?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Wastewater effluents directly discharged into nearby stream are eventually used to irrigate plants like sugarcane in Ghana. In this study, 24 triplicates sugarcane stems (sugarcane juice was extracted), 24 triplicates soil samples and 8 triplicates water samples were collected from sugarcane farms in four communities in Ashanti region of Ghana. Two of the communities were exposed to wastewater while the other two without wastewater contamination served as control. Metals (Pb, Cd, Cr, Cu, and Fe) concentration were determined in the digested samples using Spectra AA 220 flame atomic absorption spectrometer. The Pb concentration in all sugarcane juice samples ranged between 12.65 and 145.0 μg/L. The mean Cu concentration of the sugarcane juice samples varied between 11.28 and 156.00 μg/L. In general, there were decrease in metals investigated in sugarcane juice as you move away from the stream. However, the reduction was more pronounced in the hotspot sampling areas than control sampling areas. The EDI value was 9.76 × 10−4, 2.94 × 10−5, 1.09 × 10−3, and 9.07 × 10−3 (mg/kg-day) for Pb, Cd, Cu, and Fe, respectively. Mean hazard quotient (HQ) for the metals studied ranged from 0.036 (Fe) to 0.286 (Pb). The results of this study indicate that sugarcane is able to grow in soils where some metals are accumulated. High levels of metals were pronounced in sugarcane originating from wastewater polluted soils as those considered in this study. The consumption of normal quantity of sugarcane juice may not present detrimental health concerns through a lifetime based on the metals contents alone.
Public interest statement
Plant species cannot avoid taken up metals in soils they are grown. Some of these metals have no use in the body and even poison the body. In this study, sugarcane grown in areas prone to wastewater was analyzed to know the extent of contamination and whether the levels are harmful to humans. The results indicate that sugarcane is able to grow in soils which are contaminated with metal. High levels of metals were pronounced in sugarcane originating from wastewater polluted soils than areas not polluted with wastewater. However, all the concentrations were below levels recommended to be harmful to human. Hence, consumption of normal quantity of sugarcane juice from this study area may not present detrimental health concerns through a lifetime based on the metals contents alone.
Competing interests
The authors declare no competing interest.
1. Introduction
Majority of plant species grown in soils that are polluted with metals are incapable of avoiding absorbing them (Baker, Citation1981). Conversely, human activities that accompany agricultural practices, industrial processes, mineral exploration and waste management greatly contribute to the pollution of natural ecosystems by heavy metals (Alumaa, Kirso, Petersell, & Steinnes, Citation2002; Bilos, Colombo, Skorupka, & Rodrigues, Citation2001; Keane, Collier, Shann, & Rogstad, Citation2001).
Heavy metals and metalloids accumulation in agricultural soils is an alarming subject due to the associated food safety issues and potential health risks, coupled-up with the harm they cause to soil ecosystems (McLaughlin, Parker, & Clarke, Citation1999).
The harmful effects of metals have extensively been described. For instance, cadmium, mercury, arsenic, nickel, lead, etc., have a wide range of toxicity including hepatotoxic, neurotoxic, teratogenic, mutagenic, and nephrotoxic effects among others (Bucheim, Stoltenburg-Didinger, Lilienthal, & Winnike, Citation1998; Domingo, Citation1994; Hudnell, Citation1999; Kelley, Citation1999; Lai, Minski, Chan, Leung, & Lim, Citation1999; McLaughlin et al., Citation1999). In addition, chromium, cadmium, and arsenic are considered cancer-causing (Costa, Citation1998).
Contaminating agricultural soils with wastewater that contains heavy metals is a serious matter because it leads to higher heavy metal uptake by crops. This uptake affects the quality and safety of our food, and poses threat to human health (Hough, Young, & Crout, Citation2003; Mensah et al., Citation1999). Serious systemic health problems can arise when dietary heavy metals like lead (Pb), cadmium (Cd), and chromium (Cr) accumulate excessively in the human body (Oliver, Citation1997).
An earlier survey in Kumasi, Ghana, found that rivers or streams in Kumasi are heavily polluted with raw sewage (Cornish, Aidoo, & Ayamba, Citation2001) as most sewage is either discharged straight into rivers and streams or is collected from septic tanks and then disposed of into waterways (Keraita & Drechsel, Citation2004)
Routine monitoring of heavy metals concentration in soils and crops are, therefore, essential to know their levels and devise strategies to minimize contamination, in order to reduce risks to human health. The objective of this project is to identify health hazards associated with sugarcane grown in wastewater polluted wetlands by determining Pb, Cd, Cr, Cu, and Fe concentration in water, soil, and sugarcane juice collected from wastewater polluted and unpolluted sugarcane farms (control) where the stream passing through the sugarcane farm serves as the irrigation water for the sugarcane; identifying the correlation between metals concentrations in water, soil and sugarcane juice; estimating the variation in metal concentration due to seasonality, determining metals concentration in sugarcane juice as you move away from the stream and estimating daily intake of metals (EDI) and target hazard quotients (THQ) values for all metals in sugarcane juice.
2. Materials and methods
2.1. Study area
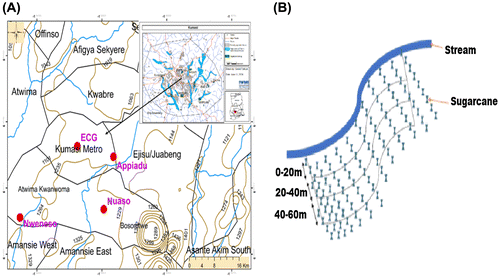
The city of Kumasi is the second largest in Ghana and lies about 150 km northwest of Accra. The population census in 2010 counted about two million inhabitants (Ghana Statistical Service, Citation2012) Kumasi is located on a drainage divide with 28% of the developed area drain to the west eventually joining the Offin River and 72% of the developed area drain to the Oda River in the south of the city. Most streams originate within the administrative boundaries of Kumasi. The only considerable inflow from outside is noted from Sisa and Wiwi Rivers to the north of Kumasi. Its area comprises 254 km2 out of which around 80% are developed. In 2005, 75 km2, or 30%, were either referred to as open space or undeveloped land such as river valleys or other unpopulated areas (Obuobie et al., Citation2006). Figure (a), shows the study area with the rivers, areas where sugarcane is grown, vegetable farms and populated areas. The study areas are wetlands, encircled by farm lands, in which sugarcane has been the planted. These sites are Nweneso, Nuaso, Adum ECG, and Appiadu
2.2. Sampling
Sampling was done in sugarcane farms in four communities in Ashanti region, Ghana. Among the four sugarcane farms under study, two were exposed to wastewater which were chosen as hotspot (Adum ECG and Appiadu) and the other two (Nweneso and Nuaso) had no wastewater inflow into the wetland. The latter served as control areas.
The stem of sugarcane plants and soils were collected along three transects established at the north (N), east (E), west (W), and south (S) directions at each farm, at 0, 20, 40, 60 m distance from the stream shown on a schematic diagram in Figure (b). The stream water flowing along the sugarcane farms was sampled. The sampling areas were demarcated using Global Positioning System (Magellan GPS 315). Dry season sampling was done in February 2016, while wet season sampling was done in October 2015. A total of 24 triplicates sugarcane stems, 24 triplicates soil samples and 8 triplicates water samples were collected for investigation. All samples were kept in labeled sample containers and transported to the Chemistry Department of KNUST for analysis.
2.2.1. Sample preparation
Concentrated nitric acid (95%) used was of analytical reagent grade (BDH Chemical Ltd, Poole, England). The nitrate salts of Cd, Pb, Cu, Cr, and Fe (bought from Merck Chemicals, Germany) were used to prepare 1,000 mg/l stock solution in distilled water. Serial dilutions were prepared from this for various elements.
Glassware for digestion and storage of digest were washed under running tap water after been soaked in detergent solution overnight. They were subsequently soaked in 10% nitric acid overnight and then rinsed with distilled water.
Sugarcane stem samples were taken from averagely matured sugarcane farm stands. The sugarcane stems were washed with distilled water to remove soil and dirt. The washed sugarcane stems were peeled and cut to smaller sizes with the help of stainless knife. With the aid of an extractor, the sugarcane juice was squeezed out into plastic vials and refrigerated before analysis (Klein, Citation1987). The pH and total dissolved sugar (Brix) were determined and are reported as supplementary Table S1.
Sugarcane juice samples were wet digested by technique defined in (AOAC, Citation2000). One-gram (1 mL) sample was taken into 50 mL digestion tube along with 5 mL 65% nitric acid (Suprapur E. Merck). The contents were heated at 200 °C for 30 min. The digest was cooled, diluted to final volume of 50 ml with distilled water and transferred to 100 mL collection bottle. The soil samples were air-dried in the laboratory for 14 days, grounded with mortar and pestle, sieved with 2 mm nylon sieve. Portion of the soil before grinding was sent to Soil Science Laboratory-KNUST for the determination of its organic carbon content, pH, conductivity, and soil particle size. The soil properties data are presented as supplementary Table S2. The soil samples were digested using aqua regia (1:3) HNO3:HCl, following a standard procedure (Vowotor et al., Citation2014). Accurately 0.5 g soil sample was taken into 50 mL digestion tube along with 10 mL aqua regia. The contents were heated at 150 °C for 30 min and cooled and diluted to final volume of 50 ml with deionized water.
A 1000 mL water sample collected at each sampling point, was acidified with 5 mL 65% nitric acid (Suprapur E. Merck) and kept in ice chest containing ice. The samples were kept in the freezer until digestion. Another 1000 mL water sample collected at each sampling point, was not acidified and used to determined physicochemical properties being; pH, conductivity, dissolved oxygen (DO) and turbidity. The result of the physicochemical parameters determined are reported as supplementary Table S3. Digestion of water samples was in accordance to standard methods (American Public Health Association, Citation2005). Aliquot of 10 mL of 65% nitric acid (Suprapur E. Merck) was added to 1000 mL water sample and boiled at 200 °C on a hot plate in a fume hood. Boiling continued until a solution obtained was about 25 mL. The digest was allowed to cool. It was then filtered into 50 mL volumetric flask and diluted to 50 mL mark with distilled water.
2.2.2. Metal analysis
Metals analysis of the digested sugarcane juice samples were done with (Spectra AA 220 AAS, UK) atomic absorption spectrometer equipped with multiple hollow cathode lamps. The instrument was calibration using different concentrations of standard solutions of Pb, Cd, Cr, Cu, and Fe. The result of metal concentration was expressed in μg/L and presented in Table .
2.3. Statistical analysis of data
Statistics was of the data were performed with GraphPad Prism (Version 6.01, San Diego, USA). Metals concentrations for sugarcane juice were subjected to one-way ANOVA and Pearson correlation analysis (Table ) to test for variation in concentrations found in various sampling points and identify any relationship with physicochemical properties of the sugarcane juice. A probability of 0.05 or less would be considered to be significant. Each experiment was repeated three times and the research findings were presented in the form of mean and standard deviation using Microsoft excel.
2.4. Quality control
Calibration curve was prepared for various elements using triplicate of serial dilution standard solution. Detection limit was determined with blank solution and spike recovery was performed for various element by adding 50 mg of each element to one of the samples and followed the same digestion procedure and determination. The percentage recovery was then calculated. Precision of the instrument was determined by running on sample for eight times and calculating the standard deviation.
2.5. Estimation of daily intake and health risk indices of metals
2.5.1. Estimated daily intake
Estimated daily intake (EDI) of contaminant like metals, depend on exposure frequency and contact time among individuals. This site-specific information was collected from a questionnaire administered to sugarcane consumers. The estimated daily intake of each heavy metal in this exposure pathway was determined by the equation:
where EF—the exposure frequency; ED—the exposure duration; IR—sugarcane juice consumption rate (g/person/day), Cm is the heavy metal concentration in foodstuffs (mg/kg); WAB—average body weight (average adult body weight was considered to be 70 kg); and TA—average exposure time for non-carcinogens (Saha & Zaman, Citation2012). All the uncertainty parameters were created in Microsoft Excel 2013 and calculated with Monte Carlo simulation at 10,000 iterations using the @ Risk 7 (Palisade Corporation) software, which is an add-on to Excel. The input parameters used for the estimating hazard quotient are present in supplementary Table S4.
2.5.2. Hazard quotient
Hazard quotients (HQ) were developed by the US Environmental Protection Agency for the estimation of health risks related with long term exposure to chemicals. The non-cancer risks were stated in terms of a HQ for a single substance as:
The HQ has been classified into < 1 being no significant risk or systemic toxicity and HQ > 1 to be potential risk. A THQ less than 1 means the exposed population is unlikely to experience obvious adverse effects
2.5.3. Health Index
With exposure involving more than one chemicals, hazard index (HRI) was determined by the sum of the individual hazard quotients for each chemical. The HRI was used as a measure of the potential for harm. HRI above 1 means that there is a chance of non- carcinogens effects, with an increasing probability as the value increases.
3. Results
3.1. Result of quality control analysis
The procedures taken to ensure the validity of the metal analysis data in this study have been described in the method section above. The recoveries, regression co-efficiencies and detection limits of the elements analyzed are presented in Table . The linearity expressed as regression coefficient values of all the metals ranged from 97 to 99%. The recoveries obtained in this study ranging from 95% to 98% were with the acceptable limits of 95 to 100.4% (Thompson, Citation2005).
Table 1. Recoveries, regression co-efficiencies and detection limits of elements
3.2. Metal levels in various environmental samples analyzed
In the water samples, the mean concentration of heavy metals, Pb, Cd, Cr, Cu, and Fe, were 31.1, 9.8, 1.4, 515.3, and 5556.1 ppb, respectively (Table ). Fe content was the highest and that of Cr was the lowest in water. The order of accumulation in water was Fe > Cu > Pb > Cd > Cr. In the soil samples analyzed, maximum concentration of 840.1 μg/L and minimum concentration of 18.4 μg/L were recorded for Pb while Cu concentration determined in the soil samples ranged from 29.7 to 1924.4 μg/L. Maximum value of 648,500.2 and minimum, 28,497.2 μg/L, was recorded for Fe in soil samples analyzed (Table ).
Table 2. Concentration of metals (µg/L) in water, soil, and sugarcane juice and their corresponding recommend limits
Table 3. Pearson correlation coefficient of metals between soil, water, and sugarcane juice analyzed
In this study, the concentration of heavy metals detected in sugarcane juice were generally lower to metals in soil. The mean concentration of Pb, Cd, Cu, and Fe, were 55.0, 2.1, 65.9, 567.0, and 931.1 ppb, respectively (Table ).
3.3. Correlation of various metals concentration in soil, water and sugarcane juice samples
In general correlation of metal concentrations in soil with water and soil with sugarcane juice were positive (Table 3). Since the Cr concentrations in sugarcane juice were below detection, there was no correlation recorded. There were strong positive correlations of Pb, Cd, Cu, and Fe metals concentration investigated in soil with concentration found in sugarcane juice. The Pearson r2 values ranged from 0.68 to 0.91. The p-values were all statistically significant being 1.4 × 10−6 for Pb, 0.0002 for Cd, 8.4 × 10−10 for Cu and 2.5 × 10−7 for Fe (Table 3). With the exception of Cu which showed strong positive correlation which is not significant (p = 0.099) between metal concentrations in water with metal concentrations in sugarcane juice, the correlation between metal concentrations in water with metal concentrations in sugarcane juice were weak positive correlation for Pb, Cd, and Fe. Additionally, Cu showed a significant (p = 0.005) strong positive correlation between metal concentrations in soil with metal concentrations in water, but Cd recorded a strong positive correlation but not significant (p = 0.054). Lead, Cr and Fe showed a weak positive correlation between metal concentrations in soil with metal concentrations in water.
3.4. Effect of pollution on metals determined in sugarcane juice
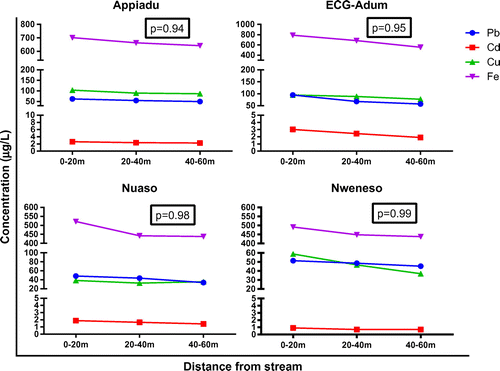
The mean values and standard deviation represent as error bars of Pb, Cd, Cu, and Fe in sugarcane juice samples from the twelve sampling sites are presented in Figure .
The hotspots, being areas polluted with solid and liquid waste (Appiadu and ECG-Adum samples) mean values of Pb, Cd, Cu, and Fe in sugarcane juice samples ranged from 24.00 to 145.00, 1.3–4, 38.38–156.00, and 261.95–1205.00 μg/L, respectively. The control sugarcane samples collected from Nweneso and Nuaso township showed mean values in the sugarcane juice samples ranged from 12.65 to 85.00 μg/L for Pb, b/d – 2.5 μg/L for Cd, 11.28–97.30 μg/L for Cu, and 149.60–855.00 μg/L for Fe. One-way ANOVA, at 95% confidence limit gave p values of 0.19, 0.02, 0.01, and 0.02 for Pb, Cd, Cu, and Fe, respectively.
3.5. Seasonal variation of parameters determined in sugarcane juice
The dry season mean values (bars) and standard deviation (error bars) of Pb (85.33 ± 22.48 μg/L), Cd (2.44 ± 0.98 μg/L), Cu (102.38 ± 38.27 μg/L), and Fe (892.17 ± 159.58 μg/L) are represented in Figure . The wet season mean and standard deviation values in the sugarcane juice samples are 24.33 ± 9.01 μg/L for Pb, 1.21 ± 0.56 μg/L for Cd, 29.34 ± 15.69 μg/L for Cu and 243.26 ± 89.18 μg/L for Fe.
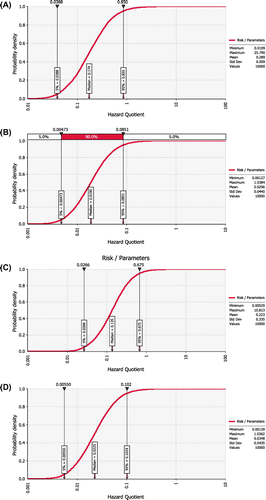
Figure 3. (a) Health quotient graph of lead, (b) Health quotient graph of cadmium, (c) Health quotient graph of copper, and (d) Health quotient graph of iron.
The p values for One-way ANOVA, at 95% confidence limit were 0.04, 0.07, 0.001, and 0.002 for Pb, Cd, Cu, and Fe, respectively
3.6. Variation from stream
In general, the concentration of metals investigated in sugarcane juice decreased as you move away from the stream. The mean Pb values of the sugarcane juice samples from the sampling sites ranged between 12.65 and 145.0 μg/L, with the highest value being recorded at ECG-Adum (0–20 m) sample (BDA) while the lowest was recorded at Nuaso (40–60 m) sample (NWC).
ECG-Adum (0–20 m) sugarcane juice sample (BDA) recorded the highest mean value of Cd determined being 4 μg/L and Nweneso and Nuaso samples collected in the wet season were b/d. Chromium values were below detection for all the samples. The mean Cu concentration of the sugarcane juice samples varied between 11.28 and 156.00 μg/L. These values were recorded in Nuaso (20–40 m) (NWB) and Appiadu (0–20 m) (ADA), respectively.
The highest value of Fe in the sugarcane juice samples was 1205.0 μg/L and was recorded at ECG-Adum (0–20 m) (BDA) while the lowest Fe concentration of 149.6 μg/L was recorded at Nweneso (20–40 m) (KWB). The p values for One- way ANOVA, at 95% confidence limit was 0.94 for Appiadu, 0.95 for ECG-Adum, 0.98 for Nuaso and 0.99 for Nweneso, respectively.
3.7. Health risk result
3.7.1. Estimated daily intake
The EDI value was 9.76 × 10−4, 2.94 × 10−5, 1.09 × 10−3 and 9.07 × 10−3 (mg/kg-day) for Pb, Cd, Cu, and Fe, respectively. The EDI showed an increasing order of Cd < Pb < Cu < Fe.
3.7.2. Health quotient
The health quotient of lead simulated over the population density shows the median and mean hazard quotient for lead was 0.175 and 0.286, respectively. At 95% probability density, hazard quotient was 0.894 which was still below 1.
Cadmium had a mean hazard quotient of 0.0297 and median hazard quotient of 0.0190. Additionally, hazard quotient at 95% probability density gave a value of 0.0860 which is by far lower than one. The median hazard quotient of copper was 0.137, with a mean of 0.223. Even at 95% probability density, the hazard quotient was 0.665. The hazard quotient at 95% probability density was 0.103 for iron. The median hazard quotient was 0.0233 and mean hazard quotient was 0.036. The health index as a sum of the individual metals hazard quotients was 0.56.
4. Discussion
4.1. Metals discussion
The maximum concentration of Pb, Cd, Cr, Cu, and Fe in the water samples are 31.1, 9.8, 1.4, 515.3, and 5556.1 μg/L, respectively. These were all below their corresponding US EPA (Citation2012) metal thresholds for reuse of wastewater being 10,000.0, 50.0, 1,000.0, 5,000.0, and 50,000 μg/L for Pb, Cd, Cr, Cu, and Fe, respectively.
In Accra, Pb contamination has been recorded in water used for vegetable irrigation to be 80 μg/L. This has been attributed to drains and untreated wastewater (diluted wastewater) joining the streams used for vegetable irrigation (Obuobie et al., Citation2006). Additionally, Azanu, Jørgensen, Darko, and Styrishave (Citation2016), recorded contamination of Pb (up to 28.7 μg/L) in waste stabilization ponds effluent entering Wiwi River in Kumasi which is used for vegetable irrigation downstream. Based on this result obtained in this study, it is clear that there is metal contamination of the streams in irrigating sugarcane farms. Wastewater used directly or indirectly for irrigation can result in metal accumulation in soils and crops eventually (Harmanescu, Alda, Bordean, Gogoasa, & Gergen, Citation2011; Singh & Agrawal, Citation2010).
Food and Agriculture Organization (FAO) and International Soil Reference and Information Centre (ISRIC) have established recommended values for Pb, Cd, Cr, Cu, and Fe metal concentrations in soils to be 150,000, 5,000, 250,000, 100,000, and 5,000,000 μg/kg (FAO and ISRIC, Citation2004). The maximum concentration of Pb, Cd, Cr, Cu, and Fe metal concentrations in soils investigated was 840.1, 15.1, 1.8, 1924.4, and 648,500.2 μg/kg respectively and were below the recommended values established by FAO and ISRIC.
Cultivation of food crops in contaminated environments is common in West Africa, as small scale farmers cultivate food crops at dumpsites to maximize yields due to the seemingly high organic contents of waste dumpsite soils. Odai, Mensah, Sipitey, Ryo, and Awuah (Citation2008) reported high levels (mg/kg) of Pb (54.6), Cd (2.87), Cu (1631.67), and Zn (2606.0) in soils used for vegetable cultivation at Kumasi waste dumpsites in Ghana. Soil contamination correlated with high concentrations of Pb (13.50) in onions, Cd (1.78) in cabbages and Cu (90.33) in lettuce grown at the dumpsites. Heavy metal contamination of agricultural soils and crops is particularly worse in developing industrialized countries such as China and India (Keraita, Jimenez, & Drechsel, Citation2008) due to extensive use of untreated industrial wastewater and Ashanti region is not an exception.
According to JECFA (Citation1983) the provisional tolerable daily intake (PMTDI) of Fe is 0.8 mg/kg and the maximum Fe concentration obtained in this study was 1205.00 ppb. The Fe concentration obtained in this study would only exceed the PMTDI when 1000 mL sugarcane juice is consumed in a day which do not happen. However the maximum Fe concentration obtained in this study was higher than concentration of Fe (352 ppb) in sugarcane juice reported in street vended sugarcane juice in Multan-Pakistan (Akhtar, Ismail, & Riaz, Citation2015). The variation could be due to pollution of Ghanaian rivers and wetlands (Cornish & Lawrence, Citation2001; Keraita, Citation2002), where these sugarcane are grown. In another study, sugarcane juice has been reported with lower Fe contents (0.266 mg/L) (Adekola & Akinpelu, Citation2002) than observed in present study.
Maximum tolerable limits of copper as contaminants in fresh vegetable juices have been defined by Codex Alimentarius Commission (Codex Alimentarius Commission, Citation2000) as 5.0 mg/L. and EU as 20 mg/kg (EC, Citation2006a).
The maximum copper concentration of 156.00 ppb was recorded among all the fresh sugarcane juices sampled. If compared with Codex Alimentarius Commission limits for Cu of 5.0 mg/L (Codex Alimentarius Commission, Citation2000), present concentrations in this particular study could not have any health risk. Mean copper contents in sugarcane juices of 150 ppb was recorded in street vended sugarcane juice in Multan-Pakistan (Akhtar et al., Citation2015) and was higher than 65.86 ppb determined in this study. The Cu concentration in street vended sugarcane in Kumasi have been reported to fluctuate between 0.192 mg/kg and dry weight (Azanu, Kyei, & Oppong, Citation2015). This indicates that sugarcane sold in the street could still contain Cu concentration and could be coming from areas polluted with metals.
Cadmium (Cd) is a toxic element of inert nature with no identified health benefits. European Commission (EC) had defined maximum acceptable level of cadmium in fresh fruit juices as 0.05 mg/L (EC, Citation2006b). Cadmium levels in all fresh sugarcane juices under study ranged from 0.4 to 4.0 ppb and was approximately 10 times lower in study under discussion to the EC limit. Fruits and beverages derived thereof have been reported with lower level of cadmium as compared to vegetables, cereals and cereals products that readily contribute to cadmium intoxication (EC, Citation2006b).
Mean concentration of Cd (45 ppb) measured in sugarcane juice from street vended sugarcane juice in Multan-Pakistan (Akhtar et al., Citation2015) and was higher than 4 ppb determined in this study. However, higher concentration of Cd (41 ppb) has been reported in sugarcane sold on the street of Kumasi (Azanu et al., Citation2015). Lead carry no health benefit rather extremely toxic if exposed beyond their safe limits that has been identified as 300 ppb for fresh fruits, vegetables and juices derived thereof (EC, Citation2006b). Codex commission recommends as 0.1 mg/L (Codex Alimentarius Commission, Citation2000). As compared to the EC limits, maximum Pb contents (145 ppb) of all sugarcane juices was approximately half in study under discussion.
Lead analysis of street vended fresh sugarcane juices of Multan-Pakistan revealed comparative level of 167 ppb (Akhtar et al., Citation2015). Wetlands in Kumasi under cultivation for sugarcane have often been reported to be contaminated with sewage water and other wastewater supplies. Hence, there could be greater probability of soil contamination with Pb and other toxic trace metals. Hence, toxic metals contamination of sugarcane juice through translocation from wastewater or sewage supplies could not be ignored.
4.2. Variation of contamination of metals determined in sugarcane juice
The hotspots sugarcane sampling areas being Appiadu and ECG-Adum, generally showed higher concentration of metals than the control areas. These differences were significant Cd (p = 0.02), Cu (p = 0.01), Fe (p = 0.02) but not for Pb (p = 0.19). In metal polluted soils a lot of vegetable species are not able to avoid the absorption of these elements (Baker, Citation1981). Concentration of Cd and Cu above EU limit have been reported in sugarcane samples from an area under the influence of a municipal landfill and a medical waste treatment system in Ribeirao, Brazil (Segura-Muñoz et al., Citation2006).
The maximum Cu concentrations in sugarcane juice were 156.0 and 97.3 μg/L for hotspot and control areas, respectively. It is assessed that in unpolluted soils, Cu concentration in vegetable tissues is between 6 and 25 mg/kg. However, in Cu-polluted soils the concentration of this element in vegetable tissues may reach 80 mg/kg (World Health Organization, Citation2001). These low values recorded in this study could be due to the use of sugarcane juice instead of the whole stem and the magnitude of pollution.
4.3. Variation from stream
The general decrease in metals concentrations investigated in sugarcane juice as you move away from the stream could be due to attenuation as you move away from point source contamination. This could be the plausible cause because the reduction was more pronounced in the hotspot sampling areas than control sampling areas.
4.4. Seasonal variation of parameters determined in sugarcane juice
Seasonal variation may cause significant difference in the availability of metals to plants. In this study, this fact was confirmed for the metals investigated. Their p values for One-way ANOVA, at 95% confidence limit were generally below 0.05 making them significantly different except Cd with p value of 0.07.
4.5. Estimation of Daily Intake and health risk indices of metals
Evidently, individual EDI’s of the various metals investigated in this study ranged from 2.94 × 10−5 to 9.07 × 10−3 mg/kg of body weight were far below RfD values of 0.7 mg/kg recommended by the international regulatory bodies (US EPA, Citation2009). The average lethal dose of iron is 200–250 mg/kg of body weight, but death has occurred following the ingestion of doses as low as 40 mg/kg of body weight (National Research Council, Citation1979). Mean hazard quotient (HQ) for each metals studied ranged from 0.036 (Fe) to 0.286 (Pb). The HQ has been defined so that if it is less than 1.0, there should be no significant risk or systemic toxicity and ratios above 1.0 could represent a potential risk. All HQ for the metals studied were below 1, hence there should be no major risk or systemic toxicity when these sugarcane juice studied are consumed.
5. Conclusions
The results of this study has demonstrated that sugarcane is able to grow well in areas where some metals in soils are accumulated. High levels of metals were found in sugarcane originating from soils polluted with wastewater in this field of study. Strong positive correlation was found between Cu concentrations in water and sugarcane juice, but Pb, Cd, and Fe showed weak correlation between metal concentrations in water and sugarcane juice. Additionally, Cu showed a significant (p = 0.005) strong positive correlation between metal concentrations in soil with metal concentrations in water, but Cd recorded a strong positive correlation but not significant (p = 0.054). The intake of normal amount of sugarcane juice may not pose any detrimental health issue through one lifetime based on the metals contents alone. Further study in this field should be considered with regards to the public health to mainly ascertain the mechanisms of metals integration during sugarcane production and also how use agricultural ways minimize heavy metals translocation in sugarcane. These studies should look on the influence of sugarcane varieties and soil type. Lastly, further studies should be focused on the interactions between metals, fertilizers, and herbicides on sugarcane and not only in aspect of risk, but also in other dimensions.
Supplementary material
Supplementary material for this article can be accessed https://doi.org/10.1080/23311843.2018.1455277.
Funding
The authors received no direct funding for this research.
OAES_1455277_Supplementary_Material.docx
Download MS Word (166.1 KB)Additional information
Notes on contributors
Agnes Oppong
Agnes Oppong is a senior technician at Department of Chemistry of Kwame Nkrumah University of Science and Technology, Ghana.
David Azanu
David Azanu is a lecturer and Head of Laboratory Services Unit at Kumasi Technical University, Ghana. Linda Aurelia Ofori is a lecturer at Department of Theoretical and Applied Biology of Kwame Nkrumah University of Science and Technology, Ghana.
References
- Adekola, F. A., & Akinpelu, A. A. (2002). Some trace elements in juice and bagasse of two varieties of sugarcane, location soil and irrigation water from Bacita Sugar Estate, Nigeria. Bioscience Research Communications, 14, 175–180.
- Akhtar, S., Ismail, T., & Riaz, M. (2015). Safety assessment of street vended juices in Multan-Pakistan: A study on prevalence levels of trace elements. International Journal of Food and Allied Sciences, 1(1), 1–10.10.21620/ijfaas.201511-10
- Alumaa, P., Kirso, U., Petersell, V., & Steinnes, E. (2002). Sorption of toxic heavy metals to soil. International Journal of Hygiene and Environmental Health, 204, 375–376.10.1078/1438-4639-00114
- American Public Health Association. (2005). Standard methods for the examination of water and waste water. (A. D. Eaton, L. S. Clesceri, E. W. Rice, & A. E. Greenberg, Eds.) (22nd ed.). Washington, DC: Author.
- AOAC (2000). Official methods of analysis, official method 999. Gaithersburg, MD: Author.
- Azanu, D., Jørgensen, S. E., Darko, G., and Styrishave, B. (2016). Simple metal model for predicting uptake and chemical processes in sewage-fed aquaculture ecosystem. Ecological Modelling, 319, 130–136. Elsevier B.V. doi:10.1016/j.ecolmodel.2015.07.023
- Azanu, D., Kyei, S. K., & Oppong, A. (2015). Street sugar cane vendors practices, metals and microbial levels of sugar cane sold in Kumasi, Ghana. International Journal of Science and Technology, 4(3), 99–104.
- Baker, A. J. M. (1981). Accumulators and excluders: Strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3, 643–654.10.1080/01904168109362867
- Bilos, C., Colombo, J. C., Skorupka, C. N., & Rodrigues, P. M. J. (2001). Sources, distribution and variability of airbone trace metal in La Plata City area, Argentina. Environmental Pollution, 111, 149–158.
- Bucheim, K., Stoltenburg-Didinger, G., Lilienthal, H., & Winnike, G. (1998). Miopathy: A possible effect of chronic low level lead exposure. Neurotoxicology, 19, 539–546.
- Codex Alimentarius Commission. (2000). Revised codex general standards for vegetable juice (CODEX STAN 179-1991). Rome.
- Cornish, G. A., Aidoo, J. B., and Ayamba, I. (2001). Informal irrigation in the Peri-urban zone of Kumasi, Ghana. An Analysis of Farmer Activities and Productivity. Report OD/TN 103, February 2001. DFID’s Water KAR Project R7132, HR Wallingford, UK, (February), 33.
- Cornish, G. A., & Lawrence, P. (2001). Informal irrigation in peri-urban areas: A summary of findings and recommendations. Wallingford: HR Wallingford.
- Costa, M. (1998). Carcinogenic metals. Science Progress, 81, 329–339.
- Domingo, J. L. (1994). Metal-induced developmental toxicity in mammals. Journal of Toxicology and Environmental Health, 42, 123–141.10.1080/15287399409531868
- EC (European Commission) (2006a). Commission Regulation (EC) No 1881/2006 Setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L, 364(1881), 5–24.
- EC (European Commission) (2006b). Setting maximum levels for certain contaminants in foodstuffs. Commission Regulation (EC) No 1881/2006 of 19 December 2006, setting, 1(1881), 5364–5365. doi: 10.1017/CBO9781107415324.004
- FAO and ISRIC. (2004). Guiding principles for the quantitative assessment of soil degradation with a focus on salinization, nutrient decline and soil pollution. Retrieved November 10, 2010, from ftp://ftp.fao.org/agl/agll/docs/misc36e.pdf
- Ghana Statistical Service. (2012). 2010 population and housing census final results. Ghana Statistical Service (pp. 1–11). Accra. Retrieved from www.statsghana.gov.gh
- Harmanescu, M., Alda, L., Bordean, D., Gogoasa, I., & Gergen, I. (2011). Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chemistry Central Journal. doi:10.1186/1752-153X-5-64
- Hough, R. L., Young, S. D., & Crout, N. M. J. (2003). Modelling of Cd, Cu, Ni, Pb and Zn uptake, by winter wheat and forage maize, from a sewage disposal farm. Soil Use and Management, 19(1), 19–27. doi:10.1111/j.1475-2743.2003.tb00275.x
- Hudnell, H. (1999). Effects from environmental Mn exposure: A review of the evidence from non- occupational exposure studies. Neurotoxicology, 20, 379–398.
- JECFA. (1983). Toxicological evaluation of certain food additives and food contaminants (Vol. 1983). Cambridge.
- Keane, B., Collier, M. H., Shann, J. R., & Rogstad, S. H. (2001). Metal content of dandelion (Taraxacum officinale) leaves in relation to soil contamination and airborne particulate matter. Science of The Total Environment, 281, 63–78.10.1016/S0048-9697(01)00836-1
- Kelley, C. (1999). Cadmium therapeutic agents. Current Parmaceutical Design, 5, 229–240.
- Keraita, B. (2002). Wastewater use in urban and peri-urban vegetable farming in Kumasi, Ghana. The Netherlands.
- Keraita, B. N., & Drechsel, P. (2004). Agricultural use of untreated urban wastewater in Ghana. In C. A. Scott et al. (Ed.), Wastewater use in irrigated agriculture: Confronting the livelihood and environmental realities (pp. 101–212). Wallingford: CABI Publ.10.1079/9780851998237.0000
- Keraita, B., Jimenez, B., and Drechsel, P. (2008, June 1). Extent and implications of agricultural reuse of untreated, partly treated and diluted wastewater in developing countries. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 3(58), 1–15. doi:10.1079/PAVSNNR20083058
- Klein, H. J. (1987). Reactions to goal setting and feedback. East Lansing, MI, Michigan State University.
- Lai, J. C., Minski, M. J., Chan, A. W., Leung, T. K., & Lim, L. (1999). Manganese mineral interactions in brain. Neurotoxicology, 20, 433–444.
- McLaughlin, M. J., Parker, D. R., & Clarke, J. M. (1999). Metals and micronutrients—food safety issues. Field Crops Research, 60, 43–163.
- Mensah, P., Owusu-Darko, K., Yeboah-Manu, D., Ablordey, A., Nkrumah, F. K., & Kamiya, H. (1999). The role of street food vendors in the transmission of enteric pathogens. Ghana Medical Journal, 33, 19–29.
- National Research Council. (1979). Iron: A report of the subcommittee on iron, committee on medical and biologic effects of environmental pollutants, division of medical sciences, assembly of life sciences. Baltimore, MD: University Park Press.
- Obuobie, E., Keraita, B., Danso, G., Amoah, P., Cofie, O. O., Raschid-sally, L., & Drechsel, P. (2006). Irrigated urban vegetable production in Ghana. Accra: IWMI-RUAF-CPWF.
- Odai, S. N., Mensah, E., Sipitey, D., Ryo, S., & Awuah, E. (2008). Heavy metals uptake by vegetables cultivated on urban waste dumpsites: Case study of Kumasi, Ghana. Research Journal of Environmental Toxicology, 2(2), 92–99.
- Oliver, M. A. (1997). Soil and human health. European Journal of Soil Science, 48, 573–592.10.1046/j.1365-2389.1997.00124.x
- Saha, N., & Zaman, M. R. (2012). Evaluation of possible health risks of heavy metals by consumpt ion of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environmental Monitoring and Assessment, 185, 3867–3878.
- Segura-Muñoz, S. I., da Silva Oliveira, a, Nikaido, M., Trevilato, T. M. B., Bocio, a, Takayanagui, a M. M., and Domingo, J. L. (2006). Metal levels in sugar cane (Saccharum spp.) samples from an area under the influence of a municipal landfill and a medical waste treatment system in Brazil. Environment International, 32(1), 52–57. doi: 10.1016/j.envint.2005.04.008
- Singh, A., & Agrawal, M. (2010). Effects of municipal waste water irrigation on availability of heavy metals and morpho-physiological characteristics of Beta vulgaris L. Journal of Environmental Biology, 31(5), 727–736. Retrieved from http://jeb.co.in/journal_issues/201009_sep10_supp/paper_01.pdf
- Thompson, M. (2005). Harmonised guidelines for the in-house validation of methods of analysis. ( Technical Report). Budapest.
- US EPA. (2012). Guidelines for water reuse. Boston, MA: CDC Smith Inc.
- US EPA. (2009). Mercury: Basic information. Retrieved May 4, 2009, from http://www.epa.gov/mercury/about.htm,
- Vowotor, M. K., Phil, M., Hood, C. O., Sackey, S. S., Tatchie, E., Osei, D. M., … Atieomo, S. M. (2014). An assessment of heavy metal pollution in sediments of a tropical Lagoon : A case study of the Benya Lagoon, Komenda Edina Eguafo Abrem municipality (KEEA) – Ghana. Journal of Health and Pollution, 4(6), 26–39.10.5696/2156-9614-4-6.26
- World Health Organization. (2001). Copper. Environmental health criteiavol. Geneva: International Programme on Chemical Safety., 200.