?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Trees on farm provide numerous supporting and regulating services. Some services are biodiversity conservation and enhancement of carbon stock storage. This depends upon the exclusion of livestock intervention to farmlands. In Ethiopia, exclosure has been implemented on communal grazing lands which brought better abundance and enhanced carbon stock than open grazing lands. Recently, the idea of exclosure has been implemented on farmlands. This study investigated the impact of farm exclosures on woody species abundance, diversity and carbon stock. Two farm types, such as farm exclosure, where there is exclusion of livestock throughout the year, and open farms, where livestock freely graze in the winter, were selected in the districts of Hawzen and Hintalo Wajirat, Tigray, Ethiopia. Eighteen farm sample plots having an area of half a hectare (100*50) were chosen randomly from each farm type, totaling 36 plots. Height, diameter at breast height and diameter at stump height were recorded for trees and shrubs found in the sample plots. Fifteen woody species representing 10 families were recorded in farm exclosures and nine species representing seven families were recorded in the open farms. Higher abundance, basal area, vegetation biomass and vegetation carbon stock were observed in the farm exclosures. Mean abundance of all woody species was 21.34 and 13.44 trees ha−1; basal area, 0.55 and 0.18 m2 ha−1; species richness, 2.72 and 2.11; Shannon diversity, 1.64 and 1.74; and vegetation carbon stock, 4.57 and 1.18 ton ha−1, for farm exclosures and open farms, respectively. The result showed that there is a significant difference in mean abundance (p < 0.05), basal area (p < 0.01) and carbon stock (p < 0.01) between the farm exclosures and open farms. Thus, exclosures applied in farmlands have a considerable contribution in increasing woody species abundance, basal area and vegetation carbon stock.
PUBLIC INTEREST STATEMENT
Trees growing on farmlands provide numerous services. They help to protect the diversity of tree species. They have also the potential to store carbon dioxide that would otherwise emit to the atmosphere. The magnitude of services we gain from trees on farm depends upon the exclusion of livestock intervention to farmlands. The objective of this study is then to quantify the difference between protected farms (no livestock intervention) and open farms (allowed livestock intervention) on tree species diversity and carbon storage. The study was conducted in two selected districts of Tigray, Ethiopia. The result showed that protected farms have a higher potential to store carbon than open farms. However, the species diversity remained the same for both farm types. This is due to farmers stick to a few species that are good for crops growing under the trees. In general, protected farms have a considerable contribution to increasing carbon storage but not diversity.
Competing Interests
The authors declare no competing interests.
1. Introduction
Nowadays, the earth is facing global warming due to anthropogenic and natural factors which causes substantial changes in the world’s climate. A gradual change in the global climate has brought visible and evident effects across the world in many aspects such as finance, health, agriculture and so on. Developing countries, specifically dry lands, experience most adverse impacts of climate change (Stern, Citation2007). Like other developing countries, Ethiopia is vulnerable to climate change due to its exposure, sensitivity and poor adaptive capacity (Conway & Schipper, Citation2011). The northern part of the country, Tigray region, is identified as a vulnerable area to climate change (Hadgu, Tesfaye, Mamo, & Kassa, Citation2015).
The level of community’s vulnerability is determined by the sensitivity and adaptive capacity of the community (Stern, Citation2007). Indeed, adaptive capacity is the ability of a system to adjust to climate change (including climate variability and extremes) to moderate potential damages, to take advantage of opportunities or to cope with the consequences (Vincent et al., Citation2013). Many pieces of literature have earlier indicated the role of trees growing in farmland coping up the climate change impacts.
Trees planted in farmlands help to adapt climate change by reducing the vulnerability to climate impacts and through reducing the effect of weather extremes (Rao, Verchot, & Laarman, Citation2007). Trees could also ensure mutual relationship with crops through decreasing nutrient loss from the soil and nitrogen fixation (Sanchez, Buresh, & Leakey, Citation1997). Incorporating trees on farmlands have often both economic and environmental benefits (Nair et al., Citation2012). Trees on farmlands improve soil fertility through control of erosion, maintenance of soil organic matter and physical properties (Kandji et al., Citation2006). Besides, some social benefits of trees on farmlands include the provision of products that include fuelwood, construction materials, fodder for livestock and additional sources of farm income generation (De Leeuw, Njenga, Wagner, & Iiyama, Citation2014). Planting trees, in general, provides an example of a set of adaptation practices that are intended to enhance productivity in a way that often contributes to climate change mitigation through enhanced carbon sequestration (Gebrehiwot & Van Der Veen, Citation2013).
However, the abundance, diversity and potential trees to sequester carbon depends upon the management practice (Chaturvedi, Raghubanshi, & Singh, Citation2011; Chaturvedi and Raghubanshi, Citation2015a; Montagnini & Nair, Citation2004). For example, exclosures have significantly higher abundance and carbon sock potential when compared to the open grazing lands (Mekuria, Citation2013; Tesfaye, Citation2011). Exclosure is an approach in which degraded lands are rehabilitated through excluding of human and livestock interventions to the enclosed area (Aerts, Nyssen, & Haile, Citation2009). Exclosures are usually established in steep, eroded and degraded areas that have been used for grazing in the past (Mekuria, Citation2013; Tucker & Murphy, Citation1997). Over the past three decades, exclosures have been widely implemented in northern Ethiopia, Tigray, and their impact on the restoration of the native vegetation, species abundance, richness, diversity and carbon stock is well documented (Birhane, Teketay, & Barklund, Citation2006; Mekuria, Veldkamp, Corre, & Mitiku, Citation2011; Mengistu, Teketay, Hulten, & Yemshaw, Citation2005; Tesfaye, Citation2011). Exclosures have many benefits such as lessen land degradation, reduce run-off, reduce soil erosion, improve the micro-climate and water infiltration, restore soil nutrients, sequester carbon, provide livestock fodder and fuelwood and enhance biodiversity and are a land management option with relatively low cost (De Leeuw et al., Citation2014; Mengistu et al., Citation2005).
However, exclosures are applied in the communal grazing land but not in the farmland where extensive grazing occurs in most of Ethiopia during the winter season (Nyssen et al., Citation2010). Therefore, implementing exclosure in grazing areas without implementing it in farmland may lead to increased grazing pressure on farmland (Baudron, Mamo, Tirfessa, & Argaw, Citation2015) which may result in degradation of biodiversity and reduced carbon stock. The concept of farmland exclosure is a new approach introduced by the Ethiopian Ministry of Agriculture, and it applies the principles of zero-grazing to farmland, with the aim of halting farmland degradation and increasing productivity (Lenaerts, Citation2013). Although the impact of exclosure in communal grazing areas on species abundance, diversity and carbon stock is well documented, studies on farmland exclosure are limited. Thus, this study was conducted to examine the impact of exclosures established in farmlands on the abundance, diversity and vegetation carbon stock.
2. Materials and methods
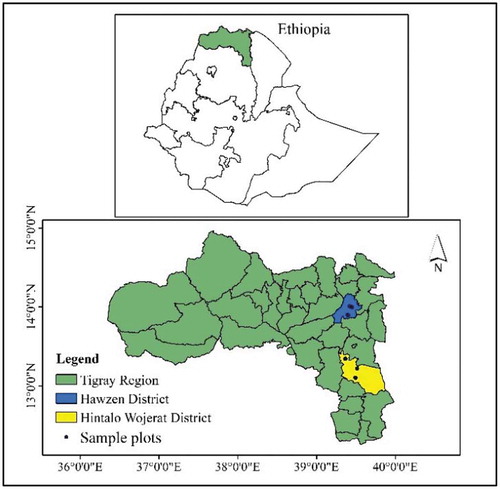
Hintalo Wajirat is a district found in Southern Tigray and geographically located at 12°54ʹ00” and 12°22ʹ00” north of latitude and at 39°17ʹ30” and 39°46ʹ00” east of longitude (Figure ). The mean monthly temperature ranges from 17.08°C to 21.9°C. The average annual rainfall of a 30-year data (1980–2009) is 568.96 mm ranging between 283.76 and 900 mm. Rainfall in the district has a unimodal pattern which covers the summer season from the end of June to mid-September with a length of growing period of 89 days. The altitude of the district ranges from 1,221 to 3,200 m above sea level. According to traditional Ethiopian agro-ecological zoning, 67.6% of the district is categorized as dry woina dega. And as to Ethiopian Ministry of Agriculture agroclimatic zoning, the district is categorized as tepid to cool sub moist mid highlands.
Hawzen is located at eastern zone of Tigray and geographically situated between 13°58ʹ39” north and 39°25ʹ45” east (Figure ). The mean monthly temperature ranges from 16.6°C to 18.2°C. The average annual rainfall of a 30-year data (1980–2009) is 543.9 mm ranging between 340.5 and 886.1 mm. Rainfall in the district has a unimodal pattern which covers the summer season from the end of June to mid-September with 100 days of Length of Growing Period (LGP). The altitude ranges from 1,554 to 2,919 m above sea level. According to traditional Ethiopian agro-ecological zoning, 85.8% of the district is categorized as dry woina dega. And as to Ethiopian Ministry of Agriculture agroclimatic zoning, the district is categorized as tepid to cool sub moist mid highlands.
2.1. Study area selection
The study was conducted in two districts, one with exclosed farms and the other with open farms. The data about the farmlands in the districts were taken from the agricultural office of each respective districts. Accordingly, in Hawzen district, the farmlands were protected from livestock intervention for 13 years (2002–2015). In Hintalo Wajirat, the farmlands were not protected from livestock intervention, and free grazing was allowed. Hence, all the farm exclosures were sampled from Hawzen district and all open farms were sampled from Hintalo Wajirat district. Accordingly, the comparison was made between farm exclosures and open farms. These two districts constituted similar agroclimatic zones, rainfall, temperature and length of the growing period. A reconnaissance field survey revealed that Acacia trees, Cordia africana, Olea europea, Carissa edulis and Maytenus senegalensis were among the trees that can naturally grow in all the study sites in common.
2.2. Sampling technique
One farm plot of 0.5 ha (50 m*100 m) belonging to an individual farmer was used as a sample plot for biomass estimation and diversity assessment. A plot of half a hectare was selected as it was a common plot area among most of the farmers in the study districts. The inventory of scattered tree on farmlands was done from randomly selected 36 plots of rectangular shape. Rectangular plots were chosen as they are more representative than circular or square plots in encompassing heterogeneity within plot (Hairiah et al., Citation2001). All tree/shrub species found within the plot were named, counted and measured their Diameter at Breast Height (DBH) (1.3 m above-ground level), Diameter at Stump Height (DSH) (30 cm above-ground level), height and crown diameter. Clinometer, caliper and tape meter were used to measure height, DBH (DSH) and crown diameter of a tree/shrub, respectively (Ponce-Hernandez, Koohafkan, & Antoine, Citation2004). Plant identification was done in the field using their local name and useful trees and shrubs for Ethiopia (Bekele-Tesemma & Tengnäs, Citation2007)
2.3. Data analysis
2.3.1. Woody species composition and diversity
Woody species density, relative density, frequency and relative frequency (RF) for each individual species were calculated according to Argaw, Teketay, and Olsson (Citation1999). The basal area (BA), relative dominancy and the importance value index (IVI) of each woody species having DBH >2.5 cm were calculated using Kent (Citation2012). The diversity values of woody plants from the three villages were calculated using the Shannon diversity index (Magurran, Citation2004; Krebs, Citation1999).
2.3.2. Living carbon stock estimation
In agricultural landscapes, biomass estimation using DBH alone has a change of 1.3% from the biomass estimated using DBH, height and specific wood density (Kuyah & Rosenstock, Citation2015). Considering the similarity in agro-climate and land use, the equation developed by Kuyah and Rosenstock (Citation2015) was adopted for this study to estimate the above-ground biomass (ABG). After having the ABG, the carbon stock was calculated assuming it constitutes 50% of the total AGB (Chaturvedi & Raghubanshi Citation2015b; Nair, Citation2012).
Equations developed by Woody Biomass Inventory and Strategic Planning Project (WBISPP, Citation2000) in Dry Weyn-adega agro-ecological zone of Ethiopia were also used for estimating tree carbon stock for trees having DBH less than 2.5 cm.
The below-ground living biomass is commonly derived from the above-ground living tree biomass using the root to shoot ratio (Nair, Citation2012). Depending on the nature of the plant and ecological conditions, the below-ground living biomass ranges from 25% to 40% of above-ground living tree biomass (Nair, Citation2012). Penman et al. (Citation2003) estimated the below-ground living biomass as 27% of the AGB.
2.4. Statistical analysis
All the data were first checked for normality and equality of variance before directly progressing to further parametric statistical analysis. The management of farms was the independent variable, while density, BA, species richness, species diversities and vegetation carbon stock were considered as dependent variables. Vegetation data of each district were compared using T-test with software package (SPSS 20). Differences were considered significant at p < 0.05 level.
3. Results and discussion
3.1. Woody species diversity
The total number of woody plant species recorded in the study area, in both the farm exclosure and open farm area, was 17, among which 7 were naturally growing species and 10 were planted. In the farm exclosure, 15 plant species representing 9 families were recorded. Out of the total woody species encountered in the study quadrats, 82.8% were trees, 14.5% fruit trees and the rest were shrubs (Table ). In the open farms, nine species were recorded representing seven families. Here, wood trees constituted 86.7%, shrubs 8.3% and fruit trees 5%. About seven species were recorded both in the exclosure and open farms, while eight species were found only in the exclosure area.
Table 1. Species composition of the sampled plots in farm exclosures and open farms
Variations were observed in terms of the RF of trees/shrubs in plots (Table ). In the exclosures, Faidherbia albida (34%) and Acacia etbaica (18%) were the most frequently found. Eucalyptus camaldulensis (32%) and Faidherbia albida (16%) were the tree species with high RF in the open farms.
IVI is a measurement of dominance. According to IVI, Faidherbia albida, Acacia etbaica, Acacia abyssinicaand Eucalyptus camaldulensis were the dominant species in the exclosures. Eucalyptus camaldulensis, Faidherbia albida and Schinus molle were the dominant species in open farms.
T-test showed that there were significant differences in the mean number of stem (abundance) per plot (p < 0.05). The farm exclosures have a significantly higher number of trees per hectare than the open farms (Table ). This is due to the exclusion of livestock to farmland encourages the vegetation to grow, through reduction of being eaten and trampled by livestock. A study in central Ethiopia also revealed that farm exclosure had far a significantly greater number of trees than that of open farms (Baudron et al., Citation2015). In Ethiopia, seedling survival and growth got to be negatively affected by the presence of livestock (Wassie, Sterck, Teketay, & Bongers, Citation2009). Exclosure has a positive impact on the density of species (Birhane et al., Citation2006). In China, Yong-Zhong, Yu-Lin, Jian-Yuan, and Wen-Zhi (Citation2005) demonstrated that excluding livestock grazing enhances vegetation recovery. BA per plot also showed a significant difference (p < 0.01). Farm exclosure had a higher BA (m2/plot) than open farms. The farm exclosure had a higher BA as it constituted a lot of Faidherbia albida tree with high DBH. Studies from other land-use types revealed that enclosed lands had better tree species abundance and BA than open grazing lands (Birhane et al., Citation2006; Mekuria, Citation2013; Mengistu et al., Citation2005).
Table 2. Mean woody species basal area, abundance, richness and Shannon index (H’)
Species richness and Shannon diversity index were not significantly different. This is due to the deliberate selection of ideal trees to integrate with crops in farm exclosures. For instance, Faidherbia albida was the dominant tree in the farm exclosures which was most preferred by farmers due to its multiple purposes in addition to improving soil fertility. In addition to this Shannon, diversity did not show significant difference as this index gives more weight to rare species (Birhane et al., Citation2006; Negash, Yirdaw, & Luukkanen, Citation2012).
3.2. Above-ground and below-ground carbon stock of trees and shrubs
In the farm exclosures, it is dominated by Faidherbia albida trees ranging DBH from 8 to 60 cm and the density ranges from 8 to 36 stems ha−1 (Table ). The estimated total tree and shrub carbon stock (TTSCS) in the farm exclosure was 4.57 ton C ha−1(Table ) This is similar to the study of Chiemela, Noulekoun, Zenebe, Abadi, and Birhane (Citation2018) in Zongi, Central Tigray, where there is a good tradition of practicing scattered trees on farm. The total biomass C stock is within the range reported for Agroforestry systems in sub-Saharan Africa (4.5–19 ton C/ha) (Unruh, Houghton, & Lefebvre, Citation1993). The TTSCS for open farms was 1.18 ton C ha−1. Here, the TTSCS was out of the specified range for AF systems in sub-Saharan Africa. This is due to livestock intervention to farms which has a negative impact on naturally regenerating trees and artificially planted trees as well. In this study, the farm exclosures had significantly (p < 0.01) higher TTSCS than open farms. This agrees with Luedeling, Sileshi, Beedy, and Dietz (Citation2011) which described carbon stock ha−1 increased with increasing tree density. In agroforestry systems, the standing stock of the carbon above ground is usually higher than the equivalent land use without trees (Altieri & Nicholls, Citation2017). Hence, the higher carbon stock in the farm exclosure is associated with higher tree abundance and higher biomass which brought as a result of the exclusion of livestock from farmlands.
Table 3. Above-ground biomass (AGB), below-ground biomass (BGB), above-ground carbon stock (AGCS) and below-ground carbon stock (BGCS) in farm types (ton C ha−1)
4. Conclusions
The vegetation on the farm exclosure has significantly higher woody vegetation abundance and BA than the open farms. This showed that exclosed farm has a positive impact on density and BA. However, it did not have a significant variation on species diversity and richness. This could be due to farmer’s deliberate selection of the ideal tree to integrate with crops. Farm exclosures have also significantly higher vegetation carbon stock than open farms. Generally, farm exclosures do positively contribute to woody vegetation abundance, BA and carbon stock. Hence, the establishment of exclosure on farmlands is needed for better woody tree resource conservation and storing carbon stock.
Additional information
Funding
Notes on contributors
H. Haftom
H. Haftom has a BSc degree in General Forestry and MSc degree in Climate and Society. Together with colleagues, he has six publications in peer-reviewed journal mainly related to species distribution model. He has also other prepared manuscripts ready to be send to journals. The research reported in this paper is part of a project that mainly promotes the adoption of climate smart agriculture. Hence, this paper has its own contribution in achieving the aim of the project which is stated as promoting climate smart agriculture to enhance agricultural productivity for improved food security and poverty reduction. Now, he is engaged in researches `related to climate science spatial autocorrelation and geostatistics.
References
- Aerts, R., Nyssen, J., & Haile, M. (2009). On the difference between” exclosures” and” enclosures” in ecology and the environment. Journal of Arid Environments, 73(8), 762–10. doi:10.1016/j.jaridenv.2009.01.006
- Altieri, M. A., & Nicholls, C. I. (2017). The adaptation and mitigation potential of traditional agriculture in a changing climate. Climatic Change, 140(1), 33–45. doi:10.1007/s10584-013-0909-y
- Argaw, M., Teketay, D., & Olsson, M. (1999). Soil seed flora, germination and regeneration pattern of woody species in an Acacia woodland of the Rift Valley in Ethiopia. Journal of Arid Environments, 43(4), 411–435. doi:10.1006/jare.1999.0532
- Baudron, F., Mamo, A., Tirfessa, D., & Argaw, M. (2015). Impact of farmland exclosure on the productivity and sustainability of a mixed crop-livestock system in the Central Rift Valley of Ethiopia. Agriculture, Ecosystems & Environment, 207, 109–118. doi:10.1016/j.agee.2015.04.003
- Bekele-Tesemma, A., & Tengnäs, B. (2007). Useful trees and shrubs of Ethiopia: Identification, propagation, and management for 17 agroclimatic zones. Eastern Africa Region: RELMA in ICRAF Project, World Agroforestry Centre.
- Birhane, E., Teketay, D., & Barklund, P. (2006). Actual and potential contribution of exclosures to enhance biodiversity of woody species in the drylands of Eastern Tigray. Journal of Drylands, 1(2), 134–147.
- Chaturvedi, R. K., & Raghubanshi, A. S. (2015a). Allometric models for accurate estimation of aboveground biomass of teak in tropical dry forests of India. Forest Science, 61(5), 938–949. doi:10.5849/forsci.14-190
- Chaturvedi, R. K., & Raghubanshi, A. S. (2015b). Assessment of carbon density and accumulation in mono-and multi-specific stands in Teak and Sal forests of a tropical dry region in India. Forest Ecology and Management, 339, 11–21. doi:10.1016/j.foreco.2014.12.002
- Chaturvedi, R. K., Raghubanshi, A. S., & Singh, J. S. (2011). Carbon density and accumulation in woody species of tropical dry forest in India. Forest Ecology and Management, 262(8), 1576–1588. doi:10.1016/j.foreco.2011.07.006
- Chiemela, S. N., Noulekoun, F., Zenebe, A., Abadi, N., & Birhane, E. (2018). Transformation of degraded farmlands to agroforestry in Zongi Village, Ethiopia. Agroforestry Systems, 92(5), 1317–1328. doi:10.1007/s10457-017-0076-7
- Conway, D., & Schipper, E. L. F. (2011). Adaptation to climate change in Africa: Challenges and opportunities identified from Ethiopia. Global Environmental Change, 21(1), 227–237. doi:10.1016/j.gloenvcha.2010.07.013
- De Leeuw, J., Njenga, M., Wagner, B., & Iiyama, M. (Eds.). (2014). Treesilience: An assessment of the resilience provided by trees in the drylands of Eastern Africa (pp. 166). Nairobi, Kenya: ICRAF.
- Gebrehiwot, T., & Van Der Veen, A. (2013). Farm level adaptation to climate change: The case of farmer’s in the Ethiopian Highlands. Environmental Management, 52(1), 29–44. doi:10.1007/s00267-013-0039-3
- Hadgu, G., Tesfaye, K., Mamo, G., & Kassa, B. (2015). Farmers climate change adaptation options and their determinants in Tigray Region, Northern Ethiopia. African Journal of Agricultural Research, 10(9), 956–964. doi:10.5897/AJAR2014.9146
- Hairiah, K., Sitompul, S., Van, N. M., & Palm, C. (2001). Methods for sampling carbon stocks above and below ground. Indonesia: ICRAF Bogor.
- Kandji, S. T., Verchot, L. V., Mackensen, J., Boye, A., Van Noordwijk, M., Tomich, T. P., … Garrity, D. P. (2006). Opportunities for linking climate change adaptation and mitigation through agroforestry systems. World Agroforestry into the future (pp. 113–121). Nairobi, Kenya.: World Agroforestry Centre-ICRAF.
- Kent, M. (2012). Vegetation description and data analysis: A practical approach (2nd ed., pp. 432). London: John Wiley & Sons.
- Krebs, C. (1999). Ecological methodology. California: Addison Wesley Longman.
- Kuyah, S., & Rosenstock, T. S. (2015). Optimal measurement strategies for aboveground tree biomass in agricultural landscapes. Agroforestry Systems, 89(1), 125–133. doi:10.1007/s10457-014-9747-9
- Lenaerts, L., 2013. Insights into agency and social interactions in natural resource management. Extended case studies from Northern Ethiopia (Doctoral dissertation). Catholic University of Leuven, Belgium.
- Luedeling, E., Sileshi, G., Beedy, T., & Dietz, J. (2011). Carbon sequestration potential of agroforestry systems in Africa. In Carbon sequestration potential of agroforestry systems (pp. 61–83). Dordrecht: Springer.
- Magurran, E. (2004). Measuring biological diversity. London: Blackwell.
- Mekuria, W. (2013). Changes in regulating ecosystem services following establishing exclosures on communal grazing lands in Ethiopia: A synthesis. Journal of Ecosystems, 2013, 12. doi:10.1155/2013/860736
- Mekuria, W., Veldkamp, E., Corre, M. D., & Mitiku, H. (2011). Restoration of ecosystem carbon stocks following exclosure establishment in communal grazing lands in Tigray, Ethiopia. Soil Science Society of American Journal, 75(1), 246–256. doi:10.2136/sssaj2010.0176
- Mengistu, T., Teketay, D., Hulten, H., & Yemshaw, Y. (2005). The role of enclosures in the recovery of woody vegetation in degraded dryland hillsides of central and northern Ethiopia. Journal of Arid Environments, 60(2), 259–281. doi:10.1016/j.jaridenv.2004.03.014
- Montagnini, F., & Nair, P. (2004). Carbon sequestration: An underexploited environmental benefit of agroforestry systems. In New vistas in agroforestry (pp. 281–295). Dordrecht: Springer.
- Nair, P. (2012). Carbon sequestration studies in agroforestry systems: A reality-check. Agroforestry Systems, 86(2), 243–253. doi:10.1007/s10457-011-9434-z
- Negash, M., Yirdaw, E., & Luukkanen, O. (2012). Potential of indigenous multistrata agroforests for maintaining native floristic diversity in the south-eastern Rift Valley escarpment, Ethiopia. Agroforestry System, 85(1), 9–28. doi:10.1007/s10457-011-9408-1
- Nyssen, J., Clymans, W., Descheemaeker, K., Poesen, J., Vandecasteele, I., Vanmaercke, M., … Walraevens, K. (2010). Impact of soil and water conservation measures on catchment hydrological response—A case in north Ethiopia. Hydrol. Processes, 24, 1880–1895. doi:10.1002/hyp.7628
- Penman, J., Gytarsky, M., Hiraishi, T., Krug, T., Kruger, D., Pipatti, R., … Tanabe, K. (2003). Good practice guidance for land use, land-use change and forestry. Japan: Institute for Global Environmental Strategies.
- Ponce-Hernandez, R., Koohafkan, P., & Antoine, J. (2004). Assessing carbon stocks and modelling win-win scenarios of carbon sequestration through land-use changes (Vol. 1). Rome: Food & Agriculture Organization.
- Rao, K., Verchot, L. V., & Laarman, J. (2007). Adaptation to climate change through sustainable management and development of agroforestry systems. Journal of SAT Agricultural Research, 4(1), 1–30.
- Sanchez, P. A., Buresh, R. J., & Leakey, R. R. (1997). Trees, soils, and food security. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 352(1356), 949–961. doi:10.1098/rstb.1997.0074
- Stern, N. (2007). The economics of climate change: The Stern review. Cambridge: Cambridge University press.
- Tesfaye, Y. (2011). Restoration of degraded semi-arid communal grazing land vegetation using the exclosure model. International Journal of Water Resources and Arid Environments, 1(5), 382–386.
- Tucker, N. I., & Murphy, T. M. (1997). The effects of ecological rehabilitations on vegetation recruitment: Some observations from the wet Tropics of North Queensland. For. Ecol. Manage., 99, 133–152. doi:10.1016/S0378-1127(97)00200-4
- Unruh, J., Houghton, R., & Lefebvre, P. (1993). Carbon storage in agroforestry: An estimate for sub-Saharan Africa. Climate Research, 3, 39–52. doi:10.3354/cr003039
- Vincent, K., Cull, T., Chanika, D., Hamazakaza, P., Joubert, A., Macome, E., & Mutonhodza-Davies, C. (2013). Farmers’ responses to climate variability and change in southern Africa—Is it coping or adaptation? Climate and Development, 5(3), 194–205. doi:10.1080/17565529.2013.821052
- Wassie, A., Sterck, F. J., Teketay, D., & Bongers, F. (2009). Effects oflivestock exclusion on tree regeneration in church forests of Ethiopia. For. Ecol. Manage., 257, 765–772. doi:10.1016/j.foreco.2008.07.032
- WBISPP (Woody Biomass Inventory and Strategic Planning Project). (2000). Manual for woody biomass inventory. Woody biomass inventory and strategic planning project. Addiss Ababa, Ethiopia: Ministry of Agriculture.
- Yong-Zhong, S., Yu-Lin, L., Jian-Yuan, C., & Wen-Zhi, Z. (2005). Influences of continuous grazing and livestock exclusion on soil properties in a degraded sandy grassland, Inner Mongolia, northern China. Catena, 59(3), 267–278. doi:10.1016/j.catena.2004.09.001