?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Fish growth analysis is a fundamental part of research in fisheries biology, aquaculture, and physiology. However, there is the question of whether the addition of mass and length depends on the amount of energy a prey can deliver. This study tested the hypothesis that there is an effect on the length/weight ratio of the predator, under the premise that fish that ingest prey with a greater amount of energy will have greater mass growth, in contrast to those that ingest food with lesser energy. The fish in this experiment was under controlled laboratory conditions under two types of food treatment and one control (low energy vs high energy amount). The energy-rich treatment had a significant effect on the mass increase (GLM; F = 2.72; P = 0.031), and the length/weight ratio was greater in the fish under the energy-rich treatment (ANCOVA; F = 3.59; P = 0.043). However, the IGR showed that fish presented low rates of intrinsic size growth (ANOVA; F = 0.189; P = 0.828).
Keywords:
PUBLIC INTEREST STATEMENT
We consider this article to be appropriate for the readers of the journal, as it describes how the energy component of the food has an effect on weight addition. These inferences can be used for good fisheries management and aquaculture patterns, especially in times when interactions between prey and predators take on real importance for sustainability.
1. Introduction
What does a species eat? How do individuals eat? What is the energy contribution of prey in the diet of predators? These are questions of research in natural life that respond to hypotheses of different levels of ecology and resource management. It is in this context that the study of trophic ecology becomes a key point for understanding trophic dynamics from individuals to ecosystems (Liedke et al., Citation2016). Specifically for fish, the dynamics between food supply and energy demand for nutrients play a crucial role in how communities are structured in the environments where fish inhabit (Albo-Gaeta et al., Citation2018; Puigserver et al., Citation2017; Wuenschel, Jugovich, & Hare, Citation2006). The environment provides the food, but also exerts a demand on the fish (Ross, Nielsen, Gislason, Nielsen, & Andersen, Citation2018) for example, low ambient temperatures increase the energy demand of fish that regulate their temperature—e.g. sunfish, tuna and some sharks—to maintain their body temperature (Wegner, Snodgrass, Dewar, & Hyde, Citation2015) other extreme, fish that do not regulate their body temperature also require higher energy to maintain their metabolic rates when the environmental temperature increases. The latter is based on the mechanism proposed by Blaxter (Citation1989) who points out that high ingestion rates increase metabolic rates as a consequence of the “extra work“ required for the capture, digestion and assimilation of prey, which is called ”Temperature Increase by Feeding” (HiE) (Gliwicz et al., Citation2018). The foundation of the theory of food preference in fish (Wainwright & Richard, Citation1995) is based on eco-morphological processes, where the combination of information on prey size and predator mandibular components allow inference of prey use and selection (Gaeta et al., Citation2018) diet composition and food preference may change throughout an individual’s life history due to different effects such as prey supply, environment, ontogeny, reproductive, behavioral and anthropogenic effects (Wootton, Citation1990). This study evaluates the premise that fish that ingest prey with a greater amount of energy will have greater mass growth, in contrast to those that ingest food with a lesser amount of energy, and that this will have an effect on the length/weight ratio of the predator. The main objective of this study was to analyze changes in body length and weight in individuals of goldfish Carassius auratus (Linnaeus, 1758) under two food treatments (high and low energy) for 30 days in controlled laboratory conditions.
2. Materials and methods
Twenty-seven specimens of Carassius auratus were used. This species was chosen because of its adaptability to experimentation under controlled conditions, its easy handling and low level of stress (Popesku et al. Citation2008). Individuals were chosen according to size (2.8 ± 0.27 cm) and weight (1.3 ± 0.25 g), which showed no significant differences among treatment group at the beginning of the experiment (ANOVA; F = 0.147; P = 0.864). The fish sample came from ornamental fish culture systems. When they arrived at the laboratory they were acclimatized for 2 days and then placed in their final aquarium. Each specimen occupied an individual aquarium (Figure ). The 27 tanks were in an experimental room was under controlled laboratory conditions with 12/12 light-dark (Zhou, Wu, Randall, & Lam, Citation2001) constant temperature of 15.3°C ± 0.5. All experimental tanks (25 L) were maintained with dissolved oxygen content 6.4 mg/L ± 0.1. Figure shows an example of the division of treatments, where all specimens were fed 0.1 g daily. The experimentation was divided into three groups: Treatment 1 (T1) (n = 9 tanks) plant-based (Spirulina sp.), treatment 2 (T2) (n = 9 tanks) animal protein base, and a control (C) (n = 9 tanks) mixture of animal and plant protein (Table ). At day 30 of experimentation each individual was euthanized with excess BZ-20 anesthetic (Fathallah, Medhioub, Medhioub, & Boussetta, Citation2010) the fish was immobile and floating on the surface of the sedation aquarium, its size and final weight were immediately measured. The values of the treatments were compared with a general lineal model (GLM) followed by an a posteriori test; an analysis of covariances (ANCOVA) test was carried out to establish the relation between size and body weight. The instantaneous growth rate (IGR) was calculated in proportion to the size and the percentage increase with respect to weight (PIW)(Persson, Leonardsson, & Alanärä, Citation2018) De (De Raedemaecker, Brophy, O’Connor, & O’Neill, Citation2012; Vega et al., Citation2015) following equations:
Table 1. Dietary values used in the different treatments. T1; treatment based on plants (Spirulina sp.). T2; treatment based on animals
LSf is the size (cm) of the individual at the end of the experiment, LSo is the size (cm) of the same individual at the beginning, t corresponds to the time interval, Wf is the final weight (g) of the individual and Wi is the initial weight. These treatment values were compared to a one-way ANOVA. All calculations and statistical analyses were done with the software R (R Core Team, Citation2018).
3. Results and discussion
The average values for each treatment after 30 days of experimentation are summarized in Table . All fish grew in weight and size with the two types of treatment (Figure ). No significant difference in body size was found among food treatments (Figure ) (GLM; F = 0.147; P = 0.864), indicating that there was no effect of diet type on fish body size. The IGR ratified the previous assertion (Table ), since the fish presented low rates of intrinsic size growth and no significant difference was observed (ANOVA; F = 0.189; P = 0.828) (Figure ).
Table 2. Results of the variation in weight and length of individuals. W: weight in grams; S: size in centimeters; IGR: instantaneous growth rate and PIW: percentage length increase with respect to weight
Figure 2. Box and whisker graphs of changes in body size (a) and weight (b) based on food treatments. T1; treatment based on vegetal (Spirulina sp.). T2; treatment based on animal.
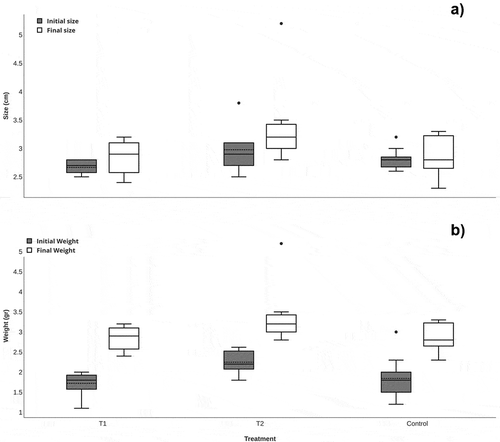
Figure 3. Violin diagram for instantaneous growth rate (IGR) in the fish studied. T1; treatment based on vegetal (Spirulina sp.). T2; treatment based on animal.
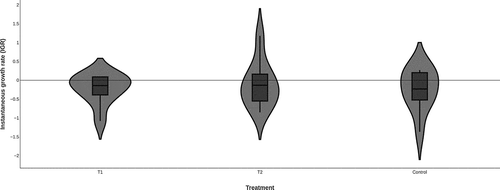
However, the fish did show changes in their final weight with respect to the initial weight (Figure ). The animal protein-based treatment (T2) had a significant effect on the increase in fish mass (GLM; F = 2.716; P = 0.0311), compared to those under the vegetable protein-based treatment (T1). Moreover, individuals under treatment T2 showed a 68.5% weight increase versus 36% of specimens under treatment T1 (Table ). The length/weight ratio of fish under different feed treatments indicated that T2 is the treatment that produced significant variation (ANCOVA; F = 3.59; P = 0.043). T1 and the control did not have a significant difference in the length/weight ratio (ANCOVA; F = 1.53; P = 0.22). Therefore, the diet based on animal protein (T2), which is also rich in energy, was a determining factor in the relationship of mass and size increase of the fish in this study.
Specimens under different food treatments all increased their body size by feeding daily. There were two situations with respect to the increase in length and mass; the energy present in the treatments did not influence the increase in fish length (Figure ), contrary to what was found in the addition of mass, where there was an effect of the treatment with greater energy (T2), resulting in a significant increase in weight (Figure ). Growth in length and weight is complementary processes but are independent; their rates of increase depend on the amount of prey energy (Wootton, Citation1990). This experiment lasted 30 days, which is a limited time to measure growth rate in fish. However, the fact that energy-rich prey had an effect on mass addition can be seen as a short-term effect and thus aid inferences in dietary studies.
This study corroborated that there is an effect of the amount of energy present in the prey with weight increase, as well as in the ratio of size and length (Figure ). An energy-rich diet ensures that fish can have a high consumption rate, thereby covering the costs of maintaining metabolism and synthesizing new tissue (Wuenschel et al., Citation2006). On the contrary, fish that do not have an energy-rich prey intake rate will allocate the energy budget to basal maintenance, affecting their consumption rates and assimilation efficiency (Buurma & Diana’, Citation1994) other authors (Pulgar et al., Citation2013; Ruohonen, Vielma, & Grove, Citation1998; Seo & Lee, Citation2008) that the diet of some fish should be complete and should include proteins, lipids, carbohydrates, vitamins, and minerals. It is important to mention that the nutritional requirements for an individual or a population are difficult to catalog because they can vary depending on environmental conditions, size, age, reproductive condition and physiological activity (Ross et al., Citation2018), especially in aquatic systems where anthropogenic activities modify and replace predator-prey dynamics. Finally, this study has just evaluated the hypothesis that there is an effect on the length/weight ratio of the predator; therefore, fish that ingest prey with a greater amount of energy will have greater mass growth.
Ethical statement
This experiment was previously approved by the bioethics committee of the College of Life Sciences at Universidad Andres Bello. The animals were adequately euthanized according to the current protocols of our institution.
Competing interests
The authors declare no competing interest.
Additional information
Funding
Notes on contributors
Sebastian A. Klarian
Dr. Sebastian A. Klarian is Marine Biologist from Universidad Andrés Bello, Chile. He´s currently Associate Professor at the School of Biosciences in Universidad Andrés Bello and Research Assistant Professor at the University of Connecticut, USA. His research focuses on fish biology, fisheries biology and trophic ecology in aquatic environments.
References
- Blaxter, K. (1989). Energy metabolism in animals and man. Cambridge: CUP Archive.
- Buurma, B. J., & Diana’, J. S. (1994). Effects of feeding frequency and handling on growth and mortality of cultured walking catfish clarias fuscus. Journal of the World Aquaculture Society, 25(2), 175–6. doi:10.1111/jwas.1994.25.issue-2
- De Raedemaecker, F., Brophy, D., O’Connor, I., & O’Neill, B. (2012). Dependence of RNA:DNA ratios and Fulton’s K condition indices on environmental characteristics of plaice and dab nursery grounds. Estuarine, Coastal and Shelf Science, 98, 60–70. doi:10.1016/j.ecss.2011.11.033
- Fathallah, S., Medhioub, M. N., Medhioub, A., & Boussetta, H. (2010). Biochemical indices (RNA/DNA ratio and protein content) in studying the nutritional status of ruditapes decussatus (Linnaeus 1758) juveniles: Nutritional status of R. decussatus juveniles. Aquaculture Research, 42(1), 139–146. doi:10.1111/j.1365-2109.2010.02614.x
- Gaeta, J. W., Ahrenstorff, T. D., Diana, J. S., Fetzer, W. W., Jones, T. S., Lawson, Z. J., … Vander Zanden, M. J. (2018). Go big or … don’t? A field-based diet evaluation of freshwater piscivore and prey fish size relationships. PloS one, 13(3), e0194092. doi:10.1371/journal.pone.0194092
- Gliwicz, Z. M., Babkiewicz, E., Kumar, R., Kunjiappan, S., Leniowski, & Leniowski, K. (2018). Warming increases the number of apparent prey in reaction field volume of zooplanktivorous fish. Limnology and Oceanography, 63, 30–43. doi:10.1002/lno.v63.S1
- Liedke, A. M. R., Barneche, D. R., Ferreira, C. E. L., Segal, B., Nunes, L. T., Burigo, A. P., … Floeter, S. R. (2016). Abundance, diet, foraging and nutritional condition of the banded butterflyfish (chaetodon striatus) along the western Atlantic. Marine Biology, 163(1), 6. doi:10.1007/s00227-015-2788-4
- Persson, L., Leonardsson, K., & Alanärä, A. (2018). Manipulation of the energetic state of Atlantic salmon salmo salar juveniles and the effect on migration speed. Journal of Fish Biology, 92(4), 961–978. doi:10.1111/jfb.13555
- Popesku, J. T., Martyniuk, C. J., Mennigen, J., Xiong, H., Zhang, D., Xia, X., ... Trudeau, V. L. (2008). The goldfish (Carassius auratus) as a model for neuroendocrine signaling. Molecular and Cellular Endocrinology, 293(1–2), 43–56.
- Puigserver, M., Muñoz, A., Navarro, J., Coll, M., Pethybridge, H., Sánchez, S., & Palomera, I. (2017). Ecological energetics of forage fish from the Mediterranean Sea: Seasonal dynamics and interspecific differences. Deep-Sea Research. Part II, Topical Studies in Oceanography, 140, 74–82. doi:10.1016/j.dsr2.2017.03.002
- Pulgar, J., Poblete, E., Alvarez, M., Morales, J. P., Aranda, B., Aldana, M., & Pulgar, V. M. (2013). Can upwelling signals be detected in intertidal fishes of different trophic levels? Journal of Fish Biology, 83(5), 1407–1415. doi:10.1111/jfb.12220
- R Core Team. (2018). R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. 2013 (version 3.5.1). Retrieved from https://www.R-project.org/
- Ross, S. D., Nielsen, J. R., Gislason, H., Nielsen, A., & Andersen, N. G. (2018). Growth and food consumption of whiting Merlangius merlangus. Journal of Fish Biology, 93(2), 334–343. doi:10.1111/jfb.13763
- Ruohonen, K., Vielma, J., & Grove, D. J. (1998). Effects of feeding frequency on growth and food utilisation of rainbow trout (oncorhynchus mykiss) fed low-fat herring or dry pellets. Aquaculture, 165(1), 111–121. doi:10.1016/S0044-8486(98)00235-X
- Seo, J.-Y., & Lee, S.-M. (2008). Effects of dietary macronutrient level and feeding frequency on growth and body composition of juvenile rockfish (sebastes schlegeli). Aquaculture International: Journal of the European Aquaculture Society, 16(6), 551–560. doi:10.1007/s10499-008-9165-y
- Vega, R., Estrada, J. M., Ramírez, D., Flores, C., Zamorano, J., Encina, F., … Dantagnan, P. (2015). Crecimiento de juveniles de congrio colorado Genypterus chilensis en condiciones de cultivo. Latin American Journal of Aquatic Research, 43(2), 344–350. Retrieved from https://scielo.conicyt.cl/scielo.php?pid=S0718-560X2015000200011&script=sci_arttext&tlng=pt
- Wainwright, P. C., & Richard, B. A. (1995). Predicting patterns of prey use from morphology of fishes. Environmental Biology of Fishes, 44(1–3), 97–113. doi:10.1007/BF00005909
- Wegner, N. C., Snodgrass, O. E., Dewar, H., & Hyde, J. R. (2015). Animal physiology. Whole-body endothermy in a mesopelagic fish, the opah, Lampris guttatus. Science, 348(6236), 786–789. doi:10.1126/science.aaa8902
- Wootton, R. J. (1990). Ecology of Teleost Fishes, xii, 404 p. London: Chapman and Hall.
- Wuenschel, M. J., Jugovich, A. R., & Hare, J. A. (2006). Estimating the energy density of fish: The importance of ontogeny. Transactions of the American Fisheries Society, 135(2), 379–385. doi:10.1577/T04-233.1
- Zhou, B. S., Wu, R. S., Randall, D. J., & Lam, P. K. (2001). Bioenergetics and RNA/DNA ratios in the common carp (Cyprinus carpio)under hypoxia. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology, 171, 49–57. doi:10.1007/s003600000149