Abstract
Considering the harmful effects of the synthetic termiticides and resistance development in the pest population, there is a need to search for economically viable, environmentally-friendly and effective termiticides. Therefore, the screening of alternative antitermiticidal activity from plant based products could be employed as a means in the termite control strategies. The present study was carried out to evaluate the anti-termitic activity of the hexane leaf extract of Prosopis juliflora against the termites of Macrotermes spp. at concentrations of 25, 50, 75 and 100 mg/L. It was found that, the LC50-LC90 values were 33.3–213.98, 12.71–66.33 and 9.41–34.71 mg/L after 24, 48 and 72 h of exposure, respectively. The phytochemical study of the crude extract showed the presence of steroids, saponins, terpenoids, alkaloids and flavonoids which might be responsible for its biocidal activity. These results suggested that hexane leaf extract of P. juliflora possessed biocidal activity aginst termites.
Public Interest Statement
Termites are the most highly destructive insects known to cause economic losses to plants, agricultural crops and buildings. Conventional insecticide has been the most common and effective method for the control of termites. However, extensive use of conventional pesticides caused serious impacts on biotic and abiotic factors of the environment. Hence, it is necessary to focus on alternative insecticides which are environmentally sound and cost effective. In this study, we evaluated the termicidal activity of Prosopis juliflora extract against termites of Macrotermes spp. It was found that P. juliflora could be used as a natural biocide to control termites.
Competing Interest
The authors declares no competing interest.
1. Introduction
Termites are highly destructive insect pests, which largely damage wooden portions of buildings, furnitures, books, utility poles, plants and agricultural crops such as sugarcane, millet, barley, cotton, wheat and paddy (Addisu, Mohamed, & Waktole, Citation2013; Elango et al., Citation2012). Termite colonies generally affects plants either by attacking the trunks and pods or by making tunnel under the plant which eventually weaken plant stems, causing them to collapse or giving access to fungus and other diseases (Badshah, Farmanullah, Salihah, Saljoqi, & Shakur, Citation2004; Elango et al., Citation2012). It is also known that termites damage a variety of materials ranging from paper fabrics to even non-cellulosic materials such as asbestos, asphalt bitumen, lead and metal foils (Bultman, Beal, & Ampong, Citation1979).
In the past, management of termites has relied on synthetic termicides including DDT, aldrin, dieldrin, chlordane, heptachlor, phosphorothioate, and BHC (Dyer, Cattani, Pisaniello, Williams, & Edwards, Citation2001). Synthetic insecticides has been successfully employed as soil treatment against termites (Elango et al., Citation2012). However, the use of synthetic termicides for a long time poses a great hazard to environment including toxicity to non-target organism and residual effects. In addition, resistance development in pest population further derives the need to search for new bioactive compounds with a wide range of new modes of action (Elango et al., Citation2012). Hence, the search for alternative economically viable, environmentaly-friendly and effective insecticides has been a concern of many researchers (Arihara et al., Citation2004; Elango et al., Citation2012).
To avoid environmental pollution and health problems caused by the use of synthetic pesticides, there is increasing interest in naturally occurring toxicants from plants (Chang, Cheng, & Wang, Citation2001). Many plants may be used as alternative sources of termite control agents because they are rich sources of bioactive compounds mainly secondary metabolites (Osbrink, Lax, & Brenner, Citation2001). Plant based insecticides might be used as alternative in pest management strategies as they are generally insect specific, relatively harmless to non-target organisms, less expensive, and biodegradable (Satti, Nasr, & Bashir, Citation2004). Manzoor et al. (Citation2011) have reported that the ethyl acetate, methanol, butanol, hexane, water and chloroform extracts of Ocimum sanctum leaves showed termicidal activity against Heterotermes indicola (Isoptera: Rhinotermitidae). Similarly, black heartwood of Cryptomeria japonica have shown good termiticidal activity against Coptotermes formosanus (Taxodiaceae) (Arihara et al., Citation2004). Oyedokun, Anikwe, Okelana, Mokwunye, and Azeez (Citation2011) tested insecticidal activity of the Phyllanthus amarus, Acassia albida and Tithonia diversifolia leaf crude extracts against the workers of Macrotermes bellicosus in vitro. The aqueous extracts of P. amarus, A. albida and T. diversifolia caused 40–56%, 24–60% and 42–88% mortality, respectively after 140 minutes of exposure to the extracts. Similarly, ethanolic extracts of P. amarus, A. albida and T. diversifolia resulted higher percentage mean mortality of 64–91%, 36.4–76% and 36–68%, respectively. In another work Elango et al. (Citation2012) reported anti-termitic activity of the crude leaf hexane, ethyl acetate, acetone and methanol extracts of medicinal plants as Andrographis lineata, Andrographis paniculata, Argemone mexicana L., Aristolochia bracteolata, Datura metel L., Eclipta prostrata L., Sesbania grandiflora and Tagetes erecta L. against Coptotermes formosanus. The present study aimed to determine the anti-termitic potential of P. juliflora leaf extract as potential bio-based materials for termite control. In addition, the phytochemical constituents of hexane leaf extract of P. juliflora were investigated.
2. Materials and methods
2.1. Plant material
P. juliflora leaves was collected from Arbaminch town, Ethiopia. The voucher specimen has been identified by botanist in the Botany Research Center and kept in the research laboratory of Arbaminch University for further reference. The leaves were washed with tap water, rinsed with distilled water and then shade dried at room temperature (28 ± 2°C) for 15 days. The dried leaves were powdered in an electric grinder and stored in airtight container under dark conditions at room temperature.
2.2. Preparation of the extract
Three hundred grams (300 g) from leaves powder was extracted with 300 mL of hexane (Fisher Scientific, USA) in Soxhlet apparatus (Technico Scientific Company, Coimbatore, India) for 8 h. The extract was filtered through a Buchner funnel with Whatman number 1 filter paper. The crude extract was evaporated to dryness in a rotary vacuum evaporator. After complete evaporation of the solvent the concentrated extract was collected and stored in a refrigerator at 4°C until required for assay. One gram of the plant residue was dissolved in 100 mL of acetone (Fisher Scientific, USA) to make stock solution. From the stock solution different concentrations; 25, 50, 75, 100 mg/L, were prepared.
2.3. Termite collection
Population of termites was collected from a termitarium from Arbaminch town, Arbaminch, Ethiopia and identified in the Entomology Research Center. Termite mounds were dug up using shovel and soil containing termites were kept in polyethylene plastic boxes. Termites were collected from the plastic sheets using camel hair brush and placed in plastic containers as described by Addisu et al. (Citation2013). Termites were fed with dry wood inside the container and the top of the container was covered with muslin cloth to allow free flow of air also to prevent the termites from escaping.
2.4. Anti-termitic activity
The no-choice bioassay method of Kang, Matsushima, Sameshima, and Takamura (Citation1990) was employed to evaluate the anti-termitic activity of the plant extract. About 1 mL of plant extract of various concentrations ranging from 25 to 100 mg/L were applied to Whatman No. 1 filter papers of 9 cm diameter. Filter paper treated with acetone was used as a control. The solvent was removed from the treated filter papers by air-drying at ambient temperature and batches of 20 worker termites were randomly selected from the stock population and kept into respective Petri dishes (10 cm in diameter 1.5 cm in height). Treated and control termites were held under laboratory conditions in darkness at 27 ± 2°C and 60–80% relative humidity. All treatments were replicated 3 times The numbers of dead termites were counted every 24 hours of exposure and the percentage mortality was calculated. A termite was considered dead when it was lying flat on its back and showing no sign of body movement after being touched with soft camel brush.
2.5. Phytochemical analysis
Phytochemical analysis of the plant extract was carried out qualitatively for the presence of various chemical compounds steroids, terpenoids, glycosides, resins and quinones according to the methodologies of Harborne (Citation1973, Citation1983).
Test for alkaloids; Mayer’s test: To a few milliliters of the filtrates, a drop of Mayer’s reagent was added along the side of the test tube. The formation of a creamy or white precipitate indicates the test is positive.
Test for carbohydrates; Benedict’s test: To 0.5 mL of the filtrate, 0.5 mL of Benedict’s reagent was added. The mixture was heated for 2 minutes in a boiling water bath. The development of a characteristic red-colored precipitate indicates the presence of carbohydrates.
Test for saponins; Foam test: about 2 mL of the extract was diluted with distilled water and made up to 20 mL. The suspension was shaken in a graduated cylinder for about 15 min. The formation of about two-centimeter layer of foam indicates the presence of saponins.
Test for phenolic compounds; Ferric chloride test: The extract was diluted to 5 ml with distilled water to which a few drops of neutral 5% ferric chloride solution were added. A dark green color indicates the presence of phenolic compounds.
Test for tannins; Ferric chloride test: About 0.5 mg of dried and powdered sample was boiled in 20 mL of water in test tubes and then filtered. A few drops of 0.1% ferric chloride was added and formation of brownish green or blue black color indicates the presence of tannins.
Test for flavonoids; Ammonia test: To a portion of the aqueous extract 5 mL of dilute ammonia solution was added. Subsequently, a few drops of concentrated sulphuric acid were added. Appearance of yellow coloration in the solution indicates the presence of flavonoids.
Test for terpenoids; Salkowski test: Five milliliters of the extract was mixed with 2 mL of chloroform and concentrated sulphuric acid to form a layer. A reddish brown coloration of the interface showed the presence of terpenoids.
2.6. Statistical analysis
The average termite mortality data were subjected to probit analysis for calculating LT50, LT90, LC50, LC90 and other statistics at 95% fiducidal limits of upper confidence limit and lower confidence limit, and χ2 values were calculated using Finney’s method (Citation1971). SPSS software package 16.0 version was used. Results with P < 0.05 were considered to be statistically significant.
3. Results
3.1. Phytochemical constituents of the P. juliflora extract
In the present study, hexane P. juliflora leaf extract was checked for the presence of phytochemicals. The results showed the presence of various phytochemicals in the extract as presented in Table .
Table 1. Phytochemical constituents of the leaf extract of P. juliflora
3.2. Anti-termitic activity of the extract
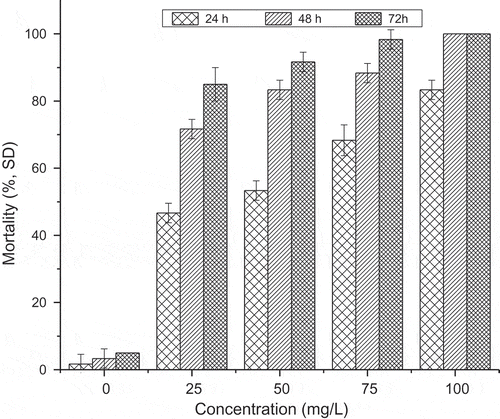
The efficacy of the P. juliflora leaf extract against the worker termites at various concentrations toghether with the different time interval (24, 48 and 72 h) is shown in Figure . The result showed that the mortality rate (±SE) was concentration and time dependent, mortality increased with the increasing in concentration and the time of exposure. Higher mortality rates were recorded at increasing concentrations of P. juliflora leaf extract and the highest mortality rates 83.33%, 100%, 100% were recorded at higher concentration of 100 mg/L within 24, 48, and 72 hours of exposure, respectively. The results indicate as that the treated termite mortality were significantly different from that of control groups (p < 0.05).
Figure 1. Mortality rates of worker termites, at three time intervals (24, 48 and 72 h) post treatments with different (25, 50, 75 and 100 mg/L) P. juliflora leaf extract.
The Probit analysis (LC50, LC90), their 95% fiducial limits at 24, 48 and 72 h are presented in Table . The result showed the LC50 and LC90 values gradually decreased with the exposure periods. LC50 values of hexane extracts from P. juliflora were 33.30, 12.71 and 9.41 mg/L after 24, 48 and 72 h exposure, respectively. Similarly, LC90 values were 213.98, 66.33 and 34.71 mg/L after 24, 48, and 72 h exposure, respectively.
Table 2. Lethal concentration (LC50, LC90) values of P. Juliflora leaf extract against worker termite at 24, 48 and 72 h post treatement
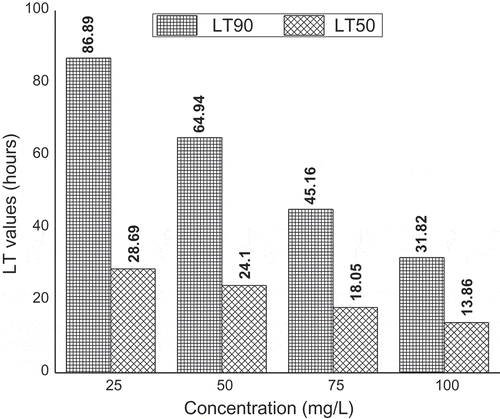
The lethal time LT50 and LT90 values and their 95% fiducial limits at various concentrations are presented in Figure . The result revealed that LT50 and LT90 values decreased when the leaf extract concentration increase. The lethal time LT50 and LT90 values were (28.69 and 86.89 h) for the lowest dose 25 mg/L and (13.86 and 31.82 h) for the highest dose 100 mg/L.
3.3. Discussion
Considering the resistance of insects to conventional synthetic insecticides and environmental safety issue, the recent trend is to search the plant based products. There are several well documented studies showing that the plant essential oils and extracts could be used as larvicides, adulticides, repellents, growth regulant and oviposition deterrent (Coria et al., Citation2008; Jeyabalan, Arul, & Thangamathi, Citation2003; Senthilkumar, Varma, & Gurusubramanian, Citation2009). In this study it was also observed that, leaf extract of P. juliflora has showed anti-termic activity against the worker termites of Macrotermes spp. The anti-termitic activities are strongly influenced by the concentration and duration of exposure.
According to various reports, the anti-termic functions of plant extract can be attributed to various secondary compounds viz., alcohols, aldehydes, fatty acid derivatives, phenolics, tannins, alkaloids, saponins, sterols, triterpenes and flavanoids which might be contributing jointly or independently (Boue & Raina, Citation2003; Cornelius, Grace, & Yates, Citation1997; Ohmura, Doi, Aoyama, & Ohara, Citation2000). It has been reported that the chemical composition of the crude plant extract and essential oils from several plants could vary significantly depending on species, plant parts, geographical origin, season, and extraction procedure (Nattudurai, Paulraj, & Ignacimuthu, Citation2012). The present studies also revealed the presence of steroids, saponins, terpenoids, alkaloids and flavonoids in leaf extract of P. juliflora which might be responsible for its insecticidal activity.
In conclusion, the results of the present study showed that the hexane leaf extract of P. juliflora is toxic for worker termites of Macrotermes spp. The termicidal activities of P. juliflora leaf extract was attributed to potent secondary metabolites in the plant extract. Hence, this study suggests that the isolation and characterization of the bioactive chemical constituents in the plant responsible for the termicidal activity are needed. Further, the field application of the extract should be evaluated for proper utilization in the management of termites. The plant extracts could be used as a potential eco-friendly pest management strategies in the future.
Additional information
Funding
Notes on contributors
Terefe Tafese Bezuneh
Terefe Tafese Bezuneh is a senior lecturer at Department of Chemistry, Arbaminch University, Ethiopia.
Hinsermu Daba Derressa
Hinsermu Daba Derressa is a senior technician at Dept. of Chemistry, Arbaminch Univ., Ethiopia.
Ramesh Duraisam
Ramesh Duraisam is a senior lecturer at Dept. of Chemistry, Arbaminch Univ., Ethiopia.
Alemu Mekonnen Tura
Alemu Mekonnen Tura is a senior lecturer at Dept. of Chemistry, Arbaminch Univ., Ethiopia.
References
- Addisu, S., Mohamed, D., & Waktole, S. (2013). Efficacy of botanical extracts against termites, Macrotermes spp., (Isoptera: Termitidae) under laboratory conditions. International Journal of Agricultural Research, 1, 1–7.
- Arihara, S., Umeyama, A., Bando, S., Imoto, S., Ono, M., & Yoshikawa, K. (2004). Three new sesquiterpenes from the black heartwood of Cryptomeria japonica. Chemical and Pharmaceutical Bulletin, 52(4), 463–465.
- Badshah, H., Farmanullah Salihah, Z., Saljoqi, A. U. R., & Shakur, M. (2004). Toxic effect of AK (Calotrpis procera) plant extracts against termites (Heterotermes indicola and Coptoterms heimi) Isoptera: Rhinotermitidae. Pakistan Journal of Biological Sciences, 7(9), 1603–1606. doi:10.3923/pjbs.2004.1603.1606
- Boue, S. M., & Raina, A. K. (2003). Effects of plant flavonoids on fecundity, survival, and feeding of the Formosan subterranean termite. Journal of Chemical Ecology, 29, 2575–2584.
- Bultman, J. D., Beal, R. H., & Ampong, F. F. K. (1979). Natural resistance of some tropical African woods to Coptotermes formosanus Shiraki. Forest Products Journal, 29, 46–51.
- Chang, S. T., Cheng, S. S., & Wang, S. Y. (2001). Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides). Journal of Chemical Ecology, 27, 717–724.
- Coria, C., Almiron, W., Valladares, G., Carpinella, C., Luduena, F., Defago, M., & Palacios, S. (2008). Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae). Bioresource Technology, 99, 3066–3070. doi:10.1016/j.biortech.2007.06.012
- Cornelius, M. L., Grace, J. K., & Yates, J. R. (1997). Toxicity of monoterpenoids and other natural products to the formosan subterranean termite (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 87, 705–708. doi:10.1093/jee/87.3.705
- Dyer, S. M., Cattani, M., Pisaniello, D. L., Williams, F. M., & Edwards, J. M. (2001). Peripheral cholinesterase inhibition by occupational chlorpyrifos exposure in Australian termiticide applicators. Toxicology, 169, 177–185. doi:10.1016/S0300-483X(01)00509-1
- Elango, G., Abdul Rahuman, A., Kamaraj, C., Bagavan, A., Abduz Zahir, A., Santhoshkumar, T., & Marimuthu, R. G. (2012). Efficacy of medicinal plant extracts against Formosan subterranean termite, Coptotermes formosanus. Industrial Crops and Products, 36, 524–530. doi:10.1016/j.indcrop.2011.10.032
- Finney, D. J. (1971). Probit analysis (3rd ed.). London: Cambridge University Press.
- Harbone, J. B. (1973). Phytochemical methods: A guide to modern techniques of plant analysis (pp. 183–195). London: Chapman and Hall Ltd.
- Harbone, J. B. (1983). Phytochemicals Methods, second (2nd ed.). London: Academic Press.
- Jeyabalan, D., Arul, N., & Thangamathi, P. (2003). Studies on effects of Pelargonium citrosa leaf extracts on malarial vector, Anopheles stephensi Liston. Bioresource Technology, 89, 185–189.
- Kang, H. Y., Matsushima, N., Sameshima, K., & Takamura, N. (1990). Termite resistance tests of hardwoods of Kochi growth I. The strong termiticidal activity of kagonoki (Litsea coreana Léveillé). Japan Wood Research Society, 36(1), 78–84.
- Manzoor, F., Beena, W., Malik, S., Naz, N., Naz, S., & Syed, W. H. (2011). Preliminary evaluation of Ocimum sanctum as toxicant and repellent against termite, Heterotermes indicola (Wasmann) (Isoptera: Rhinotermitidae). Pakistan Journal of Science, 63(1), 59–62.
- Nattudurai, G., Paulraj, M. G., & Ignacimuthu, S. (2012). Fumigant toxicity of volatile synthetic compounds and natural oils against red flour beetle Tribolium castaneum (Herbst) (Coleopetera: Tenebrionidae). Journal of King Saud University – Science, 24, 153–159. doi:10.1016/j.jksus.2010.11.002
- Ohmura, W., Doi, S., Aoyama, M., & Ohara, S. (2000). Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. The Journal of Wood Science, 46, 149–153. doi:10.1007/BF00777362
- Osbrink, W. L. A., Lax, A. R., & Brenner, R. J. (2001). Insecticide susceptibility in Coptotermes formosanus and Reticulitermes virginicus (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 94, 1217–1228.
- Oyedokun, A. V., Anikwe, J. C., Okelana, F. A., Mokwunye, I. U., & Azeez, O. M. (2011). Pesticidal efficacy of three tropical herbal plants’ leaf extracts against Macrotermes bellicosus, an emerging pest of cocoa, Theobroma cacao L. Journal of Biopesticides, 4(2), 131–137.
- Satti, A. A., Nasr, O. E., & Bashir, N. H. (2004). Technology of natural pesticides: Productionand uses in Sudan. The Second National Pest Management Conference in the Sudan, December 6–9, Faculty of Agricultural Sciences, University of Gezira-Wad Medani, Sudan.
- Senthilkumar, N., Varma, P., & Gurusubramanian, G. (2009). Larvicidal and adulticidal activities of some medicinal plants against the malarial vector, Anopheles stephensi (Liston). Parasitology Research, 104, 237–244. doi:10.1007/s00436-008-1180-4