?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigates geochemistry and ecological risk of artisanal gold mining-derived potentially harmful elements in semi-arid soils in Burkina Faso. R-mode factor analysis, which reduces the variables (elements) to few factors, was applied to explain the dominant variance in the data. Three factors, which account 80% of the total variance, described differences in soil geochemistry resulting from anthropogenic and geogenic sources. High loadings of factor 1 on As, Au, Bi, Cd, Hg, Mo, Pb, Sb, Te, W and Zn suggest that the artisanal gold mining was the most important factor controlling the soil geochemistry. Factor 2 had high loadings on Al, Fe, Mn, Ti, Co, Cr, Cu, Ni, Sc, Sr, Tl and V, representing their geogenic origin. With high loadings on Ca, Mg, S and La, factor 3 describes contribution of biogeochemical cycling to the elements’ abundance in the soils. Lead isotope compositions identified atmospheric deposition as the main source of Pb in farmland soils, whereas topsoil and soil profiles were primarily influenced by the mining activities. Mercury, As and, to a lesser degree, Cd posed the most serious ecological threat to the soils collected around the mining site relative to those of the farmland. Based on the findings of this study, a best pollution control plan of potentially harmful element loadings into soils is urgently required around artisanal gold mining sites across the country.
Public interest statement
The study describes the anthropogenic and geogenic sources of artisanal gold mining-derived potentially toxic heavy metals/metalloids and their ecological impacts on semi-arid soils in southwestern Burkina Faso. Even though artisanal gold mining contributes to poverty alleviation in rural communities, it causes several environmental problems such deforestation, soil degradation and water and air pollution. The release and accumulation of large quantities of potentially harmful elements in the surrounding soils can pose serious threat to human health and also to ecosystems. The presence of hundreds of uncontrolled artisanal gold mining sites around rural dwellings and farmlands across the country makes environmental geochemistry and ecological risk assessment of potentially harmful elements in soils highly important.
Competing interests
The authors declares no competing interests.
1. Introduction
The Birimian greenstone belts that cross Burkina Faso are endowed with polymetallic deposits of gold (Au), zinc (Zn), manganese (Mn), copper (Cu) and molybdenum (Mo) (Huot, Sattran, & Zida, Citation1987). Yet, mining in Burkina Faso has been primarily based on Au (Kiethega, Citation1983). This is mainly due to the severe drought in late 1980s and the boom in world gold price in mid-2000s that made Au extraction attractive for both industrial investors and artisanal miners (Luning, Citation2008; Mégret, Citation2011; Werthmann, Citation2000). Currently, 10 industrial gold mines and about 800 artisanal gold mining (AGM) sites are active across the country. With about 36.1 tons of Au produced in 2014, gold overtook cotton as Burkina Faso’s first export commodity, and the country became the fourth biggest Au producer in Africa after South Africa, Ghana and Mali (Andersson, Ola, & Niklas, Citation2015; Zabsonre, Agbo, Some, & Haffin, Citation2016). While the industrial gold mines are solely owned and operated by foreign companies, AGM activities are carried out by about 700,000 unskilled subsistence farmers (Fofana, Ouédraogo, & Zombré, Citation2009; Jaques, Zida, Billa, Greffié, & Thomassin, Citation2006; Mégret, Citation2011).
Although some AGM sites remain active throughout the year, this low-tech enterprise that uses rudimentary tools to extract gold from rich and near-surface deposits is largely seasonal and usually begins at the end of crop harvest (Gueye, Citation2001; Jaques et al., Citation2006). Because of low levels of agricultural mechanization, farming in Burkina Faso requires too much work for far little yields (Zhou, Citation2016). In order to compensate their inability to ensure self-sufficiency in food production, farmers combine both AGM and farming (Hilson, Citation2016). Hence, the AGM contributes to poverty alleviation and livelihood diversification of rural communities (Andersson et al., 2014). However, the unregulated AGM activities with inadequate or inexistent pollution control measures raise many environmental and human health concerns (Amegbey, Dankwa, & Al-Hasson, Citation1997; Davidson, Citation1993; Donkor, Bonzongo, Nartey, & Adotey, Citation2005).
The Bagassi South gold deposits are hosted in a metallogenic assemblage of quartz and sulfide minerals (Hein, Citation2015). These minerals are generally associated with potentially harmful elements (PHE; Plant et al., Citation1997; Salvarredy-Aranguren, Probst, Roulet, & Isaure, Citation2008), such as silver (Ag), arsenic (As), copper (Cu), lead (Pb), nickel (Ni), antimony (Sb) and zinc (Zn) that may, through mechanical removal and leaching, contaminate the surrounding soils (Cai et al., Citation2012). Artisanal miners also use metallic mercury (Hg°) to extract gold from crude ores that could contribute to mercury (Hg) emission into the atmosphere, as well as its direct release to soils (Bose-O’Reilly et al., Citation2010; Donkor et al., Citation2005; Paruchuri et al., Citation2010). The interest in the PHE abundance in soils stems from the fact that many PHE have no known biological importance and can have adverse human health effects even at trace concentrations (Drasch, Böse-O’Reilly, Beinhoff, Roider, & Maydl, Citation2001; Duruibe, Ogwuegbu, & Egwurugwu, Citation2007; Weeks, Citation2000). Furthermore, the soil-accumulated PHE can pose long-term hazards to plants, as well as to humans that consume these plants (Agbozu, Ekweozor, & Opuene, Citation2007; Nagajyoti, Lee, & Sreekanth, Citation2010; Qian, Wang, & Tu, Citation1996). Pollution caused by PHE may also induce changes in size, composition and activity of soil microbial communities, and thereby affecting organic matter decomposition rates and soil structure (Giller, Witter, & McGrath, Citation1998; McBride, Sauvé, & Hendershot, Citation1997; Merrington, Rogers, & Van, Citation2002).
The behavior of PHE in the soil solution and its downward movement within the profile are a function of a given element’s concentration and the physico-chemical properties of the soil, such as pH, grain sizes, total organic carbon content and cation exchange capacity (CEC) (Nyamangara & Mzezewa, Citation1999; Sposito, Citation1989). For example, under high pH and oxygenated conditions, PHE may be adsorbed onto the surfaces of clay particles, precipitated as sulfides, carbonate oxides or iron- and manganese-oxyhydroxides (Moral, Gilkes, & Jordan, Citation2005). In contrast, under low pH and reduced conditions, previously adsorbed PHE may be released into the soil solution, and thus the soil behaves as both sink and source for PHE (McBride, Citation1989; McBride et al., Citation1997; Sauvé, Hendershot, & Allen, Citation2000).
Several geochemical indices, such as enrichment factor (Covelli & Fantolan, Citation1997), geo-accumulation index (Müller, Citation1981) and pollution index (Tomlinson, Wilson, Harris, & Jeffrey, Citation1980) have been used to differentiate between geogenic and anthropogenic sources of PHE contamination of soils. These indices are based on total concentrations of the elements in the samples, which may be affected by pedogeochemical processes (i.e., weathering and soil formation mechanisms) and regional variations. Knowing the total concentrations of PHE alone is not sufficient for a precise identification of contamination sources and the effects of PHE on the ecosystem. Stable Pb isotope compositions (204Pb, 206Pb, 207Pb and 208Pb), which are not significantly affected by physico-chemical fractionation, have been successfully used to separate anthropogenic Pb from lithogenic sources in soils (e.g., Cicchella et al., Citation2014; Hernandez, Probst, Probst, & Ulrich, Citation2003; Soderberg & Compton, Citation2007; Steinmann & Stille, Citation1997). Therefore, a combination of elemental and isotopic geochemistry of a soil can be a good indicator of AGM-derived PHE contamination of the surrounding environment. Furthermore, in the absence of toxicological study data, the adverse effects of PHE on the ecosystem can be assessed through potential ecological risk index (RI), which includes both total concentrations and toxicological-response factors of individual PHE (Håkanson, Citation1980).
Although some environmental investigations of soil geochemistry have been carried out on AGM sites across Sub-Saharan Africa (e.g., Ako et al., Citation2014; Ekengele, Danala, Zo’o, & Myung, Citation2016; Foli, Nude, & Apea, Citation2012; Manga, Neba, & Suh, Citation2017; Mensah et al., Citation2015; Rajaee et al., Citation2015; van Straaten, Citation2000; Waziri, Citation2014), virtually, no data are available on soils affected by AGM activities in Burkina Faso. In the present study, (1) we report elemental and isotopic geochemistry of tropical semi-arid soils, (2) discuss the factors that control dispersion of PHE around an AGM site and (3) argue that the AGM activities pose serious ecological threat to the adjacent soils.
2. Materials and methods
2.1. Environmental and geological setting
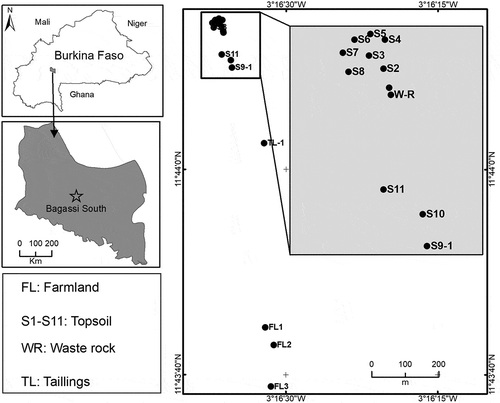
The Bagassi south AGM site was set up in 2000 following the arrival of the first wave of artisanal miners from the central plateau region of the country. This unauthorized site, located in the village of Bagassi in southwestern Burkina Faso, is part of the Bagassi exploration permits owned by Roxgold Inc. (Figure ). The average annual rainfall of this Sudano-sahelian climatic zone is about 800 mm (Roxgold, Citation2017). Vegetation cover in uncultivated areas comprises savannah woodlands with dense shrubs. The landscape is characterized by a system of plains interrupted by chains of ferruginous lateritic hills (450 m above mean sea level).
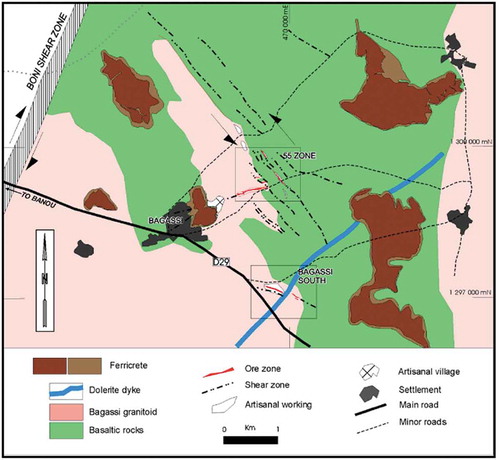
Figure 1. Geological structure of the Bagassi deposits showing the Bagassi South artisanal gold mining site (after Hein, Citation2015).
The soils of the study area are made of shallow (up to 48 cm deep) and weakly developed regosolic soils, encountered on eroded valley slopes and peripheral depression (CPCS, Citation1967). The topsoil (0–14 cm) is dark brown (7.5YR4/4) and becomes yellowish red (5YR4/6) between 14 and 48 cm. From 48 to 110 cm, the soils are composed of unconsolidated weathered schist. Therefore, the gravelly load of the soils consists of 60–90% quartzite and schist debris. The soils contain low weatherable minerals and low silt to clay ratios. Because of their high porosity, these soils are characterized by a well-developed biological activity and high organic matter content (Roxgold, Citation2017). Due to the limited availability of arable land, the soils are increasing being exploited for subsistence (e.g., maize and millet) and cash crops (mainly cotton and groundnut) agriculture (Roxgold, Citation2017). As a result, deforestation and soil nutrient depletion are widespread in the area. In recent years, direct and indirect impacts of the AGM activities have also exacerbated the soil degradation (Roxgold, Citation2017).
The Bagassi gold deposits are situated within the eastern edge of the Palaeoproterozoic Birimian Houndé greenstone belt (Lüdtke et al., Citation1998; Béziat et al., Citation2000; Figure ). The main areas of gold mineralization are the 55 Zone and Bagassi South (Figure ), both of which are hosted in the Bagassi tonalite-granidiorite at the margin of a metamorphosed basalt sequence composed of plagioclase, K-feldspar and quartz, with rare magmatic biotite, overprinted by chlorite, rutile, calcite, pyrite and rare pyrrhotite (Baratoux et al., Citation2011). These formations that occur along dextral shear zone are made up of quartz—gold ± pyrite ± pyrrholite ± chalcopyrite assemblage (Sibthorpe, Citation2012). The granitoid rocks are silicified, kaolinised and carbonate altered, whereas the meta-basalt units are massive and intercalated with hyaloclastite and rare pillowed basalt and sedimentary units (Hein, Citation2015). Both the metabasaltic rocks and the Bagassi granitoid are crosscut by a northeast trending dolerite dyke (Hein, Citation2015).
2.2. Sampling and analysis
A total of 11 topsoils (S1–S11) were sampled randomly around the Bagassi south AGM site on 21 May 2017 to assess lateral dispersion of the mine waste (Figure ). Two profiles were also sampled, according to the limits of the morphological horizons, at sites S1 and S9 (0–45 cm), to investigate a possible translocation of PHE within the soil profiles. Composites of two subsamples of waste rock (WR) and tailings (TL) were also collected from the ore processing area. A representative of the host rock of the Bagassi ore (BO) was also sampled. Three topsoils (FL1–FL3) were sampled from an adjacent cotton farm and used as control samples. All samples were sealed in clean plastic bags.
Back in the laboratory, the samples were air-dried at room temperature, protected from external contamination and subsequently sieved through a stainless steel sieve in order to isolate less than the 2-mm fraction. Electrical conductivity (EC) and pH were measured in a soil to deionized water ratio of 1:2.5 mg l−1. Grain size distribution (sand, silt and clay fractions) was determined using the hydrometer sedimentation method also known as the Robinson Pipette method (Rouiller et al., Citation1994). The carbonate content (CaCO3) was estimated after loss on ignition (LOI) by combustion at 1,000°C for two hours (1974; Bengtsson & Enell, Citation1986).
The Ag-Thiourea (AgTu) method was used to extract basic cations (Pleysier & Juo, Citation1980). Extractable cations (Ca2+, Mg2+, Na+ and K+) and exchangeable silver ion (Ag+) concentrations were measured by a Flame Atomic Absorption Spectrophotometer (Perkin Elmer AA100). The unbuffered CEC was assumed to be the total reactive site concentrations of the solids.
A subset of finely powdered samples was analyzed at Bureau Veritas Commodities Ltd (Vancouver, Canada) for Ag, aluminum (Al), As, Au, boron (B), barium (Ba), bismuth (Bi), calcium (Ca), cadmium (Cd), cobalt (Co), chromium (Cr), Cu, iron (Fe), gallium (Ga), Hg, potassium (K), lanthanum (La), magnesium (Mg), manganese (Mn), molybdenum (Mo), sodium (Na), Ni, phosphorus (P), Pb, sulfur (S), Sb, selenium (Se), scandium (Sc), strontium (Sr), tellurium (Te), thorium (Th), titanium (Ti), thallium (Tl), uranium (U), vanadium (V), tungsten (W) and Zn concentrations with an extended package for Pb isotope analysis. The samples were analyzed using the Bureau’s AQ250 package (i.e., modified aqua regia method) whereby 0.5 g of individual samples was digested in aqua regia (2:2:2 of HCl:HNO3:H2O) at 95°C for 1 h in a heating block, and the sample was made up to a final volume of 300 ml with 5% HCl. The aqua regia digestion is a “pseudo-total” concentration method for dissolution of elements bound to labile minerals, such as water soluble salts, clay particles, as well as those coated by secondary oxyhydroxide minerals (Chen & Lena, Citation2001). This method is adequate for determining the maximum element’s concentration available to plants (Vercoutere, Fortunati, Muntau, Griepink, & Maier, Citation1995).
Aliquots of digested solutions were subsequently analyzed by ulitratrace Inductively Coupled-Mass Spectrometry (ICP-MS). The accuracy of the analytical method was determined by measuring a series of certified standards (SD11, NIST-981-1Y and NIST-983-1Y) and procedural blanks in each analytical set. The results were within 95% confidence limits of the recommended values for a given standard. The relative standard deviation was between 5% and 10%.
2.3. Statistical analysis
The R-mode factor analysis was used to investigate the relationships between major and trace element concentrations in the samples. The principal component analysis technique and correlation matrix were applied in the factor analysis. The number of significant factors was selected on the basis of the Kaiser criterion, and only the factors with eigenvalues greater than or equal to 1 were taken into account (Kaiser, Citation1961). The contribution of each variable to the principal component is considered only if the loading is greater than 0.5. Statistical analyses were carried out using IBM SPSS statistics package (version 20).
2.4. Ecological risk index
To assess the potential adverse effects of the PHE on soils, the potential ecological RI proposed by Håkanson (Citation1980), which takes into account the sensitivity of the soil biological community to PHE (Liu et al., Citation2015) was used. The ecological risk (RI) estimation, usually based on Hg, Cd, As, Ni, Pb, Cr and Zn (Canli & Atli, Citation2003), was calculated as follows (Eqs. 1–3)
Where Cif is the pollution factor of an element i; Ci (mg/kg) is the measured concentration of the element i in the soil; Cin (mg/kg) represents the pre-industrial reference concentration of the element in soils. In the present study, the average concentrations of As, Cd, Cr, Cu, Hg, Pb and Zn (mg/kg) in farmland soils (i.e., control site) were used as background values for estimating the ecological risks posed by the AGM activities on the adjacent soils. Eir is the potential risk of individual element i and Tir is the biological toxic-response factor for an element taken as As = 10, Cd = 30, Cr = 2, Cu = 30, Hg = 40, Pb = 5 and Zn = 1 (Håkanson, Citation1980; Hilton, Davison, & Ochsenbein, Citation1985; Wang et al., Citation2011) and RI is the sum of potential risk of the individual metals. Håkanson defined the following five categories for Eir: Eri < 40, low potential risk; 40 ≤ Eri < 80, moderate potential ecological risk; 80 ≤ Eri < 160, considerable potential ecological risk; 160 ≤ Eri < 320, high potential ecological risk and Eri ≥ 320, very high ecological risk. Likewise, four categories describe RI: RI < 150 corresponds to low ecological risk; 150 ≤ RI < 300, moderate ecological risk; 300 ≤ RI < 600, ecological risk and RI > 600, very high ecological risk.
3. Results and discussion
3.1. Physico-chemical properties of the soils
The mobility and fate of PHE in soils is governed by their mineralogy and physico-chemical parameters, such as pH, EC, particle size, TOC and CEC (Hertz, Angehrn-Bettinazzi, & Stöckli, Citation1990). These factors are also climate-specific, and thus the mobility of PHE in temperate soils is significantly different from that encountered in tropical semi-arid soils. The physico-chemical parameters of the present soils reflect the semi-arid climatic conditions of the study area, as well as the pedogeochemical processes. Thus, the soil samples were predominately composed of sand, silt and, to a lesser degree, clay fraction (Table ). This sandy-silty texture can be attributed to intense and long-term weathering, which has resulted in leaching of Si from the silicate minerals and enrichment of primary quartz and secondary Fe and Al oxide minerals (Wilcke, Kretzschmar, Bundt, & Zech, Citation1999). This was partially explained by low CaCO3 contents (1.1–2.9%; Table ) and predominately low pH (5.4–7.0). With slightly acidic conditions, PHE are unlikely to be retained through precipitation of carbonate minerals (Plassard, Winiarski, & Petit-Ramel, Citation2000). However, the co-occurrence of inner and outer sphere sorption could be the dominant geochemical processes that control PHE retention in this semi-arid soil (Appel & Ma, Citation2002).
Table 1. Physico-chemical properties of Bagassi ore (BO), waste rock (WR), tailings (TL),soil samples from a nearby farmland and topsoils (S2–S8 and S10–S11) and soil profiles (S1-1 to S1-5 and S9-1 to S9-5) collected in the close vicinity of the Bagassi South artisanal gold mining site
The majority of the samples had CEC values in the range of that of kaolin (Halloysite: 3–57 cmolc/kg; Kabata-Pendias, Citation2011). The CEC also showed an increasing pattern with depth, which may be attributed to organic matter accumulation in deeper horizons (Hernandez et al., Citation2003). A preliminary study carried out by Roxgold (Citation2017) in this semi-arid area reported high soil organic matter content (up to 2.8%) and C/N ratios (12–15), suggesting a well-developed biological activity and high organic matter turnover. EC of the samples ranged from 370 to 854 µS/cm. Farmland soils had the lowest EC (average ± SD = 310 ± 17 µS/cm; CV = 5.5%) followed by sample BO (370 µS/cm), whereas TL and WR had the highest (824 and 704 µS/cm, respectively). EC of the soils collected around the AGM site ranged from 540 to 894 µS/cm (average ± std = 754 ± 85 µS/cm; CV = 11%), indicating the presence of high amounts of labile ions in mine waste and AGM-affected soils (Fuller, Feamebough, Mitchel, & Trueman, Citation1995). In light of the above physico-chemical properties, it can be concluded that kaolin, the dominant clay type in the local soils, will have less control over the PHE retention in the soils, whereas Fe and Al oxides and CEC are likely to play a central role in certain trace element immobilization.
3.2. Major and trace element distribution
Major and trace element concentrations of the soils varied between sites. All samples showed very low base cation content (Ca + Mg + Na + K: 0.35–4.37%), with the highest content observed in the less weathered sample BO and the lowest content in the farmland (Table and Figure ). The relatively low base cations in the farmland could be attributed to the absence of vegetation cover on cultivated soils compared to non-cultivated ones around the AGM site. A similar observation was observed in forested soils (tree plantation) and crop land in the Brazilian Amazon (Farella, Lucotte, Davidson, & Daigle, Citation2006). However, the high base cation content in BO compared to other samples clearly reflects the mineralogical compositions of the samples and the weak chemical weathering. Based on the CEC values, the soils have been identified as halloysite, which has the lowest CEC after kaolinite (Kabata-Pendias, Citation2011). That is, the soils had experienced an intensive chemical weathering leading to removal of the base cations (Sposito, Citation1989). Consequently, these soils have very low capacity to retain cations including PHE (Jordan, Citation1984). No depth-trends in base cation abundance were observed (Table ). Both farmland and soil samples collected around the AGM site had similar phosphorus content (average ± standard deviation = 0.022 ± 0.009%; CV = 42%), whereas phosphorus content was slightly higher in samples BO, WR and TL. Furthermore, sulfur concentrations were below detection limit of the instrument (IDL) (except in samples BO, WR and TL), suggesting the influence of the BO on these samples. Compared to Al concentrations (average ± SD = 1.55 ± 0.63%; CV = 41%), those of Fe (average ± SD = 4.84 ± 3.1%; CV = 64%) and Ti (average ± SD 0.019 ± 0.01%; CV = 54%) varied widely across sites. This indicates that Al was the least affected by pedogeochemical processes and AGM activities among the so-called “conservative elements”.
Table 2. Descriptive statistics of major and trace elements of topsoil samples from the Bagassi South artisanal gold mining site and the nearby farmland (n = 25)
Table 3. Major and trace element concentrations of two soil profiles collected in the close vicinity the Bagassi South artisanal gold mining site
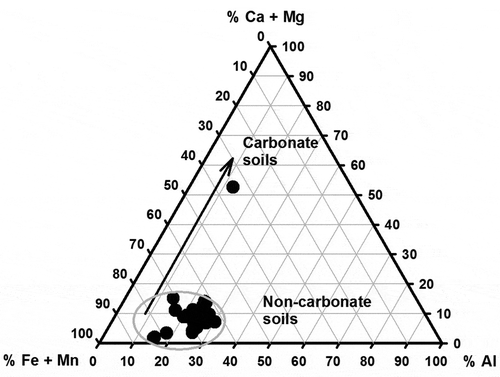
Figure 3. Ternary diagram of Bagassi South soil, illustrating major element distribution. The soil samples (except Bagassi ore) are clustered around the Fe and Mn end-members.
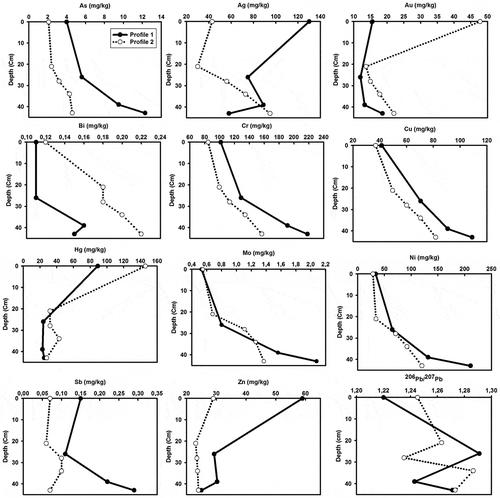
A series of trace elements, associated with gold deposits and anthropogenic pollution, had high concentrations in TL, WR and the AGM-affected soil samples relative to the farmland soils. Thus, Ag, As, Au, Bi, Cu, Cd, Hg, Sb, Te, Pb, Mo, W and Zn concentrations increased in the following order: TL> WR> topsoil> FL (Table ). Likewise, the average concentrations of chalcophile elements, such as Ag (229 and 272 µg/kg), As (9.2 and 91 mg/kg), Au (758.9 and 342.4 µg/kg), Bi (0.38 and 1.96 mg/kg), Hg (899 and 8737 µg/kg), Pb (12.54 and 17.36 mg/kg), Mo (1.83 and 2.73 mg/kg), Sb (0.22 and 0.24 mg/kg), W (15.27 and > 100 mg/kg) and Zn (165.8 and 74.6 mg/kg) were higher in TL and WR compared to the average global soil concentrations (Kabata-Pendias, Citation2011). By contrast, elements, such as Co, Cr, Ni, La, Sc, Sr, Ba, Th, U, V, Ga and Tl concentrations were within the average global soil concentrations, and their distributions were fairly homogeneous across the sampling sites, suggesting that they were not significantly affected by mining activities. Au, Hg, Pb and Zn showed a decreasing pattern with depth, indicating their addition to the soil system through anthropogenic activities (Table and Figure ). Since both farmland and non-cultivated soils around the AGM site are derived from the same parent materials and exposed to similar pedogeochemical processes, it can be concluded that the presence of certain elements were enhanced by mining and mine waste dispersion through runoff and wind deposition compared to others.
3.3. Apportionment of sources of potentially harmful elements
The total concentrations of PHE in soils do not necessarily indicate anthropogenic influence, as weathering can lead to high accumulation of PHE in soils. Consequently, the R-mode factor analysis has been commonly used in environmental geochemistry to identify geogenic versus anthropogenic sources of PHE contamination (e.g., Facchinelli, Sacchi, & Mallen, Citation2001; Micó, Recatala, Peris, & Sanchez, Citation2006). The concentrations of the elements below the IDL were assigned to 50% of the detection limits in the R-mode factor analysis (Cicchella et al., Citation2014). The distribution of 11 elements (Mg, Na, S, Au, Bi, Cd, Hg, Mo, Tl, U and W) was positively skewed, and thus they were log-transformed. Only the elements (30 elements) with high communalities close to unity were included in the analysis. After varimax rotation, three factors (F1, F2 and F3) that describe about 80% of the total variability were extracted (Figure ).
Figure 5. Loading plot of the three components of R-mode factor analysis extracted by PCA method. F1, F2 and F3 represent the influence of artisanal gold mining, chemical weathering and biogeochemical cycling, respectively, on the soil geochemistry.
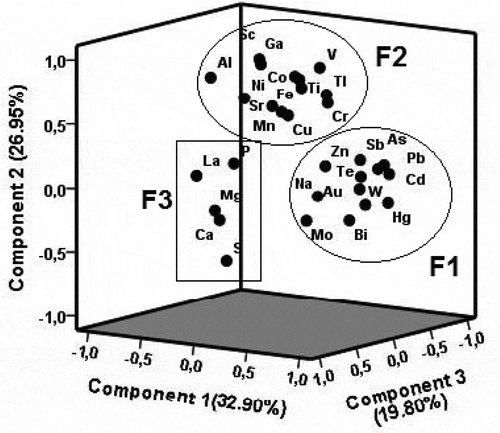
The first factor (F1: 32.94 % variance) showed high loadings on Na and Au and a series of PHE (As, Bi, Cd, Hg, Mo, Pb, Sb, Te, W and Zn). The association of these PHE with Au, As, Bi, Hg and Sb, which were particularly enriched in TL and WR relative to the farmland, is a clear indication of the AGM contribution to these element loadings to the soils through dispersion and transport (Figure ).
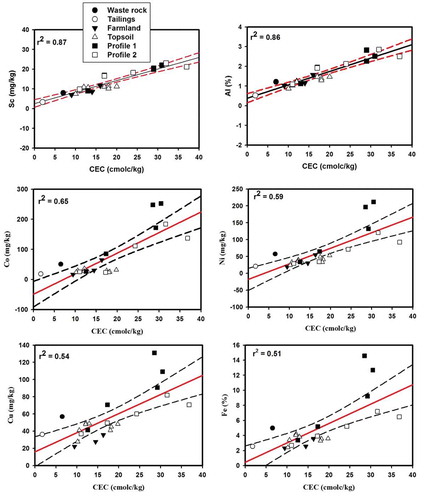
The anthropogenic origin of F1 was further investigated by normalizing certain elements of F1 to Sc. By definition, geochemical normalization consists of establishing correlations between PHE concentrations and the concentration of a reference element i.e., a geochemical normalizer (Donkor et al., Citation2005; Garcia, Barreto, Passos, & Alves, Citation2009). In order for an element to be a good geochemical normalizer, its abundance in the sample must be of lithogenic origin, be structurally linked to at least one of the PHE carriers in the soils and its concentration must not be significantly influenced by anthropogenic inputs (Alves, Passos, & Garcia, Citation2007; Loring, Citation1990). The most common geochemical normalizers are Al, Fe, Sc and Ti. In the present study, Al and Sc were the most homogeneously distributed in the samples with the lowest coefficients of variance (CV = 41% and 46%, respectively). Furthermore, most elements of lithogenic origin (e.g., Al, Sc, Fe, Cu, Ni and Co) exhibited significant (Sc, Al, Co and Ni) and moderate (Fe and Cu) linear relationships with CEC, a potential carrier of trace elements in the soils (Figure ). Because Al is expressed in percent and Sc including PHE in mg/kg, Sc was used as a geochemical normalizer to assess the selected PHE enrichment in the soils and mine waste. Eight PHE (Hg, As, Ag, Mo, Cd, Pb, Sb and Zn) plus Au that showed high loadings on F1 showed weak correlations with Sc, and the soil samples most affected by AGM activities plotted outside the 95% confident interval (Figure not shown).
Figure 6. Relationships between cation exchange capacity (CEC) and Sc, Al, Co, Ni, Cu and Fe. The solid line represents the regression line and the dotted line the prediction interval at the 95% confidence level.
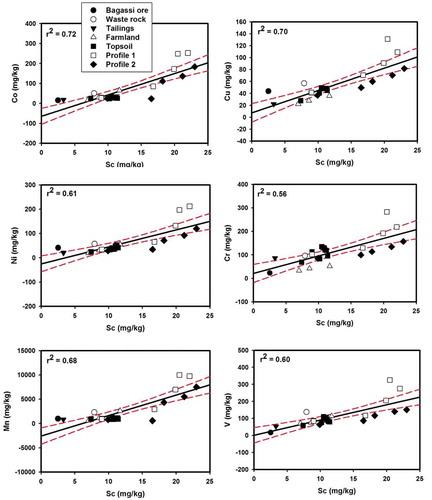
The second factor (F2: 26.95% of the variance) revealed elevated loadings on four major elements (Al, Fe, Mn and Ti) and Co, Cr, Cu, Ga, Ni, Sc, Sr, Tl and V (Figure ). Representative elements of F2 (i.e., Co, Cu, Ni, Cr, Mn and V) co-varied with Sc (Figure ), suggesting that the concentrations of these elements were below or close to those of the pedogeochemical background. Consequently, it can be implied that F2 represents the contribution of soil pedogeochemical processes to these element distribution.
Figure 7. Strong relationships between Sc and geogenically-derived PHE with a few samples lying outside the 95% confidence interval.
Factor 3 (F3: 19.80% of the variance) had high loadings on Ca, Mg, P, S and La. Although rock weathering and atmospheric deposition are the major sources of Ca, Mg and P and S, respectively, these major elements are also essential to biochemical structure and metabolism functioning of organisms (Garten, Citation1976; Reiners, Citation1986). Hence, F3 could represent the biogeochemical cycling of essential elements and La in the soil system through organic matter decomposition. This is consistent with high organic matter and C/N ratios observed in the soils around the site (Roxgold, Citation2017).
In addition to AGM activities and pedogeochemical processes, other anthropogenic sources such petrol and atmospheric deposition may contribute to PHE (particularly Pb) loadings to the soils. In the present study, Pb isotope ratios (206Pb/204/Pb, 207Pb/204Pb, 206Pb/207Pb and 208Pb/206Pb) were used to evaluate the potential sources of Pb in the soil samples (Table ).
Table 4. Lead isotope ratios and inverse lead concentrations in the Bagassi ore, waste rock, tailings, farmland soil, topsoil and soil profile samples collected around the Bagassi South artisanal gold mining site
Figure highlight the relationship between 206Pb/207Pb and 208Pb/206. Although the farmland soils fall on the mixing line of two end-members, i.e., BO and petrol, they are plotted closer to the petrol isotopic compositions than to that of BO. This result suggests that the farmland soils, a relatively less polluted environment, received anthropogenic Pb contribution other than that of the local lithology (Figure ). On the 206Pb/207Pb and 208Pb/206Pb mixing diagram, topsoil samples highly affected by AGM activities appeared to be more influenced by petrol, TL and WR (Figure ). In contrast to the farmland soils and topsoil, isotopic ratios of TL and WR samples did not plot on the petrol and BO end-member mixing line, indicating a third source of Pb. This third source could be the portion of Pb introduced to the soil system during Au extraction and processing. It is interesting to note that soil samples from deeper horizons are clustered around WR isotopic signatures (Figure ), implying migration and accumulation of mine waste, with high Pb concentrations, within soil profiles. Anthropogenic contribution of Pb to the Bagassi south soils was determined through the regression line (r2 = 0.88) between 206Pb/207Pb ratios and Pb concentrations. According to this plot, the anthropogenic contribution to 206Pb/207Pb corresponds to 1.190. The R-mode factor analysis, geochemical normalization and enrichment factors of PHE and Pb isotopic ratios successfully identified pedogeochemical processes, AGM activities and atmospheric deposition as the primary sources of PHE loadings to the adjacent soils.
Figure 8. left panel: three-isotope diagram of 206Pb/207Pb vs. 208Pb/206Pb showing the Pb isotopic composition of farmland (control soil), tailing, waste rock, topsoil (collected at the close vicinity of the mining site), the host rock of the Bagassi ore and European leaded gasoline (Monna, Lancelot, Croudace, Cundy, & Lewis, Citation1997). Right panel: three-isotope mixing diagram of 206Pb/207Pb vs. 208Pb/206Pb showing the Pb isotopic composition of soil profiles (P1 and P2) relative to those of petrol, tailings and the host rock of the Bagassi ore, and Pb isotope diagram of 206Pb/207Pb vs. 1/[Pb]. The dotted line represents the regression through the Bagassi ore, farmland soil samples and leaded gasoline.
![Figure 8. left panel: three-isotope diagram of 206Pb/207Pb vs. 208Pb/206Pb showing the Pb isotopic composition of farmland (control soil), tailing, waste rock, topsoil (collected at the close vicinity of the mining site), the host rock of the Bagassi ore and European leaded gasoline (Monna, Lancelot, Croudace, Cundy, & Lewis, Citation1997). Right panel: three-isotope mixing diagram of 206Pb/207Pb vs. 208Pb/206Pb showing the Pb isotopic composition of soil profiles (P1 and P2) relative to those of petrol, tailings and the host rock of the Bagassi ore, and Pb isotope diagram of 206Pb/207Pb vs. 1/[Pb]. The dotted line represents the regression through the Bagassi ore, farmland soil samples and leaded gasoline.](/cms/asset/cc7ed3db-b839-4006-ac9a-368a3d4ec622/oaes_a_1543565_f0008_b.gif)
3.4. Ecological risk assessment
The potential impact of the PHE on the ecological functions of the soils was evaluated through ecological risk assessment, proposed by Håkanson (Citation1980). The potential ecological risk indices for single elements (Eri) showed that the severity of AGM-derived pollution of eight PHE decreased in the following order: Hg> As> Cd> Cu = Pb> Ni> Cr> Zn (Table ). As expected, mercury in WR and TL samples posed very high ecological risk (Eri = 1,199 and 11,649, respectively). Thus, 10 topsoil samples had considerable potential ecological risk indices for Hg, whereas seven samples (WR, TL, S1-1, S1-2, S1-3, S1-4 and S1-4) exhibited considerable potential ecological risk indices for As. Although Pb and Zn showed low potential ecological risk indices at all sites, the Eri values were the highest for WR and TL.
Table 5. Results of Potential ecological risk indices () of individual potentially harmful element ecological risk indices (RI) of the samples
Based on the overall ecological risk indices, only the BO had a low RI value, whereas WR and TL posed the highest ecological risk. Three sites (S1-5, S1-4 and S5) posed considerable ecological risk and the rest of the sites posed moderate ecological risk relative to the control site. According to Pb isotope ratios, all sites are vulnerable to atmospheric transport of Pb with low ecological risk, whereas the soil around the AGM site is moderately to considerably threatened by mine waste.
4. Conclusions
Trace element distribution, R-mode factor analysis results, geochemical normalization and lead isotope compositions successfully identified anthropogenic and geogenic contributions of PHE loadings to the soils. Each technique contributed important information that was capital in investigating PHE sources in the soils. The raw data showed that elements, such as Au, Hg, As, Bi, Cu, Cd, Sb, Mo and Zn that are generally associated with gold deposits had concentrations exceeding the average world’s soil concentrations. The abundance of Au, As, Bi, Cu, Sb, Mo and Zn could be attributed to the gold mining, whereas Hg is added to the soils through gold extraction. By contrast, lithophile elements, such as Co, Cr, Ni, La, Sc, Ba, Th, U, V and Tl had concentrations closer to or lower than those of the average world’s soil.
These results were corroborated by the R-mode factor analysis, which identified the anthropogenic activities as the main source of PHE abundance in the soils. The technique also suggested that biogeochemical cycling is partially responsible for certain major element distribution in the soils. The use of geochemical normalization indicated anthropogenic origin of Au and some PHE in the soils.
According to Pb isotope ratios, the control site appear to be impacted by atmospheric inputs of leaded gasoline, whereas topsoil samples collected around the AGM site reflect both atmospheric inputs and mine waste signature. The potential ecological risk indices identified Hg, As and Cd as the elements likely to pose serious adverse ecological effects on the soils. Although AGM is recent in the area, the study demonstrated that the soils are moderately contaminated with PHE. To protect soils and ecosystems as a whole, a sound pollution control program is urgently required for the 800 AGM sites across Burkina Faso.
The influence of organic (soil organic matter) and inorganic ligands on trace element speciation, precipitation and adsorption could be investigated in a future study to ascertain the mobility and bioavailability of AGM-derive PHE in tropical semi-arid soils.
Acknowledgements
The authors thank Dr Sâga S. Sawadogo for compiling the sampling map. Comments and suggestions from two reviewers and Dr Arno Rein, the editor of the journal, greatly improved the early version of the manuscript.
Additional information
Funding
Notes on contributors
Aboubakar Sako
Aboubakar Sako is an environmental geochemist and assistant professor of environment and geosciences from University of Dédougou (Burkina Faso). He holds a PhD in Environmental Sciences from Arkansas State University (Arkansas, USA). His research involves geochemical characterization of heavy metals in surface water, groundwater and soils. Dr Sako is also interested in hydrogeochemical modeling and ecological risk assessment of industrial and artisanal gold mining sites in the West Africa region.
Mamadou Nimi
Mamadou Nimi is a geochemical analyst and presently head of the Geological Survey of Burkina Faso in the regional office of Bobo-Dioulasso. He gained about 13 year experience in natural water, soil and rock sample characterization and analysis for environmental assessment and mineral exploration.
References
- Agbozu, I. E., Ekweozor, I. K. E., & Opuene, K. (2007). Survey of heavy metals in the catfish synodontis clarias. International Journal of Environmental Science & Technology, 4(1), 93–98. doi:10.1007/BF03325966
- Ako, T. A., Onoduku, U. S., Oke, S. A., Adamu, I. A., Ali, S. E., Mamodu, A., & Ibrahim, A. T. (2014). Environmental impact of artisanal gold mining in Luku, Minna, Niger State, North Central Nigeria. International Journal of Geomatics and Geosciences, 2(1), 28–37.
- Alves, J. P. H., Passos, E. A., & Garcia, C. A. B. J. (2007). Metals and acid volatile sulfide in sediment cores from the Sergipe River Estuary, Northeast, Brazil. Journal of Brazilian Chemical Society, 18(4), 748–758. doi:10.1590/S0103-50532007000400013
- Amegbey, N. A., Dankwa, J. B. K., & Al-Hasson, S. (1997). Small scale mining in Ghana techniques and environmental considerations. International Journal of Surface Mining Reclamation and Environment, 11(11), 135–138. doi:10.1080/09208119708944077
- Andersson, M., Ola, H., & Niklas, O. (2015). Mining, economic activity and remote sensing: Case studies from Burkina Faso, Ghana, Mali and Tanzania. In World Bank Team The Centre for the Study of African Economies (CSAE), Department of economics at oxford university (pp. 1–42). Malmö University Electronic Publishing.
- Appel, C., & Ma, L. (2002). Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. Journal of Environmental Quality, 31, 581–589. doi:10.2134/jeq2002.5810
- Baratoux, L., Metelka, V., Naba, S., Jessell, M. W., Grégoire, M., & Ganne, J. (2011). Juvenile Paleoproterozoic crust evolution during the Eburnean orogeny (∼2.2–2.0 Ga), western Burkina Faso. Precambrian Research, 191(1 – 2), 18–45. doi:10.1016/j.precamres.2011.08.010
- Bengtsson, L., & Enell, M. (1986). Chemical analysis. In B. E. Berglund (Ed.), Handbook of holocene palaeoecology and palaeohydrology (pp. 423–451). Chichester: John Wiley and Sons Ldt.
- Béziat, D., Bourges, F., Debat, P., Lompo, M., Martin, F., & Tollon, F. (2000). A Paleoproterozoic ultramafic-mafic assemblage and associated volcanic rocks of the Boromo greenstone belt: Fractionates originating from island-arc volcanic activity in the West African Craton. Precambrian Research, 101, 25–47. doi:10.1016/S0301-9268(99)00085-6
- Bose-O’Reilly, S., Drasch, G., Beinhoff, C., Tesha, A., Drasch, K., & Roider, G. (2010). Health assessment of artisanal gold miners in Tanzania. Science of the Total Environment, 408(4), 796–805. doi:10.1016/j.scitotenv.2009.10.051
- Cai, L., Xu, Z., Reu, M., Guo, Q., Hu, G., Wan, H., & Peng, P. (2012). Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong, Province, China. Ecotoxicology and Environmental Safety, 78(1), 2–8. doi:10.1016/j.ecoenv.2011.07.004
- Canli, M., & Atli, G. (2003). The relationships between heavy metals (Cd, Cr, Cu, Fe, Zn) levels and size of six Mediterranean fish species. Environmental Pollution, 121(1), 129–136. doi:10.1016/S0269-7491(02)00194-X
- Chen, M., & Lena, Q. M. (2001). Comparison of three aqua regia digestion methods for twenty Florida soils. Soil Science Society of America Journal, 65(2), 491–499. doi:10.2136/sssaj2001.652491x
- Cicchella, D., De Vivo, B. A., Lima, A., Albanese, S., McGill, R. A. R., & Parrish, R. R. (2014). Heavy metal pollution and Pb isotopes in urban soils of Napoli, Italy. Geochemistry: Exploration, Environment, Analysis, 8(1), 103–112.
- Covelli, S., & Fantolan, G. (1997). Application of normalization procedure in determining regional geochemical baseline. Environmental Geology, 30(1 – 2), 34–45. doi:10.1007/s002540050130
- CPCS. (1967). Classification des sols, édition 1967 (pp. 96).
- Davidson, J. (1993). The transformation and successful development of small-scale mining enterprises in developing countries. Natural Resources Forum, 315–326. doi:10.1111/j.1477-8947.1993.tb00192.x
- Dean, W. E., Jr. (1974). Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. Journal of Sedimentary Petrology, 44(1), 242–248.
- Donkor, A. K., Bonzongo, J.-C. J., Nartey, V. K., & Adotey, D. K. (2005). Heavy metals in sediments of the gold mining impacted Pra River Basin, Ghana, West Africa. Soil and Sediment Contamination: an International Journal, 14(6), 479–503. doi:10.1080/15320380500263675
- Drasch, G. S., Böse-O’Reilly, S., Beinhoff, C., Roider, G., & Maydl, S. (2001). The Mt. Diwata study on the Philippines 1999 - assessing mercury intoxication of the population by small scale gold mining. Science of the Total Environment, 267(1–3), 151–168. doi:10.1016/S0048-9697(00)00806-8
- Duruibe, J. O., Ogwuegbu, M. O. C., & Egwurugwu, J. N. (2007). Heavy metal pollution and human biotoxic effects. International Journal of Physical Sciences, 2(5), 112–118.
- Ekengele, N. L., Danala, D. S., Zo’o, Z. P., & Myung, C. J. (2016). Physical and metals impact of traditional gold mining on soils in Kombo-Laka area (Cameroon). International Journal of Geosciences, 7(9), 1102–1121. doi:10.4236/ijg.2016.79084
- Facchinelli, A., Sacchi, E., & Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environmental Pollution, 114(3), 313–324. doi:10.1016/S0269-7491(00)00243-8
- Farella, N., Lucotte, M., Davidson, R., & Daigle, S. (2006). Mercury release from deforested soils triggered by base cation enrichment. Science of the Total Environment, 368(1), 19–29. doi:10.1016/j.scitotenv.2006.04.025
- Fofana, A., Ouédraogo, D., & Zombré, B. R. (2009). Atelier sous-régional d’information despays de l’Afrique de l’Ouest Francophone sur les problèmes liés à l’orpaillage. Communication. ONUDI, Grand Hôtel de Bamako, 8–10 Décembre 2009.
- Foli, G., Nude, P. M., & Apea, O. B. (2012). Geochemical characteristics of soils from selected districts in the upper east region, Ghana: Implication for trace element pollution and enrichment. Research Journal of Environmental and Earth Sciences, 4(2), 186–195.
- Fuller, M. A., Feamebough, W., Mitchel, D., & Trueman, I. C. (1995). Desert reclamation using yellow river irrigation watr in Ningxia, china. Soil Use and Management, 11, 77–83. doi:10.1111/j.1475-2743.1995.tb00500.x
- Garcia, C. A. B., Barreto, M. S., Passos, E. A., & Alves, J. P. H. (2009). Regional geochemical baselines and controlling factors for trace metals in sediments from the Poxim River, Northeast Brazil. Journal of the Brazilian Chemical Society, 20, 1334–1342. doi:10.1590/S0103-50532009000700019
- Garten, C. T. (1976). Correlations between concentrations of elements in plants. Nature, 261, 686–688. doi:10.1038/261686a0
- Giller, K. E., Witter, E., & McGrath, S. P. (1998). Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biology & Biochemistry, 30(10 – 11), 1389–1414. doi:10.1016/S0038-0717(97)00270-8
- Gueye, D. (2001). Étude sur les Mines Artisanales et Les Exploitations Minières à Petite Échelle au Burkina Faso. IIED and WBCSD Report.
- Håkanson, L. (1980). An ecological risk index for aquatic pollution control of sediment ecological approach. Water Research, 14(8), 975–1000. doi:10.1016/0043-1354(80)90143-8
- Hein, K. A. A. (2015). The Bagassi gold deposits on the eastern margin of the Houndé greenstone belt, Burkina Faso. Ore Geology Reviews, 78, 660–666. doi:10.1016/j.oregeorev.2015.10.028
- Hernandez, L., Probst, A., Probst, J. L., & Ulrich, E. (2003). Heavy metal distribution in some French forest soils: Evidence for atmospheric contamination. Science of the Total Environment, 312(1 – 3), 195–219. doi:10.1016/S0048-9697(03)00223-7
- Hertz, J., Angehrn-Bettinazzi, C., & Stöckli, H. (1990). Distribution of heavy metals in various litter horizons and forest soils. International Journal of Environmental Analytical Chemistry, 39(1), 91–99. doi:10.1080/03067319008027685
- Hilson, G. (2016). Artisanal and small-scale mining and agriculture. Exploring their limits in rural sub-Saharan Africa. Sustainable Market, Issue paper IIED, London.
- Hilton, J., Davison, W., & Ochsenbein, U. (1985). A mathematical model for analysis of sediment coke data. Chemical Geology, 48(1 – 4), 281–291. doi:10.1016/0009-2541(85)90053-1
- Huot, D., Sattran, V., & Zida, P. (1987). Gold in Birimian greenstone belts of Burkina Faso, West Africa. Economic Geology, 82(8), 2033–2044. doi:10.2113/gsecongeo.82.8.2033
- Jaques, E., Zida, B., Billa, M., Greffié, C., & Thomassin, J.-F. (2006). Artisanal and small-scale gold mines in Burkina Faso: Today and tomorrow’. In G. Hilson (Ed.), Small-Scale Mining, Rural Subsistence and Poverty in West Africa (pp. 115–134). Brouton on Dunsmore, Rugby: Intermediate Technology Publications Ltd.
- Jordan, C. F. (1984). Soils of the Amazon rainforest. In G. T. Prance & T. E. Lovejoy (Eds.), Key environments Amazonia (pp. 442). chap. 5. Oxford: Pergamon Press.
- Kabata-Pendias, A. (2011). Trace elements in soils and plants (4th ed.). Boca Raton London New York: Taylor and Francis Group.
- Kaiser, H. F. (1961). A note on Guttman’s lower bound for the number of the common factors. British Journal of Mathematical and Statistical Psychology, 14(1), 41–72.
- Kiethega, J.-B. (1983). L’or de la Volta Noire. Exploitation traditionnelle: Histoire et archéologie. Paris: Karthala.
- Liu, J., Zhuo, Z., Sun, S. H., Ning, X., Zhao, S., Xie, W., … Li, B. (2015). Concentrations of heavy metals in six municipal sludges in Guangzhou and their potential ecological risk assessment for agricultural land use. Polish Journal of Environmental Studies, 24(1), 165–175. doi:10.15244/pjoes/28348
- Loring, D. H. (1990). Lithium — A new approach for the granulometric normalization of trace metal data. Marine Chemistry, 29, 155–168. doi:10.1016/0304-4203(90)90011-Z
- Lüdtke, G., Hirdes, W., Konan, G., Koné, Y., Yao, C., Diarra, S., & Zamblé, Z. (1998). Géologie de la région Haute Comoé Nord–Feuilles Kong (4b et 4d) et Téhini-Bouna (3a à 3d), Direction de la Géologie Abidjan Bulletin, 178 pp.
- Luning, S. (2008). Liberalization of the gold mining sector in Burkina Faso. Review of African Political Economy, 35(117) October):387–401. Accessed October 21, 2014. doi:10.1080/03056240802411016
- Manga, V. E., Neba, G. N., & Suh, E. C. (2017). Environmental geochemistry of mine tailings soils in the artisanal gold mining district of Bétaré-Oya. Cameroon. Environment and Pollution, 6(1), 52–61. doi:10.5539/ep.v6n1p52
- McBride, M. B. (1989). Reactions controlling metal heavy solubility in soils. Advances in Soil Sciences, 10, 1–56.
- McBride, M. B., Sauvé, S., & Hendershot, W. (1997). Solubility control of Cu, Zn, Cd, and Pb in contaminated soils. European Journal of Soil Science, 48(2), 337–346. doi:10.1111/j.1365-2389.1997.tb00554.x
- Mégret, Q. (2011). Gaining access to a globally coveted mining resource: A case study in Burkina Faso. International Social Sciences Journal, 61(202), 389–398.
- Mensah, A. K., Mahiri, I. O., Owusu, O., Mireku, O. D., Wireko, I., Evans, A., & Kissi, E. A. (2015). Environmental impacts of mining: A study of mining communities in Ghana. Applied Ecology and Environmental Sciences, 3(3), 81–94. doi:10.12691/aees-3-3-3
- Merrington, G., Rogers, S. L., & Van, Z. L. (2002). The potential impact of long-term copper fungicide usage on soil microbial biomass and microbial activity in an avocado orchard. Australian Journal Soil Research, 40(5), 749–759. doi:10.1071/SR01084
- Micó, C., Recatala, L., Peris, M., & Sanchez, J. (2006). Assessing heavy metal sources in agricultural soils of a European Mediterranean area by multivariate analysis. Chemosphere, 65(5), 863–872. doi:10.1016/j.chemosphere.2006.04.056
- Monna, F., Lancelot, J., Croudace, I. W., Cundy, A. B., & Lewis, J. T. (1997). Pb isotopic composition of airborne particulate material from France and the southern United Kingdom: Implications for Pb pollution sources in urban areas. Environmental Technology, 31(8), 2277–2286.
- Moral, R., Gilkes, R., & Jordan, M. (2005). Distribution of heavy metals in calcareous and non calcareous soils in Spain. Water, Air, and Soil Pollution, 162(1 – 4), 127–142. doi:10.1007/s11270-005-5997-5
- Müller, G. (1981). Die Schwermetallbelstung der sedimente des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chemiker-Zeitung, 105, 157–164.
- Nagajyoti, P. C., Lee, K. D., & Sreekanth, T. V. M. (2010). Heavymetals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8(3), 199–216. doi:10.1007/s10311-010-0297-8
- Nyamangara, J., & Mzezewa, J. (1999). The effects of long-term sewage sludge application on Zn, Cu, Ni and Pb levels in clay loam soil under pasture grass in Zimbabwe. Agriculture, Ecosystems & Environment, 73(3), 199–204. doi:10.1016/S0167-8809(99)00056-0
- Paruchuri, Y., Siuniak, A., Johnson, N., Levin, E., Mitchell, K., & Goodrich, J. M. (2010). Occupational and environmental mercury exposure among small-scale gold miners in the Talensi-Nabdam District of Ghana’s Upper East region. Science of the Total Environment, 408(24), 6079–6085. doi:10.1016/j.scitotenv.2010.08.022
- Plant, J. A., Klaver, G., Locutura, J., Salminen, R., Vrana, K., & Fordyce, F. M. (1997). The forum of European geological surveys geochemistry task group inventory 1994–1996. Journal of Geochemical Exploration, 59(2), 123–146.
- Plassard, F., Winiarski, T., & Petit-Ramel, M. (2000). Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. Journal of Contaminant Hydrology, 42(2 – 4), 99–111. doi:10.1016/S0169-7722(99)00101-1
- Pleysier, J. L., & Juo, A. S. R. (1980). A single-extraction method using silver-thiourea for Measuring exchangeable cations and effective CEC in soils with variable charges. Soil Science, 129(4), 205–211. doi:10.1097/00010694-198004000-00002
- Qian, S., Wang, Z., & Tu, Q. (1996). Distribution and plant availability of heavy metals in different particle-size fractions of soils. Science of the Total Environment, 187(2), 131–141. doi:10.1016/0048-9697(96)05134-0
- Rajaee, M., Obiri, S., Green, A., Long, R., Cobbina, S. J., Nartey, V., … Basu, N. (2015). Integrated assessment of artisanal and small-scale gold mining in Ghana – Part 2: Natural sciences review. International Journal of Environmental Research and Public Health, 12(8), 8971–9011. doi:10.3390/ijerph120808971
- Reiners, W. A. (1986). Complementary models for ecosystems. American Naturalist, 127, 57–73. doi:10.1086/284467
- Rouiller, J., Souchier, B., Bruckert, S., Feller, C., Toutain, F., & Védy, J. C. (1994). Méthode d’analyses des sols. In M. Bonneau & B. Souchier (Eds.), Pédologie 2. Constituants et propriétés du sol (pp. 619–652). Paris: Masson.
- Roxgold, (2017). Technical report for the Yaramoko gold mine, Burkina Faso. SRK Project Number 3CR016.008.
- Salvarredy-Aranguren, M. M., Probst, A., Roulet, M., & Isaure, M. P. (2008). Contamination of surface waters by mining wastes in the Milluni valley (Cordillera Real, Bolivia): Mineralogical and hydrological influences. Applied Geochemistry, 23(5), 299–324. doi:10.1016/j.apgeochem.2007.11.019
- Sauvé, S., Hendershot, W., & Allen, H. E. (2000). Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environmental Science & Technology, 34(7), 1125–1131. doi:10.1021/es9907764
- Sibthorpe, R. (2012). Yaramoko concession, 2012 program, Investor ppt Presentation, 13 Slides.
- Soderberg, K., & Compton, J. S. (2007). Dust as a nutrient source for fynbos ecosystems, South Africa. Ecosystems, 10(4), 550–561. doi:10.1007/s10021-007-9032-0
- Sposito, G. (1989). The chemistry of soils (pp. 277). USA: Oxford University Press.
- Steinmann, M., & Stille, P. (1997). Rare earth element behavior and Pb, Sr, Nd isotope systematics in a heavy metal contaminated soil. Applied Geochemistry, 12(5), 607–623. doi:10.1016/S0883-2927(97)00017-6
- Sutherland, R. A. (2000). Bed sediment associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology, 39(6), 611–627. doi:10.1007/s002540050473
- Tomlinson, D. C., Wilson, D. J., Harris, C. R., & Jeffrey, D. W. (1980). Problem in assessment of heavy metals in estuaries and the formation of pollution index. Helgoländer Meeresunters, 33(1 – 4), 566–575. doi:10.1007/BF02414780
- van Straaten, P. (2000). Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Science of the Total Environment, 259(1–3), 105–113. doi:10.1016/S0048-9697(00)00553-2
- Vercoutere, K., Fortunati, U., Muntau, H., Griepink, B., & Maier, E. A. (1995). The certified reference materials CRM 142 R light sandy soil, CRM 143 R sewage sludge amended soil and CRM145 R sewage sludge for quality control in monitoring environmental and soil pollution. Fresenius Journal of Analytical Chemistry, 352(1 – 2), 197–202. doi:10.1007/BF00322326
- Wang, Y., Yang, Z., Shen, Z., Tang, Z., Niu, J., & Gao, F. (2011). Assessment of heavy metals in sediments from a typical catchment of the Yangtze River, China. Environmental Monitoring and Assessment, 172(1 – 4), 407–417. doi:10.1007/s10661-010-1316-8
- Waziri, N. M. (2014). Environmental geochemistry of soils and stream sediments from the Birnin-Gwari Artisanal Gold Mining Area, North-western Nigeria. Universal Journal of Geoscience, 2(1), 18–27.
- Weeks, J. M. (2000). A study of the potential risks to human health from consumption of rice cultivates in paddy fields irrigated by mercury-contaminated mine waste water, Naboc River, Philippines. Report for UNIDO Contract DP/PHI/98/00511-02.
- Werthmann, K. (2000). Gold rush in West Africa. The appropriation of “natural” resources: Non industrial gold mining in south-western Burkina Faso. Sociologus, 50(1), 90–104.
- Wilcke, W., Kretzschmar, S., Bundt, M., & Zech, W. (1999). Metal concentrations in aggregate interiors, exteriors, whole aggregates and bulk of Costa Rican soils. Soil Science Society of America Journal, 63(5), 1244–1249. doi:10.2136/sssaj1999.6351244x
- Zabsonre, A., Agbo, M., Some, J., & Haffin, I. (2016). Gold exploitation and income disparities: Case of Burkina Faso. Conference economique africaine, Abuja, Nigeria.
- Zhou, Y. (2016). Agricultural mechanization in West Africa (pp. 1–11). Syngenta Foundation for Agriculture.