Abstract
Data from National Health and Nutrition Examination Survey for 1999–2012 were used to evaluate factors that affect observed levels of blood cadmium (BCd) and urine cadmium (UCd) among never smoker adults aged 20–64 years and senior citizens aged ≥ 65 years. Over 1999–2010, adjusted levels of BCd declined for adults aged 20–64 years (β = −0.02457, p < 0.01) and senior citizens aged ≥ 65 years (β = −0.01845, p < 0.01). For UCd, this decline was observed among never smoker adults aged 20–64 years only (β = −0.01605, p < 0.01). Adjusted BCd as well as UCd levels were found to be statistically significantly higher for female never smoker adults as well as senior citizens than male never smoker adults and senior citizens, respectively, (p < 0.01). Non-Hispanic whites had statistically significantly lower levels (p < 0.01) of BCd than non-Hispanic blacks and Mexican Americans for adults as well as senior citizens (p < 0.01). For UCd, this association was not observed. Among adults, levels of both BCd increased with age and decreased with body mass index (p < 0.01). Dietary intake of cadmium was not found to affect the levels of either BCd or UCd.
Public Interest Statement
Exposure to cadmium resulting in elevated levels of urine and/or blood cadmium has been reported to be associated with prediabetes, impaired fasting glucose and diabetes, increased prevalence of peripheral arterial disease, impaired kidney function, myocardial infarction, elevated risk of osteoporosis, increased odds of the prevalence of stroke and heart failure, increased odds of breast cancer, increased odds of age-related macular degeneration, increased all-cause and cause specific mortality, and iron deficiency among children. An extensive range of adverse health effects associated with exposure to cadmium requires investment of resources in public health efforts to monitor existing levels of exposure to cadmium on an ongoing basis. This communication reports on the biomonitoring data on exposure to cadmium among never smokers for the 14-year period covering 1999–2012 for general US population of adults aged 20–64 years and senior citizens age ≥ 65 years.
Competing Interests
The author declares no competing interest. All data used in this research are available free of charge at www.cdc.gov/nchs/nhanes.htm
1. Introduction
Tobacco leaves contain high amounts of cadmium (Cd) and as such, smoking greatly increases exposure to Cd (https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf, p. 12). Among nonsmokers and children, the largest source of Cd is via consumption of leafy vegetables such as lettuce and spinach; staples such as potatoes and grains; and peanutsoaen, soybeans, and sunflower seeds. Consumption of shell fish and organ meats is also associated with increased exposure to Cd. As quoted by Agency for Toxic Substances and Disease Registry (https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf), half-life of Cd has been estimated to be 6–38 years for the human kidney and 4–19 years for human liver (p. 201). As such, Cd stays in the human body for a very long time. Concentrations of Cd in blood (BCd) and urine (UCd) provide estimates of human exposure to Cd. While, BCd can be used as a measure of the recent exposure to Cd, UCd can be used as a measure of long-term exposure to Cd (Adams & Newcomb, Citation2014). Since, estimated half-life of cadmium in the kidney is from one to four decades (https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15), UCd being a measure of long-term exposure to Cd, can be stated to represent exposure to Cd for several decades.
Elevated levels of UCd and/or BCd have been reported to be associated with prediabetes (Wallia, Allen, Badon, & El Muayed, Citation2014), impaired fasting glucose and diabetes (Schwartz, Il’yasova, & Ivanova, Citation2003), increased prevalence of peripheral arterial disease (Navas-Acien et al., Citation2004), impaired kidney function (Buser, Ingber, Raines, Fowler, & Scinicariello, Citation2016; Ferraro, Costanzi, Naticchia, Sturniolo, & Gambaro, Citation2010; Trzeciakowski, Gardiner, & Parrish, Citation2014), myocardial infarction (Everett & Frithsen, Citation2008), high prevalence of elevated C-reactive protein and fibrinogen (Lin, Rathod, Ho, & Caffrey, Citation2009), elevated risk of osteoporosis (Gallagher, Kovach, & Meliker, Citation2008), increased odds of the prevalence of stroke and heart failure (Peters, Perlstein, Perry, McNeely, & Weuve, Citation2010), modestly elevated blood pressure (Tellez-Plaza, Navas-Acien, Crainiceanu, & Guallar, Citation2008), increased odds of breast cancer (Gallagher, Chen, & Kovach, Citation2010), increased odds of age-related macular degeneration (Wu, Schaumberg, & Park, Citation2014), and iron deficiency among children (Silver, Lozoff, & Meeker, Citation2013).
Mortality linked data from National Health and Nutrition Examination Survey (NHANES) have been often used to report on associations between exposure to Cd and all-cause and cause-specific mortality. Some of the authors who have reported elevated levels of BCd and/or UCd to be associated with mortality are Aoki et al. (Citation2016), Cheung, Kang, Ouyang, and Yeung (Citation2014), Patel et al. (Citation2013), Lin et al. (Citation2013), Tellez-Plaza, Navas-Acien, Menke, et al. (Citation2012), and Wu, Chang, Lin, Caffrey, and Lin (Citation2011).
Smokers have been reported to have higher levels of Cd than nonsmokers (https://www.cdc.gov/biomonitoring/Cadmium_BiomonitoringSummary.html). Some of the studies in general population and among several groups of patients reporting higher levels among smokers as compared to nonsmokers are by Ahn, Kim, and Park (Citation2017), Yaprak et al. (Citation2017), Afridi, Kazi, Talpur, and Brabazon (Citation2015), Kazi et al. (Citation2010), Richter, Bishop, Wang, and Swahn (Citation2009), and Sorkun et al. (Citation2007).
Adverse health effects associated with occupational and environmental exposure to Cd (and other contaminants) have been extensively studied. Some of the recent studies reporting on these health adverse effects are by Thomas, Hodgson, Nieuwenhuijsen, and Jarup (Citation2009), Beveridge, Pintos, Parent, Asselin, and Siemiatycki (Citation2010), Honda et al. (Citation2010), Gil et al. (Citation2011), Haddam et al. (Citation2011), Arrandale, Beach, Cembrowski, and Cherry (Citation2015), De Franciscis et al. (Citation2015), Togawa et al. (Citation2016), and Dodd-Butera et al. (Citation2017).
Adams and Newcomb (Citation2014) used NHANES data from 1999–2010 for those aged ≥ 20 years and reported both BCd and UCd levels to be substantially higher among those aged ≥ 70 years than those aged 20–29 years. Adjusted geometric means of BCd and UCd were reported to be higher in females than males. Riederer, Belova, George, and Anastas (Citation2013) used NHANES data from 1999–2008 and reported decrease in UCd levels among children, teens, and adults over 1999–2008. CitationTellez-Plaza, Navas-Acien, Caldwell, et al. (2012) reported declining trends (p < 0.01) in UCd levels over 1988–2008 in overall US population as well as all participant subgroups. Among females of reproductive age, non-smoking Mexican American and non-Hispanic Black females were more likely to have high Cd than non-Hispanic white females (Mijal & Holzman, Citation2010).
The objectives of the proposed study were to (i) study time trends in unadjusted and adjusted levels of BCd and UCd over 1999–2012 for never smokers adults aged 20–64 years and senior citizens aged ≥ 65 years, and (ii) evaluate factors that affect adjusted levels of BCd and UCd including gender, race/ethnicity, body mass index (BMI), poverty income ratio (PIR), exposure to environmental tobacco smoke at home (ETSH) and work (ETSW), and diet. The proposed study is similar to the study by Adams and Newcomb (Citation2014) in certain aspects but different in other aspects. Adams and Newcomb (Citation2014) did consider using ETSH as a variable but excluded it from the final models because it was not found to be statistically significant. For the purpose of this study, ETSH and intensity of exposure to ETSH will be studied. In addition, ETSW and intensity of exposure to ETSW will also be used as independent variables in the models. The proposed study will fit separate regression models for BCd and UCd for both adults and senior citizens. Adams and Newcomb (Citation2014) did not consider the role of dietary intake on the levels for BCd and UCd, this study will.
2. Materials and methods
2.1. Data source and data descriptions
Data from NHANES for 1999–2012 from demographic, body measures, blood metal, urine metal, serum cotinine, home interview smoking questionnaire, recent tobacco use questionnaire, family smoking questionnaire, occupational questionnaire, and first day individual food dietary questionnaire files were downloaded and match merged. NHANES uses a complex, stratified, multistage, probability sampling designed to be representative of the civilian, non-institutionalized US population based on age, sex, and race/ethnicity. Sampling weights are created in NHANES to account for the probabilities of selection and response as well as total US population for the selected combinations of gender, age, and race/ethnicity. All analyses completed for this study used sampling weights as well as sampling design information, i.e. stratification and clustering to compute relevant statistics. Unweighted sample sizes after removing missing data for dependent and independent variables are given in Table . Percent observations at or above the limitation of detection (LOD) for BCd were found to be 71.1% for adults and 88.4% for senior citizens. Percent observations at or above LOD for UCd were found to be 92.4% for adults and 97.5% for senior citizens.
Table 1. Weighted frequency distribution of blood and urine cadmium by gender, race/ethnicity, and exposure to environmental tobacco smoke (ETS) at work and home
Data for exposure to ETS at home (ETSH) were extracted from family smoking questionnaires (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/SMQFAM_G.htm) used in NHANES. A total of three variables indicating exposure to ETSH, namely, exposure to ETSH (yes/no), number of cigarette smokers living inside home, and number of cigarettes smoked inside home every day were available. Data for exposure to ETS at work (ETSW) were extracted from occupational questionnaires (https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/OCQ_F.htm) used in NHANES. There were two variables indicating exposure to ETSW, namely, exposure to ETSW (yes/no) and number of hours the participants inhaled smoke from other people’s cigarettes, cigars, and/or pipes that were available. However, there was a change in NHANES 2011–2012 about how data on exposure to ETSW were collected. Instead of asking if the respondents inhaled tobacco smoke at work as was done for 1999–2010, the question in NHANES 2011–2012 asked the respondents if anyone smoked cigarettes, cigars, or pipes during the last two weeks in the area the respondents worked. Data on number of hours the participants inhaled smoke from other people’s cigarettes, cigars, and/or pipes were no longer collected in NHANES 2011–2012. For this reason, adjusted analyses were completed for 1999–2010 only.
2.2. Laboratory methods
For 2003–2012, BCd was determined using inductively coupled plasma mass spectrometry (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PbCd_G.htm#Description_of_Laboratory_Methodology) based on quadrupole ICP-MS technology. ICP-MS was also used to determine UCd (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/UHM_G.htm#Description_of_Laboratory_Methodology). For 1999–2002, BCd was determined on a Perkin Elmer Model SIMAA 6000 simultaneous multi-element atomic absorption spectrometer with Zeeman background correction (https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/L06_B.htm#Description_of_Laboratory_Methodology) and UCd was measured using ICP-MS based assay (https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/L06HM_B.htm) but data on UCd were corrected for interference from molybdenum oxide before being released in the public domain.
2.3. Definition of selected variables
Database for never smokers aged ≥ 20 years was generated by selecting those who self-reported not smoking 100 cigarettes in life, did not self-report using any tobacco products during the last five days and currently had their serum cotinine levels less than 10 ng/mL.
Selection of dietary variables for use in this study was guided by the Canadian study reported by Garner and Levallois (Citation2016). Garner and Levallois (Citation2016) used frequency of the consumption of selected foods in their study during the last 12 months but data on food frequency consumption for NHANES are not made available in public domain on a consistent basis. Instead, data on the consumption of selected foods in grams during the last 24 h (https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/DR1IFF_G.htm) were used for this study. Ten food groups based on the United States Department of Agriculture’s (USDA) eight-digit food codes were generated. The USDA food codes (https://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/fndds/fndds5_doc.pdf) used to generate these food groups are provided by Jain (Citation2016). Preliminarily, 10 food groups selected were: red meats, organ meats, shell fish, nuts and seeds, white bread, cereals, dark green leafy vegetables, white potatoes, fried potatoes, and lettuce based salads. However, percent participants consuming some of these foods were too low to have enough variability in the data. For this reason, an arbitrary decision was made to include only those food groups in the analyses for which there were at least 10% participants consuming them. As such, the food groups included in analyses were: white bread, cereals, white potatoes, fried potatoes, nuts and seeds, and lettuce based salads.
2.4. Outcome variables
Since, the distributions of both BCd and UCd were positively skewed, their measurements in ng/L were log10 transformed before being used as dependent variables in the models.
2.5. Covariates/independent variables
Categorical independent variables used in the models for adults were: gender (male, female), race/ethnicity (non-Hispanic white or NHW, non-Hispanic Black or NHB, Mexican American or MA, other unclassified race/ethnicities or OTH), exposure to ETS at home (ETSH) and exposure to ETS at work (ETSW). Since, there were only 32 senior citizens who had exposure to ETS at work, they were deleted from the models and as such, ETSW was not used as an independent variable in the models for senior citizens. Continuous independent variables used in the models were, age, age2, log10 transformed values of body mass index (BMI), and poverty income ratio (PIR), survey year (coded as one through seven), number of smokers smoking inside home, number of cigarettes smoked inside home every day, and for adults only, number of hours smoke was inhaled from other people’s cigarettes, cigars, and pipes as well as dietary variables as previously specified. Models also tested for significant first-order interactions between gender, race/ethnicity, ETSH, and ETSW but they were included in the final models if they were found to be statistically significant at α = 0.05.
2.6. Software and statistical analysis
All analyses conducted for this study used SAS University Edition software (www.sas.com). Proc SURVEYREG was sued to fit multivariate regression models as well as univariate models to test for time trends in unadjusted values of BCd and UCd. Pairwise comparisons were done using t-test.
3. Results
3.1. Univariate analysis
Unadjusted geometric mean levels (UGM) of BCd exhibited a decreasing trends (p < 0.01) over 1999–2012 irrespective of age (Table ). For adults, BCd levels in 2011–2012 were 67.6% of what they were in 1999–2000 and for every NHANES cycle, BCd levels declined by 5.3%. For seniors, BCd levels in 2011–2012 were 72.9% of what they were in 1999–2000 and for every NHANES cycle, BCd levels declined by 5.7%. For UCd, decreasing trends (p < 0.01) were observed for adults only (p < 0.01). UCd levels in 2011–2012 were 73% of what they were in 1999–2000 and for every NHANES cycle, UCd levels declined by 4.5%.
Table 2. Unadjusted geometric means with 95% confidence intervals for blood cadmium in μg/L and urine cadmium in μg/L by survey year by age for never smokers
UGMs of BCd as well as UCd for both adults and senior citizens were found to be higher for females when compared with males (Table , p < 0.01). The ratio of females to males UGM were: 1.4 and 1.1 for BCd for adults and seniors, respectively, and 1.3 and 1.1 for UCd for adults and seniors, respectively. The order in which BCd among three major race/ethnic categories were observed was: NHB > MA > NHW and all three pairwise differences were statistically significant for both adults and seniors (Table , p < 0.01). Racial/ethnic differences were not found to exist for UCd (Table ).
Table 3. Unadjusted geometric means with 95% confidence intervals for blood cadmium in μg/L and urine cadmium in μg/L by gender, race/ethnicity, and exposure to environmental tobacco smoke (ETS) by age for never smokers
Exposure to ETS either at home or at work did not seem to affect UGMs for BCd or UCd among adults as well as seniors (Table ). The observed difference between exposure to ETSW and no exposure to ETSW as observed for adults was probably due to small sample sizes.
Irrespective of gender, race/ethnicity, and ETS exposure, seniors had higher UGM for both BCd and UCd than adults (p < 0.01, Table ).
3.2. Multivariate analysis
3.2.1. Gender and racial/ethnic differences
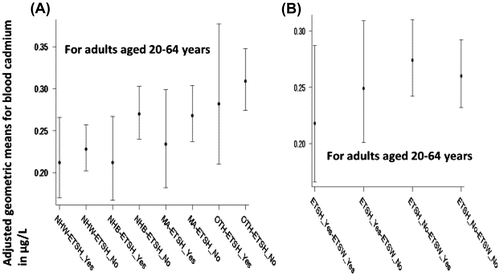
Adjusted geometric means (AGM) of BCd as well as UCd for both adults and senior citizens were found to be higher for females when compared with males (Table , p < 0.01). The ratios of females to males AGMs were: 1.4 and 1.2 for BCd for adults and seniors, respectively, and 1.2 and 1.5 for UCd for adults and seniors, respectively. The order in which BCd among three major race/ethnic categories were observed was: NHB > MA > NHW and all three pairwise differences were statistically significant for both adults and seniors (Table , p < 0.01). Racial/ethnic differences were not found to exist for UCd (Table ). However, among adults, when statistically significant interaction between race/ethnicity and ETSH was taken into account, NHW had lower AGM for BCd than NHB and MA only when there was no ETSH exposure (0.228 vs 0.270 and 0.268 μg/L, Figure , Panel A).
Table 4. Adjusted geometric means with 95% confidence intervals for blood cadmium in μg/L and urine cadmium in μg/L by gender, race/ethnicity, and exposure to environmental tobacco smoke (ETS) by age for never smokers
Figure 1. Adjusted geometric means in μg/L with 95% confidence intervals for blood cadmium for adults aged 20–64 years by (A) race/ethnicity and exposure to environmental tobacco smoke (ETS) at home (NHW = Non-Hispanic White, NHB = Non-Hispanic Black, MA = Mexican American, OTH = other unclassified race/ethnicities, ETSH_Yes = exposed to ETS at home, ETS_No = not exposed to ETS at home, and (B) exposure to ETS at home and exposure to ETS at work (ETSW_Yes = exposure to ETS at work, ETSW_No = no exposure to ETS at work).
In the presence of statistically significant interaction between gender and race/ethnicity for BCd for seniors, male–female differences were limited to NHW (0.34 vs. 0.41 μg/L, p < 0.01) and NHB (0.35 vs. 0.42 μg/L, p < 0.01) only (Figure , Panel A), and among senior males, only NHW had lower levels than MA (0.34 vs. 0.385 μg/L, p = 0.03, Figure , Panel A). For females, racial/ethnic differences among three major racial/ethnic groups for BCd for seniors were not observed (Figure , Panel A).
Figure 2. Adjusted geometric means in μg/L with 95% confidence intervals for (A) blood cadmium for adults aged 20–64 years by gender (M = male, F = female) and race/ethnicity (NHW = Non-Hispanic White, NHB = Non-Hispanic Black, MA = Mexican American, OTH = other unclassified race/ethnicities) and (B) urine cadmium for seniors aged ≥ 65 years by gender and race/ethnicity.
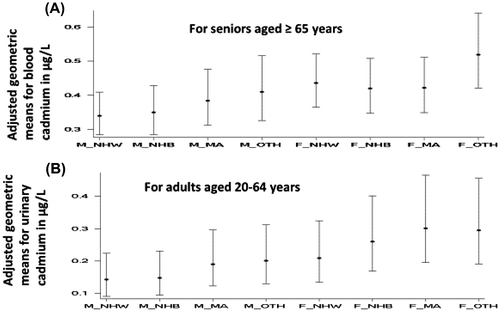
For adults, there was a statistically significant interaction for UCd, and male–female differences were observed for all four racial/ethnic groups but male–female differences varied by race/ethnicity. For NHW, the male–female difference was 0.066, 0.112 μg/L for NHB, and 0.111 μg/L for MA. Thus, male–female differences were higher for NHB and MA as compared to NHW (Figure , Panel B). Between NHW and MA and between NHB and MA, statistically significant differences were observed for both males and females but statistically significant differences between NHW and NHB were observed for females only (Figure , Panel B).
3.2.2. Effect of exposure o ETS
AGMs for those who were exposed to ETS and were not exposed to ETS at home and/or work did not differ (Table ) for either BCd or UCd. However, among adults, when statistically significant interaction between race/ethnicity and ETSH was taken into account, NHB with no ETSH had higher AGM for BCd than NHB without ETSH (0.270 vs 0.211 μg/L, Figure , Panel A). Also, when statistically significant interaction between ETSH and ETSW is taken into account for adults, among those with ETSH exposure, exposure to ETS at work was associated with lower BCd levels when compared with no exposure to ETS at work (0.218 vs. 0.274 μg/L, p = 0.04, Figure , Panel B); and among those with no ETSH, exposure to ETS at work was associated with higher BCd levels when compared with no exposure to ETS at work (0.274 vs. 0.260 μg/L, p = 0.04, Figure , Panel B).
3.2.3. Effect of age, age2, body mass index, and poverty income ratio
Among adults (Table ), levels of both BCd and UCd increased with increase in age (Table , p < 0.01). For one-year increase in age, the levels of BCd and UCd increased by 1.3% and 6.1%, respectively. For adults, there was a negative association between the levels of UCd and age2. Among seniors, there was a negative association between the levels of UCd and age (β = −0.09013, p < 0.01, Table ) and a positive association between the levels of UCd and age2 (β = 0.00063, p = 0.03, Table ).
Table 5. Regression slopes and percent change in the values of blood and urine cadmium with a unit changeTable Footnote* in the values of independent variables for those aged 20–64 years
Table 6. Regression slopes and percent change in the values of blood and urine cadmium with a unit changeTable Footnote* in the values of independent variables for those aged ≥ 65 years
For a 10% increase in body mass index, among adults, levels of BCd decreased by 2.5% and the levels of UCd decreased by 2.6% (Table ). For a 10% increase in body mass index, among seniors, levels of BCd decreased by 1.6% and the levels of UCd decreased by 3.5% (Table ). Poverty income ratio did not affect the levels of either BCd or UCd among either adults or seniors (Tables and ).
3.2.4. Impact of exposure to ETS
Number of cigarette smokers smoking inside home, number of cigarettes being smoked at home every day, or number of hours participants were exposed to ETS at work did not affect the levels of BCd or UCd among either adults or seniors (Tables and ).
3.2.5. Adjusted time trends
Among adults, for every two-year period, adjusted BCd levels decreased by 5.8% and adjusted UCd levels decreased by 3.8% (Table , p < 0.01). Among seniors, for every two-year period, adjusted BCd levels decreased by 4.3% (Table , p < 0.01) but adjusted UCd levels did not change over time (Table , p = 0.27).
3.2.6. Impact of diet
Diet did not affect the adjusted levels of BCD or UCd among either adults or seniors.
4. Discussion
4.1. Trends in unadjusted and adjusted levels of BCd and UCd
Compared to 1999–2000, there was a 32.4% decline in the unadjusted values of BCd for adults and 27% decline for seniors. For UCd, there was a 27.1% decline for adults and 16.3% decline for seniors (Table , p = 0.29). Richter et al. (Citation2009) did not report UGMs for each individual NHANES wave, for example, for 1999–2000. Percent reduction in UGMs for UCd for 2003–2008 NHANES data as compared to 1988–1994 data among those aged ≥ 20 years was 28.4% among never smokers, 35.7% among former smokers, and 32.4% among current smokers. Adams and Newcomb (Citation2014) did not report on time trends for either UCd or BCd levels.
Trends in adjusted levels of BCd paralleled trends for unadjusted levels. For 1999–2010, decline in adjusted levels of BCd was 5.8% for adults and 4.3% for seniors for every two year. Decline in adjusted levels of UCd was 3.8% for adults and 1.8% for seniors for every two year.
4.2. Age differences
Unadjusted levels of both UCd and BCd were higher for seniors than adults. While no formal statistical tests were conducted, adjusted levels of both BCd and UCd were in the order: seniors > adults (Table ). In addition, among never smoker adults, adjusted levels of BCd increased by 1.3% (p < 0.01) but not among seniors. Adjusted levels of UCd increased by 6.1% (p < 0.01) among adults but decreased by 23.1% (p < 0.01) among seniors. However, because of statistically significant negative slope for age2 (p < 0.01), this increase slowed down with increase in age for BCd as well as UCd for adults.
4.3. Gender differences
Higher Adjusted levels of both BCd and UCd for never smoker females than never smoker males are consistent with other studies, for example, Adams and Newcomb (Citation2014). Riederer et al. (Citation2013), however, did not observe these gender differences which according to these authors may be due to them controlling for a wider range of covariates. Females have been stipulated to absorb higher levels of Cd because of relatively lower iron storage than males (Gallagher, Chen, & Kovach, Citation2011; Julin et al., Citation2011).
4.4. Racial/ethnic differences
Riederer et al. (Citation2013) reported MA and NHB adults born within and outside of U.S. to have higher UCd levels than NHW born within U.S. among adult nonsmokers and MA born outside of U.S. to have higher UCd levels than NHW born within U.S. among adult smokers. Richter et al. (Citation2009) reported unadjusted levels for UCd levels among NHB to be higher than NHW. Consistent with these observations, for this study for never smoker adults and seniors, the order of adjusted levels for BCd and UCd was NHW < NHB as well as NHW < MA and almost all pairwise differences were statistically significant. Riederer et al. (Citation2013) speculated race/ethnic differences to be attributable to where different racial/ethnic groups reside. Those living in urban areas are likely to be associated with higher Cd levels than those living in the rural areas. The speculation of Riederer et al. (Citation2013) may be true to a degree because there are racial/ethnic differences in where they live but the possibility of metabolic differences in how Cd is excreted from the body among different race/ethnic groups cannot be ruled out.
4.5. Effect of environmental tobacco smoke
Exposure to ETSH or ETSW did not affect the levels of BCd or UCd in this study. These results are consistent with those reported by Adams and Newcomb (Citation2014) who did not find exposure to ETSH having any effect on the levels of either UCd or BCd.
4.6. Effect of body mass index
Negative association between BCd as well as UCd levels and BMI for never smoker adults and seniors is consistent with the results reported by Riederer et al. (Citation2013) and Padilla, Elobeid, Ruden, and Allison (Citation2010). For a 10% change in BMI, the decrease in the levels of BCd was 2.5% for adults and 1.6% for seniors. For a 10% change in BMI, the decrease in the levels of UCd was 2.6% for adults and 3.5% for seniors.
4.7. Effect of poverty income ratio
For this study, no association between poverty income ratio and BCd as well as UCd was observed for never smoker adults and seniors. Riederer et al. (Citation2013) did not find any association between UCd and PIR.
4.8. Effect of diet
Garner and Levallois (Citation2016) evaluated the association of the frequency of the yearly consumption of nine different foods with the levels of BCd and UCd among all respondents and never smokers and reported positive associations between BCd levels and (i) the consumption of shellfish, cereals, and potatoes other than french fries for all female respondents, and (ii) cereals and nuts and seeds for all male respondents. They also reported negative associations between UCd levels and potatoes other than french fries among all female respondents. In order to evaluate association between dietary intake and BCd and/or UCd, long-term dietary intake data like the one used by Garner and Levallois (Citation2016) are preferred but due to the non-availability of food frequency data from NHANES on a consistent basis, data on consumption of 10 foods consumed in grams during the last 24 h were used for this study. Since, UCd is a measure of long-term exposure to Cd and dietary data used for this study depicted recent intake of Cd via food, no association between UCd and dietary intake was observed. Also, no association between BCd levels and dietary intake was observed. However, Riederer et al. (Citation2013) also used total consumption of micronutrients like iron, magnesium, selenium, and others during the last 24 h to evaluate association between UCd levels and these micronutrients and found no associations for children and adolescents. But, they did observe a negative association between UCd levels and total calorie intake during the last 24 h and a positive association with total magnesium intake during the last 24 h for nonsmoker adults.
4.9. Strengths and limitations
A large nationally representative data over a period of 14 years add strength to the results observed in this study. However, cross-sectional nature of data also dilutes the confidence that can be placed in the observed results. No longitudinal data on the levels of exposure to Cd including those from dietary sources and no data on observed levels of BCd and/or UCd were available. UCd data were based on spot urine samples which further complicate the issue of “true” levels of Cd in these samples.
Additional information
Funding
Notes on contributors
Ram B. Jain
Ram B. Jain has been engaged in environmental health research since 2002. Since his retirement from Centers for Disease Control in 2010, he practices as a private consultant and an independent researcher from his home and is primarily involved in analyzing data using National Health and Nutrition Examination Survey. He has recently published in Biomarkers, Environmental Science and Pollution Research, and Environmental Toxicology and Pharmacology. His current research interests, among others, include studying concentrations of environmental contaminants among users of e-cigarettes as compared to the smokers of traditional tobacco products.
References
- Adams, S. V., & Newcomb, P. A. (2014). Cadmium blood and urine concentrations as measures of exposure, NHANES 1999–2010. Journal of Exposure Science and Environmental Epidemiology, 24(2), 163–170. doi:10.1038/jes.2013.55
- Afridi, H. I., Kazi, T. G., Talpur, F. N., & Brabazon, D. (2015). Evaluation of trace and toxic elements in the samples of different cigarettes and their impact on human health of Irish diabetes mellitus patients. Clinical Laboratory, 61(1–2), 123–140.
- Ahn, B., Kim, S. H., & Park, M. J. (2017). Blood cadmium concentrations in Korean adolescents: From the Korea National Health and Nutrition Examination Survey 2010-2013. International Journal of Hygiene and Environmental Health, 220(1), 37–42. doi:10.1016/j.ijheh.2016.10.003
- Aoki, Y., Brody, D. J., Flegal, K. M., Fakhouri, T. H., Axelrad, D. A., & Parker, J. D. (2016). Blood lead and other metal biomarkers as risk factors for cardiovascular disease mortality. Medicine, 95(1), e2223. doi: 10.1097/MD.0000000000002223
- Arrandale, V. H., Beach, J., Cembrowski, G. S., & Cherry, N. M. (2015). Urinary metal concentrations among female welders. Annals of Occupational Hygiene, 59(1), 52–61. doi:10.1093/annhyg/meu079
- Beveridge, R., Pintos, J., Parent, M. E., Asselin, J., & Siemiatycki, J. (2010). Lung cancer risk associated with occupational exposure to nickel, chromium VI, and cadmium in two population-based case-control studies in Montreal. American Journal of Industrial Medicine, 53(5), 476–485. doi:10.1002/ajim.20801
- Buser, M. C., Ingber, S. Z., Raines, N., Fowler, D. A., & Scinicariello, F. (2016). Urinary and blood cadmium and lead and kidney function, NHANES 2007-2012. International Journal of Hygiene and Environmental Health, 219(3), 261–267. doi: 10.1016/j.ijheh.2016.01.005
- Cheung, M. R., Kang, J., Ouyang, D., & Yeung, V. (2014). Association between urinary cadmium and all cause, all cancer and prostate cancer specific mortalities for men: An analysis of national health and nutrition examination survey (NHANES III) data. Asian Pacific Journal of Cancer Prevention, 15(1), 483–488.10.7314/APJCP.2014.15.1.483
- De Franciscis, P., Ianniello, R., Labriola, D., Ambrosio, D., Vagnetti, P., Mainini, G., … Caprio, F. (2015). Environmental pollution due to cadmium: Measure of semen quality as a marker of exposure and correlation with reproductive potential. Clinical and Experimental Obstetrics & Gynecology , 42(6), 767–770.
- Dodd-Butera, T., Quintana, P. J., Ramirez-Zetina, M., Batista-Castro, A. C., Sierra, M. M., Shaputnic, C., … Hull, S. (2017). Placental biomarkers of PAH exposure and glutathione-S-transferase biotransformation enzymes in an obstetric population from Tijuana, Baja California, Mexico. Environmental Research, 152, 360–368. doi:10.1016/j.envres.2016.04.019
- Everett, C. J., & Frithsen, I. L. (2008). Association of urinary cadmium and myocardial infarction. Environmental Research, 106(2), 284–286.10.1016/j.envres.2007.10.009
- Ferraro, P. M., Costanzi, S., Naticchia, A., Sturniolo, A., & Gambaro, G. (2010). Low level exposure to cadmium increases the risk of chronic kidney disease, analysis of the NHANES 1999-2006. BMC Public Health, 10, 668. doi: 10.1186/1471-2458-10-304
- Gallagher, C. M., Chen, J. J., & Kovach, J. S. (2010). Environmental cadmium and breast cancer risk. Aging, 2(11), 804–814.10.18632/aging.v2i11
- Gallagher, C. M., Chen, J. J., & Kovach, J. S. (2011). The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20-49 years. Environmental Research, 111(5), 702–707. doi: 10.1016/j.envres.2011.03.007
- Gallagher, C. M., Kovach, J. S., & Meliker, J. R. (2008). Urinary cadmium and osteoporosis in U.S. Women >or = 50 years of age, NHANES 1988-1994 and 1999-2004. Environmental Health Perspectives, 116(10), 1338–1343. doi: 10.1289/ehp.11452
- Garner, R., & Levallois, P. (2016). Cadmium levels and sources of exposure among Canadian adults. Health Reports, 27(2), 10–18.
- Gil, F., Hernández, A. F., Márquez, C., Femia, P., Olmedo, P., López-Guarnido, O., & Pla, A. (2011). Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Science of The Total Environment, 409(6), 1172–1180. doi:10.1016/j.scitotenv.2010.11.033
- Haddam, N., Samira, S., Dumont, X., Taleb, A., Lison, D., Haufroid, V., & Bernard, A. (2011). Confounders in the assessment of the renal effects associated with low-level urinary cadmium: An analysis in industrial workers. Environmental Health, 10, 65. doi:10.1186/1476-069X-10-37
- Honda, R., Swaddiwudhipong, W., Nishijo, M., Mahasakpan, P., Teeyakasem, W., Ruangyuttikarn, W., … Nakagawa, H. (2010). Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicology Letters, 198(1), 26–32. doi:10.1016/j.toxlet.2010.04.023
- Jain, R. B. (2016). Factors affecting the variability in the observed levels of urinary cadmium among children and nonsmoker adolescents. Environmental Sc Pollution Research. doi:10.1007/s11356-016-8008-z
- Julin, B., Vahter, M., Amzal, B., Wolk, A., Berglund, M., & Åkesson, A. (2011). Relation between dietary cadmium intake and biomarkers of cadmium exposure in postmenopausal women accounting for body iron stores. Environmental Health, 10, 201.10.1186/1476-069X-10-105
- Kazi, T. G., Wadhwa, S. K., Afridi, H. I., Kazi, N., Kandhro, G. A., Baig, J. A., … Arain, M. B. (2010). Interaction of cadmium and zinc in biological samples of smokers and chewing tobacco female mouth cancer patients. Journal of Hazardous Materials, 176(1–3), 985–991. doi:10.1016/j.jhazmat.2009.11.139
- Lin, Y. S., Caffrey, J. L., Lin, J. W., Bayliss, D., Faramawi, M. F., Bateson, T. F., & Sonawane, B. (2013). Increased risk of cancer mortality associated with cadmium exposures in older americans with low zinc intake. Journal of Toxicology and Environmental Health, Part A, 76(1), 1–15. doi: 10.1080/15287394.2012.722185
- Lin, Y. S., Rathod, D., Ho, W. C., & Caffrey, J. J. (2009). Cadmium exposure is associated with elevated blood C-reactive protein and fibrinogen in the U. S. population, the third national health and nutrition examination survey (NHANES III, 1988–1994). Annals of Epidemiology, 19(8), 592–596. doi: 10.1016/j.annepidem.2009.02.005
- Mijal, R. S., & Holzman, C. B. (2010). Blood cadmium levels in women of childbearing age vary by race/ethnicity. Environmental Research, 110(5), 505–512. doi: 10.1016/j.envres.2010.02.007
- Navas-Acien, A., Selvin, E., Sharrett, A. R., Calderon-Aranda, E., Silbergeld, E., & Guallar, E. (2004). Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation., 109(25), 3196–3201.10.1161/01.CIR.0000130848.18636.B2
- Padilla, M. A., Elobeid, M., Ruden, D. M., & Allison, D. B. (2010). An examination of the association of selected toxic metals with total and central obesity indices, NHANES 99-02. International Journal of Environmental Research and Public Health, 7(9), 3332–3347. doi: 10.3390/ijerph7093332
- Patel, C. J., Rehkopf, D. H., Leppert, J. T., Bortz, W. M., Cullen, M. R., Chertow, G. M., Ioannidis, J. P.. (2013). Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States national health and nutrition examination survey. International Journal of Epidemiology, 42(6), 1795–1810. doi: 10.1093/ije/dyt208
- Peters, J. L., Perlstein, T. S., Perry, M. J., McNeely, E., & Weuve, J. (2010). Cadmium exposure in association with history of stroke and heart failure. Environmental Research, 110(2), 199–206. doi: 10.1016/j.envres.2009.12.004
- Richter, P. A., Bishop, E. E., Wang, J., & Swahn, M. H. (2009). Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population, the National Health and Nutrition Examination Survey (NHANES) 1999–2004. The International Journal of Environmental Research and Public Health, 6 (7), 1930–1946. doi:10.3390/ijerph.6071930
- Riederer, A. M., Belova, A., George, B. J., & Anastas, P. T. (2013). Urinary cadmium in the 1999–2008 U.S. national health and nutrition examination survey (NHANES). Environmental Science & Technology, 47(2), 1137–1147. doi: 10.1021/es303556n
- Schwartz, G. G., Il’yasova, D., & Ivanova, A. (2003). Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care., 26(2), 468–470.10.2337/diacare.26.2.468
- Silver, M. K., Lozoff, B., & Meeker, J. D.. (2013). Blood cadmium is elevated in iron deficient U.S. children, a cross-sectional study. Environmental Health, 12, 50. doi: 10.1186/1476-069X-12-117
- Sorkun, H. C., Bir, F., Akbulut, M., Divrikli, U., Erken, G., Demirhan, H., … Yozgatlı, U. (2007). The effects of air pollution and smoking on placental cadmium, zinc concentration and metallothionein expression. Toxicology, 238(1), 15–22.10.1016/j.tox.2007.05.020
- Tellez-Plaza, M., Navas-Acien, A., Caldwell, K. L., Menke, A., Muntner, P., & Guallar, E. (2012b). Reduction in cadmium exposure in the United States population, 1988-2008, the contribution of declining smoking rates. Environ Health Perspect, 120(2), 204–209. doi: 10.1289/ehp.1104020
- Tellez-Plaza, M., Navas-Acien, A., Crainiceanu, C. M., & Guallar, E. (2008). Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect, 116(1), 51–56. doi: 10.1289/ehp.10764
- Tellez-Plaza, M., Navas-Acien, A., Menke, A., Crainiceanu, C. M., Pastor-Barriuso, R., & Guallar, E. (2012a). Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environmental Health Perspectives, 120(7), 1017–1022. doi: 10.1289/ehp.1104352
- Thomas, L. D., Hodgson, S., Nieuwenhuijsen, M., & Jarup, L. (2009, February). Early kidney damage in a population exposed to cadmium and other heavy metals. Environmental Health Perspectives, 117(2), 181–184. doi:10.1289/ehp.11641
- Togawa, K., Le Cornet, C., Feychting, M., Tynes, T., Pukkala, E., Hansen, J., … Schu z, J. (2016). Parental occupational exposure to heavy metals and welding fumes and risk of testicular germ cell tumors in offspring: A registry-based case-control study. Cancer Epidemiology Biomarkers & Prevention, 25(10), 1426–1434.10.1158/1055-9965.EPI-16-0328
- Trzeciakowski, J. P., Gardiner, L., & Parrish, A. R. (2014). Effects of environmental levels of cadmium, lead and mercury on human renal function evaluated by structural equation modeling. Toxicology Letters, 228(1), 34–41. doi: 10.1016/j.toxlet.2014.04.006
- Wallia, A., Allen, N. B., Badon, S., & El Muayed, M. (2014). Association between urinary cadmium levels and prediabetes in the NHANES 2005-2010 population. International Journal of Hygiene and Environmental Health, 217(8), 854–860. doi: 10.1016/j.ijheh.2014.06.005
- Wu, C. K., Chang, M. H., Lin, J. W., Caffrey, J. L., & Lin, Y. S. (2011). Renal-related biomarkers and long-term mortality in the US subjects with different coronary risks. Atherosclerosis, 216(1), 226–236. doi: 10.1016/j.atherosclerosis.2011.01.046
- Wu, E. W., Schaumberg, D. A., & Park, S. K. (2014). Environmental cadmium and lead exposures and age-related macular degeneration in U.S. adults, the national health and nutrition examination survey 2005 to 2008. Environmental Research, 133, 178–184. doi: 10.1016/j.envres.2014.05.023
- Yaprak, E., Yolcubal, İ., Sinanoğlu, A., Doğrul-Demiray, A., Guzeldemir-Akcakanat, E., & Marakoğlu, İ. (2017). High levels of heavy metal accumulation in dental calculus of smokers: A pilot inductively coupled plasma mass spectrometry study. Journal of Periodontal Research, 52, 83–88. doi:10.1111/jre.12371
