Abstract
Unlike ionic copper, chelated copper herbicides reduce fish toxicity by chaperoning copper. Application guidelines that were developed based on toxicity data from ionic copper may therefore be too strict for chelated copper herbicides, limiting their application to concentrations ineffective against target plants. Our goals were to examine the toxicity of the chelated copper herbicide NautiqueTM relative to other copper herbicides in laboratory and field trials. Our laboratory results showed that NautiqueTM was two orders of magnitude less toxic than CaptainTM and copper sulfate, indicating that it had a larger margin of safety for non-target fish. The results of our caged fish study in Greene County, NY, however, indicated that NautiqueTM can elevate fish mortality in field settings at concentrations much lower than those observed in the laboratory. Mortality risk for caged fish within the first 72 h after treatment was 2.3 times greater, on average, in the treatment sites than the reference sites. The elevated fish toxicity we observed in the field trials could have been affected by high water temperature. In our laboratory trials, a higher percentage of fathead minnows died at 28°C than at 22°C after NautiqueTM exposure. It is possible that the stress of the higher lake water temperatures (29–34°C), particularly for caged fish that could not descend to cooler waters, increased NautiqueTM toxicity. Although our laboratory results indicate that NautiqueTM may have fewer impacts on non-target fish than other copper formulations at concentrations effective for aquatic plant control, field trials indicate that the margin of safety of NautiqueTM may be lower for fish in warm lake waters. Warming climate trends may therefore increase and complicate the challenges of invasive macrophyte control.
Public Interest Statement
Copper herbicides are applied to control invasive exotic aquatic plants, but they can be toxic to fish and other non-target organisms. It is therefore essential to identify effective formulations with the lowest non-target toxicities. As the climate warms, it is also important to assess the effects of temperature on herbicide toxicity. This article describes the toxicities of three common copper herbicide formulations to fathead minnows and brook trout. Our laboratory results indicated that the chelated copper formulation NautiqueTM had a greater margin of safety for fish than other copper formulations. However, field trials indicated that the toxicity of NautiqueTM may be higher for fish in warm lake waters. Warming climate trends may therefore increase and complicate the challenges of invasive aquatic plant control.
1. Introduction
Most algaecides and many aquatic herbicides contain copper-based compounds (Timmons, Citation2005). Copper sulfate has been applied as an aquatic herbicide and algaecide for decades because, when put into an aqueous solution, the toxic copper ions dissociate and enter the targeted plant (Mastin & Rodgers, Citation2000). At low concentrations, copper is a minor nutrient for plants and animals, but at higher concentrations, ionic copper herbicides are considered fairly toxic to fish and other non-target organisms (Mastin & Rodgers, Citation2000).
Copper-complexed herbicides are putatively more selective in acting against plant species and less toxic to non-target organisms (Beste, Citation1983). NautiqueTM and CaptainTM (SePRO Corporation, Carmel, IN, USA) are popular chelated-copper aquatic herbicides and algaecides. NautiqueTM Aquatic Herbicide is composed of the chelated copper-complexes copper ethylenediamine and copper triethanolamine, and is used to control aquatic macrophytic species and some algae. CaptainTM Algaecide is composed of a mixed copper ethanolamine complex used to control algal species and a number of copper susceptible macrophytes (e.g. Hydrilla).
The toxicity of copper in aquatic environments is primarily from ionic copper (Eisler, Citation1997). Chelated copper products like and CaptainTM and NautiqueTM are expected to be less toxic to fish because they do not produce large increases in ionic copper (Willis & Bishop, Citation2016). In general, the amount of ionic copper present after a treatment of copper-based algaecides is affected by the pH, alkalinity, and hardness of the receiving water (Masuda & Boyd, Citation1993), as well as by the presence of organic ligands (Hodson, Borgmann, & Shear, Citation1979). Copper sulfate produces a substantial increase in ionic copper, especially at the beginning of a treatment (Masuda & Boyd, Citation1993). In contrast, chelated copper remains largely bound and absent in a free ionic form, which may reduce its toxicity (Azenha, Vasconcelos, & Cabral, Citation1995). However, the extent to which toxicity varies among chelated-copper compounds with different formulations is unclear.
The NautiqueTM and CaptainTM labels instruct that each product be applied up to a maximum concentration of 1 mg Cu/L; however, the New York State Department of Environmental Conservation (NYSDEC) limits the maximum concentration to 0.3 mg Cu/L (6 NYCRR 327, Citation2014). These state guidelines were based on toxicity data from copper sulfate and may render some copper-complexed herbicides ineffective against target plants. The minimum effective NautiqueTM and CaptainTM concentrations for control of low-density Hydrilla verticillata and macrophytes, for example, are 0.4–0.6 mg Cu/L (Supplemental Environmental Impact Statement Assessments of Aquatic Herbicides, Citation2000).
In this study, we examined the toxicity of a chelated copper herbicide, NautiqueTM on fathead minnows (Pimephales promelas) and brook trout (Salvelinus fontinalis) in laboratory trials. We compared these results to our published toxicity data from two other copper herbicides: copper sulfate and CaptainTM (Closson & Paul, Citation2014). In addition, we examined the effects of water temperature on the toxicity of NautiqueTM because other studies indicate that high temperatures can increase metabolic rates (Dillon, Wang, & Huey, Citation2010), potentially increasing the toxicity of copper to fish and other aquatic organisms (Khan et al., Citation2006; Lemus & Chung, Citation1998). Finally, because the results of laboratory trials can differ from ecotoxicity outcomes in the field (Kleinhenz et al., Citation2016), we also examined the effects of NautiqueTM in a field setting, comparing survival of caged fathead minnows in treatment and control sites in a lake in Greene County, NY. Our goals were to examine the toxicity of NautiqueTM relative to other copper herbicides at concentrations effective for the control of aquatic plants and to observe the effects of temperature on its toxicity.
2. Material and methods
2.1. Laboratory assays
The three herbicides tested contained different copper formulations. Copper Sulfate Pentahydrate Fine Crystal (Old Bridge Chemical Inc., Old Bridge, NJ, USA) is an algaecide containing 25.2% copper. CaptainTM is a liquid copper algaecide containing 28.2% copper ethanolamine (metallic copper equivalent 9.1%), and NautiqueTM is an aquatic herbicide containing 13.2% copper ethylenediamine and 14.9% copper triethanolamine (metallic copper equivalent 9.1%).
Toxicity tests were carried out by the NYSDEC Aquatic Toxicant Research Unit (Rome, NY) and adhered to toxicity test protocols of the USEPA (Citation2002). Juvenile New York State Rome Lab strain brook trout (S. fontinalis) were used (mean length of 53 mm, mean weight = 1.5 g). The fathead minnows (P. promelas) were shipped from Aquatic Biosystems, Inc. (Fort Collins, CO), acclimated for one week, and were 45 days old when tested (mean length = 20 mm, mean weight = 0.09 g). Neither species was fed during the toxicity tests. Spring water (pH = 8.10, hardness = 132 mg/L CaCO3) from the NYSDEC Rome Fish Hatchery (Rome, NY) was used for dilution. The hardness and pH of the water used in the toxicity test was measured before each test series and did not differ from the spring water. Dissolved oxygen (DO) was measured at each fish count interval. The test containers were only aerated for the final 48 h of the brook trout toxicity tests. No aeration was required in fathead minnow toxicity tests. The DO never dropped below 60% saturation in any of the test containers.
Experimental conditions varied between test species as appropriate for their size. For brook trout toxicity tests, 20-L glass containers were filled with 16 L of test solution and held at 13 ± 1°C. For fathead minnow toxicity tests, 2-L glass containers were filled with 1.5 L of test solution and held at 22 ± 1°C. In addition, NautiqueTM was tested for fathead minnows at 28 ± 1°C. NautiqueTM and CaptainTM were mixed directly into the glass containers, whereas copper sulfate was dissolved into 100 ml of distilled water before being added to the test containers to achieve the desired final concentration. All herbicides and stock solutions were measured and added using a certified digital pipette (precision = 0.1 μL). For brook trout, test concentrations were 0.13, 0.20, 0.30, 0.45, 0.68, and 1.00 mg Cu/L for CaptainTM, 1.0, 6.4, 10.0, 20.0, and 40.0 mg Cu/L for NautiqueTM, and 0.087, 0.13, 0.20. 0.30, 0.45, 0.68, and 1.00 mg Cu/L for copper sulfate. For fathead minnows, test concentrations were 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, and 6.4 mg Cu/L for CaptainTM and copper sulfate, and 16.9, 20.0, 21.8, 23.7, 28.2, 33.5, and 40.0 mg Cu/L for NautiqueTM. Control containers were filled with spring water.
All toxicity tests were conducted with five brook trout or five fathead minnows in each glass container. Each concentration was replicated at least six times. Mortality and behavior were observed after six hours (fathead minnow) or eight hours (brook trout) and counted at 24 h intervals thereafter. Each test was conducted for 96 h. At each interval, mortality and intoxication were documented and dead fish were removed. Fish were classified as intoxicated if they displayed uncontrolled movements or were unable to remain upright. Lethal concentrations (LC) were based on the number of dead fish; effective concentrations (EC) were based on the number of dead or intoxicated fish.
We calculated LC50s and EC50s using nominal concentrations of copper and the trimmed Spearman-Karber method (Hamilton, Russo, & Thurston, Citation1977). We also examined the relative lethal and effective toxicities in generalized linear models [GLM; binomial distribution and logit link; analyzed using library “nlme” (Pinheiro, Bates, DebRoy, Sarkar, & R Core Team, Citation2017) in R v. 3.3.1 (R Core Team, Citation2016)], with either percentage of fish that died or the percentage that were affected (intoxicated or dead) as the response variables, and time, concentration, and formulation as predictors. To examine the additive effects of temperature and chelated copper herbicides on intoxication and mortality, we ran a similar pair of models on data collected from NautiqueTM and fathead minnows, with either percentage of fish that died or the percentage that were affected as the response variables, and time, concentration, and temperature (22 or 28°C) as predictors, also using library “nlme”. Response variables of all models were weighted by number of fish exposed. Models were run in JMP 11.2.0.
2.2. Field studies
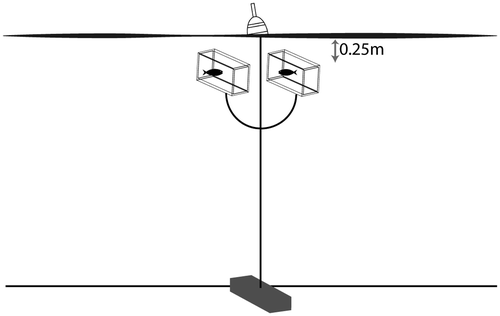
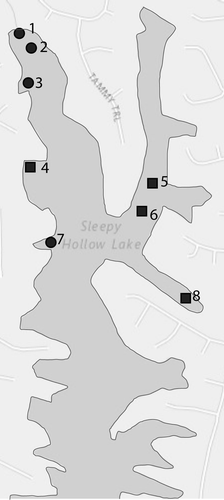
We examined the toxicity of NautiqueTM in the field in July 2013 using caged fathead minnows (43 days old when tested; mean length = 17 mm). We established three reference (control) sites and three treated sites in Sleepy Hollow Lake, Greene County, NY (42°17′43″N; 73°48′26″W; Figure ). Treatment sites in Sleepy Hollow Lake were permitted by the Bureau of Pesticides of the NYSDEC for treatment with NautiqueTM to control brittle naiad (Najas minor). NautiqueTM was mixed and spray applied, according to the pesticide label and all permit conditions, by a certified professional aquatic pesticide applicator. Four anchor lines were set at each site, and four cages were attached to each line, suspended ~0.25 m below the lake surface with a buoyed line. The 4-L plastic cages had screw-topped caps and mesh screened sides to allow water circulation. Five minnows were placed in each cage (n = 80 fish at each site) 24 h before the NautiqueTM treatment (Figure ). We checked the cages for dead and intoxicated fish 0, 5, 24, 48, 72, 96, 120 and 144 h (six days) after NautiqueTM application. Dead fish were removed at each check.
Figure 1. Map of Sleepy Hollow Lake showing sampling locations.
Dissolved oxygen and temperature profile measurements were recorded at each cage check on every day of testing. Temperature ranged from 29.2 to 34.7°C (mean = 31.3 ± 0.02) at each check. Minimum DO during testing was 6.3 ppm, corresponding to 85% saturation at 31°C. Alkalinity at Sleepy Hollow Lake was measured at 67 mg/L, conductivity at 243 μS, hardness at 85 mg/L, and pH at 7.42.
We measured copper concentrations at all treatment and reference sampling sites in 2013 (sites 1–6). In 2013, target treatment levels for NautiqueTM were 0.6 mg/L at sites 1 and 2 and 0.8 mg/L at site 3. In 2014, we measured copper concentrations in three treatment sites (1, 2, and 7) and one reference site (site 8; Figure ). Target treatment level was 0.8 mg/L in 2014. No caged fish study was conducted in 2014. Treatment sites were measured at 0, 0.5, 1, 2, 4, 8, 24, and 48 h post-treatment. Reference sites were sampled at 0.5 and 48 h post-treatment in 2013 and 0, 24 and 48 h post-treatment in 2014. Water samples were collected 0.2 m below the lake surface for analysis. Blanks (Rome Fish Hatchery spring water) were included with each submission for quality control. Copper analyses were conducted by SEPRO (Whitakers, NC) and ALS Environmental (Rochester, NY) in 2013 and ALS Environmental in 2014.
3. Results
3.1. Laboratory assays
The LC50 and EC50 values are presented in Tables and . The LC50 and EC50 values for NautiqueTM were nearly two orders of magnitude greater than CaptainTM and copper sulfate in both brook trout and fathead minnows. CaptainTM and copper sulfate LC50 and EC50 values were nearly the same for brook trout, except for in the short 8-h exposures. For fathead minnows, the LC50 and EC50 values were approximately 0.5 mg Cu/L higher for CaptainTM compared to copper sulfate, except in the short 6-h exposures. The EC50 and LC50 values were significantly lower for NautiqueTM at 28°C than at 22°C after 48 h of exposure. No fish died in any control containers.
Table 1. Lethal concentrations to 50% [and 95% CI] of test fish at various times for tested herbicides. Eight hour results for brook trout and six hour results for fathead minnow tests. Copper sulfate and CaptainTM results previously reported in Closson and Paul (Citation2014)
Table 2. Effective concentrations to 50% [95% CI] of test fish. Eight hour results for brook trout and six hour results for fathead minnow tests. Copper sulfate and CaptainTM results from Closson and Paul (Citation2014)
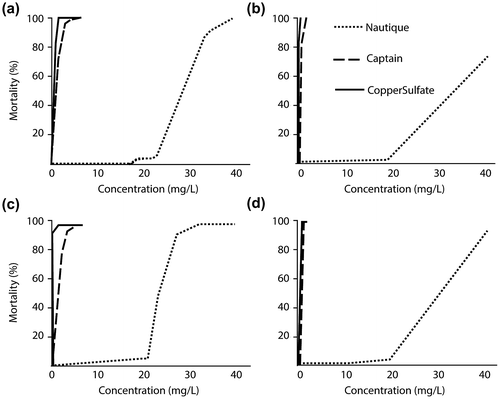
The patterns of toxicity vs. test concentrations after 24 or 96 h of exposure are presented in Figure . Similar patterns were observed at other time intervals. The concentration at which we began to observe mortality for copper sulfate or CaptainTM was less than 1 mg Cu/L, in contrast with 20 mg Cu/L for NautiqueTM.
Figure 3. Mortality percentages in response to NautiqueTM, CaptainTM and copper sulfate concentrations at 22°C for fathead minnows and 13°C for brook trout. (a) Fathead minnow mortality at 24 h; (b) Brook trout mortality at 24 h; (c) Fathead minnow mortality at 96 h; (d) Brook trout mortality at 96 h.
NautiqueTM was significantly less toxic to brook trout and fathead minnows than the other formulations (Table ). With time and concentration held constant, the percentage of brook trout that died was 25% greater for CaptainTM and copper sulfate than for NautiqueTM (p < 0.0001). Likewise, the percentage of brook trout intoxicated was greater for both CaptainTM and copper sulfate than for NautiqueTM (p < 0.0001). Not surprisingly, the percentage of brook trout or fathead minnows that died or were intoxicated increased with time and herbicide concentration.
Table 3. Output from models examining the relative lethal and effective toxicities of three copper formulations on brook trout and fathead minnows. Time and concentration were also included in the models. Effects of CaptainTM and copper sulfate are given in comparison with NautiqueTM
Temperature significantly increased the toxicity of NautiqueTM on fathead minnows (GLMs with either percentage of fish that died or were affected as the response, and time, concentration, and temperature as predictors). With concentration and time held constant, the percentage of fathead minnows that died was 23% ± 0.02 SE greater at 28°C than at 22°C (p < 0.0001). Likewise, the percentage of fish intoxicated was >18% greater at 28°C than at 22°C (p < 0.0001). However, the No Observed Effective Concentration (NOEC) value for NautiqueTM at 28°C (16.9 mg Cu/L) was still an order of magnitude higher than concentrations recommended for macrophyte control (~1.0 mg Cu/L; Supplemental Environmental Impact Statement Assessments of Aquatic Herbicides, Citation2000).
3.2. Field studies
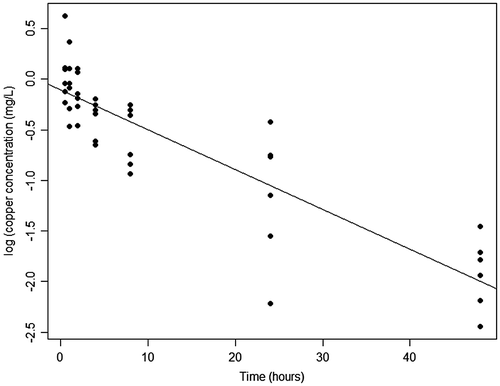
Measured copper concentrations were <0.0006 mg/L at reference sites (n = 4 sites). In 2013 (the year of the caged fish trials), mean copper concentrations in the treatment sites 30 min after NautiqueTM application ranged from 0.6 to 1.3 mg/L (mean ± SE = 0.9 ± 0.4 mg/L; n = 3). In 2014, mean copper concentrations in the treatment sites ranged from 0.8 to 4.2 mg/L (mean ± SE = 0.2.1 ± 1.1 mg/L; n = 3). Copper concentrations declined over time in the treatment sites (Figure ). In a mixed model with log-transformed copper concentration as the response, time as a fixed effect, and site as a random effect, copper concentrations declined by 3.9% per hour, on average, after treatment (log (copper) = −0.04 ± 0.003*(hours) – 0.1; p < 0.001; n = 42 observations). Copper concentrations were log-transformed to meet assumptions of normality.
Figure 4. Change over time of log (copper) after treatment at the treatment sites. Regression line based on simple linear model. Parameter estimates given in the text are based on random effects model accounting for repeated measures at each site.
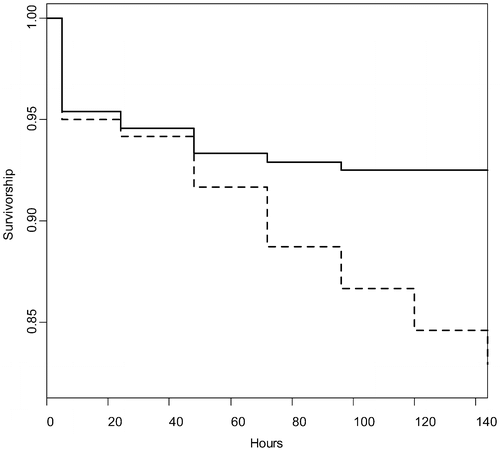
Mortality was higher for caged fish in the treatment than the reference sites 72 h after treatment in Sleepy Hollow Lake. Eighteen of 240 (8%) of the fish in the reference site died, vs. 41 of 240 (17%) in the treatment sites (Fisher exact test (one-tailed): p = 0.001). A Cox proportional hazard survival analysis indicated the hazard rate for death within 72 h after treatment was 2.3 times greater (95% CI: 1.3–4.1) for fish in the treatment sites than the reference sites (p = 0.00269; Figure ). Water temperature had no significant effect (either as a main effect or interactive effect with treatment) and was removed from the final model. Lack of temperature effect was not surprising, given that temperature did not vary substantially among sites or treatments. Elevated mortality of fish in the treatment site in comparison with the reference site was not due to warmer temperatures in those sites because temperatures did not differ between reference and treatment sites (mean reference: 31.42 ± 0.3; mean treatment: 31.26 ± 0.3°C; t (41) = 0.43; p = 0.67). Survival in treated and reference sites was compared using a Cox proportional hazard survival analysis in R v. 3.3.1.
4. Discussion
The invasive macrophyte Hydrilla has spread aggressively since its introduction to the United States in 1959 (Langeland, Citation1996; Madeira, Jacono, & Van, Citation2000) and has a potential invasion distributional area that spans much of eastern and southern North America (Peterson, Papes, & Kluza, Citation2003). Herbicides play a key role in controlling the spread of invasive aquatics, and identifying compounds that minimize impacts to non-target species and ecological risks is essential (Fedorenkova et al., Citation2012; Getsinger, Netherland, Grue, & Koschnick, Citation2008). Our laboratory results indicated that the chelated copper herbicide NautiqueTM was less toxic to fish than other copper herbicides (copper sulfate and CaptainTM). We did not observe fish mortality until NautiqueTM concentrations reached 20 mg Cu/L, well above the NYSDEC limit of 0.3 mg Cu/L (NYCRR 327.6). These laboratory data indicate that NautiqueTM concentration application limits could be increased to levels that would more effectively control Hydrilla verticillata and other macrophytes (e.g. 0.4–0.6 mg Cu/L to control low-density macrophytes; 1.0 mg Cu/L for high density macrophytes; Supplemental Environmental Impact Statement Assessments of Aquatic Herbicides, Citation2000), without a corresponding increase in fish mortality.
The results of our caged fish study in Sleepy Hollow Lake, however, indicated that NautiqueTM can elevate fish mortality in field settings at concentrations much lower than those observed in the laboratory. At target concentrations of 0.6–0.8 mg Cu/L (with actual application concentrations of 0.6–1.3 mg Cu/L), we found that the mortality risk for caged fish within the first 72 h after treatment was 2.3 times greater, on average, in the treatment sites than the reference sites.
Our laboratory trials indicated that temperature elevates the toxicity of NautiqueTM, which could explain the different outcomes from the field and lab studies. In our laboratory trials, a significantly higher percentage of fathead minnows died at 28°C than at 22°C after NautiqueTM exposure. Because we only ran temperature trials with NautiqueTM, we cannot generalize about the effects of temperature on the toxicity of copper compounds. However, our results align with other studies documenting increased toxicity of copper with temperature (e.g. Gamain et al., Citation2017; Khan et al., Citation2006; Lemus & Chung, Citation1998). In a study by Lemus and Chung (Citation1998), for example, the toxicity of copper to Petenia kraussii juveniles was lower at 22°C than it was at 30°C. The authors suggested that physiological condition of the fish was diminished at high temperatures because increased metabolic rate elevated the toxicity of the metal. Our laboratory trials of NautiqueTM indicated that temperature was unlikely to increase acute toxicity at concentrations applied in the field. The laboratory NOEC value for NautiqueTM at 28°C was 16.9 mg Cu/L, a concentration much higher than the 1.0 mg Cu/L concentrations recommended for high-density macrophyte control.
The elevated fish toxicity we observed in the field trials could have been due, at least in part, to unusually high water temperatures. In our field trials, the water temperatures observed (range 29–34°C) exceeded the “warm” temperature limit (28°C) set in our laboratory trials. It is possible that the stress of these higher water temperatures, particularly for caged fish that could not descend to cooler waters, increased the impacts of NautiqueTM on the fish at application concentrations of 0.6–1.3 mg Cu/L. Although our laboratory results indicate that NautiqueTM may have fewer impacts on non-target fish than other copper formulations at concentrations effective for aquatic plant control, the margin of safety of NautiqueTM (and other copper herbicides) may be lower for fish in warm lake waters.
The Intergovernmental Panel on Climate Change (Citation2007) has projected that global temperatures will increase 1.8–4.0°C by the end of the century. These warming temperatures may exacerbate herbicide toxicity for non-target species, reducing their margin of safety, as indicated by this study. This problem may be compounded with the benefits of warming temperatures to some of the invasive species themselves. With milder winters, invasive plants like Hydrilla could have longer growing seasons and greater fitness (Hellmann, Byers, Bierwagen, & Dukes, Citation2008). In general, warmer temperatures are predicted to increase the growth and spread of some invasive plants, many of which are limited by temperature (Owens & Madsen, Citation1995). Overall, warming climate trends may increase the challenges of invasive macrophyte control.
We focused primarily on acute toxicity of copper herbicides, but the sublethal effects of herbicides on aquatic organisms are also essential to evaluate (Villarroel, Sancho, Ferrando, & Andreu, Citation2003). Sublethal copper toxicity can change gill permeability to copper (Taylor, McGeer, Wood, & McDonald, Citation1998) and cause gill lesions, hypertrophy, hyperplasia, epithelial lifting and necrosis (Mallatt, Citation1985). Gill lesions from acute waterborne copper exposure can increase the distance of diffusion across the secondary lamellae, decreasing the amount of oxygen transferred across the gill epithelium (Tuurala, Pärt, Nikinmaa, & Soivio, Citation1984). Additionally, copper exposure can negatively impact sensory abilities and swimming performance. Coho salmon (Oncorhynchus kisutch), for example, exhibited a reduced olfactory response within 10 minutes of copper exposure (Baldwin et al., Citation2003), and critical swimming speed decreased by 48, 31, and 13% for rainbow trout, common carp (Cyprinus carpio), and gibel carp (Carassius gibelio), respectively, after exposure (De Boeck, van der Ven, Hattink, & Blust, Citation2006). Furthermore, the ecotoxicity of aquatic copper can be altered when presented in combination with other chemicals (Chia, Galadima, & Japhet, Citation2015; Hansen & Roslev, Citation2016). Further investigation into the sublethal effecsts of these copper compounds, and the extent to which factors like temperature and other herbicides mediate these effects, is therefore warranted.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standard of the institution or practice at which the studies were conducted.
Acknowledgments
We would like to thank Ben Durie, Kristin Closson and Ryan Collins for their assistance in conducting these tests.
Additional information
Funding
Notes on contributors
Jacob L. Wagner
This copper-based algaecide research is a part of a larger study by the New York State Department of Environmental Conservation’s Aquatic Toxicant Research Unit (ATRU), examining the potential non-target effects of aquatic herbicides on fish and aquatic animals. The ATRU has conducted field and laboratory aquatic toxicity investigations and research since the 1950s. These ecotoxicology studies combine controlled laboratory toxicity tests with “real life” field data to better understand the toxicity of pesticides and other contaminants on fish and other aquatic life. The studies conducted by the ATRU are specifically geared toward the habitats and the species of New York State, often using “non-traditional” species of particular concern for New York. The ATRU is directed by Eric Paul, the corresponding author.
References
- 6 NYCRR Part 327. (2014). New York State Department of the Environment. Environment Conservation Law, Chapter 6, Part 327. Use of chemicals for the control or elimination of aquatic vegetation. Accessed 11 September 2014.
- Azenha, M., Vasconcelos, M. T., & Cabral, J. P. S. (1995). Organic ligands reduce copper toxicity in Pseudomonas syringae. Environmental Toxicology and Chemistry, 14, 369–373.10.1002/etc.v14:3
- Baldwin, D. H., Sandahl, J. F., Labenia, J. S., & Scholz, N. L. (2003). Sublethal effects of copper on coho salmon: Impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environmental Toxicology and Chemistry, 22, 2266–2274.
- Beste, C. E. (1983). Herbicide Handbook of the Weed Science Society of America (5th ed.). Champaign, IL: Weed Science Society of America.
- Chia, M. A., Galadima, S. Y., & Japhet, W. S. (2015). Combined effect of atrazine and copper on the growth, biomass production, morphology and antioxidant response of Scenedesmus quadricauda. Phycologia, 54, 109–117.10.2216/14-71.1
- Closson, K. R., & Paul, E. A. (2014). Comparison of the toxicity of two chelated copper algaecides and copper sulfate to non-target fish. Bulletin of Environmental Contamination and Toxicology, 93, 660–665.10.1007/s00128-014-1394-3
- De Boeck, G., van der Ven, K., Hattink, J., & Blust, R. (2006). Swimming performance and energy metabolism of rainbow trout, common carp and gibel carp respond differently to sublethal copper exposure. Aquatic Toxicology, 80, 92–100.10.1016/j.aquatox.2006.07.017
- Dillon, M. E., Wang, G., & Huey, R. B. (2010). Global metabolic impacts of recent climate warming. Nature, 467, 704–706.10.1038/nature09407
- Eisler, R. (1997). Copper hazards to fish, wildlife, and invertebrates: A synoptic review ( U.S. Geological Survey, Biological Resources Division, Biological Science Report USGS/BRD/BSR-1997-0002, 98 pp.).
- Fedorenkova, A., Vonk, J. A., Lenders, H. J. R., Creemers, R. C. M., Breure, A. M., & Hendriks, A. J. (2012). Ranking ecological risks of multiple chemical stressors on amphibians. Environmental Toxicology and Chemistry, 31, 1416–1421.10.1002/etc.v31.6
- Gamain, P., Gonzalez, P., Cachot, J., Clérandeau, C., Mazzella, N., Gourves, P. Y., & Morin, B. (2017). Combined effects of temperature and copper and S-metolachlor on embryo-larval development of the Pacific oyster, Crassostrea gigas. Marine Pollution Bulletin, 115, 201–210.10.1016/j.marpolbul.2016.12.018
- Getsinger, K. D., Netherland, M. D., Grue, C. E., & Koschnick, T. J. (2008). Improvements in the use of aquatic herbicides and establishment of future research directions. Journal of Aquatic Plant Management, 46, 32–41.
- Hamilton, M. A., Russo, R. C., & Thurston, R. V. (1977). Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environmental Science & Technology, 11, 714–719.10.1021/es60130a004
- Hansen, L. R., & Roslev, P. (2016). Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquatic Toxicology, 179, 36–43.10.1016/j.aquatox.2016.08.010
- Hellmann, J. J., Byers, J. E., Bierwagen, B. G., & Dukes, J. S. (2008). Five potential consequences of climate change for invasive species. Conservation Biology, 22, 534–543.10.1111/j.1523-1739.2008.00951.x
- Hodson, P. V., Borgmann, U., & Shear, H. (1979). Toxicity of copper to aquatic biota. In J. O. Nriagu (Ed.), Copper in the environment. Part 2: Health effects (pp. 307–372). New York, NY: John Wiley.
- Intergovernmental Panel on Climate Change. (2007). Climate change 2007: The physical science basis. Agenda, 6, 333.
- Khan, M. A. Q., Ahmed, S. A., Catalin, B., Khodadoust, A., Ajayi, O., & Vaughn, M. (2006). Effect of temperature on heavy metal toxicity to juvenile crayfish, Orconectes immunis (Hagen). Environmental Toxicology, 21, 513–520.10.1002/(ISSN)1522-7278
- Kleinhenz, L. S., Nugegoda, D., Verspaandonk, E. R., Coombes, D. C., Howe, S., & Shimeta, J. (2016). Toxicity of an herbicide and adjuvant to saltmarsh invertebrates in the management of invasive grass; Comparative laboratory and field tests. Marine Pollution Bulletin, 109, 334–343.10.1016/j.marpolbul.2016.05.061
- Langeland, K. A. (1996). Hydrilla verticillata (LF) Royle (Hydrocharitaceae), the perfect aquatic weed. Castanea, 293–304.
- Lemus, M. J., & Chung, K. S. (1998. Effect of temperature on copper toxicity in Petenia Kraussii (Pisces: Cilclidae) juveniles. Fish Response to Toxic Environments. International Congress on the Biology of Fish, 107–120.
- Madeira, P. T., Jacono, C. C., & Van, T. K. (2000). Monitoring hydrilla using two RAPD procedures and the nonindigenous aquatic species database. Journal of Aquatic Plant Management, 38, 33–40.
- Mallatt, J. (1985). Fish gill structural changes induced by toxicants and other irritants: A statistical review. Canadian Journal of Fisheries and Aquatic Sciences, 42, 630–648.10.1139/f85-083
- Mastin, B. J., & Rodgers, J. H. (2000). Toxicity and bioavailability of copper herbicides (clearigate, cutrine-plus, and copper sulfate) to freshwater animals. Archives of Environmental Contamination and Toxicology, 39, 445–451.10.1007/s002440010126
- Masuda, K., & Boyd, C. E. (1993). Comparative evaluation of the solubility and algal toxicity of copper sulfate and chelated copper. Aquaculture, 117, 287–302.10.1016/0044-8486(93)90326-T
- Owens, C. S., & Madsen, J. D. (1995). Low-temperature limits of water-hyacinth. Journal of Aquatic Plant Management, 33, 63–68.
- Peterson, A. T., Papes, M., & Kluza, D. A. (2003). Predicting the potential invasive distributions of four alien plant species in North America. Weed Science, 51, 863–868.10.1614/P2002-081
- Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & R Core Team. (2017). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-131. Retrieved from https://CRAN.R-project.org/package=nlme
- R Core Team. (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/
- Supplemental Environmental Impact Statement Assessments of Aquatic Herbicides. (2000). Lacey, WA: Washington State Department of Ecology. Water Quality Program.
- Taylor, L. N., McGeer, J., Wood, C., & McDonald, D. (1998). Modelling chronic thresholds for toxicity–physiological effects of chronic copper exposure to rainbow trout. Fish Response to Toxic Environments, 95.
- Timmons, F. L. (2005). A history of weed control in the United States and Canada. Weed Science, 53, 748–761.10.1614/0043-1745(2005)053[0748:AHOWCI]2.0.CO;2
- Tuurala, H., Pärt, P., Nikinmaa, M., & Soivio, A. (1984). The basal channels of secondary lamellae in Salmo gairdneri gills—A non-respiratory shunt. Comparative Biochemistry and Physiology, 79, 35–39.
- USEPA. (2002). Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organism (5th ed). Washington, DC: EPA-821-R-02-012 US Environmental Protection Agency.
- Villarroel, M. J., Sancho, E., Ferrando, M. D., & Andreu, E. (2003). Acute, chronic and sublethal effects of the herbicide propanil on Daphnia magna. Chemosphere, 53, 857–864.10.1016/S0045-6535(03)00546-0
- Willis, B. E., & Bishop, W. M. (2016). Understanding fate and effects of copper pesticides in aquatic systems. Journal of Geoscience and Enviroment Protection, 4, 37–42. doi:10.4236/gep.2016.45004