Abstract
The present work describes the first attempt at cultivating corals in the northern Arabian Gulf where seawater temperature ranges from 13 to 33°C and salinity exceeds 40 psu, exceeding normal lethal limits for coral survival. Despite the environmental extremes, natural recruitment of corals occurred in Sabah Al-Ahmad Sea City water ways during 2009, this encouraged an attempt to create an in situ coral nursery and establish a coral garden. A mid-water suspended coral PVC nursery (3 m2) was installed at 3.5 m depth for rearing staghorn coral, Acropora downingi for transplantation into artificial lagoons in Sabah Al-Ahmad Sea City. Fragments of Acropora downingi colonies damaged by boat anchoring were collected (n = 240) from patch reefs at Min Al Zour and Qit At Binaya, Kuwait. Coral nubbins obtained from fragments were glued individually to discs made of powdered Electric Arc Furnace Slag (EAF Slag) and marine grade cement. Nubbins showed up to 56% survival and a mean skeletal extension of 10.6–13.4 mm (SD ± 0.8) in 10 months. The nursery served as an artificial reef ecosystem. Transplantation of 116 Acropora downingi colonies was carried out (in June 2014 and June 2015) and monitored colonies (n = 6) attained an average geometric mean diameter (GMD) of 73.6 mm (SD ± 2.91) in one year. The first batch of A. downingi transplants (June 2014) showed a survival rate of 43% but the second batch (June 2015) showed 89.5% survival. Temperature induced mortality, detachment rate reduced after using marine grade cement and additive mixture instead of epoxy.
PUBLIC INTEREST STATEMENT
Coral reefs in the Arabian/Persian gulf are very unique as they thrive in extreme environmental conditions (low–high temperature and high salinity). Pollution, coastal development, recreational activities, etc. threaten the existence of reefs, which can impact fisheries. In this study, for the first time in northern Arabian Gulf, we conducted a “coral nursery” and transplantation experiment for staghorn coral (Acropora downingi) in the largest coastal township (>70 Km2 area) project in Kuwait known as “Sabah Al-Ahmad Sea City”. Coral colonies damaged by boat anchoring were collected from nearby reefs and glued to discs made of steel slag and cement, reared in the nursery for a year and transplanted onto a rock island. Transplanted staghorn corals successfully grew in spite of extreme temperature and salinity in Kuwait waters. The results of this experiment will serve as a baseline to design future reef restoration efforts in the region.
Competing interests
The authors declare no competing interests.
1. Introduction
Growing multi-species of coral in a nursery is the first phase in coral gardening, which adopts “silviculture” methods (Rinkevich, Citation2008). Several species of corals worldwide have been successfully grown from nubbins (10–30 mm length) to a transplantable size in floating nurseries prior to transplantation onto denuded reefs (Mbije, Spanier, & Rinkevich, Citation2010; Putchim, Thongtham, Hewett, & Chansang, Citation2008; Shafir, Rijin, & Rinkevich, Citation2006a, Citation2006b; Shaish, Levy, Gomez, & Rinkevich, Citation2008). In the Arabian Gulf (hereafter Gulf), coral nursery experiments are so far limited to southern tropical waters (Sen & Yousif, Citation2016; Wilson & Marimuthu, Citation2012) with a few preliminary transplantation experiments in Iran (Vajed Samei, Koosha, & Behrooz, Citation2012) and Abu Dhabi, United Arab Emirates (Katakura, Yuriko, Sumihiro, Hiroshima, & Iwata, Citation2013; Sen & Yousif, Citation2016). The present work describes the first establishment of an in situ coral nursery in the extreme environmental conditions of the northern Gulf (Kuwait), for habitat enhancement. Corals in Kuwait survive in extreme environmental conditions with winter minimum (13°C) and summer maximum sea surface temperatures of (33°C) and salinity (>40 psu) exceeding lethal limits for corals elsewhere and challenging reef building (Carpenter, Harrison, Hodgson, Al-Saffar, & Al-Hazeem, Citation1997).
Sabah Al Ahmad Sea City (hereafter Sea City) is the largest coastal township development in Kuwait with over >70 km2 of artificial lagoons, beaches, islands and will house 100,000 people. An array of man-made marine habitats now exists in Sea City including intertidal sand beaches, rock revetments, groynes, mud flats, salt marsh, seagrass and mangrove habitats and subtidal sand, mixed rock and rock habitats. Over 1,000 species of benthic fauna and 100 species of fish (Jones & Nithyanandan, Citation2013; Jones, Nithyanandan, & Williams, Citation2012) have been recorded to date. Since 2009 natural recruitment of nine species of scleractinian corals—“Dipsastrea speciosa (=Favia speciosa), D. pallida (=F. pallida), Siderastrea savignyana, Porites sp, Cyphastrea serialia, C. microphthalma and Platygyra daedalea”—have been observed colonizing on the rock revetments of Sea City with a mean colony density of 10 (SD ± 5) colonies m−2 (Jones & Nithyanandan, Citation2015) covering an area of <200 m−2. However, no recruitment of the branching corals such as Acropora spp and Stylophora pistillata has occurred, which are essential to the creation of three dimensional habitat increasing complexity and niches for various marine organisms (Sheppard, Citation2015).
This study aims at establishing an in situ coral nursery for the mariculture of the branching coral, Acropora downingi and subsequent transplantation into Sea City waterways to enhance the environmental value. Further this study also reports the impact of sea surface temperature-SST (°C) on the survival and growth rate of A. downingi in northern gulf.
2. Materials and methods
2.1. Site selection
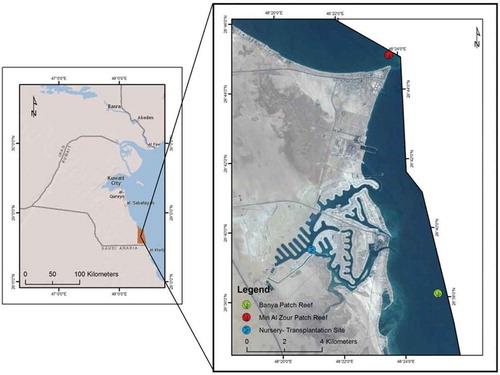
Two donor coral sites: on offshore patch reef at Min Al Zour (28°45ʹN; 48°24°E) and Qit at Binaya (28° 38°N; 48° 25°E) off Al Khiran (Figure ) were selected as they are dominated by branching and non-branching coral genera. The nursery site was selected in a shallow channel of 5 m depth with a rocky bottom, adjacent to the second rock island in phase A3 of Sea City (Figure ). The second rock island 50 m away from the nursery site with an inclined open rock surface was selected as a transplantation site (Figure ). Both nursery and transplantation sites were 8 km away from the donor sites.
Figure 1. Acropora downingi offshore donor sites (Al Zour and Qit at Binaya patch reefs) and nursery-transplantation sites at Sabah Al Ahmad Sea City.
2.1.1. Physicochemical parameters
Prior to installation of the nursery, currents (ms−1) were measured during spring tides using a Valeport ® 106 self reading current meter in April 2013. Daily sea temperature (°C) (SST), salinity (psu), dissolved oxygen (mgl−1) and pH were recorded at 1 m depth using YSI 556 multi-probe system and bi-weekly measurements of total suspended solids (TSS), nutrients—Nitrates (NO3), nitrites (NO2), phosphates (PO4), ammonia (NH3) and silicates (SiO3) following American Public Health Association (APHA, Citation1995) methods were carried out during May 2012–July 2015.
2.1.2. Nursery design and substrate preparation
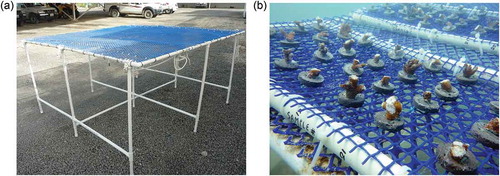
A cost-effective nursery design was adopted based on Edwards (Citation2010) and Shaish et al. (Citation2008) and modified as a “Suspended table nursery” (Figure ) with a 3 m2 frame made out of 6 × 25 mm diameter PVC pipes, six legs (1 m) with end cups and a plastic mesh (3 m × 3 m × 30 mm mesh size) stretched over the frame and secured with cable ties. Seven rearing trays were constructed using PVC (600 × 300 mm) pipes and plastic mesh (600 mm × 300 mm × 30 mm mesh size). The nursery was suspended at 3.5 m depth with marine grade ropes attached to concrete sinkers. Buoys were (4 × 1) attached to nursery to maintain buoyancy and to suspend it at 1.5 m above the lagoon bed. Although table nurseries are of a fixed type (Shaish et al., Citation2008), we suspended the table nursery in the water column and its legs offered framework support and stability. Seven rearing trays were used in the experiment and rest of the space was kept empty for further addition.
Figure 2. (a) Table nursery used in the experiment, (b) nubbins immediately after being placed in the nursery showing stress symptoms by secreting intense mucous threads from severed portions. (Photos M. Nithyanandan.)
Slag, a by-product obtained during manufacturing of steel has a proven potential as a cost-effective substrate/substrate ingredient for recent coral rehabilitation experiments worldwide (Mohammed, Hamed, Habib, Ezz El-Arab, & El-Moshley, Citation2012; Okamoto et al., Citation2010; Oyamada, Okamoto, & Iwata, Citation2014). Powdered EAF slag was mixed with marine grade cement (3:1) and the mixture was poured into PVC rings (30 mm diameter, 10 mm thick) into which four small holes were made by inserting a thick metal wire and cured overnight. Slag discs were attached using a fish line to the rearing trays in arrays of 4 × 8 rows with 20–30 mm interval between discs (Figure ), and this method is based on Kerby (Citation2014). Each rearing tray was marked with an individual number and row numbers for identification.
2.1.3. Coral nubbin preparation and transplantation
Acropora downingi ramets of 50–100 mm size broken off by boat anchoring were collected during June–August 2013 from various areas of the patch reefs (depth 5–6 m) in Min Al Zour and Qit at Binaya, Kuwait to ensure diversity in genotypes. Collecting anchor damaged colonies to serve as donors eliminated pressure on existing natural population of A. downingi. Fragments were placed in a 60 L cooler box filled with fresh seawater and aerated vigorously to minimize stress levels which were indicated by extensive mucous secretion after fragmentation. Four people were involved in cutting the fragments into nubbins (10–30 mm size) using electrical pliers and gluing to slag discs using Super Glue® (b, Shafir et al., Citation2006a). Further, two SCUBA divers placed trays with discs in the nursery and attached them using cable ties. Intensive mucous secretion by newly attached nubbins was observed as a stress reaction upon exposure to air for 5–10 min while attaching to EAF slag discs (Figure ).
After a year in the nursery, during May 2014 the first batch of 30 colonies of different genotypes were randomly removed from the nursery, cleaned of foulers and transplanted onto rock surfaces at 3 m depth using marine grade epoxy resin (Gomez, Cabaitan, Yap, & Dizon, Citation2013) in two alternate rows with 200–300 mm interval between rows and nubbins. At least a 50–60 mm thick layer of epoxy had to be used to hold the nubbins in position against gravitational and current forces on the inclined rock substrate. In June 2015, a second batch of 86 nubbins were transplanted using marine grade cement (Yap, Citation2009) mixed with a water proofing additive (3:1), Water Proof.150 BLC® from Sodamco® which cures in less than 5 min. Manufacturer specification indicates that the curing process is directly proportional to temperature for additive use, hence the transplantation was carried out in early summer (May–June). Significant difference in sample size was due to availability of extra manpower for transplantation during the second phase (June 2015) of transplantation.
2.1.4. Maintenance and monitoring
Two divers conducted weekly maintenance dives to check nursery’s structural stability, buoyancy and remove algae and foulers on the substrate and amongst the coral nubbins using a brush. Divers regularly waved their hands to remove any debris settling between nubbins (Shafir et al., Citation2006b). A similar cleaning method was also adopted for transplanted colonies.
In the nursery, 20 nubbins were randomly tagged with a code number to record growth and number of branches produced monthly from September 2013 to July 2014. A twist tie was attached to the base of each tagged nubbin and growth was recorded by measuring skeletal extension beyond the twist tie to the nearest mm using a plastic Vernier caliper. Monthly digital photographs were taken with a ruler placed beside each colony for calibration and growth analysis, using the software Adobe Acrobat Pro®. Species of colonizing benthic community and fishes observed in the nursery were recorded as abundant (>10 individuals), common (sighted during every monitoring/maintenance dive) or rare (sighted only once during the entire monitoring period). In the case of transplanted colonies (first batch, n = 30), six individuals were tagged randomly and growth was monitored for 12 months (August 2014–June 2015) by recording maximum horizontal and perpendicular length (mm) to calculate geometric mean diameter (GMD) in mm following Clark and Edwards (Citation1995), for a three dimensional growth pattern. The number of branches was counted monthly to derive mean number of branches produced. No growth monitoring was carried out for the second batch transplants. Growth rate differences between genotypes were not studied as it was beyond the scope of the present study. Nursery and transplanted colonies were also monitored for monthly survival, detachment, bleaching and mortality.
2.1.5. Statistical analysis
A two tailed t-test was performed to understand the influence of SST (°C) on nubbin mortality and detachment in the nursery and similarly salinity (psu) on mortality, detachment, skeletal extension (mm) of nubbins and average GMD (mm) of transplants. Linear regression analysis was performed for monthly mean SST (°C) against average GMD (mm) and number of branches against average GMD (mm) in MS Office Excel 2007.
3. Results
A peak flow velocity of 0.45 ms−1 was recorded during the spring tides at the nursery and transplantation sites. The suspended table nursery was moved in all directions by currents. Minimum and maximum values of physicochemical parameters (Table ) indicate that the nursery and transplantation sites are exposed to a wide temperature, salinity range, optimum nutrient levels and low total suspended solids (TSS).
Table 1. Minimum and maximum values of physicochemical parameters recorded during May 2012–July 2015. Values are expressed in ±SD
After a year in the nursery A. downingi nubbins (n = 240) showed a survival rate of 56%, detachment 37.9% and mortality 6.3% (Table ). Mortality occurred in summer (September 2013) and winter (December 2013). No significant correlation was observed between SST (°C) and nubbin mortality and detachment rates (t-test, p > 0.05). Nubbins produced axial corallites at the cut point and produced branches with radial corallites in 2 months. The first batch of transplanted colonies (n = 30) showed 43.3% survival (Figure ), 46.6% detachment and 10% mortality (Figure b), whereas the second batch (n = 86) showed 89.5% survival (Figure ), 4.7% detachment and 5.8% mortality during the post-transplantation period (Figure ). Similarly, mortality in the first batch of transplants occurred during cold temperatures in January–February 2015 and in the second batch due to a partial bleaching event in August 2015 (Figure ), however the rates of mortality in nubbins and first batch of transplants were much lower compared to detachment rate influenced by the mechanical disturbance during maintenance (in nubbins) and use of poor quality epoxy (in transplants). However the use of 3:1 marine grade cement and water proof BLC® (additive) reduced the detachment rate in the second batch transplants. In the nursery, nubbins self-attached in 30 days with tissue and skeleton encrusting the EAF slag disc (Figure ), whereas in the case of transplants self-attachment to the substrate occurred in 60 days.
Table 2. Monthly survival, mortality and detachment (%) of A. downingi nubbins in the nursery
Figure 3. Survival, mortality and detachment (%), (a) survival of transplants and (b) detachment and mortality in nursery reared nubbins and transplants.
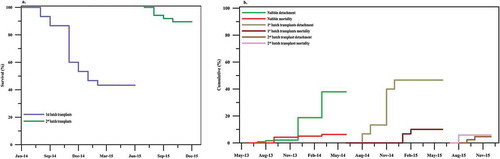
Figure 4. (a) SST (°C) impact on growth-mean skeletal extension (mm)/average GMD (mm) in A. downingi (b) Salinity (psu) impact on growth-mean skeletal extension (mm)/average GMD (mm) in A. downingi.
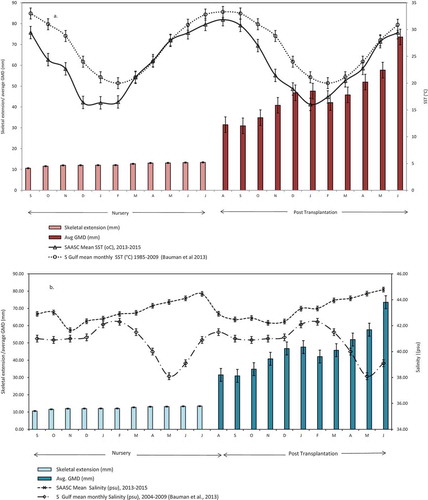
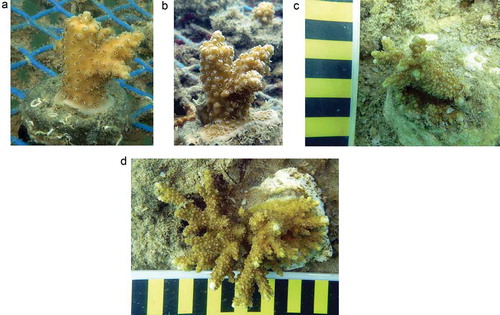
Figure 5. (a) A. downingi nubbin in the nursery self-attached to EAF slag substrate in a month; (b) one year old nubbin with impacted growth; (c, d) nubbin (average GMD 23.5 mm) transplanted onto a open inclined rock surface using epoxy (June 2014) and in a year it attained an average GMD of 102 mm (SD ± 20.08 mm). Alternate yellow and black bars in the scale is 10 mm each. (Photos R. Dinesh Kumar.)
Sea surface temperatures below 18°C and above 31°C showed an impact on growth in the nursery (Figure ). Moreover SST values recorded for the sea city nursery and transplantation sites have a mean annual difference of 3.0°C (Figure a), lower compared to southern Gulf waters reported by Bauman et al. (Citation2013). Similarly higher salinity (>42 psu) recorded in the nursery-transplantation site is 2.3 PSU higher than the southern gulf waters (Bauman et al., Citation2013). However, there is no significant impact of salinity on the survival, detachment and growth of nubbins (t-test, p > 0.05) and transplants (Figure b, t-test, p > 0.05). In the case of transplants negative growth was witnessed during hottest summer, August–September 2014 and coldest winter months, January–February 2015 (Figure ).
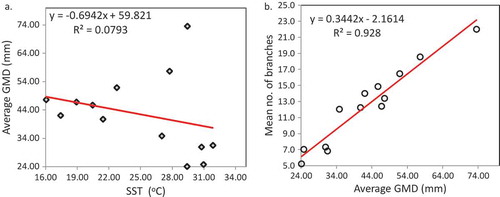
Monthly mean skeletal extension of nursery reared A. downingi ranged from 0.03 (SD ± 4.0 mm) to 0.98 mm (SD ± 3.9 mm) and the annual skeletal extension was 2.8 mm yr−1 (Figure , ). Whereas post transplantation, the monitored A. downingi colonies (n = 6) average GMD (mm) increased from 24.1 (SD ± 9.85 mm) to 73.6 (SD ± 29.09 mm) with an annual growth rate (GMD) of 50 mm yr−1 and the highest average GMD attained was from 23.5 mm to 102 mm (SD ± 20.8 mm) (by nubbin no. 8, Figure , ) in 1 year. Linear regression of mean SST (°C) against average GMD (mm) of transplants showed negative correlation (Figure ; r2 = 0.079, p > 0.05). Significant positive correlation is seen between average number of branches and average GMD (mm) of transplants (Figure ; r2 = 0.928, p < 0.05). Partial bleaching occurred in transplanted colonies during August 2014 as the mean maximum sea surface temperature (°C) reached 34.7°C (Table ); however, colonies completely recovered by November 2014.
Figure 6. Linear regression analysis of transplanted A. downingi; (a) average GMD (mm) vs. SST (°C), (b) mean number of branches vs. average GMD (mm).
The suspended nursery attracted colonization of benthic invertebrates and vertebrates which eventually established a diverse community (Table ). Barnacles and bryozoans dominated the community and intensively competed for space with the nubbins. Barnacle infestation was more intense due to the shallow nursery site. Many fish species visited the nursery for food, and a few species such as Apogon taeniatus, Pseudochromis persicus and cryptic species such as Gobies and Blennies (Laith et al., Citation2015) sought a permanent refuge in the nursery. Intensive algae cover in the nursery was grazed by herbivore top shells such as Priotrochus obscura, but as they were low in abundance several algae species continued to dominate over several periods although regular cleaning procedures were adopted. During winter (November–February) drift, algae fouled the nursery.
Table 3. List of benthic community established in the suspended nursery (A = Abundant, C = Common and R = Rare)
4. Discussion
The Kuwait Environmental Protection Society (KEPS), a voluntary organization in Kuwait, has carried out preliminary experiments adopting biorock® technique to grow coral fragments in the offshore islands of Kuwait for reef restoration (KEPS, personal communication).
The movement of nursery in all direction aided by currents helped in removal of debris and prevented accumulation of fine sediments. This increased water circulation was also beneficial to the nubbins as reported by Shafir et al. (Citation2006a). Ambient nutrient levels recorded show nutrient levels similar in those of the open sea southern waters of Kuwait (Al-Yamani, Bishop, Ramadhan, Al-Husaini, & Al-Ghadban, Citation2004).
The survival and detachment rates (%) of A. downingi in the nursery and post transplantation are comparable with results obtained for Acropora spp from Gulf of Eilat, Red Sea (Shafir et al., Citation2006a, Citation2006b), Tanzania (Mbije, Spanier, & Rinkevich, Citation2013; Mbije et al., Citation2010), Egypt (Mohammed et al., Citation2012) and Thailand (Sujirachato, Thamrongnawasawat, Thongtham, Jantrarotai, & Worachananant, Citation2013). The higher detachment rate was not due to SST variations as Acropora spp thrives in extreme temperature conditions in both the northern and southern Gulf (Al-Yamani et al., Citation2004; Bauman et al., Citation2013) and is mostly due to poor adhesion and mechanical disturbances caused by fishes and divers during maintenance (Shaish et al., Citation2006b). However, nursery survival was lower compared to A. formosa reared in a shallow site using a suspended nursery in the Philippines (Shaish et al., Citation2008). Mortality of nursery reared nubbins in summer is probably due to temperature related stress, but in post spring (March 2014) additional mortality due to drift algae covering the nubbins demanded regular cleaning. In this case, increased maintenance also lead to increased detachment rate (Table ). Mortality rate in the present study was related to stress caused by higher summer temperature (Table ) and a partial bleaching episode that occurred in summer of 2014 and 2015. Temperature extremes challenge corals physiologically, lower seawater temperature recorded during winter in the northern Gulf (Saudi Arabia) waters showed lethal effects on Acropora pharaonis and Platygyra daedalea (Coles & Fadlallah, Citation1991). In their study in Philippines, Yap, Alino, and Gomez (Citation1992) demonstrated that warm temperature can also induce mortality and bleaching in transplanted corals.
The SST (°C) values in the present study showed impact on growth of nubbins in the nursery at temperatures below 18°C and above 31°C (Figure ), which are lower than those recorded in southern gulf waters where coral community structure is much influenced by SST and recurrent bleaching events (Bauman et al., Citation2013). Corals in northern Gulf continue to survive in temperatures above the normal threshold for survival (Carpenter et al., Citation1997) reported for the region (Bauman et al., Citation2013; Riegl et al., Citation2012). Similarly mean salinity (psu) variations recorded in this study are higher compared to southern Gulf waters (Bauman et al., Citation2013) with no impact on growth (Figure ), perhaps indicating adaptability of Kuwait corals to a higher salinity regime (D’Angelo et al., Citation2015). The negative growth recorded in this study (Figure ) was probably induced by mechanical disturbances during maintenance, fishes and partial bleaching rather than SST extremes.
In the present study, use of marine grade cement mixed with water proof additive (3:1), is a new approach to shorten the curing period. Although marine epoxy is an ideal adhesive for coral transplantation experiments (Dizon, Edwards, & Gomez, Citation2008), in this study the slow curing property of 10–15 min increased detachment rate due to strong currents. Hence, adhesives with a rapid curing property can be very effective, if transplantation experiments are to be carried out in high energy environments. The curing process is directly proportional to temperature, hence the transplantation experiments were conducted during early summer (May–June).
Self-attachment time of 60 days in A. downingi transplants is longer than results obtained for Acroproa spp in Philippines (Guest, Dizon, Edwards, Franco, & Gomez, Citation2011). The colonies in this study were transplanted on an inclined rock surface and the amount of epoxy/cement layer was very thick (>5 cm), hence extra energy and time is spent to encrust and reach the rock. In coral transplants self-attachment time is influenced by a combination of factors such as growth form, growth rate and environmental parameters, etc. (Guest et al., Citation2011).
During the post-transplantation period Average Geometric Mean Diameter (mm) was much higher compared to results obtained for A. tenuis transplanted in Philippines (Guest, Baria, Gomez, Heyward, & Edwards, Citation2014). Growth rate of inversely and normally transplanted A. downingi at Hengam Island, Iran (Southern Gulf) was measured as an increment of weight (Vajed Samei et al., Citation2012), so that methods of growth measurements adopted in the present study do not allow comparison. Annual growth rate of Acropora downingi in this study indicates that growth is up to 50 mm yr−1 which is comparable to the growth rate of 50–100 mm yr−1 for Acropora spp recorded by Riegl (Citation2002) in Dubai, UAE. Survival and growth rates of A. downingi presented in this study are the first available for a coral species in the Northern Gulf where a high variation in SST in the range of 11.1–35°C is reported (Al-Yamani et al., Citation2004; Devlin et al., Citation2015). This is an even higher variation (23.9°C) than that was recorded (17°C) in Abu Dhabi during the recent nursery and transplantation experiment (Sen & Yousif, Citation2016).
Although growth impact on nubbins and transplants during hot summer and cold winter months were less prominent (Figure ), a similar trend was observed in Philippines wherein growth rates were not affected significantly by seasonal fluctuations in temperature (Yap et al., Citation1992). On the contrary a recent study on calcification rates in A. downingi in Hengam Island, Iran predicts that temperature fluctuations and irradiance levels can impact calcification rates seasonally, with winter months having higher calcifications rates than fall, spring and summer (Vajed Samei et al., Citation2016). However, environmental conditions in Kuwait waters are different from Hengam Island, Iran hence adaptability of corals to these conditions should vary considerably which needs further investigation. Other potential factors which may impact growth include biofouling, shallow nursery site and maintenance induced damage (Shafir et al., Citation2006b). Also the small size of fragments (10–30 mm) used in this study for nursery rearing could account for reduced growth rate as witnessed for Acropora crevicornis in Florida, US (Herlan & Lirman, Citation2008).
Only partial bleaching of A. downingi nubbins and transplants was observed in the summer of 2014 and 2015 despite the mean maximum SST (°C) value (Table ) being equivalent to the bleaching threshold reported for Gulf corals (Riegl et al., Citation2012). However, post bleaching recovery in November 2014 was a month earlier than that was observed for A. arabensis nubbins reared in Abu Dhabi (Wilson & Marimuthu, Citation2012). Worldwide branching corals are highly susceptible to large-scale bleaching phenomenon (Edwards, Citation2010) due to SST anomalies and the same is true for the Gulf (Riegl et al., Citation2012). However, resilience and adaptability to environmental extremes appears to be a robust trait for northern Gulf coral populations which will be helpful for targeting future restoration experiments.
In situ nursery rearing and transplantation of A. downingi in the artificial lagoons of Sea City is successful, as is the use of EAF slag as a potential artificial substrate on which to rear corals as found by Mohammed et al. (Citation2012) in Egypt and Japan (Okamoto et al., Citation2010; Oyamada et al., Citation2014). Intensive fouling by algae, barnacles, ascidians Phallusia nigra, Didemnum spp, the bryozoan Schizoporella spp and polychaete Hydroides spp was probably influenced by EAF slag that deposits a layer of CaCo3 over the upper surface upon reaction with seawater which attracts invertebrates (Mohammed et al., Citation2012), thus intensifying the maintenance regime. Hence, deeper sites with low biofouling impact are crucial for successful coral nursery operation as they yield better results (Edwards, Citation2010; Shafir et al., Citation2006a), however biofouling impacts can also be curtailed by restricted use of anti-fouling paints (Shafir, Abady, & Rinkevich, Citation2009) to reduce maintenance costs. There is scope for experimentation on nursery designs appropriate to shallow Gulf environments and selection of appropriate species for nursery rearing and transplantation.
The diverse benthic community colonization in the suspended nursery attracted several species of fish for food and shelter. Shafir and Rinkevich (Citation2010) observed that floating mid-water nurseries in Red Sea attracted colonization of several species of benthic organisms and fishes thus functioning as an artificial reef. Thus, apart from functionality in rearing corals, suspended nurseries can serve to enhance biodiversity by acting as an artificial reef.
Although the Gulf coastline has been subjected to drastic modification due to intensive coastal development (Burt, Citation2014; Sheppard et al., Citation2010; Van Leveiren et al., Citation2011), some of these man-made habitats such as Sea City get to enhance marine biodiversity and create additional marine habitats when designed carefully. The present study demonstrates for the first time in Kuwait that it is possible to enhance the environmental value of artificially created marine habitats by adopting the “coral gardening concept” (Rinkevich, Citation2008) and rear corals in situ despite the extreme environmental conditions of the northern gulf to restore reefs. Further experiments on non-branching species, nursery designs to suit extreme environmental conditions in the gulf are essential for initiating future reef restoration efforts.
Acknowledgements
We would like to thank Mr. Fawaz Al Marzouq, Chairman of La Ala Al Kuwait Real Estate Co. K.S.C. for permission to publish the data and facilities provided, M/s. Ian Williams, Project Director, Sabah Al Ahmad Sea City for his constant support and encouragement, Chris Rose for his useful suggestions, Drs. David Jones, Spain, Baruch Rinkevich Israel Oceanographic Institute, Israel, Jerald Wilson, King Abdul Aziz University, Jeddah, Saudi Arabia, R.P. Kumarran, Marine Mammal Consultant, India and N. Marimuthu, Zoological Survey of India for their valuable advice in the various stages of this study. We thoughtfully acknowledge Dr. Shaker Al-Hazeem, Kuwait Institute for Scientifc Research for his valuable comments on the manuscript. We also extend our thanks to Dr. Saif Udin, Kuwait Institute for Scientific Research for the preparation of map, M/s. Bapu Rao Sontakke, Hassan Ali Hassan and Jag Jeevan Singh for their support during fieldwork.
Additional information
Funding
Notes on contributors

Manickam Nithyanandan
M. Nithyanandan, a marine ecologist with master’s degree in Oceanography from Alagappa University, India. Interested in various aspects of marine ecology, marine biodiversity and habitat restoration in Arabian gulf. Currently working in Kuwait Institute for Scientific Research under the Ecosystem based management of marine sciences program. Recent research interest is to study the benthic ecology of Kuwait Bay and has published several articles in international peer reviewed journals.
Lewis Le Vay
Professor Lewis Le Vay, Director, Centre for Applied Marine Sciences in Bangor University, UK. An expert in marine biology and aquaculture.
Dinesh Kumar Raja
Dinesh Kumar Raja, a marine biologist and PADI divemaster experienced in studying marine ecology of Andaman islands and Kuwait, Arabian Gulf.
Ratheesh Kesavan
Ratheesh Kesavan, a marine biologist studying marine ecology of Kuwait, Arabian Gulf.
Don Pereira
Don Pereira, a Research Diver and PADI dive master with a good experience in coral and seagrass restoration techniques in Arabian Gulf.
References
- Al-Yamani, F. Y., Bishop, J., Ramadhan, E., Al-Husaini, M., & Al-Ghadban, A. (2004). Oceanographic atlas of Kuwait waters (203 p). Kuwait: Kuwait Institute for Scientific Research.
- American Public Health Association. (1995). Standard methods for the examination of water and waste water (19th ed.). Washington, DC: Author.
- Bauman, A. G., Pratchett, M. S., Baird, A. H., Riegl, B., Heron, S. F., & Feary, D. A. (2013). Variation in the size structure of corals is related to environmental extremes in the Persian Gulf. Marine Environmental Research, 84, 43–50. doi:10.1016/j.marenvres.2012.11.007
- Burt, J. A. (2014). The environmental costs of coastal urbanization in the Arabian Gulf. City, 18, 760–770. doi:10.1080/13604813.2014.962889
- Carpenter, K. E., Harrison, P. L., Hodgson, G., Al-Saffar, A. H., & Al-Hazeem, S. H. (1997). The corals and coral reef fishes of Kuwait. Kuwait: Kuwait Institute for Scientific Research.
- Clark, S., & Edwards, A. J. (1995). Coral transplantation as an aid to reef rehabilitation: Evaluation of a case study in the Maldive Islands. Coral Reefs, 14, 201–213. doi:10.1007/BF00334342
- Coles, S. L., & Fadlallah, Y. H. (1991). Reef coral survival and mortality at low temperatures in the Arabian Gulf: New species-specific lower temperature limits. Coral Reefs, 9, 231–237. doi:10.1007/BF00290427
- D’Angelo, C., Hume, B. C. C., Burt, J., Smith, E. G., Achterberg, E. P., & Wiedenmann, J. (2015). Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. The ISME Journal, 9, 2551–2560. doi:10.1038/ismej.2015.80
- Devlin, M. J., Massoud, M. S., Hamid, S. A., Al-Zaidan, A., Al-Sarawi, H., Al-Enezi, M., … Lyons, B. P. (2015). Changes in the water quality conditions of Kuwait’s marine waters: Long term impacts of nutrient enrichment. Marine Pollution Bulletin, 100, 607–620. doi:10.1016/j.marpolbul.2015.10.022
- Dizon, R. M., Edwards, A. J., & Gomez, E. D. (2008). Comparison of three types of adhesives in attaching coral transplants to clam shell substrates. Aquatic Conservation: Marine and Freshwater Ecosystems, 18, 1140–1148. doi:10.1002/aqc.v18:7
- Edwards, A. J. (2010). Reef rehabilitation manual (ii + 166 pp). St. Lucia: Coral Reef Targeted Research & Capacity Building for Management Program.
- Gomez, E. D., Cabaitan, P. C., Yap, H. T., & Dizon, R. M. (2013). Can coral cover be restored in the absence of natural recruitment and reef recovery. Restoration Ecology, 22, 142–150. doi:10.1111/rec.12041
- Guest, J. R., Baria, M. V., Gomez, E. D., Heyward, A. J., & Edwards, A. J. (2014). Closing the circle: Is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs, 33, 45–55. doi:10.1007/s00338-013-1114-1
- Guest, J. R., Dizon, R. M., Edwards, A. J., Franco, C., & Gomez, E. D. (2011). How quickly do fragments of coral “self attach” after transplantation? Restoration Ecology, 19, 234–242. doi:10.1111/rec.2011.19.issue-2
- Herlan, J., & Lirman, D. (2008). Development of a coral nursery program for the threatened coral Acropora crevicornis in Florida. In Proceedings of the 11th international coral reef symposium (pp. 1244–1249). Ft. Lauderdale, FL.
- Jones, D. A., & Nithyanandan, M. (2013). Recruitment of marine biota onto hard and soft artificially created subtidal habitats in Sabah Al-Ahmad Sea City, Kuwait. Marine Pollution Bulletin, 72, 351–356. doi:10.1016/j.marpolbul.2012.11.001
- Jones, D. A., & Nithyanandan, M. (2015). If it is possible to create and manage marine ecosystem in a desert, why is the whole gulf ecosystem under threat? In Qatar University life science symposium 2015 (Abstract). doi:10.5339/qproc.2015.qulss2015.14
- Jones, D. A., Nithyanandan, M., & Williams, I. (2012). Sabah Al-Ahmad Sea City Kuwait: Development of a sustainable man-made coastal ecosystem in a saline desert. Aquatic Ecosystem Health & Management, 15(S1), 84–92. doi:10.1080/14634988.2012.663706
- Katakura, N., Yuriko, T., Sumihiro, T., Hiroshima, H., & Iwata, I. (2013). Development and practical applications of transplantation technique for coral and seagrass in sub-tropical island. Journal of Japan Society for Civil Engineers Series B3 (Ocean Engineering), 69, 1078–1083 (In Japaense).
- Kerby, A. B. (2014). Best practices manual for Caribbean Acropora restoration (p. 39). Puntacana: Punctacana Foundation.
- Laith, A. J., Nithyanandan, M., Raja, D. K., Kesavan, R., & Pereira, D. (2015). First record of the pixie triplefin Enneapterygius pusillus (Pisces: Tripterygiidae) and confirmation of the presence of cheekspot blenny Parablennius opercularis (Blenniidae) in the north Arabian Gulf. Marine Biodiversity Records, 8, e8. doi:10.1017/S1755267214001419
- Mbije, N. E., Spanier, E., & Rinkevich, B. (2013). A first endeavour in restoring denuded, post bleached reefs in Tanzania. Estuarine and Coastal Shelf Science, 128, 41–51. doi:10.1016/j.ecss.2013.04.021
- Mbije, N. E. J., Spanier, E., & Rinkevich, B. (2010). Testing the first phase of the ‘gardening concept’ as an applicable tool in restoring denuded reefs in Tanzania. Ecological Engineering, 36, 713–721. doi:10.1016/j.ecoleng.2009.12.018
- Mohammed, T. A., Hamed, A. A., Habib, N. F., Ezz El-Arab, M. A., & El-Moshley, K. H. M. (2012). Coral rehabilitation using steel slag as a substrate. International Journal of Environment Protection, 2, 1–5.
- Okamoto, M., Yap, M., Roeroe, A. K., Nojima, S., Oyamada, K., Fujiwara, S., & Iwata, I. (2010). In situ growth and mortality of juvenile Acropora over 2 years following mass spawning in Sekisei Lagoon, Okinawa (24°N). Fisheries Science, 76, 343–353. doi:10.1007/s12562-010-0222-x
- Oyamada, K., Okamoto, M., & Iwata, I. (2014). Development of restoration technology for coral reefs using “Marine Block™”. JFE Technical Report, 19, 118–125.
- Putchim, L., Thongtham, N., Hewett, A., & Chansang, H. (2008). Survival and growth of Acropora spp. In mid-water nursery and after transplantation at Phi Phi islands, Andaman Sea, Thailand. In Proceedings of the 11th international coral reef symposium 2 (pp. 1263–1266). Ft. Lauderdale, FL.
- Riegl, B. M. (2002). Effects of the 1996 and 1998 positive sea-surface temperature anomalies on corals, coral diseases and fish in the Arabian Gulf (Dubai, UAE). Marine Biology, 140, 29–40. doi:10.1007/s002270100676
- Riegl, B. M., Purkis, S. J., Al-Cibahy, A. S., Al-Harthy, S., Grandcourt, E., Al-Sulaiti, K., … Abdel-Moati, A. M. (2012). Coral bleaching and mortality thresholds in the SE Gulf: Highest in the world. In B. M. Riegl & S. J. Purkis (Eds.), Coral reefs of the Gulf: Adaptations to climatic extremes, coral reefs of the world 3 (pp. 95–105). Netherlands: Springer Science + Business Media BV.
- Rinkevich, B. (2008). Management of coral reefs: We have gone wrong when neglecting active reef restoration. Marine Pollution Bulletin, 56, 1821–1824. doi:10.1016/j.marpolbul.2008.08.014
- Sen, S., & Yousif, O. M. (2016). Development of a coral nursery as a sustainable resource for reef restoration in Abu Al Abyad Island, Abu Dhabi, United Arab Emirates, Arabian Gulf. Galaxea, 18, 3–8. doi:10.3755/galaxea.18.1_3
- Shafir, S., Abady, S., & Rinkevich, B. (2009). Improved sustainable maintenance for mid-water coral nursery by the application of an anti-fouling agent. Journal of Experimental Marine Biology and Ecology, 368, 124–128. doi:10.1016/j.jembe.2008.08.017
- Shafir, S., Rijin, J. V., & Rinkevich, B. (2006a). A mid-water coral nursery. In Proceedings of the 10th intenational coral reef symposium (pp. 1674–1679). Okinawa.
- Shafir, S., Rijin, J. V., & Rinkevich, B. (2006b). Steps in the construction of underwater coral nursery, an essential component in reef restoration acts. Marine Biology, 149, 679–687. doi:10.1007/s00227-005-0236-6
- Shafir, S., & Rinkevich, B. (2010). Integrated long-term mid-water coral nurseries: A management instrument evolving into a floating ecosystem. University of Mauritius Research Journal, 16, 365–385.
- Shaish, L., Levy, G., Gomez, E., & Rinkevich, B. (2008). Fixed and suspended coral nurseries in the Philippines: Establishing the first step in the “gardening concept” of reef restoration. Journal of Experimental Marine Biology Ecology, 358, 86–97. doi:10.1016/j.jembe.2008.01.024
- Sheppard, C. (2015). Coral reefs in the Gulf are mostly dead now, but can we do anything about it? Marine Pollution Bulletin. doi:10.1016/j.marpolbul.2015.09.031
- Sheppard, C., Al-Husiani, M., Al-Jamali, F., Al-Yamani, F., Baldwin, R., Bishop, J., … Zainal, K. (2010). The Gulf: A young sea in decline. Marine Pollution Bulletin, 60, 13–38. doi:10.1016/j.marpolbul.2009.10.017
- Sujirachato, P., Thamrongnawasawat, T., Thongtham, N., Jantrarotai, P., & Worachananant, S. (2013). Survival rate of coral fragments transplanted by different methods. Galaxea, 15, 351–358. doi:10.3755/galaxea.15.351
- Vajed Samiei, J., Koosha, D., & Behrooz, A. (2012). Short term growth rate of Acropora downingi in the coral reef of Hengam island, the Persian Gulf. Journal of Persian Gulf (Marine Science), 3, 49–54.
- Vajed Samiei, J., Saleh, A., Shirvani, A., Fumani, N. S., Hashtroudi, M., & Pratchett, M. S. (2016). Variation in calcification rate of Acropora downingi relative to seasonal changes in environmental conditions in the northeastern Persian Gulf. Coral Reefs, 35, 1371–1382. doi:10.1007/s00338-016-1464-6
- Van Leveiren, H., Burt, J. D., Feary, D. A., Cavalcante, G., Marquis, E., Benedettii, L., … Sale, P. F. (2011). Managing the growing impacts of coastal development on fragile costal and marine ecosystems: Lessons from the Gulf. A policy report. Hamilton, ON: UNU-INWEH.
- Wilson, J., & Marimuthu, N. (2012). Post bleaching recovery of transplanted coral nubbins in Abu Al Abyad island of Abu Dhabi, United Arab Emirates. Galaxea, 14, 1–2. doi:10.3755/galaxea.14.1_1
- Yap, H. T. (2009). Local changes in community diversity after coral transplantation. Marine Ecology Progress Series, 374, 33–41. doi:10.3354/meps07650
- Yap, H. T., Alino, P. M., & Gomez, E. D. (1992). Trends in growth and mortality of three coral species (Anthozoa: Scleractinia), including effects of transplantation. Marine Ecology Progress Series, 83, 91–101. doi:10.3354/meps083091

