Abstract
This study was carried out to assess the chronic effects of both an insecticide (spirotetramat) and a herbicide (2,4-Dichlorophenoxyacetic acid) on two phytoplankton species: Monoraphidium griffithii and Coscinodiscus sp. The effects of the chemicals on an exponentially-growing population of the two species were evaluated using single compounds and a compound mixture. The experiments lasted for 27 days. The results show a decrease in the populations’ growth for both species after the application of single pesticides and the mixture. 2,4-D applied alone and the mixture of both chemicals have affected the organisms the most. Both pesticides have induced an increase in chlorophyll-a content in M. griffithii while no effect was observed on chlorophyll-a in Coscinodiscus sp. A significant effect was recorded in the cell size of both species with both pesticides. The two spirotetramat tested concentrations have induced an increase in cell size, while the opposite effect was obtained for the (2,4-D) herbicide concentrations. The mixture of compounds induced an additive effect on the cell size of both species. These results indicate the risk which the two chemicals represent in a natural environment. They are agents that can have chronic impairments at the physiology or behavior level of such phytoplankton species as M. griffithii or Coscinodiscus sp. Such impairments might consequently affect the health of the ecosystem.
PUBLIC INTEREST STATEMENT
Chemicals used in agriculture to control weeds or invasive insects often induce adverse effects in the environment. This article describes the impact of two such chemicals on two micro-algal species. Such studies are important in that microalgae are the first level of the food chain in aquatic ecosystems. Thus, any modification in their community might affect the whole ecosystem. Our results show that the herbicide (2,4-D) as well as the insecticide (spirotetramat) considered here reduce the growth rate of microalgae populations. At the same time, spirotetramat induces an increase in the microalgae cell size while 2,4-D has the opposite effect. These observations are expected to help decision-makers to control the use of these substances.
Competing interests
The authors declared that there are no potential competing interests.
1. Introduction
The proper functioning of an aquatic ecosystem provides many services that are also beneficial to humans. This functioning is dependent upon the structure of the biological community as well as on the existing relationships between the components of the community (Houssou, Agadjihouédé, Montchowui, Bonou, & Lalèyè, Citation2015; Pérez et al., Citation2007; Pratt, Melendez, Barreiro, & Bowers, Citation1997). Today, economic development in many countries has increased pressure on natural resources. This pressure leads to changes in the environment, as a result of pollution from urban wastes, industries, agriculture, tourism, and multiple uses of resources (fishing, aquaculture, water activities) which coexist (Stachowski-Haberkorn, Citation2008). All these result in change in the biology of the ecosystem with, most of the time, a loss of diversity (Houssou, Yaovi, Ahouansou Montcho, Bonou, & Montchowui, Citation2016). The community structure then appears modified and consequently the functioning of the ecosystem is impaired.
Among these environmental problems, agricultural pesticides are very important since toxicity can be observed at short term or long term. One-time pollution could impair the ecosystems at all levels of the food chain. Herbicides and insecticides are known to induce adverse effects in fresh and marine water ecosystems (Chang et al., Citation2008; Hourmant et al., Citation2004; Houssou, Daguégué, & Montchowui, Citation2017a; Rohr & Crumrine, Citation2005; Van den Brink, Hartgers, Gylstra, Bransen, & Brock, Citation2002). These effects are variable and depend on the nature of the pesticide used as well as on the potential impact of the physico-chemical parameters on the target communities (AitHsine et al., Citation2002). So, ecotoxicological researchers have to focus on the determination of observable responses of species to chemicals (Relyea & Hoverman, Citation2006).
Phytoplanktons, being the first step in the food chain of aquatic environments, are highly sensitive to environmental pollutants due to its small size and short life cycle. An affected phytoplankton community leads to changes in the upper level of the food web (as fish, shrimp, etc.). As a result, several studies which have investigated the impact of pesticides on phytoplankton species have shown that pesticides are the cause of resulting ecological damage (Sabater & Carrasco, Citation2001a, Citation2001b; DeLorenzo & Serrano, Citation2003; Liu, Wang, Zhang, Zhu, & Li, Citation2013 etc.).
The use of agricultural pesticides should be followed by an evaluation of their impacts on the receiving ecosystem. The present study has evaluated the effect of two pesticides (the herbicide (2,4-D: C8H6Cl2O3) and the keto-enol insecticide spirotetramat (C21H27NO5) commonly used in Romanian agriculture (Houssou, Cocan, Bonou, Miresan, & Montchowui, Citation2018)) on two freshwater phytoplankton species: Monoraphidium griffithii (Chlorophyceae) and Coscinodiscus sp. (Diatoms). These two species were selected due to their predominance in lake ecosystems in Cluj-Napoca (Romania) (Houssou et al., unpublished). Evaluating the impacts of pesticides on the dominant species could be very informative regarding ecosystem health. The two tested pesticides are under regulation and are highly used in Romania (Houssou et al., Citation2018). This study aims then to predict their possible impacts on Romanian freshwater ecosystems based on dominant phytoplankton species sensitivity.
The complex mixture of contaminations in natural environments is also an important factor in the communities’ responses, especially those of phytoplankton. These communities are potentially sensitive to low concentrations of pollutants, both for a single pollutant and for a combination of pollutants (Chang et al., Citation2008; Daly et al., Citation2007). In a natural environment, communities are rarely exposed to a single pollutant; the observed response in a natural community is always a result of a combination of numerous pollutants. Knowledge of the effect of a mixture of pollutants is therefore essential to efficiently monitor ecosystem performances. This aspect was also studied for the two pesticides tested.
2. Materials and methods
The experiments were conducted according to the OECD 201 protocol relative to growth inhibition tests on freshwater algae and cyanobacteria (OECD, Citation2006).
2.1. Test organisms and experimental setup
To obtain the test organisms of the two species, phytoplankton was sampled in the Gheorgheni I natural lake (46° 46ʹN, 23° 37ʹE) located near Cluj-Napoca (Romania) at about 4 km East of the town center. Samples were cultured in a batch culture system using the bold culture medium in order to have sufficient density of the species. To prepare 1 L of bold culture medium, 10 mL of solutions A and B and 1 mL of the trace solution (Table ) were deducted from stock solutions. These samples of stock solutions were mixed and completed to 1 L with distilled water. The culture temperature was maintained at about 21°C (average 20.75 ± 0.1°C) using an electronic thermostat when ambient temperature is low. The photoperiod was set to 16 h of light and 8 h darkness. In order to have the test organisms less affected by possible pollution in the natural environment, the culture was reinitiated twice. After 15 days of phytoplankton species multiplication in the bold culture medium, the target species (Monoraphidium griffithii and Coscinodiscus sp.) were isolated under a fluorescence microscope (Nikon Eclipse E600). An inoculum of each species was then used with new monospecific cultures in the same conditions described above. After 7 days, each monospecific culture was also reinitiated.
Table 1. Chemical composition of the culture solution (bold medium) modified from Barberousse (Citation2006)
Two groups of experiments (2 species) were carried out, and the experimental design of each of them was comprised of 12 aquariums (Control + 2 concentrations x 2 pesticides + Mixture, each being replicated twice) with a total volume of 12 L each. Aquariums were filled each with 10 L of bold culture medium. Since phytoplankton is a CO2 consumer, this was regularly added to the culture medium through a CO2 production device in order to maintain the pH between 7 and 8. Also, 68.8 mg of silicate (Na2SiO3+ H2O) was introduced in the diatom (Coscinodiscus sp.) culture medium preparation (for each tank) in order to have 842.5 µg L−1 of silicon (Si) since it is important for development of diatoms.
The prepared microcosms were inoculated at day 0 (D0) with phytoplankton to a density of 21103 cells L−1 of Monoraphidium griffithii and 8.7× 103 cells L−1 of Coscinodiscus sp. Cultures have evolved healthily for 4 days (D4) to allow development of populations. Thereafter, contaminants were introduced at day 4. Two concentrations of each active substance were tested on each species. The two pesticides were tested in the commercial formulation. The herbicide SDMA Super (Tellurium Chemical) was used for 2,4-D then the insecticide MOVENTO (Bayer CropScience) was used for Spirotetramat. The two formulations were chosen because of their frequent use in Romanian agriculture (Houssou et al., Citation2018). Since our study aims at determining the chronic effect of pesticides on phytoplankton species, the tested concentrations were chosen to be lower than the median Effective Concentration (EC50) on other phytoplankton species. So, for spirotetramat, we have tested nominal concentrations of 1.0 mg L−1 and 12.0 mg L−1 which represent, respectively, the 120 h No Observed Effect Concentration (NOEC) and the 80% of the median Effective Concentration (80%EC50) observed by PMRA (Citation2008) on the Navicula pelliculosa diatom species. For 2,4-D, nominal concentrations of 19.2 mg L−1 and 23.0 mg L−1 were tested. These concentrations were, respectively, the NOEC-120 h and 80% of the EC50-120 h of 2,4-D on the chlorophyta Selenastrum capricornutum (currently known as Raphidocelis subcapitata) species (INERIS, Citation2012). A compound mixture (1.0 mg L−1 of spirotetramat + 19.2 mg L−1 of 2,4-D) was also tested. The compound mixture was then composed of the lowest tested concentration of each pesticide. These concentrations being with no observed effect on other phytoplankton species, the experiments allowed to assess the effect of a combination of non-expected effect concentrations of spirotetramat and 2,4-D on Coscinodiscus sp. and Monoraphidium griffithii.
The experiments were done with two replications of each tested concentration and a control culture (no contamination) which was also replicated twice. Artificial fluorescent light was used to provide a photoperiod of 16/8 h of light/darkness. The culture medium temperature was 20.8 ± 0.02°C during the study.
2.2. Sampling and sample preparation
In order to monitor the culture’s evolution, phytoplankton was sampled every 48 h during the pre-contamination phase and every 72 h during the post-contamination phase. Then, a 500 mL sample was taken after the homogenization of the whole culture. From this sample, three subsamples each of 100 mL were taken. One of these subsamples was immediately preserved with 5% formalin and was used to determine population growth. Cells were then counted using aBürker Türk chamber under a microscope. For that, cells were first allowed to settle in the chamber for 30 min before counting. In each sample, a minimum of 400 cells were counted. Another subsample was used to determine the effect of pollution on cell size. Length and/or diameter of 200 cells randomly selected in each sample (diameter for Coscinodiscus sp.; length and diameter for M. griffithii) were measured. Measurements were taken using the digital camera of the microscope. The third subsample was immediately filtered through a Whatman glass micro-filter for analysis of chlorophyll-a concentration. This concentration was accessed using the 90% acetone dissolution method according to Ritchie (Citation2006).
2.3. Data analysis
Data on population growth and chlorophyll-a concentration are presented as mean ± standard deviation. Cell size classes were identified based on a cluster analysis done on data collected from the measurement of cell size in all samples for all tests. This analysis was performed with PAST v3.14 software using the paired group method with Pearson correlation for similarity measure. Although both cell diameter and length were measured for M. griffithii samples, it appeared that only cell length is discriminant of size classes. So, for M. griffithii, cluster analysis was done on the measured length while in Coscinodiscus sp., it was done on measured diameter. The one-way ANOVA test and the Tukey honesty test (for equivalent sample size) were performed in STATISTICA v7 software.
3. Results
3.1. Cell density and chlorophyll-a concentration in M. griffithii
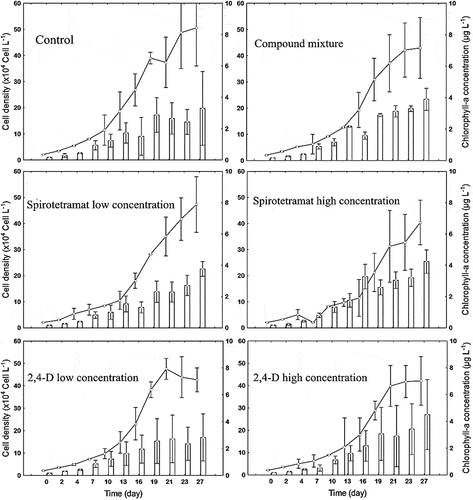
The trend of M. griffithii cell density and chlorophyll-a concentration in different treatments is shown in Figure . Exponential growth was observed until day 19 in the control treatment, day 27 in the treatment with spirotetramat low concentration (1.0 mg L-1), day 21 in the treatment with 2,4-D low concentration (19.2 mg L−1), day 23 with 2,4-D high concentration (23.0 mg L−1) and day 21 in the case of mixture contamination (1.0 mg L−1of spirotetramat + 19.2 mg L−1 of 2,4-D). Regarding the treatment with spirotetramat at high concentration (12.0 mg L−1), a decrease was found at day 7 before a growing phase until day 21. The lowest density (42.1x104 ± 108,851 cell L−1) obtained with 2,4-D high concentration is significantly different (p > 0.05) from the highest density (50.6 104 ± 145664 cells L−1) obtained in the control culture (Table ).
Table 2. Highest cell density and chlorophyll-a concentrations in Monoraphidium griffithii and Coscinodiscus sp. cultures for the various treatments
The temporal trend of chlorophyll-a concentration (Figure ) in all treatments was approximately same as that of cell density, with the exception of spirotetramat low concentration and 2,4-D low concentration. In the case of spirotetramat low concentration, opposite trends were observed between cell density and the chlorophyll-a concentration from day 21. For 2,4-D low concentration, an increase was observed in chlorophyll-a from day 21 when the cell density decreased. The Tukey honesty test showed no significant difference (p < 0.05) between mean chlorophyll-a concentration in each treatment for the test period (Table ). Nonetheless, the highest mean (4.73 ± 0.7 µg mL−1) was observed with spirotetramat high concentration, and the lowest mean (2.82 ± 1.7 µg mL−1) was found in cultures polluted with 2,4-D low concentration.
Figure 1. Cell density and Chlorophyll-a concentration in Monoraphidium griffithii cultivated under six different conditions (five polluted environments and one control). Lines and symbols indicate cell density, bars indicate chlorophyll-a concentration. Error bars indicate average ± standard deviation
3.2. Cell density and chlorophyll-a concentration in Coscinodiscus sp
Cell density and chlorophyll-a trend in Coscinodiscus sp. during the study are presented in Figure . An exponential growth in cell density was observed until day 13 in the control treatment. Beyond that time segment, growth became zero. Compared to the control treatment, cell density growth has lowered in all other treatments. 2,4-D high concentration appears to be the most inhibiting, followed by the lower concentration of 2,4-D and the compound mixture, respectively. The two spirotetramat single tested concentrations also reduced cell density growth but less than others. Regarding the highest cell density in different treatments, the most impacted population was that exposed to 2,4-D high concentration (maximum of 1.96 104 ± 7369 cells L−1 on day 23). The highest cell density (3.59 104 ± 8748 cells L−1 on day 21) was observed in the control batches (p < 0.05).
Figure 2. Cell density and Chlorophyll-a concentration in Coscinodiscus sp. cultivated under six different conditions (five polluted environments and one control). Lines and symbols indicate cell density; bars indicate chlorophyll-a concentration. Error bars indicate average ± standard deviation
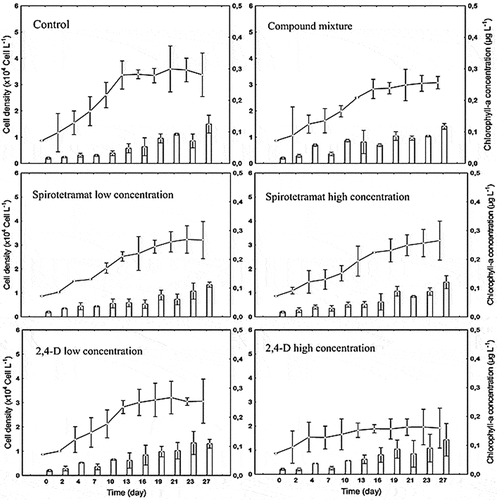
Chlorophyll-a concentration in the different treatments is shown in Figure . Despite differences in cell density, there were very little variations in chlorophyll-a concentrations between treatments. The maximum chlorophyll-a concentrations (Table ) were 0.125 (± 0.02) µg mL−1, 0.112 (± 0.01) µg mL−1, 0.121 (± 0.02) µg mL−1, 0.112 (± 0.03) µg mL−1, 0.120 (± 0.02) µg mL−1 and 0.117 (± 0.01) µg mL−1 respectively, for control, spirotetramat low and high concentration, 2,4-D low and high concentration, and compound mixture. No significant variation was observed in chlorophyll-a concentrations in the chemical treatments (p > 0.05).
3.3. Observed cell size variability
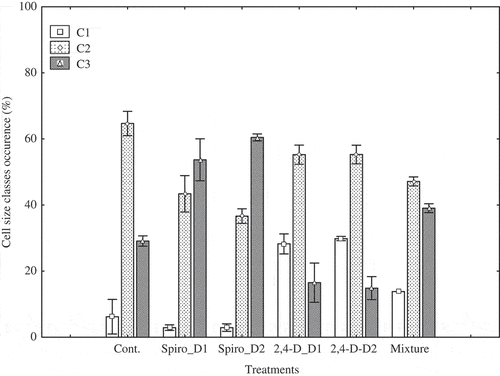
Results of the cluster analysis are shown in Figure . Three cell size classes were obtained for M. griffithii (CI—III) (Figure ) and for Coscinodiscus sp. (C1–3) (Figure ). According to the classification of M. griffithii cell size, CI is composed of cells with length between 52.6 and 70 µm, CII between 70 and 80 µm, and CIII between 80 and 89 µm. For Coscinodiscus sp., C1 is composed of cells with a diameter between 42.3 and 52.5 µm, C2 between 52.5 and 64.7 µm, and C3 between 64.7 and 76.5 µm.
Occurrence of cell size classes for the different treatments and organisms is shown in Figures and . For M. griffithii, only the first two classes were recorded in the control treatment (Figure ). Cells with moderate size (CII) were predominant (90.2% of total cell density) while large cells (CIII) were about 9.8% of total cell density. Respective to the spirotetramat low and high concentration, only two size classes were recorded (CII and CIII): large cells were predominant (56%) for both applied concentrations. For 2,4-D, the effect was opposite to that of the insecticide (spirotetramat). Significant portion of the cell density was comprised of small cells (CI) in both applied concentrations. Thus at low concentration, 38% of total cell density was small, whereas the largest (CIII) accounted for 10%. In high concentration, 28.3% was of small size. The moderate size (CII) was 63%, while the largest was only 8.7%. In the case of the compound mixture, cells with moderate size (CII) represented 62.2% of the total cell density, followed by small cells (CI) (28.7%) and large cells (CIII) (9.1%).
Regarding Coscinodiscus sp., all three cell size classes were found in each treatment (Figure ). In the control treatment, 63.5% of the total cell density was of moderate size (C2), while 30% was of large cells (C3), and the small cells (C1) represented only 6.5%. With spirotetramat single applied concentrations, large cells were the most represented. They accounted for 57% and 61%, respectively, for the low and high concentrations. Small cells were about 5% for both concentrations. Respective to 2,4-D low concentration, cells with small diameter reached 30% of the total cell density, large cells represented only about 18%, and moderate cells made up about 52%. The same observations were obtained in the cultures polluted with a high concentration of 2,4-D. In the case of the compound mixture, cells with moderate size were about 46.1%, followed by large cells (38.9%), while small cells made up 15%.
Figure 3. Phytoplankton cell size classes (CI-CIII, C1-C3) obtained from cluster analysis for the different treatments. (A): sizes identified for M. griffithiitreatments; (B): sizes identified for Coscinodiscus sp. treatments. l is length, d is diameter
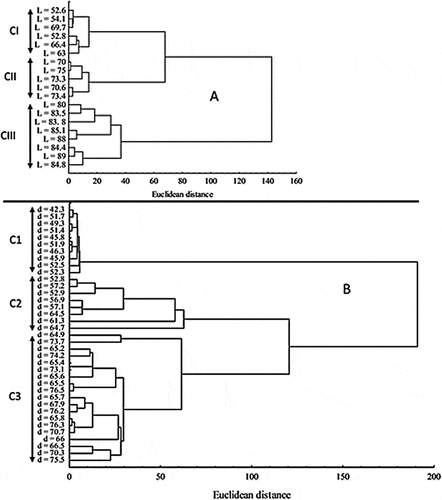
Figure 4. Occurrence of cell size classes in Monoraphidium griffithii for the various treatments. CI includes cells of 52.6–70 µm in length (small cell size), CII cells of 70–80 µm in length (moderate cell size), and CIII cells of 80–89 µm in length (large cell size). D1 and D2 indicate low and high concentrations, respectively. Cont = control, Spiro = spirotetramat. Error bars indicate average ± standard deviation
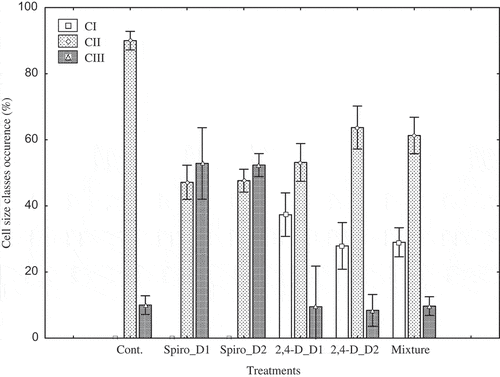
Figure 5. Occurrence of cell size classes in Coscinodiscussp. for the various treatments. C1 includes cells of 42.3–52.5 µm in diameter (small cell size), C2 cells of 52.3–64.7 µm in diameter (moderate cell size), and C3 cells of 64.7–76.5 µm in diameter (large cell size). D1 and D2 indicate low and high concentrations, respectively, Cont = control, Spiro = spirotetramat. Error bars indicate average ± standard deviation
4. Discussion
In aquatic systems, algae are the foundation of the food chain and are therefore essential to the functioning of the ecosystem (Houssou, Montchowui, & Bonou, Citation2017b). Variable responses (at community or physiological level), depending on the biological and chemical composition of the ecosystem, are observed in the microalgae community, hence their importance in aquatic ecotoxicology. This study has evaluated the chronic effects of one insecticide (spirotetramat) and one herbicide (2,4-D) on the M. griffithii and Coscinodiscus sp. freshwater phytoplankton. The results showed that these two pesticides affect both phytoplankton species. Monoraphidium griffithii showed exponential population growth with some variations among the exposure concentrations of both pesticides. In general, both pesticides have induced growth reduction compared to the control treatment; even a decrease followed by an increase was observed in the control from day 19.
This variation in the control might be related to the way the culture was handled. Observations have shown that spirotetramat and 2,4-D at concentrations of about 1.0 mg L−1and 19.2 mg L−1, respectively, might affect M. griffithii population growth by delaying cell division time. Liu et al. (Citation2013) have shown inhibition of green algae (Chlorella pyrenoidosa) growth exposed to insecticide (propoxur) and herbicides (Simetryn, Bromacil, and Hexazinone). Berard and Benninghoff (Citation2001) also found that Chlorella vulgaris, Chlamydomonas sp. (green algae) densities are inhibited by atrazine. Therefore, the results of the present study confirm that insecticides and herbicides can negatively affect the green algae population in natural aquatic systems with a variety of outcomes depending on the type of chemical considered. At the tested concentrations, the (2,4-D) herbicide has not had a greater inhibitory effect than the spirotetramat insecticide, which appears to be different from observations by Liu et al. (Citation2013) showing a greater inhibition of Chlorella pyrenoidosa by herbicides (simetryn, bromacil, hexazinone) than by the propoxur insecticide. This difference might be due to both the difference in the chemical used and the species experimented on. Regarding Coscinidiscus sp. density in the different polluted microcosms, growth inhibition due to both pesticides was also observed. The inhibition appears to be more important with 2,4-D high concentration (23.0 mg L−1). So, ecological chronic impacts can be expected in natural environment polluted with 2,4-D or spirotetramat at concentrations reaching those tested in the present study. These results confirm those of Berard and Benninghoff (Citation2001) who observed, in a laboratory study, an inhibition of central diatoms treated with atrazine. Contaminated by spirotetramat and 2,4-D, both species had different degrees of damage at the same concentrations. Some tolerance of Monoraphidium griffithii to the herbicide seems to be the case, which will eventually lead to ecological disruption, affecting the nutritional selectivity of primary consumers and therefore the entire food chain.
Exposures have induced chlorophyll-a concentration increase in M. griffithii cultures except the culture polluted with the low concentration of spirotetramat. Spirotetramat high concentration appeared to be the most effective in increasing pigment concentration. In the case of Coscinodiscus sp., no effect was observed in chlorophyll-a concentration for various concentrations of both pesticides. According to Dahl and Blanck (Citation1996) and AitHsine et al. (Citation2002), an increase in chlorophyll pigments in an ecosystem contaminated with pesticides can be a consequence of a homeostatic mechanism (an increase of chloroplast to maintain the chemical light-energy conversion). Seguin, Druart, and Le Cohu (Citation2001) reported increased chlorophyll-a in an algae community exposed to atrazine. These authors have asserted that the increase in chlorophyll-a content may be related to moderate exposure to herbicides, cells being able to maintain sufficient photosynthetic activity. Our results show no particular effect of either pesticide on chlorophyll-a content at tested concentrations on Coscinodiscus sp. So, the photosynthetic activity seems to not have been affected. Regarding the mixture’s effect on chlorophyll-a concentration, the increase was also observed in M. griffithii. The obtained chlorophyll-a concentration appears to be similar to that observed for the low concentration of 2,4-D in the same species. Since the low concentration of spirotetramat has no effect (mentioned above), this observation would indicate an additive chronic effect of the two pesticides at the lower tested concentrations in M. griffithii.
Studying M. griffithii cells size, an increase was observed in the size due to both concentrations of spirotetramat exposure. In the case of 2,4-D (both concentrations) and exposure to the mixture, a decrease in cell size was observed. The same observations were made in the Coscinodiscus sp. culture exposed to various concentrations of both the insecticide and the herbicide. Durant et al. (Citation2007) asserted that phytoplankton species cell size increases as cell division is inhibited, reflecting an important chemical stress. This chemical stress can justify our results regarding M. griffithii and Coscinodiscus exposure to spirotetramat. But in the case of 2,4-D, a decrease in cell size follows growth inhibition, showing that the mode of action and activity of the pollutant are important factors in the variation of planktonic algae cell size. Similarly, Laisne et al. (Citation2004) have observed that the reduction or increase in algae cell size is related to the mode of action and the applied concentration of the pesticide. They have reached those conclusions by studying the effect of pesticides (nicosulfuron, sulcotrione, epoxiconazole, bentazone) on the cell size of marine algae. So, the mode of action, the applied concentration, and environmental parameters are important factors in the response of an algal community to different pollutants.
The complexity of aquatic ecosystems limits the use of many existing indicators in the monitoring of the ecological status of these areas. Pollutants tested individually on communities in a laboratory setting do not respond the same way in the real environments (Houssou et al., Citation2018). Thus, several studies were carried out in recent years, trying to consider the pollutant mixture found in aquatic environments. In that sense, Relyea (Citation2009), using an outdoor mesocosm, noted that both insecticides (malathion, carbaryl, chlorpyrifos, diazinon, and endosulfan) and herbicides (glyphosate, atrazine, acetochlor, metolachlor, and 2,4-D) can affect chlorophyll pigment in microalgae. The author observed no significant effect of a compound mixture of the five insecticides compared to each of them tested alone on microalgae. In our study, the results of binary pollution (a mixture of two compounds) showed an additive effect by both pesticides in chlorophyll-a concentration and in the cell size of both species. DeLorenzo and Serrano (Citation2003) have also observed additive toxicity of a compound mixture of atrazine and chlorpyrifos on the Dunaliella tertiolecta (Chlorophyta) marine phytoplankton. Mixing atrazine and chlorothalonil, the same study has shown a synergistic effect on Dunaliella tertiolecta. So, various impacts can be observed depending on the compounds involved in the mixture. Further studies are then needed on the mixtures actually occurring in natural settings along with their impacts at all levels of the aquatic system.
5. Conclusions
Planktonic algae can exhibit varying responses depending on the species considered, the mode of action of pesticides, the concentration of the pesticides, and different compound mixtures in the environment. In this laboratory study, we have found at chronic exposition, that 2,4-D at concentrations of 19.2 mg L−1 and 23.0 mg L−1 and spirotetramat at concentrations of 1.0 mg L−1 and 12.0 mg L−1 generally reduce the growth rate of M. griffithii and Coscinodiscus sp. A higher concentration of 2,4-D may have a higher impact on Coscinodiscus sp. growth rate. Both pesticides induce an increased chlorophyll-a content in M. griffithii while no effect was observed on the chlorophyll-a content in Coscinodiscus sp. Regarding the cell size of both species, an increase was observed with both concentrations of spirotetramat while a decrease was observed with both 2,4-D concentrations. For the mixed compounds, the effect was additive on cell size in both species and on the chlorophyll-a concentration in M. griffithii. Generally, spirotetramat and 2,4-D, by affecting M. griffithii and Coscinodiscus sp. at both the community and physiological levels, could represent a risk to the health of aquatic ecosystems.
Acknowledgements
This study was conducted with the Eugen Ionescu scholarship coordinated by the Agence Universitaire de la Francophonie (AUF). We are hereby expressing our gratefulness. We thank Professor Andrea Bunea, Dr Radu Constantinescu, Dr Erol Gabor, and Călin Laţiu from the University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca (UASVM Cluj-Napoca, Romania) for their contributions to the study.
Additional information
Funding
Notes on contributors
Arsène Mathieu Houssou
Dr Arsène Mathieu Houssou is from the Republic of Benin, West Africa. His research interests are on how plankton communities (phytoplankton and zooplankton) can be used to assess the impacts of human on inland aquatic systems. His research covers both ecotoxicology and applied ecology
References
- Ait Hsine, E., Mandi, L., & Benhammou, A. (2002). Impact des pesticides sur le fonctionnement des bassins de stabilisation. Proceedings of International Symposium on Environmental Pollution Control and Waste Management, 7-10 January 2002, Tunis (EPCOWM’2002), 689–14.
- Barberousse, H. (2006). Etude de la diversité des algues et des cyanobactéries colonisant les revêtements de façade en France et recherche des facteurs favorisant leur implantation. Ecologie, Environnement. Museum national d’histoire naturelle - MNHN PARIS.
- Bérard, A., & Benninghoff, C. (2001). Pollution-induced community tolerance (PICT) and seasonal variations in the sensitivity of phytoplankton to atrazine in nanocosms. Chemosphere, 45(4–5), 427–437. doi:10.1016/S0045-6535(01)00063-7
- Chang, K. H., Sakamoto, M., Ha, J. Y., Murakami, T., Miyabara, Y., Nakano, S. I., … Hanazato, T. (2008). Comparative study of pesticide effects (Herbicide and Fungicide) on Zooplankton community. In Y. Murakami, K. Nakayama, S.-I. Kitamura, H. Iwata, & S. Tanabe (Eds.), Interdisciplinary studies on environmental chemistry (pp. 361–366). Tokyo: Terrapub.
- Dahl, B., & Blanck, H. (1996). Toxic effect of the antifouling agent 1051 on periphyton communities in Coastal water microcosms. Marine Pollution. Bulletin, 32, 342–345. doi:10.1016/0025-326X(96)84828-4
- Daly, G. L., Lei, Y. D., Teixeira, A., Muir, D. C. G., Castillo, L. E., & Wania, F. (2007). Accumulation of current use pesticides in neotropicalmontane forests. Environment Science Technology, 41, 1118–1123. doi:10.1021/es0622709
- DeLorenzo, M. E., & Serrano, L. (2003). Individual and mixture toxicity of three pesticides; atrazine, chlorpyrifos, and chlorothalonil to the marine phytoplankton species Dunaliella tertiolecta. Journal of Environmental Science and Health, Part B, 38(5), 529–538. doi:10.1081/PFC-120023511
- Durant, G., Limon, G., Arzul, G., Quiniou, F., Stachowski, S., De la Broise, D., & Hourmant, A. (2007). Impact des pesticides sur l’environnement Marin. Synthèse des résultats du projet IPEM.
- Hourmant, A., Patrick, P., Arzul, G., Durant, G., Hureau, D., Robic, A., … De la Broise, D. (2004). Influence of nutrients on the influence of nutrients on the phytotoxicity of bentazone in pure solution and formulation, to marine phytoplankton. Congrès SETAC Europe 14th Annual Meeting, 14-21 Avril 2004. Prague (République Tchèque).
- Houssou, A. M., Agadjihouédé, H., Montchowui, E., Bonou, C. A., & Lalèyè, P. (2015). Structure and seasonal dynamics of phytoplankton and zooplankton in Lake Azili, small Lake of the pond of River Ouémé, Benin. International Journal of Aquatic Biology, 3(3), 161–171.
- Houssou, A. M., Cocan, D., Bonou, C. A., Miresan, V., & Montchowui, E. (2018). Survival and reproduction of Cyclops abyssorum (freshwater copepod) exposed to spirotetramat and 2,4-D. Romanian Biotechnological Letters, 23(4), 13761–13770.
- Houssou, A. M., Daguégué, E. J., & Montchowui, E. (2017a). Lethal and sub-lethal effects of cypermethrin and glyphosate on the freshwater’s copepod, Acanthocyclops robustus. Invertebrate Survival Journal, 14, 140–148.
- Houssou, A. M., Montchowui, E., & Bonou, C. A. (2017b). Composition and structure of phytoplankton community in Ouémé River basin, Republic of Benin. International Journal of Aquatic Biology, 5(6), 413–424.
- Houssou, A. M., Yaovi, R., Ahouansou Montcho, S., Bonou, C. A., & Montchowui, E. (2016). Diversity and seasonal variation of zooplankton of Lake Hlan, Republic of Bénin (West Africa). Journal of Applied Biosciences, 102, 9723–9737.
- Institut National de l’Environnement Industriel et des Risques. (2012). Normes de qualité environnementale de 2,4-D (dont sels et esters de 2,4-D) n°CAS: 94-75-7.
- Laisne, C., Lassus, M., Arzul, G., Quiniou, F., Hureau, D., & Durant, G. (2004). Effet des pesticides sur la taille cellulaire du phytoplancton marin. 21e Forum des jeunes océanographes-1er Forum européen des jeunes océanographes, 13-14 Mai 2004. 06230 Villefranche/mer.
- Liu, S. S., Wang, C. L., Zhang, J., Zhu, X. W., & Li, W. Y. (2013). Combined toxicity of pesticide mixtures on green algae and photobacteria. Ecotoxicology and Environmental Safety, 95, 98–103. doi:10.1016/j.ecoenv.2013.05.018
- Organisation for Economic Co-operation and Development (OECD). (2006). OECD GUIDELINES FOR THE TESTING OF CHEMICALS; freshwater alga and cyanobacteria, growth inhibition test. 26p. Available on https://www.oecd-ilibrary.org/docserver/9789264069923-en.pdf?expires=1534167113&id=id&accname=guest&checksum=846E5826CC1AD5EA8B49EB5660E3576C.
- Perez, G. L., Torremorell, A., Mugni, H., Rodriguez, P., Vera, M. S., Do Nascimento, M., … Zagarese, H. (2007). Effects of the herbicide roundup on freshwater microbial communities: A mesocosm study. Ecological Applications, 17, 2310–2322. doi:10.1890/07-0499.1
- Pest Management Regulatory Agency of Canada (PMRA). (2008). Projet de decision d’homologation du Spirotétramate Ottawa (Ontario) K1A 0K9 (134p).
- Pratt, J. R., Melendez, A. E., Barreiro, R., & Bowers, N. J. (1997). Predicting the ecological effects of herbicides. Ecological Applications, 7, 1117–1124. doi:10.1890/1051-0761(1997)007[1117:PTEEOH]2.0.CO;2
- Relyea, R. A. (2009). A cocktail of contaminants: How mixtures of pesticides at low concentrations affect aquatic communities. Community Ecology, 159, 363–376.
- Relyea, R. A., & Hoverman, J. T. (2006). Assessing the ecology in ecotoxicology: A review and synthesis in freshwater systems. Ecology Letters, 9, 1157–1171. doi:10.1111/j.1461-0248.2006.00966.x
- Ritchie, R. J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynthesis Research, 89, 27–41. doi:10.1007/s11120-006-9065-9
- Rohr, J. R., & Crumrine, P. W. (2005). Effects of an herbicide and an insecticide on pond community structure and processes. Ecological Applications, 15, 1135–1147. doi:10.1890/03-5353
- Sabater, C., & Carrasco, J. M. (2001a). Effects of the organophosphorus insecticide fenitrothion on growth in five freshwater species of phytoplankton. Environmental Toxicology, 16(4), 314–320. doi:10.1002/(ISSN)1522-7278
- Sabater, C., & Carrasco, J. M. (2001b). Effects of pyridaphenthion on growth of five freshwater species of phytoplankton. A laboratory study. Chemosphere, 44(8), 1775–1781. doi:10.1016/S0045-6535(00)00575-0
- Seguin, F., Druart, J. C., & Le Cohu, R. (2001). Effects of atrazine and nicosulfuron on periphytic diatom communities in freshwater outdoor lentic mesocosms. International Journal of Limnology, 37(1), 3–8. doi:10.1051/limn/2001004
- Stachowski-Haberkorn, S. (2008). Méthodes d’évaluation de l’impact de pesticides sur le phytoplancton marin et le naissain d’huître creuse. Thèse de doctorat de l’université de Bretagne occidentale. 187p.
- Van den Brink, P., Hartgers, E. M., Gylstra, R., Bransen, F., & Brock, T. C. M. (2002). Effet of Mixture of two insecticides in freshwater Microcosms: II. Responses of Plankton and Ecological Assessment. Kluwer Academic Publichers. Ecotoxicologie, 11, 181–197.
