin N, Lettenmaier DP, Palmer RN. 2004. Effects of climate change on the hydrology and water resources of the Colorado River Basin. Climate Change.62(1-3):337–363. doi:10.1023/B:CLIM.0000013684.13621.1f
- Clarkson RW. 2004. Effectiveness of electrical fish barriers associated with the Central Arizona Project. North Am J Fish Manage. 24(1):94–105. doi:10.1577/M02-146
- Cole DN, Yung L, editors. 2010. Beyond naturalness: rethinking park and wilderness stewardship in an era of rapid change. Washington, D.C: Island Press.
- Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, et al. 2013. Long-term climate change: projections, commitments and irreversibility. Pages 1029–1136 In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Doschung J, Nauels A, Xia Y, Bex V, and Midgley PM, editors. Climate Change 2013 - The Physical Science Basis. Cambridge, UK: Cambridge University Press.
- Cooke SJ, Dunlop WI, MacClennan D, Power G. 2000. Applications and characteristics of angler diary programmes in Ontario. Fisheries Manag. 7(6):473–487. doi:10.1046/j.1365-2400.2000.00232.x
- Cooper SD. 1996. Rivers and streams. In: McClanahan RR, and Young TP, editors. East African ecosystems and their conservation. New York: Oxford University Press. p. 133–170
- Coutant CC. 1985. Striped bass, temperature, and dissolved oxygen: a speculative hypothesis for environmental risk. Trans Am Fish Soc. 114(1):31–61. doi:10.1577/1548-8659(1985)114<31:SBTADO>2.0.CO;2
- Coutant CC. 1990. Temperature-oxygen habitat for freshwater and coastal striped bass in a changing climate. Trans Am Fish Soc. 119(2):240–253. doi:10.1577/1548-8659(1990)119<0240:THFFAC>2.3.CO;2
- Covich AP, Palmer MA, Crowl TA. 1999. The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. Bioscience 49(2):119–127. doi:10.2307/1313537
- Crandall CA, Monroe M, Dutka-Gianelli J, Fitzgerald B, Lorenzen K. 2018. How to bait the hook: identifying what motivates anglers to participate in a volunteer angler data program. Fisheries 43(11):517–526. doi:10.1002/fsh.10156
- Crooks JA. 2005. Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12(3):316–329. doi:10.2980/i1195-6860-12-3-316.1
- Davidson AD, Hewitt CL, Kashian DR. 2015. Understanding acceptable level of risk: incorporating the economic cost of under-managing invasive species. PLoS One. 10(11):e0141958doi:10.1371/journal.pone.0141958
- De Stasio BT, Hill DK, Kleinhans JM, Nibbelink NP, Magnuson JJ. 1996. Potential effects of global climate change on small north-temperate lakes: physics, fish, and plankton. Limnol Oceanogr. 41(5):1136–1149. doi:10.4319/lo.1996.41.5.1136
- DEFRA (Department for Environment, Food and Rural Affairs). 2013. Impact of climate change on dams & reservoirs. Final Guidance Report prepared by Atkins for the Department for Environment, Food and Rural Affairs, London, U.K. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/399993/RFI7086_DG09_Guidance_Final.pdf.
- Donohue I, Garcia-Molinos J. 2009. Impacts of increased sediment loads on the ecology of lakes. Biol Rev Camb Philos Soc. 84(4):517–531. doi:10.1111/j.1469-185X.2009.00081.x
- Ehsani N, Vörösmarty CJ, Fekete BM, Stakhiv EZ. 2017. Reservoir operations under climate change: storage capacity options to mitigate risk. J Hydrol. 555:435–446. doi:10.1016/j.jhydrol.2017.09.008
- Faith DP. 2003. Environmental diversity (ED) as surrogate information for species-level biodiversity. Ecography. 26(3):374–379. doi:10.1034/j.1600-0587.2003.03300.x
- Fang X, Stefan HG. 1997. Simulated climate changes on dissolved oxygen characteristics in ice-covered lakes. Ecol Modell. 103(2-3):209–229. doi:10.1016/S0304-3800(97)00086-0
- Fang X, Stefan HG. 1999. Projection of climate change effects on water temperature characteristics of small lakes in the contiguous U.S. Climate Change. 42(2):377–412. [Mismatch] doi:10.1023/A:1005431523281
- Folke C, Carpenter SR, Walker B, Scheffer M, Chapin T, Rockstrom J. 2010. Resilience thinking: integrating resilience, adaptability and transformability. E&S. 15(4):20. doi:10.5751/ES-03610-150420
- Fore LS, Paulsen K, O'Laughlin K. 2001. Assessing the performance of volunteers in monitoring streams. Freshwater Biol. 46(1):109–123. doi:10.1111/j.1365-2427.2001.00640.x
- Furniss MJ, Staab BP, Hazelhurst S, Clifton CF, Roby KB, Ilhadrt BL, Larry EB, Todd AH, Reid LM, Hines SJ, et al. 2010. Water, climate change, and forests: watershed stewardship for a changing climate. General Technical Report PNW-GTR-812. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station, Portland, Oregon.
- Gaeta JW, Sass GG, Carpenter SR. 2014. Drought-driven lake level loss: effects on coarse woody habitat and fish. Can J Fish Aquat Sci. 71(2):315–325. doi:10.1139/cjfas-2013-0451
- George DG, Hewitt DP, Lund JWG, Smyly WJP. 1990. The relative effects of enrichment and climate change on the long-term dynamics of Daphnia in Esthwaite Water. Freshwater Biol. 23(1):55–70. doi:10.1111/j.1365-2427.1990.tb00253.x
- George DG, Hurley MA. 2003. Using a continuous function for residence time to quantify the impact of climate change on the dynamics of thermally stratified lakes. J Limnol. 62(1s):21–26. doi:10.4081/jlimnol.2003.s1.21
- Gregory R, Ohlson D, Arvai J. 2006. Deconstructing adaptive management: criteria for applications to environmental management. Ecol Appl. 16(6):2411–2425. doi:10.1890/1051-0761(2006)016[2411:DAMCFA]2.0.CO;2
- Haag RW, Gorham PR. 1977. Effects of thermal effluent on standing crop and net production of Elodea canadensis and other submerged macrophytes in Lake Wabamum. Alberta. J Appl Ecol.14(3):835–851. doi:10.2307/2402815
- Hallegatte S. 2009. Strategies to adapt to an uncertain climate change. Global Environ Change. 19(2):240–247. doi:10.1016/j.gloenvcha.2008.12.003
- Hamlet AF, Lettenmaier DP. 2007. Effects of 20th century warming and climate variability on flood risk in the Western U.S. Contribution 1313, Seattle: JISAO/SMA CSES Climate Impacts Group. University of Washington.
- Hansen GJA, Gaeta JW, Hansen JF, Carpenter SR. 2015. Learning to manage and managing to learn: sustaining freshwater recreational fisheries in a changing environment. Fisheries. 40(2):56–64. doi:10.1080/03632415.2014.996804
- Hansen H, Hess SC, Cole D, Banko PC. 2007. Using population genetic tools to develop a control strategy for feral cats (Felis catus) in Hawaii. Wildl Res. 34(8):587–596. doi:10.1071/WR07043
- Hartmann DL, A.M.G. Klein Tank M, Rusticucci LV, Alexander S, Brönnimann Y, Charabi FJ, Dentener EJ, Dlugokencky DR, Easterling A, Kaplan BJ, Soden, et al. 2013. Observations: atmosphere and surface. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM, editors. Climate change 2013: the physical science basis. Cambridge, U.K: Cambridge University Press. p. 159–254
- Hatcher HR, Miranda LE, Colvin ME, Coppola G, Lashley MA. 2019. Fish assemblages in a reservoir mudflat with low structural complexity. Hydrobiologia. 841(1):163–175. doi:10.1007/s10750-019-04019-w
- Hoddle MS. 2004. Restoring balance: using exotic species to control invasive exotic species. Conservation Biol. 18(1):38–49. ‐doi:10.1111/j.1523-1739.2004.00249.x
- Holling CS. 1996. Engineering resilience versus ecological resilience. In: Schulze P, editor. Engineering Within Ecological Constraints. Washington, D.C: National Academy Press. p. 31–44
- Hoyer MV, Bigham DL, Bachman RW, Canfield DE. Jr. 2014. Florida LAKEWATCH: citizen scientists protecting Florida’s aquatic systems. Florida Sci. 77:184–197.
- Hudon C. 1997. Impact of water-level fluctuations on St. Lawrence River aquatic vegetation. Can J Fish Aquat Sci. 54(12):2853–2865. doi:10.1139/f97-201
- Hulme PE. 2006. Beyond control: wider implications for the management of biological invasions. J Appl Ecol. 43(5):835–847. doi:10.1111/j.1365-2664.2006.01227.x
- Hunt LM, Fenichel EP, Fulton DC, Mendelsohn R, Smith JW, Tunney TD, Lynch AJ, Paukert CP, Whitney JE. 2016. Identifying alternate pathways for climate change to impact inland recreational fishers. Fisheries. 41(7):362–373. doi:10.1080/03632415.2016.1187015
- IPCC (Intergovernmental Panel on Climate Change) 2019. Representative Concentration Pathways (RCPs). [accessed 2019 March 2] Available online: https://sedac.ciesin.columbia.edu/ddc/ar5_scenario_process/RCPs.html.
- Jackson DA, Mandrack NE. 2002. Changing fish biodiversity: predicting the loss of cyprinid biodiversity due to global climate change. Am Fish Soc Symp. 32:89–98.
- Jacobson PC, Jones TS, Rivers P, Pereira DL. 2008. Field estimation of a lethal oxythermal niche boundary for adult ciscoes in Minnesota lakes. Trans Am Fish Soc. 137(5):1464–1474. doi:10.1577/T07-148.1
- Jain S, Hoerling M, Eischeid J. 2005. Decreasing reliability and increasing synchroneity of Western North American streamflow. J Climate.. 18(5):613–618. doi:10.1175/JCLI-3311.1
- Jeppesen E, Brucet S, Naselli-Flores L, Papastergiadou E, Stefanidis K, Nõges T, Nõges P, Attayde JL, Zohary T, Coppens J, et al. 2015. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia. 750(1):201–227. doi:10.1007/s10750-014-2169-x
- Jeppesen E, Kronvang B, Meerhoff M, Søndergaard M, Hansen KM, Andersen HE, Lauridsen TL, Liboriussen L, Beklioglu M, Özen A, et al. 2009. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J Environ Qual. 38(5):1930–1941. doi:10.2134/jeq2008.0113
- Jewgenow K, Dehnhard M, Hildebrandt TB, Goritz F. 2006. Contraception for population control in exotic carnivores. Theriogenology. 66(6-7):1525–1529. doi:10.1016/j.theriogenology.2006.01.027
- Kangur A, Kangur P, Pihu E. 2002. Long-term trends in the fish communities of Lakes Peipsi and Vortsjarv (Estonia). Aquatic Ecosyst Health Manag. 5(3):379–389. doi:10.1080/14634980290001922
- Kankaala P, Ojala A, Tulonen T, Arvola L. 2002. Changes in nutrient retention capacity of boreal aquatic ecosystems under climate warming: a simulation study. Hydrobiologia. 469(1/3):67–76. [Mismatch] doi:10.1023/A:1015563224554
- Karl, T.R., J.M. Melillo, and T.C. Peterson, editors. 2009. Global climate change impacts in the United States. New York: Cambridge University Press.
- Kerr JK, Brousseau CS, Muschett M. 2005. Invasive aquatic species in Ontario: a review and analysis of potential pathways for introduction. Fisheries. 30(7):21–30. doi:10.1577/1548-8446(2005)30[21:IASIO]2.0.CO;2
- Kerr M, Ely E, Lee V, Mayio A. 1994. A profile of volunteer environmental monitoring: national survey results. Lake Reservoir Manag. 9(1):1–4. doi:10.1080/07438149409354713
- Kirtman B, Power SB, Adedoyin JA, Boer GJ, Bojariu R, Camilloni I, Doblas-Reyes FJ, Fiore AM, Kimoto M, Meehl GA, et al. 2013. Near-term climate change: projections and predictability. Pages 953-1028 In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, and Midgley PM, editors. Climate change 2013 - The physical science basis. Cambridge, UK: Cambridge University Press
- Klapper H. 1991. Control of eutrophication in inland waters. West Sussex, UK: Ellis Horwood.
- Klein ZB, Quist MC, Miranda LE, Marron MM, Steuck MJ, Hansen KA. 2018. Commercial fisheries of the upper Mississippi River: a century of sustained harvest. Fisheries. 43(12):563–574. doi:10.1002/fsh.10176
- Knoll LB, Vanni MJ, Renwick WH. 2003. Phytoplankton primary production and photosynthetic parameters in reservoirs along a gradient of watershed land use. Limnol Oceanogr. 48(2):608–617. doi:10.4319/lo.2003.48.2.0608
- Knowles N, Dettinger MD, Cayan DR. 2006. Trends in snowfall versus rainfall in the Western United States. J Climate.. 19(18):4545–4559. doi:10.1175/JCLI3850.1
- Lacoul P, Freedman B. 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev. Rev14(2):89–136. doi:10.1139/a06-001
- Littell JS, McKenzie D, Kerns BK, Cushman S, Shaw CG. 2011. Managing uncertainty in climate-driven ecological models to inform adaptation to climate change. Ecosphere. 2(9):art102–19. doi:10.1890/ES11-00114.1
- Lynch AJ, Myers BJE, Chu C, Eby LA, Falke JA, Kovach RP, Krabbenhoft TJ, Kwak TJ, Lyons J, Paukert CP, et al. 2016. Climate change effects on North American inland fish populations and assemblages. Fisheries. 41(7):346–361. doi:10.1080/03632415.2016.1186016
- Maclean IMD, Wilson RJ. 2011. Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci USA. 108(30):12337–12342. doi:10.1073/pnas.1017352108
- Madsen JD, Owens CS. 2000. Factors contributing to the spread of Hydrilla in lakes and reservoirs. Aquatic Plant Control Technical Notes Collection (ERDC TN-APCRP-EA-01), U.S. Army Engineer Research and Development Center, Vicksburg, Mississippi.
- Magee MR, Hein CL, Walsh JR, Shannon PD, Vander Zanden MJ, Campbell TB, Hansen GJA, Hauxwell J, LaLiberte GD, Parks TP, et al. 2019. Scientific advances and adaptation strategies for Wisconsin lakes facing climate change. Lake Reservoir Manag. 35(4):364–381. doi:10.1080/10402381.2019.1622612
- Magnuson JJ, Webster KE, Assel RA, Bowser CJ, Dillon PJ, Eaton JG, Evans HE, Fee EJ, Hall RI, Mortsch LR, et al. 1997. Potential effects of climate change on aquatic systems: Laurentian Great Lakes and Precambrian Shield region. Hydrol Process. 11(8):825–871. doi:10.1002/(SICI)1099-1085(19970630)11:8<825::AID-HYP509>3.0.CO;2-G
- Mawdsley JR, O'Malley R, Ojima DS. 2009. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv Biol. 23(5):1080–1089. doi:10.1111/j.1523-1739.2009.01264.x
- McCauley R, Beitinger T. 1992. Predicted effects of climate warming on the commercial culture of the channel catfish Ictalurus punctatus. Geojournal. 28(1):61–66. doi:10.1007/BF00216407
- MDFW (Massachusetts Division of Fisheries and Wildlife). 2010. Change and Massachusetts fish and wildlife: volume 2 habitat and species vulnerability. Massachusetts Division of Fisheries and Wildlife, Westborough.
- Melillo, J.M., T.C. Richmond, and G.W. Yohe, editors. 2014. Climate change impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program. Available online: https://nca2014.globalchange.gov/downloads.
- Miranda LE, Krogman RM. 2015. Functional age as an indicator of reservoir senescence. Fisheries. 40(4):170–176. doi:10.1080/03632415.2015.1007207
- Miranda LE. 2017. Reservoir fish habitat management. Totowa, New Jersey: Lightning Press.
- Moe SJ, De Schamphelaere K, Clements WH, Sorensen MT, Van den Brink PJ, Liess M. 2013. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ Toxicol Chem. 32(1):49–61. doi:10.1002/etc.2045
- Mower EB, Miranda LE. 2013a. Evaluating changes to reservoir rule curves using historical water level data. Int J River Basin Manag. 11(3):323–328. doi:10.1080/15715124.2013.823979
- Mower EB, Miranda LE. 2013b. Frameworks for amending reservoir water management. Lake Reservoir Manag. 29(3):194–201. doi:10.1080/10402381.2013.829893
- Moyle PB, Cech JJ. 1988. Fishes: an introduction to ichthyology, 2nd ed. Englewood Cliffs, New Jersey: Prentice Hall.
- Neal JW, Noble RL. 2006. A bioenergetics-based approach to explain largemouth bass size in tropical reservoirs. Trans Am Fish Soc. 135(6):1535–1545. doi:10.1577/T05-258.1
- Nichols JD, Konef MD, Heglund PJ, Knutson MG, Seamans ME, Lyons JE, Morton JM, Jones MT, Boomer GS, Williams BK. 2011. Climate change, uncertainty, and natural resource management. J Wildl Manag. 75(1):6–18. doi:10.1002/jwmg.33
- Noatch MR, Suski CD. 2012. Non-physical barriers to deter fish movements. Environ Rev. 20(1):71–82. doi:10.1139/a2012-001
- Olden JD, Poff NL, Bestgen KR. 2006. Life-history strategies predict fish invasions and extirpations in the Colorado River Basin. Ecol Monogr. 76(1):25–40. doi:10.1890/05-0330
- Padilla DK, Williams SL. 2004. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic systems. Front Ecol Environ. 2(3):131–138. doi:10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2
- Pagano T, Garen D. 2005. A recent increase in western U.S. streamflow variability and persistence. J Hydrometeor. 6(2):173–179. doi:10.1175/JHM410.1
- Palmer MA, Reidy Liermann CA, Nilsson C, Flörke M, Alcamo J, Lake PS, Bond N. 2008. Climate change and the world’s river basins: anticipating management options. Front Ecol Environ. 6(2):81–89. doi:10.1890/060148
- Patton T, Lyday C. 2008. Ecological succession and fragmentation in a reservoir: effects of sedimentation on habitats and fish communities. Am Fish Soc Symp. 62:147–167.
- Paukert CP, Glazer BA, Hansen GJA, Irwin BJ, Jacobson PC, Kershner JL, Shuter BJ, Whitney JE, Lynch AJ. 2016. Adapting inland fisheries management to a changing climate. Fisheries 41(7):374–384. doi:10.1080/03632415.2016.1185009
- Peeters F, Livingstone DM, Goudsmit GH, Kipfer R, Forster R. 2002. Modeling 50 years of historical temperature profiles in a large central European lake. Limnol Oceanogr. 47(1):186–197. doi:10.4319/lo.2002.47.1.0186
- Penrose D, Call SM. 1995. Volunteer monitoring of benthic macroinvertebrates: regulatory biologists’ perspectives. J N Am Benthol Soc.14(1):203–209. doi:10.2307/1467735
- Peters DP, Groffman PM, Nadelhoffer KJ, Grimm NB, Collins SL, Michener WK, Huston MA. 2008. Living in an increasingly connected world: a framework for continental-scale environmental science. Front Ecol Environ. 6(5):229–237. doi:10.1890/070098
- Peters JA, Lodge DM. 2009. Invasive species policy at the regional level: a multiple weak links problem. Fisheries. 34(8):373–381. doi:10.1577/1548-8446-34.8.373
- Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC. 1997. The natural flow regime, a paradigm for river conservation and restoration. Bioscience. 47(11):769–784. doi:10.2307/1313099
- Postel S. 2001. Growing more food with less water. Sci Am. 284(2):46–51. doi:10.1038/scientificamerican0201-46
- Quevauviller P, Borchers U, Gawlik BM. 2007. Coordinating links among research, standardisation and policy in support of water framework directive chemical monitoring requirements. J Environ Monit. 9(9):915–923. doi:10.1039/b709540f
- Rahel FJ, Olden JD. 2008. Assessing the effects of climate change on aquatic invasive species. Conserv Biol. 22(3):521–533. doi:10.1111/j.1523-1739.2008.00950.x
- Rahmani V, Kastens JH, deNoyelles F, Jakubauskas ME, Martinko EA, Huggins DH, Gnau C, Liechti PM, Campbell SW, Callihan RA, et al. 2018. Examining storage capacity loss and sedimentation rate of large reservoirs in the Central U.S. Great Plains. Water. 10(2):190. doi:10.3390/w10020190
- Raines CD, Miranda LE. 2016. Role of riparian shade on the fish assemblage of a reservoir littoral. Environ Biol Fish. 99(10):753–760. doi:10.1007/s10641-016-0519-4
- Rist L, Moen J. 2013. Sustainability in forest management and a new role for resilience thinking. For Ecol Manag. 310:416–427. doi:10.1016/j.foreco.2013.08.033
- Rogers MW, Allen MS. 2009. Exploring the generality of recruitment hypotheses for largemouth bass along a latitudinal gradient of Florida lakes. Trans Am Fish Soc. 138(1):23–37. doi:10.1577/T07-178.1
- Ruebush BC, Sass GG, Chick JH, Stafford JD. 2012. In situ tests of sound-bubble-strobe light barrier technologies to prevent range expansions of Asian carp. AI. 7(1):37–48. doi:10.3391/ai.2012.7.1.005
- Sass GG, Rypel AL, Stafford JD. 2017. Fisheries habitat management: lessons learned from wildlife ecology and a proposal for change. Fisheries. 42(4):197–209. doi:10.1080/03632415.2017.1276344
- Sass GG, Shaw SL. 2020. Catch-and-release influences on inland recreational fisheries. Rev Fish Sci Aquacult. 28(2):211–227. doi:10.1080/23308249.2019.1701407
- Scheffer M, van Nes EH. 2007. Shallow lakes theory revisited: various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia. 584(1):455–466. doi:10.1007/s10750-007-0616-7
- Schneider SH, Semenov S, Patwardhan A, Burton I, Magadza CHD, Oppenheimer M, Pittock AB, Rahman A, Smith JB, Suarez A, et al. 2007. Assessing key vulnerabilities and the risk from climate change. In: Parry ML, Canziani OF, Palutikof JP, Van Der Linden PJ, and Hanson CE, editors. Climate Change 2007: Impacts, Adaptation and Vulnerability. Cambridge, UK: Cambridge University Press. p. 779–810.
- Shuter BJ, Post JR. 1990. Climate, population viability, and the zoogeography of temperate fishes. Trans Am Fish Soc. 119(2):314–336. doi:10.1577/1548-8659(1990)119<0314:CPVATZ>2.3.CO;2
- Slipke JW, Sammons SM, Maceina MJ. 2005. Importance of the connectivity of backwater areas for fish production in Demopolis Reservoir, Alabama. J Freshwater Ecol. 20(3):479–485. doi:10.1080/02705060.2005.9664763
- Slocombe DS. 1998. FORUM: Defining Goals and Criteria for Ecosystem-Based Management. Environ Manag. 22(4):483–493. doi:10.1007/s002679900121
- Stamou AI, Hadjibiros K, Andreadakis A, Katsiri A. 2007. Establishing minimum water level for Plastiras Reservoir (Greece) combining water quality modelling with landscape aesthetics. Environ Model Assess. 12(3):157–170. doi:10.1007/s10666-006-9058-2
- Stefan HG, Fang X, Eaton JG. 2001. Simulated fish habitat changes in North American lakes in response to projected climate warming. Trans Am Fish Soc. 130(3):459–477. doi:10.1577/1548-8659(2001)130<0459:SFHCIN>2.0.CO;2
- Stefan HG, Hondzo M, Fang X. 1993. Lake water quality modeling for projected future climate scenarios. J Environ Qual. 22(3):417–431. doi:10.2134/jeq1993.00472425002200030005x
- Stein BA, Staudt A, Cross MS, Dubois NA, Enquist C, Griffis R, Hansen LJ, Hellmann JJ, Lawler JL, Nelson EJ, et al. 2013. Preparing for and managing change: climate adaptation for biodiversity and ecosystems. Front Ecol Environ. 11(9):502–510. doi:10.1890/120277
- Thuiller W, Richardson DM, 2007. and GF. Midgley Will climate change promote alien plant invasions? Pages 197–211 In: Nentwig W, editor. Biological invasions. Berlin (Germany): Springer-Verlag.
- Tingley RW, Paukert C, Sass GG, Jacobson PC, Hansen GJA, Lynch AJ, Shannon PD. 2019. Adapting to climate change: guidance for the management of inland glacial lake fisheries. Lake Reservoir Manag. 35(4):435–452. doi:10.1080/10402381.2019.1678535
- Trolle D, Hamilton DP, Pilditch CA, Duggan IC, Jeppesen E. 2011. Predicting the effects of climate change on trophic status of three morphologically varying lakes: implications for lake restoration and management. Environ Model Softw.26(4):354–370. doi:10.1016/j.envsoft.2010.08.009
- USBR (U.S. Bureau of Reclamation) 2008. The effects of climate change on the operation of Boise River reservoirs, initial assessment report. U.S. Department of the Interior, Bureau of Reclamation Pacific Northwest Region, Boise, Idaho. Available online: https://www.usbr.gov/pn/programs/srao_misc/climatestudy/boiseclimatestudy.pdf.
- USEPA (U.S. Environmental Protection Agency) 2017. Multi-model framework for quantitative sectoral impacts analysis: a technical report for the Fourth National Climate Assessment. U.S. Environmental Protection Agency, EPA 430-R-17-001.
- Vander Zanden MJ, Olden JD. 2008. A management framework for preventing the secondary spread of aquatic invasive species. Can J Fish Aquat Sci. Sci65(7):1512–1522. doi:10.1139/F08-099
- Venturelli PA, Hyder K, Skov C. 2017. Angler apps as a source of recreational fisheries data: opportunities, challenges and proposed standards. Fish Fish. 18(3):578–595. doi:10.1111/faf.12189
- Vincent WF. 2009. Effects of climate change on lakes. In: Likens GE, editor. Encyclopedia of Inland Waters. Vol. 3. New York: Academic Press. p. 55–60.
- Walters CJ, Holling CS. 1990. Large-scale management experiments and learning by doing. Ecology. 71(6):2060–2068. doi:10.2307/1938620
- Walther G-R. 2003. Plants in a warmer world. Perspect Plant Ecol Evol Syst. 6(3):169–185. doi:10.1078/1433-8319-00076
- West JM, Julius SH, Kareiva P, Enquist C, Lawler JJ, Petersen B, Johnson AE, Shaw MR. 2009. US natural resources and climate change: concepts and approaches for management adaptation. Environ Manag. 44(6):1001–1021. doi:10.1007/s00267-009-9345-1
- Wisser D, Frolking S, Hagen S, Bierkens MFP. 2013. Beyond peak reservoir storage? A global estimate of declining water storage capacity in large reservoirs. Water Resour Res. 49(9):5732–5739. doi:10.1002/wrcr.20452
- Woolway RI, Merchant CJ. 2019. Worldwide alteration of lake mixing regimes in response to climate change. Nat Geosci. 12(4):271–276. doi:10.1038/s41561-019-0322-x
- Wrona FJ, Prowse TD, Reist JD, Hobbie JE, Levesque LMJ, Vincent WF. 2006. Climate change effects on aquatic biota, ecosystem structure and function. Ambio. 35(7):359–369. doi:10.1579/0044-7447(2006)35[359:CCEOAB]2.0.CO;2
- Yeh WWG. 1985. Reservoir management and operational models: a state-of-the-art review. Water Resour Res. 21(12):1797–1818. doi:10.1029/WR021i012p01797
- Zhang W, Liu P, Wang H, Chen J, Lei X, Feng M. 2017. Reservoir adaptive operating rules based on both of historical streamflow and future projections. J Hydrol. 553:691–707. doi:10.1016/j.jhydrol.2017.08.031
