Abstract
This article reviews the international literature on the natural enemies of Dreissena spp.—bivalves which internationally have strong impacts on aquatic ecosystems, industries, fisheries, and aquaculture. It represents a revised edition of the initial treatment on this topic published over two decades ago, and as in the previous publication, it reviews the biology and ecology of organisms known to be involved in the predation (143 species), parasitism and commensalism (86 species and higher taxa), and competitive exclusion (14 species) of species in the genus Dreissena. Predators can at times have major impacts on dreissenid populations, but these reductions are typically only temporal and in restricted (e.g., shallow) areas within large waterbodies. A cumulative effect of a growing suite of enemies may have a constant, but overall limited, role in suppressing Dreissena densities—one far from any likelihood of population eradication. A diverse and abundant community of natural enemies, however, is beneficial because of its positive impact on energy flow. The introduction of dreissenids has redirected energy from the planktonic to the benthic community and predators, in particular molluscivorous fish and waterfowl, have served to redistribute this energy flow back into the pelagic environment.
1. Introduction
The dreissenid bivalves Dreissena polymorpha (Pallas), the zebra mussel and D. rostriformis bugensis (Andrusov), the quagga mussel—two species native to the Ponto Caspian region—are considered among the most aggressive freshwater invaders due to the economic and ecological disruptions that have accompanied their spread across Europe and North America (Beekey et al. Citation2004; Burlakova et al. Citation2012; Higgins and Vander Zanden Citation2010; Karatayev and Burlakova Citation2022a; Karatayev et al. Citation1997, Citation2002a, Citation2007, Citation2015; Nalepa and Schloesser Citation2013; van der Velde et al. Citation2010). Numerous factors have been cited to explain their rapid spread and explosive population growth in invaded habitats, including life history traits, such as short generation time, high fecundity, a planktonic larval stage that facilitates dispersal, a byssate adult stage allowing dense aggregations, the ability to filter-feed on a wide range of plankton, high individual growth rates, and wide tolerance of a range of environmental conditions (Johnson and Carlton Citation1996; Karatayev and Burlakova Citation2022a; Karatayev et al. Citation1997, Citation2007, Citation2015). While their economic impacts typically stem from increased operating expenses resulting from their fouling of water pipes and other raw water-dependent infrastructure (Connelly et al. Citation2007; Nakano and Strayer Citation2014; O’Neill Citation2008; Pimentel Citation2005), their ecological impacts are primarily related to their effectiveness as ecosystem engineers in modifying the bottom substrates and reshaping energy and nutrient fluxes through benthic and pelagic habitats, resulting in fundamental changes in food web structures (Burlakova et al. Citation2023; Higgins and Vander Zanden Citation2010; Karatayev and Burlakova Citation2022a; Karatayev et al. Citation1997, Citation2002a, Citation2007, Citation2015; Mayer et al. Citation2002). Both economic and ecological consequences of dreissenids invasion have extremely strong impacts on fisheries and aquaculture (reviewed in Burlakova et al. Citation2023; Karatayev and Burlakova Citation2022a, Citation2022b).
Because of their significant impacts, there has been a concerted effort to better understand the biotic and abiotic factors that govern their population dynamics, in particular, to identify the factors that lead to their mortality and population control. In this regard, a recurrent question is what role do natural enemies play in controlling their population dynamics? According to the enemy release hypothesis, the relative absence of a diverse suite of native enemies (mainly predators and parasites) in newly invaded regions contributes to their rapid population growth (Gozzi et al. Citation2020; Jeschke and Heger Citation2018; MacLeod et al. Citation2010; Torchin et al. Citation2003). With time, however, native predators may adapt to become more effective at feeding on the exotic prey, either rapidly, due to phenotypic plasticity, or more slowly, via natural selection (Carlsson et al. Citation2009, Citation2011). Thus, the introduction and rapid spread of dreissenids throughout North America has been associated with the virtually complete escape of both D. polymorpha and D. r. bugensis from their species-specific endosymbionts (defined in this article as parasites and commensals) (Karatayev and Burlakova Citation2022a; Mastitsky et al. Citation2014; Molloy et al. Citation1997), and most of the predators evolutionary adapted to consume these mussels. Although the vast majority of the enemies of Dreissena in their Ponto Caspian native range are not present in North America, ecologically similar species do exist in the newly invaded areas, and these native predators, parasites, and ecological competitors are gaining the status as the new natural enemies of dreissenids (reviewed in Burlakova et al. Citation2023; Carlsson and Strayer Citation2009; Carlsson et al. Citation2011; Karatayev and Burlakova Citation2022a, Citation2022b; Molloy et al. Citation1997).
The first contribution synthesizing the international literature on the natural enemies, including predators (95 species), parasites and commensals (34 species), and competitors (11 species) of Dreissena was published over 25 years ago (Molloy et al. Citation1997).Footnote1 The rate of publications on Dreissena, including surveys of their enemies and competitors, increased greatly in the last three decades following their discovery in North America in the late 1980s (reviewed in Karatayev and Burlakova Citation2022a; Nalepa and Schloesser Citation2013; van der Velde et al. Citation2010) and their parallel spread to several European countries in the late twentieth century (Karatayev and Burlakova Citation2022a). During the last 25 years, a large number of predators were reported to feed on dreissenids both in the Old and in the New World (reviewed in Burlakova et al. Citation2023; Karatayev and Burlakova Citation2022a), and new species-specific parasites of D. polymorpha were described (Molloy et al. Citation2005, Citation2012).
The present article is an updated and significantly expanded second edition of Molloy et al. (Citation1997), summarizing the current knowledge of the diversity, impact, and geographical distribution of Dreissena’s enemies as well as the ecological interactions between dreissenids and their enemies. A total of 606 references are cited, including the 252 publications cited by Molloy et al. (Citation1997) which reported on nearly 140 years of research (from 1858 to 1996).
Although this review covers available information on the enemies of D. polymorpha and D. r. bugensis that have earned this genus its international pest status, it also includes available information (albeit relatively limited due to their non-pest status) on the enemies of other Dreissena spp. (e.g., D. carinata, endemic to Lake Ohrid, Republic of North Macedonia).
Obviously, not all enemies have been studied with equal intensity. In organizing this review, subheadings were used freely to assist the reader in identifying the subject areas where information on a specific enemy was significant. As a result, subheadings differ among sections, and some potentially important issues are missing altogether due to the lack of published information. Because of space limitations, this review has attempted to be thorough, but not exhaustive in coverage, and has focused on papers containing clear and conclusive data, as opposed to anecdotal information.
Species and common names of the fishes listed in the text follow Robins et al. (Citation1991a, Citation1991b).
2. Predators
2.1. Anti-predator adaptations: a cost-benefit tradeoff
Sedentary organisms are particularly vulnerable to predation and often have evolved morphological adaptations in response to predation threats. In molluscs, the shell is the main morphological defense against predators (Ponder et al. Citation2019), and in dreissenids and other byssate bivalves, its firm adhesion to the substrate significantly decreases their vulnerability to predation (Reimer and Tedengren Citation1997). The shell’s strength (i.e., its crush resistance) is also a major defense. Stronger shells in zebra mussel populations from European lakes with increased predation mortality indicate that higher energy investments in shell production are evolutionarily justified (Czarnoleski et al. Citation2006). Both zebra and quagga mussels respond to predation risk cues by forming thicker shells (Naddafi and Rudstam Citation2014a), but the responsiveness of mussels to predators decreases as shells grow in size and become less vulnerable to crushing (Czarnoleski and Muller Citation2013).
In addition to morphological adaptations, a wide and complex variety of behaviors (as detailed below) have been reported from experimental laboratory studies in which zebra mussels were exposed to predation risks (Coons et al. Citation2004; Czarnoleski and Muller Citation2013; Czarnoleski et al. Citation2011; Dzierzynska-Bialonczyk et al. Citation2019; Kobak Citation2013; Kobak and Rynska 2014; Kobak et al. Citation2010; Naddafi et al. Citation2007; Toomey et al. Citation2002). In the presence of predators, they are more likely to reduce their mobility and form denser aggregations (Kobak Citation2013; Kobak et al. Citation2010). In response to predation cues, zebra mussels may increase or decrease the strength of byssal attachment (Czarnoleski and Muller Citation2013; Czarnoleski et al. Citation2011; Hirsch et al. Citation2014). Their crawling tends to reflect a negative phototaxis, suggesting a strategy to decrease their vulnerability to predators by their seeking darkness or dimly lit refugia (Kobak 2002, Citation2013). Small zebra mussels often climb upwards, but light and predator scents inhibit that movement (Kobak Citation2013). In the presence of light mussels gape less and have lower feeding/clearance rates—suggesting a strategy to reduce both the release of disclosing metabolites, as well as potential injury to their exposed soft tissues (Kobak Citation2013). In the presence of crushed conspecifics, zebra mussels reduce their gaping and decrease their crawling speed resulting in traveling shorter distances (Kobak and Rynska 2014). Houghton and Janssen (Citation2013) found that in the presence of high round goby densities, dreissenids strike a balance between lowered risk of predation by occupying sheltered habitats (under rocks and in crevices), and enhanced feeding opportunities (and likely spawning) by occupying more exposed habitats. Like other byssate mussels, zebra mussels appear to adjust these antipredator strategies in relation to the spatio-temporal variability of predation pressure during their lifespan (Antol et al. 2018; Czarnoleski and Muller Citation2013; Czarnoleski et al. Citation2010, Citation2011; Dzierzynska-Bialonczyk et al. Citation2019; Reimer and Tedengren Citation1997). Zebra mussels use chemosensors to assess predation risks and differentiate between species of predators, to tune their responses to the type and intensity of the alarm cues, and to recognize the immediacy of predatory attacks (Czarnoleski and Muller Citation2013).
Anti-predator behaviors have also been investigated in quagga mussels. In laboratory experiments comparing the predator-induced responses of zebra and quagga mussels, Naddafi and Rudstam (Citation2013) observed that quagga mussels did not lower their clearance rates as much as zebra mussels, but that the strength of the other responses measured, e.g., increased aggregation, increased attachment strength, and increased use of refuge sites, was similar between the two species.
The energy resources invested in anti-predator morphological and behavioral responses can have negative long-term consequences for the bivalves themselves. Anti-predator adaptive strategies similar to those aforementioned in dreissenids have also been observed in marine bivalves, such as hard clams, Mercenaria mercenaria (Smee and Weissburg Citation2006) and blue mussels, Mytilus edulis (Reimer and Tedengren Citation1997). Anti-predator responses (in particular reduced gaping/feeding) in these non-dreissenid bivalves, although potentially enhancing their short-term survival, have been documented to also be costly, with growth and reproductive output potentially diminished when the exposure to predation is prolonged (Large et al. Citation2012; Nakaoka Citation2000). Similarly, Naddafi and Rudstam (Citation2014c) found that predator-induced increases in shell thickness in zebra and quagga mussels observed in laboratory tests were negatively correlated with their growth rates. In their study of European lakes, where zebra mussels had been gradually replaced by quagga mussels, Balogh et al. (Citation2019, Citation2022) reported that in contrast to quagga mussels, zebra mussels exhibited two better developed anti-predator strategies—more crush-resistant shells and stronger byssal attachment, but at the cost of their lower weight increments per unit length and lower glycogen content throughout their entire size range. Balogh et al. (Citation2019) concluded that the comparatively faster growth rate they observed in quagga mussels was likely enabled by their weaker development of the aforementioned anti-predation strategies and may have promoted their competitive success over zebra mussels.
2.2. Fish
Of the 77 fish species recorded as consuming dreissenids, 42 were field-documented as preying only on attached mussels, 22 on both life stages, and 13 feeding exclusively on planktonic larvae ( and ). These numbers include the 46 species listed by Molloy et al. (Citation1997), of which 23 prey on attached mussels only, 15 prey on both planktonic and attached mussels, and 8 were observed to feed on planktonic mussels only ( and ).
Table 1. Fish documented in field studies in Europe (E) and North America (NA) as predators of planktonic Dreissena.
Table 2. Fish documented in field studies in Europe (E) or North America (NA) as predators of attached (i.e., juvenile and adult) Dreissena.
2.2.1. Fish predation on planktonic dreissenid mussels
Relatively little research had been conducted on the predation of planktonic dreissenids by fish (primarily fry), and their impact on dreissenid populations is generally unknown. This literature review indicates that 11 European and 11 North American fish species have been field-documented as containing planktonic Dreissena larvae in their alimentary tracts. These fishes include several families: Atherinidae (one species); Cyprinidae (eight species), Clupeidae (four species), Gadidae (one species), Osmeridae (two species), Percidae (three species), Percichthyidae (two species), and Salmonidae (one species) (). Predation on larval dreissenids by fish is likely far more common than these few records indicate. Published reports frequently use the term “veliger” to characterize the larval stage of Dreissena observed. The mussel’s initial larval stage, the trochophore, is also likely a prey item for fish, but this has not been specifically reported.
During the summer, Dreissena veligers often comprise up to 73% of total zooplankton density and up to 40% of the zooplankton biomass and production (Bowen et al. Citation2018; David et al. Citation2009; Karatayev et al. Citation2010; Kornobis Citation1977; Lazareva et al. Citation2016; Lvova et al. Citation1994; Mitrakhovich and Karatayev Citation1986; Wiktor Citation1958; Withers et al. Citation2015). In Europe, fish predation on dreissenid larvae has not been extensively quantified but is considered to be high, for example, in the eutrophic inland waters of the Netherlands which contain dense fish stocks (van der Velde et al. Citation1994). In a Russian reservoir, Grigorash (Citation1963) reported 20–40 veligers in roach (Rutilus rutilus) fry during July. Wiktor (Citation1958), who observed up to 65 D. polymorpha larvae in one fry, reported that predation on Dreissena larvae in the Lagoon of Szczecin (Poland) by hatchlings of several fish species was typically brief (2–4 weeks) and involved primarily fry >12 mm in length. In studies in Poland, Dreissena veligers were recorded in unidentified 10–16 mm fry (Kornobis Citation1977), and in Russia in 12–16 mm roach (Spanowskaya Citation1963). In the Dnieprodzershinskoe Reservoir (Ukraine), larval Dreissena represented 63 and 37% of the biomass ingested, respectively, by fry of roach (R. rutilus) and bream (Abramis brama) (Belyaev et al. Citation1970). In the Khodorovskoe region of the Kanewskoe Reservoir (Ukraine), veligers were a regular component in the diet of fry of the silver bream (Abramis bjoerkna), rudd (Scardinius erythrophthalmus), and bleak (Alburnus alburnus) (Voronchuck et al. Citation1983). Chrisafi et al. (Citation2007), analyzing stomach contents from 240 specimens collected monthly in Trichonis Lake (Greece), found that larvae of D. polymorpha were the dominant prey for Atherina boyeri.
In North America, Limburg and Ahrend (Citation1994) reported that in the Hudson River (USA) dreissenid veligers were present in 44.2% of the feeding white perch (Morone americana) fry between 3.1 and 6.6 mm standard length. The heaviest predation of veligers occurred in the 3.5–4.4 mm size class. Of the 23 white perch analyzed, 70 and 17% contained one and four veligers, respectively (Limburg et al. Citation1997), as well as one blueback herring (Alosa aestivalis) with a single veliger. In the latter study, the authors found that zebra mussel larvae in the Hudson River were most commonly consumed by the smallest larval size class (<5 mm) of striped bass and white perch. In Lake Ontario, Mills et al. (Citation1995) observed planktivory of dreissenid veligers by young-of-the-year and adults of both alewife (Alosa pseudoharengus) and rainbow smelt (Osmerus mordax), but they concluded that consumption was not sufficient to substantially reduce veliger densities in nearshore waters. Likewise, Stanczykowska (Citation1987) concluded that fish fry (species not identified) consume only small amounts of larval D. polymorpha and that this predation does not have major effects on mussel populations.
More data on fish predation has become available in the last few decades. In Lake Ontario, veliger biomass increased between 2008 and 2014, and represented up to 39% of total zooplankton biomass in May–October (Bowen et al. Citation2018). In contrast, zooplankton biomass declined markedly in the deep Great Lakes colonized by dreissenids (reviewed in Karatayev and Burlakova Citation2022b). As a result, fish learned to prey on the abundant veligers when other more preferred food items are rare. In southeastern Lake Michigan in 2010 and 2011 dreissenid veligers comprised 69–100% of yellow perch and up to 38% of alewife diets, complementing early-feeding larvae with a relatively abundant prey source that may partially offset the apparent low consumption of other prey (Withers et al. Citation2015). In western Lake Erie, dreissenid veligers comprised 20% of larval yellow perch diet, indicating that dreissenid mussels may influence larval yellow perch foraging, growth, and survival (Marin Jarrin et al. Citation2015). In Hudson River D. polymorpha veligers made up over 68% of Alosa sapidissima diet by individual counts and 25% by dry weight (Nack et al. Citation2015). The importance of veligers as a diet item, however, greatly depends on larval fish–veliger temporal and spatial (both in-depth and local distribution) overlap, yearly shifts in veliger abundance, as well as their digestibility, nutritional quality, and the fate of veliger production incorporated into the food web—all questions in need of further study.
2.2.2. Fish predation on attached (byssate) dreissenid mussels
Consumption of dreissenids attached to the substrate has been recorded for at least 64 fish species (13 families), including 31 species (10 families) in North America and 37 species (10 families) in Europe (). Only four species—the common carp (Cyprinus carpio), pumpkinseed (Lepomis gibbosus), black carp (Mylopharyngodon piceus), and round goby (Neogobius melanostomus)—have been field-documented as predators on both continents. Some of these references, however, contain records of only occasional findings of a few dreissenids in the guts of fish species. For example, in a four year study summarizing food consumption by eels (Anguilla anguilla) in Lake Balaton (Hungary), the percentage of prey items in the gut that were bivalves (including Dreissena) averaged 0.4%, with a maximum of 5.6% (Biro Citation1974). Nine other species of fish (Acipenser sturio, Gymnocephalus cernua, Esox lucius, Notropis hudsonius, Prosopium cylindraceum, Salmo trutta, Salvelinus profundus, Salvelinus umbla, and Squalius cephalus) were reported feeding on Dreissena at very limited rates (). Dreissenids occurred in their diets very sparsely, seasonally, and in some cases, mussels could possibly have been consumed as incidental by-catch.
In Europe, Cyprinidae are the most common dreissenid predators, especially roach, and to a lesser degree, carp, silver bream, and common bream (). Among all fish species, roach is clearly the most dominant, widely reported, and aggressive predator of Dreissena in European waters. In North America, round gobies are the most commonly reported predators of dreissenids, although freshwater drum (Aplodinotus grunniens: Sciaenidae), common carp (Cyprinus carpio), and pumpkinseed (Lepomis gibbosus) are also well documented ().
2.2.3. Factors affecting fish predation
Some of the biotic and abiotic factors affecting fish predation rates on dreissenids are discussed below. This list, however, is far from complete because population-wise fish consumption of dreissenids is not well understood and is difficult to assess accurately. For example, the same fish species, such as common bream (Abramis brama), has been noted to feed on Dreissena at different intensities in various waterbodies. Dreissenids are rarely eaten by common bream in Russia’s Kuibyshev Reservoir basin (Egereva Citation1971) but are common dietary items in both the Moldovan Kuchurgansky Liman (Gontya Citation1971) and in the Ukrainian Kanevskoe Reservoir (Severenchuk and Kaftannikova Citation1983). Similarly, their consumption by round gobies varies both seasonally and with depth (French and Jude Citation2001; Schaeffer et al. Citation2005; Walsh et al. Citation2007) and differs substantially depending on the time of day, habitat, and region, suggesting a capacity to adapt to locally abundant food sources (Kornis et al. Citation2012). In lakes Huron and Ontario round gobies prey on dreissenids in shallow water (27–46 m) and on native invertebrates at greater depths (Schaeffer et al. Citation2005; Walsh et al. Citation2007).
2.2.3.1. Fish size
Fish have a clearly marked threshold body size above which they begin to feed on dreissenids (), which often relates to morphological changes in their pharynx. The presence of molariform pharyngeal teeth is the key characteristic shared by benthivorous fishes that are effective predators of molluscs, including dreissenids (French Citation1993, Citation1997). The roach, the most aggressive European predator of dreissenids, does not begin to prey on them until its teeth become well developed (at fish lengths of ca. 140–160 mm) (French Citation1993). The crushing power of the pharyngeal teeth of the roach is exceptional, thus, allowing them to ingest relatively large dreissenids (Nagelkerke and Sibbing Citation1996). Round gobies also have robust molariform teeth capable of crushing dreissenids (Ghedotti et al. Citation1995), but sometimes swallow intact specimens (Andraso et al. Citation2011; Ray and Corkum Citation1997). Ontogenetic changes in the pharyngeal morphology of round gobies at ca. 60 mm of length may contribute to their diet shift to dreissenids. The pharyngeal teeth of gobies <50 mm are small, narrow, and papilliform, consistent with a diet based on soft-bodied prey, whereas in larger individuals (∼80 mm) they develop into the molariform teeth typical for molluscivorous fish (Andraso et al. Citation2011). Dreissenids comprise up to 82% of the diet of round gobies that are 80–90 mm in total length, while larger (>100 mm) round gobies can feed almost exclusively on dreissenids (Jude et al. Citation1995). The pharyngeal morphology of round gobies can also change depending on the prevalent food: gobies preying mostly on dreissenid mussels have wider and more robust lower pharyngeal teeth than those from dreissenid-free locations, where crustaceans are their main prey (Andraso et al. Citation2017). The molariform pharyngeal teeth of the common carp are adapted to move upward to crush and grind mollusc shells against a chewing pad located on the pharyngeal roof; European studies suggest that the common carp prefers more thin-shelled molluscs than dreissenids (Ivlev Citation1961; Stein et al. Citation1975). Tucker et al. (Citation1996) provided evidence of the effectiveness of the common carp’s molariform pharyngeal teeth and chewing pads concluding that all dreissenids consumed are crushed, regardless of the size of the carp or the dreissenid.
Table 3. Relationship between predation on attached (i.e., juvenile and adult) Dreissena and fish length and/or age in field populations.
In efficient predators, form and function complement each other. In the closed position, the angle of the pharyngeal teeth of the cyprinid Vimba elongata (∼44°) is almost identical to that of the valves of D. polymorpha (∼45°), suggesting that this species is particularly well adapted for crushing the shells of dreissenids (Ritterbusch-Nauwerck Citation1991). Among the cyprinids, oral gape, pharyngeal slit, and chewing cavity are particularly well matched in size in roach, i.e., any large mussel that can reach the chewing cavity can be crushed. In contrast, although the oral gape of the common bream is large, its ability to crush large mussels in the pharyngeal region is limited, and large individuals are often rejected (Nagelkerke and Sibbing Citation1996).
In addition to the nonindigenous species like the common carp and the round goby, molariform pharyngeal teeth are only present in a limited number of native fishes in North America, including freshwater drum (A. grunniens), redear sunfish (Lepomis microlophus), pumpkinseed (L. gibbosus), copper redhorse (Moxostoma hubbsi), and river redhorse (Moxostoma carinatum) (French Citation1993). Predation of dreissenids by freshwater drum commences at a length where they first become able to crush shells (ca. 250–265 mm) and increases with the total length of drum; while 70% of the dreissenid shells in drum stomachs are shattered, no apparent damage was reported to the digestive tracts from these fragments (French and Bur Citation1993). The diet of lake whitefish in Lake Michigan changes with growth from cladoceran zooplankton to soft-bodied macroinvertebrates, and large (>350 mm) fish mainly consume molluscs, particularly D. r. bugensis (Pothoven and Nalepa Citation2006) (). In contrast, lake whitefish from Lake Champlain, invaded only by D. polymorpha, did not show a dietary shift toward dreissenid mussels, but instead fed primarily on fish eggs, Mysis diluviana, gastropods, and sphaeriids, resulting in higher fish condition and energy density compared with those of lakes Michigan, Huron, and Ontario after the dreissenid invasion (Herbst et al. Citation2013).
Since well-developed molariform pharyngeal teeth are clearly the key characteristic of highly effective molluscivores, fishes lacking these structures, although capable of preying on bivalves, will likely only be minor consumers of dreissenids. Ictalurids (e.g., the channel catfish, Ictalurus punctatus) and most centrarchids have cardiform pharyngeal teeth which are not adapted for crushing shells; these fish swallow the bivalves whole (Herod et al. Citation1997) and are likely to prey on dreissenids only when their preferred prey items are scarce (French Citation1993; McMahon Citation1991). The presence or absence of molariform pharyngeal teeth can also affect the rate of net energy intake by fish. Due to the lack of shell-crushing organs, blue catfish (Ictalurus furcatus) spend little time processing zebra mussel prey and ingest large amounts of low-energy food per unit time (dreissenids with their shells). Conversely, freshwater drum and redear sunfish spend more time processing their prey (i.e., crushing the shells), and thus, obtain less but higher-energy food (only the flesh) (Magoulick and Lewis Citation2002). Eels are known to be mollusc predators and have occasionally been reported preying on dreissenids (); since they lack well-developed pharyngeal teeth, the shells instead are crushed by their powerful jaws (French Citation1993).
2.2.3.2. Habitat overlap
Obviously, in order for predation to occur at all, the foraging habitats of a benthivorous fish must overlap areas of dreissenid presence. Pumpkinseed prefers vegetated or otherwise sheltered littoral areas in rivers and lakes, which are also suitable habitat for dreissenids. Microhabitats are also important; although common carp is known dreissenid predator, in Skadar Lake (Montenegro), dreissenids avoid intensive carp predation by attaching to large objects in the sediments (usually gravel or other shells); in addition, they are scarce in areas of soft sediment, where carps forage most frequently (Stein et al. Citation1975). Although the European eel is a relatively minor Dreissena predator, dreissenids are a more common prey item in eel stomachs in samples from open-waters than from littoral areas (Biro Citation1974).
In addition to dreissenids, Neogobius melanostomus feeds on zooplankton (as juveniles), benthic invertebrates, small fishes, and the eggs and larvae of large fishes. They exhibit a high feeding plasticity predominantly preying on molluscs, which are abundant in lentic (seas and lakes) habitats, but switching to non-mollusc benthic invertebrates in lotic habitats (streams) where molluscs are often less abundant (reviewed in Kornis et al. Citation2012).
2.2.3.3. Preferred prey items
Fish predation rates on dreissenids are likely related to the availability of preferred prey items throughout the year in any given habitat; in general, intensive consumption of bivalves seems highest when other more profitable food items become scarce. Even for fishes widely known to actively feed on molluscs (e.g., roach, freshwater drum), dreissenids are often the dominant prey item during certain seasons only, most often in summer (). In laboratory trials with rams-horn snails (Helisoma anceps) and dreissenids, redear sunfish (200–222 mm long) strongly preferred the snails, a more bioenergetically profitable prey due to their higher proportion of digestible tissue in comparison with dreissenids of similar size. Thus, in habitats where gastropods are abundant, the redear sunfish may not rely heavily on dreissenids (French and Morgan Citation1995). In Lake Dardanelle (USA) the blue catfish shows a distinct seasonal prey shift: during the summer, it chiefly feeds on the abundant but low-energy zebra mussels, whereas in the winter it feeds on shad (Dorosoma spp.). This change has been attributed to the fact that while shad is energetically more profitable, at the higher summer water temperatures it is harder to locate, pursue, capture, and ingest, whereas in winter it suffers temperature-dependent stress and mortality, thus, making it an easier and more profitable food item (Magoulick and Lewis Citation2002). In southwestern Lake Ontario, round goby guts contain mostly Dreissena spp. and Mysis relicta, but the proportions of the latter, a higher-energy prey, increase with depth, suggesting that round gobies may switch from dreissenids to more profitable prey when it is available (Walsh et al. Citation2007).
Table 4. Seasonal changes in fish predation on attached (i.e., juvenile and adult) Dreissena: a reflection of preferred prey seasonal scarcity?
The bioenergetic profitability, and thus, the feeding preferences, are likely also related to the ease of removing a prey item from the substrate. The difficulty of breaking the dreissenid’s byssus may represent a formidable obstacle for many fish species. Dreissenids and other molluscs are relatively large, shelled prey that must often be handled individually by their predators. Mussels must be detached from the substratum, positioned properly in the buccal cavity, transported to the pharynx, and finally swallowed whole or crushed by pharyngeal teeth (Prejs et al. Citation1990). In cyprinids, the feeding efficiency is largely determined by the time needed to handle the mussels, rather than by the energy required for specific feeding actions like detaching or crushing them (Nagelkerke and Sibbing Citation1996). In a laboratory study of cyprinid species, the highest individual feeding efficiency values were achieved by large white bream and roach, while common bream performed poorly, suggesting that for the latter feeding on dreissenids was possibly only of marginal profitability (Nagelkerke and Sibbing Citation1996). In experimental conditions, smaller D. polymorpha were consumed more frequently by round gobies than larger individuals because more effort was required to remove larger mussels than smaller mussels. This conclusion is supported by the fact that nearly all D. polymorpha on rock tops in a location invaded by the round goby were larger than the size range preferred by gobies, in contrast to a goby-free location, where most of the mussels on rock tops were within the size range preferred by round gobies (Djuricich and Janssen Citation2001).
It should be noted that ingestion of dreissenids by fish may also be unintentional. Marsden (Citation1997) noted that even though dreissenids were found in the stomachs of common carp, the microhabitats created by dreissenid colonies in the study area were richly inhabited by crustaceans and snails which may actually have been the primary goal of the foraging carp. In Lake Balaton, however, the common carp was found to be strongly selective in choosing dreissenids as prey (Specziár et al. Citation1997).
2.2.3.4. Dreissenid mussel density
Marsden (Citation1997) reported that, in southwestern Lake Michigan, predation on dreissenids by the common carp was greatest at the site with the highest dreissenid densities, suggesting that dreissenid consumption is proportional to their availability. Likewise, Dreissena abundance can be a determinant of habitat use by round gobies because they frequently consume dreissenid mussels and are commonly found in habitats with high mussel densities (Coulter et al. Citation2015; Johnson et al. Citation2005b; Kornis et al. Citation2012; Walsh et al. Citation2007). Higher densities of dreissenids, however, may not always result in increased predation by fishes. Sterlet and bream actively fed on dreissenids when they first colonized the Kuibyshev Reservoir (Russia), but after mussel densities rose, predation declined, probably because isolated mussels are easier to remove from the substrate than those included in compact, high-density mussel beds (Egereva Citation1971).
2.2.3.5. Dreissenid mussel size
As aforementioned, predation tends to be size-selective, and thus, also has the potential to restructure the size-frequency of the prey population, resulting in important community and ecosystem-level consequences (Strayer et al. Citation2019). Round gobies, for example, prefer foraging on smaller D. polymorpha (see above; Andraso et al. Citation2011; Djuricich and Janssen Citation2001; Ghedotti et al. Citation1995; Naddafi and Rudstam Citation2014b, Citation2014c; Ray and Corkum Citation1997) (). Fish lacking the molariform pharyngeal teeth required for crushing the shells of large mussels are generally limited to small ones. Fish mouth size is also an important trait limiting the consumption of larger dreissenids and other prey (Prejs et al. Citation1990). Consequently, fish generally exhibit prey size preferences and select molluscs in proportions that do not match their abundance and accessibility in the habitat. For example, roach ≥120 mm long in Lake Sniardwy (Poland) ingested 11–17 mm mussels, but none of the 5–8 mm mussels that were most abundant in the habitat surveyed (Prejs et al. Citation1990). Data based on laboratory and field studies suggest that dreissenids ca. >15 mm are generally less vulnerable to fish predation, except by large roaches which can crush the shells of large mussels (). Tucker et al. (Citation1996) reported that, at their study site in the Mississippi River, larger common carp tended to prey on larger dreissenids (up to 42.5 mm) (). In Kuibyshev Reservoir (Russia) ide typically consume 8–17 mm zebra mussels (Mikheev Citation1977), whereas in the Upper Volga Basin, the same fish feeds on 10–30 mm quagga mussels (Shcherbina and Buckler Citation2006).
Table 5. Size of Dreissena consumed by fish.
2.2.4. Impact of fish on dreissenid populations
Relatively few quantitative studies on the impact of fish predation on dreissenid populations have been conducted (). Soon after the discovery of Dreissena in North America in the late 1980s, McMahon (Citation1991) proposed that fishes would be the most active predators of attached D. polymorpha, and this has been borne out in several studies (Bartsch et al. Citation2005; Eggleton et al. Citation2004; Watzin et al. Citation2008). A negative relationship between D. polymorpha and roach densities and biomass has also been observed in Sweden, indicating that fish predation might be a strong regulating factor of dreissenid populations (Naddafi et al. Citation2010). Several surveys from North America showed declines in quagga mussel densities caused by the round goby (Lederer et al. Citation2006, Citation2008; Rudstam and Gandino Citation2020). Some studies in Russia indicate that high percentages (up to over 80%) of dreissenid production can be consumed by fish, with the greatest impact on mussels <15 mm long (Lvova Citation1977). In the North Caspian Sea, ∼90% of dreissenid annual production (130,000 tons) is consumed by fish (Yablonskaya Citation1985).
Table 6. Impact of fish predation on Dreissena populations.
Other studies, however, have provided evidence that few fish species are important as predators, and that dreissenid densities will not be regulated unless predator fish abundance increases significantly (Boles and Lipcius Citation1997; Mitchell et al. Citation2000; Thorp et al. Citation1998) (). In North America, long-term suppression of Dreissena populations by freshwater drums has not been considered likely (French and Bur Citation1993; Mitchell et al. Citation2000), possibly due to the fact that the drum’s preference for mussels ≤21 mm long lessens their impact in reducing dreissenid populations (French and Love Citation1995). Enclosure studies in the Mississippi and Ohio Rivers and Lake Dardanelle (USA) suggested that although fish can reduce numbers of dreissenids, current levels of fish predation seemed insufficient to regulate their densities because of the large reproductive capacity of the remaining individuals (Bartsch et al. Citation2005; Magoulick and Lewis Citation2002; Thorp et al. Citation1998). Predation, however, could potentially suppress initial zebra mussel colonization and recolonization of adult zebra mussels following temperature-dependent mortality (Magoulick and Lewis Citation2002). Studies in the Hudson River (Boles and Lipcius Citation1997) recorded a 14% reduction in dreissenids within two weeks due likely to fish predation; the authors noted, however, that the presence of experimental cages may have increased predation rates by attracting fish and concluded that dreissenid populations in the Hudson River will not be regulated by the local predator guild (including finfish) unless predator abundances increase significantly. Unfortunately, many studies that examine the diet of fish species do not include a quantitative assessment of the impact of predation on dreissenids populations.
Even though large round gobies prefer small dreissenids, they are generally not regarded to be effective enough to significantly impact dreissenid populations systemwide (Ray and Corkum Citation1997). Intensive size-selective predation of small dreissenids by round gobies was hypothesized to have occurred in Lake Erie in 2002 (i.e., 3–12 mm dreissenids were absent in the samples analyzed), resulting in a decline of overall mussel population density, but not in population biomass which actually increased from previous years due to an increase in the average size of adult mussels that apparently escaped the round goby predation (Patterson et al. Citation2005). Moreover, small mussels were present later in 2009–2012 at most of the sites sampled, suggesting that the predation impact observed in 2002 was a transient event (Karatayev et al. Citation2014). As expected, using mass balance dietary simulations and stomach content data, Campbell et al. (Citation2009) observed that amphipods and chironomids were the preferred prey of small round gobies (<11.2 cm), whereas larger individuals showed a strong preference for dreissenids. According to Bunnell et al. (Citation2005) and Johnson et al. (Citation2005b), however, round gobies in Lake Erie consume a relatively small fraction of dreissenids, whose densities are mostly limited by hypoxia (Karatayev et al. Citation2018a). In reviewing the literature, Burlakova et al. (Citation2014) pointed out that the predation of gobies on Dreissena is spatially heterogeneous (Ruetz et al. Citation2012) depending on the substratum and water clarity/visibility (Diggins et al. Citation2002). Due to the preference of round gobies for smaller dreissenids, there will always be mussels surviving predation—either very large mussels or small mussels protected in refuges (between larger zebra mussels, on the underside of rocks, in crevices, etc.). Hence, the impact of round gobies is very variable, and they are unlikely to totally remove dreissenids from a habitat (Djuricich and Janssen Citation2001). Finally, the consumption rates may not be high enough to effect dreissenid populations on a system-wide scale (Johnson et al. Citation2005b; Kornis et al. Citation2012; Pennuto et al. Citation2012).
Another important factor for the magnitude of round goby impacts on Dreissena in lakes can be lake morphometry, as dreissenids are available for round goby only at shallow to intermediate depths (<60 m) (Karatayev et al. Citation2022a). Although declines in quagga mussel densities caused by round gobies in shallow areas/lakes, lake basins, or bays have been reported (Barton et al. Citation2005; Karatayev et al. Citation2022b; Lederer et al. Citation2006, Citation2008; Naddafi and Rudstam Citation2014b; Rudstam and Gandino Citation2020), at the scale of deep Great Lakes, gobies have little lake-wide effects (Bunnell et al. Citation2005; Foley et al. Citation2017; Johnson et al. Citation2005b; Karatayev and Burlakova Citation2022b; Karatayev et al. Citation2022a). For example, in Lake Ontario, which hosts large round goby populations, between 2008 and 2018 there were no declines in dreissenid densities, despite the frequent drops at <90 m in mussels around 5–10 mm, and even up to 15 mm (which is the size-range that gobies consume most actively, ) (Karatayev et al. Citation2022a). In Lake Michigan, although Dreissena populations declined between 2010 and 2015 at depths <90 m (Mehler et al. Citation2020; Nalepa et al. Citation2020), factors other than round goby predation (e.g., food limitation) were likely involved (Karatayev et al. Citation2022a). Moreover, this decline appeared to be a temporary event as the most recent survey in 2021 recorded a substantial increase in quagga mussel densities both lake-wide and at the shallowest (<30 m) zone where small (<5 mm) mussels were most abundant (87%), suggesting a negligible predation impact (Burlakova and Karatayev Citation2023). Thus, although in shallow areas/lakes dreissenid populations can temporarily decline due to recruitment bottlenecks resulting from round goby predation on small mussels, if a large portion of the lake is deep, mussels inhabiting these areas will continue to reproduce and compensate for the losses.
To summarize, in shallow lakes large populations of predators can likely affect dreissenid densities but cannot eliminate them due to the large reproductive potential of the prey. If the density of predators or level of consumption is insufficient, or large areas of the waterbody colonized by dreissenids are not available to the predators, there will be no lake-wide effects on dreissenid populations. Most importantly, to validate these conclusions (which are based primarily on indirect evidence), more studies are needed that not only include an examination of the diet of the fish but also a quantitative assessment of the impact of predation on dreissenids populations.
2.2.5. Predation by fish: effect on fish populations
When dreissenids colonize new habitats, they can quickly become a major component of the diet of molluscivorous fishes. This has been reported repeatedly in Eurasia and North America. In general, the effect of dreissenids on the fishes varies depending on the feeding mode of the consumer, the morphology of the waterbody invaded, the time elapsed since mussel invasion, co-evolutionary history, and Dreissena species, and is different in Europe and North America (Higgins and Vander Zanden Citation2010; Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation1997, Citation2002a, Citation2015; Molloy et al. Citation1997; Strayer et al. Citation2004). Pelagic species can be negatively affected as a result of lower phytoplankton abundance and associated decreases in zooplankton, competition with benthic species, and higher larval fish predation due to increased water transparency (Francis et al. Citation1996; Higgins and Vander Zanden Citation2010; Lozano et al. Citation2001; Strayer et al. Citation2004). Benthivorous fishes are usually affected positively, even those that do not feed on dreissenids, due to the increased biomass of invertebrates associated with the mussel beds (Karatayev and Burlakova Citation1992, Citation1995, Citation2022a; Karatayev et al. Citation1997, Citation2002a; Lyakhnovich et al. Citation1988; Molloy et al. Citation1997; Strayer et al. Citation2004). Indirect negative impacts on benthivorous fishes due to declines of important prey items, such as Diporeia and sphaeriids, were also well documented, particularly in the Laurentian Great Lakes (Dermott and Kerec Citation1997; Hoyle et al. Citation1999; Lozano et al. Citation2001; Nalepa et al. Citation2009a; Pothoven et al. Citation2001).
2.2.5.1. Effects on European fishes
In Europe, the introduction of zebra mussels is often associated with increases in fish productivity and commercial catches (Karatayev and Burlakova Citation1995, Citation2022a; Lyagina and Spanowskaya Citation1963; Lyakhnovich et al. Citation1988; Poddubny Citation1966; reviewed in Karatayev et al. Citation1994; Molloy et al. Citation1997). A vivid example is the roach (Rutilus rutilus), the most prominent consumer of dreissenids, that after Dreissena´s introduction exhibited much higher individual growth rates, larger body size, and higher lipid content (Lyagina and Spanowskaya Citation1963; Poddubny Citation1966; Zheltenkova Citation1949). One of the best documented Eurasian examples of diet shift is the effect on fish populations following the introduction of D. polymorpha in the Rybinsk Reservoir in Russia (Gerasimov Citation2007, Citation2015). Following D. polymorpha’s introduction in the reservoir during 1960–1965, roaches started to feed on Dreissena in all parts of the reservoir, and by 1967 it consumed D. polymorpha in the entire waterbody, including the central part and its river reaches. As a result, the population of roaches in the Rybinsk Reservoir formed two ecological groups: the coastal group, with a mixed food spectrum, and the floodplain-bottom or migratory group, feeding mainly on dreissenids. This diet-related pressure likely drives the differentiation of two morphotypes. Roach-consuming Dreissena significantly increased their growth rates, and the biomass of roaches caught by fishermen doubled (reviewed in Gerasimov Citation2007).
A similar phenomenon was also observed in Lake Pleshcheyevo (Russia): since the introduction of D. polymorpha, a new “mollusc-eating” roach morphotype emerged, characterized by massive pharyngeal teeth, higher growth rates, larger maximum size, and a longer lifespan (Kodukhova and Karabanov Citation2017). In addition to roach, bream and silver bream became active D. polymorpha feeders in the reservoir. These latter two fish species, however, are less efficient than roach in feeding on Dreissena, because they can only handle smaller mussels (10–14 mm, as opposed to roach: 20 mm) (Shcherbina and Buckler Citation2006).
Due primarily to Dreissena consumption, 4.1 × 105 kg of black carp (Mylopharyngodon piceus) were reported to be harvested annually from the Russian Tsimlyanskoe Reservoir (Miroshnichenko Citation1990). Likewise, the rapid rate of growth of common carp in Lake Balaton was attributed in part to consuming dreissenids (Specziár et al. Citation1997).
2.2.5.2. Effects on North American fishes
In North America, predation on dreissenid mussels has been documented for many commercially important native fishes ( and ). In Oneida Lake (USA), dreissenid mussels are a substantial component of the diets of large lake sturgeon, a species of conservation concern (Jackson et al. Citation2020), although high dreissenid densities can reduce juvenile sturgeon foraging (McCabe et al. Citation2006). Dreissenids comprise a major part of the diet of the endangered silver chub (Macrhybopsis storeriana), having largely replaced formerly abundant local molluscs (Kočovský Citation2019). A similar shift from a pre-invasion diet of other benthic littoral invertebrates to zebra mussels was recorded for several species of true sunfish Lepomis (Colborne et al. Citation2015; Magoulick and Lewis Citation2002; Mercer et al. Citation1999; Molloy et al. Citation1997). In addition, after the dreissenid invasion, many other nearshore invertebrates that benefited from dreissenid-mediated benthification became the primary forage of nearly all nearshore fish species (Turschak and Bootsma Citation2015). After Lake Erie was invaded by dreissenids, benthic resources were estimated to support 75–95% of the potential fish production (Johannsson et al. Citation2000). In Lake Ida (USA) 90% of fish species increased the use of littoral carbon (from 43 to 67%) after zebra mussels established there (Morrison et al. Citation2021). Yellow perch can exploit the new prey associated with zebra mussel colonies, although it may require slightly more effort than foraging on isolated individuals in loose sediments (Cobb and Watzin Citation2002). The growth rate of yellow perch in pond enclosures with D. polymorpha is higher than in enclosures without mussels, largely due to mussel-induced changes in the benthic structure and biota (Thayer et al. Citation1997). Conversely, for several fish species (e.g., ruffe, perch) the refuges provided by complex dreissenid beds can negatively affect predation success (Dieterich et al. Citation2004).
In some waterbodies, the effects of D. polymorpha on yellow perch do not occur via benthic pathways but through modifications of water clarity and zooplankton. Thus, after the introduction of the zebra mussel in Lake Oneida, the growth rates of young of the year yellow perch increased due to increases in the size of the zooplankton resulted from factors associated with zebra mussels (those affected by water clarity) and another that is not (low yellow perch numbers relative to historic values) (Mayer et al. Citation2000).
In the food webs of the North American Great Lakes Dreissena were initially considered as a “dead end” (reviewed in Madenjian et al. Citation2010) and as a major loss of energy and potential production because food resources were withdrawn from the pelagial and incorporated into benthos (Johnson et al. Citation2005b). Dreissenid-induced loss of primary production and oligotrophication of the Great Lakes resulted in large declines in pelagic fish, including some commercially important species, like the whitefish (Coregonus clupeaformis), largely due to dramatic decreases in their main food, deep-water amphipod Diporeia (Dermott and Kerec Citation1997; Lozano et al. Citation2001; Nalepa Citation2010; Nalepa et al. Citation2009a; Pothoven et al. Citation2001), likely outcompeted by dreissenids. Despite its feeding on Dreissena, the shift from Diporeia to quagga mussels resulted in the decline of whitefish condition, growth, and abundance (Hoyle et al. Citation2008; Lumb et al. Citation2007; Nalepa et al. Citation2009b; Owens and Dittman Citation2003; Pothoven et al. Citation2001; Rennie et al. Citation2009) because Diporeia is rich in lipids (up to 54% of Diporeia dry weight, Gardner et al. Citation1985) and provides a much better source of energy than dreissenids (Owens and Dittman Citation2003). The decline in Diporeia was also associated with a decline of alewife (Alosa pseudoharengus), sculpin (Cottus cognatus), bloater (Coregonus hoyi), and other fishes that are prey for larger piscivores, including salmon and trout (reviewed in Nalepa Citation2010). The consequences of dreissenid introductions to fisheries in other inland lakes, however, have been much less significant than those in the Great Lakes (Nienhuis et al. Citation2014).
The introduction in the Great Lakes of another Ponto-Caspian invader, the round goby, added a very important previously missing trophic link between dreissenids and commercially and recreationally valuable fish species (Johnson et al. Citation2005b; Madenjian et al. Citation2011). A population explosion of the invasive round goby occurred in the early 2000s in the western part of Lake Erie where their population size was estimated at 9.9 billion (Johnson et al. Citation2005a). These high population densities, however, were not only attributable to their preying on dreissenids, since smaller round gobies prefer a variety of benthic invertebrates, including chironomids and amphipods (Campbell et al. Citation2009; Dermott et al. Citation2012; Diggins et al. Citation2002; French and Jude Citation2001; Kornis et al. Citation2012; Walsh et al. Citation2007). Nevertheless, consumption of dreissenids by round gobies substantially increased the transfer of energy stored by dreissenids in the benthos back to the pelagial and eventually increased fish productivity, including commercially important species. The round goby is actively consumed by a number of North American fishes, including lake trout (Salvelinus namaycush) (Dietrich et al. Citation2006), burbot (Lota lota) (Madenjian et al. Citation2011), yellow perch (Perca flavescens) (Weber et al. Citation2011), whitefish (Coregonus clupeaformis) (Lehrer-Brey and Kornis Citation2014; Pothoven and Madenjian Citation2013), smallmouth bass (Micropterus dolomieu) (Crane and Einhouse Citation2016), lake sturgeon (Acipenser fulvescens) (Bruestle et al. Citation2019; Jacobs et al. Citation2017), and walleye (Sander vitreus) (Pothoven et al. Citation2017). In Lake Huron, the overall percentage of adult (>400 mm in length) lake whitefish that fed on other fishes increased from 10% in 2002–2006 to 20% in 2007–2011 when round gobies accounted for 92% of all fishes consumed by lake whitefish (Pothoven and Madenjian Citation2013).
Finally, dreissenids can play a significant role in the biomagnification of organic contaminants and trace elements up through the food chain as they bioaccumulate many pollutants, toxins, and heavy metals (reviewed in Binelli et al. Citation2015), but bioaccumulation and trophic transfer of contaminants vary among species (Evariste et al. Citation2018; Matthews et al. Citation2015; Zimmermann et al. Citation1997), locations (Hanari et al. Citation2004; Kimbrough et al. Citation2013), and pollutant types (Perez-Fuentetaja et al. Citation2015). The switch of fish consumption to dreissenids, and especially the invasion of the round goby, created a new pathway through which these contaminants can be incorporated into the food webs. Hogan et al. (Citation2007) documented that in Lake Erie the concentrations of methyl mercury (MeHg) are lowest in the sediments, and increase progressively in dreissenids, round gobies, and smallmouth bass (e.g., concentrations in the smallmouth bass are 1000 times higher than those in the sediments). Conversely, concentrations of other pollutants (e.g., PBDEs—polybrominated diphenyl ethers) in mussels have been found to be lower than in zooplankton and amphipods (Perez-Fuentetaja et al. Citation2015), which may decrease the levels of these compounds in mussel-feeding fishes (Hahm et al. Citation2009). Further research is needed to assess the magnitude of dreissenid biomagnification and whether dreissenids are effectively more harmful in transferring contaminants than other native or introduced prey species. This topic, however, is too large to be comprehensively covered in this review.
2.3. Birds
Consumption of attached Dreissena has been recorded for at least 39 species, including 22 in Europe and 22 in North America (). Five species—greater scaup (Aythya marila), goldeneye (Bucephala clangula), oldsquaw (Clangula hyemalis), herring gull (Larus argentatus), and white-winged scoter (Melanitta fusca)—have been observed eating Dreissena both in Europe and North America. In Europe, the tufted duck (Aythya fuligula), greater scaup (A. marila), and pochard (Aythya ferina) are the primary predators in most situations, sometimes accompanied by goldeneye (B. clangula) and coot (Fulica atra) (Suter Citation1982a; van Eerden and de Leeuw Citation2010; van Eerden et al. Citation1997). In North America, greater scaup (Aythya marila), lesser scaup (Aythya affinis), and bufflehead (Bucephala albeola) have been most commonly recorded, with less frequent reports for goldeneye (Mazak et al. Citation1997; Petrie and Knapton Citation1999; Wormington and Leach Citation1992). A 20-year study conducted in the Lake IJsselmeer area of the Netherlands (van Eerden et al. Citation1997) is the most extensive investigation to date of the patterns of food exploitation by diving waterfowl feeding on dreissenids. This long-term study was conducted in parallel with a comprehensive series of experiments on the foraging behavior and energetics of diving ducks (Carbone et al. Citation1996; de Leeuw Citation1997a, Citation1997b, Citation1999; de Leeuw and van Eerden Citation1992).
Table 7. Birds documented to eat Dreissena in the field and where predation was observed (E—Europe, NA—North America).
2.3.1. Factors affecting bird predation
2.3.1.1. Dietary preferences of bird species
The proportion of a waterfowl’s diet that is comprised of bottom-dwelling invertebrates, particularly molluscs, can be a useful predictor of its potential importance as a dreissenid predator. Tufted duck feed almost exclusively on benthic animals and are the most aggressive avian predators of dreissenids in Europe (Draulans Citation1987). In Lake IJsselmeer (The Netherlands) between 80 and 95% of the diet of adult tufted duck consists of dreissenids (de Kock and Bowmer Citation1993). Likewise, in the brackish lagoons of the Odra River Estuary greater scaups feed almost exclusively on zebra mussels (97% in terms of biomass) (Marchowski et al. Citation2015). The availability of dreissenids, however, does not always lead to intense predation; in Szczecin Firth (Poland), even though dreissenids are abundant, their consumption by tufted ducks and other waterfowl is low (Wiktor Citation1969). Pochard (Draulans Citation1987) and coot (Borowiec Citation1975) may preferentially consume plant material when seasonally available. The relative importance of dreissenids in the diet of waterfowl was further investigated by studies of overwintering populations in the Rhine River (Swiss-German border); tufted duck and pochard fed almost exclusively on dreissenids, while coot and goldeneye used them as main and supplementary food, respectively (Suter Citation1982a, Citation1982b). Although dietary preferences are best revealed in field studies under natural conditions, controlled feeding studies can provide useful supplementary information. Dobrowolski et al. (Citation1996) carried out experiments showing that coot, mallard, and pochard readily ingest dreissenids, but the red-crested pochard feeds on plants only. Diving ducks do not seem to discriminate between D. polymorpha and D. r. bugensis (Mitchell et al. Citation2000).
A simple list of bird species observed to consume dreissenids () can be misleading, as some of these data are based on a single literature reference and include species that are not generally molluscivorous. In contrast, the number of literature citations for a particular bird species () is a much more reliable indicator of which species are true molluscivorous predators. The common merganser (Mergus merganser) and the red-breasted merganser (M. serrator), for example, feed chiefly on fish and were reported as ingesting dreissenids only once (Jacoby and Leuzinger Citation1972). Likewise, redheads (Aythya americana) mostly consume dreissenids attached to the stems of aquatic plants, suggesting that mussels are not their primary feeding target (Custer and Custer Citation1996; Petrie and Knapton Citation1999).
2.3.1.2. Dreissenid density
Birds prey on dreissenids because they are often very abundant, requiring low search and handling times (Draulans Citation1982; Kornobis Citation1977; Leuzinger and Schuster Citation1970; Suter Citation1982c; Wormington and Leach Citation1992). Regions of high dreissenid density are, thus, the preferred foraging areas for their waterfowl predators. In both European (Kornobis Citation1977; Leuzinger and Schuster Citation1970; Stanczykowska Citation1987; van Eerden et al. Citation1997) and North American (Wormington and Leach Citation1992) lakes, waterfowl flocks tend to concentrate in shallow areas where dreissenids are abundant. In the Plas Leblanc (a sand-pit pond in Belgium), predation by tufted duck flocks was observed to be most intense in areas of the highest dreissenid density (Draulans Citation1982). At the outlet of Lake Constance (Swiss-German border), overwintering waterfowl always feed first where dreissenid populations are the most dense (Suter Citation1982a). In the Netherlands, diving ducks concentrate in larger numbers at patches of high dreissenid density, and their overall pattern of mussel exploitation is clearly density-dependent (van Eerden et al. Citation1997).
Dense mussel colonies can apparently be located rather quickly by migrating waterfowl flocks. At a power plant on Lake Michigan, this behavior had unfortunate consequences when ca. 400 scaup (primarily lesser scaup) were entrained and killed as they congregated to feed on dreissenids encrusting a 4-m deep water intake structure (Mitchell and Carlson Citation1993). Not all birds, however, always feed in the areas of the highest mussel density within a waterbody (Stanczykowska et al. 1990), suggesting that the selection of the foraging area is not governed by prey density only. de Leeuw (Citation1997b) suggested that the energetic cost of searching for sites with high mussel densities, in particular in areas where mussel beds are patchy, might explain the decoupling between foraging areas and mussel densities.
2.3.1.3. Depth
Predation rates by birds are obviously higher at shallower depths. Although coots are capable of diving to 7 m, they prefer to feed in areas ≤2 m deep (Borowiec Citation1975). Tufted ducks are very good divers with the capability of sustained foraging down to 14 m (reviewed in Werner et al. Citation2005), but their predation rates decline with depth (Draulans Citation1982), with the highest activity at 1–2 m when mussels are available (Olney 1963). Pochards also usually forage at 1–2.5 m but can dive down to 4.5–5 m (reviewed in Werner et al. Citation2005). Predation by tufted ducks and greater scaup declines significantly below 4 m (van Eerden et al. Citation1997). In the Lake IJsselmeer area (the Netherlands), both tufted duck and scaup exploit the shallow, coastal zones early in winter, but feed in deeper water, farther off-shore, in late winter (de Leeuw Citation1997b). The preference for foraging at shallow depths is clearly energetically advantageous since diving effort increases with depth, the energy content of mussels decreases, and feeding in deeper waters usually requires longer flight distances from the shore-based roosts (de Leeuw Citation1997b), but could be justified considering weaker attachment and higher mussel biomass and at these depths. Thus, on sandy substrates in Lake Constance where D. polymorpha is loosely attached and can easily be dislodged, diving ducks were documented to retrieve mussels from depths of up to 11 m (Werner et al. Citation2005).
2.3.1.4. Dreissenid size
The size of the mussels ingested can relate to many factors, including water temperature, water depth, bird species, the predator’s satiation, mussel size availability, and whether mussels are actively selected (vs. passively and inadvertently ingested with vegetation). Many of these factors, of course, are related to energy profitability, since foraging decisions are largely governed by their effects on the energy balance of the predator and their fitness consequences (de Leeuw Citation1997b).
Waterfowl which actively forage on dreissenids generally appear to prefer mussels of ca. 8–20 mm (), but interspecific differences have been recorded. In lakes Erie and St. Clair, Hamilton and Ankney (Citation1994) found that larger species of diving ducks appeared to select the largest mussels, greater scaups consumed larger mussels than did lesser scaups, and both scaup species preferred larger mussels prey than did bufflehead and common goldeneye. Nevertheless, all species took a very broad and overlapping range of dreissenids. In laboratory feeding experiments, tufted ducks and greater scaups were able to swallow all mussels ≤30 mm long, but showed a slight preference for 7–16 mm mussels; selectivity decreased with biomass consumed and increased with water temperature (de Leeuw Citation1999). Other laboratory trials provided evidence that pochards select mussels smaller than tufted ducks do (Draulans Citation1987). The size-composition of Dreissena from the guts of diving ducks in Lake Erie also differed, with increasingly larger mussels consumed, respectively, by bufflehead, lesser scaup, and greater scaup (Mazak et al. Citation1997) (). Goldeneye in the upper Rhine River also tended to select smaller mussels, presumably due to the relatively weak musculature of their gizzards (Suter Citation1982a). Dreissenids consumed by waterfowl while feeding on macrophytes tend to be smaller as macrophytes die back each winter and mussels attached to vegetation are <1-year old (e.g., mean of 3 mm, Custer and Custer Citation1996), therefore, in this case, the size of mussels retrieved from the gut may be a misleading indicator of a predator’s size preferences as only small mussels were available.
Table 8. Size of Dreissena consumed by birds.
Analyses of the size of the mussels consumed must obviously take into account their availability in the area. In lakes Erie and St. Clair, the average mussel sizes taken by diving ducks differ greatly among sites, but these differences matched those of the mussel sizes available at the sites studied (Badzinski and Petrie Citation2006; Hamilton and Ankney Citation1994). In Lake Erie, greater and lesser scaup favored 11–13 mm dreissenids (Hamilton et al. Citation1994), whereas in Lake Michigan the mean length of dreissenids consumed by scaups was 4 mm. This contrast is likely influenced by the fact that, at the time, all mussels at the Lake Michigan site were <10 mm long (Mitchell and Carlson Citation1993).
Size feeding preferences by diving ducks have been the subject of several analyses. Why, for example, do tufted duck prefer medium-sized mussels when larger, higher-profitability mussels are available? The calorific value of a mussel increases exponentially with shell length, but shells also thicken as mussels grow, so there probably is a tipping point where shell thickness and the amount of digestible tissue are optimal, which has been suggested to occur in medium-sized mussels (Hamilton and Ankney Citation1994). Further, the size and number of mussels that a diving bird can retrieve in a single dive may also play a major role. Draulans (Citation1982) suggested that the preference for medium-sized mussels is a reflection of optimum energetic gain per dive. For example, in one dive a tufted duck can swallow a maximum of either two 16 mm mussels (total energy content ca. 400 calories) or one 21 mm mussel (ca. 275 calories). The relation of the preference for medium-sized dreissenids to energy profitability was also suggested by de Leeuw and van Eerden (Citation1992) in their study of the tufted duck. van Eerden et al. (Citation1997) reported that even though tufted duck, pochard, greater scaup, and goldeneye all consumed mussels ≤27 mm long, they tended to take mussels <15 mm, especially at depths of <2.5 m. They suggested that size preference was apparently operating only at depths <2.5 m because of the time constraint on the foraging ducks set by water depth. Likewise, at lower temperatures, the dive duration of tufted ducks is shorter and less time is spent selecting small mussels, with the result that larger mussels are ingested (de Leeuw et al. Citation1999). Diving ducks feeding in the Great Lakes, however, appear to feed on mussels of widely varying sizes, and although larger ducks consume larger mussels when available, even very small mussels are sufficiently profitable and are common in the diet (Hamilton and Ankney Citation1994). Finally, the size selection of mussels by birds may be impacted by kleptoparasitism. According to Marchowski and Neubauer (Citation2019), mallards attempt to steal zebra mussels from other mallards and coots when large or intermediate-sized prey items are involved. The probability of success of a kleptoparasitic attack is lowest when the attacked bird holds small prey items, but higher if the prey is intermediate or large.
2.3.1.5. Season
High rates of bird predation have been most commonly reported between autumn and spring, when flocks are either temporarily present during their migration (Hamilton et al. Citation1994; Mitchell and Carlson Citation1993), or overwintering (bij de Vaate Citation1991; Cleven and Frenzel Citation1993; van Eerden et al. Citation1997). During these seasons predation on dreissenids can be enhanced by the absence of some other food items. Plants, for example, are a major food item for coots, but are less available in winter; Stempniewicz (Citation1974) observed that in the winter dreissenids represent 93% of coot’s food, but decline to 63% in summer due to their grazing on plants.
In cool climates, winter ice formation precludes predation activities and results in duck emigration (van Eerden et al. Citation1997). If ice formation is hindered (for example, due to the discharge of heated water by electric power stations), flocks may overwinter on site. This occurs in Lukomskoe Lake (Belarus), where large flocks of mallard regularly overwinter and consume large numbers of Dreissena in shallow areas (Karatayev et al. Citation1994; Kozulin Citation1995). Similarly, in an ice-free hole generated by a discharge of coolant water at Nanticoke, Lake Erie, predation by wintering waterbirds causes dramatic, but very localized, declines in the abundance of D. polymorpha (Mitchell et al. Citation2000).
2.3.2. Bird diving and feeding behavior
2.3.2.1. Diel patterns
Waterfowl predation on dreissenids can take place during both day and night. Both goldeneye and coot locate their prey visually, and thus, are only active during the day, spending, respectively, up to 10 and 16 hr/day feeding (Borowiec Citation1975; Suter Citation1982a). In contrast, tufted ducks and pochards are primarily active at night, especially in autumn and early winter when dreissenids are abundant due to recent reproduction and settlement; yet they become more active during the day to locate their prey visually when dreissenid densities decline (Suter Citation1982a). Werner et al. (Citation2005) found that in October and November, diving ducks (tufted ducks and pochards) and coots are mainly active at night; as winter progresses, the birds forage increasingly during the daytime as well. This change is likely due to the fact that with decreasing food supply tactile foraging becomes less effective (Suter Citation1982a), forcing the birds to resort to search visually for the scarcer remaining mussels.
de Leeuw and Renema (Citation1997) provide observations on how feeding behavior may be impacted by kleptoparasitism. They suggest that night feeding by tufted ducks that rely on tactile cues when feeding on mussels, may be partly driven by the need to avoid food stealing behavior of other birds, such as coots and gulls (Larus spp.). Goldeneye and coot use visual cues for feeding and are, thus, active during the day, despite the risk of food stealing. Swallowing the prey underwater (e.g., goldeneye) and social feeding in dense flocks (e.g., coots) are likely alternative tactics to avoid food stealing.
2.3.2.2. Dive duration
Dive duration differs among species Coots prefer feeding in shallow waters, and thus, typically have a short diving time (ca. 5 sec) (Borowiec Citation1975). In Lake Constance (Swiss-German border), the mean time for the deeper-diving goldeneye is about 14 sec (Suter Citation1982a). The mean diving time of tufted ducks increases from ca. 19 sec at 2 m, to ca. 27 sec at 4–6 m depth (Draulans Citation1982). Dive duration can also be affected by prey density (Draulans Citation1982); the mean diving time of the tufted duck declines with increasing prey density (ca. 30–20 sec), but increases slightly again at the highest prey densities; this may be due to their tendency to be most size-selective at the highest dreissenid densities.
Birds that dive to consume dreissenids are physiologically well adapted for this activity (Woakes and Butler Citation1983). The diving energy budgets of tufted ducks and pochards have been comprehensively analyzed by Carbone et al. (Citation1996), chiefly centering on the problem of the limited oxygen supply during breathhold, particularly in deep water.
2.3.2.3. Consumption patterns
Diving ducks typically swallow dreissenids whole and crush the shells in the gizzard. van Eerden et al. (Citation1997) observed that diving ducks relied particularly on individual, unattached mussels, but were also able to take mussels in clumps. In laboratory trials, for tufted ducks, the food intake rates decreased with the degree of byssal thread attachment of the mussels, while intake rates of scaups were only affected when mussels grew in tightly attached clumps (de Leeuw Citation1999). In Lake Constance, zebra mussels attached to rocks are consumed mostly from shallow (1–3 m depth) areas, while loosely attached mussels on sand are retrieved from depths down to 11 m (Werner et al. Citation2005).
The details of how birds eat dreissenids have been particularly well studied in the tufted duck (de Leeuw and van Eerden Citation1992; de Leeuw et al. Citation1999; Draulans Citation1982; Olney 1963). Tufted ducks sieve mussels in a manner similar to how puddle ducks (Anas spp.) filter seeds. While at the bottom, tufted ducks collect mussels ≤16 mm in length in a water-suction-flow generated by repeated tongue movements. Kooloos et al. (Citation1989) provided a detailed anatomical and functional analysis of this mechanism in the tufted duck. Longer mussels (maximum of 25 and 30 mm, respectively, for tufted duck females and males) are typically picked up individually. While underwater, tufted ducks generally feed on small mussels, but may pick up a large mussel before returning to the surface. Consequently, feeding observations based on birds at the surface only are likely to yield biased prey-size preferences. In laboratory trials, feeding activity was observed to consist of short feeding bouts involving several dives in quick succession to fill the esophagus with mussels, followed by longer resting periods of 5–10 min to crush mussel shells in the gizzard and digest the flesh (de Leeuw Citation1999). For tufted ducks, much time can be spent at the surface orienting large mussels in the bill to achieve a suitable position for swallowing them whole. In their Lake Erie study, Hamilton et al. (Citation1994) observed bufflehead, greater scaup, and lesser scaup returning to the surface with several mussels in their bills and manipulating them one by one before swallowing them.
Feeding on mussels in open waters is apparently not the only strategy among diving birds. Kornobis (Citation1977) has reported that coots also recover other larger bivalves like Unio and Anodonta bivalves (Unionidae), transport them to the shore, and remove the dreissenids attached to their shells.
Gulls with limited diving abilities developed two kinds of interspecific relationships with ducks—food commensalism and interspecific kleptoparasitism. Gulls steal mussels from ducks emerging with a clump of mussels or pick up lost dreissenids lying on the water surface, thus, obtaining otherwise inaccessible food (Marchowski et al. Citation2016). As a result, the diet of gulls in Szczecin Lagoon (Poland) changes dramatically from predominantly fish parts to almost exclusively mussels when large numbers of Aythya ducks arrive in November (Marchowski et al. Citation2016).
2.3.3. Impact of bird predation on dreissenid mussel populations
The interactions of birds and their dreissenid prey have been studied more intensively than that of any other Dreissena enemy. Impacts of bird predation on dreissenid populations can include a reduction in mussel density and biomass, an alteration of mussel distribution within a waterbody, and a shift in mussel size-frequency distributions.
2.3.3.1. Documented reductions in dreissenid populations
Reductions in mussel abundance can be one of the most dramatic impacts of bird predation. Birds can consume up to 30% of the annual zebra mussel production in shallow areas (Smit et al. Citation1993) and up to 70–97% of their biomass (Mikulski et al. Citation1975; Stempniewicz Citation1974; Werner et al. Citation2005). In a 20-year study, diving ducks (e.g., tufted duck, pochard, greater scaup, and goldeneye) annually reduced dreissenid biomass by 5–22% throughout the entire Lake IJsselmeer area of the Netherlands and in some shallow areas by up to over 90% (van Eerden et al. Citation1997). In the littoral zones of Lake Constance, overwintering waterbirds can have a severe impact on zebra mussel populations, with only 2–3% of the D. polymorpha biomass remaining in shallower water (Werner et al. Citation2005). In Goplo Lake (Poland), coots annually consume 20–70% of the yearly Dreissena production (Mikulski et al. Citation1975; Stempniewicz Citation1974). Intense predation by overwintering diving ducks at an ice-free site in Lake Erie (Canada) resulted in reductions of 74–93% for D. r. bugensis and 86–100% for D. polymorpha, depending on mussel length (Mitchell et al. Citation2000). In a Lake Erie bay, diving duck predation, in combination with reduced food resources (phytoplankton), contributed to a 67% decline in dreissenid density over a three year period (Petrie and Knapton Citation1999). Other studies have recorded smaller impacts (reviewed in Stanczykowska et al. Citation1975). In Skoszewska Cove of the Odra River Estuary (Poland) greater scaups alone consumed about 38% of the zebra mussel population (Marchowski et al. Citation2015).
In studies where significant declines in mussel populations have been documented, the reductions tend to be temporary, with mussel densities rebounding the next year. During their migration passage, diving ducks (bufflehead, greater scaup, and lesser scaup) foraging at Point Pelee (Lake Erie, Canada) reduced dreissenid biomass by 57% during November, which was their period of heaviest feeding; differences between study and control areas, however, had disappeared by the following spring (Hamilton et al. Citation1994). In a four-year study of the Rhine River at the outflow of Lake Constance (Swiss-German border), one of the highest biomasses of dreissenids recorded in Europe (up to 12 kg/m2) was annually reduced by 97% by overwintering waterfowl flocks; immigrant mussels from adjacent areas, however, recolonized the site each spring and restored mussel biomass to previous levels (Suter Citation1982b). In the Seerhein River, which connects the two main basins of Lake Constance, from autumn 1988 through spring 1989 overwintering waterfowl (mainly tufted duck and pochard) reduced the dreissenid standing crop (ash-free dry weight) by 91% (i.e., from 56,400/m2 to 5,100/m2); however, subsequent springtime immigration of large numbers of dreissenids from deeper parts of Lake Constance, i.e., areas out of the reach of the diving ducks, combined with summertime reproduction, completely restored population densities by fall 1989 (i.e., 59,800/m2) (Cleven and Frenzel Citation1993).
The above studies demonstrate that significant long-term (i.e., multi-year) reductions in mussel densities are most likely to occur only in localized areas where waterfowl overwinter (rather than just stage during their fall migrations) and mussel recruitment is limited. During 1975–1985, throughout Lake IJsselmeer, diving ducks reduced dreissenid biomass annually by 10–13% on average, but because mussel recruitment was successful throughout this period, dreissenid populations actually increased over this decade (van Eerden et al. Citation1997). In contrast, in nearby Lake Markermeer, years of irregular larvae fall, in combination with intense duck predation, eventually led to a collapse of the mussel population, thus, providing the clearest evidence to date that successful annual recruitment of dreissenids is a key factor in determining to what degree predation by overwintering ducks impacts mussel populations. Using data from their experimental enclosure studies in Lake Erie (Canada), Mitchell et al. (Citation2000) also concluded that reductions in Dreissena densities caused by diving ducks are typically short-lived due to recolonization by small mussels migrating from refugia in the spring and settling of larval mussels during the following summer.
The extent of avian predation on dreissenid populations depends on several factors, including the predator density, depth, substrate, mussel accessibility (see discussion above), and Dreissena species. Considering lake morphology, waterfowl predation is likely to be more intense in shallow polymictic lakes than in deep dimictic lakes. Since zebra mussels are largely limited to the littoral zone, they are probably more vulnerable to waterfowl predation than quagga mussels which are usually more abundant in the profundal than in the littoral zones (Karatayev et al. Citation2021a). Conversely, the survival of quagga mussels may be hindered by their thinner, more fragile shells that are more easily crushed (Bowers et al. Citation2005; Casper and Johnson Citation2010).
Because of their depth feeding preferences, diving waterfowl can alter the distribution of dreissenid populations within a water body. The average depth of dreissenid colonies in Lake Zurich (Switzerland) increased from 4 to 5 m, due in part to predation by large populations of tufted duck and coot (Burla and Lubini-Ferlin Citation1976).
Waterfowl predation can also significantly affect dreissenid population size-structure due to size feeding preferences (). From autumn 1988 through spring 1989 in the Seerhein River (Germany and Switzerland) overwintering waterfowl, mainly tufted duck and pochard, completely eliminated their preferred size class (1+ cohort mussels, >5 mm) (Cleven and Frenzel Citation1993). Likewise, in the shallow zone of Lake Constance, almost all mussels >1 year (>5 mm) were consumed by tufted ducks, pochards, and coots in the winter of 2001/2002 (Werner et al. Citation2005). In Lake Neuchâtel (Switzerland), entire cohorts of dreissenids disappeared in years following heavy mussel predation (Pedroli Citation1977). The effect of tufted duck predation on the size composition of mussel populations is most pronounced at high prey densities; in theory, increased prey densities reduce the diving time required for tufted duck to locate dreissenids, thereby reducing the effort in prey-size selection (Draulans Citation1982).
2.3.4. Importance of dreissenids as food for birds and their effects on bird populations
2.3.4.1. Benefits from dreissenids as a food source
Dreissenids can be a valuable food source for waterfowl, and their consumption by migrating or overwintering birds is well documented both in Europe and in North America. In the Lake IJsselmeer area of the Netherlands, dreissenids were reported as the main food item for over 300,000 overwintering diving ducks (tufted duck, pochard, greater scaup, and goldeneye) (bij de Vaate Citation1991; de Leeuw Citation1997a). In these Dutch lakes during the winter, in terms of biomass, dreissenids can make up to over 90% of the birds’ diets (van Eerden et al. Citation1997). The daily consumption of mussels can be extremely high, up to 2–3 times the bird’s body mass (de Leeuw et al. Citation1999). In the brackish lagoons of the Odra River Estuary (Baltic Sea), an important resting area for greater scaup (A. marila) during the non-breeding season, the birds consume an average of 5400 tons of zebra mussels annually (Marchowski et al. Citation2015). The declining European populations of A. marila, thus, now depend on the non-native zebra mussels that constitute >90% of their food (in terms of biomass). Their dietary importance for coots and greater scaup in Poland (Marchowski et al. Citation2015; Mikulski et al. Citation1975), for tufted ducks in Belgium (Draulans Citation1982) and the British Isles (Olney 1963), and diving ducks in the Great Lakes region (Custer and Custer Citation1996; Hamilton et al. Citation1994; Mazak et al. Citation1997; Petrie and Knapton Citation1999) has been well documented.
2.3.4.2. Increases in flock sizes and overwintering
Because of their importance as a prey item, dramatic increases in flock sizes can occur following dreissenid colonization of a waterbody, as observed in France (Géroudet Citation1966), Switzerland (Jacoby and Leuzinger Citation1972; Leuzinger and Schuster Citation1970; Pedroli Citation1981b; Suter and Schifferli Citation1988), Germany (Hiller Citation1997; Ziegler Citation1987), and North America (Luukkonen et al. Citation2013; Petrie and Knapton Citation1999; Wormington and Leach Citation1992). Dreissenids are not the only molluscs in North America to attract large flocks of molluscivorous waterfowl; high densities of fingernail clams (Sphaerium transversum) have made the Keokuk Pool on the Mississippi River (Iowa) a favored annual migration stopover for nearly 20 million diving ducks including lesser scaup and goldeneye (Thompson Citation1973). Before the appearance of dreissenids in Swiss lakes, birds fed on aquatic macrophytes in the fall, and after plant die-back, they migrated to the south. Following the buildup of dreissenid densities, thousands of birds began to overwinter locally (Leuzinger and Schuster Citation1970). In western Lake Constance, soon after the arrival of dreissenids in the late 1960s, 45,000 tufted ducks, pochards, and coots were observed overwintering, representing a 10- to 50-fold increase over previous levels (Suter Citation1982a). Following the establishment of dreissenids in western Lake Constance, goldeneye began to arrive at overwintering areas earlier (Suter Citation1982a). The densities of D. polymorpha larvae in Lake Walensee (Switzerland), which were assumed to be a reflection of the densities of adult mussels present the previous winter, were found to correlate with the size of winter populations of the tufted duck, the common goldeneye, and the common coot. Between 1982 and 1990, a 9-fold increase in larval numbers resulted in a doubling in common goldeneye and a 3-fold increase in common coot and tufted duck populations. Further, these increases were much smaller than those reported for other Swiss lakes (5- to 10-fold), because the densities of Dreissena in Lake Walensee were still relatively low due to nutrient-poor waters and because only a small area of the lake was accessible to diving waterbirds (Marti et al. Citation2004). Midwinter counts of diving ducks in the Rhone River near Lake Geneva (France) increased dramatically following the arrival of dreissenids (Géroudet Citation1978).
Conversely, the decline of dreissenids may trigger decreases in wetland use by waterfowl. Thus, in the Inner Long Point Bay of eastern Lake Erie, colonization by zebra mussels in the early 1990s increased water clarity, which in turn favored the growth of submerged aquatic vegetation (SAV) and increased the usage of the wetland by waterfowl (Petrie and Knapton Citation1999). In the 2020s, however, increased eutrophication, sediment loads, and predation of the mussels by both fish and waterfowl led to a >90% decline in filter-feeding dreissenid populations. Enhanced phytoplankton densities reduced light penetration and SAV, lowering the bay’s carrying capacity for waterfowl, fish, and other wildlife (Churchill et al. Citation2016).
2.3.4.3. Changes in the timing and routes of waterfowl migrations
The location and density of dreissenid populations cannot only affect waterfowl distribution and overwintering (see above) but also the timing and routes of their migration. The geographical range of tufted duck in England expanded due in part to the spread of dreissenids (Olney 1963). Food abundance and availability, particularly Dreissena, were suggested as the main factor governing lake choice by overwintering diving ducks in Switzerland (Suter Citation1994).
The arrival of dreissenids in North America has resulted in changes in migration routes and increases in flock sizes of diving ducks (Wormington and Leach Citation1992). The combined lesser and greater scaup use (i.e., waterfowl days) of Long Point Bay, one of the most important waterfowl staging areas in North America, increased 92-fold between 1986 and 1997, despite a substantial decline in the North American scaup population (Petrie and Knapton Citation1999). Waterfowl days for bufflehead in Long Point Bay increased 14-fold during the same period (Petrie and Knapton Citation1999). The number of waterfowl, including scaup, canvasback (Aythya valisineria), and redhead ducks (Aythya americana) that use Lake St. Clair (in its USA section) during their fall migrations increased from 1.1 million use-days before dreissenids arrived to 2.1 million after dreissenid establishment (Luukkonen et al. Citation2013). Conversely, in areas where dreissenid populations declined, diving birds show a tendency to leave overwintering areas earlier (Suter Citation1982c) or not return the following winter (van Eerden et al. Citation1997). Likewise, the establishment of dreissenids in Lake Neuchâtel (Switzerland) modified the migratory phenology of tufted ducks, pochards, and greater scaup (Pedroli Citation1981a).
2.3.4.4. Positive indirect effects of dreissenids on birds
In addition to the direct consumption of dreissenids, waterfowl also prey on the invertebrates facilitated by the mussels, as well as on macrophytes and bottom algae that benefit from dreissenid-enhanced water clarity. Thus, in unprotected enclosures in shallow areas of Lake Constance, the abundance of macroinvertebrates associated with Dreissena colonies (mostly Oligochaeta, Chironomidae, and Ephemeroptera) were significantly reduced, presumably due to waterfowl predation (Mörtl et al. Citation2010). The number of waterfowl, including canvasbacks, that do not directly prey on Dreissena, increased after the colonization of Lake St. Clair by dreissenid mussels, likely due to increased submerged aquatic macrophyte food associated with the enhanced water clarity following mussel colonization (Luukkonen et al. Citation2013). The bay of Lucerne (Switzerland) has become an internationally important overwintering site for the red-crested pochard (Netta rufinadue) due to its recolonization by stoneworts (Characeae) after the introduction of zebra mussels in the 1980s (Schwab et al. Citation2001). In some lakes, both Chara and zebra mussels are now considered keystone species that control ecosystem resilience, and careful management of these species has been suggested to be as important as the control of nutrients (Ibelings et al. Citation2007). Declines in dreissenid densities can diminish wetland quality and usage by reducing water transparency and the concomitant decline in submerged aquatic vegetation (Churchill et al. Citation2016).
While generally advantageous, reliance on dreissenids as the main prey item can involve hazards. For example, in the unusually cold winter of 1986 in Europe, dreissenid populations were severely affected, leading to the starvation deaths of thousands of diving birds which depended on this resource (Suter and van Eerden Citation1992).
Because of their efficient filter feeding, dreissenids can concentrate various pollutants from the water column (Binelli et al. Citation2015), which are subsequently transferred to mussel-eating birds. Accumulation of PCBs derived from their food was reported in tufted ducks in the Linth Canal in Switzerland (Zimmermann et al. Citation1997) as was the presence of organic contaminants in North American diving ducks (Mazak et al. Citation1997). In a European study where caged tufted ducks fed on dreissenids contaminated with cadmium and organochlorine compounds, these toxins were carried over into their eggs with teratogenic effects (de Kock and Bowmer Citation1993). Dreissenids can also accumulate and transfer selenium to waterfowl (Custer and Custer Citation2000; Schummer et al. Citation2010; Weegman and Weegman Citation2007), yet the short- and long-term impacts of this element on bird health and reproduction are unknown. These are only a few examples from the extensive literature that was not reviewed comprehensively due to limited space.
Ingestion of dreissenids by birds can also lead to infections with trematode parasites of the family Echinostomatidae, such as Echinoparyphium recurvatum. The role of dreissenids and waterfowl in the life cycle of these trematodes is discussed later in this review.
2.4. Crustaceans
2.4.1. Cladocerans
Their small size, patchy distribution, and slow locomotion (Karatayev and Burlakova Citation2022a) make dreissenid larvae an attractive and easily obtainable food for various planktonic predators. Lazareva et al. (Citation2016) estimated that, in Rybinsk Reservoir (Russia), up to 90% of veliger production is consumed by pelagic invertebrate predators. In laboratory tests with Great Lakes zooplankton, Pichlová-Ptáčníková and Vanderploeg (Citation2009) observed that the invasive Ponto-Caspian cladoceran Cercopagis pengoi feeds efficiently on D. polymorpha veligers, but they also noted that densities of C. pengoi in Lake Michigan are comparatively low and that these cladocerans are very unlikely to have a significant impact, if any, on dreissenid larvae.
2.4.2. Copepods
Laboratory experiments by Karabin (Citation1978) in Poland suggest that the predatory copepod Mesocyclops may feed on planktonic larvae of D. polymorpha. Mogilchenko (Citation1986) reported that in the Kanewskoe Reservoir (Ukraine) copepods feed on dreissenid veligers, but they do not actively hunt for this prey, consuming veligers when they circumstantially come into contact with them. The North American laboratory experiments of Liebig and Vanderploeg (Citation1995) with the calanoid copepods Diaptomus sicilis, Limnocalanus macrurus, and Epischura lacustris indicated that both the trochophore (the initial shell-less larval stage) as well as the D-stage of D. polymorpha could be successfully preyed upon, but it was the trochophore that was especially vulnerable to this predation. Although consumption of trochophores by a wide variety of predators must be common in nature, these laboratory experiments apparently still remain the only record of any organism preying specifically on this dreissenid larval stage.
2.4.3. Amphipods
There is no clear evidence that the amphipod Dikerogammarus villosus—well-known for its aggressive, carnivorous nature—preys on dreissenids. There is a single laboratory observation of D. villosus feeding on the byssal threads of D. polymorpha when both were held in experimental containers (Platvoet et al. Citation2009). In contrast, extensive laboratory observations of D. polymorpha by Kobak et al. (Citation2012) and Dzierzynska-Bialonczyk et al. (Citation2019) did not report any evidence of predation by D. villosus. Kobak et al. (Citation2012) did indicate, however, that when in the presence of D. villosus, mussels moved less and increased their byssal attachment strength—two behavioral reactions suggested to indicate inadvertent mechanical irritation of the exposed mussels’ soft tissues by the amphipod’s appendages. Dzierzynska-Bialonczyk et al. (Citation2019) observed that when exposed to D. villosus, D. polymorpha reduced its gaping activity, and they suggested that the mechanical irritation to the mussel’s siphons and mantle caused by the appendages of the crawling D. villosus was likely involved. They further noted that although it is unlikely that D. villosus could pose a direct predatory threat to dreissenids, if the amphipod’s populations are sufficiently high, the repeated mechanical irritation and resulting reduced gaping/feeding might negatively affect D. polymorpha’s body condition.
2.4.4. Mysids
Mysids are a major component of estuarine and coastal zooplankton communities. In trials involving multi-prey assemblages, two native mysid species from the St. Lawrence River middle estuary, Neomysis americana and Mysis stenolepis, exhibited high predation rates on zebra mussel veligers (Winkler et al. Citation2007). Another mysid, Mysis diluviana actively consumed veligers in Lake Michigan in summer, with up to 50% of Mysis stomachs examined containing dreissenid larvae (O’Malley and Bunnell Citation2014).
2.4.5. Crabs
Both laboratory and field data suggest that blue crabs, Callinectes sapidus, were responsible for a 1992 population crash of 2–3 cm long dreissenids in the Hudson River near Catskill, New York (Molloy et al. Citation1994). Laboratory trials confirmed that these crabs can aggressively consume such large dreissenids (). Further supportive evidence of the blue crab predation hypothesis was gained in subsequent summers when blue crabs did not migrate into the Catskill area and no massive declines in 2–3 cm mussels occurred. Cage experiments in the Hudson River suggest that blue crabs could be more effective in reducing dreissenid abundance than either local fish or other invertebrate predators, but that mussel populations would not be regulated unless predator abundance, including blue crabs, increased significantly (Boles and Lipcius Citation1997). Later observations conducted in the Hudson River estuary in control areas open to predation and in exclosures inaccessible to large predators provided further evidence that blue crabs cause high mortality of zebra mussels (Carlsson et al. Citation2011). In Oklahoma and Texas, two invasive species, zebra mussels and Harris mud crabs (Rhithropanopeus harrisii) now coexist in a novel predator–prey relationship (Hallidayschult and Hambright Citation2018). Laboratory experiments showed that Harris mud crabs consume zebra mussels and may play an important role as a predator of dreissenids. In nature, however, the consumption may be lower: in the Odra estuary (Poland), R. harrisii tridentatus was found to feed mainly on detritus (61% of the gut content), while animal food (13%) contained remains of copepods, insects, fragments of the blue mussel (Mytilus edulis) and the zebra mussel (D. polymorpha) (Czerniejewski and Rybczyk Citation2008).
2.4.6. Crayfish
Most crayfish species are omnivorous, and mollusks are often one of their main prey items (Lodge et al. Citation1994; Nyström 2002). It is generally accepted that consumption of dreissenids by crayfish (Decapoda: Astacoidea) occurs widely in nature, but to date, only the following five species have been field-documented as predators: Orconectes rusticus in North America (Green et al. Citation2008; Perry et al. Citation1997, Citation2000), and four species in Europe: Astacus leptodactylus (Grinbart and Suprunovitch Citation1981; Malinowskaya Citation1976; Sebestyén 1937), Cambarus affinis (Pieplow Citation1938), Orconectes limosus (Kornobis Citation1977; Piesik Citation1974; Smit et al. Citation1993; Szlauer Citation1974), and Procambarus clarkii (Chucholl Citation2013) (). Laboratory feeding trials showed that nine other crayfish species consume dreissenids as well (). Martin and Corkum (Citation1994) and Schreiber et al. (Citation1998) stressed, however, that laboratory results do not necessarily accurately predict feeding habits in the wild where other prey is available and that future research needs to focus on crayfish foraging under field conditions.
Table 9. Crayfish species documented eating Dreissena.
2.4.6.1. Feeding preferences
Although crayfish are omnivores (Nystrom 2002), they do have feeding preferences. In laboratory trials, the rate of predation on dreissenids by Orconectes decreased when crayfish were concurrently offered either macrophytes (MacIsaac Citation1994) or trout eggs (Love and Savino Citation1993). Malinowskaya (Citation1976) observed that in the Kyshunskoe Reservoir (Kazakhstan) dreissenids were a predominant food item for Astacus leptodactylus females, while males ate mainly vegetation. In laboratory trials, MacIsaac (Citation1994) observed a higher predation rate in O. propinquus females and Piesik (Citation1974) in O. limosus females; the latter author considered that the lower rate of male predation was a short-term effect related to their reproductive cycle. The laboratory trials of Perry et al. (Citation1997) recorded no difference in the maximum size of dreissenids consumed by male and female O. rusticus. Laboratory trials with O. virilis and Cambarus robustus indicated that feeding on dreissenids may involve odor cues (Hazlett Citation1994).
2.4.6.2. Prey handling techniques and size preference
Laboratory trials provided extensive details on the handling techniques used by crayfish preying on dreissenids (MacIsaac Citation1994; Reynolds and Donohoe Citation2001; Schreiber et al. Citation1998). Such trials have also consistently demonstrated that crayfish prefer small dreissenids, with a positive correlation between predator and prey sizes (MacIsaac Citation1994; Martin and Corkum Citation1994; Naddafi and Rudstam Citation2014c; Perry et al. Citation1997; Piesik Citation1974; Reynolds and Donohoe Citation2001; Schreiber et al. Citation1998; zu Ermgassen and Aldridge Citation2011). Martin and Corkum (Citation1994) observed that O. propinquus consumed mussels up to 17 mm in length, with a preference for mussels ≤8 mm long. MacIsaac (Citation1994) determined that O. propinquus can consume small to medium-sized mussels (3–14 mm long), but preferred that 3–5 mm in length; he suggested that the high predation rates on these small mussels were related to the relative ease with which they are handled, i.e., small mussels require significantly less manipulation time (median: 68 sec) than medium-sized mussels (median: 456 sec) before they are ingested. Piesik (Citation1974) observed that 90 mm long O. limosus, although capable of consuming Dreissena up to 12 mm long, also preferred small mussels (1–5 mm long). Schreiber et al. (Citation1998) observed that all Pacifastacus leniusculus showed a clear preference for the smallest mussels offered, but also that when breaking the shell was no longer a barrier for the crayfish (as in the case when dead and crushed mussels were offered as prey), there was no size-selectivity, leading to the conclusion that dead mussels are likely more attractive than live mussels. In laboratory experiments, Reynolds and Donohoe (Citation2001) observed that white-clawed crayfish Austropotamobius pallipes predominantly feed on mussels <11 mm in length, with 3–7 mm mussels consumed in the highest numbers, but when eaten mussels were not replaced, crayfish shifted to larger sizes. Larger crayfish consumed more mussels of a wider size range, but females consumed on average both 50–80% less than their male counterparts and smaller mussels than males. While in the presence of alternative prey experienced crayfish ate mussels and alternative food items in similar amounts, whereas those that had no prior experience with zebra mussels nearly always chose the alternative items first (Reynolds and Donohoe Citation2001).
The intensity of crayfish predation decreases with water temperature (Piesik Citation1974). Although Cambarus affinis in Germany consumes dreissenids from at least April through December (the entire period of the field study), the highest predation rates are in July and August (Pieplow Citation1938). Feeding rates on attached mussels are also lower compared to feeding rates on detached mussels (Schreiber et al. Citation1998).
2.4.6.3. Impact of crayfish predation
Information on the impact of crayfish predation on dreissenid populations in nature is very limited. Predation leading to significant declines in a European dreissenid population was reported in a non-controlled study (Piesik Citation1974). In caged field studies in Lake Erie, Orconectes rusticus had a negligible effect on Dreissena’s density and shell-length frequency distribution, likely due to their feeding preferences on other macroinvertebrate prey (Stewart et al. Citation1998). In North American stream trials, however, crayfish did reduce dreissenid recruitment and density in enclosures (Perry et al. Citation1997, Citation2000). These results suggested that relative to lakes, predation by crayfish in streams may be a more important population density regulating mechanism since mussel recruitment in streams is already constrained by water velocity and other factors (reviewed in Karatayev and Burlakova Citation2022a). Even at high predation pressures, it is unlikely that in streams crayfish can reduce dreissenid populations below densities that are ecologically important (Perry et al. Citation1997). Although zebra mussels can be important food items for predators, such as O. rusticus which use chemical cues, their vulnerability to predators appears to be directly related to the integrity of the mussel shell and/or their byssus threads and is less pronounced in nature (lakes) than in the laboratory, probably owing to the presence in nature of alternative prey items (Green et al. Citation2008). Therefore, although zebra mussels may represent a substantial food source, only local and temporary reductions of mussels may occur in the wild, and crayfish are unlikely to be able to significantly impact established zebra mussel populations.
2.4.6.4. Impact of dreissenids on crayfish
Dreissenids have been observed on the exoskeleton of Astacus leptodactylus, particularly older specimens which normally do not molt as frequently as juveniles, e.g., >7 cm length (Lamanova Citation1971). Sebestyén (1937) considered that such overgrowth has only a temporary negative effect on Astacus leptodactylus, but Lamanova (Citation1971) reported chitinous sores (≤1 cm diameter) on this species, with potentially adverse effects on crayfish vision and feeding. Anwand (Citation1996) reported that O. limosus neither suffered apparent damage nor benefited from dreissenid colonization, but that the mussels likely benefited from the additional substrate, enlarged activity area, and better feeding conditions. Brazner and Jensen (Citation2000) reported observing six rusty crayfish (O. rusticus) colonized with D. polymorpha near Green Bay (Lake Michigan, USA). The mean length of attached mussel was 3.6 mm, and the number of mussels ranged from 16 to 431 per crayfish. The authors suggested that the energetic costs or physical constraints caused by the attached dreissenids might be detrimental to the infested crayfish.
2.5. Other predator groups
2.5.1. Coelenterates
Conn and Conn (Citation1993) documented Hydra americana preying on Dreissena veligers in the St. Lawrence River (United States-Canadian border); these hydrae immobilized their prey with tentacles containing stinging nematocysts. Both attached and planktonic hydra fed on dreissenid veligers, and some were observed to have several veligers in their gastrovascular cavities (Conn and Conn Citation1993). Predation on dreissenid planktonic larvae by Cordylophora was reported from the Bay of Szczecin (Poland) (Wiktor Citation1969).
2.5.2. Rotifers
Veligers up to 300 μm in diameter were found comparatively often (13% of the specimens analyzed) in the stomachs of the predatory Asplanchna herricki, the largest pelagic rotifer species in Rybinsk Reservoir (Russia) (Lazareva Citation2004).
2.5.3. Annelids
Reports of leeches feeding on molluscs are rare. Consumption of juvenile dreissenids in Europe by Glossiphonia complanata (Hirudinea: Glossiphoniidae) (Smit et al. Citation1993) is the only record available. Predation on North American dreissenids is likely since G. complanata has been reported feeding on molluscs in Iowa, including the freshwater bivalve Lampsilis siliquoidea (Waffle Citation1963).
2.5.4. Turtles
Laboratory studies with map turtles, Graptemys geographica, collected from the St. Lawrence River suggest that these reptiles forage on dreissenids in nature: turtles 6–9 cm (plastron length) in size consumed mussels 4–32 mm in length. The authors noted, however, that dreissenids can be important only when more desirable prey (e.g., snails) are scarce (Serrouya et al. Citation1995). In contrast, Bulté and Blouin-Demers (Citation2008) documented that in Lake Opinicon (Canada) zebra mussels constitute up to 36% of the diet of the map turtle and estimated that turtles can consume over 3000 kg of zebra mussels per year.
In addition to map turtles G. geographica, the stinkpot turtle Sternotherus odoratus was also found to prey heavily on invasive mussels in the Laurentian Great Lakes (Lindeman Citation2006; Patterson and Lindeman Citation2009). While juvenile and male map turtles fed on zebra and quagga mussels occasionally (33–44% occurrence), 100% of the adult females consumed dreissenids compared to only 20% of the adult males. The Index of relative importance (IRI) of dreissenids in adult females was 98%, compared to 1% in males (Lindeman Citation2006). In contrast, no differences between the sexes were found for the stinkpot turtle: dreissenids were the most prevalent food item consumed, with similar values of IRI (62 for males, 60 for females) (Patterson and Lindeman Citation2009). Therefore, both map and stinkpot turtles exhibited shifts toward increased molluscivory having switched to heavy consumption of invasive dreissenids.
2.5.5. Rodents
Consumption of dreissenids by the Norway rat, Rattus norvegicus, has been reported from Italy (Bedulli and Franchini Citation1978). Although muskrats, Ondatra zibethicus, prefer plant food, they have been observed to consume dreissenids in Germany (Reichholf Citation1985) and Poland (Wolk Citation1979). Muskrats have been documented to have a significant impact on other bivalves before dreissenid introduction: thus, in Narrow Lake (Canada) they annually consume 3% of the Anodonta grandis simpsoniana (Unionidae) population, which represents 31% of its annual tissue production. The authors speculate that muskrat consumption of the largest mussels (>55 mm) reduced the reproductive capacity of the mussel population (Hanson et al. Citation1989). After some waterbodies were invaded by dreissenids, muskrats began preying on zebra mussels attached to unionids but left the native unionids untouched and alive (Sietman et al. Citation2003). All live unionids from middens left by the muskrats had zebra mussel byssal threads or in some cases, live zebra mussels on their shells. The change in muskrat prey selection from native unionids to zebra mussels is probably due to the fact that the latter may be easier to open and consume than unionids (Sietman et al. Citation2003).
2.5.6. Other animals
Dreissenids were found in the guts of a declining Laurentian Great Lakes native species, the large mudpuppy salamander (Necturus maculosus), but at low frequency (2–6%), likely due to difficulties in the consumption of the hard shells (Beattie et al. Citation2017). To evaluate possible customers for potential zebra mussel farming in Germany, feeding experiments were carried out in The Zoological Garden in Osnabrück with mussels harvested in the Oder Lagoon (Schernewski et al. Citation2019). The trials showed that mongooses (Mungos mungo) and the oriental small-clawed otters (Aonyx cinerea) immediately accepted zebra mussels as food, and raccoons (Procyon lotor) even showed a preference for zebra mussels. Further studies are needed to confirm if otters and racoons feed on dreissenids in the wild.
2.5.7. Intraspecific predation on dreissenid larvae
The cannibalism of planktonic larvae by sessile Dreissena has been documented in Europe (Mikheev Citation1966; Shevtsova et al. Citation1986) and in North America (MacIsaac et al. 1991). MacIsaac et al. (1991, Citation1995) conducted laboratory and field studies addressing the impact of this intraspecific predation and suggested that it may be a density-dependent population regulatory mechanism. They observed that rates of clearance of veligers from the water column increased with mussel size, with maximum prey sizes likely constrained by the diameter of the inhalant siphon. They proposed that larval mortality in North America was initially substantially lower than today due to the scarcity of predatory adult mussels. The percentage of dreissenid plankton that are drawn into the mantle cavity but are rejected and survive has not been documented, although in other bivalve species (e.g., Mytilus edulis) conspecific larvae have been observed to emerge alive after having passed through the digestive tract of adult predators (Voskresensky Citation1973).
3. Endosymbionts
Seventy-five species and higher taxa of endosymbionts (commensals and parasites) have been found within the mantle cavity and/or associated with D. polymorpha tissue, including ciliates, trematodes, nematodes, chironomids, oligochaetes, mites, and leeches in Europe, and 21 in North America ( and ). All these organisms have been reported living within attached mussels. There have been no records of endosymbionts reported from planktonic larvae, but this is likely due in large part to the absence of research on this topic. While some species are highly specific and found exclusively within a particular Dreissena species (e.g., certain ciliates and trematodes), others have a broader range of hosts (e.g., the oligochaete Chaetogaster limnaei and the trematode Echinoparyphium recurvatum). Although some parasites and commensals use dreissenids as the only host in their life cycle, others, such as digenetic trematodes, may use them as intermediate hosts, developing into adults typically in fish or waterfowl (reviewed in Molloy et al. Citation1997). In addition to obligate endosymbionts, a large range of free-living benthic species are occasionally reported from dreissenid mantle cavities, including chironomid larvae (Karatayev et al. Citation2000a; Mastitsky and Samoilenko Citation2005; Ricciardi Citation1994), nematodes (Karatayev et al. Citation2000a, Citation2003a; Mastitsky and Gagarin Citation2004), and leeches (Karatayev et al. Citation2000a; Kuperman et al. Citation1994).
Table 10. Endosymbionts including commensal (C) and parasitic (P) reported from attached Dreissena polymorpha and D. r. bugensis populations in Europe.
Table 11. Endosymbionts reported from the attached Dreissena polymorpha and D. r. bugensis populations in North America.
Reports of Dreissena endosymbionts vary from extremely common, recorded from virtually all European populations examined (e.g., Conchophthirus spp., Ciliophora), to those recorded from very few locations (e.g., haplosporidians) (). In addition, many more parasites and commensals were reported for D. polymorpha than for D. r. bugensis, and more from Europe than from North America ( and ). These differences, however, could be due to the actual differences in their occurrence or to sampling bias. Some species like Conchophthirus spp. and Ophryoglena spp. can be easily identified when mussels are dissected, and therefore historically they were reported from numerous waterbodies (reviewed in Karatayev et al. Citation2000a, Citation2007; Molloy et al. Citation1997), whereas other endosymbionts, particularly microbial parasites, such as haplosporidians and prokaryote bacteria, require histological analyses, and therefore, will almost certainly be missed when only dissections are performed. The massive research efforts initiated by Molloy et al. during the 1990s, based on samples from 12 European countries and United States where almost 5800 mussels were dissected and over 3300 mussels were histologically analyzed, have greatly increased the information on Dreissena endosymbionts, especially those whose identification requires histological analysis (Molloy et al. unpublished data, ).
3.1. Ciliates
Four species of host-specific ciliates (Conchophthirus acuminatus, Hypocomagalma dreissenae, Sphenophrya dreissenae, and S. naumiana) are known from the mantle cavity of D. polymorpha, and at least two ophryoglenine species (Ophryoglena hemophaga and an undescribed Ophryoglena sp.) from the digestive gland (). The nature of the symbiotic relationships of these species with their dreissenid hosts is usually poorly known but appears to range from commensalism to parasitism (reviewed in Molloy et al. Citation1997). In her review of protozoans in molluscs, Bradbury (Citation1994) noted that a healthy mollusc in a low-stress environment is usually in equilibrium with the ciliates in its mantle cavity and that typically the hosts are not seriously affected unless protist populations increase beyond control. As with ciliates in Dreissena, most ciliates in molluscs, in general, retain the cilia that identify them as members of the phylum, but with or without cilia, all of them possess dimorphic nuclei and an infraciliature at some point in their life history—the basic characteristics of the phylum (reviewed in Molloy et al. Citation1997). Ciliates occupying the mantle cavity are also present in the gill water tubes and the suprabranchial cavities, suggesting that they can exit into surrounding waters via the exhalant siphon and colonize other individuals (Laruelle et al. Citation1999).
Figure 2. Ciliates from Dreissena spp.: A, Conchophthirus acuminatus; B, Conchophthirus klimentinus (both adapted from Raabe Citation1971); C, Sphenophrya naumiana; D, Sphenophrya dreissenae (both adapted from Raabe Citation1966); E, Hypocomagalma dreissenae (adapted from Fenchel Citation1965); F, Ancistrumina limnica (adapted from Raabe Citation1947); G, H, trophont and theront of Ophryoglena hemophaga (adapted from Molloy et al. Citation2005).
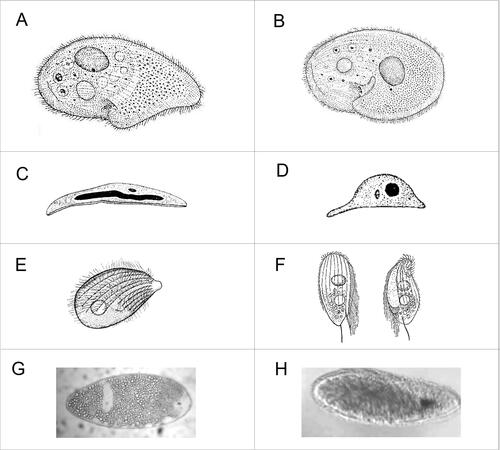
3.1.1. Conchophthirus (Scuticociliatida: Conchophthiridae)
3.1.1.1. General description and biology
The mantle cavities of lamellibranchs are infected with Conchophthirus spp. worldwide, both in marine and freshwater habitats (Kirby Citation1941). The species Conchophthirus acuminatus has been reported from D. polymorpha and D. r. bugensis, while C. klimentinus was found exclusively in D. carinata (=D. stankovici) (). The most common endosymbiont of D. polymorpha in Europe is C. acuminatus, and it is typically reported to have the highest prevalence (i.e., percent of mussels with endosymbionts) and intensity (i.e., number of endosymbionts per infected mussel) of infection (Burlakova Citation1998; Burlakova et al. Citation1998; Karatayev et al. Citation2000a, Citation2007; Molloy et al. Citation1997). Please note that the term “infection” is used for convenience and consistency for all symbionts even if some of them are commensals.
As is typical for the Conchophthiridae, C. acuminatus and C. klimentinus are strongly laterally compressed and lie on their flattened left side (i.e., the side in contact with the substrate is not their ventral side). The body of C. acuminatus (ca. 50–120 µm in length, 30–60 µm wide) is slightly tapered anteriorly and rounded posteriorly (Raabe Citation1971). In contrast to C. acuminatus, the body of C. klimentinus is slightly rounded at both ends, has a more ovoid outline, and its left side is uniformly concave along almost its entire length; L = 60–130 µm (mean ca. 100 µm) and W = 40–100 µm (mean ca. 55 µm) (Raabe Citation1966, Citation1971).
Little is known about their biology, and conjugation has rarely been observed (Raabe Citation1971). They live primarily on the gills, the visceral mass, or the walls of the mantle cavity, with some species preferring specific regions (Kirby Citation1941). In unionids, for example, C. anodontae is primarily attached to the nonciliated surface of the oral palps (Kidder Citation1934). In Dreissena, C. acuminatus can be found in a variety of locations (Laruelle et al. Citation1999) but is most frequently observed on the visceral mass and gills, where they creep about using their short, dense cilia. According to Kidder (Citation1934), all Conchophthirus spp. have an obligate association with bivalves and likely can tolerate only short periods outside their hosts, as during their transfer to new hosts. Karatayev et al. (Citation2003b), however, demonstrated in laboratory trials that C. acuminatus can survive outside a host for up to 6 days, but most ciliates died within 48 hr.
3.1.1.2. Host specificity
Both in North America (Antipa and Small Citation1971; Kidder Citation1934) and in Europe (Raabe Citation1950), Conchophthirus spp. tend to be fairly host-specific. The Conchophthirus spp. occurring in North American unionids, for example, have not been reported from dreissenids on this continent (Molloy et al. Citation1997). Dreissenid endosymbiont C. acuminatus, whose range is limited to Europe, in addition to D. polymorpha, has been reported in D. r. bugensis from the Dnieper River, Ukraine (Karatayev et al. Citation2000b; Yuryshynets Citation2019), Volga River reservoirs, Russia (Pryanichnikova et al. Citation2011; Tyutin et al. Citation2013a), and in D. carinata from Lake Ohrid, Macedonia (Molloy et al. Citation2010; Raabe Citation1966). Although in the Dnieper River and Volga reservoirs both Dreissena species coexist, the prevalence of infection in D. r. bugensis is consistently significantly lower than in D. polymorpha. Because in the Dnieper River, both Dreissena species were sampled at the same locations, Karatayev et al. (Citation2000b) suggested that D. r. bugensis may not be as suitable a host as D. polymorpha, or possibly D. r. bugensis may just be an accidental host in which C. acuminatus cannot survive and/or reproduce. In Lake Ohrid, where only one dreissenid species is present (D. carinata) (Lvova and Starobogatov Citation1982; Molloy et al. Citation2010), two species of Conchophthirus were reported: C. klimentinus (endemic to this lake), and C. acuminatus (Molloy et al. Citation2010; Raabe Citation1966). Recent data, however, suggest that ciliates reported as C. acuminatus from Lake Ohrid are actually an undescribed Conchophthirus species (Molloy, unpublished data).
3.1.1.3. Nature of the relationship
The Conchophthirus spp. in bivalves are generally considered commensals (reviewed in Molloy et al. Citation1997). No evidence exists of any detrimental effects on unionid bivalves (Antipa and Small Citation1971) or D. polymorpha (reviewed in Karatayev et al. Citation2007), even at the highest densities. Fenchel (Citation1965) reported that the ciliature near the cytostome (mouth) of Conchophthirus spp. was very much reduced, suggesting that these ciliates are incapable of ingesting suspended particles and that they likely feed primarily on items present on the epithelial tissues within the mantle cavity. Food vacuoles in Conchophthirus spp. typically contain algae, bacteria, and sloughed-off epithelial cells (Kirby Citation1941). Kidder (Citation1933), for example, concluded that in the saltwater mussel, Mytilus edulis, Conchophthirus mytili was “no doubt” a commensal since its food vacuoles were filled primarily with algae. A year later, however, the same author (Kidder Citation1934) reported observing well-preserved unionid epithelial cells in Conchophthirus magna, which suggested active consumption of the host’s tissues. In examining the food vacuoles of C. curtus from unionids, Antipa and Small (Citation1971) found host cilia and host tissue nuclei but concluded that C. curtus had only consumed sloughed cells and other cellular debris. Although Laruelle et al. (Citation1999) occasionally observed dreissenid sperm in food vacuoles of C. acuminatus, epithelial tissues in contact with high densities of these ciliates showed no evidence of pathology, thus, providing further evidence of this species’ commensal nature. There is also a generally accepted parasitological concept that the prevalence of infection is inversely related to pathogenicity (Anderson and May Citation1981). Therefore, the ubiquitous high prevalence of infection in European D. polymorpha, which often reaches 100%, and the infection intensity that often exceeds thousands of ciliates per mussel (see below) are indirect evidence suggesting that C. acuminatus is commensal rather than parasitic.
3.1.1.4. Prevalence and infection intensity
High prevalence rates of Conchophthirus infection in D. polymorpha have been commonly reported, with 100% infection observed in Denmark (Fenchel Citation1965), Belarus (Burlakova et al. Citation1998), and Ukraine (Yuryshynets et al. Citation2008). Overall, the prevalence of infection in 37 European populations of D. polymorpha ranged from 75 to 100% (Karatayev et al. Citation2007; Tyutin et al. Citation2013a). In the Dnieper River (Ukraine), Karatayev et al. (Citation2000b) recorded C. acuminatus in both D. polymorpha and D. r. bugensis, but the prevalence and intensity of infection were significantly lower for D. r. bugensis, suggesting lower susceptibility. Intensity of C. acuminatus infection in D. polymorpha correlates directly with mussel length (r2 = 0.83–0.92), and when infection prevalence is 100%, it is not uncommon to find from 500 to 2000 C. acuminatus in the mantle cavity of a single individual (Burlakova et al. Citation1998; Karatayev et al. Citation2000b). The smallest infected mussel ever reported was 2-mm long and contained only a single C. acuminatus, whereas the maximum number (14,035 ciliates/mussel) was recorded in a 26.4-mm D. polymorpha (Karatayev et al. Citation2000b).
The laboratory experiments of Burlakova et al. (Citation1998) showed that dying mussels are swiftly left by the ciliate and authors hypothesized that the dying mussels were likely a major source for the spread of C. acuminatus infections. These ciliates commonly leave their hosts when they are still alive, with the rate of emergence being temperature dependent and episodic, with periods of no emergence followed by periods of high emergence (up to 720 ciliates per mussel per day) (Karatayev et al. Citation2003b). During a 24-day experiment, the average number of C. acuminatus that emerged from each experimental mussel at 21 °C (207 ciliates/mussel) was significantly higher than the number that emerged at 14 °C (29 ciliates/mussel) (Karatayev et al. Citation2003b). Field observations also demonstrated that the presence of live mussels with high intensity of infection could also serve as a source to initiate and subsequently amplify the infection of nearby dreissenids. Similarly, Karatayev et al. (Citation2000b) stressed that since infection intensity and prevalence were strongly correlated with mussel size, the presence of large, infected mussels was likely important to serve as a reservoir for maintaining infection in the overall population. They also suggested that mass emigration of C. acuminatus into surrounding waters might be synchronized to occur when new potential hosts, i.e., juvenile mussels, become abundant. In a study conducted in 2001–2002 in Drozdy Reservoir in Belarus, Karatayev et al. (Citation2003a) recorded clear infection peaks in August, positively correlated with water temperature. In addition, the mean size of C. acuminatus was negatively correlated with temperature, and the timing of temperature increase was positively correlated with asexual reproduction, with a peak in cell division in April (5% of fusion pairs in the population) when water temperature increases (Karatayev et al. Citation2003a).
3.1.1.5. Geographical distribution
This ciliate is widespread in European D. polymorpha populations and is the most common of all known symbionts (). In Belarus, for example, this species was observed in all 31 D. polymorpha populations sampled (reviewed in Karatayev et al. Citation2007). It was recorded in dreissenid populations from all 16 European countries studied ().
Because C. acuminatus is present in virtually all European freshwater populations of D. polymorpha and has not been found in North America yet, Karatayev et al. (Citation2000b) hypothesized that: (1) planktonic larvae, rather than attached Dreissena, invaded North America, and (2) the European waterbodies invaded by Dreissena where C. acuminatus-infestations are found were either colonized by adult mussels or, if a waterbody was colonized by planktonic larvae, it is connected to an upstream source with infested adults and the ciliates were transported as free-living individuals, since they can survive in the water-column for up to 6 days (Karatayev et al. Citation2003b). Ireland, being an island, is not directly connected by freshwater to any previously existing source population of D. polymorpha. The presence of C. acuminatus and other species-specific endosymbionts of D. polymorpha in the River Shannon system (Burlakova et al. Citation2006a) supports the hypothesis proposed by Pollux et al. (Citation2003) that Ireland was colonized by adult D. polymorpha (infested with C. acuminatus). Therefore, analyses of Dreissena endosymbionts may help reconstruct the mechanisms of invasion of these mussels (reviewed in Karatayev et al. Citation2007).
Zebra mussels can live in fresh and brackish (<6%o) waters, but C. acuminatus may be less tolerant to salinity: in a Polish Bay, the prevalence of C. acuminatus was noted to decline from 100 to 0% with increasing salinity (Raabe Citation1956 but see Chuševė et al. Citation2012). In order to confirm whether D. polymorpha and C. acuminatus have different tolerance to salinity, Karatayev et al. (Citation2007) suggested to check for the presence of C. acuminatus or related ciliates in other species and subspecies of Dreissena from their native area in the Caspian Sea and the Azov seas. Such studies may help to explain whether D. polymorpha and C. acuminatus have different origins and will shed light on their coevolutionary history.
3.1.2. Hypocomagalma (Rhynchodida: Ancistrocomidae)
Only one species of ancistrocomid ciliate, Hypocomagalma dreissenae, has been reported from Dreissena spp. (reviewed in Molloy et al. Citation1997) (). The body of H. dreissenae is almost entirely covered with cilia, elongated, banana-shaped, has reduced ciliation, and typically has a rounded posterior end (Fenchel Citation1965; Raabe Citation1966, Citation1970). Its dimensions (L × W × H) are 32–50 × 14–19 × 10–15 µm (Jarocki and Raabe Citation1932; Raabe Citation1970). In Dreissena this ciliate was most frequently observed attached to epithelial cells lining the outer gill surfaces, but also occasionally on the visceral mass, the mantle cavity epithelium, in gill water tubes, and rarely on labial palps and within the suprabranchial cavities (Laruelle et al. Citation1999). The mouth in all Hypocomagalma spp. has been functionally replaced by a suctorial tentacle (attachment knob) at the anterior end, which is inserted into the cytoplasm of the host’s epithelial cell. Material from the epithelial cell passes into the ciliate through this tentacle, damaging the penetrated host cell in the process (reviewed in Molloy et al. Citation1997). Three dreissenid species: D. polymorpha, D. r. bugensis, and D. carinata have been reported as hosts of H. dreissenae. This ciliate species does appear to be specific to Dreissena and is widely distributed in Europe (). In contrast, records of H. dreissenae from D. r. bugensis exist in Ukraine only (Yuryshynets et al. Citation2003; Molloy et al. unpublished data). In addition, H. dreissenae was reported from D. carinata in Lake Ohrid (Molloy et al. Citation2010; Raabe Citation1966).
While prevalence rates of up to 100% have been reported (Fenchel Citation1965), they usually are much lower (Molloy et al. Citation2010; Raabe Citation1966). Salinity may affect their distribution: in a Polish bay, the prevalence of H. dreissenae has been observed to rise from 2 to 80% with increasing salinity (Raabe Citation1956). In bivalves, Hypocomagalma spp. are clearly parasitic in nature, their infections are typically of low intensity, with little pathological effect (Bradbury Citation1994), which is also true for H. dreissenae (reviewed in Laruelle et al. Citation1999; Molloy et al. Citation1997, Citation2010).
3.1.3. Sphenophrya (Rhynchodida: Sphenophryidae)
3.1.3.1. General description and biology
Ciliates in the genus Sphenophrya spp. are parasites on bivalve gills (Bradbury Citation1994; Fenchel Citation1965). Two species, Sphenophrya dreissenae and S. naumiana, have been described from dreissenids (reviewed in Molloy et al. Citation1997) (). Adult S. dreissenae are frequently shaped like an elongate helmet (their dimensions range around 30 × 24–40 × 34 µm), slightly flattened laterally, and with one or two distinctly protruding processes on the body margin (Dobrzanska Citation1958; Raabe Citation1970). The ciliate S. dreissenae was frequently recorded attached to the mantle cavity epithelium and outer gill surfaces, within the gill water tubes, occasionally on the visceral mass, and rarely in the suprabranchial cavities. Depending on how S. dreissenae is attached to the gill epithelium, however, its shape may vary considerably; if adults attach by the “sucoir” located at their anterior end, they may be pear-shaped; if they attach by their entire inferior surface, they may appear flat or even concave (Dobrzanska Citation1958). In contrast, S. naumiana is elongate to canoe-shaped (L × W = 60–80 × 12–18 µm)—a form more typical of other species in this genus (Raabe Citation1966, Citation1970). Whereas adult S. dreissenae lives on the gills, their immature “tomit” forms, which are produced by budding, can be found either on the gills or on the epithelium lining the mantle cavity (Dobrzanska Citation1958). Immature Sphenophrya resemble species of the suborder Ancistrocomina (e.g., Hypocomagalma) in the pattern of their ciliature, the shape of their bodies, and the presence of an anterior suctorial tentacle (Bradbury Citation1994). In S. dreissenae, both asexual budding and sexual reproduction (conjugation) are relatively synchronous in individual mussels (Dobrzanska Citation1961).
3.1.3.2. Nature of the relationship, prevalence, and infection intensity
Although S. dreissenae has always been considered as a parasite (Dobrzanska Citation1958, Citation1961), the histological observations of Laruelle et al. (Citation1999) provided the first conclusive evidence of its pathology. They reported that foci of high ciliate presence frequently show tissue damage, including epithelial hyperplasia, cell hypertrophy, and extensive vacuolization.
In Dreissena from Lake Ohrid, Raabe (Citation1966) reported ≤1% prevalence and low infection intensity by both S. dreissenae and S. naumiana. In contrast, Dobrzanska (Citation1961) recorded high intensity and prevalence (up to 100%) with S. dreissenae in littoral areas of Polish lakes but noted marked reductions in low density mussel populations in sublittoral areas. She found that the highest intensities occurred generally in spring and autumn, and in younger dreissenids (as has been reported for Sphenophrya spp. in other freshwater bivalves).
3.1.3.3. Geographical distribution
So far Sphenophrya naumiana has been reported only from Lake Ohrid (Raabe Citation1966) where D. carinata is the only documented dreissenid species. Historically (before the 1990s), in addition to the Republic of North Macedonia (Raabe Citation1966), S. dreissenae was reported only from Poland (the type locality of S. dreissenae), where it is common in both flowing and standing waters (Dobrzanska Citation1958, Citation1961). Over the last few decades, S. dreissenae has also been reported in D. polymorpha in Russia (Laruelle et al. Citation1999), France (Minguez et al. Citation2009), Belarus, Denmark, Germany, Italy, and in the Netherlands (Molloy et al. unpublished data), as well as in both D. polymorpha and D. r. bugensis in Ukraine (Molloy et al. unpublished data; Yuryshynets et al. Citation2003). As is often the case with host records, however, genetic analyses are needed to confirm that these are truly valid records of S. dreissenae and not new undescribed, morphologically similar species.
3.1.4. Ophryoglena spp. (Ophryoglenida: Ophryoglenidae)
3.1.4.1. General description and biology
Ciliates in the genus Ophryoglena were repeatedly observed living inside the digestive gland of D. polymorpha (Karatayev et al. Citation2000a, Citation2002b, Citation2003a; Molloy et al. Citation1996, Citation2005; Zdun et al. Citation1994). Two morphotypes have been described: large and small. The large forms were observed primarily inside the digestive gland ducts of D. polymorpha and were described as the new species Ophryoglena hemophaga (Molloy et al. Citation2005). When released from the digestive gland ducts during dissection, O. hemophaga is nearly cylindrical and have a mean L × W of 278 × 77 µm (). In contrast, the small Ophryoglena form is found exclusively in the digestive gland tubules of D. polymorpha and represents an undescribed species (Molloy, unpublished data). In infected D. polymorpha populations, dozens of these relatively small undescribed Ophryoglena are often found during dissections, but O. hemophaga rarely exceeds 10 per host (reviewed in Molloy et al. Citation1997).
The ciliate O. hemophaga is the first ophryoglenine species recorded as a molluscan parasite, with some evidence of pathological effects on their host (Molloy et al. Citation1997; Zdun et al. Citation1994). As is typical of ciliates in the suborder Ophryoglenina, O. hemophaga exhibits a polymorphic life history with encystment and reproduction by palintomy (Molloy et al. Citation2005). The presence of a single, longitudinal tract of multiple contractile vacuoles represents O. hemophaga’s most unique morphological feature and distinguishes it from all other described Ophryoglena spp. Its life cycle includes the parasitic trophont (96–288 μm in length), an emerging protomont, the encysted tomont (50–150 μm in diameter), and the infective theront (96–131 μm in length) ().
Figure 3. Hypothetical life cycle of Ophryoglena hemophaga (adapted from: Canella and Rocchi-Canella Citation1976; Morton Citation1993; Owen Citation1955; Thorp and Covich Citation1991). In laboratory studies (Molloy et al. Citation2005), (1) the presence of “trophonts” (the feeding stage) was repeatedly observed in the ducts of the digestive gland (right image); (2) well-fed trophonts were subsequently commonly found to have recently emerged as “protomonts” from infected D. polymorpha (left image), but it is unknown if they emerged via the exhalant siphon (as pictured) or the inhalant siphon; (3) likewise, protomont encystment into the “tomont” stage, followed by “tomite” production within the tomont, followed by the exit of these tomites from the cyst as free swimming “theronts” was also commonly observed, but it is unknown if these theronts immediately initiate reinfection by entering through the inhalant siphon (as pictured) or possibly go through another encystment-tomont-tomite-theront production cycle again outside the mussel before initiating infection again.
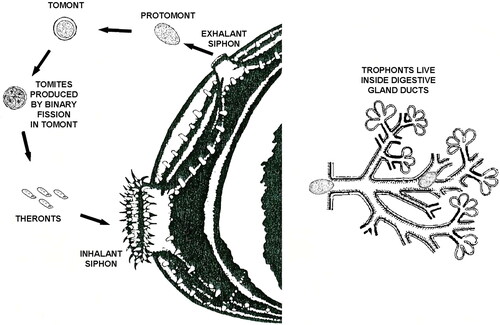
3.1.4.2. Prevalence and infection intensity
In the Dnieper-Bug Canal in Belarus the prevalence and intensity of infection of D. polymorpha with the small undescribed Ophryoglena sp. varied, respectively, from 11 to 62%, and from 0.9 to 24.1 ciliates/mussel (Karatayev et al. Citation2002a). This canal is believed to be the main route of invasion of D. polymorpha into Western Europe and likely also a corridor for the western migration of their symbionts, including this undescribed small Ophryoglena sp. Moderate to high prevalence (43–100%) and low to moderate intensity (1.4 − 65.8 ciliates/mussel) of Ophryoglena infection were reported from the Svisloch River (Belarus) (Karatayev et al. Citation2003a). A high prevalence of infection (100%) of D. polymorpha with O. hemophaga was reported from the Shannon River (Ireland) (Burlakova et al. Citation2006a), low to high (up to 97%) Ophryoglena infection in the Meuse River (Minguez and Giambérini Citation2012), and low to moderate (17.5 − 82.5%) infection in the brackish Curonian Lagoon (Chuševė et al. Citation2012).
In two separate studies of seasonal dynamics of D. polymorpha symbionts in Belarus, Karatayev et al. (Citation2000a, Citation2003a) demonstrated that both prevalence and intensity of infection with the undescribed small Ophryoglena sp. were negatively correlated with temperature and were considerably lower in summer as compared to winter. A similar seasonal pattern in the prevalence of Ophryoglena infection was observed in D. polymorpha in France (Minguez and Giambérini Citation2012).
Karatayev et al. (Citation2002b) reported that a transinfection of D. polymorpha by the undescribed small Ophryoglena sp. was achieved in the laboratory where initially infected and uninfected mussels were kept together. In 15 days, specimens initially free from the ciliate reached a mean prevalence of 86.7% and intensity of 8.3 ciliates/mussel, which were similar to those of initially infected animals.
3.1.4.3. Geographical distribution
Infection of Dreissena with Ophryoglena has been reported in many European populations of D. polymorpha (). In addition, O. hemophaga is the only parasite specific to Dreissena that was also reported from North America. This ciliate was found in D. polymorpha from the Mohawk River (Minguez et al. Citation2013; Molloy, unpublished data) and Lake Erie (Mastitsky, personal communication). Toews et al. (Citation1993) observed Ophryoglena inside “the shells” (i.e., mantle cavity) of living and dead D. polymorpha from Lake Erie. Because Toews et al. (Citation1993), however, did not report these Ophryoglena from the digestive gland or any other organ, Molloy et al. (Citation1997) suggested that they are not the same species as the one found in European D. polymorpha and may simply have been a free-living histophagous species.
In addition to D. polymorpha, observations of Ophryoglena spp. have also been reported from D. r. bugensis and D. carinata. Tyutin and Scherbina (Citation2006) reported Ophryoglena sp. from D. r. bugensis in the Volga River reservoirs and mentioned that the prevalence of infection was much lower than in D. polymorpha. The low prevalence (ca. 1%) of infection in D. r. bugensis from Volga reservoirs was confirmed by Pryanichnikova et al. (Citation2011). Therefore, compared to D. polymorpha where the prevalence of infection may reach 100% (see above), the prevalence of D. r. bugensis infection is always much lower, suggesting lower susceptibility.
An Ophryoglena sp. was frequently found by Raabe (Citation1966) in the mantle cavity of Dreissena in Ohrid Lake. More recently, Molloy et al. (Citation2010) found large (151–160 μm) and small (ca. 40 μm) Ophryoglena sp. in D. carinata from Lake Ohrid. It is likely that these ophryoglenids are new undescribed species (Molloy, unpublished data).
3.1.5. Ancistrumina (Scuticociliatida: Ancistridae)
The ciliate Ancistrumina (=Ancistrina) limnica is a non-host-specific symbiont of freshwater lamellibranchs and gastropods (Raabe Citation1947, Citation1959, Citation1965) (). Its dimensions (L × W) are 35 × 18 µm (Raabe Citation1947). In D. polymorpha, A. limnica was recorded frequently within gill water tubes, occasionally on the outer gill epithelium, and rarely within the suprabranchial cavities (Laruelle et al. Citation1999). Ancistrumids typically feed on bacteria, diatoms, and other material retrieved from water currents (Kirby Citation1941), and thus, are not considered parasitic (Laruelle et al. Citation1999).
The geographic range of A. limnica includes European populations of D. polymorpha in Poland (Raabe Citation1956), the Netherlands and Greece (Laruelle et al. Citation1999), Belarus (Karatayev et al. Citation2000a), Ireland (Burlakova et al. Citation2006a), and Russia and Ukraine (Molloy et al. unpublished data) (). This species was also reported from D. r. bugensis in Ukraine (Yuryshynets Citation2019; Molloy et al. unpublished data). In Belarus, A. limnica was reported in the mantle cavities of D. polymorpha in 11 of the 17 waterbodies studied, with a prevalence around 0.3–21.6% (Karatayev et al. Citation2000a), but occasionally as high as 94% (Karatayev et al. Citation2003a). The highest intensity of infection was observed in a single mussel (299 A. limnica in a 25 mm long individual) from the Svisloch River, Belarus (Karatayev et al. Citation2000a). Both prevalence and intensity of infection were positively correlated with temperature, being considerably higher in summer and fall than in winter and spring (Karatayev et al. Citation2003a).
3.1.6. Peritrichia
Laruelle et al. (Citation1999) reported ciliates in the subclass Peritrichia in the mantle cavity of European D. polymorpha. Although peritrichs have been previously reported from bivalve mantle cavities (Fenchel Citation1965), this was the first report of these commensal ciliates within dreissenids. It is likely that these ciliates were attached to the epithelium of the visceral mass and not simply free-floating. No signs of negative effects were found in the adjacent epithelium. Since peritrich populations were observed on the shells of D. polymorpha, these ciliates were likely carried passively by water currents into the mantle cavity where they reattached. Records of Peritrichia from European populations of D. polymorpha include Belarus, Greece, the Netherlands, and Ukraine; in Ukraine, they were also found in D. r. bugensis (Molloy et al. unpublished data) ().
3.2. Trematodes
Seven genera of trematodes have been reported as parasites of Dreissena spp. (Molloy et al. Citation1997; Stunženas et al. 2004). In their life cycles, dreissenids can serve as the first intermediate host (e.g., Bucephalus polymorphus and Phyllodistomum macrocotyle), second intermediate host (Echinoparyphium recurvatum, Echinoparyphium paraulum, and Echinoparyphium echinatoides), or the only host (Aspidogaster spp.). Most of these trematodes are Digenea—a subclass in which species require more than one host to complete their life cycle. While trematodes like B. polymorphus and P. macrocotyle have been reported in Dreissena exclusively from Europe (Molloy et al. Citation1997; Stunženas et al. 2004), nonspecific trematodes like E. recurvatum are also known from North American dreissenids (Karatayev et al. Citation2012; Toews et al. Citation1993). Chernogorenko and Nizovskaya (Citation1986) suggested that trematodes exerted antagonism toward each other in their hosts (e.g., inhibition of one species by the other) and suggested that this may explain why two trematode species were rarely recorded in the same mussel; the only double infection they observed was a Dreissena with both B. polymorphus and echinostomatid cysts.
3.2.1. Bucephalus polymorphus (Digenea: Bucephalidae)
3.2.1.1. Life cycle
Three hosts are required to complete the life cycle of Bucephalus polymorphus (). Infection commences in a dreissenid when the earliest larval stage, the miracidium, hatches from an egg, enters a mussel’s visceral mass, and gives rise to the sporocyst stage (reviewed in Molloy et al. Citation1997). The sporocyst of B. polymorphus appears as an entanglement of branching, white tubules of knotty, irregular diameter, within which cercariae develop. Located primarily in the gonads, where they typically induce sterility, these tubules may extend out into other tissues, such as the digestive gland, the gills, the mantle epithelium lining the internal surface of the shells, and the connective tissue between the bundles of adductor muscles (Baturo Citation1977; de Kinkelin et al. Citation1968b; Laruelle et al. Citation2002; Molloy et al. Citation1996). Heavy infections can lead to host castration, with the entire gonadal space often occupied by the sporocysts (Laruelle et al. Citation2002; vom Scheidt Citation1984). Digestive gland tubules in infected D. polymorpha, however, are as numerous and full-bodied as in uninfected specimens (Laruelle et al. Citation2002; Molloy et al. Citation1996).
Figure 4. Life cycle of Bucephalus polymorphus (adapted from Molloy et al. Citation1997).
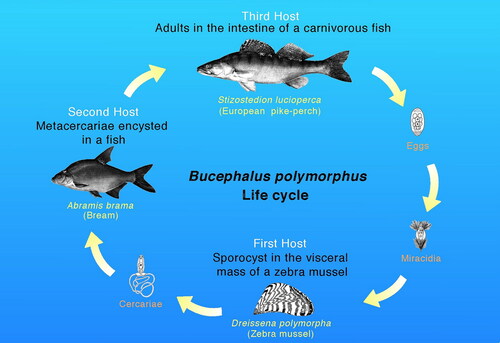
Cercariae released into the surrounding waters attach to fish fry, encyst in their tissues, and transform into metacercariae. Although specifically for B. polymorphus observations are lacking, European research on bucephalid species that infect other freshwater bivalves (Taskinen et al. Citation1994) has indicated that the infection is carried over from year to year. Thus, it is likely that once a dreissenid is infected with B. polymorphus, its gonads will annually produce cercariae, rather than gametes, for the rest of its life (reviewed in Molloy et al. Citation1997).
The cercarial release is seasonal and appears to have diurnal periodicity. Laboratory studies indicate that in a 12:12 hr light:dark cycle, the highest cercarial releases occur during darkness, whereas under a 16:8 hr light:dark cycle they peak during the first hour of illumination (Wallet et al. Citation1985). These patterns possibly increase the chances of contact with their fish hosts. In the Seine Basin (France), cercarial release was recorded from June through October (de Kinkelin et al. Citation1968b). In two lakes in the Konin region of Poland, cercarial emission occurred throughout the entire sampling period from April to November, with a peak between June and October (Baturo Citation1978). In the latter study, the lakes are artificially heated by waters from hydroelectric plants, which likely contributes to the longer duration of cercarial release. In the Drava River, Croatia, young cercariae are dominant in D. polymorpha in autumn and winter while mature cercariae are dominant in spring and summer (Lajtner et al. Citation2008). Cercarial release from dreissenids overlaps periods of fish hatching, thus, facilitating infection. In Goslawickie Lake (Poland), B. polymorphus metacercariae were found as early as April in fish fry 8–11 mm long, with prevalence in fry reaching a maximum in summer and early fall (Baturo Citation1978). This trematode species appears to be widely distributed in European D. polymorpha populations ().
Although a wide range of fish species can serve as hosts for the metacercarial stage of B. polymorphus, cyprinids are the most common (Baturo Citation1978), and also the only hosts reported to be adversely affected. In an epizootic in the Seine River Basin (France), 20 species in six families (Cyprinidae, Percidae, Esocidae, Centrarchidae, Cobitidae, and Gasterosteidae) were observed with B. polymorphus metacercariae, but serious pathologies and deaths were only recorded in cyprinids. The presence of encysted metacercariae in the fins, eyes, and mouths of cyprinids causes a hemorrhagic and necrotic syndrome, which is usually chronic and sometimes fatal (de Kinkelin et al. Citation1968a). Cyprinids in Polish lakes have metacercariae in almost all organs, but the majority are in somatic muscles, the head, and the body cavities (Baturo Citation1978).
Apparently, the pathological impact of B. polymorphus on fish cannot be predicted solely from the prevalence of dreissenid infection. The degree of pathology in cyprinids noted in the Seine Basin (de Kinkelin et al. Citation1968a) was not observed in cyprinids in waterbodies in southern France, even though dreissenid populations had about the same infection prevalence (Wallet and Lambert Citation1986).
From the extensive studies in Poland by Baturo (Citation1978) it would appear that the severe, adverse effects on cyprinids observed in the Seine Basin (de Kinkelin et al. Citation1968a) are an exception. In the Polish lakes investigated by Baturo, no adverse effects were observed on cyprinids, except possibly for bleak A. alburnus (Baturo Citation1978).
The final hosts of B. polymorphus are predatory fish that had consumed fish infected with metacercariae. European records include northern pike (Esox lucius), Eurasian perch (Perca fluviatilis), zander (Stizostedion lucioperca), brown bullhead (Ictalurus nebulosus), burbot (Lota lota), and Wels catfish (Silurus glanis) (Baturo Citation1978; de Kinkelin et al. Citation1968b; Kvach and Mierzejewska Citation2011). Adult worms are located in the intestines (Dubinin Citation1952; de Kinkelin et al. Citation1968b), and prevalence and intensity rates can be high. In Goslawickie Lake (Poland), all 14 zanders sampled were infected and contained a mean of 483 adult parasites, but no evidence of pathological effects (Baturo Citation1978). The absence of ill effects was also noted during the Seine Basin epizootic event, where zander individuals hosted up to several thousand B. polymorphus adults (de Kinkelin et al. Citation1968b). The presence of fish species that are highly suitable as hosts for adult B. polymorphus may be critical for epizootic outbreaks. The explosive development of B. polymorphus populations in the Seine Basin during the 1960s was attributed to the recent introduction of zander (de Kinkelin et al. Citation1968b). Recently, infections by metacercariae of B. polymorphus have increased in several water systems in Europe, due to the expansion of both the first intermediate host, D. polymorpha, and the second intermediate hosts, gobiid fishes from the Ponto-Caspian region, which are heavily preyed upon by piscivorous fish, an essential condition for the completion of the parasite’s life cycle (Kvach and Mierzejewska Citation2011; Ondračková et al. Citation2015). Although D. polymorpha has been present in the River Morava basin (Czech Republic) for more than 40 years, a marked increase in B. polymorphus abundance was observed only after the introduction of the tubenose goby (reviewed in Ondračková et al. Citation2015). In the Vistula drainage (Poland), the life cycle of B. polymorphus includes three non-indigenous organisms: D. polymorpha, the source of cercariae; gobiids (Babka gymnotrachelus and Neogobius fluviatilis), recent invasive fish that could play a more important role in the life cycle than the commonly occurring cyprinids as their small sizes might be favorable for parasite transmission up the food-web; and the last component of the life cycle of B. polymorphus, the Chinese sleeper fish (Perccottus glenii), which is a new accidental definitive host of this parasite (Kvach and Mierzejewska Citation2011).
3.2.1.2. Host specificity
Infections of D. polymorpha by B. polymorphus have been reported frequently (reviewed in Karatayev et al. Citation2000a; Molloy et al. Citation1996, Citation1997; Ondračková et al. Citation2015; Stunžėnas et al. Citation2004). It appears that B. polymorphus is specific to D. polymorpha, as this trematode is usually absent in D. r. bugensis sampled from waterbodies with infected D. polymorpha populations (Pryanichnikova et al. Citation2011; Tyutin and Scherbina Citation2006; Tyutin et al. Citation2005). According to Tyutin et al. (Citation2013b), replacement of D. polymorpha by D. r. bugensis in the Upper Volga reservoirs led to a decrease in the parasitic load of B. polymorphus on their fish hosts. So far, there is only one record of the infection of D. r. bugensis with B. polymorphus (Chernogorenko and Boshko Citation1992).
In Lake Ohrid, B. polymorphus was not found in D. carinata (Molloy et al. Citation2010). In the past, B. polymorphus was reported from Anodonta and Unio (Unionidae) (Chernogorenko and Boshko Citation1992; Combes et al. Citation1980; Kulczycka Citation1939; Smirnova and Ibrasheva Citation1967). The accuracy of the unionid host data is doubtful, however, because the sporocysts and cercariae of B. polymorphus are very similar to those of the bucephalid trematode Rhipidocotyle. Evidence is accumulating that B. polymorphus is a host specific to Dreissena and that the “B. polymorphus” recorded from unionids were actually Rhipidocotyle spp. (reviewed in Molloy et al. Citation1997; Petkevičiūtė et al. Citation2014). Based on a careful examination of cercarial morphology, Baturo (Citation1977) recorded B. polymorphus only in Dreissena and in the unionid R. campanula (=R. illense). Gibson et al. (Citation1992) concluded that the bucephalid cercariae with long filamentous furcae (typical of B. polymorphus) recorded in a unionid population in Finland are actually Rhipidocotyle fennica.
3.2.1.3. Prevalence of infection
Although widely distributed geographically, infection in dreissenid populations is not common, and the prevalence of infection can vary widely. In the most extensive field study conducted to date, very high prevalence rates (up to 73%) were recorded in D. polymorpha from southeastern France (Wallet and Lambert Citation1986). This four-year study also showed seasonal fluctuations: maximum prevalence occurred each year at the warmest water temperatures, with subsequent shedding of cercariae 1–2 months later. Prevalence rates, however, are typically low (0.4%) to moderate (28%) (Aristanov Citation1986; Baturo Citation1977; Chernogorenko and Boshko Citation1992; de Kinkelin et al. Citation1968b; Karatayev et al. Citation2002b; Kuperman et al. Citation1994; Lajtner Citation2012; Lajtner et al. Citation2008; Minguez and Giambérini Citation2012; Molloy et al. Citation1996; Smirnova and Ibrasheva Citation1967; Tyutin et al. Citation2013a).
According to Aristanov (Citation1992), the prevalence of infection varies strongly with mussel size and season of the year. He found a strong increase in the prevalence of infection with D. polymorpha length, from 0.7% in mussels 5–10 mm, to 17.9% in mussels 11–16 mm, and to 51% in mussels 17–23 mm. There was also a substantial increase in prevalence from May (4.3%) to July (31.7%), and a decline in October (19.5%). In contrast, no clear seasonal patterns in the prevalence of infection were found by Minguez and Giambérini (Citation2012). In the only record of B. polymorphus infection in D. bugensis, a prevalence of 6% was reported from Ukraine (Chernogorenko and Boshko Citation1992).
3.2.2. Phyllodistomum macrocotyle (Digenea: Gorgoderidae)
3.2.2.1. Life cycle
Historically, Phyllodistomum trematodes found in D. polymorpha were referred to as P. dogeli, P. angulatum, or P. folium. Recent molecular evidence (Petkevičiūtė et al. Citation2015, Citation2020), however, showed that P. macrocotyle is the only valid Phyllodistomum species documented, thus, far observed in D. polymorpha. Only one other host, a fish, is required for P. macrocotyle to complete its life cycle (). A free-swimming miracidium which has hatched from an egg is drawn into the mantle cavity of D. polymorpha where it penetrates a gill demibranch by peristaltic contractions. Following its transformation into a mother sporocyst, 12–14 daughter sporocysts are produced. Each daughter sporocyst migrates out of the mother sporocyst to another location within the gills, transforms into a parent sporocyst, and gives rise to additional sporocysts which embed themselves elsewhere in the gills (). Several such non-synchronous multiplications lead to a total of ca. 200–300 yellow sporocysts between the lamellae of the four gill demibranchs (reviewed in Molloy et al. Citation1997). Mature free-swimming sporocysts, each containing metacercariae, are shed from the gills and float to the water surface where they are consumed by fish. Although many fish species were reported as hosts of adult P. folium trematodes that were presumably shed from D. polymorpha (Molloy et al. Citation1997; Pietrock et al. Citation1999), a recent study (Petkevičiūtė et al. Citation2020) showed that many of them are P. folium (rather than P. macrocotyle) shed from other bivalves (Sphaeriidae). According to Petkevičiūtė et al. (Citation2020), P. macrocotyle develops into adults in the ureters of fish like rudd (Scardinius erythrophthalmus) and ide (Leuciscus idus). A detailed description of adult P. macrocotyle was recently produced by Petkevičiūtė et al. (Citation2020).
Figure 5. Life cycle of Phyllodistomum macrocotyle (adapted from Molloy et al. Citation1997 with information in Petkevičiūtė et al. Citation2020).
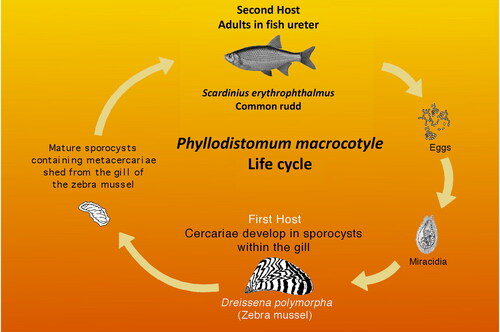
Figure 6. Sporocysts (yellow bodies) within the gills of a Phyllodistomum sp. in Dreissena carinata from Lake Ohrid, Republic of North Macedonia (Credit: D. P. Molloy).
In choosing an intermediate host, P. macrocotyle appears quite specific since they have only been reported from D. polymorpha. In Rybinsk and Gorky reservoirs (Russia), for example, P. macrocotyle parasitizes D. polymorpha, but not D. r. bugensis (Pryanichnikova et al. Citation2011; Tyutin et al. Citation2005). In Lake Ohrid, sporocysts containing metacercariae of Phyllodistomum sp. were observed in the gills of 7% of the dissected and 3% of the histologically examined D. carinata (Molloy et al. Citation2010).
No adverse effects on the fish hosts have been reported. D. polymorpha infected with P. macrocotyle, however, have been reported as having 30% less dry weight than uninfected mussels, presumably due to diminished feeding caused by the presence of sporocysts in the gills. Some toxicants (e.g., Cd and Pb) were also more concentrated in infected mussels, due apparently to the loss of soft tissues (Kraak and Davids 1991). Although reduced gonadal tissue has also been reported (Davids and Kraak Citation1993), some infected mussels are still capable of producing gametes (Molloy et al. Citation1996).
3.2.2.2. Prevalence and infection intensity
Infections by P. macrocotyle are not common in dreissenid populations, and when present, the prevalence is low to moderate (<1–33%) (Karatayev et al. Citation2000a; Kraak and Davids 1991; Kuperman et al. Citation1994; Lyakhnovich et al. Citation1983; Minguez et al. Citation2013; Molloy et al. Citation1996; Peribáñez et al. Citation2011; Smirnova and Ibrasheva Citation1967; Tyutin et al. Citation2013a; Zdun Citation1965). Water temperature can affect the prevalence of infection in dreissenid populations; in Lukomskoe Lake (Belarus), higher rates occur in areas where waters are artificially heated by the outflow of electricity generation plants (Karatayev Citation1983; Lyakhnovich et al. Citation1983). The prevalence of infection also tends to be higher in larger mussels: in the Volga Basin infection is restricted to mussels 23–29 mm in length (Kuperman et al. Citation1994). In Lukomskoe Lake (Belarus), infection is absent in mussels <8 mm long and increases steadily in mussels up to 28 mm long; intensity also increases, from ca. 40 to ca. 200 sporocysts/mussel as mussel length increases from 10 to 26 mm (Lyakhnovich et al. Citation1983). A maximum of 222 sporocysts were reported from D. polymorpha in the Volga Basin (Kuperman et al. Citation1994). In the Netherlands, infected D. polymorpha with all sporocyst developmental stages are present throughout the year (Kraak and Davids 1991).
The trematode species P. macrocotyle (including those previously identified as either P. folium, P. dogieli, or P. angulatum) appears to be widely distributed among European D. polymorpha populations ().
3.2.3. Echinoparyphium recurvatum and related species (Digenea: Echinostomatidae)
3.2.3.1. Life cycle
Three species of echinostomatids were reported from dreissenids, including Echinoparyphium recurvatum (Ginezinskaja Citation1959; Kochnev Citation1977; Lajtner Citation2012; Mastitsky and Veres Citation2010; Molloy et al. Citation1997; Pryanichnikova et al. Citation2011), E. echinatoides (Chernogorenko and Boshko Citation1992; Molloy et al. Citation1997; Pryanichnikova et al. Citation2011; Tyutin et al. Citation2013a), and Echinostoma paraulum (Kochnev Citation1977; Molloy et al. Citation1997). Identification of the echinostomatid species found in Dreissena is problematic due to the morphological similarity of E. recurvatum with species from the Echinostoma revolution complex and usually requires additional identification approaches, such as enzymatic and molecular techniques (Le et al. Citation2020; Saijuntha et al. Citation2011a, Citation2011b; Tkach et al. Citation2016). Therefore, in most publications these trematodes are not identified at the species level (e.g., Karatayev et al. Citation2000a, Citation2012; Minguez and Giambérini Citation2012; Yuryshynets 1999, Citation2019). Rearing of metacercariae to the adult stage is sometimes used for their identification. Using this technique, infected dreissenids in Russia were fed to ducklings, and the adult trematodes identified as E. recurvatum and Echinostoma paraulum (Kochnev Citation1977).
Three hosts are required for the completion of the life cycle of E. recurvatum (reviewed in Molloy et al. Citation1997). In contrast to other trematodes, Dreissena is not the first host, but rather one of several possible second intermediate hosts. Its life cycle details have been summarized by McDonald (Citation1969) and Yamaguti (Citation1971, Citation1975) (). Miracidia hatching from eggs invade snails (sometimes tadpoles), where sporocysts and rediae develop. Free-swimming cercariae emerge from infected snails in about 32 days and form a cyst (ca. 120–165 µm in diameter) in a second host—typically an aquatic animal, such as a snail, tadpole, or Dreissena. These cysts containing metacercariae are double-walled, transparent, spherical, and enclosed in a very thin sheet of host connective tissue.
Figure 7. Life cycle of Echinoparyphium recurvatum (adapted from Molloy et al. Citation1997).
In dreissenids, cysts containing metacercariae found upon dissection are those loosely attached to the epithelia of either the mantle cavity, gills, or the visceral mass, indicating that many cercariae do not succeed in penetrating the epithelium to become embedded in mussel tissue. Histological examinations, however, demonstrated that some cercariae had successfully encysted internally within the kidney, gonadic tubule, gills, gonads, visceral mass epithelia, and pericardial cavity (Laruelle et al. Citation2002; Molloy et al. Citation1997, Citation2010).
Adults of echinostomatids are parasites of the small intestines of waterfowl (e.g., anatid ducks), and occasionally of mammals, including humans (Saijuntha et al. Citation2011b). These final hosts become infected by eating animals harboring the encysted metacercariae. Following the rupture of the cyst, the metacercariae mature in 8–22 days within the intestines of the consumer (reviewed in Molloy et al. Citation1997).
The effect of echinostomatids on Dreissena and other intermediate hosts is usually benign (Laruelle et al. Citation2002; Molloy et al. Citation1997, Citation2010). A North American laboratory trial in which Echinoparyphium sp. cercariae were successfully transmitted from field-collected Physa snails to D. polymorpha indicated that no damage to the host’s tissues was apparent (Conn and Conn Citation1995). Infection of amphibian kidneys by metacercariae, however, can result in severe pathologies (Martin and Conn Citation1990). The trematode E. recurvatum is a common and occasionally fatal helminth parasite of North American waterfowl (McDonald Citation1969; Roscoe and Huffman Citation1982). Other echinostomatids could also be moderately to highly pathogenic to their definitive hosts (McDonald Citation1981). Since infection with this species (as well as metacercariae of other unidentified species) has been field documented in D. polymorpha and D. r. bugensis in the Lower Great Lakes region (Karatayev et al. Citation2012), a potential increase in echinostomatid infections in North American water birds preying on dreissenids is of concern.
3.2.3.2. Geographical distribution
In Europe, metacercariae of E. recurvatum were reported from D. polymorpha from Russia, Belarus, and Croatia; E. echinatoides from Ukraine and Russia, and E. paraulum from Russia (). In addition, unidentified metacercariae of Echinostomatidae were reported from Ukraine, France, Belarus, Denmark, Germany, Greece, Italy, the Netherlands, and the United States (). Echinostomatids were also reported from D. r. bugensis in Russia, Ukraine, and United States, as well as from D. carinata in the Republic of North Macedonia ().
3.2.3.3. Prevalence and infection intensity
Echinostomatid (E. recurvatum, E. echinatoides) prevalence of infection in D. polymorpha is usually low, from <1 to 4.7% (Chernogorenko and Boshko Citation1992; Ginezinskaja Citation1959; Pryanichnikova et al. Citation2011; Tyutin et al. Citation2013a). For unspecified Echinostomatidae species prevalence of infection has been reported as low (1–5%, Minguez and Giambérini Citation2012), moderate (0.3–28.5%, Karatayev et al. Citation2000a), and high (up to 68.9%) (Karatayev et al. Citation2012; Mastitsky and Vezhnovets Citation2002). In Lake Naroch (Belarus), the prevalence and intensity of infection for E. recurvatum in D. polymorpha varies with depth, with peak values at 2 m, as well as seasonally, being much higher in October (prevalence: 36.5%; intensity: 6.1 ± 1.2 metacercariae/mussel) than in May (prevalence: 10.3%; intensity: 2.7 ± 0.6 metacercariae/mussel), but occasionally the maximum prevalence reaches 100% with the infection intensity up to 190 metacercaria/mussel (Mastitsky and Veres Citation2010).
In Russia, the prevalence of infection of D. r. bugensis with E. recurvatum was 0.5%, and 2.1% with E. echinatoides (Pryanichnikova et al. Citation2011). In the Dnieper River (Ukraine) in 1996, the average prevalence of infection in D. polymorpha (14.8 ± 5.5%) was similar to that in D. r. bugensis (16.3 ± 7.9%) (authors’ unpublished data). In contrast, in Oneida Lake (USA) the echinostomatid prevalence of infection in D. polymorpha was substantially higher (63.6%) than in D. r. bugensis (24.2%) (Karatayev et al. Citation2012). In D. carinata from Lake Ohrid cysts containing metacercariae of echinostomatid trematodes were observed in 47% of the dissected mussels (Molloy et al. Citation2010).
Prevalence of echinostomatid infection in D. polymorpha in a cooling water reservoir (Russia) correlated positively with temperatures within a waterbody; although unheated zones lacked infection, waters of 12.3, 16.4, and 19.0 °C had, respectively, 3.7, 7.8, and 8.5% infection (Kochnev Citation1977).
3.2.4. Aspidogastrea
3.2.4.1. Life cycle
In Europe, two Aspidogaster species are known from D. polymorpha, including Aspidogaster limacoides (Kuperman et al. Citation1994; Molloy et al. Citation1996; Nagibina and Timofeeva Citation1971; Popova and Biochino Citation2001; Tyutin et al. Citation2013a) and A. conchicola (Chernogorenko and Boshko Citation1992; Kulczycka Citation1939). There are also records of A. limacoides from D. r. bugensis (Popova and Biochino Citation2001). It should be mentioned that A. conchicola is the only European dreissenid parasite also native to North America, where it has been observed in D. polymorpha (Toews et al. Citation1993). Like other trematode parasites of Dreissena, these worms are quite small (the maximum size of A. limacoides is 1.3 × 0.9 mm) (Kuperman et al. Citation1994). Their shape is quite distinctive, with an oval, muscular, attachment disk on their ventral surface. In bivalve hosts, Aspidogaster spp. are typically found in the pericardial and renal cavities, where they feed on blood cells and hemolymph (Bakker and Davids Citation1973). In D. polymorpha, A. limacoides has been reported in the gonads, the pericardial cavity, and on the outer epithelium of the visceral mass (Kuperman et al. Citation1994; Molloy et al. Citation1996). Records of the encapsulation of this trematode in connective tissue within the digestive gland of D. polymorpha represents the first report of a host defense reaction to an aspidogastrid infection in zebra mussels (Laruelle et al. Citation2002).
Only a single host is needed for Aspidogaster spp. to complete their life cycle since eggs containing larvae infect molluscs and give rise to adult worms (Rohde Citation1994). In addition to Dreissena, A. limacoides has been recorded from other bivalves including Unionidae, Sphaeriidae, Cardium, and Adacna (Bakker and Davids Citation1973; Brian and Aldridge Citation2021; Nagibina and Timofeeva Citation1971; Pauley and Becker Citation1968). Vertebrates, including fish, may also become hosts by ingesting infected molluscs (Evlanov Citation1990; Kuperman et al. Citation1994; Nagibina and Timofeeva Citation1971; Roitman et al. Citation1981; Zhokhov and Kasyanov Citation1995; Zhokhov Citation2001).
3.2.4.2. Effect of infection
The species A. conchicola feeds on blood cells and hemolymph (Gentner Citation1971). According to Huehner et al. (Citation1989), A. conchicola graze on the epithelium of the freshwater mussel Anodonta grandis using its ventral sucker disk to disrupt the host’s tissues. In D. polymorpha, aspidogastrids were observed to induce hemocyte hemorrhage, suggesting that blood cells may be at least part of its diet (Laruelle et al. Citation2002). Bakker and Davids (Citation1973) observed no visible damage to the freshwater lamellibranchs Unio and Anodonta experimentally infected with A. conchicola. In contrast, Michelson (Citation1970) examining snails infected with A. conchicola, noted pathological changes at the cellular level. Renal metaplasia was observed in Anodonta spp. (Unionidae) which were heavily infected with A. conchicola, but this did not represent a severe pathological condition (Pauley and Becker Citation1968).
The colonization of Rybinsk Reservoir by D. polymorpha was followed by the introduction of A. limacoides (discovered in 1980, Roitman et al. Citation1981), which infect many cyprinid fish and became the most common parasite of roach. The switch in roach to feeding on D. polymorpha led to a dramatic increase in its infection intensity with A. limacoides (Zhokhov and Kasyanov Citation1995; Zhokhov Citation2001).
3.2.4.3. Prevalence and infection intensity
Prevalence and infection intensity in D. polymorpha with A. limacoides is always low: 0.1–7.6% (Kuperman et al. Citation1994; Nagibina and Timofeeva Citation1971; Pryanichnikova et al. Citation2011; Tyutin and Scherbina Citation2006; Tyutin et al. Citation2013a; Zhokhov Citation2001). For A. conchicola the values for D. polymorpha are similar: 0.2% (Chernogorenko and Boshko Citation1992), as well as those for the unidentified Aspidogaster: 2.7% with ≤2 worms/host (Toews et al. Citation1993).
3.2.4.4. Geographical distribution
The species A. limacoides has been reported from D. polymorpha in Russia and Croatia, and A. conchicola from Poland, Ukraine, and North America (). In addition, A. limacoides has also been reported in D. r. bugensis in Russia (Popova and Biochino Citation2001). Unidentified Aspidogaster species were reported in D. polymorpha from Belarus (Karatayev et al. Citation2000a), France (Minguez et al. Citation2011), and the Netherlands (Molloy et al. unpublished data) ().
3.2.5. Other digenetic trematodes
Other digenetic trematodes were briefly mentioned in Molloy et al. (Citation1997) as having been reported from dreissenids, including representatives of two families (Brachylaemidae and Sanguinicolidae) from Europe and one from North America (Plagiorchiidae). In dreissenids, however, these three families rarely have been observed (Molloy et al. Citation1997).
According to Chernogorenko (personal communication in Molloy et al. Citation1997), Leucochloridiomorpha constantiae (Digenea: Brachylaemidae) was found in dreissenids from the Dnieper Delta and Dnieper-Bug Liman (Ukraine). Their cysts (≤1 mm in diameter) contained freely moving, relatively small metacercariae. The intensity of infection ranged from 1 to 15 cysts per mussel, with a prevalence of ca. 35%. Chernogorenko and Boshko (Citation1992) reported a Leucochloridiomorpha sp. in 6% of the D. polymorpha sampled in the Dniester River (Ukraine).
Plagiorchiid (Digenea) metacercariae were observed in 2.9% of D. polymorpha at Port Colborne on Lake Erie (Toews et al. Citation1993). The Plagiorchiidae develop into adults in the intestines, or occasionally the bile duct/gall bladder, of vertebrates, especially birds and mammals (Yamaguti Citation1971).
Metacercariae of Psilostomatidae (Digenea), tentatively identified as Sphaeridiotrema sp., were found in D. r. bugensis from Lake Oneida (New York, NY, USA). The prevalence of infection in mussels with these trematodes was 3%, and the infection intensity was 2 metacercariae/mussel (Karatayev et al. Citation2012). In addition, in Lake Erie Karatayev et al. (Citation2012) reported frequent infections of dreissenids with large and small unidentified trematode metacercariae. The prevalence of infection with large metacercariae in D. polymorpha varied from 25 to 70% and in D. r. bugensis from 6.7 to 100%. Prevalence of infection with small metacercariae ranged from 4.5 to 80%, and from 60 to 90% in D. polymorpha and D. r. bugensis, respectively.
3.3. Other parasites
3.3.1. Haplosporidium raabei (Haplosporidia: Haplosporidiidae)
3.3.1.1. Life cycle
Haplosporidians are spore-forming, endoparasitic protists that are well documented as pathogens of marine invertebrates, especially of commercially important bivalves (Burreson and Ford Citation2004; Ford et al. Citation2018; Lauckner Citation1983; Sparks Citation1985). For example, Haplosporidium nelsoni, responsible for the MSX disease, has devastated oyster populations of the Delaware and Chesapeake Bays and other regions of the eastern United States coast (Burreson and Ford Citation2004; Ford and Tripp Citation1996; Ford et al. Citation2018; Haskin and Andrews Citation1988). Reports of haplosporidian infections in freshwater invertebrates, however, are rare (Burreson and Ford Citation2004), and the first case of a haplosporidian in D. polymorpha was reported from the Netherlands (Bowmer and van der Meer Citation1991; de Kock and Bowmer Citation1993). In 1988 Bowmer and van der Meer (Citation1991) observed a putatively lethal infection in the blood system and all key organs of D. polymorpha. Based on genetic sequencing, morphology, and host, this protist was described as a new species—Haplosporidium raabei, representing the first haplosporidian species from a freshwater bivalve (Molloy et al. Citation2012, ).
Figure 8. Histological section of advanced infection with the haplosporidian Haplosporidium raabei in gill of Dreissena polymorpha (adapted from Molloy et al. Citation2012).
In D. polymorpha, infections with multinucleate plasmodia and sporocysts were observed systemically in connective tissues and organs, such as gills, gonads, and digestive gland often exhibited the most severe infections, but there was no evidence of haplosporidian cells in epithelial or muscle tissue (Molloy et al. Citation2012). Plasmodia, sporocysts, and mature spores were observed in spring through fall with no evidence of seasonality. Plasmodial development and sporogenesis within individual mussels are relatively synchronous. Multinucleate plasmodia are irregular in shape with a maximum dimension of 73 μm. Sporocysts are generally spherical and typically ≤40 μm in diameter. Mussels containing spores liberated from sporocysts or nearing completion of sporogenesis were observed in the Baggersee Reeserward, Meuse, and Moselle rivers, with mean spore dimensions (L × W) of 8.9 × 6.5, 7.5 × 5.2, and 8.0 × 5.4 μm, respectively (Molloy et al. Citation2012).
3.3.1.2. Effect of infection
Hemocyte infiltration is associated with infection, and plasmodia were observed being phagocytosed by hemocytes (Molloy et al. Citation2012). Sporogenesis is accompanied by degeneration of host tissues, but no major signs of disease are evident during dissection. Histological examinations revealed that gills can be very swollen in advanced infections. Extensive connective tissue lysis is a common consequence of advanced infection by H. raabei as sporogenesis is accompanied by systemic tissue degeneration. When sporogenesis is completed, mature spores typically appear densely packed throughout much of the host’s connective tissues, with their subsequent liberation into the environment dependent on host death. Bowmer and van der Meer (Citation1991) observed that advanced infection of D. polymorpha can be severe, completely destroying the gonads, and appears to be lethal since there is little to no functional tissue left in the digestive gland. According to Molloy et al. (Citation2012), gross signs of disease were not observed in infected mussels and their dissected tissues appear normal. Only two epizootics have been documented in D. polymorpha: in 1990 Bowmer and van der Meer (Citation1991) reported infection in 26% of the mussels in Lake Volkerak, and in 1992 Molloy et al. (Citation2012) described an infection prevalence of 20% in the Hollands Diep. Both waterbodies, located in the Netherlands, are part of the freshwater confluence of the Meuse and Rhine basins.
3.3.1.3. Geographical distribution and prevalence
Although D. polymorpha populations from 11 countries across Europe were examined by histological analyses, H. raabei infections were detected only in the Rhine and Meuse river basins in France, Germany, and The Netherlands. It is likely that the spread of H. raabei between these two river basins was facilitated by the freshwater estuary they share in the Netherlands. Moreover, infection outbursts were only intermittently observed in this region during the 17-year sampling period, and when H. raabei was detected, the prevalence of infection was typically <4%, with a maximum of 20% (Molloy et al. Citation2012).
3.3.2. Bacteria
3.3.2.1. General description and biology
Molloy et al. (Citation2001) characterized intracytoplasmic infections by prokaryote microorganisms in a Dreissena sp. from Lake Volvi (northeastern Greece). Light microscope observations of stained tissues revealed basophilic, cytoplasmic inclusions in 87.5% of the mussels sectioned. Inclusions in epithelial cells and connective tissues were noted in 34.4 and 71.9% of the specimens, respectively, with five mussels (15.6%) having both tissue types infected. Epithelial cell infections were observed in histological sections only in digestive gland tubules and ducts; within the tubules, inclusions were present more often in secretory than in digestive cells. Connective tissue infections, however, were systemic; among the 32 mussels sectioned, inclusions were found in the gills (65.6%), foot (12.5%), mantle (9.4%), labial palps (6.3%), digestive gland (6.3%), stomach (6.3%), and gonads (3.1%). In the gills, cytoplasmic inclusions were prominent enough to be visible in 17.0% of the 247 mussels dissected. Ultrastructurally, prokaryote cells in gill connective tissues were characteristic of Chlamydiales-like organisms, with each intracytoplasmic inclusion containing a loosely packed mixture of elementary, reticulate, intermediate bodies, and blebs. Prokaryotes in the epithelial cells of the digestive gland contained only one of the four morphological cell types and were considered Rickettsiales-like organisms (RLO) (Minguez and Giambérini Citation2012; Minguez et al. Citation2012, Citation2013). Hexagonal, virus-like particles were present in the cytoplasm of the largest of these prokaryotes.
3.3.2.2. Effect of infection
Although host stress was evident from localized cell necrosis and dense hemocyte infiltration, overall infection with RLO was fairly benign, with no major adverse impacts on the body condition of the mussels analyzed. A possible negative effect was the partial constriction of gill water tubes, but at the infection intensity observed (typical range 1–7 inclusion bodies per section), significant interference with respiration and other metabolic functions of the gills was considered highly unlikely. Host response was observed in D. carinata tissues only in intense infections. In such cases, connective tissues of the digestive gland, the mantle, and the gills exhibited cell necrosis. These connective tissues were infiltrated with numerous hemocytes, but only in regions containing relatively numerous inclusions (Molloy et al. Citation2001).
3.3.2.3. Geographical distribution and prevalence
Intracytoplasmic infections by prokaryote microorganisms in Dreissena have been reported in Greece (Molloy et al. Citation2001), France (Minguez and Giambérini Citation2012; Minguez et al. Citation2012, Citation2013), and United States (Minguez et al. Citation2013) (). The prevalence of infection varied from low to moderate: 9–22% (Minguez and Giambérini Citation2012; Molloy et al. Citation2001, Citation2010).
3.3.3. Nematodes
3.3.3.1. General description and biology
Among Dreissena endosymbionts, nematodes are likely the most common ( and ). They have been reported from all dreissenid species examined: D. polymorpha (Karatayev et al. Citation2000a, Citation2003a; Kuperman et al. Citation1994; Mastitsky and Gagarin Citation2004; Mastitsky et al. Citation2008), D. r. bugensis (Pryanichnikova et al. Citation2011; Reid et al. Citation2012), and D. carinata (Molloy et al. Citation2010). Evidence to date suggests that they are likely free-living species, without any obligate association with dreissenids (Karatayev et al. Citation2003a; Mastitsky and Gagarin Citation2004; Mastitsky et al. Citation2008; Molloy et al. Citation1997; Reid et al. Citation2012). Free-living nematodes probably enter the mantle cavity unintentionally by crawling along the byssal threads (Karatayev et al. Citation2003a; Molloy et al. Citation1997) or carried with water currents through the inhalant siphon (Mastitsky and Gagarin Citation2004). Nematodes have been frequently reported in the mantle cavity of Dreissena populations both in Europe (Karatayev et al. Citation2000a, Citation2003a; Kuperman et al. Citation1994; Mastitsky and Gagarin Citation2004; Mastitsky et al. Citation2008; Pryanichnikova et al. Citation2011) and in North America (Conn et al. Citation1994; Reid et al. Citation2012; Toews et al. Citation1993).
As specific identification of this group is challenging, their identifications vary greatly, from species (Karatayev et al. Citation2003a; Mastitsky and Gagarin Citation2004; Mastitsky et al. Citation2008; Reid et al. Citation2012) to phylum (Karatayev et al. Citation2000a; Pryanichnikova et al. Citation2011). Currently, 25 species and genera of nematodes have been reported from D. polymorpha from Europe and 10 taxa from D. r. bugensis species in North America. In Europe, the highest diversity (22 species) was reported by Mastitsky and Gagarin (Citation2004) in D. polymorpha from Narochanskie Lakes (Belarus). In North America, 10 species and higher taxa of nematodes were reported in D. r. bugensis from the Colorado River Aqueduct (Copper Basin Reservoir and Lake Skinner, USA) (Reid et al. Citation2012). All these nematodes are free-living organisms common in benthic and/or periphytic freshwater communities. It is interesting to notice that in all studies that reported nematodes in Dreissena, the most common species was Chromadorina bioculata. The relative abundance of this species among the nematodes found in the mantle cavity of D. polymorpha is 24–90% in Narochanskie Lakes (Belarus) (Mastitsky and Gagarin Citation2004), 82% in Drozdy Reservoir (Belarus) (Karatayev et al. Citation2003a), and 96.9% in Lake Erken (Sweden) (Mastitsky et al. Citation2008). Surprisingly, C. bioculata is also the most common species in the mantle cavity of D. r. bugensis from the Colorado River Aqueduct (Reid et al. Citation2012). These data suggest that, in contrast to other nematode species reported from dreissenids, C. bioculata may have a rather high affinity with the mussels (Mastitsky et al. Citation2008). Further investigations are required to understand the nature of C. bioculata/Dreissena relationships.
3.3.3.2. Geographical distribution
In European populations of D. polymorpha nematodes were reported from 15 countries (). In addition to D. polymorpha, nematodes were reported from D. r. bugensis in Russia (Pryanichnikova et al. Citation2011; Tyutin et al. Citation2013a) and Ukraine (Yuryshynets Citation2019), and from D. carinata in the Republic of North Macedonia (Molloy et al. Citation2010). In North America, nematodes were reported from both D. polymorpha (Conn et al. Citation1994; Toews et al. Citation1993) and D. r. bugensis (Reid et al. Citation2012).
3.3.3.3. Prevalence and infection intensity
Nematodes are among the most common organisms found within the mantle cavity of dreissenids. Reported prevalence usually ranges from moderate to high: 10–15% (Chuševė et al. Citation2012), 10–50% (Burlakova et al. Citation2006a), 40% (Conn et al. Citation1994), 52% (Karatayev et al. Citation2000a), 50–72% (Mastitsky and Gagarin Citation2004), and 17–82% (Mastitsky et al. Citation2008). In contrast to prevalence, the number of worms per host is almost always low, from 1 to 4.2 worms/mussel (Burlakova et al. Citation2006a; Chuševė et al. Citation2012; Conn et al. Citation1994; Karatayev et al. Citation2000a; Mastitsky and Gagarin Citation2004; Mastitsky et al. Citation2008). Similar values of prevalence (31%) and infection intensity (1–3 worms/mussel) were reported for D. carinata from the Republic of North Macedonia (Molloy et al. Citation2010), although higher prevalence (85%), but low intensities (5.3 worms/mussels) were reported in D. r. bugensis from North America (Reid et al. Citation2012).
There is only one report of seasonal dynamics of the prevalence and intensity of nematode infection in D. polymorpha (Karatayev et al. Citation2003a). In this two-year study in Svisloch River (Belarus) the mean prevalence ranged from 6.7 to 76.7%, and mean intensity from 1 to 3.7 worms/mussel. This survey found a significant negative correlation between temperature and both infection prevalence and intensity, with the highest numbers of nematodes in mussels in January–February. This suggests that during the winter either more nematodes may actively use zebra mussels for shelter or that free-living nematode population densities are simply higher in the benthos during winter (Karatayev et al. Citation2003a).
3.3.4. Oligochaetes
Oligochaetes (Annelida: Oligochaeta) have been frequently reported from the mantle cavities of freshwater bivalves in North America, but the nature of this relationship remains unclear (Conn et al. Citation1994, Citation1996; Curry Citation1979; Karatayev et al. Citation2000a; Klemm Citation1976; Sickel and Lyles Citation1981). In many instances, these oligochaetes are likely free-living species that have unintentionally entered the mantle cavity and could be considered as commensals (reviewed in Molloy et al. Citation1997). Among oligochaetes Chaetogaster limnaei, however, is one of the very few species that has likely evolved in association with gastropods and bivalves. According to Conn et al. (Citation1994, Citation1996), in dreissenids the vast majority of C. limnaei inhabit the mantle cavity of their hosts, frequently occurring between the gill lamellae with only slight evidence of pathologic effects. Only once a C. limnaei was found inside the ovary of a mussel, where it had caused appreciable damage by feeding on the host’s oocytes and gonadal tissues (Conn et al. Citation1994, Citation1996). The relationship could actually be in part mutualistic, since C. limnaei was reported to eat miracidia in snails in Europe, thus, possibly reducing trematode infections (Conn et al. Citation1996).
In Europe, Chernogorenko and Boshko (Citation1992) reported C. limnaei in 8% of D. polymorpha from the Dniester River/Liman (Ukraine). Karatayev et al. (Citation2000a) found C. limnaei in 11 of 17 D. polymorpha populations in Belarus with a prevalence and intensity ranging from 1.4 to 11.9%, and 1 to 1.5 worms/mussel, respectively. In addition, C. limnaei was also recorded in D. polymorpha from Germany (Molloy et al. unpublished data). Kuperman et al. (Citation1994) observed the free-living species Psammoryctides baebatus and P. moldavensis in D. polymorpha in the Volga Basin (Russia). Unidentified oligochaetes were reported from European populations of D. polymorpha from Belarus (Karatayev et al. Citation2000a), Ireland (Burlakova et al. Citation2006a), Denmark, and Ukraine (Molloy et al. unpublished data, ). In addition, there are two reports of unidentified oligochaetes from European populations of D. r. bugensis in Russia (Pryanichnikova et al. Citation2011; Tyutin et al. Citation2013a) and Ukraine (Yuryshynets Citation2019). According to Pryanichnikova et al. (Citation2011), the prevalence of infection in D. r. bugensis with oligochaetes is 0.53%.
In North America C. limnaei was reported in both Dreissena species from the St. Lawrence River (USA) (Conn et al. Citation1996). The prevalence of infection ranged from 0 to 80%, depending on the collection site and date. The mean prevalence and intensity of infection in D. r. bugensis were 27.4% and 2.7 worms/mussel, and in D. polymorpha—25.5% and 2.9 worms/mussel. The mean and maximum intensity of infection were ca. 3 and 18 worms, respectively, and peaks of prevalence and intensity occurred during July and August (Conn et al. Citation1996).
3.3.5. Leeches
At least a dozen leech species have evolved in close association with Unionidae since the Miocene (Bolotov et al. Citation2019). In contrast, there are no reports of any obligate associations between Dreissena and leeches. In fact, only two studies, both from Europe, reported leeches from dreissenids (Karatayev et al. Citation2000a; Kuperman et al. Citation1994). Two species Caspiobdella fadejewi and Helobdella stagnalis (Annelida: Hirudinea) were found associated with “internal organs” of D. polymorpha in the Volga Basin, Russia (Kuperman et al. Citation1994). Leeches Erpobdella octoculata and H. stagnalis were observed in the mantle cavity of molluscs from one and three, respectively, of a total of 17 D. polymorpha populations sampled in Belarus (Karatayev et al. Citation2000a). The prevalence was <1%, and typically only one leech per infested mussel was present. Young specimens of Erpobdella sp. and Helobdella sp. were reported from D. r. bugensis in Russia (Tyutin et al. Citation2013a). Since both these leech species are free-living predators, they were considered to have inadvertently entered the mantle cavity.
3.3.6. Chironomids
Chironomid larvae (Insecta: Chironomidae) have been observed in the mantle cavities of Dreissena both in Europe and in North America (Conn et al. Citation1994; Kuperman et al. Citation1994; Mastitsky and Samoilenko Citation2005; Ricciardi Citation1994, Citation1994). In Europe, Chironomus bathophilus was observed in D. polymorpha in the Volga Basin (Kuperman et al. Citation1994), and 14 species and higher taxa were reported from the mantle cavity of D. polymorpha from seven Belarussian lakes with Limnochironomus gr. nervosus being the most common () (Mastitsky and Samoilenko Citation2005). All chironomids reported from dreissenids are free-living species commonly found in the benthos (Mastitsky and Samoilenko Citation2005). In addition, unidentified chironomids were recorded from D. polymorpha populations in Belarus (Karatayev et al. Citation2000a), Ireland (Burlakova et al. Citation2006a), Sweden (Mastitsky et al. Citation2008), Russia (Pryanichnikova et al. Citation2011), Czech Republic, Denmark, Germany, Greece, and Ukraine (Molloy et al. unpublished data, ). Chironomid larvae were also reported from D. r. bugensis in Ukraine (Yuryshynets Citation2019).
In North America, Paratanytarsus sp. was reported from both Dreissena species in the St. Lawrence River (New York State and Quebec Province) (Conn et al. Citation1994; Ricciardi Citation1994). Up to 38% of D. polymorpha and 10% of D. r. bugensis hosted larvae of Paratanytarsus sp. Dreissenids are typically colonized by either the third or the fourth instar larvae and are more common in larger mussels. Chironomid larvae Paratanytarsus sp. inhabits the mantle cavity of dreissenid mussels around the gills, gonads, and siphonal tissue, and are most frequently found near the inhalant siphon. They were never observed feeding on dreissenid tissues, and no tissue damage was ever detected (Ricciardi Citation1994), which indicates that these associations are commensal (Mastitsky and Samoilenko Citation2005; Ricciardi Citation1994). The chironomid larvae probably benefit from the association since the mussels might provide refuge from predation, as well as a source of oxygenated water and suspended food particles (Mastitsky and Samoilenko Citation2005; Ricciardi Citation1994).
3.3.7. Mites
In Ukraine, Unionicola sp. (Arachnida: Hydrobatidae) was observed in 3.2% of the specimens of D. polymorpha examined (Chernogorenko and Boshko Citation1992). At several locations in the Volga Basin (Russia), Unionicola eggs were present on the gills of both D. polymorpha (Kuperman et al. Citation1994) and D. r. bugensis (Tyutin et al. Citation2013a). In Belarus, unidentified hydracarinid mites were recorded from 6 of 17 D. polymorpha populations; the prevalence ranged from 0.4 to 1.8%, with typically one mite per host (Karatayev et al. Citation2000a). A single unidentified aquatic mite was found in the mantle cavity of a D. polymorpha from Sweden (Mastitsky et al. Citation2008). In the St. Lawrence River, one D. polymorpha had one mite in its mantle cavity, but many mussels had hydracarinid egg masses glued to their shells (Conn et al. Citation1994). Adult Unionicola spp. are known to be common parasites of freshwater mussels, living on the gills, mantle, and foot of their hosts and using these tissues as sites of oviposition (Edwards and Vidrine Citation2006). Therefore, in Dreissena, Unionicola are likely parasitic (rather than commensal) as well, but their impact on host populations is probably negligible.
4. Ecological competitors
Dreissenids are sessile bivalves feeding on seston from the water column, and therefore potentially vulnerable to competitors for substrate and/or for food suspended in the water-column. Although both D. polymorpha and D. r. bugensis belong to the same genus and have similar life history characteristics, they are not identical and have different invasion dynamics and distribution patterns within waterbodies (Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation2015, Citation2021a; Nalepa et al. Citation2010). In shallow lakes, both species can colonize the entire bottom, if appropriate substrate for attachment is available, but in deep lakes, D. polymorpha is largely limited to the shallow well-mixed warm littoral zone, while D. r. bugensis, in addition to the littoral fringe, can also colonize the entire profundal zone. In nearshore environments, attachment to a suitable substrate is essential for both dreissenids to withstand wave activity, but here they can be outcompeted by other organisms, including sponges, amphipods, algae, bryozoans, hydrozoan coelenterates, and other bivalves (reviewed in Balogh et al. Citation2008, Citation2018; Jantz and Schöll Citation1998; Lauer and Spacie Citation2000, Citation2004; Molloy et al. Citation1997) (). In contrast to the nearshore, in deep, cold, and calm offshore environments, where wave action does not reach the bottom, D. r. bugensis do not require hard substrates for attachment and can form large aggregations on soft sediments (Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation2021a). Here, the role of the aforementioned competitors for space is negligible, but competition for food resources can be important. In this regard, in dreissenids both intraspecific and interspecific competition can play an extremely important role in affecting their population size and dynamics (reviewed in bij de Vaate et al. Citation2014; Hetherington et al. Citation2019; Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation2011a, Citation2015, Citation2021a; Strayer et al. Citation2019).
Table 12. Competitors of Dreissena spp. reported from Europe (E) and North America (NA).
4.1. Sponges
The ability of sponges (Porifera) to overgrow and kill dreissenids was first reported in Europe by Arndt (Citation1937) for Ochridaspongia rotunda in Lake Ohrid, inhabited by D. carinata. In Lake Balaton (Hungary) Sebestyén (1937) reported Spongilla carteri and Eunapius (=Spongilla) fragilis to overgrow and embed D. polymorpha’s colonies. Local negative impacts of the sponge Ephydatia fluviatilis on D. polymorpha were also reported in Poland (Piesik Citation1983) and in Italy (Lancioni and Gaino Citation2009) ().
In the first comprehensive North American (Great Lakes-St. Lawrence River system) investigation, Ricciardi et al. (Citation1995) found that sponges (E. fragilis, Ephydatia muelleri, and Spongilla lacustris) are always successful in overgrowing dreissenids. Overgrowth occurred either by lateral growth from an existing nearby sponge colony or by settlement of sponge larvae on the shells. Overgrowth starts as early as in May (at water temperatures ca. 10 °C) and typically declines in late autumn. Lauer (Citation1997) also observed that in the Great Lakes, sponge overgrowth was generally limited to the May to October period, with the most severe fouling from June through August. Ricciardi et al. (Citation1995) observed that adhesion of gemmule (overwintering structures) patches occur in autumn, giving rise to new colonies the following year. Surviving mussels are, thus, subject to annual overgrowth from successive sponge generations, resulting in a slow death, with 50–79% of the sponge-covered mussels dying, respectively, after 1–3 to 4–6 months. No significant differences in mortality rates were observed between small and large mussels, or between Dreissena species (D. polymorpha and D. r. bugensis). Because of the adverse effects of siltation on sponge colonies, high growth rates are generally restricted to vertical surfaces. Thus, sponges may control mussel abundance only locally, particularly on vertical surfaces, such as in canals. Ricciardi et al. (Citation1995) concluded that the overall impact of sponges on Dreissena populations in the Great Lakes-St. Lawrence River system is negligible due to the high rate of mussel recruitment and the environmental constraints on sponge growth.
The actual causes of mussel death due to sponge overgrowth are likely a combination of factors. Ricciardi et al. (Citation1995) hypothesized that sponge overgrowth blocks the mussel’s siphons, seriously impairing mussel feeding and respiration. In overgrowth studies with Eunapius fragilis, Early and Glonek (Citation1999) reported that dreissenid adenosine triphosphate (ATP) was depleted suggesting anoxic stress. Lauer and Spacie (Citation2000, Citation2004) observed that fouled mussels in Lake Michigan suffer significant reductions in both their soft tissue weight and glycogen content, suggesting that in addition to starvation and reduction in energy stores, other processes likely contribute to mortality, including the increased metabolic demand and inhibition of gas exchange or waste excretion.
4.2. Amphipods
Amphipod Chelicorophium curvispinum is a small (adult length 2.5–7.0 mm), filter-feeding species that build mud tubes on hard substrates, and therefore can potentially compete for space with dreissenids. Native to the Ponto-Caspian basin, C. curvispinum began to expand its range into Europe at the beginning of the twentieth century via various river systems and interconnecting canals (Paffen et al. Citation1994). According to Sebestyén (1937), the establishment of C. curvispinum in Lake Balaton (Hungary) was facilitated by the suitable substratum offered by the numerous crevices in dreissenid colonies, and in addition, he observed that dreissenids were sparse where this amphipod’s population was dense. Recently, Balogh et al. (Citation2008, Citation2018) reported that D. polymorpha and C. curvispinum populations in Lake Balaton, both with a long history of coexistence characterized by competition and intermittent population fluctuations, currently suffer population reductions due to the introduction of another competitor, D. r. bugensis.
After having been discovered in 1987 in the River Rhine, in the 1990s C. curvispinum underwent a population explosion causing the displacement of D. polymorpha from hard substrates in the Lower Rhine (Jantz and Schöll Citation1998; Smit et al. Citation1993; van den Brink et al. Citation1993; van der Velde et al. Citation1994). Since both C. curvispinum and D. polymorpha are filter-feeders, competition may have existed for suspended particles, but it was in the battle for attachment sites where D. polymorpha appears to have been no match for C. curvispinum (reviewed in Molloy et al. Citation1997). This amphipod typically covers available stones in the Lower Rhine (both breakwaters and riverbanks) with a 1–4 cm thick layer of their mud tubes, thereby smothering dreissenids. Moreover, mussel larvae are unable to settle successfully on these tubes. In some sections of the Dutch Rhine, C. curvispinum became the dominant macroinvertebrate species, and densities of dreissenids were dramatically reduced (Paffen et al. Citation1994). In 2001, however, C. curvispinum decreased in numbers, most likely due to top–down regulation caused by the increased predatory pressure of the recent invasion by the Ponto-Caspian amphipod Dikerogammarus villosus (van Riel et al. Citation2006).
4.3. Other competitors for surface
Other competitors with a documented negative impact on D. polymorpha include the green alga Cladophora, the hydroid Cordylophora caspia, and the bryozoans Plumatella repens, Pectinatella magnifica, and Lophopodella carteri (reviewed in Lauer et al. Citation1999; Molloy et al. Citation1997). In Lake Balaton, dense colonies of D. polymorpha are occasionally entirely smothered by Cladophora (Sebestyén 1937). Zebra mussel is also absent from stones covered with Cladophora in the Rhine and Meuse rivers (Smit et al. Citation1993). In the Netherlands, the colonial hydroid Cordylophora caspia was observed to compete for substrate with D. polymorpha on stones in the Rhine River and in Lake Markermeer (Smit et al. Citation1993). This species was reported as a substrate competitor along with the bryozoan Plumatella repens in an Oder River canal in Poland (Piesik Citation1983). In the St. Lawrence River (USA), adult dreissenids can be overgrown and killed by the bryozoan Pectinatella magnifica (Conn and Conn Citation1993). In Lake Michigan (USA), colonies of the bryozoan Lophopodella carteri on pier posts inhibit the successful settling of mussel larvae, but mussels >10 mm long are not affected by this overgrowth (Lauer et al. Citation1999).
4.4. Bivalves
4.4.1. Mytilaster lineatus
The marine, byssus-producing bivalve Mytilaster (=Brachyodontes) lineatus, which is widespread in the Mediterranean and Black Sea, was inadvertently introduced into the Caspian Sea in 1919, where it colonized the entire sea, except its less saline northern reaches (reviewed in Karpinsky et al. Citation2005). By 1934–1938, its biomass rose from 11 to 42% of the overall benthos (up to 7 kg/m2 in wet weight). New bivalve invaders started competing with the endemic Caspian dreissenids D. elata and D. caspia, which were still present in 1938, but disappeared by 1955–1957 (Kostianoy and Kosarev Citation2005) and are now considered extinct (Leroy et al. Citation2020). According to Karpinsky et al. (Citation2005), the mechanism of Dreissena replacement is in the ability of M. lineatus to create high biomass and deplete dissolved oxygen to levels intolerable for dreissenids. It should be noticed, however, that M. lineatus coexists with D. rostriformis. Surveys conducted in 1986–1987 showed that near the Middle Caspian eastern coast, the biomass of M. lineatus peaks at depths of 10–25 m, where it exceeds the biomass of the second most abundant species, D. rostriformis by a factor of 5–8 (Karpinsky Citation2010). The fact that the dreissenid is still very abundant indicates that it can coexist with M. lineatus, although the competition involved is likely very strong.
4.4.2. Intraspecific dreissenid competition
Could Dreissena be its own worst enemy? Intraspecific competition in dreissenids can be a significant mortality factor and a major density-dependent, population-regulating mechanism. Population abundance of invasive species often exhibits an invasion cycle characterized by very strong initial growth in abundance and a subsequent marked decline (Karatayev et al. Citation2015; Simberloff and Gibbons Citation2004; Strayer et al. Citation2017). This cyclicity might be due to various factors, including density-dependent changes in the abundance of the invader. For example, when mussels attach to each other and colonies become several centimeters thick, the ones at the bottom may die (Lewandowski Citation1982). In Uchinskoe Reservoir (Russia), substrate limitation for D. polymorpha attachment due to the accumulation of feces and pseudofeces was concluded to be the major reason for the decline in mussel biomass in 1960s (Lvova Citation1977). Likewise, adults in high-density populations may compete with their planktonic larvae for limited food resources, thus, reducing settling rates and/or survival of postveligers. Strayer et al. (Citation1996) provided evidence that adult dreissenids may outcompete their pelagic larvae for phytoplankton in the Hudson River (USA) and suggested that such food-limited dreissenid populations may be especially frequent in rivers and estuaries, where the ratio of food supply to available substratum is small. Dreissenids are known to reduce their food resources (e.g., seston and chlorophyll) through their feeding and filtering activities (reviewed in Higgins and Vander Zanden Citation2010; Karatayev et al. Citation1997, Citation2002a; Pothoven and Fahnenstiel Citation2013; Rowe et al. Citation2015), especially below the thermocline (Karatayev et al. Citation2018b, Citation2021a). In the eastern basin of Lake Erie, early in D. r. bugensis invasion (before 2006), seston concentrations were similar at the surface and in the near bottom layers, and mean mussel length and recruitment rates were also similar in both zones (Karatayev et al. Citation2018b). After 2006 however, in the deepest zone, there was a 3-fold decline in near bottom summer seston concentrations, suggesting a strong depletion of food resources in the hypolimnion during stratification due to grazing by D. r. bugensis, which coincided with a strong increase in average mussel length as recruitment and growth of young-of-the-year mussels became unsuccessful in this zone in 2007–2021 (Karatayev and Burlakova Citation2022b).
4.4.3. Interspecific dreissenid competition
There are a multitude of reports, both from North America and from Europe, indicating that there is interspecific competition between D. polymorpha and D. r. bugensis, with the latter generally displacing the former (Balogh et al. Citation2018; bij de Vaate et al. Citation2014; Heiler et al. Citation2013; Hetherington et al. Citation2019; Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation2011a, Citation2015, Citation2021a, Citation2021b; Noordhuis et al. Citation2016; Orlova et al. Citation2004, Citation2005; Strayer et al. Citation2019). The outcome of this competition, however, may vary from an almost complete extirpation of D. polymorpha to the co-existence of both species and sometimes even reversals to a predominance of D. polymorpha (Karatayev et al. Citation2011a, Citation2021c; Rudstam and Gandino Citation2020; Strayer and Malcom Citation2006; Strayer et al. Citation2019; Zhulidov et al. Citation2006, Citation2010). Among the most important factors determining the outcome of the competition are lake morphometry, prevalent substrate types, food availability, predation, and others (Balogh et al. Citation2022; bij de Vaate et al. Citation2014; Burlakova et al. Citation2006b; Hecky et al. Citation2004; Hunter and Simons Citation2004; Jackson et al. Citation2020; Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation1997, Citation2002a, Citation2011a, Citation2021a; Rudstam and Gandino Citation2020). In general, in shallow polymictic lakes, D. r. bugensis becomes dominant 4–12 years after coexisting with previously established D. polymorpha, but it does not appear to fully replace the zebra mussels (Balogh et al. Citation2018; Hetherington et al. Citation2019; Karatayev et al. Citation2014, Citation2021a, Citation2021c; Noordhuis et al. Citation2016; Orlova et al. Citation2004). There are several examples suggesting that the dominance of D. r. bugensis could be reversed due to species-selective predation (Rudstam and Gandino Citation2020; Zhulidov et al. Citation2006, Citation2010), periodic mass mortality due to hypoxic events, or other factors (Karatayev et al. Citation2021c). Due to its thinner shells, weaker aggregation behavior, and lower attachment strength, D. r. bugensis is more vulnerable to predation than D. polymorpha (Balogh et al. Citation2019, Citation2022; Czarnoleski and Muller Citation2013; Kobak and Kakareko Citation2009; Naddafi and Rudstam Citation2013; Peyer et al. Citation2009, Citation2010). After mass die-offs induced by hypoxia or other factors, D. polymorpha can recolonize faster areas in waterbodies with mixed dreissenid populations (Karatayev et al. Citation2021c). Interestingly, within their native range in the Dnieper-Bug Liman, Ukraine, a shallow productive polymictic waterbody, the two species have been intermittently dominant in different areas and/or periods for thousands of years since the last glaciation (reviewed in Karatayev et al. Citation2011a).
In a recent study, Karatayev et al. (Citation2021a, Citation2022b) compared the outcome of the competition between these two dreissenid species in the Laurentian Great Lakes. All these lakes and embayments were initially colonized by D. polymorpha and later by D. r. bugensis. In shallow polymictic lakes and lake basins (Lake St. Clair, western basin of Lake Erie, and Saginaw Bay), although D. r. bugensis is currently dominant, both species still coexist after 30 years since the initial invasion and in the western basin in 2019 the reverse trend was recorded with zebra mussels again becoming the dominant species (Karatayev et al. Citation2021a, Citation2021c). In contrast, in deep, well-stratified lakes (lakes Michigan, Huron, Ontario, and eastern basin of Lake Erie), D. r. bugensis became dominant, spread to greater depths, reached much higher densities, and drove D. polymorpha to virtual extirpation (Karatayev et al. Citation2021a; Madenjian et al. Citation2015; Nalepa et al. Citation2010).
Several hypotheses have been suggested to explain the displacement of D. polymorpha by D. r. bugensis (reviewed in Karatayev et al. Citation2013, Citation2015, Citation2021a; Zhulidov et al. Citation2006). Although the mechanisms and conditions under which D. r. bugensis displace D. polymorpha still remain incompletely known (Strayer et al. Citation2019), it is most likely that they are associated with the higher physiological activity of D. r. bugensis, including higher filtration rates at low food concentrations, greater assimilation efficiency (Baldwin et al. Citation2002; Diggins Citation2001; Stoeckmann Citation2003), and greater plasticity in shell production allowing more energy allocation to growth and reproduction (Karatayev et al. Citation2011b; Mills et al. Citation1999; Nalepa et al. Citation2010; Pryanichnikova Citation2012). Other studies reported higher growth rates (Baldwin et al. Citation2002; Casper et al. Citation2014; Karatayev et al. Citation2011b; Marescaux et al. Citation2015; Metz et al. Citation2018; Stoeckmann Citation2003), a larger size of larvae at settlement (Martel et al. Citation2001), and longer reproduction period, especially for mussels below the thermocline (Nalepa et al. Citation2010; Wong et al. Citation2012). These advantages allow D. r. bugensis to outcompete D. polymorpha in many lakes and reservoirs and make them especially successful in deep stratified waterbodies (Karatayev and Burlakova Citation2022a, Citation2022b; Karatayev et al. Citation2021a).
5. Conclusions and future research needs
For a freshwater bivalve, dreissenids can create unusually high biomasses, often exceeding the combined biomass of all pelagic and benthic invertebrates in a waterbody by an order of magnitude. Therefore, it is not surprising that in their native range, 156 species of predators, parasites, and commensals use these bivalves as a food resource or shelter. A diverse and abundant array of natural enemies is beneficial not only because of its controlling effect on dreissenid population growth but also because of its enhancement of energy flow. The introduction of these filter-feeding bivalves into new waterbodies has modified this flow by redirecting large amounts of energy from planktonic to benthic communities. Predators, in particular molluscivorous fish and waterfowl, redirect this energy flow back to the pelagic environment.
In agreement with the enemy release hypothesis, more natural enemies of Dreissena spp. were initially present in their native range in Europe compared to North America. During the early years after the invasion, North American native species were likely “naïve” to the new potential prey, which may at least in part explain why fewer natural enemies were reported from the New World (19 species of fish) than from Europe (30 species). Interestingly, even in the 1990s, virtually no difference was found between the number of birds recorded preying on Dreissena in Europe (21 species) compared to North America (20). With time, however, more indigenous North American fishes were observed feeding on dreissenids, and currently, their respective numbers are very similar (Europe: 42 predators; North America: 39) (). Likewise, some indigenous North American parasites with a broad host range have also been reported to be capable of infecting Dreissena (Karatayev et al. Citation2012; Toews et al. Citation1993), and the inventory is expected to increase as more studies are conducted.
Table 13. Total number of Dreissena spp. natural enemies recorded in Europe and North America according to Molloy et al. (Citation1997) and current study (2022).
5.1. Fish
Twenty-two species of fish were documented to feed on planktonic larvae of Dreissena, 11 in the Old World, and 11 in North America. During the summer, Dreissena veligers often comprise a large part of the zooplankton biomass and production, and are likely utilized by many species of fish, especially when other food items are rare. Relatively little research, however, had been conducted to quantify the importance of this resource for their consumers and the potential impact on dreissenid populations.
A total of 64 fish species have been documented to feed on attached dreissenids, including 37 species in Europe, 31 in North America, and 4 in both continents (). Generally, smaller (<15 mm) mussels are most vulnerable to fish predation. Only a few fish species have been reported to consume large zebra mussels (e.g., ide, up to 30 mm, and large carp, up to 42.5 mm). Although fish predators, including round gobies, may cause declines in dreissenid densities, these declines are usually limited to shallow lakes or the shallow areas of deep lakes, are likely temporary, and have little lake-wide effects at the scale of large, deep lakes like the Laurentian Great Lakes. More studies are needed not only on fish diets but especially on quantitative assessment of the impact of predation on dreissenids populations.
In general, the effect on fish varies depending on the feeding mode of the consumer, the morphology of the waterbody invaded, time since mussel invasion, co-evolutionary history, and Dreissena species, and is different in Europe and in North America.
5.2. Birds
An identical number of birds (22 species) are known to consume attached dreissenids in Europe and in North America (). Birds prey on dreissenids because they are often very abundant, requiring low search and handling times. Since diving effort increases with depth (and depth increases with the distance from shore) and the energy content of mussels decreases with depth, predation rates tend to be higher in shallow, coastal areas. Therefore, shallow waterbodies with dense mussel populations are the most attractive foraging areas for waterfowl, where they can exert high predation pressure on dreissenid populations. High rates of bird predation have been most commonly reported between autumn and spring when flocks are either temporarily present on waterbodies during their migration or overwintering and can be enhanced by the seasonal absence of other food items.
The intensity of avian predation on dreissenid populations depends on several factors, including predator densities, depth, substrate, mussel accessibility, and Dreissena species. Multiple studies documented significant declines in mussel populations due to bird predation, but these reductions tend to be temporary. Significant long-term reductions in mussel densities are most likely to occur only in localized areas where waterfowl overwinter (not just stage during the fall migration), and mussel recruitment is limited. Birds benefit from direct consumption of dreissenids as a reliable and abundant food source, and from feeding on the invertebrates, macrophytes, and bottom algae facilitated by and associated with the mussels. As a result, waterfowl flock sizes increase, and overwintering, timing, and routes of bird migration change.
In summary, while predation has been cited as one of the factors responsible for declines in dreissenid population densities and/or biomass, its effectiveness in steadily reducing Dreissena densities over the long term has yet to be demonstrated. The high fecundity and recruitment rates are precisely the traits that preclude its enemies from causing steady, long-term declines in dreissenid populations. The notion that predators or other enemies can succeed in extirpating the mussels, even in limited areas, seems unrealistic. As in Europe, there will likely be isolated reports of major impacts, but in general, the cumulative effects of a suite of enemies will likely have a constant, but limited, role in suppressing dreissenid populations.
5.3. Endosymbionts
Seventy-five species and higher taxa of endosymbionts have been found within the mantle cavity and/or associated with D. polymorpha in Europe, and 21 in North America (). The number of Dreissena-specific endosymbionts in a given population depends on many factors, in particular the Dreissena species (D. polymorpha hosts more symbionts than D. r. bugensis), the life stage of the mussel (attached or planktonic), as well as the number of mussels initially introduced, and/or the number of introduction events involved. The fact that C. acuminatus is present in virtually all European freshwater populations of D. polymorpha, but has not been found in North America, supports the hypothesis that planktonic larvae (rather than attached Dreissena) invaded North America. Therefore, analysis of Dreissena endosymbionts may help us to reconstruct the mechanisms of Dreissena invasion. Several records of O. hemophaga from North America, however, conflict with the hypothesis of the introduction of D. polymorpha at the planktonic stage, and additional investigation is required.
Among the most significant ecological and economic impacts of exotic species are those associated with their role as vectors for the introduction of parasites and commensals into the areas invaded, as well as new hosts for native parasites that promote preexisting diseases. Both processes were documented for D. polymorpha. For example, in Belarus—a country colonized by D. polymorpha in the early 1800s—at least six species-specific endosymbionts were introduced with the mussel, while in Ireland, colonized only in 1997, only two species-specific endosymbionts have been recorded so far. In addition to ciliates that spend their entire life within their host, D. polymorpha may host trematodes that can increase parasite loads on fish. Thus, increases in infection by metacercariae of B. polymorphus were documented in several water systems in Europe for many fish species. These infections were due to the expansion of both the first intermediate B. polymorphus host, D. polymorpha, and the second intermediate host, gobiid fishes, which in turn are heavily preyed upon by piscivorous fish. Dreissenids can also become a new reservoir for indigenous parasites, promoting native diseases in fish and waterfowl populations that otherwise would not have happened in the waterbodies invaded. To determine the level of endosymbiont specificity requires additional studies since it is not entirely clear if endosymbionts reported from D. polymorpha are species-specific or genus-specific.
In strong contrast to the evidence presented in the “Predators” section of this article, suggesting that predators can be responsible for some declines in dreissenid populations, even if these declines are limited in space and time, there is no published evidence to date that parasites can control dreissenid populations to any extent. Usually, infectious diseases are mentioned as a possible cause only when the decline studied has no explanation, and the putative infection is speculation unsupported by actual data. This does not imply that parasites capable of killing dreissenids have not been observed—they exist and occasionally their lethal effects have been thoroughly documented. Compared to the wide diversity of virulent parasites known from other bivalves, particularly commercially valuable marine species, dreissenids appear to have relatively few serious diseases. As a result, clear evidence of lethal parasites killing a sizable portion (e.g., >20%) of a dreissenid population has never been reported. Such infections leading to mass mortalities (e.g., >20%) potentially may occur in dreissenid populations, but they go unreported. This is most likely because these rare events require a very swift research response and the availability of uncommon experience and skills, as well as considerable financial resources. The identification of parasitic, infectious organisms among the myriad of creatures swarming in the body cavities and inside the tissues of dying or recently dead mussels, and distinguishing the parasites from the hordes of saprophytic microscopic organisms that invade and proliferate in the animal is a very difficult task that few scholars are able and willing to undertake. In short, so far the understanding of the role that infectious diseases play in dreissenid population declines is extremely limited. Intensive research efforts employing histological techniques to detect diseases are currently underway both in Europe and in North America (Molloy, unpublished data). These investigations will likely reveal a much broader range of parasites, particularly microbial parasites, and shed more light on their pathogenicity.
5.4. Ecological competitors
Fourteen competitors were reported to have some impact on dreissenids. Although some of them have been shown to be able to occasionally outcompete D. polymorpha, but the effect was always very limited in space and time, and no significant impacts on entire populations were found. In contrast, impacts on D. polymorpha by its congener D. r. bugensis were shown to be able to cause dramatic declines of the former in many European and North American water bodies, especially in deep stratified lakes.
Acknowledgments
The deepest gratitude to Dr. Demetrio Boltovskoy (Universidad de Buenos Aires, Argentina) for many thoughtful suggestions that greatly improved this manuscript. Special thanks to thank Pamela Bolton and Administrative Assistant Susan Dickinson (Great Lakes Center) for proofreading the manuscript, and the Editor and two anonymous reviewers for helpful comments. The views expressed in this publication are those of the authors and do not necessarily represent the views or policies of the U.S. EPA. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. EPA.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
1 In Molloy et al. (Citation1997), the total numbers of predators and competitors were erroneously reported as 176 and 10.
References
- Aleksenko TL. 2004. Molluscs of Dnieper-Bug estuary region and their role in fishes feeding. Gidrobiol Zh. 40(1):56–62.
- Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Philos Trans R Soc Lond B Biol Sci. 291:451–524.
- Andraso G, Blank N, Shadle MJ, DeDionisio JL, Ganger MT. 2017. Associations between food habits and pharyngeal morphology in the round goby (Neogobius melanostomus). Environ Biol Fish. 100(9):1069–1083. doi: 10.1007/s10641-017-0623-0.
- Andraso GM. 2005. Summer food habits of pumpkinseeds (Lepomis gibbosus) and bluegills (Lepomis macrochirus) in Presque Isle Bay, Lake Erie. J Great Lakes Res. 31(4):397–404. doi: 10.1016/S0380-1330(05)70271-9.
- Andraso GM, Ganger MT, Adamczyk J. 2011. Size-selective predation by round gobies (Neogobius melanostomus) on dreissenid mussels in the field. J Great Lakes Res. 37(2):298–304. doi: 10.1016/j.jglr.2011.02.006.
- Antipa GA, Small EB. 1971. The occurrence of thigmotrichous ciliated protozoa inhabiting the mantle cavity of unionid molluscs of Illinois. Trans Am Microsc Soc. 90:63–472.
- Antoł A, Kierat J, Czarnoleski M. 2018. Sedentary prey facing an acute predation risk: testing the hypothesis of inducible metabolite emission suppression in zebra mussels, Dreissena polymorpha. Hydrobiologia. 810(1):109–117. doi: 10.1007/s10750-017-3144-0.
- Anwand K. 1996. Dualismus von Orconectes limosus (Raf.) (Crustacea) und Dreissena polymorpha Pallas (Mollusca) Pallas (Mollusca). Limnologica. 26(4):423–426.
- Aristanov E. 1986. Parasite fauna of the molluscs of the southern Aral Sea. In: Biological resources of Aral. Tashkent (Uzbekistan): Academy of Sciences of the Republic of Uzbekistan “Fan” Press. p. 155–168.
- Aristanov E. 1992. The role of Dreissena polymorpha Pallas in the life cycle of Bucephalus polymorphus Baer 1827. Uzb Biol Zh. 1992:75–76.
- Arndt W. 1937. Ochridaspongia rotunda n. g. n. sp., ein neur Susswasserschwamm aus dem Ochridasee. Arch Hydrobiol. 31:36–677.
- Badzinski SS, Petrie SA. 2006. Diets of lesser and greater scaup during autumn and spring on the Lower Great Lakes. Wildl Soc Bull. 34(3):664–674. doi: 10.2193/0091-7648(2006)34
- Baer J, Spiessl C, Auerswald K, Geist J, Brinker A. 2022. Signs of the times: isotopic signature changes in several fish species following invasion of Lake Constance by quagga mussels. J Great Lakes Res. 48(3):746–755. doi: 10.1016/j.jglr.2022.03.010.
- Baer JC, Spiessl C, Brinker A. 2022a. Size matters? Species- and size-specific fish predation on recently established invasive quagga mussels Dreissena rostriformis bugensis Andrusov 1897 in a large, deep oligotrophic lake. J Fish Biol.
- Baer JC, Spiess C, Brinker A. 2022b. Size matters? Species- and size-specific fish predation on recently established invasive quagga mussels Dreissena rostriformis bugensis Andrusov 1897 in a large, deep oligotrophic lake. J Fish Biol. 100(5):1272–1282.
- Bakker KE, Davids C. 1973. Notes on the life history of Aspidogaster conchicola Baer, 1826 (Trematoda: Aspidogastridae). J Helminthol. 47(3):269–276. doi: 10.1017/S0022149X00026547.
- Baldwin BS, Mayer MS, Dayton J, Pau N, Mendilla J, Sullivan M, Moore A, Ma A, Mills EL. 2002. Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Can J Fish Aquat Sci. 59(4):680–694. doi: 10.1139/f02-043.
- Balkuvienė G, Pernaravičiūté B. 1994. Growth rates of roach (Rutilus rutilus (L)) in a cooling water reservoir under different thermal conditions. Int Revue Ges Hydrobiol Hydrogr. 79(1):139–142. doi: 10.1002/iroh.19940790113.
- Balogh C, Muskó IB, G.-Tóth L, Nagy L. 2008. Quantitative trends of zebra mussels in Lake Balaton (Hungary) in 2003–2005 at different water levels. Hydrobiologia. 613(1):57–69. doi: 10.1007/s10750-008-9472-3.
- Balogh C, Serfőző Z, de Vaate AB, Noordhuis R, Kobak J. 2019. Biometry, shell resistance and attachment of zebra and quagga mussels at the beginning of their co-existence in large European lakes. J Great Lakes Res. 45(4):777–787. doi: 10.1016/j.jglr.2019.05.011.
- Balogh C, Serfőző Z, Kobak J. 2022. Factors determining selective predation of the common carp on quagga versus zebra mussels. Freshw Biol. 67(4):619–629. doi: 10.1111/fwb.13867.
- Balogh C, Vláčilová A, G.-Tóth L, Serfőző Z. 2018. Dreissenid colonization during the initial invasion of the quagga mussel in the largest Central European shallow lake, Lake Balaton, Hungary. J Great Lakes Res. 44(1):114–125. doi: 10.1016/j.jglr.2017.11.007.
- Barton DR, Johnson RA, Campbell L, Petruniak J, Patterson M. 2005. Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. J Great Lakes Res. 31:252–261. doi: 10.1016/S0380-1330(05)70318-X.
- Bartsch MR, Bartsch LA, Gutreuter S. 2005. Strong effects of predation by fishes on an invasive macroinvertebrate in a large floodplain river. J N Am Benthol Soc. 24(1):168–177. doi: 10.1899/0887-3593(2005)024<0168:SEOPBF>2.0.CO;2.
- Baturo B. 1977. Bucephalus polymorphus Baer, 1827 and Rhipidocotyle illense (Ziegler, 1883) (Trematoda, Bucephalidae): morphology and biology of developmental stages. Acta Parasitol Pol. 24:203–220 + Plates I–IV.
- Baturo B. 1978. Larval bucephalosis in artificially heated lakes of the Konin region, Poland. Acta Parasitol Pol. 25:307–321.
- Beattie AM, Whiles MR, Willink PW. 2017. Diets, population structure, and seasonal activity patterns of mudpuppies (Necturus maculosus) in an urban, Great Lakes coastal habitat. J Great Lakes Res. 43(1):132–143. doi: 10.1016/j.jglr.2016.11.004.
- Bedulli D, Franchini DA. 1978. Primi rinvenimenti nel fiume Po e predazione su di essa da parte di Rattus norvegicus. Quad Civ Staz Idrobiol Milano. 6:85–92.
- Beekey MA, McCabe DJ, Marsden JE. 2004. Zebra mussels affect benthic predator foraging success and habitat choice on soft sediments. Oecologia. 141(1):164–170. doi: 10.1007/s00442-004-1632-1.
- Belyaev LD, Galinsky VL, Nikitin VF, Fatovenko MA. 1970. Fry in the Dnieprodzershinskoe water storage and its feed base. In: Biological processes in sea and continental water reservoirs. Abstracts of the Second Congress of the All-Union Hydrobiological Society. Kishinev (Moldova): Academy of Sciences of the Republic of Moldova Press. p. 42–43.
- Berchtold AE, Colborne SF, Longstaffe FJ, Neff BD. 2015. Ecomorphological patterns linking morphology and diet across three populations of pumpkinseed sunfish (Lepomis gibbosus). Can J Zool. 93(4):289–297. doi: 10.1139/cjz-2014-0236.
- bij de Vaate A. 1991. Distribution and aspects of population dynamics of the zebra mussel, Dreissena polymorpha (Pallas, 1771), in the Lake IJsselmeer area (The Netherlands). Oecologia. 86(1):40–50. doi: 10.1007/BF00317387.
- bij de Vaate A, Van der Velde G, Leuven RSEW, Heiler KCM. 2014. Spread of the quagga mussel, Dreissena rostriformis bugensis, in Western Europe. In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. 2nd ed. Boca Raton (FL): CRC Press. p. 83–92.
- Binelli AC, Della Torre C, Magni S, Parolini M. 2015. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environ Pollut. 196:386–403. doi: 10.1016/j.envpol.2014.10.023.
- Biro P. 1974. Observations on the food of eel (Anguilla anguilla L.) in Lake Balaton. Ann Inst Biol. 41:133–152.
- Boles LC, Lipcius RN. 1997. Potential for population regulation of the zebra mussel by finfish and the blue crab in North American estuaries. J Shellfish Res. 16:179–186.
- Bolotov IN, Klass AL, Kondakov AV, Vikhrev IV, Bespalaya YV, Gofarov MY, Filippov BY, Bogan AE, Lopes-Lima M, Lunn Z, et al. 2019. Freshwater mussels house a diverse mussel-associated leech assemblage. Sci Rep. 9(1): 16449. doi: 10.1038/s41598-019-52688-3.
- Borowiec E. 1975. Food of the coot (Fulica atra L.) in different phenological periods. Pol Arch Hydrobiol. 22:157–166.
- Botnariuc N, Spataru P, Erhan E. 1964. The diet of the carp in the Flax Lake Crapina-Jijila assemblage. Hidrobiologiya. 5:197–215.
- Bowen KL, Conway AJ, Currie WJS. 2018. Could dreissenid veligers be the lost biomass of invaded lakes? Freshw Sci. 37(2):315–329. doi: 10.1086/697896.
- Bowers R, Sudomir JC, Kershner MW, de Szalay FA. 2005. The effects of predation and unionid burrowing on bivalve communities in a Laurentian Great Lake coastal wetland. Hydrobiologia. 545(1):93–102. doi: 10.1007/s10750-005-2212-z.
- Bowmer CT, van der Meer M. 1991. Reproduction and histopathological condition in first year zebra mussels (Dreissena polymorpha) from the Haringvliet, Volkerakmeer and Hollands Diep Basins. Delft: TNO Institute of Environmental Sciences Report R91/132.
- Bradbury PC. 1994. Parasitic protozoa of molluscs and crustacea. In: Kreier JP, editor. Parasitic protozoa. Vol. 8. 2nd ed. San Diego (CA): Academic Press. p. 139–263.
- Brazner JC, Jensen DA. 2000. Zebra mussel (Dreissena polymorpha (Pallas)) colonization of rusty crayfish (Orconectes rusticus (Girard)) in Green Bay, Lake Michigan. Am Midl Nat. 143(1):250–256. doi: 10.1674/0003-0031(2000)143[0250:ZMDPPC.2.0.CO;2]
- Brian JI, Aldridge DC. 2021. Abundance data applied to a novel model invertebrate host shed new light on parasite community assembly in nature. J Anim Ecol. 90(5):1096–1108. doi: 10.1111/1365-2656.13436.
- Bruestle EL, Karboski C, Hussey A, Fisk AT, Mehler KC, Pennuto C, Gorsky D. 2019. Novel trophic interaction between lake sturgeon (Acipenser fulvescens) and non-native species in an altered food web. Can J Fish Aquat Sci. 76(1):6–14. doi: 10.1139/cjfas-2017-0282.
- Budzynska H, Romaniszyn W, Romanski J, Rubisz A, Stangenberg M, Stangenberg W. 1956. The growth and the summer food of the economically most important fishes of the Goplo Lake. Zool Pol. 7:63–120.
- Bulté G, Blouin-Demers G. 2008. Northern map turtles (Graptemys geographica) derive energy from the pelagic pathway through predation on zebra mussels (Dreissena polymorpha). Freshwater Biol. 53(3):497–508. doi: 10.1111/j.1365-2427.2007.01915.x.
- Bunnell DB, Davis BM, Chriscinske MA, Keeler KM, Mychek-Londer JG. 2015. Diet shifts by planktivorous and benthivorous fishes in northern Lake Michigan in response to ecosystem changes. J Great Lakes Res. 41 (Suppl. 3):161–171. doi: 10.1016/j.jglr.2015.07.011.
- Bunnell DB, Johnson TB, Knight CT. 2005. The impact of introduced round gobies (Neogobius melanostomus) on phosphorus cycling in central Lake Erie. Can J Fish Aquat Sci. 62(1):15–29. doi: 10.1139/f04-172.
- Burkett EM, Jude DJ. 2015. Long-term impacts of invasive round goby Neogobius melanostomus on fish community diversity and diets in the St. Clair River, Michigan. J Great Lakes Res. 41(3):862–872. doi: 10.1016/j.jglr.2015.05.004.
- Burla H, Lubini-Ferlin V. 1976. Bestandesdichte und verbreitungsmuster von wandermuscheln im Zürichsee. Vierteljahrsschr Naturforsch Ges Zürich. 121:187–199.
- Burlakova LE. 1998. Ecology of Dreissena polymorpha (Pallas) and its role in the structure and function of aquatic ecosystems [dissertation]. Minsk: Zoology Institute of the Academy of Science Republic Belarus. p. 1–167.
- Burlakova LE, Karatayev AY. 2023. Lake Michigan Benthos Survey Cooperative Science and Monitoring Initiative 2021. Technical Report. USEPA-GLRI GL00E02254. Buffalo (NY): Great Lakes Center, SUNY Buffalo State University. https://greatlakescenter.buffalostate.edu/sites/greatlakescenter.buffalostate.edu/files/uploads/Documents/Publications/LakeMichiganBenthosSurveyCSMI2021FinalReport.pdf
- Burlakova LE, Karatayev AY, Boltovskoy D, Correa NM. 2023. Ecosystem services provided by the exotic bivalves Dreissena polymorpha, D. rostriformis bugensis, and Limnoperna fortunei. Hydrobiologia. 850(12–13):2811–2854. doi: 10.1007/s10750-022-04935-4.
- Burlakova LE, Karatayev AY, Karatayev VA. 2012. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia. 685(1):121–134. doi: 10.1007/s10750-011-0791-4.
- Burlakova LE, Karatayev AY, Molloy DP. 1998. Field and laboratory studies of zebra mussel (Dreissena polymorpha) infection by the ciliate Conchophthirus acuminatus in the Republic of Belarus. J Invertebr Pathol. 71(3):251–257. doi: 10.1006/jipa.1997.4728.
- Burlakova LE, Karatayev AY, Padilla DK. 2006b. Changes in the distribution and abundance of Dreissena polymorpha within lakes through time. Hydrobiologia. 571(1):133–146. doi: 10.1007/s10750-006-0234-9.
- Burlakova LE, Karatayev AY, Pennuto C, Mayer C. 2014. Changes in Lake Erie benthos over the last 50 years: historical perspectives, current status, and main drivers. J Great Lakes Res. 40(3):560–573. doi: 10.1016/j.jglr.2014.02.008.
- Burlakova LE, Padilla DK, Karatayev AY, Minchin D. 2006a. Endosymbionts of Dreissena polymorpha in Ireland: evidence for the introduction of adult mussels. J Molluscan Stud. 72(2):207–210. doi: 10.1093/mollus/eyi067.
- Burreson EM, Ford SE. 2004. A review of recent information on the Haplosporidia, with special reference to Haplosporidium nelsoni (MSX disease). Aquat Living Resour. 17(4):499–517. doi: 10.1051/alr:2004056.
- Camp JW, Blaney LM, Barnes DK. 1999. Helminths of the round goby, Neogobius melanostomus (Perciformes: Gobiidae), from southern Lake Michigan, Indiana. J Helminthol Soc Wash. 66(1):70–72.
- Campbell LM, Thacker R, Barton D, Muir DCG, Greenwood D, Hecky RE. 2009. Re-engineering the eastern Lake Erie littoral food web: the trophic function of non-indigenous Ponto-Caspian species. J Great Lakes Res. 35(2):224–231. doi: 10.1016/j.jglr.2009.02.002.
- Canella MF, Rocchi-Canella I. 1976. Biologie des Ophryoglenina (Ciliés: Hymenostomes: Histophages): L ‘Epoque des ultrastructures de la ciliatologie pourra-t-elle aboutir a une systematique phylogenetique ou s’agit-il d’une flatteuse illusion? Ann Univ Ferrara. 3(Suppl. 2):1–510.
- Carbone C, DE Leeuw JJ, Houston AI. 1996. Adjustments in the diving time budgets of Tufted Duck and Pochard: is there evidence for a mix of metabolic pathways. Anim Behav. 51(6):1257–1268. doi: 10.1006/anbe.1996.0130.
- Carlsson NOL, Bustamante H, Strayer DL, Pace M. 2011. Biotic resistance on the increase: native predators structure invasive zebra mussel populations. Freshw Biol. 56(8):1630–1637. doi: 10.1111/j.1365-2427.2011.02602.x.
- Carlsson NOL, Sarnelle O, Strayer DL. 2009. Native predators and exotic prey – an acquired taste? Front Ecol Environ. 7(10):525–532. doi: 10.1890/080093.
- Carlsson NOL, Strayer DL. 2009. Intraspecific variation in the consumption of exotic prey – a mechanism that increases biotic resistance against invasive species? Freshw Biol. 54(11):2315–2319. doi: 10.1111/j.1365-2427.2009.02263.x.
- Casper AF, Johnson LE. 2010. Contrasting shell/tissue characteristics of Dreissena polymorpha and Dreissena bugensis in relation to environmental heterogeneity in the St. Lawrence River. J Great Lakes Res. 36(1):184–189. doi: 10.1016/j.jglr.2009.10.001.
- Casper AF, Johnson LE, Glémet H. 2014. In situ reciprocal transplants reveal species-specific growth pattern and geographic population differentiation among zebra and quagga mussels. J Great Lakes Res. 40(3):705–711. doi: 10.1016/j.jglr.2014.06.005.
- Chernogorenko MI, Boshko EG. 1992. Parasite fauna of aquatic organisms of the Dniester and Dniesetr Liman. In: Nesluzhenko VE, editor. Hydrobiological condition of the Dniester and its reservoirs. Kiev: Naukova Dunka Publishers. p. 321–329.
- Chernogorenko MI, Nizovskaya LV. 1986. Parasitocenosis of bivalve mollusks of unionidae and dreissenidae families in Dnieper and Dnieper-Bug Liman In: Parasity i bolezni wodnyh bespozvonochnyh (Tez. Dokl. IV Vsesojuzn. Sympoz.). Moscow (USSR): Nauka Publishing House. p. 147–149.
- Chrisafi E, Kaspiris P, Katselis G. 2007. Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). J Appl Ichthyol. 23(3):209–214. doi: 10.1111/j.1439-0426.2006.00824.x.
- Chucholl C. 2013. Feeding ecology and ecological impact of an alien ‘warm-water’ omnivore in cold lakes. Limnologica. 43(4):219–229. doi: 10.1016/j.limno.2012.10.001.
- Chucholl F, Chucholl C. 2021. Differences in the functional responses of four invasive and one native crayfish species suggest invader-specific ecological impacts. Freshw Biol. 66(11):2051–2063. doi: 10.1111/fwb.13813.
- Churchill RLT, Schummer ML, Petrie SA, Henry HAL. 2016. Long-term changes in distribution and abundance of submerged aquatic vegetation and dreissenid mussels in Long Point Bay, Lake Erie. J Great Lakes Res. 42(5):1060–1069. doi: 10.1016/j.jglr.2016.07.012.
- Chuševė R, Mastitsky SE, Zaiko A. 2012. First report of endosymbionts in Dreissena polymorpha from the brackish Curonian Lagoon, Baltic Sea. Oceanologia. 54(4):701–713. doi: 10.5697/oc.54-4.701.
- Claparède E, Lachmann J. 1858. Etudes sur les Infusoires et les Rhizopodes. Geneva: Messman.
- Cleven EJ, Frenzel P. 1992. Population dynamics and production of Dreissena polymorpha in the River Seerhein, the outlet of Lake Constance In: Neumann D, Jenner HA, editors. The Zebra Mussel Dreissena polymorpha. Ecology, biology monitoring and first applications in the water quality management. New York (NY): Gustav Fischer. p. 45–47.
- Cleven EJ, Frenzel P. 1993. Population dynamics and production of Dreissena polymorpha (Pallas) in River Seerhein, the outlet of Lake Constance (Obersee). Hydrobiologie. 127(4):395–407. doi: 10.1127/archiv-hydrobiol/127/1993/395.
- Cobb SE, Watzin MC. 2002. Zebra mussel colonies and yellow perch foraging: spatial complexity, refuges, and resource enhancement. J Great Lakes Res. 28(2):256–263. doi: 10.1016/S0380-1330(02)70581-9.
- Colborne SF, Clapp ADM, Longstaffe FJ, Neff BD. 2015. Foraging ecology of native pumpkinseed (Lepomis gibbosus) following the invasion of zebra mussels (Dreissena polymorpha). Can J Fish Aquat Sci. 72(7):983–990. doi: 10.1139/cjfas-2014-0372.
- Combes C, Albaret JL, Arvy L, Bartoli P, Bayssade-Dufour C, Deblock S, Durette-Desset MC, Gabrion C, Jourdane J, Lambert A, et al. 1980. Atlas mondial des cercaires. Mem Mus Nat Hist Nat Ser A Zool. 115(6–59):186–197.
- Conn DB, Babapulle MN, Klein KA, Rosen DA. 1994. Invading the invaders: infestation of zebra mussels by native parasites in the St. Lawrence River. Proceedings: Fourth International Zebra Mussel Conference. Madison (WI): Wisconsin Sea Grant Institute. p. 515–523.
- Conn DB, Conn DA. 1993. Parasitism, predation, and other biotic associations between dreissenid mussels and native animals in the St. Lawrence River. Proceedings: Third International Zebra Mussel Conference. Boston (MA): Stone and Webster Environmental Technology & Services. p. 2/24–2/34.
- Conn DB, Conn DA. 1995. Experimental infection of zebra mussels Dreissena polymorpha (Mollusca: Bivalvia) by metacercariae of Echinoparyphium sp. (Platyhelminthes: Trematoda). J Parasitol. 81(2):304–305. doi: 10.2307/3283939.
- Conn DB, Ricciardi A, Babapulle MN, Klein KA, Rosen DA. 1996. Chaetogaster limnaei (Annelida: Oligochaeta) as a parasite of the zebra mussel Dreissena polymorpha, and quagga mussel Dreissena bugensis (Mollusca: Bivalvia). Parasitol Res. 82(1):1–7. doi: 10.1007/s004360050058.
- Conn DB, Simpson SE, Minchin D, Lucy FE. 2008. Occurrence of Conchophthirus acuminatus (Protista: Ciliophora) in Dreissena polymorpha (Mollusca: Bivalvia) along the River Shannon, Ireland. Biol Invasions. 10(2):149–156. doi: 10.1007/s10530-007-9118-9.
- Connelly NA, O’Neill CR, Knuth BA, Brown TL. 2007. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environ Manag. 40(1):105–112. doi: 10.1007/s00267-006-0296-5.
- Coons K, McCabe DJ, Marsden JE. 2004. The effects of strobe lights on zebra mussel settlement and movement patterns. J Freshw Ecol. 19(1):1–8. doi: 10.1080/02705060.2004.9664505.
- Coulter DP, Murry BA, Uzarski DG. 2015. Relationships between habitat characteristics and round goby abundance in Lakes Michigan and Huron. J Great Lakes Res. 41(3):890–897. doi: 10.1016/j.jglr.2015.06.001.
- Crane DP, Einhouse DW. 2016. Changes in growth and diet of smallmouth bass following invasion of Lake Erie by the round goby. J Great Lakes Res. 42(2):405–412. doi: 10.1016/j.jglr.2015.12.005.
- Creque SM, Czesny SJ. 2012. Diet overlap of non-native alewife with native yellow perch and spottail shiner in nearshore waters of southwestern Lake Michigan, 2000–2007. Ecol Freshw Fish. 21(2):207–221. doi: 10.1111/j.1600-0633.2011.00538.x.
- Curry MG. 1979. New freshwater unionid clam hosts for three glossiphoniid leeches. Wassman J Biol. 37:89–92.
- Custer CM, Custer TW. 1996. Food habits of diving ducks the in the Great Lakes after the zebra mussel invasion. J Field Ornithol. 67:86–99.
- Custer CM, Custer TW. 2000. Organochlorine trace element contamination in wintering migrating diving ducks in the southern Great Lakes, USA, since the zebra mussel invasion. Environ Toxicol Chem. 19(11):2821–2829. doi: .
- Czarnołęski M, Kozłowski J, Kubajak P, Lewandowski K, Müller T, Stańczykowska A, Surówka K. 2006. Cross-habitat differences in crush resistance and growth pattern of zebra mussels (Dreissena polymorpha): effects of calcium availability and predator pressure. Hydrobiologie. 165(2):191–208. doi: 10.1127/0003-9136/2006/0165-0191.
- Czarnoleski M, Muller T. 2013. Antipredator strategy of zebra mussels (Dreissena polymorpha) from behavior to life history. In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. Boca Raton (FL): CRC Press. p. 345–357.
- Czarnołęski M, Müller T, Adamus K, Ogorzelska G, Sog M. 2010. Injured conspecifics alter mobility and byssus production in zebra mussels Dreissena polymorpha. Fundam Appl Limnol. 176(3):269–278. doi: 10.1127/1863-9135/2010/0176-0269.
- Czarnoleski M, Müller T, Kierat J, Gryczkowski L, Chybowski Ł. 2011. Anchor down or hunker down: an experimental study on zebra mussels’ response to predation risk from crayfish. Anim Behav. 82(3):543–548. doi: 10.1016/j.anbehav.2011.06.008.
- Czerniejewski P, Rybczyk A. 2008. Body weight, morphometry, and diet of the mud crab, Rhithropanopeus harrisii tridentatus (Maitland, 1874) in the Odra estuary, Poland. Crustaceana. 81(11):1289–1299. doi: 10.1163/156854008X369483.
- Daoulas CC, Economidis PS. 1984. The feeding of Rutilus rubilio (Bonaparte) (Pisces, Cyprinidae) in Lake Trichonis, Greece. Cybium. 8:29–38.
- David KA, Davis BM, Hunter RD. 2009. Lake St. Clair zooplankton: evidence for post-Dreissena changes. J Freshw Ecol. 24(2):199–209. doi: 10.1080/02705060.2009.9664284.
- Davids C, Kraak MHS. 1993. Trematode parasites of the zebra mussel (Dreissena polymorpha). In: Nalepa TF, Schloesser DW, editors. Zebra mussels: biology, impacts, and control. Boca Raton (FL): Lewis Publishers. p. 749–759.
- de Kinkelin P, Besse P, Tuffery G. 1968a. Une nouvelle affection nécrosante des téguments et des nageoires: La Bucéphalose larvaire à Bucephalus polymorphus (Baer, 1827). Bull Off Int Epizoot. 69:1207–1230.
- de Kinkelin P, Tuffery G, Leynaud G, Arrignon J. 1968b. Étude épizootiologique de la Bucéphalose larvaire a Bucephalus polymorphus, (Baer 1827) dans le peuplement piscicole du bassin de la Seine. Rech Vet. 1:77–98 + plate.
- de Kock WC, Bowmer CT. 1993. Bioaccumulation, biological effects, and food chain transfer of contaminants in the zebra mussel (Dreissena polymorpha). In: Nalepa TF, Schloesser DW, editors. Zebra mussels: biology, impacts, and control. Boca Raton (FL): Lewis Publishers. p. 503–533.
- de Leeuw JJ. 1997a. Introduction. In: de Leeuw JJ, editor. Demanding divers: ecological energetics of food exploitation by diving ducks. Vol. 61. Lelystad: Van Zee Tot Land. p. 9–20.
- de Leeuw JJ. 1997b. Synthesis: an energetic approach to habitat use and food exploitation limits of diving ducks in the IJsselmeer area. In: de Leeuw JJ, editor. Demanding divers: ecological energetics of food exploitation by diving ducks. Vol. 61. Lelystad: Van Zee Tot Land. p. 147–168.
- de Leeuw JJ. 1999. Food intake rates and habitat segregation of Tufted Duck Aythya fuligula and Scaup Aythya marila exploiting zebra mussels Dreissena polymorpha. Ardea. 87:15–31.
- de Leeuw JJ, Renema W. 1997. Dwingt kleptoparasitisme Kuifeenden Aythya fuligula tot nachtelijk foerageren? [Do tufted ducks Aythya fuligula feed by night to avoid kleptoparasitism?]. Limosa. 70(1):1–4.
- de Leeuw JJ, van Eerden MR. 1992. Size selection in diving tufted ducks Aythya fuligula explained by differential handling of small and large mussels, Dreissena polymorpha. Ardea. 80:353–362.
- de Leeuw JJ, van Eerden MR, Visser GH. 1999. Wintering Tufted Ducks Aythya fuligula diving for zebra mussels Dreissena polymorpha balance feeding costs within narrow margins of their energy budget. J Avian Biol. 30(2):182–192. doi: 10.2307/3677128.
- de Nie HW. 1982. A note on the significance of larger bivalve molluscs (Anodonta spp. and Dreissena sp.) in the food of the eel (Anguilla anguilla) in Tjeukemeer. Hydrobiologia. 95(1):307–310. doi: 10.1007/BF00044491.
- Dermott R, Kerec D. 1997. Changes to the deep water benthos of eastern Lake Erie since the invasion of Dreissena: 1979–1993. Can J Fish Aquat Sci. Sci. 54(4):922–930. doi: 10.1139/f96-332.
- Dermott R, Martchenko D, Johnson M. 2012. Changes in the benthic community of the Bay of Quinte, Lake Ontario, over a 40 year period. Aquat Ecosyst Health Manag. 15(4):410–420. doi: 10.1080/14634988.2012.728480.
- Dieterich A, Mörtl M, Eckmann R. 2004. The effects of zebra mussels (Dreissena polymorpha) on the foraging success of Eurasian perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus). Internat Rev Hydrobiol. 89(3):229–237. doi: 10.1002/iroh.200310693.
- Dietrich JP, Morrison BJ, Hoyle JA. 2006. Alternative ecological pathways in the Eastern Lake Ontario food web-round goby in the diet of lake trout. J Great Lakes Res. 32(2):395–400. doi: 10.3394/0380-1330(2006)32[395:AEPITE.2.0.CO;2]
- Diggins TP. 2001. A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. J Great Lakes Res. 27(4):457–466. doi: 10.1016/S0380-1330(01)70660-0.
- Diggins TP, Kaur J, Chakraborti RK, Depinto JV. 2002. Diet choice by the exotic round goby (Neogobius melanostomus) as influenced by prey motility and environmental complexity. J Great Lake Res. 28(3):411–420. doi: 10.1016/S0380-1330(02)70594-7.
- Djuricich P, Janssen J. 2001. Impact of round goby predation on zebra mussel size distribution at Calumet Harbor, Lake Michigan. J Great Lakes Res. 27(3):312–318. doi: 10.1016/S0380-1330(01)70646-6.
- Dmitrenko MA. 1967. The food of roach (Rutilus rutilus caspicus Jar.) in the Volga delta. Tr Kazan Nauchno-Issled Inst Rybn Khoz. 23:227–232.
- Dobrowolski KA, Leznicka B, Halba R. 1996. Natural food of ducks and coots in shallow, macrophyte dominated lake: Lake Luknajno (Masurian Lakeland, Poland). Ekol Pol. 44:271–287.
- Dobrzanska J. 1958. Sphenophrya dreissenae sp. n. (Ciliata, Holotricha, Thigmotrichida) living on the gill epithelium of Dreissena polymorpha Pall., 1754. Bull Acad Pol Sci Ser Sci Biol. 6:173–178 + Fig. 6–10 on unnumbered pages.
- Dobrzanska J. 1961. Further study on Sphenophrya dreissenae Dobrzanska, 1958 (Ciliata, Thigmotricha). Acta Parasitol Pol. 9:117–139.
- Draulans D. 1982. Foraging and size selection of mussels by the tufted duck Aythya fuligula. J Anim Ecol. 51(3):943–956. doi: 10.2307/4015.
- Draulans D. 1987. Do Tufted duck and Pochard select between differently sized mussels in a similar way? Wildfowl. 38:49–54.
- Draulans D, De Bont AF. 1980. An analysis of the diving for food of the Tufted Duck, Aythya fuligula, outside of the breeding season. Le Gerfaut. 70:251–260.
- Draulans D, Wouters R. 1988. Density, growth and calorific value of Dreissena polymorpha (Mollusca: Bivalvia) in a pond created by sand extraction, and its importance as food for fish. Ann Soc R Zool Belg. 118:51–60.
- Dubinin VB. 1952. Fauna of larval parasitic worms of vertebrates of the Volga River delta. Parazitol Sbor. 14:213–265.
- Dzierżyńska-Białończyk A, Jermacz Ł, Zielska J, Kobak J. 2019. What scares a mussel? Changes in valve movement pattern as an immediate response of a byssate bivalve to biotic factors. Hydrobiologia. 841(1):65–77. doi: 10.1007/s10750-019-04007-0.
- Early TA, Glonek T. 1999. Zebra mussel destruction by a Lake Michigan sponge: populations, in vivo 31P nuclear magnetic resonance, and phospholipid profiling. Environ. Sci. Technol. 33(12):1957–1962. doi: 10.1021/es980874+.
- Edwards DD, Vidrine MF. 2006. Host specificity among Unionicola spp. (Acari: Unionicolidae) parasitizing freshwater mussels. J Parasitol. 92(5):977–983. doi: 10.1645/GE-3565.1.
- Egereva IV. 1971. The feeding and feeding relations of fishes in the Kuibyshev reservoir. In: Volga I. Problems of studying and rational use of biological resources of waterbodies – materials of the First Conference on Studying Waterbodies of the Volga Basin. Kuibyshev: Kuibyshev Publishing House. p. 268–273.
- Eggleton MA, Miranda LE, Kirk JP. 2004. Assessing the potential for fish predation to impact zebra mussels (Dreissena polymorpha): insight from bioenergetics models. Ecology Freshwater Fish. 13(2):85–95. doi: 10.1111/j.1600-0633.2004.00033.x.
- Eppehimer DE, Bunnell DB, Armenio PM, Warner DM, Eaton LA, Wells DJ, Rutherford ES. 2019. Densities, diets, and growth rates of larval Alewife and Bloater in a changing Lake Michigan ecosystem. Trans Am Fish Soc. 148(4):755–770. doi: 10.1002/tafs.10171.
- Essian DA, Chipault JG, Lafrancois BM, Leonard JBK. 2016. Gut content analysis of Lake Michigan waterbirds in years with avian botulism type E mortality, 2010–2012. J Great Lakes Res. 42(5):1118–1128. doi: 10.1016/j.jglr.2016.07.027.
- Evariste L, David E, Cloutier P-L, Brousseau P, Auffret M, Desrosiers M, Groleau PE, Fournier M, Betoulle S. 2018. Field biomonitoring using the zebra mussel Dreissena polymorpha and the quagga mussel Dreissena bugensis following immunotoxic reponses. Is there a need to separate the two species? Environ Pollut. 238:706–716. doi: 10.1016/j.envpol.2018.03.098.
- Evlanov IA. 1990. Distribution of Aspidogaster limacoides in the population of Rutilus rutilus depending on the sex and age of the host. Zool Zh. 69:132–134.
- Evtushenko NY, Potrokhov AS, Zinkovskii OG. 1994. The black carp as a subject for acclimatization: a review. Hydrobiol J. 30:1–10.
- Fenchel T. 1965. Ciliates from Scandinavian molluscs. Ophelia. 2(1):71–174. doi: 10.1080/00785326.1965.10409598.
- Ferreira-Rodriguez N, Gessner J, Pardo I. 2016. Assessing the potential of the European Atlantic sturgeon Acipenser sturio to control bivalve invasions in Europe. J Fish Biol. 89(2):1459–1465. doi: 10.1111/jfb.13019.
- Filuk J, Zmudzinski L. 1965. Feeding of Vistula Firth ichthyofauna. Pr Morskiego Inst Rybackiego Ser A. 13:43–55.
- Fokin SI, Giamberini L, Molloy DP, bij de Vaate A. 2003. Bacterial endocytobionts within endosymbiotic ciliates in Dreissena polymorpha (Lamellibranchia: Mollusca). Acta Protozool. 42:31–39.
- Foley CJ, Andree SR, Pothoven SA, Nalepa TF, Höök TO. 2017. Quantifying the predatory effect of round goby on Saginaw Bay dreissenids. J Great Lakes Res. 43(1):121–131. doi: 10.1016/j.jglr.2016.10.018.
- Ford SE, Stokes NA, Alcox KA, Kraus BSF, Barber RD, Carnegie RB, Burreson EM. 2018. Investigating the life cycle of Haplosporidium nelsoni (Msx): a review. J Shellfish Res. 37(4):679–693. doi: 10.2983/035.037.0402.
- Ford SE, Tripp MR. 1996. Diseases and defense mechanisms. In: Kennedy VS, Newell RIE, Eble AF, editors. The Eastern Oyster Crassostrea virginica. College Park (MD): Maryland Sea Grant. p. 581–660.
- Francis JT, Robillard SR, Marsden JE. 1996. Yellow perch management in Lake Michigan: a multi-jurisdictional challenge. Fisheries. 21:18–20.
- French JRP. 1993. How well can fishes prey on zebra mussels in eastern North America? Fisheries. 18(6):13–19. doi: 10.1577/1548-8446(1993)018<0013:HWCFPO>2.0.CO;2.
- French JRP. 1997. Pharyngeal teeth of the freshwater drum (Aplodinotus grunniens) a predator of the zebra mussel (Dreissena polymorpha). J Freshw Ecol. 12(3):495–498. doi: 10.1080/02705060.1997.9663561.
- French JRP, Bur MT. 1993. Predation of the zebra mussel (Dreissena polymorpha) by freshwater drum in western Lake Erie. In: Nalepa TF, Schloesser DW, editors. Zebra mussels: biology, impacts, and control. Boca Raton (FL): Lewis Publishers. p. 453–464.
- French JRP, Jude DJ. 2001. Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River, Michigan. J Great Lakes Res. 27(3):300–311. doi: 10.1016/S0380-1330(01)70645-4.
- French JRP, Love JG. 1995. Size limitation on zebra mussels consumed by freshwater drum may preclude the effectiveness of drum as a biological controller. J Freshw Ecol. 10(4):379–383. doi: 10.1080/02705060.1995.9663460.
- French JRP, Morgan MN. 1995. Preference of redear sunfish on zebra mussels and rams-horn snails. J Freshw Ecol. 10(1):49–55. doi: 10.1080/02705060.1995.9663416.
- Gardner WS, Nalepa TF, Frez WA, Cichocki EA, Landrum PF. 1985. Seasonal patterns in lipid content of Lake Michigan macroinvertebrates. Can J Fish Aquat Sci. 42(11):1827–1832. doi: 10.1139/f85-229.
- Gavlena FK. 1977. The bighead goby Neogobius kessleri in the Volgograd reservoir Russian SFSR USSR. Vopr Ikhtiol. 17:359–360.
- Gaygusuz O, Gaygusuz CG, Tarkan AS, Acipinar H, Turer Z. 2007. Preference of zebra mussel, Dreissena polymorpha in the diet and effect on growth of gobiids: a comparative study between two different ecosystems. Ekoloji. 17(65):1–6. doi: 10.5053/ekoloji.2007.651.
- Gentner HW. 1971. Notes on the biology of Aspidogaster conchicola and Cotylaspis insignis. Zeitschrift Für Parasitenkunde. 35:263–269.
- George EM, Roseman EF, Davis BM, O’Brien TP. 2013. Feeding ecology of pelagic larval burbot in Northern Lake Huron, Michigan. Trans Am Fish Soc. 142(6):1716–1723. doi: 10.1080/00028487.2013.788561.
- Gerasimov YV. 2007. Intrapopulation spatial segregation in bream (Abramis brama) and roach (Rutilus rutilus) in the Rybinsk Reservoir. Russ J Ecol. 38(6):430–435. doi: 10.1134/S1067413607060094.
- Gerasimov YV. 2015. Population dynamics of the Rybinsk Reservoir fishes throughout the whole period of its existence: role of natural and anthropogenic factors. Trudy VNIRO (Proceedings of Russian Federal Research Institute of Fisheries and Oceanography). Vol. 156. p. 67–90.
- Géroudet P. 1966. Premières consequences ornithologiques de l’introduction de la “moule zébrée” Dreissena polymorpha dans le Lac Leman. Nos Oiseaux. 28:301–307.
- Géroudet P. 1978. L’evolution du peuplement hivernal des oiseaux d’eau dans le canton de Geneve (Leman et Rhone) de 1951 a 1977. Nos Oiseaux. 34(5):207–221.
- Ghedotti MJ, Smihula JC, Smith GR. 1995. Zebra mussel predation by round gobies in the laboratory. J Great Lakes Res. 21(4):665–669. doi: 10.1016/S0380-1330(95)71076-0.
- Gibson DI, Taskinen J, Valtonen ET. 1992. Studies on bucephalid digeneans parasitizing molluscs and fishes in Finland. II. The description of Rhipidocotyle fennica n. sp. and its discrimination by principal components analysis. Syst Parasitol. 23(1):67–79. doi: 10.1007/BF00008011.
- Ginezinskaja TA. 1959. About cercarial fauna of molluscs from Rybinskoye Dam Reservoir. II. Ecological factors influencing infection in molluscs by parthenogenic trematodes. West Leningr Univ Ser Biol. 4:62–77.
- Golikova MN. 1960. Ecological-parasitological study of the biocoenosis of some lakes in the Kaliningrad district. III. Parasitofauna of fish. Vestn Leningr Univ Biol. 15:110–121.
- Gonçalves V, Gherardi F, Rebelo R. 2016. Modelling the predation effects of invasive crayfish, Procambarus clarkii (Girard, 1852), on invasive zebra mussel, Dreissena polymorpha (Pallas, 1771), under laboratory conditions. Ital J Zool. 83(1):59–67. doi: 10.1080/11250003.2016.1138558.
- Gonçalves V, Gherardi F, Rebelo R. 2017. Bivalve or gastropod? Using profitability estimates to predict prey choice by P. clarkia. Acta Ethol. 20(2):107–117. doi: 10.1007/s10211-017-0251-x.
- Gontya FA. 1971. Mollusks of Kuchurgansky Liman. In: Mollusks: the mechanisms, methods and results of their study (abstracts of collected conference papers). Leningrad, Russia: Nauka Publishing House. p. 82–83.
- Gozzi AC, Lareschi M, Navone GT, Guichón ML. 2020. The enemy release hypothesis and Callosciurus erythraeus in Argentina: combining community and biogeographical parasitological studies. Biol Invasions. 22(12):3519–3531. doi: 10.1007/s10530-020-02339-w.
- Green NS, Hazlett BA, Pruett-Jones S. 2008. Attachment and shell integrity affects the vulnerability of zebra mussels (Dreissena polymorpha) to predation. J Freshw Ecol. 23(1):91–99. doi: 10.1080/02705060.2008.9664560.
- Grigorash VA. 1963. Twenty-four hour feeding regime of roach at early stages of development. In: Uchinskoe and Mozhaiskoe water storages. Moscow: Moskovskii Universitet Press. p. 235–261.
- Grinbart SB, Suprunovitch AV. 1981. Feeding resources of zoobenthos in the Dnestrovsky Liman and feed of crayfish (Astacidae). In: Abstracts of the Fourth Congress of the All-Union Hydrobiological Society (Part IV). Kiev. p. 103–110.
- Guillemette M, Bolduc F, Desgranges J-L. 1994. Stomach contents of diving and dabbling ducks during fall migration in the St Lawrence River, Quebec, Canada. Wildfowl. 45:167–175.
- Hahm J, Manchester-Neesvig JB, DeBord D, Sonzogni WC. 2009. Polybrominated diphenyl ethers (PBDEs) in Lake Michigan forage fish. J Great Lakes Res. 35(1):154–158. doi: 10.1016/j.jglr.2008.12.001.
- Hallidayschult TC, Hambright KD. 2018. Harris Mud Crabs (Rhithropanopeus Harrisii) as a Novel Invasive Predator of Zebra Mussels (Dreissena polymorpha) Chapter 4 in Hallidayschult TC. Population dynamics and long-term impacts of the invasive zebra mussel (Dreissena polymorpha) in a subtropical reservoir [PhD thesis]. Norman (OK): Department of Biology, University of Oklahoma. https://hdl.handle.net/11244/54342.
- Hamilton DL, Ankney D. 1994. Consumption of zebra mussels Dreissena polymorpha by diving ducks in Lakes Erie and St. Clair. Wildfowl. 45:159–166.
- Hamilton DJ, Ankney CD, Bailey RC. 1994. Predation of zebra mussels by diving ducks: an exclosure study. Ecology. 75(2):521–531. doi: 10.2307/1939555.
- Hanari N, Kannan K, Horii Y, Taniyasu S, Yamashita N, Jude DJ, Berg MB. 2004. Polychlorinated naphthalenes and polychlorinated biphenyls in benthic organisms of a Great Lakes food chain. Arch Environ Contam Toxicol. 47(1):84–93. doi: 10.1007/s00244-003-3106-6.
- Hanson JM, Mackay WC, Prepas EE. 1989. Effect of size-selective predation by muskrats (Ondatra zebithicus) on a population of unionid clams (Anodonta grandis simpsoniana). J Anim Ecol. 58(1):15–28. doi: 10.2307/4983.
- Hartmann J. 1982. Hierarchy of niches of the fishes of Lake Constance, a lake undergoing eutrophication. Schweiz Z Hydrol. 44(2):315–323. doi: 10.1007/BF02502296.
- Haskin HH, Andrews JD. 1988. Uncertainties and speculations about the life cycle of the eastern oyster pathogen Haplosporidium nelsoni (MSX). Am Fish Soc Spec Publ. 18:5–22.
- Hazlett BA. 1994. Crayfish feeding responses to zebra mussels depend on microorganisms and learning. J Chem Ecol. 20(10):2623–2630. doi: 10.1007/BF02036196.
- Hecky RE, Smith REH, Barton DR, Guildford SJ, Taylor WD, Charlton MN, Howell ET. 2004. The near shore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can J Fish Aquat Sci. 61(7):1285–1293. doi: 10.1139/f04-065.
- Heiler KCM, bij de Vaate A, Ekschmitt K, von Oheimb PV, Albrecht C, Wilke T. 2013. Reconstruction of the early invasion history of the quagga mussel (Dreissena rostriformis bugensis) in Western Europe. Aquat Invasions. 8(1):53–57. doi: 10.3391/ai.2013.8.1.06.
- Herbst SJ, Marsden JE, Lantry BF. 2013. Lake whitefish diet, condition, and energy density in Lake Champlain and the lower four Great Lakes following dreissenid invasions. Trans Am Fish Soc. 142(2):388–398. doi: 10.1080/00028487.2012.747991.
- Herod JJ, Frey TL, Sickel JB. 1997. Blue catfish (Ictalurus furcatus: Ictaluridae) predation on the zebra mussel in the Ohio River near Paducah, Kentucky. Trans Ky Acad Sci. 58:96.
- Hetherington A, Rudstam LG, Schneider RL, Holeck KT, Hotaling CW, Cooper JE, Jackson JR. 2019. Invader invaded: population dynamics of zebra mussels (Dreissena polymorpha) and quagga mussels (Dreissena rostriformis bugensis) in polymictic Oneida Lake, NY, USA (1992–2013). Biol Invasions. 21(5):1529–1544. doi: 10.1007/s10530-019-01914-0.
- Higgins SN, Vander Zanden MJ. 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr. 80(2):179–196. doi: 10.1890/09-1249.1.
- Hiller W. 1997. Waterfowl dynamics at Lake Tegernsee (upper Bavaria) from 1973 to 1997. Ornithol Anz. 36:143–158.
- Hirsch PE, Cayon D, Svanbäck R. 2014. Plastic responses of a sessile prey to multiple predators: a field and experimental study. PLOS One. 9(12):e115192. doi: 10.1371/journal.pone.0115192.
- Hogan LS, Marschall E, Folt C, Stein RA. 2007. How non-native species in Lake Erie influence trophic transfer of mercury and lead to top predators. J Great Lakes Res. 33(1):46–61. doi: 10.3394/0380-1330(2007)33
- Houghton CJ, Janssen J. 2013. Variation in predator-prey interactions between round gobies and dreissenid mussels. In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. Boca Raton (FL): CRC Press. p. 359–366.
- Hoyle JA, Bowlby JN, Morrison BJ. 2008. Lake whitefish and walleye population responses to dreissenid mussel invasion in eastern Lake Ontario. Aquat Ecosyst Health Manag. 11(4):403–411. doi: 10.1080/14634980802530392.
- Hoyle JA, Schaner T, Casselman JM, Dermott R. 1999. Changes in lake whitefish (Coregonus clupeaformis) stocks in eastern Lake Ontario following Dreissena mussel invasion. Great Lakes Res Rev. 4:5–10.
- Huehner MK, Hannan K, Garvin M. 1989. Feeding habits and marginal organ histochemistry of Aspidogaster conchicola (Trematoda: Aspidogastrea). J Parasitol. 75(6):848–852. doi: 10.2307/3282862.
- Hunter RG, Simons KA. 2004. Dreissenids in Lake St. Clair in 2001: evidence for population regulation. J Great Lakes Res. 30(4):528–537. doi: 10.1016/S0380-1330(04)70368-8.
- Ibelings BW, Portielje R, Lammens EHRR, Noordhuis R, van den Berg MS, Joosse W, Meijer ML. 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems. 10(1):4–16. doi: 10.1007/s10021-006-9009-4.
- Ivlev VS. 1961. Experimental ecology of the feeding of fishes. New Haven (CT): Yale University Press.
- Jackson JR, VanDeValk AJ, Brooking TE, Holeck KT, Hotaling C, Rudstam LG. 2020. The fisheries and limnology of Oneida Lake 2019. Albany (NY): New York State Department of Environmental Conservation. p. 1–25.
- Jacobs GR, Bruestle EL, Hussey A, Gorsky D, Fisk AT. 2017. Invasive species alter ontogenetic shifts in the trophic ecology of Lake Sturgeon (Acipenser fulvescens) in the Niagara River and Lake Ontario. Biol Invasions. 19(5):1533–1546. doi: 10.1007/s10530-017-1376-6.
- Jacoby H. 2005. Der Bodensee als Lebensraum fur Wasservogel, insbesondere als Zugrastplatz und Winterquartier. In: Klötzli F, editor. Der Rhein: Lebensader Einer Region. p. 239–245.
- Jacoby H, Leuzinger H. 1972. Die Wandermaschel (Dreissena polymorpha) als Nahrung der Wasservogel am Bodensee. Anz Ornithol Ges Bayern. 11:26–35.
- Jantz B, Schöll F. 1998. Größenzusammensetzung und Altersstruktur lokaler Bestände einer Zebramuschel-Flußpopulation Untersuchungen am Rhein zwischen Basel und Emmerich (Rh-km 168-861). Limnologica. 28(4):395–413.
- Jarocki A, Raabe Z. 1932. Über drei neue Infusorien-Genera der Familie Hypocomidae (Ciliata: Thigmotricha), Parasiten in Süßwassermuscheln. Bull Acad Pol Sci Lett. 1:29–45.
- Jeschke JM, Heger T. 2018. Enemy release hypothesis. In: Jeschke JM, Heger T, editors. Invasion biology: hypotheses and evidence. Boston (MA): Cabi. p. 92–102.
- Johannsson OE, Dermott R, Graham DM, Dahl JA, Millard ES, Myles DD, LeBlanc J. 2000. Benthic and pelagic secondary production in Lake Erie after the invasion of Dreissena spp. with implications for fish production. J Great Lakes Res. 26(1):31–54. doi: 10.1016/S0380-1330(00)70671-X.
- Johnson LE, Carlton JT. 1996. Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology. 77(6):1686–1690. doi: 10.2307/2265774.
- Johnson TB, Allen M, Corkum LD, Lee VA. 2005a. Comparison of methods needed to estimate population size of round gobies (Neogobius melanostomus) in western Lake Erie. J Great Lakes Res. 31(1):78–86. doi: 10.1016/S0380-1330(05)70239-2.
- Johnson TB, Bunnell DB, Knight CT. 2005b. A potential new energy pathway in central Lake Erie: the round goby connection. J Great Lakes Res. 31:238–251. doi: 10.1016/S0380-1330(05)70317-8.
- Jude DJ, Janssen J, Crawford G. 1995. Ecology, distribution and impact of the newly introduced round and tubenose gobies on the biota of the St. Clair and Detroit Rivers. In: Munawar M, Edsall T, Leach J, editors. The Lake Huron ecosystem: ecology, fisheries and management. Ecovision World Monograph Series. Amsterdam: S. P. B. Academic Publishers. p. 447–460.
- Jude DJ, Reider RH, Smith GR. 1992. Establishment of Gobiidae in the Great Lakes basin. Great Lakes Res Rev. 3:27–34.
- Karabin A. 1978. The pressure of pelagic predators of the genus Mesocyclops (Copepoda, Crustacea) on small zooplankton. Ekol Pol. 26:241–257.
- Karatayev A. 1983. Ecology of Dreissena polymorpha Pallas in its role in macrobenthos of the cooling waterbody of a thermal power plant [PhD thesis]. Minsk: Belarusian State University.
- Karatayev AY, Burlakova LE. 1992. Changes in tropic structure of macrozoobenthos of an eutrophic lake, after invasion of Dreissena polymorpha. Biol Vnutr Vod Inform Bull. 93:67–71.
- Karatayev AY, Burlakova LE. 1995. The role of Dreissena in lake ecosystems. Russian J Ecol. 26(3):207–211. Translated from Ecologiya 1995, 26(3):232–236.
- Karatayev AY, Burlakova LE. 2022a. What we know and don’t know about the invasive zebra (Dreissena polymorpha) and quagga (Dreissena rostriformis bugensis) mussels. Hydrobiologia. doi: 10.1007/s10750-022-04950-5.
- Karatayev AY, Burlakova LE. 2022b. Dreissena in the Great Lakes: what have we learned in 30 years of invasion. Hydrobiologia. doi: 10.1007/s10750-022-04990-x.
- Karatayev AY, Burlakova LE, Daniel SE, Hrycik AR 2021b. Lake Ontario Benthos Survey Cooperative Science and Monitoring Initiative 2018. Technical Report. USEPA-GLRI GL00E02254. Buffalo (NY): Great Lakes Center, SUNY Buffalo State. p. 1–37. https://greatlakescenter.bufa lostate.edu/sites/greatlakescenter.bufalostate.edu/fles/uploads/Documents/Publications/LakeOntarioBenthosSu rveyCSMI2018FinalReport.pdf.
- Karatayev AY, Burlakova LE, Hrycik AR, Daniel SE, Mehler K, Hinchey EK, Dermott R, Kennedy GW, Griffiths R. 2022b. Long-term dynamics of Lake Erie benthos: one lake, three distinct communities. J Great Lakes Res. 48(6):1599–1617. doi: 10.1016/j.jglr.2022.09.006.
- Karatayev AY, Burlakova LE, Mastitsky SE, Padilla DK, Mills EL. 2011a. Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. J Shellf Res. 30(3):923–931. doi: 10.2983/035.030.0334.
- Karatayev AY, Burlakova LE, Mehler K, Bocaniov SA, Collingsworth PD, Warren G, Kraus RT, Hinchey EK. 2018a. Biomonitoring using invasive species in a large lake: Dreissena distribution maps hypoxic zones. J Great Lakes Res. 44(4):639–649. doi: 10.1016/j.jglr.2017.08.001.
- Karatayev AY, Burlakova LE, Mehler K, Elgin AK, Rudstam LG, Watkins JM, Wick M. 2022a. Dreissena in Lake Ontario 30 years post-invasion. J Great Lakes Res. 48(2):264–273. doi: 10.1016/j.jglr.2020.11.010.
- Karatayev AY, Burlakova LE, Mehler K, Hinchey EK, Wick M, Bakowska M, Mrozinska N. 2021c. Rapid assessment of Dreissena population in Lake Erie using underwater videography. Hydrobiologia. 848(9):2421–2436. doi: 10.1007/s10750-020-04481-x.
- Karatayev AY, Burlakova LE, Molloy DP. 2000b. Seasonal dynamics of Conchophthirus acuminatus (Ciliophora, Conchophthiridae) infection in Dreissena polymorpha and D. bugensis (Bivalvia, Dreissenidae). Eur J Protistol. 36(4):397–404. doi: 10.1016/S0932-4739(00)80045-0.
- Karatayev AY, Burlakova LE, Molloy DP, Mastitsky SE. 2007. Dreissena polymorpha and Conchophthirus acuminatus: what can we learn from host-commensal relationships. J Shellf Res. 26(4):1153–1160. doi: 10.2983/0730-8000(2007)26[1153:DPACAW.2.0.CO;2]
- Karatayev AY, Burlakova LE, Molloy DP, Volkova LK. 2000a. Endosymbionts of Dreissena polymorpha (Pallas) in Belarus. Internat Rev Hydrobiol. 85(5–6):543–559. doi: 10.1002/1522-2632(200011)85:5/6<543::AID-IROH543>3.0.CO;2-3.
- Karatayev AY, Burlakova LE, Molloy DP, Volkova LK, Volosyuk VV. 2002a. Field and laboratory studies of Ophryoglena sp. (Ciliata: Ophryoglenidae) infection in zebra mussels, Dreissena polymorpha (Bivalvia: Dreissenidae). J Invertebr Pathol. 79(2):80–85. doi: 10.1016/s0022-2011(02)00021-6.
- Karatayev AY, Burlakova LE, Padilla DK. 1997. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. J Shellfsh Res. 16:187–203.
- Karatayev AY, Burlakova LE, Padilla DK. 2002b. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers In: Leppakoski E, Gollach S, Olenin S, editors. Invasive aquatic species of Europe: distribution, impacts and management. Dordrecht: Kluwer Academic Publishers. p. 433–446.
- Karatayev AY, Burlakova LE, Padilla DK. 2010. Dreissena polymorpha in Belarus: history of spread, population biology and ecosystem impacts. In: Van Der Velde G, Rajagopal S, bij de Vaate A, editors. The zebra mussel in Europe. Leiden: Backhuys Publishers. p. 101–111.
- Karatayev AY, Burlakova LE, Padilla DK. 2015. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia. 746(1):97–112. doi: 10.1007/s10750-014-1901-x.
- Karatayev AY, Burlakova LE, Pennuto C, Ciborowski J, Karatayev VA, Juette P, Clapsadl M. 2014. Twenty five years of changes in Dreissena spp. populations in Lake Erie. J Great Lakes Res. 40(3):550–559. doi: 10.1016/j.jglr.2014.04.010.
- Karatayev AY, Karatayev VA, Burlakova LE, Mehler K, Rowe MD, Elgin AK, Nalepa TF. 2021a. Lake morphometry determines Dreissena invasion dynamics. Biol Invasions. 23(8):2489–2514. doi: 10.1007/s10530-021-02518-3.
- Karatayev VA, Karatayev AY, Burlakova LE, Padilla DK. 2013. Lakewide dominance does not predict the potential for spread of dreissenids. J Great Lakes Res. 39(4):622–629. doi: 10.1016/j.jglr.2013.09.007.
- Karatayev AY, Karatayev VA, Burlakova LE, Rowe MD, Mehler K, Clapsadl MD. 2018b. Food depletion regulates the demography of invasive dreissenid mussels in a stratified lake. Limnol Oceanogr. 63(5):2065–2079. doi: 10.1002/lno.10924.
- Karatayev AY, Mastitsky SE, Burlakova LE, Karatayev VA, Hajduk MM, Conn DB. 2012. Exotic molluscs in Great Lakes host epizootically important trematodes. J Shellf Res. 31(3):885–894. doi: 10.2983/035.031.0337.
- Karatayev AY, Mastitsky SE, Burlakova LE, Molloy DP, Vezhnovets GG. 2003a. Seasonal dynamics of endosymbiotic ciliates and nematodes in Dreissena polymorpha. J Invertebr Pathol. 83(1):73–82. doi: 10.1016/s0022-2011(03)00043-0.
- Karatayev AY, Mastitsky SE, Molloy DP, Burlakova LE. 2003b. Patterns of emergence and survival of Conchophthirus acuminatus (Ciliophora: Conchophthiridae) from Dreissena polymorpha (Bivalvia: Dreissenidae). J Shellfish Res. 22:495–500.
- Karatayev AY, Mastitsky SE, Padilla DK, Burlakova LE, Hajduk MM. 2011b. Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia. 668(1):183–194. doi: 10.1007/s10750-010-0533-z.
- Karatayev AY, Mikheev VP, Afanasev AS, Kirpichenco MY, Protasov AA, Shevtsova LV, Kharchenko TG. 1994. Practical significance, use, and outgrowth prevention of hydrotechnological constructions. In: Starobogatov JI, editor. Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae): systematics, ecology, practical meaning. Moscow: Nauka. p. 206–221.
- Karpinsky MG. 2010. Review: the Caspian Sea benthos: unique fauna and community formed under strong grazing pressure. Mar Pollut Bull. 61(4–6):156–161. doi: 10.1016/j.marpolbul.2010.02.009.
- Karpinsky MG, Shiganova TA, Katunin DN. 2005. Introduced Species Hdb Env Chem. Vol. 5, Part P. Berlin; Heidelberg: Springer-Verlag. p. 175–190.
- Kasyanov AN, Klevakin AA. 2011. Stellate tadpole-goby Benthophilus stellatus (Sauvage, 1874) in the Cheboksary Reservoir. Russ J Biol Invasions. 2(4):242–244. doi: 10.1134/S2075111711040047.
- Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM. 2009. Parasite spillback: a neglected concept in invasion ecology? Ecology. 90(8):2047–2056. doi: 10.1890/08-1085.1.
- Kharchenko TA. 1975. Dnieper sea-roach as a regulatory factor of Dreissena numbers in channels. In: Biological autopurification and formation of water quality. Moscow: Nauka. p. 73–74.
- Kidder GW. 1933. Studies on Conchophthirus mytili De Morgan. I. Morphology and division. II. Conjugation and nuclear reorganization. Arch Protistenkd. 79:1–49 + Plates 41–49.
- Kidder GW. 1934. Studies on the ciliates from fresh water mussels. I. the structure and neuromotor system of Conchophthirus anodontae Stein. C. curtus Engl., and C. magna sp. nov. Biol Bull. 66(1):69–90. doi: 10.2307/1537464.
- Kimbrough KL, Johnson WE, Jacob AP, Lauenstein GG. 2013. Contaminant concentrations in dreissenid mussels from the Laurentian Great Lakes: a summary of trends from the Mussel Watch Program. In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. Boca Raton (FL): CRC Press. p. 273–284.
- Kirby H. 1941. Relationships between certain protozoa and other animals. In: Calkins GN, Summers FM, editors. Protozoa in biological research. New York (NY): Columbia University Press. p. 890–1008.
- Klemm DJ. 1976. Leeches (Annelida: Hirudinea) found in North American mollusks. Malacol Rev. 9:63–76.
- Kobak J. 2013. Behavior of juvenile and adult zebra mussels (Dreissena polymorpha). In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. Boca Raton (FL): CRC Press. p. 331–344.
- Kobak J, Kakareko T. 2009. Attachment strength, aggregation and movement of the zebra mussel (Dreissena polymorpha, Bivalvia) in the presence of potential predators. Fundam Appl Limnol. 174(2):193–204. doi: 10.1127/1863-9135/2009/0174-0193.
- Kobak J, Kakareko T, Poznańska M. 2010. Changes in attachment strength and aggregation of zebra mussel, Dreissena polymorpha in the presence of potential fish predators of various species and size. Hydrobiologia. 644(1):195–206. doi: 10.1007/s10750-010-0113-2.
- Kobak J, Poznańska M, Kakareko T. 2012. Behavioural changes of zebra mussel Dreissena polymorpha (Bivalvia) induced by Ponto-Caspian gammarids. Biol Invasions. 14(9):1851–1863. doi: 10.1007/s10530-012-0197-x.
- Kobak J, Ryńska A. 2014. Environmental factors affecting behavioural responses of an invasive bivalve to conspecific alarm cues. Anim Behav. 96:177–186. doi: 10.1016/j.anbehav.2014.08.014.
- Kochnev SA. 1977. Infection with trematode metacercariae of Dreissena polymorpha in a reservoir warmed by waters of the thermo-electric station. In: Ekologiya Gelmintov. Yaroslavl: Yaroslavl State University. p. 46–52.
- Kočovský PM. 2019. Diets of endangered silver chub (Macrhybopsis storeriana, Kirtland, 1844) in Lake Erie and implications for recovery. Ecol Freshw Fish. 28(1):33–40. doi: 10.1111/eff.12424.
- Kodukhova YV, Karabanov DP. 2017. Morphological changes in the population of roach (Rutilus rutilus, Cyprinidae) in Lake Pleshcheevo as a result of the introduction of the mollusk, Dreissena polymorpha (Bivalvia) Zool. Zhurnal. 96:1069–1077.
- Kogan AV. 1970. The food resources of Tsimlyansk Reservoir and the extent of their utilization by mass fish species. J Ichthyol. 10:667–674.
- Königstein PJ. 1986. Zur Nahrungsökologie des Bläßhuhns Fulica atra L. auf West-Berliner Kanälen unter besonderer Berücksichtigung des städtischen Einflusses. Verh Ornithol Ges Bayern. 24:209–247.
- Konradt AG, Mukhammedova AF. 1974. Prospects of the black amur settlement in Tsimlyanskoe Reservoir. In: Studies of continental water bodies proceeding from the needs of industrial fishery. Vol. 12. p. 48–56. Moscow, USSR: GosNIORKh Press.
- Kooloos JGM, Kraaijeveld AR, Langenbach GEJ, Zweers GA. 1989. Comparative mechanics of filter feeding in Anas platyrhynchos, Anas clypeata and Aythya fuligula (Aves, Anseriformes). Zoomorphology. 108(5):269–290. doi: 10.1007/BF00312160.
- Kornis MS, Mercado-Silva N, Vander Zanden MJ. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. J Fish Biol. 80(2):235–285. doi: 10.1111/j.1095-8649.2011.03157.x.
- Kornobis S. 1977. Ecology of Dreissena polymorpha (Pall.). (Dreissenidae, Bivalvia) in lakes receiving heated water discharges. Pol Arch Hydrobiol. 24:531–546.
- Kostianoy AG, Kosarev AN. 2005. The Caspian Sea environment. Berlin: Springer.
- Kozulin A. 1995. Ecology of mallards Anas platyrhynchos wintering in low temperature conditions in Belarus. Acta Ornithol. 30:125–134.
- Kraak MHS, Davids C. 1990. The effect of the parasite Phyllodistomum macrocotyle (Trematoda) on heavy metal concentrations in the freshwater mussel Dreissena polymorpha. Neth J Zool. 41(4):269–276. doi: 10.1163/156854291X00199.
- Krasutska NO. 2017. Temperature influence on structural-functional characteristics of symbiosis of some species of molluscs [Dissertation]. Kiiv: Institute of Hydrobiology. National Academy of Science of Ukraine. p. 1–170.
- Krauß M. 1979. Zur Nahrungsökologie des Bläßhuhns Fulica atra auf den Berliner Havelseen und der Einfluß von Bläßhuhn und Bisamratte Ondatra zibethicus auf das Schilf Pharagmites communis. Anz Ornithol Ges Bayern. 18:105–144.
- Krepis OI, Mikhalchenko MM, Kantsur AI. 1981. Growth and feeding of black amur in experimental ponds of the zoostation based at the Moldavian electric water-power station. In: Complex use of Moldavian water bodies: introduction of fish and invertebrates. Kishivev: Shtiintsa Publishers. p. 61–65.
- Kublitskas A. 1959. Feed of benthophage fish in the Kurshyu Mares bay. In: Kurshyu Mares. Vilnius, Lithuania: Academy of Sciences of the Republic of Lithuania Press. p. 463–519.
- Kuhns LA, Berg MB. 1999. Benthic invertebrate community responses to round goby (Neogobius melanostomus) and zebra mussel (Dreissena polymorpha) invasion in southern Lake Michigan. J Great Lakes Res. 25(4):910–917. doi: 10.1016/S0380-1330(99)70788-4.
- Kulczycka A. 1939. Contributions to the study of larval trematode forms in the lamellibranchs near Warsaw. CR Seances Soc Sci Lett Varsovie Class IV. 32:80–82.
- Kuperman BI, Zhochov AE, Popova LB. 1994. Parasites of Dreissena polymorpha (Pallas) molluscs of the Volga basin. Parazitologiya, 28:396–402.
- Kvach Y, Mierzejewska K. 2011. Non-indigenous benthic fishes as new hosts for Bucephalus polymorphus Baer, 1827 (Digenea: Bucephalidae) in the Vistula River basin, Poland. Knowl Manag Aquat Ecosyst. 400:2.
- Lajtner J. 2012. Presence of Bucephalus polymorphus, Echinoparyphium recurvatum and Aspidogaster limacoides (Platodes, Trematoda) in the visceral mass of Dreissena polymorpha (Mollusca, Bivalvia). Helminthologia. 49(3):147–153. doi: 10.2478/s11687-012-0030-1.
- Lajtner J, Luciæ A, Marušiæ M, Erben R. 2008. The effects of the trematode Bucephalus polymorphus on the reproductive cycle of the zebra mussel Dreissena polymorpha. Acta Parasitol. 53(1):85–92.
- Lamanova AI. 1971. Attachment by zebra mussels and acorn barnacles on crayfish. Hydrobiol J. 6:89–91.
- Lancioni T, Gaino E. 2009. Competition between the freshwater sponge Ephydatia fluviatilis and the zebra mussel Dreissena polymorpha in Lake Trasimeno (central Italy). Ital J Zool. 72(1):27–32. doi: 10.1080/11250000509356649.
- Large SI, Torres P, Smee DL. 2012. Behavior and morphology of Nucella lapillus influenced by predator type and predator diet. Aquat Biol. 16(2):189–196. doi: 10.3354/ab00452.
- Laruelle F, Molloy DP, Fokin SI, Ovcharenko MA. 1999. Histological analysis of mantle-cavity ciliates in Dreissena polymorpha: their location, symbiotic relationship and distinguishing morphological characteristics. J Shellfish Res. 18:251–257.
- Laruelle F, Molloy DP, Roitman VA. 2002. Histological analysis of trematodes in Dreissena polymorpha: their location, pathogenicity, and distinguishing morphological characteristics. J Parasitol. 88(5):856–863. doi: 10.1645/0022-3395(2002)088[0856:HAOTID.2.0.CO;2]
- Lauckner G. 1983. Diseases of mollusca: Bivalvia. In: Kinne O, editor. Diseases of marine animals. Vol. II. Hamburg: Biologische Anstalt Helgoland. p. 477–961.
- Lauer TE. 1997. Space as a limiting resource among sessile benthic invertebrates: zebra mussels, freshwater sponges, and bryozoans [PhD thesis]. West Lafayette, Indiana: Purdue University.
- Lauer TE, Barnes DK, Ricciardi A, Spacie A. 1999. Evidence of recruitment inhibition of zebra mussels (Dreissena polymorpha) by a freshwater bryozoan (Lophopodella carteri). J N Am Benthol Soc. 18(3):406–413. doi: 10.2307/1468453.
- Lauer TE, Spacie A. 2000. The effects of sponge (Porifera) biofouling on zebra mussel (Dreissena polymorpha) fitness: reduction of glycogen, tissue loss, and mortality. J Freshw Ecol. 15(1):83–92. doi: 10.1080/02705060.2000.9663724.
- Lauer TE, Spacie A. 2004. Space as a limiting resource in freshwater systems: competition between zebra mussels (Dreissena polymorpha) and freshwater sponges (Porifera). Hydrobiologia. 517(1–3):137–145. doi: 10.1023/B:HYDR.0000027342.31716.9a.
- Lazareva VI. 2004. Seasonal life-cycle and feeding of predatory rotifer of genus Asplanchna in the Rybinsk reservoir. Biol Inland Waters. 4:59–68.
- Lazareva VI, Kopylov AI, Sokolova EA, Pryanichnikova EG. 2016. Veliger larvae of dreissenids (Bivalvia, Dreissenidae) in the plankton foodweb of Rybinsk Reservoir. Biol Bull Russ Acad Sci. 43(10):1313–1321. doi: 10.1134/S1062359016100083.
- Le TH, Pham LTK, Doan HTT, Le XTK, Saijuntha W, Rajapakse RPVJ, Lawton SP. 2020. Comparative mitogenomics of the zoonotic parasite Echinostoma revolutum resolves taxonomic relationships within the ‘E. revolutum’ species group and the Echinostomata (Platyhelminthes: Digenea). Parasitology. 147(5):566–576. doi: 10.1017/S0031182020000128.
- Lederer AM, Janssen J, Reed T, Wolf A. 2008. Impacts of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan. J Great Lakes Res. 34(4):690–697. doi: 10.1016/S0380-1330(08)71611-3.
- Lederer A, Massart J, Janssen J. 2006. Impact of round gobies (Neogobius melanostomus) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and the associated macroinvertebrate community across an invasion front. J Great Lakes Res. 32(1):1–10. doi: 10.3394/0380-1330(2006)32[1:IORGNM.2.0.CO;2]
- Lehrer-Brey G, Kornis MS. 2014. Winter distributional overlap facilitates lake whitefish (Coregonus clupeaformis) piscivory on invasive round gobies (Neogobius melanostomus) in Green Bay, Lake Michigan. J Freshw Ecol. 29(1):153–156. doi: 10.1080/02705060.2013.815663.
- Leroy SAG, Lahijani HAK, Crétaux JF, Aladin NV, Plotnikov IS. 2020. Past and current changes in the largest lake of the world: the Caspian Sea. In: Mischke S, editor. Large Asian lakes in a changing world. Cham: Springer. p. 65–107.
- Leuzinger H, Schuster S. 1970. Auswirkungen der massenvermehrung de wandermuschel Dreissena polymorpha auf die Wasservögel des Bodensees. Ornithol Beob. 67:269–274.
- Lewandowski K. 1982. The role of early developmental stages in the dynamics of Dreissena polymorpha (Pall.) (Bivalvia) populations in lakes. 2. Settling of larvae and the dynamics of numbers of settled individuals. Ekol Pol. 30:223–286.
- Liebig JR, Vanderploeg HA. 1995. Vulnerability of Dreissena polymorpha larvae to predation by Great Lakes calanoid copepods: the importance of the bivalve shell. J Great Lakes Res. 21(3):353–358. doi: 10.1016/S0380-1330(95)71046-2.
- Limburg KE, Ahrend K. 1994. Zebra mussel veligers observed in larval fish guts. Vol. 5. Brockport (NY): Dreissena! Zebra Mussel Information Clearinghouse Newsletter. p. 4.
- Limburg KE, Pace ML, Fischer D, Arend KK. 1997. Consumption, selectivity, and use of zooplankton by larval striped bass and white perch in a seasonally pulsed estuary. Trans Am Fish Soc. 126(4):607–621. doi: 10.1577/1548-8659(1997)126<0607:CSAUOZ>2.3.CO;2.
- Lindeman PV. 2006. Zebra and quagga mussels (Dreissena spp.) and other prey of a Lake Erie population of common map turtles (Emydidae: Graptemys geographica). Copeia. 2006(2):268–273. doi: 10.1643/0045-8511(2006)6[268:ZAQMDS.2.0.CO;2]
- Linzmaier SM, Jeschke JM. 2020. Towards a mechanistic understanding of individual-level functional responses: invasive crayfish as model organisms. Freshw Biol. 65(4):657–673. doi: 10.1111/fwb.13456.
- Locke SA, Bulté G, Marcogliese DJ, Forbes MR. 2014. Altered trophic pathway and parasitism in a native predator (Lepomis gibbosus) feeding on introduced prey (Dreissena polymorpha). Oecologia. 175(1):315–324. doi: 10.1007/s00442-014-2898-6.
- Lodge DM, Kershner MW, Aloi JE, Covich AP. 1994. Effects of an omnivorous crayfish (Orconectes rusticus) on a freshwater littoral food web. Ecology. 75:1265–1281. doi: 10.2307/1937452.
- Logvinenko BM, Starobogatov YI. 1968. Mollusca. In: Birshstein YA., editors. Atlas of invertebrates of the Caspian Sea. Moscow: Pishchevaya Promyshlennost Press. p. 308–385 plate 5.
- Love J, Savino JF. 1993. Crayfish (Orconectes virilis) predation on zebra mussels (Dreissena polymorpha). J Freshw Ecol. 8(3):253–259. doi: 10.1080/02705060.1993.9664861.
- Lozano SJ, Scharold JV, Nalepa TF. 2001. Recent declines in benthic macroinvertebrate densities in Lake Ontario. Can J Fish Aquat Sci. 58(3):518–529. doi: 10.1139/f01-002.
- Lumb CE, Johnson TB, Cook HA, Hoyle JA. 2007. Comparison of lake whitefish (Coregollus clupeaformis) growth, condition, and energy density between lakes Erie and Ontario. J Great Lakes Res. 33(2):314–325. doi: 10.3394/0380-1330(2007)33[314:COLWCC.2.0.CO;2]
- Luukkonen DR, Kafcas EN, Shirkey BT, Winterstein SR. 2013. Impacts of dreissenid mussels on the distribution and abundance of diving ducks on Lake St. Clair. In: Nalepa TF, Schloesser DW, editors. Quagga and zebra mussels: biology, impacts, and control. Boca Raton (FL): CRC Press. p. 647–660.
- Lvova AA. 1977. Ecology of Dreissena polymorpha Pallas in the Uchinski Reservoir [PhD thesis]. Moscow: Moscow State University.
- Lvova AA, Karatayev AY, Makarova GE. 1994. Planktonic larva. In: Starobogatov, JI, editor. Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Moscow: Nauka Press. p. 149–155.
- Lvova AA, Starobogatov YI. 1982. A new species of the genus Dreissena (Bivalvia, Dreissenidae) from Lake Ohrid. Zool Zh. 61:1749–1752.
- Lyagina TN, Spanowskaya VD. 1963. Morphological peculiarities of some fish in the Uchinski Reservoir. In: Uchinskoe and Mozhaiskoe reservoirs: hydrobiological and ichthyological studies. Moscow: Moscow State University. p. 269–310.
- Lyakhnovich VP, Karataev AY, Antsipovich NN. 1983. The effect of water temperature on the rate of infection of Dreissena polymorpha with larvae of Phyllodistomum folium Olfers in Lake Lukoml’skoe. Biol Vnutr Vod Inf Byull. 58:35–38.
- Lyakhnovich VP, Karatayev AY, Mitrakhovich PA, Guryanova LV, Vezhnovets GG. 1988. Productivity and prospects for utilizing the ecosystem of Lake Lukoml, thermoelectric station cooling reservoir. Soviet J Ecol. 18:255–259.
- MacIsaac HJ. 1994. Size-selective predation of zebra mussels (Dreissena polymorpha) by crayfish (Orconectes propinquus). J N Am Benthol Soc. 13(2):206–216. doi: 10.2307/1467239.
- MacIsaac HJ, Lonnee CJ, Leach JH. 1995. Suppression of microzooplankton by zebra mussels: importance of mussel size. Freshwater Biol. 34(2):379–387. doi: 10.1111/j.1365-2427.1995.tb00896.x.
- MacLeod CJ, Paterson AM, Tompkins DM, Duncan RP. 2010. Parasites lost—do invaders miss the boat or drown on arrival? Ecol Lett. 13(4):516–527. doi: 10.1111/j.1461-0248.2010.01446.x.
- MacLsaac HJ, Sprules WG, Leach JH. 1991. Ingestion of small-bodied zooplankton by zebra mussels (Dreissena polymorpha): can cannibalism on larvae influence population dynamics? Can J Fish Aquat Sci. 48(11):2051–2060. doi: 10.1139/f91-244.
- Madenjian CP, Bunnell DB, Warner DM, Pothoven SA, Fahnenstiel GL, Nalepa TF, Vanderploeg HA, Tsehaye I, Claramunt RM, Clark RD. 2015. Changes in the Lake Michigan food web following dreissenid mussel invasions: a synthesis. J Great Lakes Res. 41(Suppl. 3):217–231. doi: 10.1016/j.jglr.2015.08.009.
- Madenjian CP, Pothoven SA, Schneeberger PJ, Ebener MP, Mohr LC, Nalepa TF, Bence JR. 2010. Dreissenid mussels are not a “dead end” in Great Lakes food webs. J Great Lakes Res. 36:73–77. doi: 10.1016/j.jglr.2009.09.001.
- Madenjian CP, Stapanian MA, Witzel LD, Pothoven SA, Einhouse DW, Whitford HL. 2011. Evidence for predatory control of the invasive Round Goby. Biol Invasions. 13(4):987–1002. doi: 10.1007/s10530-010-9884-7.
- Magoulick DD, Lewis LC. 2002. Predation on exotic zebra mussels by native fishes: effects on predator and prey. Freshw Biol. 47(10):1908–1918. doi: 10.1046/j.1365-2427.2002.00940.x.
- Malinowskaya AS. 1976. Feeding of long-fingered crayfish. In: Abstracts of the Third Congress of the All-Union Hydrobiological Society. Vol. 3. Riga, Lithuania: Riga Zinantis Press. p. 284–287.
- Marchowski D, Jankowiak L, Wysocki D. 2016. Newly demonstrated foraging method of Herring Gulls and Mew Gulls with benthivorous diving ducks during the nonbreeding period. Ornithology. 133(1):31–40. doi: 10.1642/AUK-15-62.1.
- Marchowski D, Neubauer G. 2019. Kleptoparasitic strategies of mallards towards conspecifics and Eurasian coots. Ardea. 107(1):110–114. doi: 10.5253/arde.v107i1.a7.
- Marchowski D, Neubauer G, Ławicki Ł, Woźniczka A, Wysocki D, Guentzel S, Jarzemski M. 2015. The importance of non-native prey, the zebra mussel Dreissena polymorpha, for the declining Greater Scaup Aythya marila: a case study at a key European staging and wintering site. PLOS One. 11(3):e0145496. Correction: PLOS One 2016 11(3):e0152543. doi: 10.1371/journal.pone.0152543.
- Marescaux J, Boets P, Lorquet J, Sablon R, Van Doninck K, Beise J-N. 2015. Sympatric Dreissena species in the Meuse River: towards a dominance shift from zebra to quagga mussels. Aquat Invasions. 10(3):287–298. doi: 10.3391/ai.2015.10.3.04.
- Marin Jarrin JR, Pangle KL, Reichert JM, Johnson TB, Tyson J, Ludsin SA. 2015. Influence of habitat heterogeneity on the foraging ecology of first feeding yellow perch larvae, Perca flavescens, in western Lake Erie. J Great Lakes Res. 41(1):208–214. doi: 10.1016/j.jglr.2014.12.024.
- Marsden JE. 1997. Common carp diet includes zebra mussels and lake trout eggs. J Freshw Ecol. 12(3):491–492. doi: 10.1080/02705060.1997.9663559.
- Martel AL, Baldwin BS, Dermott RM, Lutz RA. 2001. Species and epilimnion/hypolimnion-related differences in size at larval settlement and metamorphosis in Dreissena (Bivalvia). Limnol Oceanogr. 46(3):707–713. doi: 10.4319/lo.2001.46.3.0707.
- Marti J, Gammeter S, Schifferli L. 2004. Effects of the colonization by Dreissena polymorpha on wintering waterbirds in a lake on the northern edge of the Swiss alps, 1967 to 2003. Ornithol Beobachter. 101:125–134.
- Martin GW, Corkum LD. 1994. Predation of zebra mussels by crayfish. Can J Zool. 72(11):1867–1871. doi: 10.1139/z94-254.
- Martin TR, Conn DB. 1990. The pathogenicity, localization and cyst structure of echinostomatid metacercariae (Trematoda) infecting the kidneys of the frogs Rana clamitans and Rana pipiens. J Parasitol. 76(3):414–419. doi: 10.2307/3282677.
- Martyniak A, Jerzyk MS, Adamek Z. 1987. The food of bream (Abramis brama) in the Pierzchaly Reservoir (Poland). Folia Zool. 36:273–280.
- Mastitsky SE. 2012. Infection of Dreissena polymorpha (Bivalvia: Dreissenidae) with Conchophthirus acuminatus (Ciliophora: Conchophthiridae) in lakes of different trophy. BIR. 1(3):161–169. doi: 10.3391/bir.2012.1.3.02.
- Mastitsky SE, Gagarin VG. 2004. Nematodes, which infect the mollusc Dreissena polymorpha (Bivalvia: Dreissenidae) in Narochanskie Lakes. Vestn Beloruss Gos Univ Ser. 2(3):22–25.
- Mastitsky SE, Karatayev AY, Burlakova LE. 2014. Parasites of aquatic exotic invertebrates: identification of hazards posed to the great lakes. Hum Ecol Risk Assess. 20(3):743–763. doi: 10.1080/10807039.2013.774576.
- Mastitsky SE, Lucy F, Gagarin VG. 2008. First report of endosymbionts in Dreissena polymorpha from Sweden. Aquat Invasions. 3(1):83–86. doi: 10.3391/ai.2008.3.1.13.
- Mastitsky SE, Samoilenko VM. 2005. Larvae of chironomids (Insecta, Diptera) encountered in the mantle cavity of zebra mussel, Dreissena polymorpha (Bivalvia, Dreissenidae). Int Rev Hydrobiol. 90(1):42–50. doi: 10.1002/iroh.200410746.
- Mastitsky SE, Veres JK. 2010. Field evidence for a parasites spillback caused by exotic mollusc Dreissena polymorpha in an invaded lake. Parasitol Res. 106(3):667–675. doi: 10.1007/s00436-010-1730-4.
- Mastitsky SE, Vezhnovets GG. 2002. Zebra mussel as a source of echinostomatosis in waterfowl of the Reservoir Komsomolskoe (Belarus). Abstr. of the XII International Conference of Young Scientists “Biology of Inland Waters: problems of Ecology and Biodiversity”; Borok, Russia, 23–26 September 2002. p. 82–83.
- Matthews J, Schipper AM, Hendriks AJ, Le TTY, de Vaate AB, van der Velde G, Leuven RSEW. 2015. A dominance shift from the zebra mussel to the invasive quagga mussel may alter the trophic transfer of metals. Environ Pollut. 203:183–190. doi: 10.1016/j.envpol.2015.03.032.
- Mayer CM, Keats RA, Rudstam LG, Mills EL. 2002. Scale-dependent effects of zebra mussels on benthic invertebrates in a large eutrophic lake. J N Am Benthol Soc. 21(4):616–633. doi: 10.2307/1468434.
- Mayer CM, VanDeValk AJ, Forney JL, Rudstam LG, Mills EL. 2000. Response of yellow perch (Perca flavescens) in Oneida Lake, New York, to the establishment of zebra mussels (Dreissena polymorpha. Can J Fish Aquat Sci. 57(4):742–754. doi: 10.1139/f00-009.
- Mazak EJ, MacIsaac HJ, Servos MR, Hesslein R. 1997. Influence of feeding habits on organochlorine contaminant accumulation in waterfowl on the Great Lakes. Ecol Appl. 7(4):1133–1143. doi: 10.1890/1051-0761(1997)007[1133:IOFHOO.2.0.CO;2]
- McCabe DJ, Beekey MA, Mazloff A, Marsden JE. 2006. Negative effect of zebra mussels on foraging and habitat use by lake sturgeon (Acipenser fulvescens). Aquatic Conserv Mar Freshw Ecosyst. 16(5):493–500. doi: 10.1002/aqc.754.
- McDonald ME. 1969. Catalogue of helminths of waterfowl (Anatidae): Bureau of Sport Fisheries and Wildlife Special Scientific Report – Wildlife No. 126, vol 46. Washington (DC): U.S. Fish and Wildlife Service.
- McDonald ME. 1981. Keys to trematodes reported in waterfowl. Resource publication 142. Washington (DC): U.S. Fish and Wildlife Service.
- McMahon RF. 1991. Mollusca: Bivalvia. In: Thorp JH, Covich AP, editors. Ecology and classification of North American freshwater invertebrates. New York (NY): Academic Press. p. 315–399.
- Mehler K, Burlakova LE, Karatayev AY, Elgin AK, Nalepa TF, Madenjian CP, Hinchey E. 2020. Long-term trends of Lake Michigan benthos with emphasis on the southern basin. J Great Lakes Res. 46(3):528–537. doi: 10.1016/j.jglr.2020.03.011.
- Mercer JL, Fox MG, Metcalfe CD. 1999. Changes in benthos and three littoral zone fishes in a shallow, eutrophic Ontario Lake following the invasion of the zebra mussel (Dreissena polymorpha). Lake Reserv Manag. 15(4):310–323. doi: 10.1080/07438149909354126.
- Metz O, Temmen A, von Oheimb KCM, Albrecht C, Schubert P, Wilke T. 2018. Invader vs. invader: intra and interspecific competition mechanisms in zebra and quagga mussels. Aquat Invasions. 13(4):473–480. doi: 10.3391/ai.2018.13.4.05.
- Miano A, Leblanc JP, Farrell JM. 2021. Diet, trophic position of upper St. Lawrence River round goby giants reveals greater dependence on dreissenids with increasing body size. J Great Lakes Res. 47(4):1126–1134. doi: 10.1016/j.jglr.2021.04.004.
- Michelson EH. 1970. Aspidogaster conchicola from freshwater gastropods in the United States. J Parasitol. 56(4):709–712. doi: 10.2307/3277717.
- Mikheev VP. 1963. Dreissenids in the diet of fishes in the shallow parts of Kuibyshev Reservoir in 1961 In: Materials on the biology and hydrology of Volga Reservoirs. Moscow: Academy Nauk U.S.S.R. p. 80–83.
- Mikheev VP. 1966. Dreissena’s water filtration fate: the plankton and benthos of inland water reservoirs. Trans Inst Biol Inland Water Acad Sci USSR. 12:134–138.
- Mikheev VP. 1977. Animal matter as feed in water reservoirs of industrial fish-ponds. Moscow: All-Union Research Institute for Industrial Fishery Publishers.
- Mikulski JS, Adamczak B, Bittel L, Bohr R, Bronisz D, Donderski W, Gizinski A, Luscinska M, Rejewski M, Strzelczyk E, et al. 1975. Basic regularities of productive processes in the Ilawa Lakes and in the Goplo Lake from the point of view of utility values of the water. Pol Arch Hydrobiol. 22:101–122.
- Millane M, O’Grady MF, Delanty K, Kelly-Quinn M. 2012. An assessment of fish predation on the zebra mussel, Dreissena polymorpha (Pallas 1771) after recent colonisation of two managed brown trout lake fisheries in Ireland. Biol Environ. 112B(1):1–9. doi: 10.1353/bae.2012.0035.
- Mills EL, Chrisman JR, Baldwin B, Owens RW, O’Gorman R, Howell T, Roseman E, Raths MK. 1999. Changes in the dreissenid community in the lower Great Lakes with emphasis on southern Lake Ontario. J Great Lakes Res. 25(1):187–197. doi: 10.1016/S0380-1330(99)70727-6.
- Mills EL, O’Gorman R, Roseman EF, Adams C, Owens RW. 1995. Planktivory by alewife (Alosa pseudoharengus) and rainbow smelt (Osmerus mordax) on microcrustacean zooplankton and dreissenid (Bivalvia: Dreissenidae) veligers in southern Lake Ontario. Can J Fish Aquat Sci. 52(5):925–935. doi: 10.1139/f95-092.
- Minguez L, Devin S, Molloy DP, Guérold F, Giambérini L. 2013. Occurrence of zebra mussel parasites: modelling according to contamination in France and the USA. Environ Pollut. 176:261–266. doi: 10.1016/j.envpol.2013.01.031.
- Minguez L, Giambérini L. 2012. Seasonal dynamics of zebra mussel parasite populations. Aquat Biol. 15(2):145–151. doi: 10.3354/ab00418.
- Minguez L, Lang A-S, Beisel J-N, Giambérini L. 2012. Is there a link between shell morphology and parasites of zebra mussels? J Invertebr Pathol. 109(2):229–234. doi: 10.1016/j.jip.2011.11.010.
- Minguez L, Meyer A, Molloy DP, Giambérini L. 2009. Interactions between parasitism and biological responses in zebra mussels (Dreissena polymorpha): importance in ecotoxicological studies. Environ Res. 109(7):843–850. doi: 10.1016/j.envres.2009.07.012.
- Minguez L, Molloy DP, Guérold F, Giambérini L. 2011. Zebra mussel (Dreissena polymorpha) parasites: potentially useful bioindicators of freshwater quality? Water Res. 45(2):665–673. doi: 10.1016/j.watres.2010.08.028.
- Miroshnichenko MP. 1990. Growth, production and importance of Dreissena polymorpha (Pallas) in forage resources of the Tsimlyanskoe Reservoir. In: Species in the area: biology, ecology and productivity of aquatic invertebrates. Minsk: Navuka Tehnika. p. 170–175.
- Mitchell CA, Carlson J. 1993. Lesser scaup forage of zebra mussels at Cook Nuclear Plant. Michigan. J Field Ornithol. 64:219–222.
- Mitchell JS, Bailey RC, Knapton RW. 2000. Effects of predation by fish and wintering ducks on dreissenid mussels at Nanticoke, Lake Erie. Ecoscience. 7(4):398–409. doi: 10.1080/11956860.2000.11682610.
- Mitrakhovich PA, Karatayev AY. 1986. The role of Dreissena polymorpha Pallas larvae in zooplankton of the cooling reservoir of the Lukoml thermal power plant. Vestn Beloruss Univ Ser. 2(1):38–41.
- Mogilchenko VI. 1986. Some feeding aspects of the larvae of food fish in the Kanewskoe Reservoir. Gidrobiol Zh. 22:36–41.
- Molloy DP, Giamberini L, Burlakova LE, Karatayev AY, Cryan JR, Trajanovski SL, Trajanovska SP. 2010. Investigation of the endosymbionts of Dreissena stankovici with morphological and molecular confirmation of host species. In: Van Der Velde G, Rajagopal S, bij de Vaate A, editors. The zebra mussel in Europe. Leiden: Backhuys Publishers. p. 227–237.
- Molloy DP, Giamberini L, Morado JF, Fokin SI, Laruelle F. 2001. Characterization of intracytoplasmic prokaryote infections in Dreissena sp. (Bivalvia: Dreissenidae). Dis Aquat Organ. 44(3):203–216. doi: 10.3354/dao044203.
- Molloy DP, Giamberini L, Stokes NA, Burreson EM, Ovcharenko MA. 2012. Haplosporidium raabei n. sp. (Haplosporidia): a parasite of zebra mussels, Dreissena polymorpha (Pallas, 1771). Parasitology. 139(4):463–477. doi: 10.1017/S0031182011002101.
- Molloy DP, Karatayev AY, Burlakova LE, Kurandina DP, Laruelle F. 1997. Natural enemies of zebra mussels: predators, parasites, and ecological competitors. Rev Fisheries Sci. 5(1):27–97. doi: 10.1080/10641269709388593.]
- Molloy DP, Lynn DH, Giamberini L. 2005. Ophryoglena hemophaga n. sp. (Ciliophora: Ophryoglenidae): a parasite of the digestive gland of zebra mussels Dreissena polymorpha. Dis Aquat Organ. 65(3):237–243. Corrections in: 2006. Dis Aquat Org. 70:181 and 2007. Dis Aquat Org. 77:259. doi: 10.3354/dao065237.
- Molloy DP, Powell J, Ambrose P. 1994. Short-term reduction of adult zebra mussels (Dreissena polymorpha) in the Hudson River near Catskill, New York: an effect of juvenile blue crab (Callinectes sapidus) predation? J Shellfish Res. 13:367–371.
- Molloy DP, Roitman VA, Shields JD. 1996. Survey of the parasites of zebra mussels (Bivalvia: Dreissenidae) in northwestern Russia, with comments on records of parasitism in Europe and North America. J Helminthol Soc Wash. 63:251–256.
- Morrison AL, Thelen MA, Howe SE, Zimmer KD, Herwig BR, Staples DF, McEachran MC. 2021. Impacts of zebra mussels (Dreissena polymorpha) on isotopic niche size and niche overlap among fish species in a mesotrophic lake. Biol Invasions. 23(9):2985–3002. doi: 10.1007/s10530-021-02553-0.
- Morrison TW, Lynch WEJr., Dabrowski K. 1997. Predation on zebra mussels by freshwater drum and yellow perch in western Lake Erie. J Great Lakes Res. 23(2):177–189. doi: 10.1016/S0380-1330(97)70895-5.
- Mörtl M, Werner S, Rothhaupt KO. 2010. Effects of predation by wintering water birds on zebra mussels and on associated macroinvertebrates. In: Van Der Velde G, Rajagopal S, bij de Vaate A, editors. The zebra mussel in Europe. Leiden: Backhuys Publishers. p. 239–249.
- Morton B. 1993. The anatomy of Dreissena polymorpha and the evolution and success of the heteromyarian form in Dreissenoidea. In: Nalepa TF, Schloesser DW, editors. Zebra mussels: biology, impacts, and control. Boca Raton (FL): Lewis Publishers. p. 55–77.
- Mukhammedova AF, Aksenov SV, Shapovalov NP. 1989. Black amur in the Tsimlyanskoe Reservoir. In: Collection of Papers of the National Research Institute for the Lake and River industry. Vol. 301. p. 149–158. Moscow, USSR: GosNIORKk Press.
- Muzzall PM, Peebles CR, Thomas MV. 1995. Parasites of the round goby, Neogobius melanostomus, and tubenose goby, Proterorhinus marmoratus (Perciformes: Gobiidae), from the St. Clair River and Lake St. Clair, Michigan. J Helminthol Soc Wash. 62:226–228.
- Naberezhny AI, Krivtsova OT, Valkowskaya OI. 1971. Reserves of feed bioproduction in the Kuchurgansky Liman cooling reservoir of the Moldavian electric water-power station and their use by mass fish species. In: Biological resources of the Moldavian waterbodies. Vol. 9. Kishinev, Moldova: Academy of Sciences of the Republic of Moldova Press. p. 88–97.
- Nack CC, Limburg KE, Schmidt RE. 2015. Diet composition and feeding behavior of larval American Shad, Alosa sapidissima (Wilson), after the introduction of the invasive zebra mussel, Dreissena polymorpha (Pallas), in the Hudson River Estuary, NY. Northeast Nat. 22(2):437–450. doi: 10.1656/045.022.0216.
- Naddafi R, Eklov P, Pettersson K. 2007. Non-lethal predator effects on the feeding rate and prey selection of the exotic zebra mussel Dreissena polymorpha. Oikos. 116(8):1289–1298. doi: 10.1111/j.0030-1299.2007.15695.x.
- Naddafi R, Pettersson K, Eklov P. 2010. Predation and physical environment structure the density and population size structure of zebra mussels. J N Am Benthol Soc. 29(2):444–453. doi: 10.1899/09-071.1.
- Naddafi R, Rudstam LG. 2013. Predator-induced behavioural defences in two competitive invasive species: the zebra mussel and the quagga mussel. Anim Behav. 86(6):1275–1284. doi: 10.1016/j.anbehav.2013.09.032.
- Naddafi R, Rudstam LG. 2014a. Predator-induced morphological defences in two invasive dreissenid mussels: implications for species replacement. Freshw Biol. 59(4):703–713. doi: 10.1111/fwb.12297.
- Naddafi R, Rudstam LG. 2014b. Does differential predation explain the replacement of zebra by quagga mussels? Freshw Sci. 33(3):895–903. doi: 10.1086/676658.
- Naddafi R, Rudstam LG. 2014c. Predation on invasive zebra mussel, Dreissena polymorpha, by pumpkinseed sunfish, rusty crayfish, and round goby. Hydrobiologia. 721(1):107–115. doi: 10.1007/s10750-013-1653-z.
- Nagelkerke LAJ, Sibbing FA. 1996. Efficiency of feeding on zebra mussel (Dreissena polymorpha) by common bream (Abramis brama), white bream (Blicca bjoerkna), and roach (Rutilus rutilus): the effects of morphology and behavior. Can J Fish Aquat Sci. 53(12):2847–2861. doi: 10.1139/f96-229.
- Nagibina LF, Timofeeva TA. 1971. True hosts of Aspidogaster limacoides Diesing, 1834 (Trematoda, Aspidogastrea). Dokl Biol Sci. 200:677–678.
- Nakano D, Strayer DL. 2014. Biofouling animals in fresh water: biology, impacts, and ecosystem engineering. Front Ecol Environ. 12(3):167–175. doi: 10.1890/130071.
- Nakaoka M. 2000. Nonlethal effects of predators on prey populations: predator-mediated change in bivalve growth. Ecology. 81(4):1031–1045. doi: 10.1890/0012-9658(2000)081[1031:NEOPOP.2.0.CO;2]
- Nalepa TF. 2010. An overview of the spread, distribution, and ecological impacts of the Quagga Mussel, Dreissena rostriformis Bugensis, with possible implications to the Colorado river system. Proceedings, Colorado river basin science and resource management symposium. Vol. 412. Publications, Agencies and Staf of the U.S. Department of Commerce. p. 121. https://digit alcommons.unl.edu/usdeptcommercepub/412.
- Nalepa TF, Burlakova LE, Elgin AK, Karatayev AY, Lang GA, Mehler K. 2020. NOAA Tech memo GLERL-175. Abundance and biomass of benthic macroinvertebrates in Lake Michigan in 2015, with a summary of temporal trends.Ann Arbor (MI): Tech. Memo, NOAA Great Lakes Environmental Research Laboratory, NOAA Great Lakes Environmental Research Laboratory. doi: 10.25923/g0d3-3v41.
- Nalepa TF, Fanslow DL, Lang GA. 2009a. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to the invasive mussel Dreissena rostriformis bugensis. Freshwater Biol. 54(3):466–479. doi: 10.1111/j.1365-2427.2008.02123.x.
- Nalepa TF, Fanslow DL, Pothoven SA. 2010. Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. J Great Lakes Res. 36:5–19. doi: 10.1016/j.jglr.2010.03.013.
- Nalepa TF, Pothoven SA, Fanslow DL. 2009b. Recent changes in benthic macroinvertebrate populations in Lake Huron and impact on the diet of lake whitefish (Coregonus clupeaformis). Aquat Ecosyst Health Manag. 12(1):2–10. doi: 10.1080/14634980802715175.
- Nalepa TF, Schloesser DW, editors. 2013. Quagga and zebra mussels: biology, impacts, and control. 2nd ed. Boca Raton (FL): CRC Press.
- Nebolsina TK, Ermolin VP, Zakora LP, Mosiyash SS. 1991. Significance of a complex program for rationalization of industrial fishery at the Volgogradskoe water storage. In: Abstracts of the Sixth Congress of the All-Union Hydrobiological Society. Murmansk: Polyarnaya Pravda Press. p. 58–59.
- Nienhuis S, Haxton TJ, Dunkley TC. 2014. An empirical analysis of the consequences of zebra mussel invasions on fisheries in inland, freshwater lakes in Southern Ontario. Manag Biol Invasions. 5(3):287–302. doi: 10.3391/mbi.2014.5.3.12.
- Nikitenko EV, Shcherbina GK. 2016. Feeding of benthophagous fish in the Chogray Reservoir. J Ichthyol. 56(3):383–389. doi: 10.1134/S0032945216030115.
- Noordhuis R, van Zuidam BG, Peeters ETHM, van Geest GJ. 2016. Further improvements in water quality of the Dutch Border lakes: two types of clear states at different nutrient levels. Aquat Ecol. 50(3):521–539. doi: 10.1007/s10452-015-9521-8.
- Nyström P. 2002. Ecology. In: Holdich DM, editor. Biology of freshwater crayfish. Oxford: Blackwell Science. p. 192–235.
- Okgerman HC, Yardimci CH, Dorak Z, Yilmaz N. 2013. Feeding ecology of vimba (Vimba vimba L. 1758) in terms of size groups and seasons in Lake Sapanca, Northwestern Anatolia. Turk J Zool. 37:288–297.
- Olney PJS. 2008. The food and feeding habits of tufted duck Aythya fuligula. Ibis. 105(1):55–62. doi: 10.1111/j.1474-919X.1963.tb02474.x.
- Olszewski Z. 1978. Reconstruction of the size of mollusc shells in studies on the food of fish. Bull Acad Pol Sci Ser Sci Biol. 26:87–91.
- O’Malley BP, Bunnell DB. 2014. Diet of Mysis diluviana reveals seasonal patterns of omnivory and consumption of invasive species in offshore Lake Michigan. J Plankton Res. 36(4):989–1002. doi: 10.1093/plankt/fbu038.
- Ondračková M, Hudcová I, Dávidová M, Adámek Z, Kašný M, Jurajda P. 2015. Non-native gobies facilitate the transmission of Bucephalus polymorphus (Trematoda). Parasit Vectors. 8:382.
- O’Neill CRJr. 2008. The silent invasion: finding solutions to minimize the impacts of invasive quagga mussels on water rates, water infrastructure and the environment. Washington (DC): Hearing of the U.S. House of Representatives Committee on Natural Resources – Subcommittee on Water and Power; [accessed 2021 Dec 20]. http://naturalresources.house.gov/uploadedfiles/ oneilltestimony06.24.08.pdf.
- Orlova MI, Muirhead JR, Antonov PI, Shcherbina GK, Starobogatov YI, Biochino GI, Therriault TW, MacIsaac HJ. 2004. Range expansion of quagga mussels Dreissena rostriformis bugensis in the Volga River and Caspian Sea basin. Aquat Ecol. 38(4):561–573. doi: 10.1007/s10452-004-0311-y.
- Orlova MI, Therriault TW, Antonov PI, Shcherbina GK. 2005. Invasion ecology of quagga mussels (Dreissena rostriformis bugensis): a review of evolutionary and phylogenetic impacts. Aquat Ecol. 39(4):401–418. doi: 10.1007/s10452-005-9010-6.
- Owen G. 1955. Observations on the stomach and digestive diverticula of the Lamellibranchia. I. The Anisomyaria and Eulamellibranchia. Q J Microsc Sci. 96(36):517–537.
- Owens RW, Dittman DE. 2003. Shifts in the diets of slimy sculpin (Cottus cognatus) and lake whitefish (Coregonus clupeaformis) in Lake Ontario following the collapse of the burrowing amphipod Diporeia. Aquat Ecosys Health Manag. 6(3):311–323. doi: 10.1080/14634980301487.
- Paffen BGP, van den Brink FWB, van der Velde G, bij de Vaate A. 1994. The population explosion of the amphipod Corophium curvispinum in the Dutch lower Rhine. Water Sci Technol. 29(3):53–55. doi: 10.2166/wst.1994.0061.
- Patterson JC, Lindeman PV. 2009. Effects of zebra and quagga mussel (Dreissena spp.) invasion on the feeding habits of Sternotherus odoratus (Stinkpot) on Presque Isle, northwestern Pennsylvania. Northeast Nat. 16(3):365–374. doi: 10.1656/045.016.n305.
- Patterson MWR, Ciborowski JJH, Barton DR. 2005. The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. J Great Lakes Res. 31:223–237. doi: 10.1016/S0380-1330(05)70316-6.
- Pauley GB, Becker CD. 1968. Aspidogaster conchicola in mollusks of the Columbia River system with comments on the host’s pathological response. J Parasitol. 54(5):917–920. doi: 10.2307/3277119.
- Peczalska A. 1961. Research on Coregonus lavaretus lavaretus L. in the Gulf of Bothnia and Szczecin Lagoon in 1952–1958. Pr Morskiego Inst Rybackiego Ser A. 11:287–320.
- Pedroli J-C. 1977. Relation entre les oiseaux aquatiques et Dreissena polymorpha dans le lac de Neuchâtel. Ornithol Beob. 74:86–87.
- Pedroli J-C. 1981a. La phénologie des Fuligules hivernants sur le lac de Neuchâtel. Nos Oiseaux. 36:157–163.
- Pedroli J-C. 1981b. Le régime alimentaire des oiseaux aquatiques hivernants se nourrissant de moules zébrées. Nos Oiseaux. 36:143–150.
- Pennuto CM, Howell ET, Makarewicz JC. 2012. Relationships among round gobies, Dreissena mussels, and benthic algae in the south nearshore of Lake Ontario. J Great Lakes Res. 38:154–160. doi: 10.1016/j.jglr.2012.02.002.
- Perez-Fuentetaja A, Mackintosh SA, Zimmerman LR, Clapsadl MD, Alaee M, Aga DS. 2015. Trophic transfer of flame retardants (PBDEs) in the food web of Lake Erie. Can J Fish Aquat Sci. 72(12):1886–1896. doi: 10.1139/cjfas-2015-0088.
- Peribáñez MA, Elrío ML, Gracia MJ, Fernández de Luco D, Castillo JA, Lucientes J, Cia I. 2006. Phyllodistomum folium (Trematoda: Gorgoderidae) infecting zebra mussels (Dreissena polymorpha) in the Ebro River, Spain. Parasitol Int. 55(2):143–145. doi: 10.1016/j.parint.2005.12.002.
- Peribáñez MA, Ordovas L, Benito J, Benejam L, Gracia MJ, Rodellar C. 2011. Prevalence and sequence comparison of Phyllodistomum folium from zebra mussel and from freshwater fish in the Ebro River. Parasitol Int. 60(1):59–63. doi: 10.1016/j.parint.2010.10.004.
- Perry WL, Lodge DM, Lamberti GA. 1997. Impact of crayfish predation on exotic zebra mussels and native invertebrates in a lake-outlet stream. Can J Fish Aquat Sci. 54(1):120–125. doi: 10.1139/f96-255.
- Perry WL, Lodge DM, Lamberti GA. 2000. Crayfish (Orconectes rusticus) impacts on zebra mussel (Dreissena polymorpha) recruitment, other macroinvertebrates and algal biomass in a lake-outlet stream. Am Midl Nat. 144(2):308–316. doi: 10.1674/0003-0031(2000)144[0308:CORIOZ.2.0.CO;2]
- Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G. 2014. Differentiation of European freshwater bucephalids (Digenea: Bucephalidae) based on karyotypes and DNA sequences. Syst Parasitol. 87(2):199–212. doi: 10.1007/s11230-013-9465-0.
- Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G, Zhokhov AE. 2015. European Phyllodistomum (Digenea, Gorgoderidae) and phylogenetic affinities of Cercaria duplicata based on rDNA and karyotypes. Zool Scr. 44(2):191–202. doi: 10.1111/zsc.12080.
- Petkevičiūtė R, Zhokhov AE, Stunzenas V, Poddubnaya LG, Staneviciute G. 2020. Phyllodistomum kupermani n. sp. from the European perch, Perca fluviatilis L. (Perciformes: Percidae), and redescription of Phyllodistomum macrocotyle (Luhe, 1909) with notes on the species diversity and host specificity in the European Phyllodistomum spp. (Trematoda: Gorgoderidae). Parasit Vectors. 13(1):561.
- Petrie SA, Knapton RW. 1999. Rapid increase and subsequent decline of zebra and quagga mussels in Long Point Bay, Lake Erie: possible influence of waterfowl predation. J Great Lakes Res. 25(4):772–782. doi: 10.1016/S0380-1330(99)70776-8.
- Peyer SM, Hermanson JC, Lee CE. 2010. Developmental plasticity of shell morphology of quagga mussels from shallow and deep-water habitats of the Great Lakes. J Exp Biol. 213(Pt 15):2602–2609. doi: 10.1242/jeb.042549.
- Peyer SM, McCarthy AJ, Lee CE. 2009. Zebra mussels anchor byssal threads faster and tighter than quagga mussels in flow. J Exp Biol. 212:2026–2035.
- Pichlová-Ptáčníková R, Vanderploeg HA. 2009. The invasive cladoceran Cercopagis pengoi is a generalist predator capable of feeding on a variety of prey species of different sizes and escape abilities. Fundam Appl Limnol. 173(4):267–279. doi: 10.1127/1863-9135/2009/0173-0267.
- Pieplow U. 1938. Fischereiwissenschaftliche Monographie von Cambarus affinis Say. Z Fisch. 36:349–440.
- Piesik Z. 1974. The role of the crayfish Orconectes limosus (Raf.) in extinction of Dreissena polymorpha (Pall.) subsisting on steelon net. Pol Arch Hydrobiol. 21:401–410.
- Piesik Z. 1983. Biology of Dreissena polymorpha (Pall.) settling of stylon nets and the role of this mollusc in eliminating the seston and the nutrients from the water-course. Pol Arch Hydrobiol. 30:353–361.
- Pietrock M, Mattheis T, Kriiger R, Meinelt T. 1999. Seasonal dynamics of Phyllodistomumfolium (von Olfers, 1816) (Trematoda, Gorgoderidae) in blue bream, Abramis ballerus (L.) and ruffe, Gymnocephalus cernuus (L.) from the Oder River (Germany/Poland). Acta Parasitol. 44(3):165–169.
- Pimentel D. 2005. Aquatic nuisance species in the New York State Canal and Hudson River systems and the Great Lakes Basin: an economic and environmental assessment. Environ Manage. 35(5):692–702. doi: 10.1007/s00267-004-0214-7.
- Pirozhnikov PL. 1971. The bioproductive effect of large rivers damming and its fishery significance. In: Volga-I. Problems of studying and rational use of biological resources of water bodies: materials of the first conference on studying water bodies of the Volga Basin. Kuibyshev: Kuibyshev Publishing House. p. 193–208.
- Platvoet D, van der Velde G, Dick JTA, Li S. 2009. Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda) – Amphipod Pilot Species Project (AMPIS) Report 5. Crustaceana. 82(6):703–720. doi: 10.1163/156854009X423201.
- Pliszka FR. 1953. The dynamics of feeding relations in Lake Harsz. Pol Arch Hydrobiol. 1:271–300.
- Poddubny AG. 1966. A note on an adaptive response of a roach population to a change in environmental conditions. Tr Inst Biol Vnutr Vod Akad Nauk SSSR. 10:131–138.
- Pollux B, Minchin D, Van Der Velde G, Van Alen T, Moon-Van Der Staay S, Hackstein J. 2003. Zebra mussels (Dreissena polymorpha) in Ireland, AFLP-fingerprinting and boat traffic both indicate an origin from Britain. Freshw Biol. 48(6):1127–1139. doi: 10.1046/j.1365-2427.2003.01063.x.
- Ponder WF, Lindberg DR, Ponder JM. 2019. Biology and evolution of the Mollusca. Vol. 1. Boca Raton (FL): CRC Press.
- Popova LB, Biochino GI. 2001. То the occurrence and parasite fauna of Dreissena bugensis in the Rybinsk reservoir. Parasitology. 35(4):356–359.
- Pothoven SA, Fahnenstiel GL. 2013. Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. J Great Lakes Res. 39(2):287–294. doi: 10.1016/j.jglr.2013.02.005.
- Pothoven SA, Madenjian CP. 2013. Increased piscivory by lake whitefish in Lake Huron. N Am J Fish Manag. 33(6):1194–1202. doi: 10.1080/02755947.2013.839973.
- Pothoven SA, Madenjian CP, Hook TO. 2017. Feeding ecology of the walleye (Percidae, Sander vitreus), a resurgent piscivore in Lake Huron (Laurentian Great Lakes) after shifts in the prey community. Ecol Freshw Fish. 26(4):676–685. doi: 10.1111/eff.12315.
- Pothoven SA, Nalepa TF. 2006. Feeding ecology of lake whitefish in Lake Huron. J Great Lakes Res. 32(3):489–501. doi: 10.3394/0380-1330(2006)32[489:FEOLWI.2.0.CO;2]
- Pothoven SA, Nalepa TF, Schneeberger P, Brandt S. 2001. Changes in diet and body condition of lake whitefish in southern Lake Michigan associated with changes in benthos. N Am J Fish Manag. 21(4):876–883. doi: 10.1577/1548-8675(2001)021<0876:CIDABC>2.0.CO;2.
- Poulton BC, Kroboth PT, George AE, Chapman DC, Bailey J, McMurray SE, Faiman JS. 2019. First examination of diet items consumed by wild-caught black carp (Mylopharyngodon piceus) in the U.S. Am Midl Nat. 182(1):89–108. doi: 10.1674/0003-0031-182.1.89.
- Prejs A. 1976. Fishes and their feeding habits. In: Pieczyenska E, editor. Selected problems of lake littoral ecology. Warsaw: University of Warsaw Press. p. 155–171.
- Prejs A, Lewandowski K, Stańczykowska-Piotrowska A. 1990. Size-selective predation by roach (Rutilus rutilus) on zebra mussel (Dreissena polymorpha): field studies. Oecologia. 83(3):378–384. doi: 10.1007/BF00317563.
- Pryanichnikova EG. 2012. Structure and function of dreissenid populations in Rybinsk Reservoir. Abstract of the candidate dissertation. Russia: Papanin Institute for Biology of Inland Waters. Russian Academy of Sciences. p. 1–20.
- Pryanichnikova EG, Tyutin AV, Shcherbina GKh. 2011. Comparative analysis of the structure and fauna of endosymbionts of communities of two dreissenid species (Mollusca, Dreissenidae) in the Upper Volga Reservoirs. Inland Water Biol. 4(2):203–210. doi: 10.1134/S1995082911020179.
- Raabe Z. 1934. Weitere Untersuchungen an einigen Arten des Genus Conchophthirus Stein. Mem Acad Pol Sci Lett Ser B Sci Nat. 1934:221–235.
- Raabe Z. 1947. Recherches sur les cilies Thigmotriches (Thigmotricha Ch. Lw.) II. Espece nouvelle d’eau douce d u genre Ancistrina Cheissin. Ann Univ M Curie-Sklod Sectio C Lublin Polonia. 2(3):111–120.
- Raabe Z. 1950. Recherches sur les ciliés Thigmotriches (Thigmotricha Ch. Lw.). V. Ciliés Thigmotriches du lac Balaton (Hongrie). Ann Univ Mariae Curie-Sklodowska Sect C Biol. 5:197–215.
- Raabe Z. 1956. Investigations on the parasitofauna of freshwater molluscs in the brackish waters. Acta Parasitol Pol. 4:375–406.
- Raabe Z. 1959. Recherches sur les cilies Thigmotriches (Thigmotricha Ch. Lw.). VI. Sur les genres “Ancistruma”, “Ancistrina” et les genres voisins [Research on the thigmotrich ciliates (Thigmotricha Ch. Lw.). VI. On the genera “Ancistruma”, “Ancistrina” and related genera]. Acta Parasitol Pol. 7:215–247.
- Raabe Z. 1965. The parasitic ciliates of Gastropods in the Ohrid Lake. Acta Protozool. 3:311–320.
- Raabe Z. 1966. The parasitic ciliates of Dreissena polymorpha and other Bivalvia in the Ohrid Lake. Acta Protozool. 4:1–14.
- Raabe Z. 1970. Ordo Thigmotricha (Ciliata-Holotricha). III. Familiae Ancistrocomidae et Sphenophryidae. Acta Protozool. 7:385–463.
- Raabe Z. 1971. Ordo Thigmotricha (Ciliata-Holotricha). IV. Familia Thigmophryidae. Acta Protozool. 9:121–170.
- Rakauskas V, Bacevičius E, Pūtys Ž, Ložys L, Arbačiauskas K. 2008. Expansion, feeding and parasites of the round goby, Neogobius melanostomus (Pallas, 1811), a recent invader in the Curonian Lagoon. Acta Zool Litu. 18(3):180–190. doi: 10.2478/v10043-008-0030-z.
- Rask M. 1989. A note on the diet of roach, Rutilus rutilus L., and other cyprinids at Tvärminne, northern Baltic Sea. Aqua Fenn. 19:19–27.
- Ray WJ, Corkum LD. 1997. Predation of zebra mussels by round gobies, Neogobius melanostomus. Environ Biol Fishes. 50(3):267–273. doi: 10.1023/A:1007379220052.
- Reichholf J. 1985. Wandermuscheln Dreissena polymorpha (Pallas) als Zusatznahrung der Bisamratte Ondatra zibethicus L. Saeugetierkd Mitt. 32:83–84.
- Reid NJ, Holovachov O, Anderson MA. 2012. Nematodes associated with the invasive quagga mussel (Dreissena rostriformis bugensis) in the Colorado River Aqueduct reservoirs, southern California, USA. Nematology. 14(7):827–837. doi: 10.1163/156854112X627345.
- Reimer O, Tedengren M. 1997. Predator-induced changes in byssal attachment, aggregation and migration in the blue mussel, Mytilus edulis. Mar Freshw Behav Physiol. 30(4):251–266. doi: 10.1080/10236249709379029.
- Rennie MD, Johnson YB, Sprules WG. 2012. Energy acquisition and allocation patterns of lake whitefish (Coregonus clupeaformis) are modified when dreissenids are present. Can J Fish Aquat Sci. 69(1):41–59. doi: 10.1139/f2011-126.
- Rennie MD, Sprules WG, Johnson TB. 2009. Factors affecting the growth and condition of lake whitefish (Coregonus clupeaformis). Can J Fish Aquat Sci. 66(12):2096–2108. doi: 10.1139/F09-139.
- Reynolds JD, Donohoe R. 2001. Crayfish predation experiments on the introduced zebra mussel, Dreissena polymorpha, in Ireland, and their potential for biocontrol. Bull Fr Pêche Piscic. 361(361):669–681. doi: 10.1051/kmae:2001012.
- Rezsu E, Specziár A. 2006. Ontogenetic diet profiles and size-dependent diet partitioning of ruffe Gymnocephalus cernuus, perch Perca fluviatilis and pumpkinseed Lepomis gibbosus in Lake Balaton. Ecol Freshwater Fish. 15(3):339–349. doi: 10.1111/j.1600-0633.2006.00172.x.
- Ricciardi A. 1994. Occurrence of chironomid larvae (Paratanytarsus sp.) as commensals of dreissenid mussels (Dreissena polymorpha and D. bugensis). Can J Zool. 72(6):1159–1162. doi: 10.1139/z94-155.
- Ricciardi A, Snyder FL, Kelch DO, Reiswig HM. 1995. Lethal and sublethal effects of sponge overgrowth on introduced dreissenid mussels in the Great Lakes-St. Lawrence River System. Can J Fish Aquat Sci. 52(12):2695–2703. doi: 10.1139/f95-858.
- Ritterbusch-Nauwerck B. 1991. The coincidence between the shape of the pharyngeal bones of Vimba elongata (Valenciennes) (Pisces, Cyprinidae) and of its prey Dreissena polymorpha (Pallas) (Bivalva, Dreissenidae). J Fish Biol. 38(2):325–326. doi: 10.1111/j.1095-8649.1991.tb03121.x.
- Robins CR, Bailey RM, Bond CE, Brooker JR, Lachner EA, Lea RN, Scott WB. 1991a. Common and scientific names of fishes from the United States and Canada (Fifth Ed). Am Fish Soc Spec Publ. 20:1–183.
- Robins CR, Bailey RM, Bond CE, Brooker JR, Lachner EA, Lea RN, Scott WB. 1991b. World fishes important to North Americans exclusive of species from the continental waters of the United States and Canada. Am Fish Soc Spec Publ. 21:1–243.
- Rohde K. 1994. The minor groups of parasitic Platyhelminthes. Adv Parasitol. 33:145–234.
- Roitman VA, Voejkov JA, Spirin SL. 1981. The finding of Aspidogaster limacoides (Diesing, 1834) (Aspidogastrea) in fishes from the Rybinsk Water Reservoir. Parazitologiya. 15:332–337.
- Roscoe DE, Huffman JE. 1982. Trematode (Sphaeridiotrema globulus)-induced ulcerative hemorrhagic enteritis in wild mute swans (Cygnus olor). Avian Dis. 26(1):214–224. doi: 10.2307/1590046.
- Rowe MD, Obenour DR, Nalepa TF, Vanderploeg HA, Yousef F, Kerfoot WC. 2015. Mapping the spatial distribution of the biomass and filter-feeding effect of invasive dreissenid mussels on the winter-spring phytoplankton bloom in Lake Michigan. Freshw Biol. 60(11):2270–2285. doi: 10.1111/fwb.12653.
- Rudstam LG, Gandino CJ. 2020. Zebra or quagga mussel dominance depends on trade-offs between growth and defense-Field support from Onondaga Lake, NY. PLOS One. 15(6):e0235387. doi: 10.1371/journal.pone.0235387.
- Ruetz CR, Reneski MR, Uzarski DG. 2012. Round goby predation on Dreissena in coastal areas of eastern Lake Michigan. J Freshw Ecol. 27(2):171–184. doi: 10.1080/02705060.2011.644702.
- Rybczyk A, Czerniejewski P, Keszka S, Janowicz M, Brysiewicz A, Wawrzyniak W. 2020. First data of age, condition, growth rate and diet of invasive Neogobius melanostomus (Pallas, 1814) in the Pomeranian Bay, Poland. J Water Land Dev. 47(3):142–149.
- Saijuntha W, Sithithaworn P, Duenngai K, Kiatsopit N, Andrews RH, Petney TN. 2011a. Genetic variation and relationships of four species of medically important echinostomes (Trematoda: Echinostomatidae) in South-East Asia. Infect Genet Evol. 11(2):375–381. doi: 10.1016/j.meegid.2010.11.009.
- Saijuntha W, Tantrawatpan C, Sithithaworn P, Andrews RH, Petney TN. 2011b. Genetic characterization of Echinostoma revolutum and Echinoparyphium recurvatum (Trematoda: Echinostomatidae) in Thailand and phylogenetic relationships with other isolates inferred by ITS1 sequence. Parasitol Res. 108(3):751–755. doi: 10.1007/s00436-010-2180-8.
- Schaeffer JS, Bowen A, Thomas M, French IIJ, Curtis GL. 2005. Invasion history, proliferation, and offshore diet of the round goby Neogobius melanostomus in western Lake Huron, USA. J Great Lakes Res. 31(4):414–425. doi: 10.1016/S0380-1330(05)70273-2.
- Schernewski G, Friedland R, Buer A-L, Dahlke S, Drews B, Höft S, Klumpe T, Schadach M, Schumacher J, Zaiko A. 2019. Ecological-social-economic assessment of zebra-mussel cultivation scenarios for the Oder (Szczecin) Lagoon. J Coast Conserv. 23(5):913–929. doi: 10.1007/s11852-018-0649-2.
- Schreiber S, Odelstrom T, Pettersson K, Eichelberg D. 1998. The zebra mussel Dreissena polymorpha as a food source for the signal crayfish Pacifastacus leniusculus in Lake Erken – laboratory experiments. Arch Hydrobiol Spec Issue Advanc Limnol. 51:169–176.
- Schummer ML, Badzinski SS, Petrie SA, Chen Y-W, Belzile N. 2010. Selenium accumulation in sea ducks wintering at Lake Ontario. Arch Environ Contam Toxicol. 58(3):854–862. doi: 10.1007/s00244-009-9370-3.
- Schwab A, Bornhauser-Sieber U, Keller V. 2001. Wintering waterbirds in the Lucerne part of Vierwaldstättersee (Switzerland) 1954/55-2000/01. Ornithol Beob. 98:179–208.
- Sebestyén O. 1938. Colonization of two new fauna-elements of Pontus-origin (Dreissena polymorpha Pall. and Corophium curvispinum G. O. Sars forma devium Wundsch) in Lake Balaton. Verh Int Ver Limnol. 8(3):169–182. doi: 10.1080/03680770.1937.11898641.
- Serrouya R, Ricciardi A, Whoriskey FG. 1995. Predation on zebra mussels (Dreissena polymorpha) by captive-reared map turtles (Graptemys geographica). Can J Zool. 73(12):2238–2243. doi: 10.1139/z95-265.
- Severenchuk NS, Kaftannikova OG. 1983. Usage of feed resources by benthophagous fish of the Kanewskoe water storage. Gidrobiol Zh. 19:26–30.
- Shcherbina GK, Buckler DR. 2006. Distribution and ecology of Dreissena polymorpha (Pallas) and Dreissena bugensis (Andrusov) in the Upper Volga basin. J Astm Int. 3(4):13256. doi: 10.1520/JAI13256.
- Shemonaev EV, Kirilenko EV. 2009. Some features of biology of the round goby Neogobius melanostomus (Perciformes, Gobiidae) in waters of Kuibyshev Reservoir. J Ichthyol. 49(6):454–459. doi: 10.1134/S0032945209060046.
- Sherstyuk VV, Severenchuk NS. 1989. Invertebrates as fodder objects for fish. In: Invertebrates and fish from Dnepr and its reservoirs. Kiev: Naukova Dumka. p. 117–135.
- Shevtsova LV, Zhdanova GA, Movchan VA, Primak AB. 1986. Experimental interrelationship between Dreissena and planktic invertebrates. Hydrobiol J. 22:36–39.
- Shields RC, Beckman DW. 2015. Assessment of variation in age, growth, and prey of freshwater drum (Aplodinotus grunniens) In the Lower Missouri River. Southwest Nat. 60(4):360–365. doi: 10.1894/0038-4909-60.4.360.
- Sickel JB, Lyles MB. 1981. Chaetogaster limnaei (Oligochaeta: Naididae) inhabiting the mantle cavity of the Asiatic clam Corbicula fluminea. Vol. 23. Barkley Lake (KY): Veliger. p. 361–362.
- Sietman BE, Dunn HL, Tucker JK, Kelner DE. 2003. Muskrat (Ondatra zibethicus) predation on zebra mussels (Dreissena polymorpha) attached to unionid bivalves. J Freshw Ecol. 18(1):25–32. doi: 10.1080/02705060.2003.9663948.
- Simberloff D, Gibbons L. 2004. Now you see them, now you don’t! – population crashes of established introduced species. Biol Invasions. 6(2):161–172. doi: 10.1023/B:BINV.0000022133.49752.46.
- Smee DL, Weissburg MJ. 2006. Hard clams (Mercenaria mercenaria) evaluate predation risk using chemical signals from predators and injured conspecifics. J Chem Ecol. 32(3):605–619. doi: 10.1007/s10886-005-9021-8.
- Smirnov AI. 1986. Fauna Ukrainy, 8. Ryby 5. Kijev: Naukova Dumka. p. 112–114.
- Smirnova VA, Ibrasheva SI. 1967. Larval trematodes from freshwater molluscs in the western Kazakhstan. Tr Inst Zool Akad Nauk Kaz USSR. 27:53–87.
- Smit H, bij de Vaate A, Reeders HH, van Nes EH, Noordhuis R. 1993. Colonization, ecology, and positive aspects of zebra mussels (Dreissena polymorpha) in The Netherlands. In: Nalepa TF, Schloesser DW, editors. Zebra mussels: biology, impacts, and control. Boca Raton (FL): Lewis Publishers. p. 55–77.
- Spanowskaya VD. 1963. Ichthyofauna of the Uchinskoe water storage and its characteristics. In: Uchinskoe and Mozhaiskoe reservoirs: hydrobiological and ichthyological studies. Moscow: Moscow University. p. 269–310.
- Sparks AK. 1985. Synopsis of invertebrate pathology exclusive of insects. Amsterdam: Elsevier Science Publishers B. V.
- Spataru P. 1967. Nutrition and some trophic relationships in Lepomis gibbosus (Linnaeus) 1758 in the Crapina-Jijila Pond complex, Danube flood area. An Univ Bucuresti Ser Stiint Nat Chim. 16:151–159.
- Specziár A, Tolg L, Biro P. 1997. Feeding strategy and growth of cyprinids in the littoral zone of Lake Balaton. J Fish Biol. 51(6):1109–1124. doi: 10.1111/j.1095-8649.1997.tb01130.x.
- Stanczykowska A. 1977. Ecology of Dreissena polymorpha (Pall.) (Bivalvia) in lakes. Pol Arch Hydrobiol. 24:461–530.
- Stanczykowska A. 1987. The place of mussel Dreissena polymorpha (Pall.) in the food web of lake ecosystems. Haliotis. 16:129–135.
- Stanczykowska A, Schenker HJ, Fafara Z. 1975. Comparative characteristics of populations of Dreissena polymorpha in 1962 and 1972 in 13 Mazurian Lakes, Poland. Bull Acad Pol Sci Ser Sci Biol. 23:383–390.
- Stańczykowska A, Zyska P, Dombrowski A, Kot H, Zyska E. 1990. The distribution of waterfowl in relation to mollusc populations in the man-made Lake Zegrzynskie. Hydrobiologia. 191(1):233–240. doi: 10.1007/BF00026056.
- Stein RA, Kitchell JF, Knezzevic B. 1975. Selective predation by carp (Cyprinus carpio L.) on benthic molluscs in Skadar Lake, Yugoslavia. J Fish Biol. 7(3):391–399. doi: 10.1111/j.1095-8649.1975.tb04613.x.
- Stempniewicz L. 1974. The effect of feeding of the coot (Fulica atra L.) on the character of the shoals of Dreissena polymorpha Pall. in the Lake Goplo. Acta Univ Nicolai Copernici Ser Mat Przyr. 34:83–103.
- Stewart TW, Miner JG, Lowe RL. 1998. An experimental analysis of crayfish (Orconectes rusticus) effects on a Dreissena-dominated benthic macroinvertebrate community in western Lake Erie. Can J Fish Aquat Sci. 55(4):1043–1050. doi: 10.1139/f98-022.
- Stoeckmann A. 2003. Physiological energetics of Lake Erie dreissenid mussels: a basis for the displacement of Dreissena polymorpha by Dreissena bugensis. Can J Fish Aquat Sci. 60(2):126–134. doi: 10.1139/f03-005.
- Strayer DL, Adamovich BV, Adrian R, Aldridge DC, Balogh C, Burlakova LE, Fried-Petersen HG, Tóth L, Hetherington AL, Jones TS, et al. 2019. Long-term population dynamics of dreissenid mussels (Dreissena polymorpha and D. rostriformis): a cross-system analysis. Ecosphere. 10(4):e02701. doi: 10.1002/ecs2.2701.
- Strayer DL, D’Antonio CM, Essl F, Fowler MS, Geist J, Hilt S, Jarić I, Jöhnk K, Jones CG, Lambin X, et al. 2017. Boom-bust dynamics in biological invasions: towards an improved application of the concept. Ecol Lett. 20(10):1337–1350. doi: 10.1111/ele.12822.
- Strayer DL, Hattala KA, Kahnle AW. 2004. Efects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Can J Fish Aquat Sci. 61(6):924–941. doi: 10.1139/f04-043.
- Strayer DL, Malcom HM. 2006. Long-term demography of a zebra mussel (Dreissena polymorpha) population. Freshw Biol. 51(1):117–130. doi: 10.1111/j.1365-2427.2005.01482.x.
- Strayer DL, Powell J, Ambrose P, Smith LC, Pace ML, Fischer DT. 1996. Arrival, spread, and early dynamics of a zebra mussel (Dreissena polymorpha) population in the Hudson River estuary. Can J Fish Aquat Sci. 53(5):1143–1149. doi: 10.1139/f96-038.
- Stunžėnas V, Cryan JR, Molloy DP. 2004. Comparison of rDNA sequences from colchicine treated and untreated sporocysts of Phylodistomum folium and Bucephalus polymorphus (Digenea). Parasitol Int. 53(3):223–228. doi: 10.1016/j.parint.2003.12.003.
- Suter W. 1982a. Vergleichende Nahrungsökologie von überwinternden Tauchenten Bucephala, Aythya und Bläßhuhn Fulica atra am Untersee-Ende/Hochrhein (Bodensee). Ornithol Beob. 79:225–254.
- Suter W. 1982b. Der Einfluss von Wasservögeln auf Populationen der Wandermuschel (Dreissena polymorpha Pall.) am Untersee/Hochrhein (Bodensee). Schweiz Z Hydrol. 44(1):149–161. doi: 10.1007/BF02502194.
- Suter W. 1982c. Die Bedeutung von Untersee-Ende/Hochrhein (Bodensee) als wichtiges Überwinterungsgewässer für Tauchenten Aythya, Bucephala und Bläßhuhn Fulica atra. Ornithol Beob. 79:73–96.
- Suter W. 1994. Overwintering waterfowl on Swiss lakes: how are abundance and species richness influenced by trophic status and lake morphology? Hydrobiologia. 279–280(1):1–14. doi: 10.1007/BF00027836.
- Suter W, Schifferli L. 1988. Uberwinternde Wasservogel in der Schweiz und ihren Grenzgebieten: 1967-1987 im internationalen Vergleich [Waterfowl wintering in Switzerland and on adjacent waters: numbers and trends 1967-1987 in a European context]. Ornithol Beob. 85:261–298.
- Suter W, van Eerden MR. 1992. Simultaneous mass starvation of wintering diving ducks in Switzerland and the Netherlands: a wrong decision in the right strategy? Ardea. 80:229–242.
- Szlauer L. 1974. Use of steelon net veils for protection of the hydro-engineering works against Dreissena polymorpha Pall. Pol Arch Hydrobiol. 21:391–400.
- Taskinen J, Urbańska M, Ercoli F, Andrzejewski W, Ożgo M, Deng B, Choo JM, Riccardi N. 2021. Parasites in sympatric populations of native and invasive freshwater bivalves. Hydrobiologia 848(12–13):3167–3178. doi: 10.1007/s10750-020-04284-0.
- Taskinen J, Valtonen ET, Makela T. 1994. Quantity of sporocysts and seasonality of two Rhipidocotyle species (Digenea: Bucephalidae) in Anodonta piscinalis (Mollusca: Bivalvia). Int J Parasitol. 24(6):877–886. doi: 10.1016/0020-7519(94)90014-0.
- Thayer SA, Haas RC, Hunter RD, Kushler RH. 1997. Zebra mussel (Dreissena polymorpha) effects on sediment, other zoobenthos, and the diet and growth of adult yellow perch (Perca flavescens) in pond enclosures. Canadian Journal of Fisheries and Aquatic Sciences. 54:1903–1915.
- Thompson D. 1973. Feeding ecology of diving ducks on Keokuk Pool, Mississippi River. J Wildl Manage. 37(3):367–381. doi: 10.2307/3800128.
- Thorp JH, Covich AP, editors. 1991. Ecology and classification of North American freshwater invertebrates. San Diego (CA): Academic Press.
- Thorp JH, Delong MD, Casper AF. 1998. In situ experiments on predatory regulation of a bivalve mollusc (Dreissena polymorpha) in the Mississippi and Ohio Rivers. Freshw Biol. 39(4):649–661. doi: 10.1046/j.1365-2427.1998.00313.x.
- Tkach VV, Kudlai O, Kostadinova A. 2016. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int J Parasitol. 46(3):171–185. doi: 10.1016/j.ijpara.2015.11.001.
- Toews S, Beverley-Burton M, Lawrimore T. 1993. Helminth and protist parasites of zebra mussels, Dreissena polymorpha (Pallas, 1771), in the Great Lakes region of southwestern Ontario, with comments on associated bacteria. Can J Zool. 71(9):1763–1766. doi: 10.1139/z93-250.
- Toomey MB, McCabe D, Marsden JE. 2002. Factors affecting the movement of adult zebra mussels (Dreissena polymorpha). J N Am Benthol Soc. 21(3):468–475. doi: 10.2307/1468483.
- Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM. 2003. Introduced species and their missing parasites. Nature. 421(6923):628–630. doi: 10.1038/nature01346.
- Travina OV, Bespalaya YV, Aksenova OV, Shevchenko AR, Sokolova SE. 2019. Infection of Dreissena polymorpha (Bivalvia: Dreissenidae) with Phyllodistomum macrocotyle (Digenea: Gorgoderidae) in the Northern Dvina River Basin, Northern Russia. Biharean Biol. 13 (1):49–51.
- Travina OV, Bespalaya YV, Kondakov AV, Aksenova OV, Khrebtova IS, Kropotin AV. 2021. Molecular data on Phyllodistomum macrocotyle (Digenea: Gorgoderidae) from an intermediate host Dreissena polymorpha (Bivalvia: Dreissenidae) in the Northern Dvina River Basin, Northwest Russia. Ecol Monten. 39:69–75. doi: 10.37828/em.2021.39.7.
- Tucker JK, Cronin FA, Soergel DW, Theiling CH. 1996. Predation of zebra mussels Dreissena polymorpha by common carp Cyprinus carpio. J Freshw Ecol. 11(3):363–372. doi: 10.1080/02705060.1996.9664459.
- Turschak BA, Bootsma HS. 2015. Lake Michigan trophic structure as revealed by stable C and N isotopes. J Great Lakes Res. 41(Suppl. 3, Sp. Iss. SI):185–196. doi: 10.1016/j.jglr.2015.04.004.
- Tyutin AV, Medyantseva EN, Pryanichnikova EG. 2013a. Parasites and endosymbionts of mollusks of the genus Dreissena in the Upper Volga basin. p. 113–118. https://www.researchgate.net/publication/274579857.
- Tyutin AV, Shcherbina GH, Medyantseva EN. 2005. Long-term dynamics of infection with Dreissena polymorpha (Bivalvia, Dreissenidae) by trematode parthenites in Upper Volga Reservoirs. In: Rivier IK, Shcherbina GH, Krylov AV, editors. Biological resources of fresh water: invertebrates. Rybinsk: Rybinsk Printing House. p. 374–384.
- Tyutin AV, Scherbina GK. 2006. Parasites of molluscs of the genus Dreissena in reservoirs of the Volga River. Proceedings of the 5th Belarusian Republican Scientific-Practical Conference “Achievements and perspectives of development of the modern parasitology”; Vitebsk, Belarus. p. 344–348.
- Tyutin AV, Verbitsky VB, Verbitskaya TI, Medyantseva EN. 2013b. Parasites of alien aquatic animals in the Upper Volga basin. Russ J Biol Invasions. 4(1):54–59. doi: 10.1134/S2075111713010098.
- van den Brink FWB, van der Velde G, bij de Vaate A. 1993. Ecological aspects, explosive range extension and impact of a mass invader, Corophium curvispinum Sars, 1895 (Crustacea: Amphipoda), in the lower Rhine (The Netherlands). Oecologia. 93(2):224–232. doi: 10.1007/BF00317675.
- van der Velde G, Paffen BGP, van den Brink FWB, bij de Vaate A, Jenner HA. 1994. Decline of zebra mussel populations in the Rhine: comparison between two mass invaders (Dreissena polymorpha and Corophium curvispinum). Naturwissenschaften. 81(1):32–34. doi: 10.1007/BF01138559.
- van der Velde G, Rajagopal S, bij de Vaate A. editors. 2010. The zebra mussel in Europe. Leiden: Backhuys Publishers.
- van Eerden M, de Leeuw J. 2010. How Dreissena sets the winter scene for water birds: dynamic interactions between diving ducks and zebra mussels. In: Van Der Velde G, Rajagopal S, bij de Vaate A, editors. The zebra mussel in Europe. Leiden: Backhuys Publishers. p. 251–264.
- van Eerden M, de Leeuw J, Slager BB, de Vaate A. 1997. A field test of the carrying capacity concept in wintering diving ducks: do high foraging costs delimit exploitation of zebra mussels? In: de Leeuw J, editor. Demanding divers: ecological energetics of food exploitation by diving ducks. Vol. 61. Lelystad: Van Zee Tot Land. p. 21–51.
- van Riel MC, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, bij de Vaate A. 2006. Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia. 565(1):39–58. doi: 10.1007/s10750-005-1904-8.
- vom Scheidt A. 1984. Etude de la Bucéphalose a Bucephalus Polymorphus Baer 1827 Dans Les Rivières, Fleuves et Canaux du Nordest de la France [Thése Doctorat Vétérinaire]. Vol. 111. Alfort: Ecole Nationale Vétérinaire D’Alfort.
- von Wicht U. 1972. Erstmals kolonieartiges Brüten des Höckerschwans (Cygnus olor) am Bodensee. Anz Ornithol Ges Bayern. 11:164–167.
- Voronchuck LV, Kudrinskaya OI, Matchinskaya SF. 1983. Feeding base and feed of fry in a zone affected by the Tripolskaya electric water-power station. Gidrobiol Zh. 19:26–30.
- Voskresensky KA. 1973. Trophology and biofilters of water reservoirs. In: Trophology of aquatic animals: results and problems. Moscow: Nauka. p. 361–377.
- Waffle EL. 1963. An ecological study of the Iowa Glossiphoniidae (Annelida: Hirudinea) with emphasis on feeding and reproductive habits [MS thesis]. Ames (IA): Iowa State University.
- Wallet M, Lambert A. 1986. Enquête sur la répartition et l’évolution du parasitisme a Bucephalus polymorphus Baer, 1827 chez le mollusque Dreissena polymorpha dans le sud-est de la France. Bull Fr Peche Piscic. 300:19–24.
- Wallet M, Theron A, Lambert A. 1985. Rythme d’émission des cercaires de Bucephalus polymorphus Baer, 1827 (Trematoda, Bucephalidae) en relation avec l’activité de Dreissena polymorpha (Lamellibranche, Dreissenidae) premier hôte intermédiaire. Ann Parasitol Hum Comp. 60(6):675–684. doi: 10.1051/parasite/1985606675.
- Walsh MG, Dittman DE, O’Gorman R. 2007. Occurrence and food habits of the round goby in the profundal zone of southwestern Lake Ontario. J Great Lakes Res. 33(1):83–92. doi: 10.3394/0380-1330(2007)33[83:OAFHOT.2.0.CO;2]
- Watzin MC, Joppe-Mercure K, Rowder J, Lancaster B, Bronson L. 2008. Significant fish predation on zebra mussels Dreissena polymorpha in Lake Champlain, USA. J Fish Biol. 73(7):1585–1599. doi: 10.1111/j.1095-8649.2008.02033.x.
- Weber MJ, Dettmers JM, Wahl DH, Czesny SJ. 2011. Size preferences and behaviors of native yellow perch foraging on invasive round gobies. J Great Lakes Res. 37(3):584–587. doi: 10.1016/j.jglr.2011.04.008.
- Weegman MD, Weegman MM. 2007. Chromium and selenium in invertebrate prey of lesser scaup. J Wildl Manag. 71(3):778–782. doi: 10.2193/2006-196.
- Werner S, Mortl M, Bauer H-G, Rothhaupt K-O. 2005. Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at Lake Constance, Germany. Freshw Biol. 50(8):1412–1426. doi: 10.1111/j.1365-2427.2005.01411.x.
- Wiktor K. 1958. Larvae of Dreissena polymorpha Pall. as a food for fish spawn. Przegl Zool. 2:182–184.
- Wiktor J. 1969. The biology of Dreissena polymorpha (Pall.) and its ecological importance in the Firth of Szczecin. Stud Mat Morsk Inst Ryb Gdynia Ser A. 5:1–88.
- Wilkońska H, Strelnikova A. 2000. Feeding patterns of some cyprinid fish larvae in Licheńskie and Gosławskie lakes in 1994. Arch Ryb Pol. 8(2):213–224.
- Winkler G, Martineau C, Dodson JJ, Vincent WF, Johnson LE. 2007. Trophic dynamics of two sympatric mysid species in an estuarine transition zone. Mar Ecol Prog Ser. 332:171–187. doi: 10.3354/meps332171.
- Wisniewski WL. 1957. Parasitofauna of Lake Goldapiwo. Wiad Parazytol. 3:261–272.
- Withers JL, Sesterhenn TM, Foley CJ, Troy CD, Höök TO. 2015. Diets and growth potential of early stage larval yellow perch and alewife in a nearshore region of southeastern Lake Michigan. J Great Lakes Res. 41:197–209. doi: 10.1016/j.jglr.2015.08.003.
- Włodarczyk R, Janiszewski T. 2014. Can expansion of zebra mussel, Dreissena polymorpha (Bivalvia) influence the numbers and behaviour of traditionally herbivorous mute swan, Cygnus olor (Aves)? Acta Zool Bulg. 66:235–238.
- Woakes AJ, Butler PJ. 1983. Swimming and diving in tufted ducks, Aythya fuligula, with particular reference to heart rate and gas exchange. J Exp Biol. 107(1):311–329. doi: 10.1242/jeb.107.1.311.
- Wolk K. 1979. Mollusks (Bivalvia) as food of muskrat (Ondatra zibethica L.) on Wigry Lake in Augustów Forests. Przegl Zool. 23:248–250.
- Wong WH, Gerstenberger S, Baldwin W, Moore B. 2012. Settlement and growth of quagga mussels (Dreissena rostriformis bugensis Andrusov, 1897) in Lake Mead, Nevada-Arizona, USA. Aquat Invasions. 7(1):7–19. doi: 10.3391/ai.2012.7.1.002.
- Wormington A, Leach JH. 1992. Concentrations of migrant diving ducks at Point Pelee National Park, Ontario, in response to invasion of zebra mussels, Dreissena polymorpha. Can Field-Nat. 106:376–380.
- Yablonskaya EA. 1985. The Caspian Sea: Fauna and biological productivity. Moscow: Nauka.
- Yamaguti S. 1971. Synopsis of the digenetic trematodes of vertebrates. Vol. 1 and 2. Tokyo: Keigaku Publishing Company.
- Yamaguti S. 1975. A synoptical review of life histories of digenetic trematodes of vertebrates. Tokyo: Keigaku Publishing Co.
- Yuryshynets VI, Ivasyuk YS, Krasutskaya NA. 2008. Experimental Infestation of the mollusk Dreissena polymorpha (Bivalvia: Dreissenidae) by the Ciliate Conchophthirus acuminatus (Ciliophora: Oligohymenophorea). Hydrob J. 44(1):104–112. doi: 10.1615/HydrobJ.v44.i1.90.
- Yuryshynets VI. 2009. The life cycle of the ciliate Ophryoglena hemophaga (Oligohymenophorea, Ophryoglenida): observations and hypotheses. Vestn Zool. 23:213–216.
- Yuryshynets VI. 2019. The symbiotic community of Dreissena bugensis (Andrusov, 1897) in the waterbody of Ukraine. Biol Ecol. 5(2):19–23. doi: 10.33989/2414-9810.2019.5.2.194420.
- Yuryshynets VI, Ovcharenko MO, Kurandina DP, Nizovska LV. 2003. Symbiofauna of mollusks of the genus Dreissena in the water bodies of Ukraine. Tavriyskyi Naukovyi Visnyk. 29:255–258.
- YuV K, Karabanov DP. 2017. Morphological changes in the population of roach (Rutilus rutilus, Cyprinidae) in Lake Pleshcheevo as a result of the introduction of the mollusk, Dreissena polymorpha (Bivalvia). Zool Zhurnal. 96:1069–1077.
- Zdun VI. 1965. Trematode larvae parasitizing dreissenids in the lower Danube. The Conference on Dreissenid Biology and the Protection of Hydroconstructions from Dreissena Growth; Tolyatti, Russia. p. 14–15.
- Zdun VI, Kiselene VK, Karatayev AY, Makarova GE. 1994. Parasites. In: Starobogatov JI, editor. Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning. Moscow: Nauka. p. 196–205.
- Zhdanova GA, Gusynskaya SL. 1985. Distribution and seasonal dynamics of Dreissena larvae in Kiev and Kremenchug reservoirs. Hydrobiol J. 3:35–40.
- Zheltenkova MV. 1949. Food and growth in genus Rutilus. Zool Zh. 28:257–267.
- Zheltenkova MV. 1955. Feeding and usage of feed base by benthic fish of the Sea of Azov. Trans All-Union Res Inst Fish. 1955:306–336.
- Zhokhov AE. 2001. The study of the transition of Cyprinidae fish to feeding on mollusk Dreissena polymorpha (Bivalvia, Dreissenidae) in the Rybinsk Reservoir using parasite Aspidogaster (Aspidogastrea, Aspidogastridae). J Ichthyol. 41:620–624.
- Zhokhov AE, Kasyanov AN. 1995. On the possibility of using parasites as biological markers to identify ecomorphs of roach, Rutilus rutilus, in Rybinsk Reservoir. J Ichthyol. 35(1):44–51.
- Zhulidov AV, Kozhara AV, Scherbina GH, Nalepa TF, Protasov A, Afanasiev SA, Pryanichnikova EG, Zhulidov DA, Gurtovaya TY, Pavlov DF. 2010. Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biol Invasions. 12(7):1923–1940. doi: 10.1007/s10530-009-9641-y.
- Zhulidov AV, Nalepa TF, Kozhara AV, Zhulidov DA, TYu G. 2006. Recent trends in relative abundance of two dreissenid species, Dreissena polymorpha and Dreissena bugensis in the Lower Don River system, Russia. Hydrobiologie. 165(2):209–220. doi: 10.1127/0003-9136/2006/0165-0209.
- Ziegler G. 1987. Zur Entstehung eines Mauserplatzes der Reiherente (Aythya fuligula) von überregionaler Bedeutung mi nördlichen Westfalen. Vogelwelt. 108:67–70.
- Zimmermann G, Dietrich DR, Schmid P, Schlatter C. 1997. Congener-specific bioaccumulation of PCBs in different water bird species. Chemosphere. 34(5–7):1379–1388. doi: 10.1016/S0045-6535(97)00435-9.
- zu Ermgassen PSE, Aldridge DC. 2011. Predation by the invasive American signal crayfish, Pacifastacus leniusculus Dana, on the invasive zebra mussel, Dreissena polymorpha Pallas: the potential for control and facilitation. Hydrobiologia. 658(1):303–315. doi: 10.1007/s10750-010-0500-8.
- Zuur B, Suter W, Krämer A. 1983. Zur Nahrungsökologie auf dem Ermatinger Becken (Bodensee) unberwinternder Wasservögel. Ornithol Beob. 80:247–262.

