?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The growth-inhibitory activity of extracts obtained from Sugi (Cryptomeria japonica) effective against Microcystis aeruginosa, an alga that causes harmful algal blooms in freshwater environments, was examined. Each sample of the inner bark, outer bark, heartwood, and leaves of Sugi was extracted with hexane, ethyl acetate, and methanol successively. Inhibitory activities were observed in the inner bark ethyl acetate and methanol extracts, the heartwood hexane extract, the outer bark hexane extract, and the leaf ethyl acetate extract. The inner bark ethyl acetate and methanol extracts showed stronger activities than the other extracts. Catechin, epicatechin, and procyanidin B3 were identified as the active components in the inner bark ethyl acetate and methanol extracts. The terpenoids 1-epicubenol, 4-epicubebol, cubenol, δ-cadinol, sandaracopimarinal, and sandaracopimarinol were the active components of the heartwood hexane extract. Thus, it was indicated that not only the polyphenolic components but also non-phenolic terpenoids had growth inhibition activity against M. aeruginosa.
Public Interest Statement
The algal blooms severely impact on the fishing industry and cause environmental problems. We examined the growth-inhibitory activities of extracts obtained from the bark, wood, and leaves of Japanese cedar against Microcystis aeruginosa, an alga that causes harmful algal blooms in freshwater environments, and we identified the active components in the extracts. Inhibitory activities were observed in the inner bark ethyl acetate and methanol extracts, the heartwood hexane extract, the outer bark hexane extract, and the leaf ethyl acetate extract. From analysis of the active extracts, it was indicated that not only the flavanols but also the non-phenolic compounds of terpenoids had growth inhibition activity against M. aeruginosa.
Competing interests
The authors declare no competing interests.
1. Introduction
Microcystis aeruginosa is a cyanobacterium that can cause harmful algal blooms in freshwater ecosystems. The algal blooms severely impact on the fishing industry and cause environmental problem such as malodours (Paerl & Huisman, Citation2008; Shirai, Citation1996). Various chemical and physiological countermeasures have been investigated for these algal blooms (Jancula & Marsalek, Citation2011), for example, dispersion of copper sulfate or pesticides (Libertad et al., Citation2004) and UV radiation (Wang, Wang, & Wang, Citation2017; Zamir, Otaki, Furumai, & Ohgaki, Citation2001). However, since chemical treatments cause concerns for organisms other than the cyanobacteria, more environmentally safe methods are required to inhibit algal growth. Plant extract components that show inhibitory activity against algal growth include tannins of barley straw (Ball, Williams, Vincent, & Robinson, Citation2000; Mecina et al., Citation2017; Park et al., Citation2006), a naphthoquinone isolated from Juglans nigra (Kessler, Citation1988), phenolic compounds from Acacia decurrens (Yin et al., Citation2010), and a quinone compound from Salvia miltiorrhiza (Zang, Yi, Hao, Liu, & Wang, Citation2013). Inhibitory activity was also detected in some phenolic and terpenoid components released from waterweed (Nakai, Inoue, Hosomi, & Murakami, Citation2000; Nakai, Inoue, Lee, & Hosomi, Citation2002). These plant components may be relatively safe agents for inhibition of algal growth.
Sugi (Cryptomeria japonica) is an important plantation tree in Japan. The bark and residue generated by wood mills are regarded as woody waste products and are available in large quantities. Several allelochemical components are known in Sugi bark, leaves, and heartwood (Cheng et al., Citation2009; Kofujita, Fujino, Ota, & Takahashi, Citation2006; Yamashita et al., Citation2015; Zhu et al., Citation2016). In particular, components of Sugi bark showed growth-inhibitory activity against red tide plankton in our previous studies (Saijo, Tsuruta, Kusumoto, Ashitani, & Takahashi, Citation2013; Tsuruta et al., Citation2011). Sugi bark and the active components show potential for use as inhibitory agents effective against M. aeruginosa. Therefore, in this study, we examined the growth-inhibitory activities of extracts obtained from the bark, wood, and leaves of Sugi against M. aeruginosa, and we identified the active components in the extracts.
2. Material and methods
2.1. Plant and algal materials
Sugi trees were felled from the Yamagata Field Science Center (Faculty of Agriculture, Yamagata University, Japan) in Tsuruoka City. Bark and heartwood were sampled from the trunk logs. The bark was separated into inner and outer bark samples. The heartwood was ground using a Wiley mill. Leaves were cut into pieces approximately 2 cm in length, and each bark piece was cut into approximately 1–2 cm2 pieces.
Microcystis aeruginosa (strain NIES-87) was obtained from the National Institute for Environmental Studies (NIES, Japan) and was maintained under 2000 lux (12 h:12 h light:dark cycle) at 25°C in an NK system BIOTRON (LH-60(S)FL3/12-DT: Nippon Medical & Chemical Instruments Co., Ltd, Tokyo, Japan). The cells were cultured in MA medium (distilled water, 1 L; Ca(NO3)2∙4H2O, 50 mg; NaNO3, 50 mg; Na2SO4, 40 mg; MgCl2∙6H2O, 50 mg; β-Na2 glycerophate∙5H2O, 100 mg; Na2EDTA∙2H2O, 5 mg; FeCl3∙6H2O, 0.5 mg; MnCl2∙4H2O, 0.8 mg; ZnCl2, 0.5 mg; CoCl2∙6H2O, 5 mg; NaMoO4∙2H2O, 0.8 mg; H3BO3, 20 mg; and bicine, 500 mg (pH 8.6)) in accordance with a previously reported method (Watanabe & Oishi, Citation1985).
2.2. Analysis of extracts and compounds
The gas chromatography–mass spectrometry (GC-MS) data were collected with a SHIMADZU QP-2010 ultra GC-MS (Kyoto, Japan). The GC-MS analysis was performed under the following conditions: a DB-1 capillary column (0.32 mm i.d. × 30 m; 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA) or Rtx-5 ms capillary column (0.25 mm i.d. × 30 m; 0.25 μm film thickness; Restek, Bellefonte, PA, USA), column temperature ranging from 50°C (1 min) to 320°C (5 min) at 5°C/min or from 100°C (2 min) to 320°C (5 min) at 5°C/min for trimethylsilyl (TMS) derivatives, injection temperature of 250°C, interface temperature of 320°C, and acquisition mass range of 600–50 amu using helium as the carrier gas. Heneicosane and veratric acid were used as internal standards in the GC analysis for neutral and flavonoid compounds, respectively. The catechin and epicatechin concentrations in inner bark extracts were calculated from calibration curves prepared using veratric acid and standard samples of (+)-catechin and (–)-epicatechin (Sigma Chemical Co., St Louis, MO, USA) in the GC analysis after TMS derivatization. Nuclear magnetic resonance (NMR) spectra were measured on a JEOL JNM-ECZ-600 (1H 600 MHz/13C 150 MHz) spectrometer. Flavanol contents of the extracts were determined using a vanillin–HCl method based on a previously reported procedure using catechin as a standard (Broadhurst & Jones, Citation1978).
2.3. Extraction and isolation
Each sample of Sugi was extracted at ambient temperature for 9 days by successive extraction with n-hexane, ethyl acetate, and methanol to obtain from low-polar (n-hexane) to high-polar (methanol) extracts. Solvents were removed by evaporation to obtain each extract, and the yield of each extract was calculated based on dry sample weights (Table ). The ethyl acetate extract of the inner bark was separated by silica gel column chromatography (60N, spherical 63–210 μm, neutral; Kanto Chemical Co., Inc., Tokyo, Japan) using a gradient elution from 100% ethyl acetate to 100% methanol to yield seven fractions with thin-layer chromatography analysis results as an index. Procyanidin B3 was separated and identified by comparison of its NMR data with reference data (Samejima & Yoshimoto, Citation1979). The hexane extract of heartwood was also separated by silica gel column chromatography using a gradient elution from 100% hexane to 100% ethyl acetate, and then to 100% methanol to yield seven fractions with GC-MS analysis results as an index. Ferruginol, phyllocradanol, and cubebol were identified by comparison of their GC-MS spectra to those of standard samples isolated during our previous study (Saijo et al., Citation2013). Cubenol, 1-epicubenol, 4-epicubebol, sandaracopimarinol, sandaracopimarinal, and δ-cadinol were identified by comparison of their GC-MS data with reference data (Adams, Citation2012) and their NMR data with reference data (Matsushita et al., Citation2008; Rezvukhin, Khan, & Dubovenko, Citation1975; Sugimoto et al., Citation2006; Weyerstahl, Marschall, Thefld, & Subba, Citation1998). The isolated components and ferruginol sample obtained from our previous study (Saijo, Kofujita, Takahashi, & Ashitani, Citation2015) were used as samples for the M. aeruginosa bioassay.
Table 1. Yield* (%) of each extract obtained from successive extraction of Sugi bark, wood, and leaf samples
2.4. Growth-inhibitory activity
Growth-inhibitory activity against M. aeruginosa was measured in accordance with previous reports (Saijo et al., Citation2013; Tsuruta et al., Citation2011). Each sample of a specified quantity was dissolved in 1 mL acetone or dimethylsulfoxide (DMSO). Five microliters of these solutions were added to 5 mL volumes of MA medium to obtain solutions that were adjusted to have final concentrations of 5 mg/L for extracts and 0.1–5 mg/L for components. The component solutions were prepared at three concentrations to calculate EC50 values (0.1, 0.5, and 1.0 mg/L for catechin and epicatechin, and 1.0, 3.0, and 5.0 mg/L for procyanidin B3 and terpenoids). In the control assay, 5 µL of pure solvent (acetone or DMSO) was added to MA medium. Microcystis aeruginosa was added to each test tube. The initial cell density was set to ca. 8 × 105 cells/mL per test tube. The volume was adjusted with culture medium to 5 mL/test tube. The cell count of each test tube was measured daily for 8 days. Cells were counted microscopically using a Thoma haemocytometer (Hirschmann Laborgerate, Eberstadt, Germany). The number of cells (N) in 400 masses in the hemocytometer was determined, and the counts were converted to cell densities per 1 mL (the scored area of the haemocytometer was 0.0025 mm2 × 0.10 mm). The cell density (D) per 1 mL and inhibitory activity were calculated with the following equations:
where Ds is the cell density of each sample test tube and Dc is the cell density of the control test tube.
2.5. Statistical analysis
Test samples were compared using one-way analysis of variance, and means were evaluated using Tukey–Kramer’s honestly significant difference comparisons (p < 0.01; JMP version 9.0.3, SAS Institute Inc., Cary, NC, USA).
3. Result and discussion
3.1. Growth-inhibitory activity against M. aeruginosa of successive extracts from Sugi
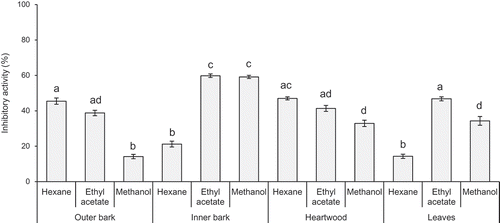
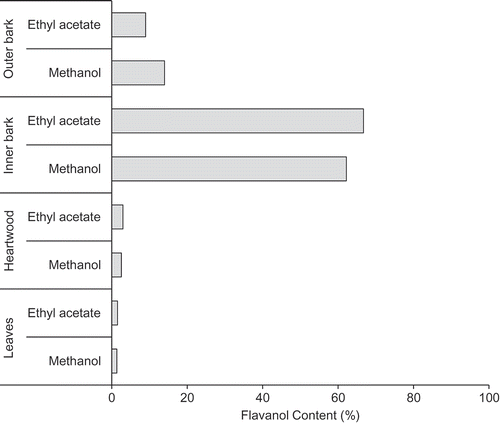
Figure shows the growth-inhibitory activity of each extract obtained from Sugi bark, wood, and leaves against M. aeruginosa. Inhibitory activity was predominantly observed in the inner bark ethyl acetate and methanol extracts, the outer bark hexane extract, the heartwood hexane extract, and the leaf ethyl acetate extract. The inner bark ethyl acetate and methanol extracts showed stronger activity than the other extracts. Flavonoid compounds are known to be active inhibitors of M. aeruginosa cell growth (Nakai et al., Citation2000, Citation2002). Therefore, the flavanol concentrations of the ethyl acetate and methanol extracts were measured (Figure ). The ethyl acetate and methanol extracts of the inner bark contained high flavanol concentrations and thus showed high inhibitory activity. However, although the leaf ethyl acetate extract exhibited inhibitory activity, the flavanol concentration in the extract was low. In addition, relative to the polar extracts of the inner bark, the hexane extracts of the heartwood and outer bark showed high inhibitory activity against M. aeruginosa. A previous study (Ashitani, Ujike, Nagahama, Ueno, & Sakai, Citation2001) revealed that the hexane extracts consist mainly of terpenoids. Flavanols, a class of polyphenols, were not detected in the hexane extracts. Therefore, the potent active extracts of the inner bark and heartwood were investigated to identify the active flavonoid and terpenoid components.
3.2. Identification of the active compounds in inner bark extracts
Catechin and epicatechin were detected in the ethyl acetate and methanol extracts of the inner bark in a GC-MS analysis. The 50% effective inhibition concentration (EC50) values of the standard samples of catechin and epicatechin in this experiment were 0.92 and 0.29 mg/L, respectively. Concentrations of catechin and epicatechin in the extracts were calculated using a calibration curve prepared from standard samples by GC-MS analysis. The ethyl acetate extract contained 5.0 wt% catechin and 1.9 wt% epicatechin, whereas the methanol extract contained 7.2 wt% catechin and 3.0 wt% epicatechin. The ethyl acetate extracts contained other active components in addition to catechin and epicatechin. Subsequently, the ethyl acetate extracts were separated by column chromatography. All fractions showed inhibitory activity, of which the activity of Fr. I4 was highest (Table ). The fraction Fr. I4 was separated further by chromatography to obtain procyanidin B3, a catechin dimer. The isolated procyanidin B3 showed concentration-dependent inhibitory activity and its EC50 value was 1.5 mg/L. Therefore, the active components in the ethyl acetate extract of the inner bark were catechin, epicatechin, and their dimer. The mechanism of inhibitory activity of the catechin was related to generate reactive oxygen species such as hydroxyl radical via o-quinone formation at B-ring of catechin structure in redox cyclers after uptake by organisms (Wang et al., Citation2011). Since procyanidin compounds of catechin polymers have catechol structure, the mechanism of inhibitory activity of the procyanidin B3 is considered as same with catechin. It was reported (Samejima & Yoshimoto, Citation1979) that the flavanol compounds of Sugi inner bark were almost procyanidins. Thus, high inhibitory activities of inner bark ethyl acetate and methanol extracts were related to high procyanidin contents in the extracts.
Table 2. Yield and growth-inhibitory activity against M. aeruginosa of fractions separated from ethyl acetate extract of Sugi inner bark
3.3. Identification of the active compounds in heartwood extracts
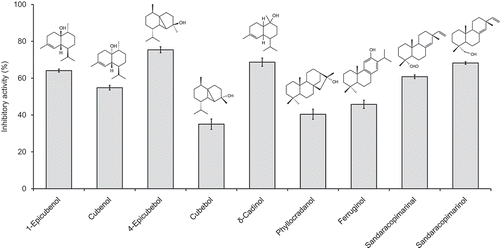
The hexane extract was separated by column chromatography into seven fractions, and the inhibitory activity of each fraction was investigated (Table ). Each active fraction was further separated by column chromatography to isolate ferruginol, 1-epicubenol, 4-epicubebol, sandaracopimarinal, cubenol, and cubebol from Fr. H3; phyllocradanol and sandaracopimarinol from Fr. H4; and δ-cadinol from Fr. H5. Figure shows the inhibitory activity and structures of the isolated components. 1-Epicubenol, 4-epicubebol, cubenol, δ-cadinol, sandaracopimarinal, and sandaracopimarinol showed strong inhibitory activity as isolated components against M. aeruginosa growth. The EC50 values of these active components, excluding δ-cadinol for which only a small amount was purified, based on the isolated samples were as follows: 1-epicubenol, 2.3 mg/L; 4-epicubebol, 2.6 mg/L; cubenol, 3.2 mg/L; sandaracopimarinal, 3.2 mg/L; and sandaracopimarinol, 2.1 mg/L. It is interesting to note that the activities of the different diastereomers of cubebol were quite different, whereas the activities of the different diastereomers of cubenol were almost identical (Figure ). This finding suggests that the stereochemistry of the components is an important determinant of their activity. Of the non-phenolic diterpenes, sandaracopimarinal and sandaracopimarinol showed strong inhibitory activity, whereas phyllocradanol showed weak activity. Sandaracopimarinol and sandaracopimarinal were the main non-phenolic diterpenes in the hexane extract of heartwood, and phyllocradanol and ferruginol were the main components in the hexane extract of the inner bark (Ashitani et al., Citation2001). In our previous reports (Saijo et al., Citation2013; Tsuruta et al., Citation2011), the inner bark extract, containing ferruginol and phyllocradanol as the main components, showed high inhibitory activity against the marine algae Heterosigma akashiwo and Skeletonema costatum. However, the hexane extract of the inner bark showed lower growth-inhibitory activity than that of the heartwood extract against M. aeruginosa, and the activity of isolated ferruginol and phyllocradanol was also lower than that of sandaracopimarinol and sandaracopimarinal in this study. In addition, the low-polarity extract of heartwood contained high amounts of active sesquiterpene alcohols, whereas the inner bark extract did not. This finding indicated that the active extracts and components were different for each alga.
Table 3. Yield and growth-inhibitory activity against M. aeruginosa of fractions separated from hexane extract of Sugi heartwood
4. Conclusion
Notable growth-inhibitory activities of Sugi extracts against M. aeruginosa were observed for the inner bark ethyl acetate and methanol extracts, the heartwood hexane extract, the outer bark hexane extract, and the leaf ethyl acetate extract. The inner bark ethyl acetate and methanol extracts showed stronger activity than the other extracts. The hexane extract of heartwood showed the next strongest activity. These results indicate that Sugi extracts could be used as inhibitory reagents against M. aeruginosa.
The active components of the inner bark extracts were flavanol compounds, such as catechin, epicatechin, and procyanidin B3. The active components in the hexane extract of the heartwood were sesquiterpene alcohols, diterpene alcohols, and an aldehyde. Potent activity was observed in isolated samples of 1-epicubenol, 4-epicubebol, cubenol, δ-cadinol, sandaracopimarinal, and sandaracopimarinol. Given that the activity of the two diastereomers of cubebol differed, the growth-inhibitory activity is likely to be influenced by the stereochemical structure of the components.
In this study, it was indicated that not only the flavanols but also the non-phenolic compounds of terpenoids had growth inhibition activity against M. aeruginosa. These results lead to further expansion of potential to control algal bloom by using plant components.
Acknowledgements
The authors thank Mr Kazuya Tsuruta, a graduate student of Yamagata University, for preliminary experiments in this study. We thank Robert McKenzie, PhD, from Edanz Group, for editing a draft of this manuscript.
Additional information
Funding
Notes on contributors
Hiromi Saijo
Hiromi Saijo is a research associate in Institute of Wood Technology, Akita Prefectural University, Japan. Her research interests are forest chemistry and wood science.
Part of this report was presented at the International Chemical Ecology Meeting 2015, Stockholm, July 2015, and at the 66th Annual Meeting of the Japan Wood Research Society in Nagoya, March 2016.
Tatsuya Ashitani
Tatsuya Ashitani is a professor of Forest Product Laboratory in Faculty of Agriculture, Yamagata University, Japan. His research interests are forest chemistry and wood chemistry. Especially, he studies about reaction and bio-activity of terpenoid components in woody plants.
References
- Adams, R. P. (2012). Identification of essential oil components by gas chromatography/mass spectrometry (4th ed.). Carol Stream, IL: Alluredbooks.
- Ashitani, T., Ujike, M., Nagahama, S., Ueno, T., & Sakai, K. (2001). Characterization of sugi (Cryptomeria japonica) bark extracts. Mokuzai Gakkaishi, 47, 276–281.
- Ball, S. A., Williams, M., Vincent, D., & Robinson, J. (2000). Algal growth control by a barley straw extract. Bioresource Technology, 77, 177–181. doi:10.1016/S0960-8524(00)00148-6
- Broadhurst, B. R., & Jones, T. W. (1978). Analysis of condensed tannins using acidified vanillin. Journal of the Science of Food and Agriculture, 29, 788–794. doi:10.1002/jsfa.2740290908
- Cheng, S. S., Chua, M. T., Chang, E. H., Huang, C. G., Chen, W. J., & Chang, S. T. (2009). Variations in insecticidal activity and chemical compositions of leaf essential oils from Cryptomeria japonica at different ages. Bioresource Technology, 100, 465–470. doi:10.1016/j.biortech.2007.11.060
- Jancula, D., & Marsalek, B. (2011). Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere, 85, 1415–1422. doi:10.1016/j.chemosphere.2011.08.036
- Kessler, C. T. (1988). Effect of juglone on freshwater algal growth. Journal of Chemical Ecology, 15, 2127–2134. doi:10.1007/BF01207443
- Kofujita, H., Fujino, Y., Ota, M., & Takahashi, K. (2006). Antifungal diterpenes from the bark of Cryptomeria japonica D. Don. Holzforschung, 60, 20–23.
- Libertad, G. V., Marcos, R., Marıa, A., Laura, S. M., Victoria, L. R., & Eduardo, C. (2004). Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: Characterisation and future implications in the use of copper sulphate as algaecide. Water Research, 38, 2207–2213. doi:10.1016/j.watres.2004.01.036
- Matsushita, Y., Sugamoto, K., Miyakubo, K., Kurogi, C., Matsui, T., Oda, H., & Fujimoto, H. (2008). Chemical changes in terpenes of sugi (Cryptomeria japonica) woos during steam drying in kiln at high temperature. Journal of Wood Science, 54, 476–482. doi:10.1007/s10086-008-0980-6
- Mecina, G. F., Dokkedal, A. L., Saldanha, L. L., Chia, M. A., Cordeiro-Araujo, M. K., Bittencourt-Oliveira, M. C., & Silva, R. M. G. (2017). Response of Microcystis aeruginosa BCCUSP 232 to barley (Hordeum vulgare L.) straw degradation extract and fractions. Science of the Total Environment, 599–600, 1837–1847. doi:10.1016/j.scitotenv.2017.05156
- Nakai, S., Inoue, Y., Hosomi, M., & Murakami, A. (2000). Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Research, 34, 3026–3032. doi:10.1016/S0043-1354(00)00039-7
- Nakai, S., Inoue, Y., Lee, B. D., & Hosomi, M. (2002). Inhibitory effects of plant-produced phenols on algal growth. Japanese Journal of Limnology, 63, 201–207. doi:10.3739/rikusui.63.201
- Paerl, H. W., & Huisman, J. (2008). Blooms like it hot. Science, 320, 57–58. doi:10.1126/science.1155398
- Park, M. H., Han, M. S., Ahn, C. Y., Kim, H. S., Yoon, B. D., & Oh, H. M. (2006). Growth inhibition of bloom- forming cyanobacterium Microcystis aeruginosa by rice straw extract. Journal Compilation, 43, 307–312.
- Rezvukhin, A. I., Khan, V. A., & Dubovenko, Z. V. (1975). NMR-13C specteoscopy of natural compounds. Communication 1. Investigation of the conformation of the sesquiterpene alcohol δ-cadinol. Russian Chemical Bulletin, 24(6), 1208–1211. doi:10.1007/BF00922047
- Saijo, H., Kofujita, H., Takahashi, K., & Ashitani, T. (2015). Antioxidant activity and mechanism of the abietane-type diterpene ferruginol. Natural Product Research, 29(18), 1739–1743. doi:10.1080/14786419.2014.997233
- Saijo, H., Tsuruta, K., Kusumoto, N., Ashitani, T., & Takahashi, K. (2013). Growth inhibition activities of Sugi bark components against Heterosigma akashiwo. Journal of Wood Science, 59(3), 238–242. doi:10.1007/s10086-013-1328-4
- Samejima, M., & Yoshimoto, T. (1979). The chemical structures of proanthocyanidins from coniferous bark. Bulletin University Tokyo Forest, 71, 153–175.
- Shirai, M. (1996). Toxicity of natural blooms and Mycrocystis species. Mycrotox, 42, 3–5. doi:10.2520/myco1975.1996.3
- Sugimoto, N., Kuroyanagi, M., Kato, T., Sato, K., Tada, A., Yamazaki, T., & Tanamoto, K. (2006). Indentification of the main constisuents in sandarac resin, a natural gum base. Journal of the Food Hygienic Society of Japan, 47(2), 76–79. doi:10.3358/shokueishi.47.76
- Tsuruta, K., Yoshida, Y., Kusumoto, N., Sekine, N., Ashitani, T., & Takahashi, K. (2011). Inhibition activity of essential oils obtained from Japanese trees against Skeletonema costatum. Journal of Wood Science, 57, 520–525. doi:10.1007/s10086-011-1209-7
- Wang, J., Zhu, J., Liu, S., Liu, B., Gao, Y., & Wu, Z. (2011). Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere, 85, 977–982. doi:10.1016/j.chemosphere.2011.06.076
- Wang, N., Wang, K., & Wang, C. (2017). Comparison of different algicides on growth of Microcystis aeruginosa and microcystin release, as well as its removal pathway in riverways. Frontiers of Environmental Science & Engineering, 11, 1–8. doi:10.1007/s11783-017-0963-1
- Watanabe, M., & Oishi, S. (1985). Effects of environmental factors on toxicity of cyanobacterium (Microcystis aeruginosa) under culture conditions. Applications of Environmental Microbiology, 49(5), 1342–1344.
- Weyerstahl, P., Marschall, H., Thefld, K. C., & Subba, C. G. (1998). Constituents of the essential oil from the rhizomes of Hedychium gardnerianum Roscoe. Flavour Fragr Journal, 13, 377–388. doi:10.1002/(SICI)1099-1026(199811/12)13:6<377::AID-FFJ755>3.0.CO;2-F
- Yamashita, Y., Hashimoto, N., Kusumoto, N., Saijo, H., Goto, I., Kobayashi, H., … Ashitani, T. (2015). Acaricidal activity of components of Cryptomeria japonica against spider mites. Journal of Wood Science, 61, 60–64. doi:10.1007/s10086-014-1445-8
- Yin, A. Q., Lee, H. W., Watanabe, K., Nakamura, K., Park, H. D., & Ban, S. (2010). Inhibitory effects of Acacia extract on growth of blue-green algae Microcystis spp. Bulletin Plankton Social Japanese, 57(2), 73–78.
- Zamir, B. A., Otaki, M., Furumai, H., & Ohgaki, S. (2001). Direct and indirect inactivation of Microcystis aeruginosa by UV-radiation. Water Research, 35, 1008–1014.
- Zang, C., Yi, Y. L., Hao, K., Liu, G. L., & Wang, G. X. (2013). Algicidal activity of salvia miltiorrhiza Bung on Microcystis aeruginosa-Towards identification of algicidal substance and determination of inhibition mechanism. Chemosphere, 93, 997–1004. doi:10.1016/j.chemosphere.2013.05.068
- Zhu, Q., Ishikawa, H., Ohnuki, K., Kakino, K., Horiuchi, N., Naito, T., … Shimizu, K. (2016). Multiple uses of essential oil and by-products from various parts of the yakushima native cedar (Cryptomeria Japonica). Journal of Wood Chemistry and Technology, 36(1), 42–55. doi:10.1080/02773813.2015.1057648