Abstract
Over the last 50 years there has been an increased frequency and severity of negative impacts affecting marine fishery and aquaculture sectors, which claimed significant economic losses due to the interference of stinging gelatinous organisms with daily operational activities. Nevertheless, original scientific information on jellyfish-related incidents, their consequences, and potential preventative and mitigation countermeasures is limited and scattered across gray literature, governmental technical reports, and communication media. A literature scan searching for records of any interactions between jellyfish and the marine fishery/aquaculture sectors was carried out. Out of 553 papers, 90 contained original information, referring to more than 130 cases worldwide of negative impacts of jellyfish on marine fishery/aquaculture over the last century. Calling attention on too often neglected socio-economic and ecological impacts of jellyfish blooms, the purpose of this paper is to review and analyze the most up-to-date research on this subject and to provide a global perspective on the importance of jellyfish impacts and their cascading effects on marine fishery and aquaculture sectors.
Introduction
Jellyfish aggregations are a natural feature of healthy pelagic ecosystems (Graham et al. Citation2001). Periodic fluctuations in occurrence and abundance, alternating rarity to population blooms, are known for a number of gelatinous zooplankton taxa, including medusozoan cnidarians and ctenophores (Arai Citation1997; Shiganova Citation1998; Boero et al. Citation2002, Citation2008; Hamner and Dawson Citation2009). In the last few decades, high-density jellyfish populations have been observed in many coastal areas around the world (Goy et al. Citation1989; Brotz and Pauly Citation2012; Brotz et al. Citation2012; Condon et al. Citation2013; Canepa et al. Citation2014). Global warming is considered to have caused expansions in some jellyfish populations, facilitated recruitment of invasive native and non-indigenous species (Boero et al. Citation2016) and migration of tropical species to sub-tropical and temperate latitudes (Boero et al. Citation2016). Water temperature may regulate jellyfish physiological performances, controlling polyp and medusae budding and growth rate of newly liberated medusae (Arai Citation1997; Purcell Citation2007; Hubot et al. Citation2017). Ecosystem eutrophication has also been associated with increased jellyfish blooms (Parsons and Lalli Citation2002) and overfishing has been associated with some increases in jellyfish blooms due to the removal of jellyfish predators and competitors (Purcell et al. Citation2007; Richardson et al. Citation2009; Utne-Palm et al. Citation2010). Moreover, the proliferation of hard artificial substrates in the marine environment (ocean sprawl) provides additional suitable habitats for the polyps, the sessile life stage of many medusozoan taxa (Richardson et al. Citation2009; Duarte et al. Citation2013; Bosch-Belmar et al. Citation2019). Conversely, massive blooms (or outbreaks) and large aggregation (swarms) of gelatinous organisms may have broad negative consequences on many sea-based human activities, including tourism and leisure, fishery and aquaculture, and coastal industrial installations (e.g., energy or desalination plants) (Purcell et al. Citation2013; De Donno et al. Citation2014; Lucas et al. Citation2014).
In recent years, negative interactions between jellyfish aggregations and marine human activities are apparently increasing in frequency and severity (Purcell et al. Citation2007; Brotz and Pauly, Citation2012; Bosch-Belmar, Azzurro et al. Citation2017). Many of these negative jellyfish interactions may simply be due to the increased spatial footprint of these coastal industries in recent decades regardless of any jellyfish increase (Bastian et al. Citation2011), however, many are also likely due to increases in jellyfish blooms. Net clogging, catch deterioration and increased fishing time and fuel consumption are among the most frequently reported impacts on fishing fleets worldwide (Purcell et al. Citation2007; Dong et al. Citation2010; Palmieri et al. Citation2014). Indirect effects of jellyfish include predation on eggs and larvae, impairing recruitment in fish populations of commercial interest, and competition (especially with juvenile fish) for crustacean plankton, contributing to the shrinkage of fish stocks (Purcell and Arai Citation2001; Milisenda et al. Citation2018; Tilves et al. Citation2018).
Marine aquaculture underwent a rapid expansion, now representing a key source of food production worldwide (FAO Citation2020). The number of reports of jellyfish blooms interfering with finfish mariculture facilities or farmed fish health has increased over the last 10–15 years. Whole jellyfish, or broken pieces of jellyfish including tentacles, can be swept through finfish cages by water currents, where they can sting fishes leading to gill and skin injuries and eventually fish death (Baxter, Albinyana et al. Citation2011; Bosch-Belmar, Milisenda et al. Citation2017). Moreover, hydrozoan colonies, a key component of fouling assemblages in aquaculture cages, are also known as a threat for marine aquaculture due to direct contact envenomation or through budding and release of free-living medusae, medusoid stages, or larvae armed with stinging cells, too (Fitridge et al. Citation2012; Bosch-Belmar et al. Citation2019).
So far, information on jellyfish bloom impacts on fisheries and aquaculture has been mostly collected from sector workers through direct personal communication and/or technical reports, and it is scattered in scientific papers usually focused on different topics, or in gray literature and media. Nevertheless, to develop adequate frameworks of marine spatial planning, coastal governance and sustainable development of human activities at sea requires understanding the complexity of the mechanisms of functioning of marine communities through an ecosystem-based approach, as adopted and recommended by the EU Marine Strategy Framework Directive (MSFD; EU-COM, Citation2008). The purpose of this paper is therefore to gather, review and analyze the state-of-art information on jellyfish impacts, and their cascading effects, on marine fishery and aquaculture sectors at global scale.
Materials & methods
An in-depth literature scan was carried out across Web of Science (WOS) and Google Scholar (GS) databases searching for records of all kind of jellyfish interactions with fisheries or marine aquaculture sectors. The main search terms used, singly or in combination were “jellyfish” or “jellyfish bloom*”, or “gelatinous plankton*”; “impact” or “affect-ed” or “damage”; “fisheries” or “fishermen”; “aquaculture” or “fish farm” or “facility”; “global” or “worldwide” or “Mediterranean” or “Atlantic” or “Pacific.” The literature search aimed to sort out every kind of finding/reporting on the occurrence of direct impacts of jellyfish on fishery or aquaculture sectors all over the world. This type of information rarely comes in scientific publications as a targeted research objective or result; instead, it is more often encountered in the introduction or discussion sections, delivered as “personal communication,” or as a reference to previous research or technical reports, or as information from newspapers or from popular social media (e.g., Facebook, Instagram), or sometimes as part of citizen science campaigns.
Papers were examined and divided in two different groups/matrices, depending on the activity sector they referred to (fishery vs. aquaculture). Within each group, only those papers reporting direct impact of jellyfish blooms (e.g., impediment of common harvesting operations, displacement of fishing fleet from traditional fishing grounds, damage to gears or net cages, damage or killing of harvested fish) were selected for (indirect effects such as fish larvae predation/competition were discarded). Information obtained from manuscripts was organized in two different groups. The fishery group included nine categories: “report year” and “paper publication date,” “geographic area” (Mediterranean Sea, North and South Atlantic, North and South Pacific), “jellyfish species” (as presence/absence record), “jellyfish number or biomass” (when available), “fishing gear,” “reported impact” (damage on fishing nets, on the catch and on the fishermen), “economic impact” (when available) and “other information” considered relevant for the study. The aquaculture group also included nine categories: “record date” and “paper publication date,” “geographic area,” “jellyfish species” (as presence/absence record), “jellyfish number or biomass” (when available), “farmed organisms” (salmon, trout, seabass, seabream and bivalves), “reported impact” (injuries on fish gills, on fish skin, effects on fish growth, mortality and damage to the facility installations), “economic impact” (when available), and “other information.”
To search for significant differences between jellyfish taxa and type of impacts acting on fisheries and on marine aquaculture, one-way PERMANOVA (Anderson Citation2001) was performed on the similarity matrices constructed using the Jaccard index.
Differences in the total number of reports among “geographic area” for both fishery and aquaculture matrices were assessed using Chi-squared analyses. Moreover, Chi-squared test analysis was used to investigate differences in the total number of reports among different “fishing gear” and among different “reported impact,” for the fishery sector; and differences in the total number of reports among different “farmed species” and among different “reported impact” for the aquaculture sector. Finally, one-way PERMANOVA was chosen to study if there were significant differences among the jellyfish species reported in the different “geographic area” within each sector matrix (fishery and aquaculture).
Systematic review and metanalysis of jellyfish impacts on fishery and aquacultureThis literature scan throughout a period of almost a century (1922–2019) retrieved 553 articles (published on scientific journals, or as gray literature, e.g., technical reports) including information on jellyfish impacts on fishery and aquaculture activities worldwide. In-depth examination disclosed 90 manuscripts reporting original information (never published elsewhere). Out of 55 articles from 1922 to 2019, up to 94 negative interactions between different jellyfish species and fishing operations were reported. With reference to marine aquaculture, 35 articles published from 1954 to 2019 listed up to 45 episodes of jellyfish impacts.
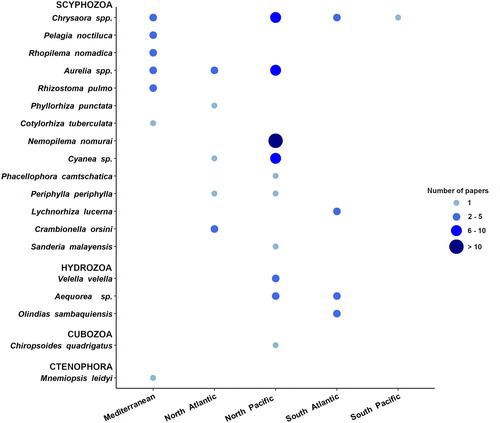
Data mining showed an increasing number of records of jellyfish direct interference with fishing activities in the last 30 years (1990–2020) reaching the highest values in the 2000s (). Aquaculture is a relatively more recent industrial activity that has undergone a faster growth in the last decades than any other major food production sectors (FAO Citation2020). Accordingly, the number of reports of jellyfish impacts on aquaculture operations has considerably increased over the last 20 years (). With respect to medusozoan jellyfish, 18 taxa (14 Scyphozoa, 1 Cubozoa, 3 Hydrozoa; ) are known to exert direct impacts on fishery operations around the world, while 13 taxa (5 Scyphozoa, 8 Hydrozoa; ) caused direct impacts on aquaculture activities. Moreover, 2 ctenophore species were both reported in 2015: Mnemiopsis leidyi (class Tentaculata) interfered with the European eel fisheries from S’Ena Arrubia Lagoon (Italy) (Diciotti et al. Citation2016) whereas Beroe cucumis (class Nuda) together with other gelatinous organisms fouled and clogged the fishing nets in a north Norwegian fjord (Knutsen et al. Citation2018).
Figure 1. Temporal trend of published papers including reports on jellyfish interference with fishery (blue) and aquaculture (red) sectors.
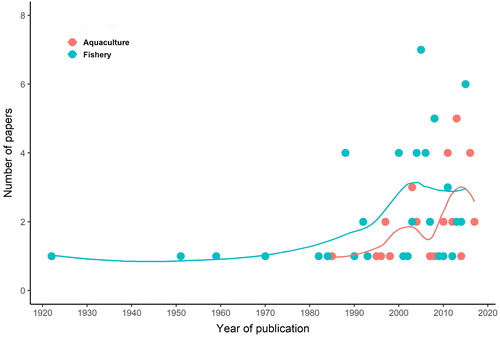
Table 1. Reported jellyfish species interfering with fishery and aquaculture sectors worldwide.
Fishing and marine farming activities were affected by different jellyfish species (F = 4.62, df = 1, p-value = 0.001), with Aurelia spp., Chrysaora spp., and Nemopilema nomurai as the most common jellyfish taxa directly interfering with fishing activities whereas Pelagia noctiluca and Aurelia spp. represented more than 50% of reports on aquaculture ().
Figure 2. Percentage of reports where every jellyfish species and cnidarian class have been involved: (A) for fishery; (B) for aquaculture sector.
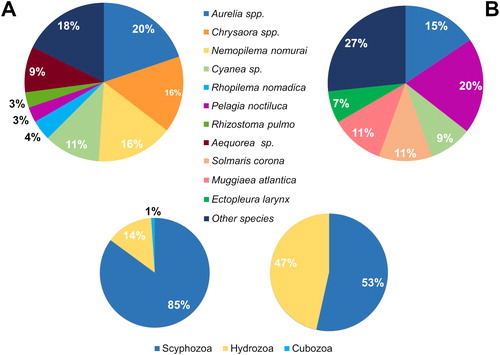
Jellyfish and fishery sector interactions
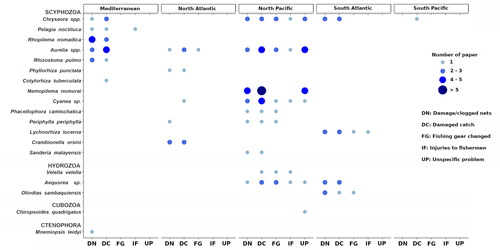
The majority (82%) of reports of jellyfish direct impacts on fishing activities refers to (at least) 8 taxa, (namely the scyphozoan representatives of Aurelia spp., Chrysaora spp., Cyanea sp., and the species Pelagia noctiluca, Nemopilema nomurai, Rhopilema nomadica, and Rhizostoma pulmo and the hydrozoan jellyfish Aequorea sp.) (). Species of the worldwide common jellyfish taxa Aurelia and Chrysaora, as well as the mauve stinger P. noctiluca, may form very large population outbreaks with millions of individuals that may spoil fishing nets with stinging mucus, broken pieces of umbrellas, oral arms and tentacles that enter in contact and damage the fish catch (Doyle et al. Citation2008). Furthermore, N. nomurai, Cyanea sp., R. nomadica, and R. pulmo are jellyfish with large, heavy bodies. By occurring in mass, they can easily clog the nets and may impair the stability of the fishing boats while hauling the nets, up to capsize them (Kawahara et al. Citation2006; Palmieri et al. Citation2014).
Fishing areas
Jellyfish are ancient organisms and ubiquitous in nearly all marine ecosystems from the poles to tropics (Parsons and Lalli Citation2002; Boero et al. Citation2008). They constantly interact with several marine human activities, but effects on fisheries are frequently reported impacts worldwide (Purcell et al. Citation2007; Palmieri et al. Citation2014). From the analysis, the number of jellyfish-related issues among geographical areas was significantly different (X2 = 45.966, p-value = 2.504−09), as well as the jellyfish species involved (F = 2.43, df = 5, p-value = 0.001).
The geographical areas most affected by direct jellyfish impacts were also different relative to fishery or the aquaculture sectors. Most of the reported interactions between jellyfish and fishery sector were recorded in the North Pacific (approx. 60%) (), where the highest marine capture production is concentrated (more than 22 million tons in 2016; FAO Citation2018). China is by far the world’s leading producer, reporting more than 15 MT of marine captures in 2016 (20% of world total marine catch). Japan is also among the top producer countries, with more than 3 MT produced in the same year (FAO Citation2018). The jellyfish Nemopilema nomurai was involved in the 41.7% of reported events in North Pacific, followed by Aurelia sp., Cyanea sp. and Chrysaora sp., identified as problematic taxa in the 30.6%, 27%, and 25% of the recorded events, respectively.
Figure 3. Percentage of reports related to: interactions between jellyfish and fishing activities by area (A); fishing gear affected by jellyfish blooms (B); jellyfish direct impacts on fishery activities (C); jellyfish interference on marine aquaculture activities by area (no reports were found from the South Atlantic region (D); most affected farmed species or group by jellyfish blooms occurrence on worldwide marine aquaculture facilities (E); jellyfish direct impacts on farmed species health and facility structures (F).
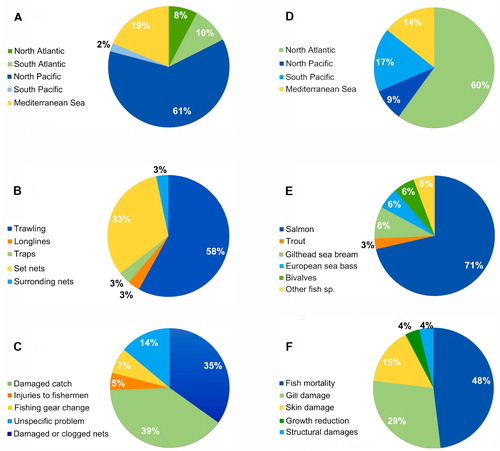
Available information indicates the Mediterranean Sea is the second most affected area in terms of jellyfish impacts on fishery (nearly 18% of the total reported events). The scyphozoans Aurelia sp. and Rhopilema nomadica were responsible of more than 50% of jellyfish reported issues in Mediterranean fishing operations, while P. noctiluca and R. pulmo represented approx. additional 35% of the total reports (). Research performed in the other areas showed a significant lower number of described events, especially for the Indian Ocean, where a single report was found (related to the endemic species Crambionella orsini). Only seven reports were from the North Atlantic area, most of them involving Aurelia sp.; in South Atlantic and South Pacific areas Chrysaora sp. (C. hysoscella and C. plocamia) and Lychnorhiza lucerna are reported as the main species interfering with fishing activities (). In South Atlantic, L. lucerna recurrently impacts the fishery sector in Brazil and Argentina, mainly by clogging nets and reducing total fish captures and catch quality (Schiariti et al. Citation2008; Nagata et al. Citation2009).
Fishing gears
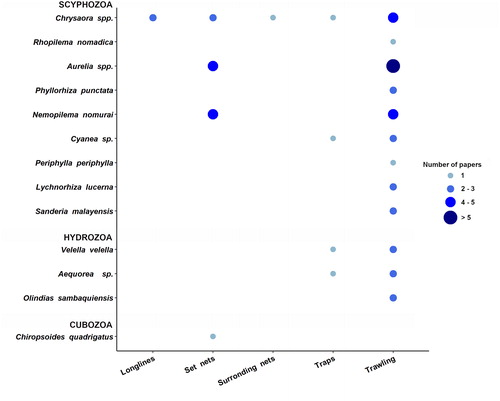
Information regarding fishing gears subject to interactions with jellyfish (31 reports) was available only in 28 out of 55 papers. Different fishing gears were differently affected by jellyfish (X2 = 37.871, p-value = 1.19−07). Out of reported interactions, 58.1% were related to bottom trawls, being the most affected fishing gear type; followed by set nets (32.3%). Other mentioned fishing gears impacted by jellyfish were longlines, traps and surrounding nets ().
In the North Pacific area, where the majority of jellyfish/fishery interactions were concentrated jellyfish impacted on a diverse range of fishing techniques including trawling, set nets, longline, surrounding nets and traps. The jellyfish Chrysaora spp. interfered in this area with all five fishing gears types, while Aurelia sp. and N. nomurai outbreaks significantly associated with damages to trawl and set nets. Bottom trawling was impacted by various jellyfish species (including scyphozoans and hydrozoans) (), involving Aurelia sp. and R. nomadica in the Mediterranean (Lotan et al. Citation1994; Öztürk and İşinibilir Citation2010; Palmieri et al. Citation2014), Phyllorhiza punctata in the subtropical eastern Atlantic coasts (Graham et al. Citation2003) and Periphylla periphylla in the North Atlantic (Conley and Sutherland Citation2015), and Olindias sp. and Lychnorhiza lucerna in the South Atlantic (Schiariti et al. Citation2008; Nagata et al. Citation2009).
Trawling represents the most common and extended fishing activity all over the world (FAO Citation2018), with hauls covering large area and filtering large volumes of water at different depths (midwater and bottom trawl). Trawling is associated to a comparatively low catch size and low species selectivity with respect to others fishing gears, resulting in large by-catch quantities and dramatic, deep impacts on habitat integrity and biodiversity (FAO Citation2018). Throughout trawling operations, hundreds of jellyfish may be trapped in the cone-shape net together with target species and other by-catch, increasing processing time during sorting operations aboard and compromising catch quality. Jellyfish by-catch in trawling fishing operations is a well-known issue, so that Norwegian shrimp fishermen developed a special net (Nordmøre grid) to reduce unwanted by-catches of jellyfish (Graham Citation2003).
Direct impacts of jellyfish blooms on fishing operations
Jellyfish blooms may impact fisheries not only through interference with fishing operations, but also indirectly, affecting marine food webs and eventually reducing fish catches due to food competition or direct predation on fish eggs and larvae (Purcell and Arai Citation2001; Richardson et al. Citation2009; Ruzicka et al. Citation2012; Tilves et al. Citation2018). A paradigmatic case is represented by the non-indigenous ctenophore Mnemiopsis leidyi in the Black Sea, which led anchovy fishery to collapse (Kideys Citation1994). As noted previously, yet this review is focused on the direct impacts of jellyfish blooms on fishery and aquaculture sectors.
Information regarding types of jellyfish impacts on fishery was available in 78% of selected scientific articles. Statistical analysis showed a significant difference among the kind of reported damages (X2= 43.69, p-value = 7.45−09). Problems were mainly related with the quantity and quality of capture fish (46%), as well as with the net manage and maintenance (40.5%) (). Specifically, fishermen worldwide, reported intense clogging and bursting of the nets (Lotan et al. Citation1992; Schiariti et al. Citation2008; Fuentes et al. Citation2011; Uye Citation2011; Palmieri et al. Citation2015), increasing by-catch sorting time aboard (Uye and Shimauchi Citation2005; Kawahara et al. Citation2006; Conley and Sutherland Citation2015), and injuries to fishermen during sorting and net cleaning operations (Uye and Shimauchi Citation2005; Mariottini et al. Citation2008; Conley and Sutherland Citation2015). Clogging shortened the hauls fishing time and increased the risk of capsizing trawls boats (Kawahara et al. Citation2006). The catch presented a high mortality of finfish by nematocyst envenomation, with significant reduction in annual mean fish catch in most cases, and a lower commercial value of captures (Graham et al. Citation2003; Chen et al. Citation2004, Citation2007; Yan et al. Citation2004; Kim et al. Citation2012; Nastav et al. Citation2013; Palmieri et al. Citation2014, Citation2015). Some fishermen displaced the hauls to areas further away from the landing ports trying to avoid the jellyfish blooms, increasing travel times and fuel expense (Palmieri et al. Citation2014); and in some cases, they temporarily shift the fishing gears to other fishing practices (ex. anchored gillnet and drift net) (Nagata et al. Citation2009; Conley and Sutherland Citation2015).
The interference of jellyfish blooms in fishery operations has been reported and documented several times but the information on the economic consequences of these events is scant. Up to 25% of examined papers included data related to economic losses suffered by fishing sector and the majority came from the North Pacific area (). In Japan, the giant scyphomedusa Nemopilema nomurai was responsible of huge economic losses between 2005 and 2006 (ca. US$270 million in one year), with more than 100,000 complaints from fishermen to the Japanese fishery agency (Kawahara et al. Citation2006; Uye Citation2011). Korean fishery industry observed significant catch decrease and product value due to jellyfish interaction with fishing activities. The annual direct damage caused by jellyfish was estimated to be between US$68.2 million and US$204.6 million, depending on fishery gear type (Kim et al. Citation2012). The impact of Chrysaora fuscescens from the Northern California Current on Oregon’s salmon and pink shrimp fisheries was estimated over US$650,000 during the peak jellyfish season (June– September) in 2012 (Conley and Sutherland Citation2015).
Figure 6. Reports (n) related to the most frequent interferences/impacts caused by jellyfish to worldwide fishery activities.
South America also has experienced the negative relationship between jellyfish and fisheries. The by-catch of Chrysaora plocamia generated economic losses mainly to artisanal and commercial purse seine fisheries. In only 35 days of fishing, this jellyfish caused more than US$200,000 economic losses to a single port of the Peruvian fishing fleet. Moreover, fishery factories refused to receive the catch if jellyfish by-catch was > 40% of the catch in weight (Quiñones et al. Citation2013; Mianzan et al. Citation2014).
Regarding the Atlantic Ocean and the Mediterranean Sea, only two reports contained information on economic losses. The Gulf of Mexico commercial shrimp industry is among the most valuable fishing activity in the United States. Total losses recorded in 2000 due to P. punctata interference could have been as high as US$10 million (Graham et al. Citation2003). The most complete study focusing on the economic impact of jellyfish blooms on Mediterranean fisheries was published by Palmieri et al. (Citation2014) regarding Italian northern Adriatic Sea. Economic losses due to reduction in fish catches were estimated to be as much as US$9.7 million per year, only for the Italian northern Adriatic trawling fleet. Other costs included additional fuel costs due to displacement of fishing activities, representing an increase of over US$542,000 per year. Jellyfish responsible of these interference with fishing operations were mainly Aurelia sp. and R. pulmo.
Jellyfish and aquaculture interactions
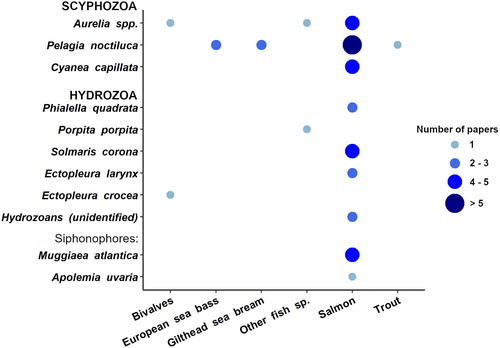
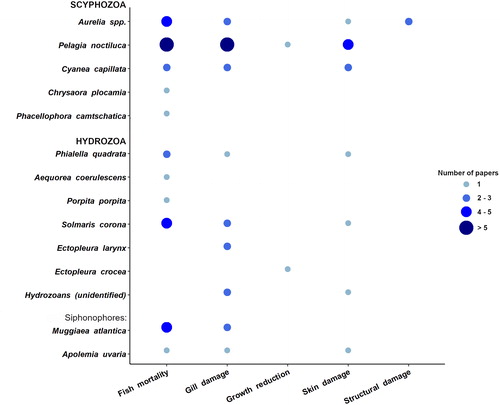
Out of 35 papers, a total of 45 events were found related to jellyfish interfering with aquaculture activities in the scientific literature: the majority of cases involved 5 jellyfish taxa (): three scyphomedusae (Pelagia noctiluca, Aurelia sp., Cyanea capillata), one hydromedusa (Solmaris corona), and one siphonophore (Muggiaea atlantica). Whereas jellyfish interfering with fishery are mostly scyphozoans, nearly 50% of gelatinous zooplankton affecting fish farming activities are represented by hydrozoan taxa (). Their polyp colonies are indeed a dominant component of the biological fouling of cages and other submerged structures (e.g., floating pontoons, piers, anchors, buoys, ropes) and can impact farmed fish in different ways (Baxter et al. Citation2012; Fitridge et al. Citation2012): the reduction of water flow through net occlusions; the seasonal budding of free-living propagules, such as medusae, medusoids, or larvae of progenetic hydroids (e.g., Tubularia spp.), all well equipped with stinging cells; or by direct contact of fish with the large polyp colonies growing on the inner border of the cages.
Marine farming area
Until recently, cnidarians were not considered as possible harmful agent for aquaculture, and low levels of mortality and unspecific gill pathology of unknown etiology were generally referred to as generalized “waterborne irritant damage” (Marcos-López et al. Citation2016; Bosch-Belmar, Azzurro et al. Citation2017). Only very large mortality events that coincided with conspicuous jellyfish observations were correlated with farmed fish health problems. Well-known examples of this are those reported in Northern Europe (Scotland and Ireland mainly), where huge blooms of P. noctiluca and Aurelia sp. caused severe gill disorders to thousands of farmed salmons and were responsible for several fish mortality events in the last 15 years (Doyle et al. Citation2008; Baxter, Sturt et al. Citation2011; Mitchell and Rodger Citation2011). In parallel, tiny hydrozoans, which are less visible organisms, are equally affecting aquaculture facilities, especially in Northern Atlantic and South Pacific (up to 45% of total reports). Medusae of Solmaris corona and Phialella quadrata and the polyp colonies of Ectopleura sp. (E. larynx and E. crocea) were cited in the 34% and 16% of the problematic events, respectively. Over the last decade, the hydroid Ectopleura larynx has become one of the most common fouling species in northern Europe aquaculture, causing increasing problems for fish farmers (Guenther et al. Citation2010; Baxter et al. Citation2012; Bosch-Belmar et al. Citation2019); together with Pennaria disticha, it has been identified as a problematic species for Mediterranean marine aquaculture facilities, too (Bosch-Belmar, Azzurro et al. Citation2017; Bosch-Belmar et al. Citation2019).
The number of jellyfish-related detrimental events for aquaculture activities was significantly different among geographical areas (X2 = 39.82, p-value = 4.70−08) (), as well as concerning the involved jellyfish (either medusa or polyp stage) taxa (F = 2.29, df = 3, p-value = 0.005).
Figure 7. Number of reports of impacts on aquaculture facilities caused by different jellyfish species across different geographical areas.
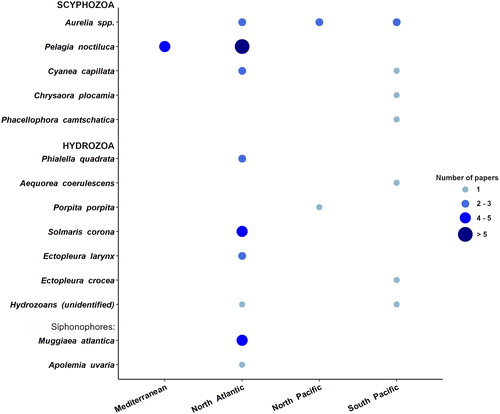
The mauve stinger Pelagia noctiluca was the most reported species, being involved in the 23% of reported events. Aurelia sp. interfered in the 16% of reported cases and represented a problem to aquaculture in all studied areas except for the Mediterranean Sea, where P. noctiluca was the only scyphozoan identified as a threat for aquaculture activities (approximately in 50% of reported incidents in this area). This species is the most common and conspicuous jellyfish in Mediterranean waters, and recent studies from the Gulf of Tunis, the Balearic Islands (Daly Yahia et al. Citation2010), and Ligurian Sea (Bernard et al. Citation2011; Ferraris et al. Citation2012), indicated that blooms may now be occurring more frequently in the western Mediterranean Sea (Canepa et al. Citation2014). The hydroid Ectopleura larynx and the siphonophore Muggiaea atlantica represented the other 50% of reports in the Mediterranean Sea. Both species were significant related with European sea bass mortalities recorded in Spanish aquaculture facilities during 2012–2014 years (Bosch-Belmar, Milisenda et al. Citation2017; Bosch-Belmar et al. Citation2019).
More than 60% of reported interactions between jellyfish and aquaculture facilities were recorded in the North Atlantic region () and involved the highest number of jellyfish taxa. The scyphomedusae Pelagia noctiluca occurred with the highest incidence, followed by the hydromedusae Solmaris corona and the siphonophore Muggiaea atlantica (). All of them have been previously identified in literature as potentially harmful species for farmed fish (Baxter, Albinyana et al. Citation2011; Fitridge and Keough Citation2013; Purcell et al. Citation2013) and together with Phialella quadrata caused the death of > 700,000 farmed fish in northern Europe (Fosså et al. Citation2003; Rodger et al. Citation2011).
North and South Pacific marine aquaculture farms were mainly impacted by Aurelia spp. jellyfish, but facilities from the southern Pacific area reported single negative interactions with many different jellyfish species (N = 8), summing up to near 20% of total reports for jellyfish-related issues in the aquaculture sector ().
Marine farmed species
Salmon fish (Salmo salar in most cases) was the farmed species most affected by jellyfish abundances (>70% reports), followed by gilthead seabream (Sparus aurata), European seabass (Dicentrarchus labrax) and bivalves (Mytilus sp.) (X2= 75.91, p-value = 5.99 −15) (). For salmon farm facilities, 84% and 16% of reports occurred from North Atlantic and South Pacific, respectively. Indeed, Norway and Chile are the second and the fourth marine and coastal aquaculture finfish major producers in the world (1.3 million tons and 726.9 thousand tons of fish live weight per year, respectively) (FAO Citation2018) with salmon representing over 80% of the harvested product (Norambuena and González Citation2020). Differently, jellyfish impacts on European seabass and gilthead seabream came only from aquaculture farms in the Mediterranean Sea, where these two species represent the most important marine aquaculture fish product (DANAQ Citation2020).
Interestingly, bivalve culture facilities have also experienced problems with stinging gelatinous zooplankton. Two Mytilus galloprovincialis mussel farms from the eastern coast of Japan and Australia were impacted by mass growth of the hydrozoan polyp colonies of Ectopleura crocea. Farmed mussels presented reduced shell length and flesh weight, coupled with an impairment of the overall physiological condition of young developing mussels (Sievers et al. Citation2013; Fitridge and Keough Citation2013). Aurelia taxa jellyfish were also reported to impact Japanese marine aquaculture, causing mass mortalities of fish and bivalves (Yasuda Citation1988, in Purcell et al. Citation2007) ().
Direct impacts of jellyfish blooms on marine aquaculture
A significant difference was detected in the type of damages caused to farming activities (X2= 36.65, p-value = 2.12−07). The most common negative impacts of jellyfish on fish farming concerned fish mortality (74% of reports), macroscopic damage on gill epithelia (44%), and skin ulcerations (24%) ().
Complex gill disorders have become one of the most serious causes of mortality in marine farmed salmon in Ireland, with average losses of 12% per year (Baxter, Rodger et al. Citation2011; Marcos-López et al. Citation2016; Herrero et al. Citation2018). Jellyfish nematocyst discharge and venom injection usually lead to local inflammatory response, cell toxicity and histopathology (Helmholz et al. Citation2010; Rodger et al. Citation2011; Marcos-López et al. Citation2016). Prolonged nematocyst discharges in fish tissues may often cause secondary bacterial infections and associated systemic reactions, including respiratory and osmoregulatory distress, altered behavior, and death (Bruno and Ellis Citation1985; Seaton Citation1989; Baxter, Sturt et al. Citation2011; Rodger et al. Citation2011). In addition, some jellyfish species can act as vectors of Tenacibaculum maritimum, the causative agent of tenacibaculosis (Småge et al. Citation2018), or as potential reservoirs of Neoparamoeba perurans (Downes et al. Citation2018), the causal agent for amoebic gill disease. Both major pathogens affect fish farming worldwide and may heavily exacerbate the impacts of jellyfish injuries (Ferguson et al. Citation2010; Delannoy et al. Citation2011; Floerl et al. Citation2016; Clinton et al. Citation2020).
Some records reported also on fish reduced growth rate (Baxter, Albinyana et al. Citation2011; Fitridge and Keough Citation2013; Bosch-Belmar et al. Citation2016) (). Interference of functioning or damage of facility infrastructures was also reported: Mitchell et al. (Citation2013) described the blockage of boat pumping system in a salmon farm in northwest Ireland clogged by hundreds of adults and juvenile ephyrae of Aurelia sp.; Bosch-Belmar, Azzurro et al. (Citation2017) reported on two different finfish mariculture farms along the Spanish Mediterranean coast whose nets had to be replaced after P. noctiluca swarms were smashed by currents against the fish cages. Similar episodes have been repeated in these farms during 2016 and 2018 (Bosch-Belmar pers. comm).
Figure 9. Number of reports relative to different jellyfish species and type of impacts caused on farmed organisms health and facility structural integrity.
Farmed fish stocks mortality, which is the most noteworthy direct impact of jellyfish on marine aquaculture and the most reported one in literature, entails significant and sometime dramatic consequences for the industry. Economic losses due to jellyfish outbreaks have been significant in aquaculture sector (Cronin et al. Citation2004; Purcell et al. Citation2007; Bosch-Belmar, Azzurro et al. Citation2017). The last scientific reports included different events in northern Europe and the Mediterranean Sea: Irish and Scottish aquaculture repeatedly suffered huge economic losses (up to US$1.3 million) due to mass salmon mortalities caused by recurring P. noctiluca invasions (Doyle et al. Citation2008; Bosch-Belmar, Azzurro et al. Citation2017). In 2009 fish mortalities in Tunisian farming facilities entailed dramatic economic losses, leading to near bankruptcy. Since 2011, seabass marine farms from Spain recorded mortality events due to different jellyfish species leading to relevant economic losses (Bosch-Belmar, Azzurro et al. Citation2017; Bosch-Belmar, Milisenda et al. Citation2017).
The number of reports related to jellyfish interactions with marine aquaculture increased in the last years, and several events were detected across web-based blogs and digital media, gray literature, also reported as “personal communications” from aquaculture facilities staff (). This new information integrates and updates the table of reports provided by Purcell et al. (Citation2013).
Table 2. Records of jellyfish blooms interference with aquaculture farming activities found in the web media (not previously reported in scientific literature).
Conclusions
Fishery and aquaculture are two of the most important industrial marine activities worldwide, annually providing nearly 178.5 million tons of food, i.e., 17% of global animal proteins available to the world’s human population (Ababouch Citation2015; FAO Citation2020). The negative impact of jellyfish blooms on fishing activities is known, nevertheless much information is lost every year. Fishermen rarely report to port authorities the presence of jellyfish in their catches, which are considered as discard and returned to the sea together with other unwanted species. Until recently, the majority of oceanographic and scientific fishing cruise reports did not incorporate information on jellyfish catches since these were not research target species. Even when jellyfish presence is recorded, information regarding species ID, number of individuals or biometric data is usually missing. Given the significant impact of jellyfish on fisheries worldwide, information on their occurrence should be included in commercial fishing reports, as performed for other species. Equally, scientific cruises should collect as much as possible information on jellyfish, facilitating the creation of large temporal databases. This will be key to understanding the environmental conditions favoring increased jellyfish blooms, modeling jellyfish growth rate, predicting abundance and spatio-temporal distribution at regional and subregional levels, and eventually to develop adequate marine spatial planning and mitigation countermeasures to reduce impacts and consequences of jellyfish abundance.
Negative interactions of jellyfish with marine aquaculture traditionally went unnoticed or received little attention; however, in the last decades awareness of the risk and threats associated to jellyfish blooms is rising among fish farmers, scientists, and associated sectorial stakeholders.
Future scenarios of climate change and ocean warming now foresee increasing jellyfish as a potential driver of negative impacts on fishery and aquaculture (Boero et al. Citation2016; Barange et al. Citation2018). Jellyfish may also establish different links between the two sectors. For example, overfishing may release food and ecological space to jellyfish populations (Purcell et al. Citation2007). Increasing occurrence of jellyfish, as currently observed in many places, may be a manifest symptom of marine ecosystems in distress, which will eventually affect human activities at sea including sea food harvesting. Therefore, more responsible fishery activities (i.e., reducing overfishing, responsible use of sustainable resources) may gradually keep jellyfish blooms in check (by increased fish predation and competition for zooplankton food), so reducing jellyfish-related pressures and risks for mariculture. In the last years global aquaculture production showed the potential to replace reduced fish captures and to overcome total fishery landings. Despite this, more aquaculture facilities might have a counterintuitive, paradoxical effect of boosting outbreaks of jellyfish species with planula-polyp-medusa life stages, by increasing ocean sprawl, i.e., substrate availability for the polyp benthic stages.
Outreach and training programs to fish farms staff and fishermen would help raise awareness on this emerging issue and assist in evaluating the feasibility of action plans, with a case-by-case definition of management guidelines and countermeasure protocols for prevention, mitigation and adaptation against recurrent jellyfish abundances in coastal area.
Lastly, jellyfish represent a valuable fishery target in most of the Southeast Asian countries and the limited data about the impacts of jellyfish bloom on local fisheries represent a significant gap in this analysis. In addition, also several non-Asiatic jellyfish species are showing the potential to be a target for fishery and aquaculture even in countries outside of Asia, including Western countries (López-Martínez and Álvarez-Tello Citation2013; Brotz et al. Citation2017). Mediterranean jellyfish species are proving to have a great potential as novel and sustainable food or feed ingredient source whose acceptance is growing also in European countries’ young consumers (Bleve et al. Citation2019; Torri et al. Citation2020). Sustainable fishing of jellyfish produced by polyps living and asexually reproducing in a different habitat may represent a truly resource, where the medusa stage represents the harvested fruit produced by a long-living, large tree (the benthic, polyp clonal population). Jellyfish may also be regarded as a target species for aquaculture, either for food production or because jellyfish are known to support enhanced secondary production in extensive or semi-extensive polyculture coastal pens with shrimps, bivalves, and sea cucumber (Guo et al. Citation2014; Li et al. Citation2014). Moreover, jellyfish have been shown to be a treasure trove with regards to the content of bioactive molecules of great interest for pharmacology, nutraceutics and cosmeceutics (Leone et al. Citation2013, Citation2015; De Domenico et al. Citation2019). A more realistic picture of the impacts of jellyfish on fishery and aquaculture could be obtained taking all those aspects in consideration. This would lay the foundation for a more helpful assessment of the comprehensive impacts of these organisms on fishery and aquaculture, possibly leading to a planning for fishing diversification and a possible more effective management of this sector. Future study on the biology of jellyfish are needed as they relate to understanding population fluctuation, which in turn can shed light on both negative impacts on productive human activities and on possible sustainable exploitation of jellyfish morphotype as fishery resource.
Based on available information, this is the most updated review on the impact of jellyfish blooms on fishery and marine aquaculture sectors worldwide. A major limitation of this review is that only English literature or reports with English abstracts were taken into consideration and consequently any information published in other languages remained out of the analysis. Further efforts will be required to promote additional search of information at global level, uncovering experience and knowledge of fishery and aquaculture operators and stakeholders from non-English speaking countries. This will represent a prerequisite understanding toward the development of action plans for the management of jellyfish abundance and the valorization of sustainable fishery and aquaculture in coastal waters.
Additional information
Funding
References
- Ababouch L. 2015. Fisheries and aquaculture in context of blue economy. In: Feeding Africa. Dakar, Senegal: Abdou Diouf International Conference Center. p. 21–23.
- Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26(1):32–46. doi:10.1046/j.1442-9993.2001.01070.x
- Arai MN. 1997. A functional biology of Scyphozoa. London (UK): Chapman and Hall.
- Barange M, Bahri T, Beveridge MCM, Cochrane KL, Funge-Smith S, Poulain F, Eds. 2018. Impacts of climate change on fisheries and aquaculture: synthesis of current knowledge, adaptation and mitigation options. FAO Fisheries and Aquaculture Technical Paper No. 627. Rome, FAO. 628 pp.
- Bastian T, Stokes D, Kelleher JE, Hays GC, Davenport J, Doyle TK. 2011. Fisheries bycatch data provide insights into the distribution of the mauve stinger (Pelagia noctiluca) around Ireland. ICES J Mar Sci. 68:436–443.
- Baxter EJ, Albinyana G, Girons A, Isern MM, García AB, Lopez M, Canepa A, Olariaga A, Gili J-M, Fuentes V. 2011. Jellyfish-inflicted gill damage in marine-farmed fish: an emerging problem for the Mediterranean? In: XIII Congreso Nacional de Acuicultura, Barcelona.
- Baxter EJ, Rodger HD, McAllen R, Doyle TK. 2011. Gill disorders in marine-farmed salmon: investigating the role of hydrozoan jellyfish. Aquacult Environ Interact. 1(3):245–257. doi:10.3354/aei00024
- Baxter EJ, Sturt MM, Ruane NM, Doyle K, Mcallen R, Rodger HD. 2012. Biofouling of the hydroid Ectopleura larynx on aquaculture nets in Ireland: implications for finfish health. Fish Vet J 13:17–29.
- Baxter EJ, Sturt MM, Ruane NM, Doyle TK, McAllen R, Harman L, Rodger HD. 2011. Gill damage to Atlantic salmon (Salmo salar) caused by the common jellyfish (Aurelia aurita) under experimental challenge. PLoS One. 6(4):e18529. doi:10.1371/journal.pone.0018529
- BBC. 2014. Jellyfish swarm kills 300,000 salmon at Uist fish farm. [accessed 2020 Jul 14]. https://www.bbc.com/news/uk-scotland-highlands-islands-30493457.
- Bernard P, Berline L, Gorsky G. 2011. Long term (1981-2008) monitoring of the jellyfish Pelagia noctiluca (Cnidaria, Scyphozoa) on Mediterranean coasts (Principality of Monaco and French Riviera). J Oceanogr Res Data. 4:1–10.
- Bleve G, Ramires FA, Gallo A, Leone A. 2019. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 8(7):263. doi:10.3390/foods8070263
- Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S. 2008. Gelatinous plankton: irregularities rule the world (sometimes). Mar Ecol Prog Ser. 356:299–310. doi:10.3354/meps07368
- Boero F, Bouillon J, Piraino S, Schmid V. 2002. Asexual reproduction in the Hydrozoa (Cnidaria) In: Hughes RN, editor. Reproductive biology of invertebrates—progress in asexual reproduction. New Delhi (India): Oxford & IBH Publishing. p 141–158.
- Boero F, Brotz L, Gibbons M, Piraino S, Zampardi S. 2016. Impacts and effects of ocean warming on jellyfish. In: Laffoley D, Baxter JM, editors. Explaining ocean warming: causes, scale, effects and consequences. Gland (Switzerland): IUCN. p 213–237.
- Bosch-Belmar M, Azzurro E, Pulis K, Milisenda G, Fuentes V, Kéfi-Daly Yahia O, Micallef A, Deidun A, Piraino S. 2017. Jellyfish blooms perception in Mediterranean finfish aquaculture. Mar Policy. 76:1–7. doi:10.1016/j.marpol.2016.11.005
- Bosch-Belmar M, Escurriola A, Milisenda G, Fuentes VL, Piraino S. 2019. Harmful fouling communities on fish farms in the SW Mediterranean Sea: composition, growth and reproductive periods. J Mar Sci Eng. 7:1–17.
- Bosch-Belmar M, M’Rabet C, Dhaouadi R, Chalghaf M, Daly Yahia MN, Fuentes V, Piraino S, Kéfi-Daly Yahia O. 2016. Jellyfish stings trigger gill disorders and increased mortality in farmed Sparus aurata (Linnaeus, 1758) in the Mediterranean Sea. PLoS One. 11(4):e0154239. doi:10.1371/journal.pone.0154239
- Bosch-Belmar M, Milisenda G, Girons A, Taurisano V, Accoroni S, Totti C, Piraino S, Fuentes V. 2017. Consequences of stinging plankton blooms on finfish mariculture in the Mediterranean Sea. Front Mar Sci. 4:240. doi:10.3389/fmars.2017.0024
- Brotz L, Cheung W, Kleisner K, Pakhomov E, Pauly D. 2012. Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia 690(1):3–20. doi:10.1007/s10750-012-1039-7
- Brotz L, Pauly D. 2012. Jellyfish populations in the Mediterranean Sea. Acta Adriat. 53:211–230.
- Brotz L, Schiariti A, López-Martínez J, Álvarez-Tello J, Peggy Hsieh YH, Jones RP, Quiñones J, Dong Z, Morandini AC, Preciado M, et al. 2017. Jellyfish fisheries in the Americas: origin, state of the art, and perspectives on new fishing grounds. Rev Fish Biol Fisheries. 27(1):1–29. doi:10.1007/s11160-016-9445-y
- Bruno DW, Ellis AES. 1985. Mortalities in farmed Atlantic salmon associated with the jellyfish Phialella quadrata. Bull Eur Ass Fish Pathol. 5:1984–1985.
- Canepa A, Fuentes V, Sabatés A, Piraino S, Ferdinando B, Josep-María G. 2014. Pelagia noctiluca in the Mediterranean Sea. In: Pitt KA, Lucas CH, editors. Jellyfish blooms. Dordrecht (The Netherlands): Springer Science + Business Media. p 237–266.
- Chen J, Li S, Ding F, Yan L. 2004. Primary analysis on the jellyfish bloom and its cause in the East China Sea and the Yellow Sea. Mod Fish Inf. 19:10–12.
- Chen W, Bo Z, Zhou W, Xue L. 2007. Investigation of the familiar jellyfish species in Zhejiang Sea area and their influence on fishery in flourishing year. J Zhejiang Ocean Univ (Nat Sci). 26:266–271.
- Clinton M, Kintner AH, Delannoy CMJ, Brierley AS, Ferrier DEK. 2020. Molecular identification of potential aquaculture pathogens adherent to cnidarian zooplankton. Aquaculture. 518:734801. doi:10.1016/j.aquaculture.2019.734801
- Condon RH, Duarte CM, Pitt KA, Robinson KL, Lucas CH, Sutherland KR, Mianzan HW, Bogeberg M, Purcell JE, Decker MB, et al. 2013. Recurrent jellyfish blooms are a consequence of global oscillations. Proc Natl Acad Sci USA. 110(3):1000–1005. doi:10.1073/pnas.1210920110
- Conley KR, Sutherland KR. 2015. Commercial fishers’ perceptions of jellyfish interference in the Northern California Current. ICES J Mar Sci. 72(5):1565–1575. doi:10.1093/icesjms/fsv007
- Cronin M, Caroline C, Fiona G. 2004. Salmon mortalities at Inver Bay and McSwyne’s Bay finfish farms, County Donegal, Ireland, during 2003. Mar Environ Heal Ser. 14(129).
- Daly Yahia MN, de Puelles MLF, Licandro P, Malej A, Molinero JC, Frangou IS, Zervoudaki S, Prieto L, Goy J, Kéfi-Daly YO. 2010. Are the outbreaks of Pelagia noctiluca (Forsskål, 1775) more frequent in the Mediterranean basin? Proc “Joint ICES/CIESM Work to Comp Zooplankt Ecol Methodol between Mediterr North Atl (WKZEM)” 300.
- DANAQ. 2020. Cultured aquatic species information programme. Rome: FAO.
- Delannoy CMJ, Houghton JDR, Fleming NEC, Ferguson HW. 2011. Mauve Stingers (Pelagia noctiluca) as carriers of the bacterial fish pathogen Tenacibaculum maritimum. Aquaculture 311(1-4):255–257. doi:10.1016/j.aquaculture.2010.11.033
- Diciotti R, Culurgioni J, Serra S, Trentadue M, Chessa G, Satta CT, Caddeo T, Luglie A, Sechi N, Fois N. 2016. First record of Mnemiopsis leidyi (Ctenophora, Bolinopsidae) in Sardinia (S’Ena Arrubia Lagoon, Western Mediterranean): a threat to local fishery? Medit Mar Sci. 17(3):714–719. doi:10.12681/mms.1719
- De Domenico S, De Rinaldis G, Paulmery M, Piraino S, Leone A. 2019. Barrel Jellyfish (Rhizostoma pulmo) as source of antioxidant peptides. Mar Drugs. 17(2):134. doi:10.3390/md17020134
- Dong Z, Liu D, Keesing JK. 2010. Jellyfish blooms in China: dominant species, causes and consequences. Mar Pollut Bull. 60(7):954–963. doi:10.1016/j.marpolbul.2010.04.022
- De Donno A, Idolo A, Bagordo F, Grassi T, Leomanni A, Serio F, Guido M, Canitano M, Zampardi S, Boero F, et al. 2014. Impact of stinging jellyfish proliferations along south Italian coasts: human health hazards, treatment and social costs. Int J Environ Res Public Health. 11(3):2488–2503. doi:10.3390/ijerph110302488
- Downes JK, Yatabe T, Marcos-Lopez M, Rodger HD, MacCarthy E, O’Connor I, Collins E, Ruane NM. 2018. Investigation of co-infections with pathogens associated with gill disease in Atlantic salmon during an amoebic gill disease outbreak. J Fish Dis. 41(8):1217–1227. doi:10.1111/jfd.12814
- Doyle TK, De Haas H, Cotton D, Dorschel B, Cummins V, Houghton JDR, Davenport J, Hays GC. 2008. Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. J Plankton Res. 30(8):963–968. doi:10.1093/plankt/fbn052
- Duarte CM, Pitt KA, Lucas CH, Purcell JE, Uye SI, Robinson K, Brotz L, Decker MB, Sutherland KR, Malej A, et al. 2013. Is global ocean sprawl a cause of jellyfish blooms? Front Ecol Environ. 11(2):91–97. doi:10.1890/110246
- EU-COM 2008. Directive 2008/56/EC of the European Parliament and of The Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive), EU-COM. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:164:0019:0040:EN:PDF
- FAO. 2018. The State of World Fisheries and Aquaculture. Rome.
- FAO. 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome.
- Ferguson HW, Christian MJ, Delannoy SH, Nicolson J, Sutherland D, Crumlish M. 2010. Jellyfish as vectors of bacterial disease for farmed salmon (Salmo salar). J Vet Diagn Invest. 22(3):376–382. doi:10.1177/104063871002200305
- Ferraris M, Berline L, Lombard F, Guidi L, Elineau A, Mendoza-Vera JM, Lilley MKS, Taillandier V, Gorsky G. 2012. Distribution of Pelagia noctiluca (Cnidaria, Scyphozoa) in the Ligurian Sea (NW Mediterranean Sea). J Plankt Res.34(10):874–885. doi:10.1093/plankt/fbs049
- Fitridge I, Dempster T, Guenther J, de Nys R. 2012. The impact and control of biofouling in marine aquaculture: a review. Biofouling 28(7):649–669. doi:10.1080/08927014.2012.700478
- Fitridge I, Keough MJ. 2013. Ruinous resident: the hydroid Ectopleura crocea negatively affects suspended culture of the mussel Mytilus galloprovincialis. Biofouling 29(2):119–131. doi:10.1080/08927014.2012.752465
- Floerl O, Sunde L, Bloecher N. 2016. Potential environmental risks associated with biofouling management in salmon aquaculture. Aquacult Environ Interact. 8:407–417. doi:10.3354/aei00187
- Ford S. 2019. Jellyfish sting Huon’s Aquaculture’s profits, production. [accessed 2020 Jul 14]. https://www.theadvocate.com.au/story/5919024/moon-jellyfish-sting-huons-aquacultures-profits-production/.
- Fosså J, Flood P, Olsen A, Jensen F. 2003. Småog usynlige, men plagsomme maneter av arten Muggiaea atlantica (Small and invisible, but troublesome jellyfish of the species Muggiaea Atlantica). Fisk og Havet (Fish Sea) 2:99–103.
- Fuentes V, Straehler-Pohl I, Atienza D, Franco I, Tilves U, Gentile M, Acevedo M, Olariaga A, Gili JM. 2011. Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the Mar Menor (Spain, NW Mediterranean). Mar Biol. 158(10):2247–2266. doi:10.1007/s00227-011-1730-7
- Goy J, Morand P, Etienne M. 1989. Long-term fluctuations of Pelagia noctiluca (Cnidaria, Scyphomedusa) in the western Mediterranean Sea. Prediction by climatic variables. Deep Res. 36(2):269–279. doi:10.1016/0198-0149(89)90138-6
- Graham N. 2003. By-catch reduction in the brown shrimp, Crangon crangon, fisheries using a rigid separation Nordmøre grid (grate). Fish Res. 59(3):393–407. doi:10.1016/S0165-7836(02)00015-2
- Graham WM, Martin DL, Felder DL, Asper VL, Perry HM. 2003. Ecological and economic implications of a tropical jellyfish invader in the Gulf of Mexico. Biol Invasions. 5(1/2):53–69. doi:10.1023/A:1024046707234
- Graham WM, Pagès F, Hamner WM. 2001. A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia. 451(1/3):199–212. doi:10.1023/A:1011876004427
- Guenther J, Misimi E, Sunde LM. 2010. The development of biofouling, particularly the hydroid Ectopleura larynx, on commercial salmon cage nets in Mid-Norway. Aquaculture 300(1-4):120–127. doi:10.1016/j.aquaculture.2010.01.005
- Guo K, Zhao W, Wang S, Dong SL. 2014. Study of food web structure and trophic level in the sea ponds of an optimized culture model (jellyfish-shellfish-fish-prawn). Aquacult Int. 22(6):1783–1791. doi:10.1007/s10499-014-9782-6
- Hamner WM, Dawson MN. 2009. A review and synthesis on the systematics and evolution of jellyfish blooms: advantageous aggregations and adaptive assemblages. Hydrobiologia 616(1):161–191. doi:10.1007/s10750-008-9620-9
- Helmholz H, Johnston B, Ruhnau C, Prange A. 2010. Gill cell toxicity of northern boreal scyphomedusae Cyanea capillata and Aurelia aurita measured by an in vitro cell assay. Hydrobiologia 645(1):223–234. doi:10.1007/s10750-010-0216-9
- Herrero A, Thompson KD, Ashby A, Rodger HD, Dagleish MP. 2018. Complex gill disease: an emerging syndrome in farmed Atlantic salmon (Salmo salar L.). J Comp Pathol. 163:23–28. doi:10.1016/j.jcpa.2018.07.004
- Hubot N, Lucas CH, Piraino S. 2017. Environmental control of asexual reproduction and somatic growth of Aurelia spp. (Cnidaria, Scyphozoa) polyps from the Adriatic Sea. PLoS One. 12(6):e0178482. doi:10.1371/journal.pone.0178482
- IntraFish. 2012. Jellyfish attacks in Chiloé cause more salmon deaths. [accessed 2020 Jul 14]. https://www.intrafish.com/news/jellyfish-attacks-in-chilo-cause-more-salmon-deaths/1-1-513564.
- Kawahara M, Uye SI, Ohtsu K, Iizumi H. 2006. Unusual population explosion of the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) in East Asian waters. Mar Ecol Prog Ser. 307:161–173. doi:10.3354/meps307161
- Kideys AE. 1994. Recent dramatic changes in the Black Sea ecosystem: the reason for the sharp decline in Turkish anchovy fisheries. J Mar Syst. 5(2):171–181. doi:10.1016/0924-7963(94)90030-2
- Kim DH, Seo JN, Yoon WD, Suh YS. 2012. Estimating the economic damage caused by jellyfish to fisheries in Korea. Fish Sci. 78(5):1147–1152. doi:10.1007/s12562-012-0533-1
- Knutsen T, Hosia A, Falkenhaug T, Skern-Mauritzen R, Wiebe PH, Larsen RB, Aglen A, Berg E. 2018. Coincident mass occurrence of gelatinous zooplankton in Northern Norway. Front Mar Sci. 5:158. doi:10.3389/fmars.2018.00158
- Leone A, Lecci RM, Durante M, Meli F, Piraino S. 2015. The bright side of gelatinous blooms: nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar Drugs. 13(8):4654–4681. doi:10.3390/md13084654
- Leone A, Lecci RM, Durante M, Piraino S. 2013. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar Drugs. 11(5):1728–1762. doi:10.3390/md11051728
- Li J, Dong S, Gao Q, Wang F, Tian X, Zhang S. 2014. Total organic carbon budget of integrated aquaculture system of sea cucumber Apostichopus japonicus, jellyfish Rhopilema esculenta and shrimp Fenneropenaeus chinensis. Aquac Res. 45:1825–1831.
- López-Martínez J, Álvarez-Tello J. 2013. The jellyfish fishery in Mexico. Agric Sci. 04(06):57–61. doi:10.4236/as.2013.46A009
- Lotan A, Ben-Hillel R, Loya Y. 1992. Life cycle of Rhopilema nomadica: a new immigrant scyphomedusan in the Mediterranean. Mar Biol. 112(2):237–242. doi:10.1007/BF00702467
- Lotan A, Fine M, Ben-Hillel R. 1994. Synchronization of the life cycle and dispersal pattern of the tropical invader Scyphomedusan Rhopilema nomadica is temperature dependent. Mar Ecol Prog Ser. 109:59–66. doi:10.3354/meps109059
- Lucas CH, Gelcich S, Uye S-I. 2014. Living with jellyfish: management and adaptation strategies. In: Pitt KA, Lucas C, editors. Jellyfish blooms. Dordrecht (The Netherlands): Springer Science + Business Media. p. 129–150.
- Marcos-López M, Mitchell SO, Rodger HD. 2016. Pathology and mortality associated with the mauve stinger jellyfish Pelagia noctiluca in farmed Atlantic salmon Salmo salar L. J Fish Dis. 39(1):111–115. doi:10.1111/jfd.12267
- Mariottini GL, Giacco E, Pane L. 2008. The Mauve Stinger Pelagia noctiluca (Forsskål, 1775). Distribution, ecology, toxicity and epidemiology of stings. A review. Mar Drugs. 6(3):496–513. doi:10.3390/md20080025
- Mianzan H, Quiñones J, Palma S, Schiariti A, Acha EM, Robinson KL, Graham WM. 2014. Chrysaora plocamia: a poorly understood jellyfish from South American waters. In: Pitt KA, Lucas CH, editors. Jellyfish blooms. Dordrecht (The Netherlands): Springer Science + Business Media. p. 219–236.
- Milisenda G, Martinez-Quintana A, Fuentes VL, Bosch-Belmar M, Aglieri G, Boero F, Piraino S. 2018. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar Coast Shelf Sci. 201:29–39. doi:10.1016/j.ecss.2016.01.002
- Mitchell SO, Baxter EJ, Rodger HD. 2013. Gill pathology in farmed salmon associated with the jellyfish Aurelia aurita. Vet Rec Case Rep. 1(1):e100045. doi:10.1136/vetreccr.100045rep
- Mitchell SO, Rodger HD. 2011. A review of infectious gill disease in marine salmonid fish. J Fish Dis. 34(6):411–432. doi:10.1111/j.1365-2761.2011.01251.x
- Nagata RM, Haddad MA, Nogueira M. 2009. The nuisance of medusae (Cnidaria, Medusozoa) to shrimp trawls in central part of southern Brazilian Bight, from the perspective of artisanal fishermen. Panam J Aquat Sci. 4:312–325.
- Nastav B, Malej M, Malej Jr A, Malej A. 2013. Is it possible to determine the economic impact of jellyfish outbreaks on fisheries? A case study—Slovenia. Medit Mar Sci. 14(1):214–223. doi:10.12681/mms.382
- Norambuena R, González L. 2020. National aquaculture sector overview—Chile. FAO.
- O’Sullivan K. 2017. Stinger jellyfish swarms wipe out farmed salmon in west of Ireland. [accessed 2020 Jul 14]. https://www.irishtimes.com/news/environment/stinger-jellyfish-swarms-wipe-out-farmed-salmon-in-west-of-ireland-1.3247245.
- Öztürk B, İşinibilir M. 2010. An alien jellyfish Rhopilema nomadica and its impacts to the Eastern Mediterranean part of Turkey. J Black Sea/Mediterranean Environ. 16:149–156.
- Palmieri MG, Barausse A, Luisetti T, Turner K. 2014. Jellyfish blooms in the Northern Adriatic Sea: Fishermen’s perceptions and economic impacts on fisheries. Fish Res. 155:51–58. doi:10.1016/j.fishres.2014.02.021
- Palmieri MG, Schaafsma M, Luisetti T, Barausse A, Harwood A, Sen A, Turner RK. 2015. Jellyfish blooms and their impacts on welfare benefits: recreation in the UK and fisheries in Italy. In: Turner RK, Schaafsma M, editors. Coastal zones ecosystem services. Springer. p. 219–240.
- Parsons TR, Lalli CR. 2002. Jellyfish population explosions: revisiting and hypothesis of possible causes. Société Fr D’océanographie 40:111–121.
- Practical F. 2014. Jellyfish devastate salmon farm. [accessed 2020 Jul 14]. https://www.practicalfishkeeping.co.uk/fishkeeping-news/jellyfish-devastate-salmon-farm/.
- Purcell JE. 2007. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar Ecol Prog Ser. 348:183–196. doi:10.3354/meps07056
- Purcell JE, Arai MN. 2001. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia. 451(1/3):27–44. doi:10.1023/A:1011883905394
- Purcell JE, Baxter EJ, Fuentes VL. 2013. Jellyfish as products and problems of aquaculture. In: Allan G, Burnell G, editors. Advances in aquaculture hatchery technology. 1st ed. Oxford (UK): Woodhead Publishing. p 404–430.
- Purcell JE, Uye S, Lo W-TL. 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Mar Ecol Prog Ser. 350:153–174. doi:10.3354/meps07093
- Quiñones J, Monroy A, Acha EM, Mianzan H. 2013. Jellyfish bycatch diminishes profit in an anchovy fishery off Peru. Fish Res. 139:47–50. doi:10.1016/j.fishres.2012.04.014
- Richardson AJ, Bakun A, Hays GC, Gibbons MJ. 2009. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol Evol. 24(6):312–322. doi:10.1016/j.tree.2009.01.010
- Rodger HD, Murphy K, Mitchell SO, Henry L. 2011. Gill disease in marine farmed Atlantic salmon at four farms in Ireland. Vet Rec. 1:1–4.
- Ruzicka JJ, Brodeur RD, Emmett RL, Steele JH, Zamon JE, Morgan CA, Thomas AC, Wainwright TC. 2012. Progress in oceanography interannual variability in the Northern California current food web structure: changes in energy flow pathways and the role of forage fish, euphausiids, and jellyfish. Prog Oceanogr. 102:19–41. doi:10.1016/j.pocean.2012.02.002
- Schiariti A, Kawahara M, Uye S, Mianzan HW. 2008. Life cycle of the jellyfish Lychnorhiza lucerna (Scyphozoa: Rhizostomeae). Mar Biol. 156(1):1–12. doi:10.1007/s00227-008-1050-8
- Seaton DD. 1989. Fish kills by planktonic organisms. Aquac Inf Ser. 9:1–10.
- Shiganova TA. 1998. Invasion of the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic community structure. Fish Oceanogr. 7(3‐4):305–310. doi:10.1046/j.1365-2419.1998.00080.x
- Sievers M, Fitridge I, Dempster T, Keough MJ. 2013. Biofouling leads to reduced shell growth and flesh weight in the cultured mussel Mytilus galloprovincialis. Biofouling. 29(1):97–107. doi:10.1080/08927014.2012.749869
- Siggins L. 2013. Jellyfish ‘bloom’ kills thousands of farmed salmon off Co Mayo. [accessed 2020 July 14]. https://www.irishtimes.com/news/ireland/irish-news/jellyfish-bloom-kills-thousands-of-farmed-salmon-off-co-mayo-1.1567468.
- Småge SB, Brevik ØJ, Frisch K, Watanabe K, Duesund H, Nylund A. 2018. Correction: concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms. PLoS One. 13 (1):e0190762. doi:10.1371/journal.pone.0190762
- Tilves U, Fuentes V, Milisenda G, Parrish CC, Vizzini S, Sabatés A. 2018. Trophic interactions of the jellyfish Pelagia noctiluca in the NW Mediterranean: evidence from stable isotope signatures and fatty acid composition. Mar Ecol Prog Ser. 591:101–116. doi:10.3354/meps12332
- Torri L, Tuccillo F, Bonelli S, Piraino S, Leone A. 2020. The attitudes of Italian consumers towards jellyfish as novel food. Food Qual Prefer. 79:103782. doi:10.1016/j.foodqual.2019.103782
- Utne-Palm AC, Salvanes AGV, Currie B, Kaartvedt S, Nilsson GE, Braithwaite VA, Stecyk JAW, Hundt M, Van Der Bank M, Flynn B, et al. 2010. Trophic structure and community stability in an overfished ecosystem. Science. 329(5989):333–336. doi:10.1126/science.1190708
- Uye S, Shimauchi H. 2005. Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. J Plankton Res. 27(3):237–248. doi:10.1093/plankt/fbh172
- Uye SI. 2011. Human forcing of the copepod-fish-jellyfish triangular trophic relationship. Hydrobiologia. 666(1):71–83. doi:10.1007/s10750-010-0208-9
- Yan L, Li S, Ding F. 2004. The preliminary studies on the dynamics of macrojellyfish resources and their relationship with fisheries in the East China Sea and Yellow Sea. Mar Fish. 26:9–12.
- Yasuda T. 1988. Studies on the common jelly-fish, Aurelia aurita (Linné). Jpn Fish Resour Cons Assoc, Tokyo, 139 pp. (in Japanese with English abstract)
- Zaki A. 2018. Salmon spooked by jellyfish can breathe easy in bigger cages. [accessed 2020 July 14]. https://www.stuff.co.nz/business/farming/aquaculture/101067707/salmon-spooked-by-jellyfish-can-breathe-easy-in-bigger-cages.