Abstract
We detected arsenic near the Tolimique Dam, an archaeological ecotourism area in Aguascalientes, México. Fortunately, the concentration range of this metalloid (0.001 to 0.003 mg L−1) did not exceed the total arsenic in water allowed by NOM-127-SSA1-1994 (0.025 mg L−1). However, because arsenic is a high-risk priority pollutant at low concentrations in aquatic ecosystems, we studied the acute toxicity, bioaccumulation, and endocrine disruption of arsenic using native species of Rotifera (Brachionus calyciflorus Pallas, 1766, Brachionus rubens Ehrenberg, 1838, and Lecane cornuta Müller, 1786), Oligochaeta (Aeolosoma hemprichi Ehrenberg, 1838), and fish (Oreochromis nilotica Linnaeus, 1758). We found 20 species in the Tolimique Dam, including 13 rotifer species, 4 cladocerans species, and one of each of the following species: copepods, oligochaetes, and fish. Environmental Risk Assessment (ERA) was performed using the Predicted No-Effect Concentration (PNEC) and the Measured Environmental Concentration (MEC) of arsenic. In conclusion, arsenic produced cellular necrosis in A. hemprichi at a concentration of 5.0 mg L−1 and endocrine disruption in B. calyciflorus at a concentration of 0.05 mg L−1. The detection of arsenic at an average of 2.05 ± 0.30 mg Kg−1 in O. niloticus fish suggests a probable biomagnification of arsenic.
PUBLIC INTEREST STATEMENT
The working group of Ecology and Dynamic of Aquatic Ecosystems of the Water Sciences Unit in the Center of Scientific Research of Yucatan is engaged in producing health, water quality and ecologic indicators in collaboration with national and international as well as inter- and intra-institutional research groups for the purpose of offering important information to relevant water-management agencies, decision-making regarding public policies, species conservation and genetic resources and as regards water security.
Competing Interests
The authors declare no competing interests.
1. Introduction
Arsenic contamination from anthropogenic and natural sources is a worldwide occurrence (Bundschuh et al., Citation2012). Arsenic is toxic in all four oxidation states (+V, +III, 0, -III), and trivalent and pentavalent arsenic are the most common species found in ecosystems (Rahman et al., Citation2014). Microorganisms biotransform arsenic as a mechanism of detoxification (Rahman & Hassler, Citation2014). The typical arsenic concentration in freshwater is less than 0.01 mg L−1, and frequently concentrations of less than 0.001 mg L−1 are recorded. In contrast, much higher concentrations have been found in groundwater, particularly in Argentina, Chile, Mexico, China, Hungary, and India (Smedley & Kinniburg, Citation2002). In general, arsenic concentrations in the United States range between 0.001 and 4.8 mg L−1 (Bundschuh et al., Citation2012). Concentrations ranging from 0.001 to 0.98 mg L−1 in Europe and from 0.0001 to 2.308 mg L−1 in Asia have been found in drinking water, well water, groundwater, and sediments (Sharma & Sohn, Citation2009). High concentrations of arsenic have also been detected in the drinking water of 13 out of the 31 Mexican states, including Durango, Coahuila, Zacatecas, Morelos, Aguascalientes, Chihuahua, Sonora, Puebla, Nuevo León, Jalisco, Oaxaca, Guanajuato, and San Luis Potosí (Bundschuh et al., Citation2012). In fact, it has been estimated that the anthropogenic load to the atmosphere due to arsenic has increased in total emissions by a factor of 1.6, nowadays. Arsenic is relatively strongly retained in watersheds, and human activities since industrialization have likely contributed to the dramatic increase in the burden of arsenic in surface soils (Mason, Laporte, & Andres, Citation2000).
Arsenic is persistent in the environment and is a risk to biota and human health. This metalloid enters the biota in primary consumers and then biomagnifies to higher order organisms (Rahman, Hasegawa, & Lim, Citation2012). For example, human exposure to low to moderate levels of arsenic exposure (10–300 µg L−1) through drinking water has adverse effects, such as skin lesions, circulatory disorders, neurological complications, diabetes, respiratory complications, hepatic and renal dysfunction, and mortality due to chronic diseases (Young-Seoub, Ki-Hoon, & Jin-Yong, Citation2014). An estimated 100 million people worldwide are exposed to arsenic levels of greater than 50 µg L−1 (Moon, Guallar, & Navas-Acien, Citation2012) via drinking water and through industrial processes (Vahter, Citation2008).
In contrast, arsenic is toxic to aquatic biota and has adverse effects at higher concentrations in cladocerans, ostracods, copepods, rotifers, and oligochaetes. Bryant, Newbery, McLusky, and Campbell (Citation1985) obtained LC50 values of 6 to 60 mg L−1 in Corophium corophium Pallas, 1766 (Amphipod) and 85 to 520 mg L−1 in Macoma balthica Linnaeus, 1758 (Marine Bivalve). Furthermore, the toxicity of pentavalent arsenic was increased at a culture temperature of 5°C in Tubifex costatus Claparede, 1863 (Annelida: Oligochaeta). Rios-Arana, Gardea-Torresdey, Webb, and Walsh (Citation2005) showed a reduction in the expression of HSP60 proteins involved in processes of cell damage in rotifer Plationus patulus Müller, 1786 (Rotifera) exposed to arsenic. Aránguiz-Acuña and Serra (Citation2016) reported a high accumulation of arsenic in Brachionus calyciflorus (Rotifera) exposed to a constant concentration after several generations, suggesting that arsenic may be accumulated transgenerationally. Actually, arsenic concentrations in living organisms are usually less than 1 µg g−1 f wt in terrestrial flora and fauna, birds, and freshwater biota (Rahman et al., Citation2012).
Our study focused on an aquatic reservoir contaminated with arsenic at the Tolimique Dam in Aguascalientes, México. This dam is 2030 m above sea level and has an annual temperature minimum of 23.1°C and maximum of 37.0°C, an average annual precipitation of 654.8 mm, and a total of 66.3 days of rain. The community near Tolimique Dam has fewer than 1000 inhabitants, but the Aguascalientes state government has promoted ecotourism activities since 2003 (Cano-Contreras & Delgado, Citation2009). Because the Tolimique Dam reservoir water contains arsenic and arsenic levels are probably biomagnified by biota, we studied the adverse effect of arsenic in native species and then conducted a risk assessment with the information obtained. The goal of the study was to provide important information for the protection of aquatic biota and prevention of adverse effects on human health.
2. Material and methods
2.1. Sample collection, arsenic quantification, and zooplankton identification
Six sampling stations were included in the quantification of arsenic at the Tolimique Dam, in the State of Aguascalientes, Mexico. Sampling was conducted only during the dry season (from May to June 2014). Sites were distributed around the Tolimique Dam and were chosen according to surface runoff near the dam and the availability of access to sampling stations. We use polypropylene bottles (previously rinsed first with 30% HNO3 and then three times with deionized water) for the water samples. Water samples were fixed with 2 mL of Instra HNO3: to maintain dissolved heavy metals in solution. The digestion of water, in this case, not required of H2O2, due to the matrix is not very complex; we do not observed turbidity and does not have a high content of solid suspended organic matter. On the other hand, the muscle tissue of fish was allowed to sit with the concentrated HNO3 grade 24 h before the digestion in microwave. These hours favored the dissolution and disintegration of the muscle tissue of the fish, which allowed do without the addition of oxidizing agents (H2O2). Finally, the zooplankton samples were made exactly like the fish tissues.
Additionally, we sampled the zooplankton from the limnetic and benthic areas using a Wisconsin type net with a 54-µm mesh opening. The net mesh opening was pulled three times to check the density of the sample, and the sample was subsequently emptied into a Nasco Whirl-Pak bag (Nasco, Fort Atkinson, WI, USA). Samples were then preserved with 5% formalin and stored in the laboratory for further analysis. Species identification was carried out with specialized taxonomic keys found in the literature (Ahlstrom, Citation1940; Osorio-Tafall, Citation1942; Ruttner-Kolisko, Citation1974; Koste, Citation1978; Koste & Shiel, Citation1987, Citation1989; Nogrady, Wallace, & Snell, Citation1993; Segers, Citation1995, Brinkhurst Citation1971; Elías-Gutiérrez et al., Citation2008).
The level of arsenic in water was determined by atomic absorption using a PE Analyst 800 Spectrometer with a transversely heated graphite furnace, longitudinal Zeeman-effect background correction, and an AS-60 auto-sampler. The detection limit for arsenic was 0.2 μg L−1. Deionized water was used for all analytical work, and glassware and all material used for metal analysis were soaked in 10% nitric acid, rinsed with deionized water, and dried before use. Analytical spike recovery was of 80 to100%. Relative percent difference between replicate analyses of subsamples was less than 10%. The sample was analyzed with transverse graphite-coated tube pyrolytic and platform. The limit of quantification according to equipment 0.005 ug/L; while the detection limit according to calibration curve was 0.2 ug/l, with three repetitions.
2.2. Culture of rotifers and oligochaeta
The rotifers Brachionus calyciflorus, Brachionus rubens, and Lecane cornuta were placed in Petri dishes with EPA medium (US Environmental Protection Agency, 1985), and fed with green algae Nannochloris oculata Droop, 1955 (LB2194 strain from the University of Texas collection) cultivated in the middle Bold´s (Nichols, Citation1973). The oligochaete Aelosoma hemprichi was cultivated in Petri dishes and fed with green algae N. oculata and with pieces of less than 1 cm2 of lemon tree leaves (Citrus sp.).
All organisms were cultivated in bioclimatic chambers with a photoperiod of 16:8 h (light/dark), at a temperature of 25 ± 2°C. In experiments with rotifers, infants under 24 h were used. In experiments with the oligochaete A. hemprichi, newly detached organisms were used from their mothers and small organisms (unlike rotifers, no asexual eggs are obtained to control their emergence in this oligochaete).
2.3. Arsenic chronic assay using Brachionus calyciforus
Arsenic [atomic absorption standards of arsenic dissolved in 1% HNO3(As2O3), Sigma Co., Saint Louis MO, USA] was used to determine effects on asexual and sexual reproduction in B. calyciflorus using a 4-day reproduction test. We placed five neonates (<24 h old) in 2 mL EPA medium (negative control) with 5 × 106 cells mL−1 of the green alga N. oculata (strain LB2164 from University of Texas Collection). This protocol is a slight modification of that of Snell and Moffat (Citation1992) because we used 24-well polystyrene plates (Costar Co., USA) instead of test tubes. Test conditions were 25°C, pH 7.4–7.8, and hardness 80–100 mg CaCO3 L−1. Six replicates were performed per control, and the arsenic concentrations were 0.025, 0.05, 0.075, 0.1, 0.5, 1.0, 2.0, 3.0, 5.0, and 7.0 mg L−1 (nominal concentrations). At the end of the test, we counted the number of nonovigerous females, ovigerous asexual females, ovigerous unfertilized sexual females, ovigerous fertilized sexual females, males, parthenogenetic (asexual or amictic) eggs, unfertilized sexual eggs, and fertilized sexual eggs (cysts). The intrinsic growth rate (r) of females was expressed according to the formula r = (ln Nt—No)/T, where ln Nt = natural logarithm of the number of female in the plate after 4 days, and ln No = natural logarithm of the initial number of rotifers in each plate (we only used the No number for females because the initial organisms were five). Parthenogenetic (asexual or amictic) eggs, unfertilized sexual eggs, fertilized sexual eggs (resting eggs) were expressed as total found at the end of the experiments for nonovigerous females, ovigerous asexual females, ovigerous unfertilized sexual females, ovigerous fertilized sexual females, and males.
2.4. Acute toxicity and risk assessment in Brachionus rubens and lLcane cornuta
Ten neonates (less than 24-h-old hatching from parthenogentic eggs) of the species B. rubens and L. cornuta were placed per well in a 24-well polystyrene plate with a final test volume of 1 mL. Next, the rotifers were incubated at 25°C, using a photoperiod 16:8 (light:dark). Three replicates were performed in the species B. rubens and L. cornuta. The As concentrations used for B. rubens were 1.0, 3.0, 5.0 8.0, and 13.0 mg L−1 (nominal concentrations), and those for L. cornuta were 1.0, 3.0, 5.0, 8.0, 13.0, and 21.0 mg L−1 (nominal concentrations). At the end of incubation, dead animals were counted using a stereomicroscope. A one-way analysis of variance (ANOVA) and Duncan’s test were used to compare mortality percentages for each toxicant concentration to that of the control. We also calculated NOEC (No Observed Effect Concentration) and LOEC (Lowest Observed Effect Concentration). The LC50 values were calculated using regression between probit units and the logarithm of each toxicant concentration using the software Statistica 6.0 (StatSoft Inc. 2001). The environment risk assessment is a tool for evaluation and management of environmental pollution. From the LC50 values, we also obtained the Environmental Quality Standard expressed as the maximum permissible concentration for Predicted No-Effect Concentration (PNEC). The PNEC values can be used to estimate environmental risk assessments, according to Cruzeiro, Amaral, Rocha, and Rocha (Citation2017). Using LC50 minimum values, we calculated the Environmental Quality Standards that were assumed to be the Maximum Permissible Concentration, expressed as PNEC values for arsenic. The PNEC was determined by applying an appropriate assessment factor (AF = 1000) to compensate for the intra- and inter-laboratory variation, biological variance, and extrapolation from lab to field (Beyer et al. Citation2014). Interpretation of risk assessment used values of >1 to indicate a high-risk assessment and values of <1 to indicate a low-risk assessment.
3. Quantification of arsenic in Oreochromis niloticus
A 0.5-g sample (muscular tissue) was taken from the medial region of the fish (which is the most fleshy part of the fish) and digested by placing in 10 mL of concentrated HNO3 and microwaving (microwave CEM Model Mars5X) at a maximum 300 W, potency of 100%, ramp of 25 min, pressure of 200 psi, temperature of 210°C, and hold time of 10 min. Then, HNO3 was added to the sample to achieve a final volume of 100 mL. The protocol of the Mexican Norm (NOM-218-SSA1-201 2011) was followed. Atomic absorption readings were recorded in mg per L and then calculated as mg per kg.
4. Necrosis cellular in aelosoma hemprichi
The oligochaetes were exposed to different concentrations (mg L−1) of arsenic as follows: 0.1 (n = 7), 0.2 (n = 11), 0.3 (n = 11), 0.5 (n = 15), 0.8 (n = 9), 0.9 (n = 4), 1.0 (n = 19), 1.5 (n = 10), 2.0 (n = 15), 3.0 (n = 11), 5.0 (n = 5) and 8.0 (n = 5). An organism was placed in a final volume of 1 mL in 24-well polystyrene plates (Costar Co., USA). A total of 109 oligochaetes were analyzed. The exposure periods were 24 h each with the exceptions of. concentrations of 0.1 to 2.0 mg L−1, for which exposure periods were revised to 12 h and 24 h, and concentrations of 3.0 to 8.0 mg L−1, for which exposure periods were revised at to 30 min only because necrosis was noted in the first 15 min. The experiments were all conducted inside the bioclimatic chamber at a temperature of 25°C, before we visualized the results using an optical microscope. Cell necrosis was identified when we observed a light coffee color change to dark brown in the body of the oligochaete. Photographs were taken of the most toxic concentrations for oligochaete from 1.0 to 8.0 mg L−1.
5. Results
5.1. Arsenic quantification and aquatic biota
The measured environmental concentrations (MEC) of arsenic in the Tolimique Dam ranged from 0.00120 to 0.00301 mg L−1. The highest concentration was in the curtain of the dam, and the specific values for each point where 1) 0.00196, 2) 0.00173, 3) 0.00120, 4) 0.00185, 5) 0.00301, and 6) 0.00196 mg L−1. These concentrations do not exceed the permissible value according to the Mexican norm NOM-127-SSA1-1994 (0.025 mg L−1) of total arsenic in water. They also do not exceed the NOM-001-ECOL-1996, which establishes permissible limits of arsenic for use in agricultural irrigation (0.2 mg L−1 monthly average, 0.4 mg L−1 daily average) and urban public use (0.1 mg L−1 monthly average, 0.2 mg L−1 daily average), and for protection of aquatic life (0.1 mg L−1 monthly average, 0.2 mg L−1 daily average). In tilapia Oreochromis nilotica, the average dry weight concentration of arsenic was 2.05 ± 0.30 mg Kg−1.
The following species of zooplankton comprise the micro aquatic fauna of the Tolimique Dam: four species of cladocerans, one species of copepod (Cypridopsis vidua Müller, 1776), one species of ostracods (Microcyclops sp.), 13 species of rotifers, and 1 species of oligochaetes (Aelosoma hemprichi). The rotifers are the most diverse, consisting of six genera; namely, Asplanchna, Brachionus, Filinia, Keratella, Lecane, and Trichocerca. Second in terms of species diversity are the cladocerans of the genera Ceriodaphnia; namely, Chydorus, Daphnia, and Disparalona.
5.2. Toxicity of arsenic and risk assessment in zooplankton
Arsenic was toxic in high concentrations during acute exposures in rotifers and oligochaetes species. According to LC50 values, L. cornuta was more tolerant to arsenic than B. rubens, and oligochaete was more tolerant to arsenic than L. cornuta and B. rubens (Table ). These values are using to obtain the PNEC (Table ), which we used to calculate the Risk Quotient (RQ = MEC/PNEC). We performed a preliminary tentative environmental risk assessment (ERA) based in our results using LC50 of B. rubens and L. cornuta: arsenic had a high risk based on a value ≥1 indicating a high-risk assessment and values ≤1 indicating a low-risk assessment (Table ). The rotifers died within a period of 24 h of exposure. However, a very interesting fact is the effect of cell necrosis in the oligochaete when exposed to high doses of arsenic equal to 8.0 mg L−1 (Figure ). No cell necrosis was observed in rotifers species.
Table 1. Comparison of sensitivity to arsenic in rotifers and oligochaetes
Table 2. Risk assessment of arsenic in two rotifer species1
Figure 1. Cellular necrosis in the oligoqueto Aelosoma hemprichi caused by acute arsenic exposure within 30 min.
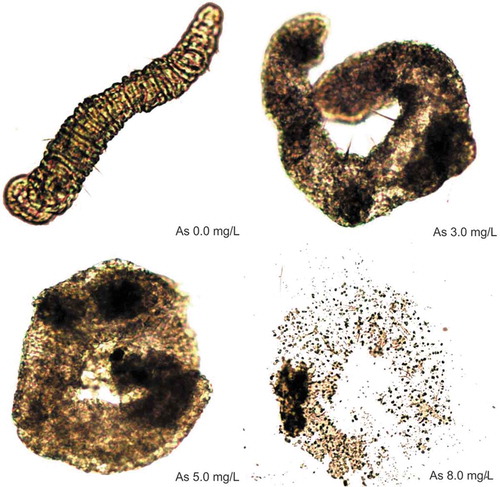
Figure 2. Intrinsic growth rate (r) of females of the Rotifer Brachionus calyciflorus exposed to arsenic for 4 days. N = 6.
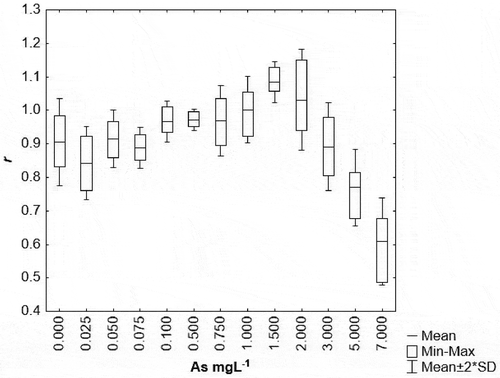
As for the chronic toxicity of arsenic in species B. calyciflorus, we observed arsenic to have the typical behavior of an endocrine disruptor (Figure ) because the rotifer can be reproduced in concentrations ranging from 0.025 to 7.0 mg L−1 of arsenic without any problems (intrinsic growth rate values of >0.5). The adverse effect of arsenic on the rate of production of females and males is described as follows: at arsenic concentrations of 0.025, 0.05, 0075, 0.1, and 0.5 mg L−1, there were no significant differences with respect to control. Arsenic increased the total production of females. However, in this same range of arsenic concentrations, only the 0.025 mg L−1 level of exposure resulted in a significant increase in the production of total males. Interestingly, there is a significant increase in the production of females and males in the exposure concentrations of 1.0, 1.5, and 2.0 mg L−1 arsenic. As the exposure increased to 7.0 mg L−1, a decrease was obtained in the rate of production of females and non-production of males. Arsenic decreases the production rate of females with parthenogenetic eggs (hhp) at concentrations of 0.025 and 7.0 mg L−1, but conversely increases the production of females with parthenogenetic eggs (hhp) at the concentration of 1.5 mg L−1. Interestingly, there is an increase in the production of females with unfertilized sex eggs (that originate males HHM) at a concentration of 1.5 mg L−1, which coincides with an increase of females with parthenogenetic eggs. Therefore, up to a concentration of 1.5 mg L−1 there is a strong induction of sexual reproduction without the production of cysts. In the production of females with cysts (HQ), only a slight increase at the concentration of 1.0 mg L−1 exposure was observed and none was seen at 1.5 mg L−1.
6. Discussion
Several years ago, Dodson and Silva-Briano (Citation1996) mentioned that rotifers, oligochaete, cladocerans, and fish cohabitate in the aquatic ecosystem of Aguascalientes, Mexico. Although the richness of zooplankton was suitable for a freshwater ecosystem in the reservoir at the Tolimique Dam according to this previous report for the region (Rico-Martinez & Silva-Briano, Citation1993), we found arsenic in the water. The presence of arsenic in the Tolimique Dam reservoir represents a hazard for the aquatic biota and humans both acutely and chronically. We found that L. cornuta is more sensitive than B. rubens with regard to the acute toxicity of arsenic in rotifers, suggesting that the species of the genus Lecane are more sensitive than the species of the genus Brachionus. However, acute toxicity data for arsenic to support this hypothesis are scarce.
The chronic effects observed in B. calyciflorus are typical of an endocrine disruptor. Arsenic adverse effects include cell necrosis and biomagnification. A study by Aránguiz-Acuña and Serra (Citation2016) shows that arsenic is accumulated and transferred from generation to generation in the rotifer B. calyciflorus, causing transgenerational effects. In contrast, in our study of the oligochaete A. hemprichi, a devastating effect of arsenic (induction of cell necrosis) was seen within 24 h of exposure time. According to our results, therefore, arsenic is more toxic for oligochaetes than rotifers.
According to Rahman et al. (Citation2012): arsenic is biotransformed in water by the biota and exists in different chemical forms, in aquatic systems, the dominant iAs form is incorporated into microorganisms such as phytoplankton and then converted to methylarsenicals and/or higher-order orgAs forms such as arseno sugars. OrgAs is mineralized to iAs and methylarsenicals by bacteria. Thus, aquatic microorganisms such as phytoplankton and bacteria play important roles in arsenic speciation, distribution, and cycling in aquatic systems. The effects of arsenic in zooplankton are related to the mechanism of toxicity in the cells. For example, although arsenic is not mutagenic per se, some deleterious effects on DNA have been reported. In addition, arsenic can reportedly promote the generation of reactive oxygen species and induce signal transduction pathways as a result of its transport, transformation, and binding inside cells (Huang, Ke, Costa, & Shi, Citation2004; Thomas, Citation2007).
In our study, we detected total arsenic and used arsenic dissolved in 1% HNO3(As2O3), which is known as arsenic trioxide (As2NO3). A study of López-Gutiérrez et al. (Citation2018) using arsenic trioxide shows that one species of rotifer Plationus patulus did not accumulate arsenic, but that Daphnia cf prolata and the ostracod Heterocypris incongruens did accumulate arsenic under experimental conditions. These authors concluded that the mechanism of accumulation and detoxification of arsenic in zooplankton is specific and that the high toxicity correlates with the high bioconcentration factor.
In zooplankton species like cladocerans, the maximum concentration of arsenic tolerated is 3.9 mg L−1 and the most sensitive is 0.55 mg L−1. In contrast, some microalgae tolerate up to 29.91 mg L−1 (Rahman et al., Citation2014). The toxicity values of arsenic in its oxidation state III in Cladocerans are as follows: Daphnia carinata LC50 = 0.55 mg L−1; D. pulex, 1758, LC50 = 2.5 mg L−1, Ceriodaphnia cf. dubia EC50 = 1.58 mg L−1. For arsenic V, the values are Daphnia carinata LC50 = 2.44 mg L−1, D. pulex LC50 = 3.9 mg L−1, and Ceriodaphnia cf. dubia EC50 = 1.72 mg L−1. According to these aquatic biota data, arsenic III it is more toxic than arsenic form V. The toxicity of arsenic III and V oscillates between 0.15 and 29.91 mg L−1 in microalgae such as Chlorella sp. and the Macrophytes Elodea candesis, Lemna minor, and Monoraphidium arcuantum (Rahman et al., Citation2014). In view of these reports and our findings, arsenic has an adverse effect on phytoplankton, zooplankton, and fish populations, and this pattern suggests that arsenic is biomagnified. According to the above, for example, a study by Ning-Xing, Yue-Yue, Zhong-Bo, Liu-Yan, and Ai-Jun (Citation2018), about toxicity of arsenic in Daphnia magna, reported that waterborne versus dietborne exposure. Unfortunately, there are no studies of risk of acute toxicity for cladoceran species in the Tolimique Dam reservoir, of biomagnification or bioaccumulation, however, our study represented the first report of biomagnification in fish.
According to our study findings, fish bioaccumulated arsenic. This phenomenon represents a hazard. The high risk of arsenic bioaccumulation in the Tolimique Dam reservoir is exemplified by the average arsenic concentration of 2.05 ± 0.30 mg Kg−1 weight found in O. nilotica compared with a report by Olmedo et al. (Citation2013) on various species of fresh fish and shellfish with concentrations of arsenic from 0.002 to 0.739 mg Kg−1 wet weight (ww). The high concentration of arsenic in fish from the Tolimique Dam reservoir poses an imminent health risk for humans if consumed because arsenic in low concentrations for long time periods and high concentrations for short exposure times has adverse effects on human health, including skin lesions, circulatory disorders, neurological complications, hepatic and renal dysfunctions, and mortality due chronic diseases (Mohammed-Abdul, Sammanthi-Jayasinghe, Chandana, Jayasumana, & De Silva, Citation2015). For example, studies by Falcó, Llobet, Bocio, and Domingo (Citation2006) and Martínez-Gómez et al. (Citation2012) found arsenic levels in red mullet, a fish preferred by humans for consumption, ranging from 15.39–17.77 mg kg−1 ww to 19.8–6.9 mg kg−1 ww. These levels of arsenic represent a hazard for human (Olmedo et al., Citation2013).
Our ERA data indicate that, arsenic represents a hazard for aquatic biota in the Tolimique Dam reservoir. To determine the mechanisms of response and acclimation to arsenic in zooplankton, it is also necessary to study the processes of bioaccumulation and biomagnification in detail in order to prevent adverse effects on human health. We therefore recommend a continuous monitoring of the concentrations of arsenic, especially in fish in the Tolimique Dam reservoir, Aguascalientes, México. As a priority related to maintaining the health of ecosystems and preserving resources for future generations, biomonitoring of contaminated and uncontaminated water bodies is critical, using ecotoxicological assessment, morphologic changes and the frequency of their occurrence as important toxicological parameters. Establishing a species or battery of species that provide indications of toxicity, and structural damage or morphology changes associated with toxicant exposure is an important priority. The results of these studies could be used to create a database enabling researchers to answer questions such as: What toxicants cause morphologic alterations? In addition, what is the minimum concentration necessary for causing structural damage and transgenerational effects? Additional research should focus on risk analysis, specifically as related to bioaccumulation and biomagnification of arsenic.
Ethical approval
The authors followed all applicable international, national, and/or institutional guidelines for the care and use of animals.
Acknowledgements
The authors appreciate the support provided by Natura Mundi A. C, as well as Fernando Lopez-Gutiérrez, Marcelo Silva Briano, and Roberto Vizcaya in the collection and identification of organisms.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
Notes on contributors
Jesús Alvarado-Flores
Jesús Alvarado-Flores, specialist in ecotoxicology and diversity of rotifer species. Areas of study: a) ecotoxicology; b) rotifers’ reproductive behavior; c) studies on endocrine disruption; d) structural damage in rotifers exposed to pollutants; and, e) description and taxonomic-genetic characterization of culturable rotifer species from the coastal zone of the Yucatan Peninsula. At present, he is part of Unidad de Ciencias del Agua [Water Sciences Unit] of Centro de Investigación Científica de Yucatán A.C. [Center of Scientific Research of Yucatan], engaged in Ecology and Dynamic of Aquatic Ecosystems, as a Catedrático CONACYT. Coordinator of the Ecotoxicology Laboratory in the institution above. The goal of the current research is to produce databases on the diversity of species of microorganisms in the Yucatan Peninsula and to establish indicators to acknowledge biological connectivity and the contamination of karstic systems’ groundwater.
References
- Ahlstrom, E. H. (1940). A revision of the rotatorian genera Brachionus and Platyias with description of one new species and two new varieties. Bulletin of the American Museum of Natural History, 77, 143–11.
- Aránguiz-Acuña, A., & Serra, M. (2016). Diapause as escape strategy to exposure to toxicants: Response of Brachionus calyciflorus to arsenic. Ecotoxicology, 25(4), 708–719. doi:10.1007/s10646-016-1629-7
- Beyer, J., Petersen, K., Song, Y., Russ, A., Grung, M., Bakke, T., & Tollefsen, KE. (2014). Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Mar Environ Res, 96, 81–91.
- Brinkhurst, RO. 1971. A guide for the identification of British aquatic oligochaeta. Freshwater biological association. Scientific publication No. 22. University of Toronto.
- Bryant, V., Newbery, D. M., McLusky, D. S., & Campbell, R. (1985). Effect of temperature and salinity on the toxicity of arsenic to three estuarine invertebrates (Corophium volutator, Macoma balthica, Tubifex costatus). Marine Ecology, 24, 129–137.
- Bundschuh, J., Litter, M. I., Parvez, F., Román-Ross, G., Nicolli, H. B., Jiin-Shun, J., … Toujaguez, R. (2012). One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Science of the Total Environment, 429, 2–35. doi:10.1016/j.scitotenv.2011.06.024
- Cano-Contreras, E. J., & Delgado, M. E. S. (2009). Aproximación al huerto familiar de clima semiárido: Caracterización del solar en el ocote, Aguascalientes, México. Etnobiologia, 7, 45–55.
- Cruzeiro, C., Amaral, S., Rocha, E., & Rocha, M. J. (2017). Determination of 54 pesticides in water of the Iberian Douro River estuary and risk assessment of environmentally relevant mixtures using theoretical approaches and Artemina salina and Daphnia magna bioassays. Ecotoxicology and Environmental Safety, 145, 126–134. doi:10.1016/j.ecoenv.2017.07.010
- Dodson, S. I., & Silva-Briano, M. (1996). Crustacean zooplankton species richness and association in reservoir and ponds of Aguascalientes State, Mexico. Hydrobiologia, 325(2), 163–172. doi:10.1007/BF00028277
- Elías-Gutiérrez, M., Suárez-Morales, E., Gutiérrez Aguirre, M. A., Silva-Briano, M., Granados Ramírez, J. G., & Garfias Espejo, T. (2008). Cladócera y Copépoda de las aguas continentales de México. G. ilustrada Ed.. J.M. Ávila Valdivieso. México.
- Falcó, G., Llobet, J. M., Bocio, A., & Domingo, J. L. (2006). Daily intake of arsenic, cadmium, mercury, and lead by consumption of edible marine species. Journal of Agricultural and Food Chemistry, 54(16), 6106–6112. doi:10.1021/jf0610110
- Huang, C., Ke, Q., Costa, M., & Shi, X. (2004). Molecular mechanisms of arsenic carcinogenesis. Molecular and Cellular Biochemistry, 255, 57–66.
- Koste, W. (1978). Rotatoria Die Rädertiere Mitteleuropas (pp. 234). Alemania: Gebrüder Borntraeger.
- Koste, W., & Shiel, R. J. (1987). Rotifera from Australian Inland Waters, II. Epiphanidae and Brachionidae (Rotifera: Monogononta). Invertebrate Taxonomy, 7, 949–1021. doi:10.1071/IT9870949
- Koste, W., & Shiel, R. J. (1989). Rotifera from Australian Inland Waters, III. Euchlanidae, Mytilinidae and Trochotriidae (Rotifera: Monogononta). Transactions of the Royal Society of South Australia, 113, 85–114.
- López-Gutiérrez, L. F., Rubio-Franchini, I., Rico-Martínez, R., Mesquita-Joanes, F., Ramírez-López, E. M., Arredondo-Figueroa, J. L., & Silva-Briano, M. 2018. Inter and Intraspecific Variability in Invertebrate Acute Toxicity Response to Arsenic and Fluoride Exposure. Journal of Environment and Health Science, 4(1), 1–10. Ommega publishers.
- Martínez-Gómez, C., Fernández, B., Benedicto, J., Valdés, J., Campillo, J. A., León, V. M., & Vethaak, A. D. (2012). Health status of red mullets from polluted areas of the Spanish Mediterranean coast, with special reference to Portmán (SE Spain). Marine Environmental Research, 77, 50–59. doi:10.1016/j.marenvres.2012.02.002
- Mason, R. P., Laporte, J. M., & Andres, S. (2000). Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Archives of Environmental Contamination and Toxicology, 38, 283–297. doi:10.1007/s002449910038
- Mohammed-Abdul, K. S., Sammanthi-Jayasinghe, S., Chandana, E. P. S., Jayasumana, C., & De Silva, P. M. C. S. (2015). Arsenic and human health effects. A review. Environmental Toxicology and Pharmacology, 40, 828–846. doi:10.1016/j.etap.2015.09.016
- Moon, K., Guallar, E., & Navas-Acien, A. (2012). Arsenic exposure and cardiovascular disease: And updated systematic review. Current Atherosclerosis Reports, 14(6), 542–555. doi:10.1007/s11883-012-0280-x
- Nichols, H. W. (1973). Growth media-freshwater (pp. 7–24). Cambridge: Cambrigde University Press.
- Ning-Xing, W., Yue-Yue, L., Zhong-Bo, W., Liu-Yan, Y., & Ai-Jun, M. (2018). Waterborne and dietborne toxicity of inorganic arsenic to the freshwater zooplankton Daphnia magna. Environmental Science & Technology, 52, 8912–8919. doi:10.1021/acs.est.8b02600
- Nogrady, T., Wallace, R. L., & Snell, T. W. (1993). Rotifera Vol 1: Biology, ecology and systematics. In J. Green, K. Walter, P. Birger, & D. HJF (Eds.), Guides of the identification of Microinvertebrates of the Continental Waters of the World 4 (pp. 142). The Netherlands: SPB Academic Publishing.
- Olmedo, P., Pla, A., Hernández, A. F., Barbier, F., Ayouni, L., & Gil, F. (2013). Determination of toxic elements (mercury, cadmium, lead, tin and arsenic) in fish and shellfish. Risk assessment for the consumers. Environment International, 59, 63–72. doi:10.1016/j.envint.2013.05.005
- Osorio-Tafall, B. F. (1942). Rotíferos planctónicos de México I, II y III. Revista de la Sociedad Mexicana de Historia Natural, 3, 23–81.
- Rahman, M. A., Hasegawa, H., & Lim, R. P. (2012). Bioccumulation, biotransformation and trophic transfer or arsenic in the aquatic food chain. Environmental Research, 116, 118–135. doi:10.1016/j.envres.2012.03.014
- Rahman, M. A., & Hassler, C. (2014). Is arsenic biotransformation a detoxification mechanism for microorganisms? Aquatic Toxicology, 146, 212–219. doi:10.1016/j.aquatox.2013.11.009
- Rahman, M. A., Hogan, B., Duncan, E., Doyle, C., Krassoi, R., Rahman, M. M., … Hassler, C. (2014). Toxicity or arsenic species to three freshwater organisms and biotranformation or inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicology and Environmental Safety, 106, 126–135. doi:10.1016/j.ecoenv.2014.03.004
- Rico-Martínez, R., & Silva-Briano, M. (1993). Contribution to the knowledge of the rotifera of Mexico. Hydrobiología, 255/256, 467–474. doi:10.1007/BF00025875
- Rios-Arana, J. V., Gardea-Torresdey, J. L., Webb, R., & Walsh, E. J. (2005). Heat shock protein 60 (HSP60) response of Plationus patulus (Rotifera: Monogononta) to combined exposures of arsenic and heavy metals. Hydrobiologia, 546, 577–585. doi:10.1007/s10750-005-4308-x
- Ruttner-Kolisko, A. (1974). Plankton rotifers biology and taxonomy (pp. 146). Germany: Gebrüder Ranz, Dieteheim.
- Segers, H. (1995). Rotifera Vol: 2 The lecanidae (Monogononta). In T. Nogrady, R. Chengalath, R. Shiel, & D. HJF (Eds.), Guides to the Identification of the microinvertebrates of the continental waters of the world 6 (pp. 225). The Nederlands: SPB Academic Publishing.
- Sharma, V. K., & Sohn, M. (2009). Aquatic arsenic: Toxicity, speciation, trasnformations, and remediation. Environment International, 35, 743–759. doi:10.1016/j.envint.2009.01.005
- Smedley, P. L., & Kinniburg, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568.
- Snell, T. W., & Moffat, B. D. (1992). A 2-d life cycle test with the rotifer Brachionus calyciflorus. Environmental Toxicology and Chemistry / SETAC, 11, 1249–1257. doi:10.1002/etc.v11:9
- Thomas, D. J. (2007). Molecular processes in cellular arsenic metabolism. Toxicology and Applied Pharmacology, 222, 365–373. doi:10.1016/j.taap.2007.02.007
- Vahter, M. (2008). Health effects of early life exposure to arsenic. Basic & Clinical Pharmacology & Toxicology, 102(2), 204–211. doi:10.1111/j.1742-7843.2007.00168.x
- Young-Seoub, H., Ki-Hoon, S., & Jin-Yong, C. (2014). Health effects of chronic arsenic exposure. Journal of Preventive Medicine & Public Health, 47, 245–252. doi:10.3961/jpmph.14.035
