Abstract
It is not clear how woody species, especially nitrogen fixers will respond to the combined effect of increased N fertilisation and reduced rainfall amount in savannas in a changing environment. A field experiment was set up at a southern African savanna site to investigate the interaction effects of increased N deposition and reduced rainfall amount on the growth of Vachellia karroo saplings in the presence of grass competition. Rainout shelters were erected around experimental plants to mimic the projected decrease in rainfall in southern Africa while N was added as ammonium nitrate over four growing seasons. The experiment uncovered significant but transient effects of rainfall suppression alone (F1 = 5.171, P = 0.031) and its interaction with N fertilisation (F1 = 6.369, P = 0.017) on the height of V. karroo saplings in the second growing season but not in the first, third and fourth season (P > 0.05). Rainfall suppression significantly reduced sapling height. The interaction of fertilisation and rainfall suppression increased stem height of the study species. In contrast, the interaction effects of N supply and rainfall suppression significantly (F1 = 4.213, P = 0.049) increased diameter of saplings during the first season but not thereafter. Conversely, grass competition did not significantly influence the growth of V. karroo either alone or in interaction with the main treatments though saplings growing in competition with grass had relatively higher growth than the control. Overall, results suggest that N fertilisation may cancel out the predicted negative effect of rainfall decrease on woody species growth thereby enabling the persistence of these species under global environmental changes.
PUBLIC INTEREST STATEMENT
We manipulated rainfall and nitrogen to understand how juvenile Sweet thorn plants will respond to the expected changes in rainfall and nitrogen deposition in savannas of Zimbabwe over four years. The height of plants was affected by rainfall alone as well by the combination of rainfall and nitrogen in the second season but not in the first, third and fourth growing season. Rainfall suppression reduced tree height while the combination of fertilisation and rainfall suppression increased the stem height of target plants. Nitrogen supply and rainfall suppression increased the diameter of juveniles during the first season but not thereafter. In contrast, grass competition did not influence the growth of juveniles either alone or in interaction with rainfall suppression though plants growing in competition with grass had relatively higher growth than the control. Overall, results suggest that N fertilisation may cancel out the predicted negative effect of rainfall decrease on woody species growth thereby enabling their persistence under global environmental changes.
Competing Interest
No conflicts of interests were declared.
1. Introduction
Rainfall and nitrogen (N) are known to limit woody species recruitment, productivity and diversity in savanna ecosystems (Scholes and Archer, Citation1997, Vadigi and Ward, Citation2013, van der Waal et al., Citation2009). Rainfall, in particular water, is considered the primary determinant of savanna structure (Sankaran, Ratnam, & Hanan, Citation2008) since water has a direct effect on the physiological processes of photosynthesis, respiration, and plant growth (Huston and Wolverton, Citation2009). Previous work has shown that maximum woody cover in savannas is regulated by mean annual rainfall (MAR). In semi-arid savannas receiving less than 650 mm of MAR water availability constrains woody cover resulting in a stable co-existence of trees and grasses (Sankaran et al., Citation2005, Sankaran et al., Citation2008). In contrast, in humid savannas receiving ≥650 mm of MAR woody cover is not limited by water availability. As a result and in the absence of disturbances such as fire and herbivory, these humid savannas can easily transit into a woodland system, and hence they are considered unstable (Sankaran et al., Citation2005).
With regard to plant nutrient requirements, N is the most important element required by plants in the largest amounts and savanna vegetation is no exception (Vitousek and Field, Citation1999, Chapin, Citation1980). However, in tropical soils where savannas are distributed, the availability of most essential plant nutrients including N is low due to the higher rates of nutrient loss and immobilisation under warm wet conditions, as well as the old age of tropical soils (Huston and Wolverton, Citation2009). This picture is however changing in the light of the observed global increase in N deposition (WallisDeVries and Bobbink, Citation2017, Valliere et al., Citation2017). For example, in semi-arid savannas of southern Africa, atmospheric N deposition has increased three- to four-fold between 1955 and 2003 (Scholes, Scholes, Otter, & Woghiren, Citation2003) and is likely to accelerate in future (Galloway et al., Citation2004, Sheffield and Wood, Citation2008; Miyazaki, Eskes, & K., Citation2012). The key drivers of this increase include industrialisation (Conradie et al., Citation2016) and land-use change (Adams et al., Citation2004, Galy-Lacaux & Delon, Citation2014). The observed changes in N deposition into savanna systems are occurring against the backdrop of a drying trend in rainfall patterns for southern Africa (Davis & Vincent, Citation2017, Hoerling, Hurrell, Eischeid, & Phillips, Citation2006, Hulme, Citation1996, IPCC, Citation2007). While there are uncertainties in rainfall projections over southern Africa, decreases of between 10 and 30% are expected in this region (Serdeczny et al., Citation2017). Currently, not much is known about the effect of the combined decrease in soil moisture availability and increased N deposition on tree–grass interactions in savannas. Yet, this knowledge is critical for savanna management in a changing world.
Although a number of observational and experimental studies investigated the effects of N addition on plant growth and competition interactions of woody and grassy species in savanna ecosystems, conflicting results have been reported. For instance, Sankaran et al. (Citation2008) conducted a meta-analysis of determinants of woody cover in African savannas and found negative effects of increased soil N availability on woody cover owing to increased grass competitiveness. Similarly, in a semi-arid Mediterranean savanna, Trubat et al., (Citation2011) reported a decrease in survival and performance of woody seedlings in an N addition study. In a bush-encroached South African savanna, an experimental study by Kraaij and Ward (Citation2006) detected significant negative effects of N enrichment on woody species growth with amplified negative effects reported in plots with herbaceous competition. Overall, these studies demonstrate that an increase in soil available N enhances the growth of herbaceous plants resulting in the suppression of woody species recruitment and growth (Walker & Langridge, Citation1997, Moshe et al., Citation2000). This effect is amplified in N poor environments (Kraaij & Ward, Citation2006, van der Waal et al., Citation2009). Contrary to these studies, Ingestad (Citation1980) reported that nitrogen enrichment stimulated woody species growth in a greenhouse experiment. Data from field experimental work in North American savannas by Siemann and Rogers (Citation2003) demonstrated that the growth of woody species increased with N supply. In addition, results from a meta-analysis by Xia and Wan (Citation2008) concluded that N fertilisation stimulates woody plant growth with non-legumes being more sensitive to fertilisation than legumes.
In contrast to the above studies reporting either significant negative or positive effects of increasing N of woody species performance, Holdo (Citation2013) did not detect significant effects of increasing N on the above ground biomass of a dominant woody species. Holdo’s experimental work was conducted in a South African savanna targeting a dominant woody species—Colophospermum mopane. Similarly, in a short-term greenhouse experiment, van Auken, Gese, and Connors (Citation1985) previously found that N fertilisation did not significantly affect the growth of N fixer woody species—Acacia smallii. In addition, Van der Waal et al. (Citation2009) and Mopipi, Trollope, and Scogings (Citation2009) showed that woody seedling growth was not significantly affected by N fertilisation in a nutrient poor semi-arid southern African savanna. Recently, in a study done in a southern African savanna, Barbosa et al. (Citation2014a, Citation2014b) found out that N addition did not induce significant growth in above-ground biomass production of woody species regardless of whether they were N-fixers or non-fixers. Put together the above studies suggest that N fertilisation could have neutral, positive and negative effect on woody species recruitment. The lack of clear outcome on the effects of increased nitrogen on woody species requires further investigation especially in the context of increased N deposition in savannas across the globe.
So far most experimental studies performed to test the effects of changes in rainfall and N on woody species growth have manipulated either resource in isolation (Beier et al., Citation2012, Fensham et al., Citation2005, Ludwig et al., Citation2001, Vadigi and Ward, Citation2013, van Auken et al., Citation1985, February, Higgins, Bond, & Swemmer, Citation2013, Berry and Kulmatiski, Citation2017). The few studies that have manipulated both resources have done so over short time scales (Barbosa et al., Citation2014a, Kraaij & Ward, Citation2006, Ludwig et al., Citation2001, Trubat et al., Citation2011, van der Waal et al., Citation2009; van Langevelde et al., Citation2003). In addition, some of the studies have manipulated these two resources in glass houses (van Auken et al., Citation1985). While glasshouse studies are important in eliminating confounding effects associated with field experiments they often fail to mimic the real world conditions (Limpens et al., Citation2012). In the light of the shortcomings of these studies, our current understanding of the combined effects of key resources on woody species recruitment and growth remains limited (Higgins et al., Citation2007). This gap needs to be closed considering that the observed widespread increases in nitrogen deposition and decreasing rainfall amounts are likely to significantly alter tree–grass interactions with far-reaching consequences on savanna structure and functioning.
To bridge this knowledge gap, a field experiment was conducted over four growing seasons to investigate the main and interaction effects of increasing N and reducing water availability on the growth of a common nitrogen fixer common in African savannas (Vachellia karroo). Fixed rainout shelters were erected around V. karroo saplings to reduce rainfall availability while N was added as ammonium nitrate in the presence and absence of grass competition in a southern African savanna. The study tests the following hypotheses:
Since tree growth is currently limited by rainfall, a 15% decrease will further constrain woody sapling growth.
As grasses are more efficient in utilising resources in the upper soil layer than woody species, N addition will stimulate growth of grass species at the expense of woody species, thereby amplifying their suppressive effects on woody species.
N addition combined with rainfall reduction may dampen the competitive effects of grasses on woody species.
2. Materials and methods
2.1. Study site
The experimental study was performed at Kyle Game Reserve located at 20°11ʹ20.01″ of latitude and 30°58′58.17″ of longitude. The area is located at an average height of 1050 m above sea level with an MAR of 638 mm. The climate of the study area is semi-arid characterised by three seasons: namely (1) hot and wet season from November to April, (2) cool and dry season between May and August and (3) hot and dry season between August and October (Shoko et al., Citation2015). Average maximum daily temperatures range from 21°C in June to 29°C in October, while the average minimum daily temperatures range between 5°C and 17°C in July and January, respectively (Masocha & Dube, Citation2017). The study area is characterised by highly variable rainfall and frequent droughts, which occur on annual to decadal scales (Shekede et al., Citation2016). Soils at the experimental site are mainly clays derived from the basement complex of banded ironstone and gold-belt schist (Wilson, Citation1964). Woody vegetation at the study site is dominated by Dichrostachys cinerea, V. karroo and the invasive Lantana camara (Masocha & Skidmore, Citation2011). The herbaceous layer at the study site mainly consists of Hyparrhenia filipendula and Hyperthelia dissoluta (Vincent and Thomas, Citation1960). Key animal species at the study site include Phacochoerus africanus, Syncerus caffer, Ceratotherium simum, Aepyceros melampus and Giraffa camelopardalis. Our species of interest for this study was V. karroo which constitutes a significant proportion of vegetation cover in the Game Reserve and is one of the key encroacher species in southern African savannas (O’connor, Puttick, & Hoffman, Citation2014).
2.2. Selection of experimental plants
Prior to setting up the field experiment, five candidate sites dominated by V. karroo adult trees interspersed with a continuous herbaceous layer were identified. At each candidate site measuring approximately 100 m by 100 m, 1-m belt transects were laid out in October 2012. Transects were placed 10 m apart. In each transect, three observers identified and counted the number of V. karroo seedling and saplings considered to belong to the same age cohort on the basis of similarity in height. An individual was classified as a seedling if it was less than 5 cm in height, whereas those above 5 cm and with a diameter at the base greater than 1 cm were classified as saplings. The field experiment targeted seedlings or saplings because these growth stages experience intense competition with grasses whose outcome can be mediated by resource availability and environmental changes leading to either woody species recruitment success or failure. Due to the failure to identify sufficient number of seedlings (>50 individuals at each candidate site), a decision was made to use V. karroo saplings as the experimental units. One site with more than 50 A. karroo saplings was randomly chosen to guarantee sufficient number of replicates for the experiment. The site was fenced off to exclude herbivory by small and large mammals.
Prior to starting the experiment, each V. karroo sapling identified was assigned a unique label and measured for stem height, basal stem diameter and number of secondary branches. Then, a total of 48 saplings that were similar in size located at least 2 m from the edge of fence were selected at random to minimize the influence of treatments among plots. To reduce the confounding effect of size, one-way analysis of variance (ANOVA) was used to test and confirm that there were no significant differences in sapling basal diameter and height. ANOVA results indicated that there were no significant differences in the stem basal diameter (F40 = 0.402, P = 0.895) as well as sapling height (F40 = 0.768, P = 0.617) at the start of the experiment. An 1 m × 1 m plot with the focal sapling centrally located was established around each selected sapling before random assignment of treatments.
2.3. Experimental design
To investigate how nitrogen (N) fertilisation and changes in rainfall reduction affect the growth of V. karroo, a field experiment in which N and rainfall amount were manipulated in the presence and absence of grass competition was set up. The field experiment consisted of three main treatments each with two levels: (1) rainfall suppression versus natural rain, (2) nitrogen addition versus no nitrogen and (3) grass competition versus no grass competition. The experiment followed a fully crossed design to yield a total of eight different treatment combinations:
(1) Adding N in the presence of competition from herbaceous vegetation;
(2) Adding N in the absence of competition from herbaceous vegetation;
(3) Reducing rainfall amount by 15% in the presence of competition from herbaceous vegetation;
(4) Reducing rainfall amount by 15% in the absence of competition from herbaceous vegetation;
(5) Herbaceous vegetation left intact to allow competition;
(6) Simultaneously adding N and reducing rainfall in the absence of competition from herbaceous vegetation;
(7) Simultaneously adding N and reducing rainfall in the presence of competition from herbaceous vegetation; and the
(8) Control in which no N was added, rainfall was not reduced and herbaceous vegetation was removed.
2.4. Treatments
Each treatment combination was replicated six times to yield a total of 48 experimental units at the start of the experiment. N was applied as a top dressing at a rate of 4 g of N m−2 at the start of the rainfall season in October. Nitrogen fertiliser was added in the form of ammonium nitrate (NH4NO3) granules evenly sprinkled within the plot by hand. This application rate is three times the N deposition rates projected for southern Africa for the 2050s (Phoenix et al., Citation2006).
To mimic IPCC (Citation2007) rainfall projections for southern Africa, rainfall was reduced by 15%. This was achieved by constructing a fixed-location rainout shelters around each 1 m × 1 m plot experimental plot following the design used by Yahdjian and Sala (Citation2002). The rainout shelter with had arcyllic bands on the roof designed to direct excess rainfall to a storage container that was emptied after rainfall event. The sides of the rainout shelter were left open to allow free air circulation and to minimise the confounding effects of temperature and humidity (Fiala, Tůma, & Holub, Citation2009). The rainout shelter was maintained throughout the duration of the experiment. Control plants had no rainout shelters and thus received normal rainfall. During the experimental period, the amount of rainfall suppressed was variable with as little as 50 mm (for a rainfall total = 337 mm) in 2012 to as much as 125 mm in 2014 (for a rainfall total 832 mm)
Competition between A. karroo saplings and herbaceous vegetation was manipulated by clipping to ground level all above-ground herbaceous biomass present in control plots at the start of the experiment and monthly thereafter. The experiment ran from October 2012 to October 2016. However, some 11 saplings died during the course of the experiment resulting in an unequal number of experimental units across treatments.
2.5. Monitoring Vachellia karroo sapling growth
Vachellia karroo saplings were monitored from October 2012 to September 2016. To quantify the treatment effects on V. karroo growth, basal diameter and stem height were measured on each individual at the end of every month. Here, only the results for the month of May of each of the four growing season are presented as these represent the growth accumulated during the growing season. At the study site, the growing season stretches from November to April. Basal diameter was measured using a veneer caliper at a height of 2 cm from the ground level. Sapling height was measured using a tape measure and height was considered as the highest point of the plant from the ground surface.
2.6. Statistical analysis
Factorial ANOVA was used to test whether the means differed significantly among the eight treatments. Post-hoc multiple comparisons based on the Fischer’s LSD test were done when the null hypothesis that the means were the same was rejected by ANOVA. Statistical analysis was done separately for stem height and basal diameter. Prior to statistical analyses data on stem height and diameter data were log transformed and square rooted, respectively, to reduce skewness. The data were then tested for normality using Shapiro–Wilk’s test. All statistical analyses were performed in STATISTICA version 10 (StatSoft, Inc., Tusla, Oklahoma, USA) and the results are reported for four growing seasons separately. The seasonal approach was adopted in this study to reduce the confounding effects of negative growth that may occur during dry season.
3. Results
3.1. Effects of N addition, rainfall suppression and grass competition on the variation in height of Vachellia karroo saplings
Figure illustrates the effects of N fertilisation, rainfall suppression and grass competition as well as their interactions on height increment of V. karroo saplings across four growing seasons. We observe that the height of V. karroo saplings exposed to different treatments generally increased across the four growing seasons. N fertilisation in the absence of grass competition had negative but non-significant effect on the growth of V. karroo saplings across the four seasons (Table ). In contrast, N fertilisation in the presence of grass competition resulted in larger height increment than fertilisation alone. However, the height differences in plots with and without grass completion were not statistically significant across the duration of the experiment (Table ).
Table 1. Effects of N addition, rainfall suppression, grass competition and their interaction on height increment of Vachellia karroo saplings.
Figure 1. Effects of N fertilisation, rainfall suppression and grass competition on the mean log height of Vachellia karroo saplings after (i) first growing season, (ii) second growing season, (iii) third growing season and (iv) fourth growing season C = control, F = N addition, G = grass competition and R = rainfall suppression. Error plots represent log mean ±95% confidence interval.
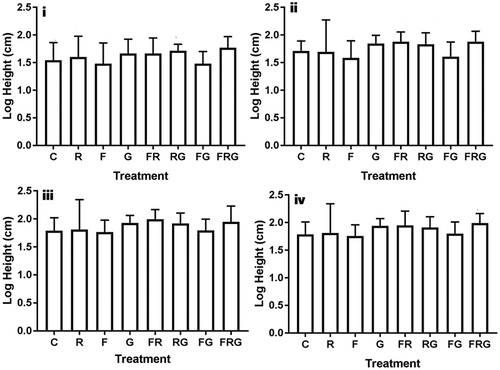
Results for ANOVA illustrate that rainfall suppression alone did not have significant effects on the height of V. karroo saplings across all growing seasons except in the second growing season (Tables 5–1). Specifically, in the second season, rainfall suppression significantly and positively influenced the stem height of V. karroo saplings with plants grown under rainfall suppression having a greater stem height (log mean 1.81 ± 0.12 cm (standard error) than those in the control (log mean 1.79 ± 0.08 cm). Nonetheless, the effects of these treatments are not detectable at the end of the third and fourth growing season (Tables 5–1). A comparison of growth patterns of V. karroo saplings illustrates that rainfall suppression induced more height increment in the presence of grass competition than without. In particular, plants exposed to rainfall suppression in the presence of grass competition increased in height 6% more than those that were exposed to rainfall suppression alone.
The interactive effects N fertilisation and rainfall suppression had significant effects on the height of V. karroo saplings only in the second season. However, these effects were more enhanced in plots with grass competition than without (Figure and Table ). Table shows the results of the post-hoc analysis performed to test which means significantly differ from each other.
Table 2. Results of the post-hoc analysis based on the least significant difference test on tree height growth in the second season.
3.2. Effects of N addition, rainfall suppression and grass completion on diameter increment of Vachellia karroo saplings
The variations in diameter growth of V. karroo saplings exposed to N addition, rainfall suppression, grass competition and their interactions are shown in Figure . Results illustrate that, similar to height growth patterns, N addition alone and in combination with grass competition did not significantly affect the diameter of V. karroo saplings though plants subjected to N addition had larger diameter than those under a combination of fertilisation and grass competition (Tables 5–2)
Figure 2. Effects of N fertilisation, rainfall suppression and grass competition on diameter of Vachellia karroo saplings after (a) first growing season, (b) second growing season, (c) third growing season and (d) fourth growing season. C = control, F = N addition, G = grass competition and R = rainfall suppression. Error plots represent mean ± CI.
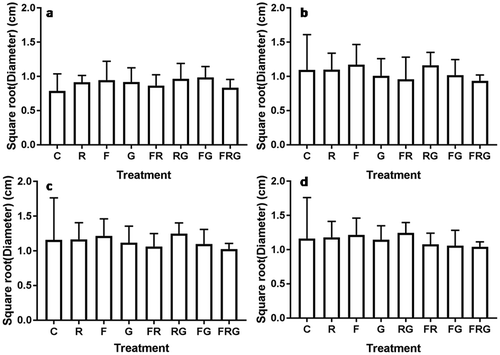
Furthermore, we observe that rainfall suppression alone and in combination with grass competition did not appear to significantly affect the diameter of V. karroo saplings. A comparison of the growth patterns of these plants illustrates that saplings growing under rainfall suppression had smaller diameter than those exposed to a combination of rainfall suppression and grass competition. We also observe that by the end of the first growing season, the interaction of rainfall suppression and N addition is the only treatment that negatively and significantly affected diameter of V. karroo saplings. Specifically, the diameter of V. karroo saplings exposed to the interaction of rainfall suppression and N treatment had significantly smaller diameter (1.14 ± 0.12 cm) than the control (1.24 ± 0.19 cm). Moreover, we did not observe any significant effects of main treatments or their interactions thereafter (Table ).
Table 3. Effects of N addition, rainfall suppression, grass competition and their interaction on stem diameter growth of Vachellia karroo saplings.
4. Discussion
The aim of this study was to test whether N fertilisation and rainfall suppression mediated by grass competition have significant effects on the growth of V. karroo saplings growing in a semi-arid savanna ecosystem. Our results indicate that interactions between N fertilisation and rainfall suppression have significant but transient effects on the growth of woody saplings. In addition, rainfall suppression significantly reduced height but not diameter of our focal species. On the other hand, grass competition did not significantly influence the growth of our focal species either alone or in interaction with the main treatments. However, saplings growing in competition with grass had relatively higher growth than the control.
4.1. Effects of nitrogen on Vachellia karroo sapling growth
Results of our short-term experiment indicate that N fertilisation alone had negative but non-significant effects on the growth of V. karroo, a nitrogen fixer. This is contrary to the expectation that nitrogen addition would significantly increase the growth of our focal species based on the premise that the ready availability of N would result in the reallocation of energy from N fixation to other processes such as growth. Results of this study are consistent with previous studies that observed a 20% reduction in woody species growth as a result of N addition (Schuster and Dukes, Citation2017). Similar studies (Mopipi et al., Citation2009, Van Der Waal et al., Citation2009, Xia and Wan, Citation2008) found no significant effects of N addition on the growth of woody seedlings in semi-arid savannas. In recent studies, Barbosa et al. (Citation2014a, Citation2014b) found out that N addition did not induce significant growth in above-ground biomass production of woody species regardless of whether they were N-fixers or non-fixers in a southern African savanna. Indeed, the growth of leguminous woody species especially Vachellia can be insensitive to N addition but may respond positively to other mineral nutrients (van Auken et al., Citation1985).
Although the mechanism explaining the growth pattern found in this study is not clear, we speculate that under increased N conditions, other factors such as water and phosphorous may be limiting resulting in investment of energy towards the root system of the plants to increase water and nutrient foraging capability (Mokany, Raison, & Prokushkin, Citation2006, Vitousek et al., 2002, Xia and Wan, Citation2008). These results are confirmed by previous research that have documented negative relationship between soil available N and woody biomass (Sankaran et al., Citation2008, Walker & Langridge, Citation1997). The negative effects of increased N availability on woody biomass growth have been explained through competition-based mechanism. Through this mechanism, herbaceous species are hypothesised to directly pre-empt N or indirectly reduce critical resources such as light or water available to woody species as a result of enhanced herbaceous species growth (Kraaij & Ward, Citation2006, Sankaran et al., Citation2008). However, in plots with N addition alone, we removed grass competition thereby ruling out the competitive effects of grasses on V. karroo saplings.
Nitrogen fertilisation led to the increase in the basal diameter of V. karroo species, though the effects were not significant. Our results are confirmed by previous studies that observed investments in basal diameter under conditions of increased nutrient availability (Vadigi and Ward, Citation2013). Conversely, nitrogen addition had negative effects on the stem height of our focal species. These two contrasting results of the response of V. karroo saplings to increased nitrogen availability could be reflecting unique and time-variant resource allocation strategy aligned to addressing limitations to plant growth imposed by the environment. Previous studies have demonstrated a change in biomass allocation to shoots or storage organs in response to increased nitrogen availability (Ingestad & Agren, Citation1991, Miranda, Kozovits, & Bustamante, Citation2014, Poorter & Nagel, Citation2000, Poorter et al., Citation2012). Our results fit well with the optimal partitioning models that suggest that plants act in response to environmental variation through partitioning biomass among organs to optimise resource acquisition and maximise growth (McCarthy and Enquist, Citation2007).
4.2. Effects of rainfall suppression on the growth of Vachellia karroo saplings
Contrary to Hypothesis 1 that expected rainfall suppression to reduce the growth of V. karroo saplings, the result from this study indicate that rainfall suppression significantly increased height but not diameter of V. karroo saplings in the second season after the start of the experiment. Specifically, saplings growing under 15% rainfall suppression had a larger mean height but smaller mean diameter than the control suggesting some compensatory behaviour in the growth patterns of our focal species. A possible mechanism explaining the larger height increment in the second season of saplings under rainfall suppression compared with the control could be increased water use efficiency. Recent studies have shown that increased water use efficiency of woody species associated with increasing aridity helps in overcoming precipitation-driven constraints (Devine, Mcdonald, Quaife, & Maclean, Citation2017). Consequently, plants growing in environments with moderate reduction in precipitation can exhibit similar growth patterns as the control as long as the reduction in precipitation does not result in severe moisture stress (Padilla, Miranda, Jorquera, & Pugnaire, Citation2009, Poorter et al., Citation2012). In fact, aridity is cited as a key factor in explaining variance in nitrogen-fixer abundance across the globe (Pellegrini, Staver, Hedin, Charles‐Dominique, & Tourgee, Citation2016). Our results are also consistent with findings by February et al. (Citation2013) who observed that woody species establishment was more pronounced during periods of short-term droughts when grass competition is reduced. Overall, our finding implies the possibility of persistence of nitrogen-fixing woody plants in a drying environment that is anticipated in southern African savannas in future.
4.3. Interactive effects of rainfall suppression and nitrogen addition on Vachellia karroo saplings’ growth
Results of this study have shown that N fertilisation coupled with rainfall suppression significantly and positively increased sapling height and diameter of V. karroo saplings. This suggests that the potential negative effects of relative moisture stress imposed on V. karroo saplings’ growth could be counteracted by nitrogen fertilisation. In fact, N fertilisation under reduced rainfall could increase plant tolerance to soil moisture stress (Kinugasa, Tsunekawa, & Shinoda, Citation2012) through, for example, increasing below ground biomass (Van den Driessche, Citation1992). In addition, studies elsewhere have shown that N-fixing plants are capable of sustaining high foliar N concentrations leading to efficient water use (Adams, Turnbull, Sprent, & Buchmann, Citation2016). This may result in larger growth rates for sapling growing in water-suppressed environment compared to plants growing under natural rainfall. Our results are confirmed by previous research that observed significant interactive effects of nutrient addition and water manipulation on woody seedling growth suggesting mediating effect of rainfall reduction on the growth of V. karroo saplings (Adams et al., Citation2016).
4.4. Effect of grass competition on Vachellia karroo sapling growth
Contrary to findings from previous studies, our results showed that grass competition alone and in interaction with either N fertilisation or rainfall suppression did not have significant effects on the growth (diameter and height increment) of V. karroo saplings. Similar studies have observed the suppressive effects of grass competition on woody species especially at the early stages of growth when grasses and woody species have intense competition for resources in the upper layers as predicted by Walter’s competition model (Cramer, Chimphango, VAN CAUTER, Waldram, & Bond, Citation2007, Knoop & Walker, Citation1985, Vadigi and Ward, Citation2013, Van Der Waal et al., Citation2009). However, it is not unusual to observe lack of suppressive effects of grasses on woody species especially N-fixers as previously documented by O’connor (Citation1995). In fact, several ecological studies have emphasised the increasing importance of facilitation over competition under conditions of increased abiotic stress (Callaway, Citation2007, Dohn et al., Citation2013, He, Bertness, & Altieri, Citation2013, Maestre et al., Citation2009); with the largest facilitative effects being realised at intermediate stressful conditions (Holmgren & Scheffer, Citation2010). A recent study on understanding tree–grass interactions along a rainfall gradient by Mazía et al. (Citation2016) has shown that facilitation could even extend up to 1800 mm. In our case, a 15% reduction in rainfall might have been marginally stressful and thus enhanced facilitation between grasses and woody saplings for nitrogen since N-fixers have been shown to transfer fixed N to non-legume species (Sierra and Nygren, Citation2006, Zhang et al., Citation2016). Our study could be providing new insights into the role of facilitation in shaping plant communities even under unstressful conditions. In fact, our study could be confirming that facilitation does not necessarily need to occur under severe environments as previously thought.
The lack of significant interactive effects of N addition and grass competition on V. karroo saplings could also suggest the scale dependence nature of the growth of our experimental plants. Previous studies have demonstrated that plants respond differently to coarse versus fine-scale nutrient application (Hutchings et al. Citation2003, Birch and Hutchings Citation1994; Fransen et al. Citation1998, Kume et al. Citation2006, van der Waal, Citation2010). For the N addition and grass competition treatment, fertiliser was sprinkled by hand, thereby making it available as scattered grains instead of large patches. Consequently, we speculate that grasses might have been more efficient in exploiting fertiliser applied at fine scale application than woody species thereby lowering growth rate of saplings exposed to N addition in the presence of grass competition than N addition alone.
An important finding of this study is that while rainfall suppression and its interaction with N addition significantly affected the growth of V. karroo saplings, these effects may be transient. In fact, results indicate that 15% rainfall suppression alone and its interaction with fertiliser addition had significant effects on the diameter of our focal species only in the second growing season. Similarly, the interaction of rainfall suppression and N addition only had significant effect on the diameter of V. karroo saplings in the first growing season but could not be detected thereafter. To the best of our knowledge, our study is among the few that have reported transient effects of rainfall suppression and N addition woody nitrogen fixers.
The novelty of this study lies in manipulating rainfall, nitrogen and grass competition under field conditions to test whether these factors significantly influence the growth of V. karroo saplings in a semi-arid savanna. Previous studies on effects on nitrogen deposition have either been carried out on relatively short time scales (Barbosa et al., Citation2014a, Holdo, Citation2013, Kraaij & Ward, Citation2006, Mopipi et al., Citation2009, Van Der Waal et al., Citation2009) or were performed in green houses, and thus may not provide useful insights into the response of savanna woody species to changes in key resources. This therefore underscores the importance of long-term studies in understanding the effects of global nutrient enrichment and changes in rainfall patterns on savanna ecosystem structure and function over longer time scales (Bhadouria, Singh, Srivastava, & Raghubanshi, Citation2016). In addition, our study is among a few that has manipulated key resources limiting growth of key woody species in the presence and absence of grass competition. We assert that such interactive studies are critical to the understanding of the combined effects of global environmental change and herbaceous competition on tree–grass ratios in tropical ecosystems.
While useful insights have been gained from assessing the effects of key limiting resources and grass competition in savannas based on a leguminous woody species, it is important that future work focuses on the response of multiple functional species typical of savannas. This is especially important considering that non-leguminous plant species are incapable of fixing nitrogen and may thus respond differently to un/availability of key resources. For instance, it has often been hypothesised that nitrogen-fixer plants may lose their comparative advantage over non-nitrogen-fixer plants in the event of increased nitrogen deposition. In addition, nitrogen fixers are also predicted to increase their water use efficiency under a drying climate as a result of increase in carbon fertilisation. It may therefore be important to manipulate multiple resources on several plant species with different functional traits in order to enhance our understanding of tree–grass interactions in savannas that are undergoing transformation due to global environmental changes.
Additional information
Funding
Notes on contributors
Munyaradzi D. Shekede
Munyaradzi D. Shekede was born on the 9th of May 1981 in Marondera, Zimbabwe. He completed his BA Honours degree in Geography at the University of Zimbabwe in 2003 and a Masters degree in Environmental Policy and Planning from the same university in 2005. Currently, Munyaradzi is studying for a PhD at the University of Zimbabwe focusing on the effects of nitrogen deposition and precipitation decline on tree-grass interactions in southern African savannas. From 2007 to date, he has been working as a lecturer at the University of Zimbabwe, Department of Geography and Environmental Science. Some of the courses he teaches include Remote Sensing, Geographic Data and Analysis, Global Environmental Change and Biodiversity Conservation. Munyaradzi is interested in understanding global changes on society and the environment including flood modelling. He has published more than fourteen peer-reviewed articles and three book chapters.
References
- Adams, M., Ineson, P., Binkley, D., Cadlsch, G., Tokuchi, N., Scholes, M., & Hicks, K. (2004). Soil function responses to excess nitrogen inputs at global scale. Ambio, 33, 530–536. doi:10.1579/0044-7447-33.8.530
- Adams, M. A., Turnbull, T. L., Sprent, J. I., & Buchmann, N. (2016). Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proceedings of the National Academy of Sciences of the United States of America, 113, 4098–4103. doi:10.1073/pnas.1523936113
- Barbosa, E. R., Tomlinson, K. W., Carvalheiro, L. G., Kirkman, K., De Bie, S., Prins, H. H., & Van Langevelde, F. (2014a). Short-term effect of nutrient availability and rainfall distribution on biomass production and leaf nutrient content of savanna tree species. PLoS One, 9, e92619. doi:10.1371/journal.pone.0092619
- Barbosa, E. R., van Langevelde, F., Tomlinson, K. W., Carvalheiro, L. G., Kirkman, K., de Bie, S., & Prins, H. H. (2014b). Tree species from different functional groups respond differently to environmental changes during establishment. Oecologia, 174(4), 1345–1357.
- Beier, C., Beierkuhnlein, C., Wohlgemuth, T., Penuelas, J., Emmett, B., Körner, C., & Hansen, K. (2012). Precipitation manipulation experiments–challenges and recommendations for the future. Ecology Letters, 15(8), 899-911.
- Berry, R. S., & Kulmatiski, A. (2017). A savanna response to precipitation intensity. PloS one, 12(4), e0175402.
- Bhadouria, R., Singh, R., Srivastava, P., & Raghubanshi, A. S. (2016). Understanding the ecology of tree-seedling growth in dry tropical environment: A management perspective. Energy, Ecology and Environment, 1, 296–309. doi:10.1007/s40974-016-0038-3
- Birch, C. P. D., & Hutchings, M. (1994). Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology, 653–664.
- Callaway, R. M. (2007). Positive interactions and interdependence in plant communities. Dordrecht: Springer.
- Chapin III, F. S. (1980). The mineral nutrition of wild plants. Annual Review of Ecology and Systematics, 11(1), 233-260.
- Conradie, E., Van Zyl, P., Pienaar, J., Beukes, J., Galy-Lacaux, C., Venter, A., & Mkhatshwa, G. (2016). The chemical composition and fluxes of atmospheric wet deposition at four sites in South Africa. Atmospheric Environment, 146, 113–131. doi:10.1016/j.atmosenv.2016.07.033
- Cramer, M. D., Chimphango, S. B., Van Cauter, A., Waldram, M., & Bond, W. (2007). Grass competition induces N2 fixation in some species of African Acacia. Journal of Ecology, 95, 1123–1133. doi:10.1111/jec.2007.95.issue-5
- Davis, C. L., & Vincent, K. (2017). Climate risk and vulnerability: A handbook for southern Africa (2nd ed.) Pretoria, South Africa: CSIR.
- Devine, A. P., Mcdonald, R. A., Quaife, T., & Maclean, I. M. (2017). Determinants of woody encroachment and cover in African savannas. Oecologia, 183, 939–951. doi:10.1007/s00442-017-3807-6
- Dohn, J., Dembélé, F., Karembé, M., Moustakas, A., Amévor, K. A., & Hanan, N. P. (2013). Tree effects on grass growth in savannas: Competition, facilitation and the stress‐gradient hypothesis. Journal of Ecology, 101, 202–209. doi:10.1111/1365-2745.12010
- February, E. C., Higgins, S. I., Bond, W. J., & Swemmer, L. (2013). Influence of competition and rainfall manipulation on the growth responses of savanna trees and grasses. Ecology, 94, 1155–1164.
- Fensham, R. J., Fairfax, R. J., & Archer, S. R. (2005). Rainfall, land use and woody vegetation cover change in semi‐arid Australian savanna. Journal of Ecology, 93(3), 596-606.
- Fiala, K., Tůma, I., & Holub, P. (2009). Effect of manipulated rainfall on root production and plant belowground dry mass of different grassland ecosystems. Ecosystems (New York, N.Y.), 12, 906–914. doi:10.1007/s10021-009-9264-2
- Fransen, B., de Kroon, H., & Berendse, F. (1998). Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia, 115(3), 351-358.
- Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., … Vörösmarty, C. (2004). Nitrogen cycles: Past, present, and future. Biogeochemistry, 70, 153–226. doi:10.1007/s10533-004-0370-0
- Galy-Lacaux, C., & Delon, C. (2014). Nitrogen emission and deposition budget in West and Central Africa. Environmental Research Letters, 9, 125002. doi:10.1088/1748-9326/9/12/125002
- He, Q., Bertness, M. D., & Altieri, A. H. (2013). Global shifts towards positive species interactions with increasing environmental stress. Ecology Letters, 16, 695–706. doi:10.1111/ele.12080
- Higgins, S. I., Bond, W. J., February, E. C., Bronn, A., Euston-Brown, D. I., Enslin, B.,… & Scheiter, S. (2007). Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology, 88(5), 1119-1125.
- Hoerling, M., Hurrell, J., Eischeid, J., & Phillips, A. (2006). Detection and attribution of twentieth-century northern and southern African rainfall change. Journal of Climate, 19, 3989–4008. doi:10.1175/JCLI3842.1
- Holdo, R. M. (2013). Effects of fire history and N and P fertilization on seedling biomass, Specific Leaf Area, and root: Shoot ratios in a South African savannah. South African Journal of Botany, 86, 5–8. doi:10.1016/j.sajb.2013.01.005
- Holmgren, M., & Scheffer, M. (2010). Strong facilitation in mild environments: The stress gradient hypothesis revisited. Journal of Ecology, 98, 1269–1275. doi:10.1111/jec.2010.98.issue-6
- Hulme, M. (1996). Recent climatic change in the world’s drylands. Geophysical Research Letters, 23, 61–64. doi:10.1029/95GL03586
- Huston, M. A., & Wolverton, S. (2009). The global distribution of net primary production: Resolving the paradox. Ecological Monographs, 79(3), 343-377.
- Hutchings, M. J., John, E. A., & Wijesinghe, D. K. (2003). Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology, 84(9), 2322-2334.
- Ingestad, T. (1980). Growth, nutrition, and nitrogen fixation in grey alder at varied rate of nitrogen addition. Physiologia Plantarum, 50(4), 353-364.
- Ingestad, T., & Agren, G. I. (1991). The influence of plant nutrition on biomass allocation. Ecological Applications, 1, 168–174. doi:10.2307/1941809
- IPCC. 2007. Climate change 2007: The physical science basis. In S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller (Eds.), Contribution of Working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (pp. 847-940). Cambridge and New York, NY.
- Kinugasa, T., Tsunekawa, A., & Shinoda, M. (2012). Increasing nitrogen deposition enhances post-drought recovery of grassland productivity in the Mongolian steppe. Oecologia, 170, 857–865. doi:10.1007/s00442-012-2354-4
- Knoop, W., & Walker, B. (1985). Interactions of woody and herbaceous vegetation in a southern African savanna. The Journal of Ecology, 235–253. doi:10.2307/2259780
- Kraaij, T., & Ward, D. (2006). Effects of rain, nitrogen, fire and grazing on tree recruitment and early survival in bush-encroached savanna, South Africa. Plant Ecology, 186, 235–246. doi:10.1007/s11258-006-9125-4
- Kume, T., Sekiya, N., & Yano, K. (2006). Heterogeneity in spatial P-distribution and foraging capability by Zea mays: Effects of patch size and barriers to restrict root proliferation within a patch. Annals of Botany, 98(6), 1271-1277.
- Limpens, J., Granath, G., Aerts, R., Heijmans, M. M., Sheppard, L. J., Bragazza, L.,… & Rochefort, L. (2012). Glasshouse vs field experiments: Do they yield ecologically similar results for assessing N impacts on peat mosses? New Phytologist, 195(2), 408-418.
- Ludwig, F., de Kroon, H., Prins, H. H., & Berendse, F. (2001). Effects of nutrients and shade on tree‐grass interactions in an East African savanna. Journal of Vegetation Science, 12(4), 579-588.
- Maestre, F. T., Bowker, M. A., Puche, M. D., Hinojosa, M. B., Martinez, I., Garcia-Palacios, P., … Escudero, A. (2009). Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecology Letters, 12, 930–941. doi:10.1111/j.1461-0248.2009.01352.x
- Masocha, M., & Dube, T. (2017). Relationship between native and exotic plant species at multiple savannah sites. African Journal of Ecology, 56(1), 81–90.
- Masocha, M., & Skidmore, A. K. (2011). Integrating conventional classifiers with a GIS expert system to increase the accuracy of invasive species mapping. International Journal of Applied Earth Observation and Geoinformation, 13, 487–494. doi:10.1016/j.jag.2010.10.004
- Mazía, N., Moyano, J., Perez, L., Aguiar, S., Garibaldi, L. A., & Schlichter, T. (2016). The sign and magnitude of tree–Grass interaction along a global environmental gradient. Global Ecology and Biogeography, 25, 1510–1519. doi:10.1111/geb.2016.25.issue-12
- McCarthy, M. C., & Enquist, B. J. (2007). Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Functional Ecology, 21(4), 713-720.
- Miranda, V. T., Kozovits, A. R., & Bustamante, M. M. (2014). Competition alters responses of juvenile woody plants and grasses to nitrogen addition in Brazilian savanna (Cerrado). Nitrogen Deposition, Critical Loads and Biodiversity, Springer.
- Miyazaki, K., Eskes, H. J., & K., S. (2012). Global NOx emission estimates derived from an assimilation of OMI tropospheric NO2 columns. Atmospheric Chemistry and Physics, 12, 2263–2288. doi:10.5194/acp-12-2263-2012
- Mokany, K., Raison, R., & Prokushkin, A. S. (2006). Critical analysis of root: Shoot ratios in terrestrial biomes. Global Change Biology, 12, 84–96. doi:10.1111/gcb.2006.12.issue-1
- Mopipi, K., Trollope, W. S. W., & Scogings, P. F. (2009). Effects of moisture, nitrogen, grass competition and simulated browsing on the survival and growth of Acacia karroo seedlings. African Journal of Ecology, 47, 680–687. doi:10.1111/j.1365-2028.2008.01022.x
- Moshe, D., Bailey, C. L., & Scholes, R. J. (2000). The effect of elevated atmospheric carbon dioxide on selected savanna plants. In Proceedings: Natural forests and savanna woodlands symposium II: towards sustainable management based on scientific understanding of natural forests and woodlands. Department of Water Affairs and Forestry, Knysna, South Africa (pp. 142–144).
- O’connor, T. (1995). Transformation of a savanna grassland by drought and grazing. African Journal of Range & Forage Science, 12, 53–60. doi:10.1080/10220119.1995.9647864
- O’connor, T. G., Puttick, J. R., & Hoffman, M. T. (2014). Bush encroachment in southern Africa: Changes and causes. African Journal of Range & Forage Science, 31, 67–88. doi:10.2989/10220119.2014.939996
- Padilla, F. M., Miranda, J. D., Jorquera, M. J., & Pugnaire, F. (2009). Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecology, 204, 261–270. doi:10.1007/s11258-009-9589-0
- Pellegrini, A. F., Staver, A. C., Hedin, L. O., Charles‐Dominique, T., & Tourgee, A. (2016). Aridity, not fire, favors nitrogen‐fixing plants across tropical savanna and forest biomes. Ecology, 97, 2177–2183. doi:10.1002/ecy.1504
- Phoenix, G. K., Hicks, W. K., Cinderby, S., Kuylenstierna, J. C. I., Stock, W. D., Dentener, F. J., … Ineson, P. (2006). Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Global Change Biology, 12, 470–476. doi:10.1111/j.1365-2486.2006.01104.x
- Poorter, H., & Nagel, O. (2000). The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Functional Plant Biology, 27, 1191. doi:10.1071/PP99173
- Poorter, H., Niklas, K. J., Reich, P. B., Oleksyn, J., Poot, P., & Mommer, L. (2012). Biomass allocation to leaves, stems and roots: Meta-analysesof interspecific variation and environmental control. New Phytologist, 193, 30–50. doi:10.1111/j.1469-8137.2011.03952.x
- Sankaran, M., Hanan, N. P., Scholes, R. J., Ratnam, J., Augustine, D. J., Cade, B. S.,… & Ardo, J. (2005). Determinants of woody cover in African savannas. Nature, 438(7069), 846.
- Sankaran, M., Ratnam, J., & Hanan, N. P. (2008). Woody cover in African savannas: The role of resourcs, fire and herbivory. Global Ecology and Biogeography, 17, 236–245. doi:10.1111/j.1466-8238.2007.00360.x
- Scholes, M. C., Scholes, R. J., Otter, L. B., & Woghiren, A. J. (eds.). (2003). Biochemistry: The cycling of elements. Washington: The Kruger experience Island Press.
- Scholes, R. J., & Archer, S. R. (1997). Tree-grass interactions in savannas. Annual Review of Ecology and Systematics, 28(1), 517–544.
- Schuster, M. J., & Dukes, J. S. (2017). Rainfall variability counteracts N addition by promoting invasive Lonicera maackii and extending phenology in prairie. Ecological Applications, 27, 1555–1563. doi:10.1002/eap.2017.27.issue-5
- Serdeczny, O., Adams, S., Baarsch, F., Coumou, D., Robinson, A., Hare, W., … Reinhardt, J. (2017). Climate change impacts in Sub-Saharan Africa: From physical changes to their social repercussions. Regional Environmental Change, 17, 1585–1600. doi:10.1007/s10113-015-0910-2
- Sheffield, J., & Wood, E. F. (2008). Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Climate Dynamics, 31, 79–105. doi:10.1007/s00382-007-0340-z
- Shekede, M. D., Murwira, A., Masocha, M., & Zengeya, F. M. (2016). Decadal changes in mean annual rainfall drive long-term changes in bush-encroached southern African savannas. Austral Ecology, 41, 690–700. doi:10.1111/aec.12358
- Shoko, C., Masocha, M., & Dube, T. (2015). A new potential method to estimate abundance of small game species. African Journal of Ecology, 53, 406–412. doi:10.1111/aje.12211
- Siemann, E., & Rogers, W. E. (2003). Changes in light and nitrogen availability under pioneer trees may indirectly facilitate tree invasions of grasslands. Journal of Ecology, 91, 923–931. doi:10.1046/j.1365-2745.2003.00822.x
- Sierra, J., & Nygren, P. (2006). Transfer of N fixed by a legume tree to the associated grass in a tropical silvopastoral system. Soil Biology and Biochemistry, 38, 1893–1903. doi:10.1016/j.soilbio.2005.12.012
- Trubat, R., Cortina, J., & Vilagrosa, A. (2011). Nutrient deprivation improves field performance of woody seedlings in a degraded semi-arid shrubland. Ecological Engineering, 37(8), 1164-1173.
- Vadigi, S., & Ward, D. (2013). Shade, nutrients, and grass competition are important for tree sapling establishment in a humid savanna. Ecosphere, 4, 1–27. doi:10.1890/ES13-00239.1
- Valliere, J. M., Irvine, I. C., Santiago, L., & Allen, E. B. (2017). High N, dry: Experimental nitrogen deposition exacerbates native shrub loss and nonnative plant invasion during extreme drought. Global Change Biology, 23(10), 4333-4345.
- Van Auken, O. W., Gese, E. M., & Connors, K. (1985). Fertilization response of early and late successional species: Acacia smallii and Celtis laevigata. Botanical. Gazzette, 1, 564–569. doi:10.1086/337561
- Van den Driessche, R. (1992). Changes in drought resistance and root growth capacity of container seedlings in response to nursery drought, nitrogen, and potassium treatments. Canadian Journal of Forest Research, 22, 740–749. doi:10.1139/x92-100
- Van der Waal, C., De Kroon, H., De Boer, W. F., Heitkãnig, I., Skidmore, A. K., De Knegt, H. J., … Page, B. R. (2009). Water and nutrients alter herbaceous competitive effects on tree seedlings in a semi-arid savanna. Journal of Ecology, 97, 430–439. doi:10.1111/j.1365-2745.2009.01498.x
- van der Waal, C. (2010). Nutrients in an African Savanna: the consequences of supply heterogeneity for plants and animals. (Doctoral dissertation). Retrieved from https://library.wur.nl/WebQuery/wurpubs/fulltext/146149.
- Van Langevelde, F., Van De Vijver, C. A., Kumar, L., Van De Koppel, J., De Ridder, N., Van Andel, J.,... & Prins, H. H. (2003). Effects of fire and herbivory on the stability of savanna ecosystems. Ecology, 84(2), 337-350.
- Vincent, V., & Thomas, R. G. (1960). An agricultural survey of Southern Rhodesia: Part I: Agro-ecological survey. Salisbury: Government Printers.
- Vitousek, P. M., & Field, C. B. (1999). Ecosystem constraints to symbiotic nitrogen fixers: a simple model and its implications. In New Perspectives on Nitrogen Cycling in the Temperate and Tropical Americas (pp. 179-202). Springer, Dordrecht.
- Vitousek, P. M., Cassman, K., Cleveland, C., Crews, T., Field, C. B., Grimm, N. B., & Rastetter, E. B. (2002a). Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry, 57, 1–45. doi:10.1023/A:1015798428743
- Walker, B. H., & Langridge, J. L. (1997). Predicting savanna vegetation structure on the basis of plant available moisture (PAM) and plant available nutrients (PAN): A case study from Australia. Journal of Biogeography, 24, 813–825. doi:10.1046/j.1365-2699.1997.00123.x
- WallisDeVries, M. F., & Bobbink, R. (2017). Nitrogen deposition impacts on biodiversity in terrestrial ecosystems: Mechanisms and perspectives for restoration. Biological Conservation, 212, 387-389.
- Wilson, J. F. (1964). The geology of the country around Fort Victoria. Bulletin – Rhodesia Geological Survey, 58.
- Xia, J., & Wan, S. (2008). Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist, 179, 428–439. doi:10.1111/j.1469-8137.2008.02488.x
- Yahdjian, L., & Sala, O. E. (2002). A rainout shelter design for intercepting different amounts of rainfall. Oecologia, 133, 95–101. doi:10.1007/s00442-002-1024-3
- Zhang, H.-Y., Yu, Q., Lü, X.-T., Trumbore, S. E., Yang, J.-J., & Han, X.-G. (2016). Impacts of leguminous shrub encroachment on neighboring grasses include transfer of fixed nitrogen. Oecologia, 180, 1213–1222. doi:10.1007/s00442-015-3538-5
