?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Food production is adversely affected by numerous biotic and abiotic factors that lead to reduction in yield and poor quality of the food products. Use of commercially synthetic pesticides was the most common method for pest control in many agricultural crops during recent decades. These synthetic chemicals have effects on all living organism when they consume such crops treated with pesticides. This research attempted is a green regulation technology as an alternative method to control cabbage moth Plutella xylostella towards the reduction of release of toxic chemical and residue. Virulence studies on different strains (Bb6, Bb11, Bb115, Bb116 and Bb362) of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) were evaluated. Various doses at 0 (control) 104, 105, 106, 107, 108 and 109 conidies ml−1 of five strains were applied topically on the third stage larvae of P. xylostella. Different parameters of larvae were measured in terms of mortality, sporulation rates, the number of pupae that emerged as adult, number of eggs laid between survived adults and the survival rate of larvae were examined at different doses, statistical analysis was performed using Cox-regression. We found that Bb11 strain of Beauveria bassiana (Balsamo) produced highest virulence compared to other strains at 109 conidia/ml while, Bb6 strains showed lower virulence effect at 109 conidia/ml as compared to control dose. Due to the larvicidal effect of different fungus strains, the percentage weight of female adult decreased significantly as compare to the control.
PUBLIC INTEREST STATEMENT
Diamondback moth is considered as the main insect pest of brassica crops, particularly cabbages. The economic impact of diamondback moth is difficult to assess since it occurs in diverse small-scale and large-scale production areas, but it has been known to completely destroy cabbage and kale crops. It is considered a major pest in all countries of the eastern and southern African region. Beauveria bassiana offers potential for use as biopesticides for control of different pests in agriculture. In the present study, we compared the effect of five strains of Beauveria bassiana that could be an alternative to chemical pesticides.
1. Introduction
The boom in urbanization resulted in a steep increase in the demand for food, especially high nutritional value crops such as fruits, vegetables and other horticultural crops (Kahane et al., Citation2013). Food production is no longer simple as providing nutrition for a growing population, but also as contributing to poverty reduction, better health outcomes and conservation of natural resources (Sayer & Cassman, Citation2013). Urban agriculture has gained importance as a viable strategy the people with limited resources to generate additional income and to reduce their reliance on cash income for food for growing their own (Vidogbéna et al., Citation2015). However, the pressure of a growing urban population demanding fresh food, food production, and urban agriculture in particular, are inevitability linked to indiscriminate use of pesticides. These both harm the environment and expose many people to toxic pesticides when they use such contaminated crops treated with pesticides (De Bon et al., Citation2014). Vegetable production in Africa is now highly dependent on insecticides, not only in places dominated by large-scale cash crops, but also in small-holder production systems (Migwi, Citation2016). Inappropriate application and handling of often banned pesticides can damage the environment and impinge both on the farmer’s health and the consumers of the crops and vegetables (Vidogbéna et al., Citation2015). Brassica vegetables, like cabbage (Brassicaceae), are one of the most frequently consumed exotic vegetables in the tropical and subtropical regions of West Africa, cutting across a wide range of culture and agro-ecologies (Lohr & Kfir, Citation2004). These vegetables are grown although throughout the year, but mostly affected by insects (Thibaud et al., Citation2015; Vidogbéna et al., Citation2016). Studies carried out in southern West Africa by Simon et al. (Citation2014) revealed that 70% of cabbage growers apply four to five chemical treatments per month, doubling or tripling the recommended dosage. Despite the availability of many registered pesticides and increased pesticide treatment frequencies and dosages, vegetable yields continue to decline due to the development of insecticide resistance among major insects. Biopesticide is gaining interest because of its advantages associated with the environmental safety, target-specificity, efficacy, biodegradability and suitability in the integrated pest management (IPM) programs (Kumar & Singh, Citation2015).
Entomopathogenic fungi, whose infection occurs through the cuticle, offer a unique opportunity and this characteristic is being exploited. Such fungi are able to kill the insects and can be transferred from one individual to another by simple contact (Kaaya & Hassan, Citation2000). Research conducted by International Institute of Tropical Agriculture Benin (IITA-Benin) concluded that biopesticides were readily accepted by farmers to reduce the dangers of many synthetic pesticides that are being used by farmers in Africa (Cherry, Citation2006). Thus, biopesticide is one of the promising alternatives to manage environmental pollutions and can be used as a component of IPM in agriculture. In recent years, Beauveria bassiana has been a resurgence of interest in the use of fungi for the control of insect pests. This revival of interest has led to the large-scale production of several promising fungi candidates with a broad natural distribution; its potential to control more than 70 insect pests has been responsible for a substantial increase in interest in the large-scale production of the fungus for applications in the field. This research investigated the effect of five of B. bassiana strains applied on the third stage (L3) of P. xylostella larvae to determinate their efficacy.
2. Materials and methods
2.1. Area of study
This research was carried out in greenhouse at the International Institute of Tropical Agriculture Benin, under controlled experimental conditions: Temperature = 26 ± 1°C and Relative Humidity = 65.5 ± 5%.
2.2. Plant material, insect colonies and fungus strains
The cabbage seed variety KK-cross was used for this experiment (Figure ). Beauveria bassiana strain (Table ) and P. xylostella larvae were kindly provided by Entomopathology Lab and the Insects section at International Institute of Tropical Agriculture-Benin respectively.
Table 1. Five fungus strains of B. bassiana, used for this experiment
Table 2. Cox regression models of the various doses of B. bassiana strains
2.3. Virulence of B. bassiana strains
Virulence of Beauveria bassiana isolates were tested at third instar stages of the cabbage moth Plutella xylostella. The fungal inoculum was cultured on Potato Dextrose Agar (PDA; Difco) in separate Petri dishes. Briefly, 39 grams of PDA powder was dissolved in one litre of distilled water in an Erlenmeyer flask, steamed for 30 min and autoclaved for 15 min at 121°C. Ten milliliter of chloramphenicol solution (0.05 g of antibiotic cholramphenicol plus 10 ml of 90% of alcohol) was added to the autoclaved media and the media poured in 9 cm Petri dishes in lamina air-flow safety cabinet for solidification (Figure ).
Figure 2. Preparation of MS media, (A) commercial packaging of PDA, (B) MS- media dissolved in distilled water and autoclaved, (C) PDA pouring into Petri dishes, (D) fungal inoculum cultured on PDA.

Conidia culture was extracted from each culture by adding 20 ml sterile distilled water (SDW) with 0.05% Tween 80 suspension. Conidia in each Petri dish were harvested from the suspension with 90 µm mesh sieve. The concentration in each suspension was determined by using Hemacytometer (Superior, Marienfeeld, Germany) and a light microscope, and adjusted to the following concentration 0 (control), 104 105 106 107 108 and 109 conidies.ml−1 109 conidia/ml to use as inoculums in the experiments. The conidia viability was determined by calculating the rate of germination using the formula:
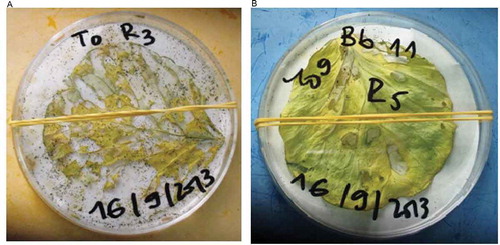
P. xylostella inoculated with the various concentration (Figure ) and strains of fungal conidia were maintained on 21-day old cabbage leaves (width = 2 cm and length = 2 cm) that were first disinfected with 10% sodium hypochlorite and washed three time with SDW. A batch of 21 disinfected leaves and 210 third stage of P. xylostella were used for each fungal strain. Daily observation was taken and dead larvae were recorded after each treatment. Dead larvae were incubated on moist tissue paper in Petri dishes for external growth of the fungus.
2.4. Effect of B. bassiana strain doses on sex ratio of P. xylostella survived by the treatments
The influence of the various fungus strains on the survived larvae after the treatment and the number of emerged adult (male and female) was recorded. Three couples of adults that steadily survived to doses 107 and 108 conidia/ml of each strain were placed in cages (20 cm × 20 cm × 20 cm) in greenhouse (Figure ). Each couple of P. xylostella was fed with a solution of saccharose (10%). A young cabbage leaf was introduced into each cage to allow the females of P. xylostella to sprawl and renewed every 24 h.
The reduction rate of eggs laid, L3 larvae alive and viable pupae were calculated from formulas (1), (2) and (3), respectively:
With:
RP: Reduction of eggs laid by P. xylostella
NOT: number of eggs laid by the control
NOI: number of eggs laid by female survived to B. bassiana
%L: percentage of third stage of P. xylostella viable
NL: number of P. xylostella L3 viable
NO: number of eggs lay
%P: percentage de pupa viable
2.5. Statistical analysis
Data were taken on the number of eggs laid, alive larvae L3, chrysalises and emerged adults were analyse for The lethal dose (LD50) (X) analysis with SPSS 16.0 software using Cox-Regression with time dependent variables. Cox-Regression formula:
where
X = Survival probability
ho (t) = baseline hazard
B = beta-coefficient (B)
and the test of Chi-square at the 5% threshold was used to compare the average of sporulation rates odead larvae.
3. Results
3.1. Estimation of the DL50 of B. bassiana strains
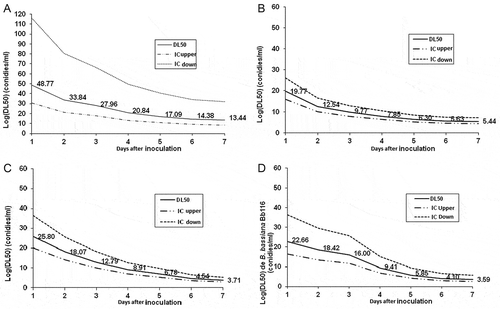
Cox regression (B) value range from 0.072 to 0.209 (Table 2). Table showed increase mortality rate observed all the dose applied with a the highest percentage lava death 109 conidia/ml in Bb11 of cox regression value 0.209 indicating a reliable control of P. Xylostella (Figure ).
Table 3. Khi-two analysis of B. bassiana strains using SPSS 16.0 software
3.2. Evolution of LD50 after application of fungus isolates
Results showed lethal doses of the different strains of B. bassiana. 107.85, 108.91, 109.41, 1020.85 conidia/ml effectively to kill 50% of P. xylostella after inoculation respectively for Bb11, Bb115, Bb116 at four days (Figure ). But Bb6 strain conidia/ml was very low and the Cox regression for LD50 could not be estimated.
3.3. Sporulation of dead larvae
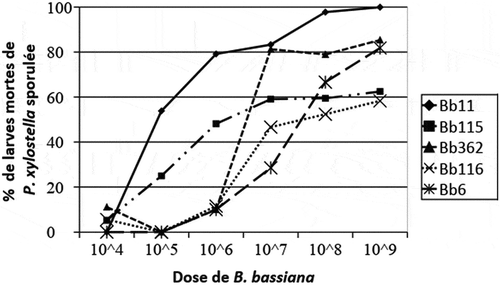
Bb11 growth rate on P. xylostella larvae increased according to the dose applied but sporulation rate was not stable for Bb362, Bb116 and Bb6 strains especially between 104 and 105 conidia/ml. Bb11 sporulate on all larva at 80% and 100% at 106 conidia/ml and 109 conidia/ml respectively with strongest sporulation rate from the dose 105 conidia/ml. However low growth of Bb116, Bb115, Bb6 and Bb362 was observed on the larva at 109 conidia/ml (Figure ).
At the 109 conidia/ml dose, the sporulation percentage of Bb116 strain is low and was not statistically different from Bb115 strain (χ2 = 0.174; p = 0.676) (Table ). Same result was also observed for Bb6 and Bb362 isolates which, at the same concentration, gave comparable sporulation rates mainly 81.80 ± 0.021% and 85.40 ± 0.021% (χ2 = 0.135; p = 0.713). The rate of sporulation appears to be limited from the conidia/ml dose for the Bb115 and Bb362 isolates. The success of sporulation observed for the isolates studied during the present work represent an indicator of choice for the mass production of the fungus. Studies have indicated that B. bassiana infects its host by simple contact, all the stages of the insect are potentially susceptible to be attacked on field.
Table 4. Estimation of the effect of dose of B. bassiana strains on the sex ratio of larvae survival. (A) Influence of Bb362 strain dose on sex ratio, (B) influence of Bb11 strain dose on sex ratio, (C) influence of Bb115 strain dose on sex ratio, (D) influence of Bb116 strain dose on sex ratio
3.4. Effect of the various strains on the sex ratio of larvae survived after innoculation
Table showed that the females are more sensitive to all the strains compared to the males, as there was reduction in the percentage survival. However, only Bb362 shows a significant change in the male to female ratio at 107 and 108 conidia/ml.
Table 5. Effect of B. bassiana strains and control on the adult of P. xylostella emerged after inoculation
3.5. Effect of B. bassiana strains on the adult of P. xylostella emerged after innoculation
The larvae survived were 52.22, 61.18, 69.38, 74.82 and 83.90 percent respectively for Bb115, Bb11, Bb116, Bb362 and Bb6. Although, the proportion of viable larvae in the control was between 88.35 and 99.31 percent, the viable chrysalises obtained from the viable larvae were less influenced by B. bassiana with between 75.70 and 92.74% for the B. bassiana strain compared to the control as indicated in Table 6.
4. Discussion
This study demonstrated that the strains of B. bassiana hold great promise as biological agent in IPM measures against the diamond-back moth, P. xylostella in cabbage. All the strains show high average germination above the recommended 85% (Lomer et al., 1997), with mortality in cabbage moth population (Godonou et al., Citation2009; Loc & Chi, Citation2007; Mehinto, Atachi, Kpindou, Dannon, & Tamò, Citation2014). Although the mortality rate observed was strain and dose dependence. Bb11 strain caused more mortality of larvae than Bb6 and Bb362 which could be as a result of difference in capacity of the B. bassiana strains to produce more enzymes and other metabolites responsible for the death of the host. The pathogenicity of a fungal strain is associated with the production of enzyme and mycotoxines during the infection of an insect (Roddam & Rath, Citation1997; Rosell, Quero, Coll, & Guerrero, Citation2008). Among all the strains, Bb11 strain show most virulence compared with the other strains, however, their is no significant different in the mortality rate when compare with Bb115 and Bb116 at the same concetration. This suggests that both strains of B. bassiana might have similar pathogenic effect.
The sporulation of P. xylostella larvae depends on the quantity of concentation of conidia applied and the highest mortality was recored at 105 and 108 conidia/ml. This suggests that fungi adhesion to the host cuticle is important for penetration. Since outgrowth and sporulation of entomopathogenic fungi on cadavers is important for proliferation and spread of the disease within a pest population in nature. The spore growth shows a positive correlation with the mortality rate, this agrees with (T. Butt & Goettel, Citation2000; T. M. Butt, Citation2002) studies.
Due to the high mortality rates observed at the level of larvae and their chrysalises, lower number of females emerged at 108 conidia/ml comparing to 104 and 105 conidia/ml. The higher the dose, the less females emerged, this suggests that female chrysalises are more sensitive. Although most of the infected larvae died at first four days of the treatment, the infection acquired in the evolving embryo distrupts the life cylce of the survival. These studies demonstrated that the infectious caused by B. bassiana strains can negatively influence the number of the adults in subsequent generations. This resembles to (Kpindou, Djegui, Glitho, & Tamò, Citation2012), when 3rd stage larvae of H. armigera was treated with the M. anisopliae and B. Bassiana.
5. Conclusion
This study demonstraded Beauveria bassiana strain B11 could be an effective broad spectrum biocontrol agent for Plutella xylostella and various other pests. The fungal spores infect the ants, which soon die. The fungus is not toxic or infective to mammals, and exposure to the public and the environment will be minimal to non-existent. Therefore, no adverse effects are expected on children, adults, pets, or the environment when the bait stations are used as directed. The interest in organic farming and agriculture products with no pesticides residue would certainly warrant increased adoption of biopesticides by the farmers. As environmental safety is a global concern, we need to concentrate more to create awareness among the farmers, manufacturers, government agencies, policy makers and the common men to switch-over to biopesticides for pest management requirements as alternate of chemical pesticides to produce healthy agricultural products. It is also believed that biological pesticides may be less vulnerable to genetic variations in plant populations that cause problems related to pesticide resistance. If deployed appropriately, biopesticides have potential to bring sustainability to global agriculture for food and feed security.
Competing interests
The author declares no competing interests.
Acknowledgements
The authors are grateful to their academic councils and the International Insttitute of Tropical Agriculture (IITA-Benin) for support in the form of infrastructural facilities made available for undertaking the present study.
Additional information
Funding
Notes on contributors
Anicet Batcho
Anicet BATCHO, M.Sc in Crop Protection. Currently, Ph.D Scholar in Plant Molecular Biology/Centre of Excellence in Molecular Biology, University of the Punjab. In my research, I have focused on using microorganisms like fungi to protect plants against insect pests and also identifying superior traits in plants.
Mohsin Ali
Mohsin Ali, Ph.D Scholar/School of Life Sciences, University of Science and Technology of China (USTC).
Adeyinka Olawale Samuel
Adeyinka Olawale Samuel, Masters of Science in Crop Improvement from University of Ibadan, Nigeria (2014). Ph.D scholar in Molecular Biology at the prestigious University of the Punjab, Pakistan.
Kamran Shehzad
Kamran Shahzad (Ph.D), Plant Breeding and Genetics and Molecular Biology.
Bushra Rashid
Bushra Rashid (Ph.D), Assistant Professor in charge of MPhil/PhD Programme, Centre of Excellence in Molecular Biology, University of The Punjab, Lahore, Pakistan.
References
- Butt, T. M. & Goettel, M. S. (2000). Bioassays of Entomogenous Fungi. In A. Navon, and K. R. S. Ascher (Eds.), Bioassays of Entomopathogenic Microbes and Nematodes (pp. 141–195). Wallingford: CAB International. doi: http://dx.doi.org/10.1079/9780851994222.0141
- Butt, T. M. (2002). Use of entomogenous fungi for the control of insect pests. In F. Kempken (Ed.), The Mycota XI. Agricultural applications (pp. 111–134). Berlin: Springer-Verlag.
- Cherry, A. (2006). Development for biopesticide registration and risk assessment guideline for Ghana R8430 (ZA 0659), Final Technical Report 1 feb 2005- 31 Dec 2005, National Ressource Institute at the University of Greenwich 15 Jan 2006.
- De Bon, H., Huat, J., Parrot, L., Sinzogan, A., Martin, T., Malézieux, E., & Vayssières, J.-F. (2014). Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agronomy for Sustainable Development, 34(4), 723–736. doi:10.1007/s13593-014-0216-7
- Godonou, I., James, B., Atcha-Ahowé, C., Vodouhè, S., Kooyman, C., Ahanchédé, A., & Korie, S. (2009). Potential of Beauveria bassiana and Metarhizium anisopliae isolates from Benin to control Plutella xylostella L. (Lepidoptera: Plutellidae). Crop Protection, 28(3), 220–224. doi:10.1016/j.cropro.2008.10.009
- Kaaya, G. P., & Hassan, S. (2000). Entomogenous fungi as promising biopesticides for tick control. Experimental & Applied Acarology, 24(12), 913–926. doi:10.1023/A:1010722914299
- Kahane, R., Hodgkin, T., Jaenicke, H., Hoogendoorn, C., Hermann, M., Hughes, J. D. A., … Looney, N. (2013). Agrobiodiversity for food security, health and income. Agronomy for Sustainable Development, 33(4), 671–693. doi:10.1007/s13593-013-0147-8
- Kpindou, O. D., Djegui, D. A., Glitho, I. A., & Tamò, M. (2012). Reponse des stades larvaires de Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) à l’application de champignons entomopathogènes Metarhizium anisopliae et Beauveria bassiana. Biotechnologie, Agronomie, Société et Environnement, 16(3), 283–293.
- Kumar, S., & Singh, A. (2015). Biopesticides: Present status and the future prospects. Journal of Biofertilizers & Biopesticides, 6(2). doi:10.4172/2471-2728.1000e129
- Loc, N. T., & Chi, V. T. B. (2007). Biocontrol potential of Metarhizium anisopliae and Beauveria bassiana against diamondback moth, Plutella xylostella. Omonrice, 15, 86–93.
- Lohr, B., & Kfir, R. (2004). Diamondback moth Plutella xylostella (L.) in Africa. A review with emphasis on biological control. Improving Biocontrol of Plutella Xylostella, 2(87614), 5707.
- Mehinto, J. T., Atachi, P., Kpindou, O. K. D., Dannon, E. A., & Tamò, M. (2014). Mortality of Maruca vitrata (Lepidoptera: Crambidae) larval stages induced by different doses of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. International Journal, 2(4), 273–285.
- Migwi, B. G. (2016). Assessment of farmers’ perceptions of and willingness to pay for Aflasafe KE01, a biological control for aflatoxins in Kenya. University of Nairobi.
- Roddam, L. F., & Rath, A. C. (1997). Isolation and characterization of Metarhizium anisopliae and Beauveria bassiana from Subantarctic Macquarie Island. Journal of Invertebrate Pathology, 69(3), 285–288. doi:10.1006/jipa.1996.4644
- Rosell, G., Quero, C., Coll, J., & Guerrero, A. (2008). Biorational insecticides in pest management. Journal of Pesticide Science, 33(2), 103–121. doi:10.1584/jpestics.R08-01
- Sayer, J., & Cassman, K. G. (2013). Agricultural innovation to protect the environment. National Academy of Sciences.
- Simon, S., Komlan, F. A., Adjaïto, L., Mensah, A., Coffi, H. K., Ngouajio, M., & Martin, T. (2014). Efficacy of insect nets for cabbage production and pest management depending on the net removal frequency and microclimate. International Journal of Pest Management, 60(3), 208–216. doi:10.1080/09670874.2014.956844
- Thibaud, M., Serge, S., Laurent, P., Komlan Françoise, A., Faustin, V., Anselme, A., …, Mathieu, N. (2015). Eco-friendly nets to improve vegetable production and quality in sub-Saharan Africa. In Hale, C., Hunter, D., Roberts, W., Ikin, R., McMaugh, S. (Eds.), XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): International Symposia on Innovative Plant Protection in Horticulture, Biosecurity, Quarantine Pests, and Market Access (pp. 221-228). Leuven: ISHS [Belgique]. ISBN 978-94-62610-99-6
- Vidogbéna, F., Adégbidi, A., Tossou, R., Assogba-Komlan, F., Martin, T., Ngouajio, M., … Zander, K. K. (2015). Consumers’ willingness to pay for cabbage with minimized pesticide residues in Southern Benin. Environments, 2(4), 449–470. doi:10.3390/environments2040449
- Vidogbéna, F., Adégbidi, A., Tossou, R., Assogba-Komlan, F., Martin, T., Ngouajio, M., … Zander, K. K. (2016). Exploring factors that shape small-scale farmers’ opinions on the adoption of eco-friendly nets for vegetable production. Environment, Development and Sustainability, 18(6), 1749–1770. doi:10.1007/s10668-015-9717-z