?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The main objective of this study was to assess the current pollution status of environmentally concerned heavy metals in the surface soil within the vicinity of the Katima Mulilo urban open land wastewater disposal centre. Multiple top soil samples (at 0–10 cm depth) were collected at four different points fortnightly over five sampling days from June to July 2018. The samples collected at each point were pooled together, homogenized and 12 sub-samples (<2 mm soil fraction) were obtained for laboratory digestion according to EPA method 3050B. Then, the digestates were analyzed for the heavy metals’ concentrations using ICP-OES (Perkin Elmer Optima 7000 DV). The results revealed consistent pattern with Fe recording the highest mean concentration (mg/kg) at each sampling point while Cd recorded the lowest mean concentration. Result of analysis of variance of the metals’ mean concentrations differed statistically (p < 0.05). The sites contamination revealed that the Point source > A200m > B400m > Control site, but the potential ecological risk indices revealed environmental low-risk levels (Er <40). Apart from Arsenic which showed moderate contamination at the point source, the geo-accumulation indices of the heavy metals mainly revealed uncontaminated to moderately contaminated levels (0 < Igeo < 1). Generally, the mean concentrations of the heavy metals were lower than the WHO’s maximum permissible limits for the protection of human and ecosystem’s health. However, due to their environmental persistence, non-degradable and bio-accumulative characteristics, heavy metals are potential toxins. Therefore, we recommend periodic monitoring of the metals levels and advising precautionary measures to minimize unintended human exposures to excessive metal contents.
PUBLIC INTEREST STATEMENT
Owing to the capabilities of several heavy metals to cause both acute and chronic toxicities, their presence at any level in the human ecosystem constitutes safety concern. This study reported the current pollution status of environmentally concerned heavy metals in the surface soils within the vicinity of the Katima Mulilo urban open land wastewater disposal centre. Katima Mulilo urban has recently witnessed exponential infrastructural development leading to increasing presence of human activities in the vicinity of the urban wastewater disposal centre. This is creating increasing safety concern, especially for the vulnerable children. Despite this obvious concern, no study has documented the heavy metals pollution status of the immediate environment of the wastewater disposal centre. Thus, considering Namibia’s special interest in both environmental and human health, this is an important baseline study which provides reference data for future monitoring of heavy metals pollution of the study area and advising precautionary mitigation measures.
Competing Interests
The authors declare no competing interests.
1. Introduction
Due to the disturbance and acceleration of nature’s slowly occurring geochemical cycle of metals by man, most soils of rural and urban environments may accumulate one or more heavy metals above defined background values high enough to pose risks to human health, plants, animals, ecosystems, or other media (D’Amore, Al-Abed, Scheckel, & Ryan, Citation2005). In different studies on trace metals in soils and plants, the authors reported that heavy metals occur naturally in the soil environment from the pedogenetic processes of weathering of parent materials at levels that are regarded as trace (<1000 mg kg−1) and rarely toxic (Kabata-Pendias & Pendias, Citation2001; Pierzynski, Sims, & Vance, Citation2000). However, heavy metals essentially become contaminants in the soil environments because: (i) their rates of generation via man-made cycles are more relative to natural ones, (ii) they become transferred from point source to random environmental locations where higher potentials of direct exposure occur, (iii) the concentrations of the metals in discarded products are relatively high compared to those in the receiving environment, and (iv) the chemical form (species) in which a metal is found in the receiving environmental system may render it more bioavailable (D’Amore et al., Citation2005).
As noted by Li et al. (Citation2013), soils serve as the most important sink for heavy metal pollutants in the terrestrial ecosystems. High concentrations of heavy metals in surface soil can threaten human health via inhalation, ingestion and dermal contact absorption (Sun, Zhou, Xie, & Liu, Citation2010; Xie et al., Citation2011). Most metals do not undergo microbial or chemical degradation (Kirpichtchikova et al., Citation2006), and their total concentration in soils persists for a long time after their introduction (Adriano, Citation2003). According to Mapanda, Mangwayana, Nyamangara, and Giller (Citation2005), heavy metals can continue to contaminate the surrounding environment for hundreds or thousands of years, even after they are no longer being added to the soil. This kind of pollution not only degrades the quality of the food crops, atmosphere, and water, but also threatens the health of human and animals (Dong et al., Citation2011). Heavy metals can be harmful to the biota and human beings when present above certain tolerable levels in the ecosystem (Anietie & Labunmi, Citation2015).
Several research reports have shown that human activities are a major cause of heavy metals contamination in the ecosystem (Kasassi et al., Citation2008). Metal-bearing solids at contaminated sites could originate from a wide variety of anthropogenic sources such as metal mine tailings, disposal of high metal wastes in improperly protected landfills, leaded gasoline and lead-based paints, land application of fertilizer, animal manures, bio-solids (sewage sludge), compost, pesticides, coal combustion residues, petrochemicals, and atmospheric deposition (Basta, Ryan, & Chaney, Citation2005; Khan, Cao, Zheng, Huang, & Zhu, Citation2008; Zhang, Liu, & Wang, Citation2010).
Although heavy metals contamination does not explicitly damage the environment within a short period, however, when it exceeds the environmental tolerance, or when environmental conditions changed, it may be activated and cause serious ecological damage (Su, Jiang, & Zhang, Citation2014). Unlike other pollutants such as petroleum, hydrocarbons, and litters that visibly accumulate on soils, heavy metals can go unnoticed while accumulating at concentrations that are toxic to plants and animals (Taghipour, Mosaferi, Armanfar, & Gaemmagami, Citation2013). Each heavy metal shows specific signs of its toxicity; for instance, Pb, As, Hg, Zn, Cu, and Al poisoning have been implicated with gastrointestinal disorders, diarrhoea, stomatitis, tremor, hemoglobinuria, ataxia, paralysis, vomiting and convulsion, depression, and pneumonia (Taghipour et al., Citation2013). Some effects of heavy metals can be toxic (acute, chronic or sub-chronic), neurotoxic, or even carcinogenic, mutagenic or teratogenic (European Union, Citation2002; Singh et al. Citation2010). For example, excessive intake of Pb into human body can damage the nervous, skeletal, endocrine, enzymatic, circulatory, and immune system (Lin et al., Citation2016). The chronic effects of Cd consist of lung cancer, pulmonary adenocarcinomas, prostatic proliferative lesions, kidney dysfunction, bone fractures, and hypertension (Brevik et al., Citation2015).
In recent years, Katima Mulilo urban has witnessed tremendous expansion of human, infrastructural and economic activities with the resultant increase in wastewater generation. Unfortunately, the town has no capacity and facilities for wastewater treatment and segregation before disposal and thus, all wastewaters collected from the residential areas, schools, hospitals, hotels, industries, abattoir, urban storm runoff, office and business establishments in the area are deposited into one central open land system. These may have unintended effects on both the types and contents of heavy metals in the immediate vicinity. More also in recent years, the infrastructural development of Katima Mulilo urban has led to the increasing presence of different human activities in the vicinity of the wastewater disposal centre. Despite this, no documented study has been done to establish the heavy metals pollution status of the immediate environment of the open land wastewater disposal centre. Thus, this study has the main objective of assessing the current pollution status of environmentally concerned heavy metals in the surface soils within the vicinity of the Katima Mulilo urban open land wastewater disposal centre and compare the values with health regulatory guideline limits. This is a baseline study which provided important reference data for future monitoring of the impact of the open land wastewater disposal centre on the heavy metals content of the immediate surrounding environment and hence, useful in advising precautionary measures that could limit unintended exposure of the human populace to metal pollutants in the area.
2. Materials and methods
2.1. Study area
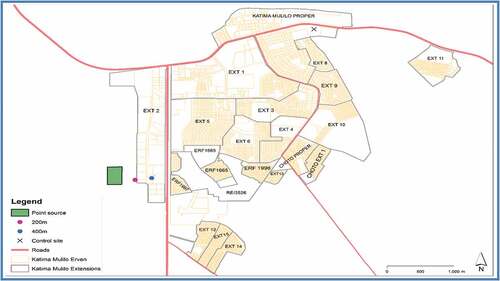
The area of the study is the Katima Mulilo urban open land wastewater disposal centre and the immediate vicinity. Katima Mulilo urban is located on latitude 17°50ʹS and longitude 24°25ʹE based on Google data of geographical coordinates (WGS84) reference system. The open land wastewater disposal centre is the central collection point for all the wastewaters generated from residential, office, institution and business establishments in the Katima Mulilo urban. Based on the information available in the 2011 Population and Housing Census Regional Profile of Zambezi Region, Katima Mulilo urban has an estimated population of 28,362 inhabitants, an annual growth rate of 1.3% and a population density of 6.2 persons per sq. km. The recent expansions of residential, office, institutions and business establishments in the Katima Mulilo urban have overstretched the capacity of the open land wastewater disposal centre. This is more worrisome owing to the fact there are now increasing presence of informal settlements and cottage industries within the periphery of the wastewater disposal centre and the town has no facilities for wastewater;/ treatment and segregation before disposal. Thus, the immediate environment might be affected by trans-boundary pollution owing to emission of several chemical pollutants such as heavy metals from the wastewater disposal centre. Figure shows the map of the study area showing the sampling points.
2.2. Sample collection and pre-treatment
Top soil samples (at depth of 0–10 cm) were collected on five different days with each sampling day occurring every fortnight from June to July 2018. On each sampling day, three soil samples each were randomly collected at four different points: Point source (Katima Mulilo urban open land wastewater disposal centre), A200m away from point source, B400m away from point source and control site. The chosen control site was a remote environment located 7.5 km north of the wastewater disposal centre. All samples were collected during the still morning weather (between 6:00 − 8:00) and packed in pre-labelled polyethylene bags (Abah, Mashebe, Onjefu & Malu, Citation2015).
The total samples collected at each sampling point were pooled together and mixed thoroughly to ensure homogeneity. Then, three representative samples were taken and filtered through < 2 mm stainless steel sieve (Abah et al., Citation2015). Thus, for the four different samplings points, 12 sub-samples were obtained and conveyed to Analytical Laboratory Services, Windhoek Namibia for further processing and analyses for the levels of heavy metals. All materials used for holding samples, homogenization and sieving were pre-cleaned to minimize the potential of cross-contamination (Abah et al., 2015).
2.3. Samples digestion and analysis
The soil samples were digested according to EPA method 3050B for Inductively Coupled Plasma-Optical Emission Spectrophotometer (ICP-OES) analysis (Abah, Mashebe, & Onjefu, Citation2014). A known amount (1.00g) of each sieved soil was transferred into a digestion vessel and 10 mL of 1:1 nitric acid (HNO3) was added, mixed thoroughly and covered with a watch glass (EPA, Citation1996). Then, the samples were heated to 90°C and refluxed at this temperature for 10 minutes after which they were allowed to cool for 5 minutes under room temperature. Thereafter, 5 mL of concentrated HNO3 was added to each, covered and refluxed again at 90°C for 30 minutes (EPA, Citation1996). Then, the solutions were allowed to evaporate without boiling to approximately 5 mL each and cooled again for 5 minutes. This was followed by the addition of 2 mL of deionised water plus 3 mL of 30% hydrogen peroxide (H2O2) to each. The vessels were covered with watch glasses and heated just enough to warm the solutions for the peroxide reaction to start (EPA, Citation1996). This was continued until effervescence subsided and the solutions were cooled. The acid-peroxide digestates were covered with watch glasses and heated until the volume reduced to approximately 5 mL again. Then, 10 mL of concentrated hydrochloric acid (HCl) was added to each, covered and heated on a heating mantle, then refluxed at 90°C for 15 minutes. After cooling, each digestate was filtered through Whatman No. 41 filter paper into a 100 mL volumetric flask and the volume made up to the mark with deionised water (EPA, Citation1996).
Ten (10) mL of each digestate was taken and mixed with equal volume of matrix modifier (EPA, Citation1996). Then, they were analyzed using ICP-OES (ICP: Perkin Elmer Optima 7000 DV) for the levels of lead (Pb), cadmium (Cd), chromium (Cr), arsenic (As), cobalt (Co), nickel (Ni), vanadium (V), copper (Cu), zinc (Zn), Iron (Fe) and manganese (Mn).
2.4. Data analysis
Descriptive statistics was used to analyze the mean of the data obtained from five replicate analyses of the samples. Furthermore, inter-elemental correlation analysis was performed to determine the degree of association between the heavy metals in the soil samples from the four sampling points: Point source (wastewater disposal centre), Point A (200 m), Point B (400 m) and the control site.
2.5. Assessment of site contamination
The heavy metals concentrations recorded in the soil samples were first, compared with their corresponding Maximum Permissible Concentrations (MPC) which are widely used as regulatory guideline limits based on which informed decision about the site’s quality was made. The MPC is the concentration of a substance in air, water, soil or sediment that should protect all species in ecosystems from adverse effects of that substance (Janssen, Traas, Rila, & van Vlaardingen, Citation2004).
Further assessment of the site’s contamination was done using the contamination factor (Cf), which expresses the single element pollution index in order to determine the individual contribution of the heavy metals to the site’s pollution (Abah et al., Citation2015), degree of contamination (Cd), aimed at providing a measure of the degree of overall contamination in the surface soil layers at a particular sampling site (Rahman et al., Citation2012), ecological risk factor [Er] (Hakanson, Citation1980), used here to provide an indicator of each element’s ecological risk index, and potential ecological risk factors (Perf), which give insight into the heavy metals toxicity and environment response (Hakanson, Citation1980). The potential ecological risk factor does not only consider the heavy metal level in the soil, but also associate ecological and environmental effects with toxicology, and evaluates pollution using comparable and equivalent property index grading method (Qui, Citation2010). Also used in the site contamination assessment is the index of geo-accumulation. Each of these assessment criteria was calculated using the following equations:
where Cn in Equations (1) and (Equation5(5)
(5) ) refers to the concentration of a particular metal element in the surface soil, PMC in equation 1 is the soil permissible maximum concentration of a particular metal element, Tr in EquationEquation (3)
(3)
(3) refers to the toxic response factor of the heavy metal (Hakanson, Citation1980), Bn in EquationEquation (5)
(5)
(5) is the geochemical background value, and 1.5 is a constant which allows us to analyze the natural fluctuations in the content of each metal in the surface soil and to detect very small anthropogenic influence (Barbieri, Citation2016).
3. Results and discussion
3.1. Mean concentrations of the heavy metals in the surface soil
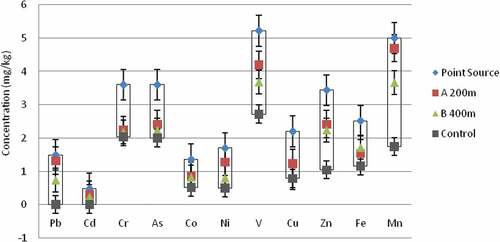
The results in Figure present the mean concentrations of lead (Pb), cadmium (Cd), chromium (Cr), arsenic (As), cobalt (Co), nickel (Ni), vanadium (V), copper (Cu), zinc (Zn), Iron (Fe) and manganese (Mn) determined in the surface soil samples collected in the vicinity of the Katima Mulilo urban open land wastewater disposal centre. The results showed that at the point source (within the open land wastewater disposal centre), Fe recorded the highest mean concentration of 2,516.00 mg/kg while Cd recorded the lowest mean concentration of 0.48 mg/kg. Within the same sampling points, the other results showed that Cr and As recorded 3.60 mg/kg each, Co recorded 1.36 mg/kg, Ni recorded 1.70 mg/kg, V recorded 5.22 mg/kg while Cu, Zn and Mn recorded 2.20 mg/kg, 3.44 mg/kg, and 50.00 mg/kg, respectively.
At the sampling point located 200 m away from the open land wastewater disposal centre, Fe also recorded the highest mean concentration of 1537.00 mg/kg while Cd still recorded the lowest mean concentration of 0.30 mg/kg. The other results obtained within this sampling point showed that Cr recorded 2.24 mg/kg, As recorded 2.42 mg/kg, Co recorded 0.86 mg/kg, Ni recorded 1.28 mg/kg, V recorded 4.20 mg/kg while Cu, Zn and Mn recorded 1.24 mg/kg, 2.42 mg/kg, and 47.00 mg/kg, respectively.
At the sampling point located 400 m away from the open land wastewater disposal centre, the results showed similar pattern to the ones earlier reported. Fe still recorded the highest mean concentration of 1715.00 mg/kg while Cd recorded the lowest mean concentration of 0.24 mg/kg.
The other results obtained within this sampling point showed that Cr recorded 2.18 mg/kg, As recorded 2.24 mg/kg, Co recorded 0.82 mg/kg, Ni and Cu recorded 0.80 mg/kg each, while V, Zn and Mn recorded 3.68 mg/kg, 2.24 mg/kg, and 36.60 mg/kg, respectively.
At the control site sampling point located 7.5 km north of the open land wastewater disposal centre, Pb and Cd were not detected in the surface soil samples collected. However, Fe still recorded the highest mean concentration of 1160.00 mg/kg while Ni (0.50 mg/kg) was the lowest mean concentration recorded. The other results obtained at this sampling point showed that Cr recorded 2.04 mg/kg, As recorded 2.00 mg/kg, Co recorded 0.52 mg/kg, Ni and Cu recorded 0.80 mg/kg each, while Cu, Zn and Mn recorded 0.78 mg/kg, 1.04 mg/kg, and 17.40 mg/kg, respectively. Generally, the concentrations of the heavy metals recorded across the sampling sites were lower than their corresponding WHO’s recommended maximum permissible concentrations in soil (Table ). However, as reported by Abah, Mashebe, and Onjefu (Citation2017), the presence of heavy metals in soils, especially where humans and livestock traverse intensively constitutes health concern due to frequent exposure to the contaminant-bearing dusts emanating from the soil. Heavy metal contamination of soil may pose risks and hazards to humans and the ecosystem through: direct ingestion or contact with contaminated soil, the food chain (soil-plant-human or soil-plant-animal-human), drinking of contaminated ground water, reduction in food quality (safety and marketability), reduction in land usability for agricultural production causing food insecurity, and land tenure problems (Ling, Shen, Gao, Gu, & Yang, Citation2007; McLaughlin, Hamon, McLaren, Speir, & Rogers, Citation2000). Pollutants entering soil interact with its active phase (clay minerals, oxides and hydroxides of iron and manganese, and organic substance) and change their own activity either increasing or decreasing their hazard (Vodyanitskii, Citation2016). The build-up of hazardous chemical pollutant such as heavy metals constitutes a major safety concern due to metal’s known non-degradable characteristics.
Table 1. Standard criteria used for the assessment of the heavy metals pollution
The result of analysis of variance (ANOVA) between the mean heavy metals concentrations in the surface soil at the different sampling sites (Table ) was statistically significant (P < 0.05). This result might be due to varying degrees of metal inputs from anthropogenically derived sources across the sampling points (Abah et al., Citation2017). However, as identified by Long et al. (Citation2002), irrespective of the origin of heavy metals in soils, excessive levels of many metals can result in soil quality degradation as well as posing significant hazards to plants, humans, animals, and ecosystem’s health. Over the last three decades, there has been increasing global concern over the public health impacts attributed to environmental pollution (Kimani, Citation2007). Environmental pollution is the contamination of the physical, chemical and biological components of the earth system to such an extent that normal environmental processes are adversely affected (Musoke, Ndejjo, Atusingwize, & Halage, Citation2016). Apart from directly affecting human’s health, especially among children who are the most vulnerable group, heavy metals in urban soils also affect environmental qualities and damage human health indirectly through polluting the food, water and atmosphere (Chao, LiQin, & WenJun, Citation2014). This is even as research report has indicated that environmental pollution data tend to vary extensively and to be subjected to various types of uncertainties due to several factors such as distance from pollution sources and pathways, natural background variation, and pollution build-up or accumulation over time (Rashad & Shalaby, Citation2007). According to the World Health Organization’s report, about a quarter of the diseases facing mankind today occur due to prolonged exposure to environmental pollution (Kimani, Citation2007). Of particular concern, heavy metals toxicity in the human ecosystem has been identified as a clinically significant condition (Ferner, Citation2001), and if unrecognized or inappropriately treated, the toxicity can result in significant illness and reduced quality of life (Amirah, Afiza, Faizal, Nurliyana, & Laili, Citation2013). Thus, for the protection of human and ecosystem’s health from unintended exposure to heavy metal pollution, it is important to evaluate and document the presence and sources of heavy metals in every environmental component that affects human health (Abah et al., Citation2017).
Table 2. ANOVA single factor between the mean concentration of the heavy metals in the surface soil at the different sampling sites
3.2. Contamination factors of the heavy metals in the surface soil
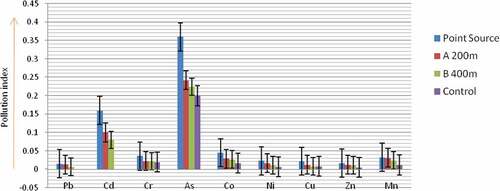
Figure shows the contamination factors of the heavy metals determined in the surface soil samples. The trend of the results revealed that As> Cd > Co > Cr >Mn> Ni > Cu > Zn >Pb. Higher contamination indices were recorded for As (0.20 to 0.36) and Cd (0.08 to 0.16) in the surface soil (0–10 cm depth). This finding suggests that the surface soil zone could accumulate the metals to levels high enough to threaten the ecosystem’s health after prolonged period. Because heavy metal contamination is colourless and odourless, it cannot be noticed easily (Chao et al., Citation2014). Moreover, it does not explicitly damage the environment in a short period but when it exceeds the environmental tolerance, heavy metals in the soil may be activated and cause serious ecological damage (Chao et al., Citation2014). This is the more reason why Wood (Citation1974),described heavy metal contamination as chemical Time Bombs (CTBs). Moreover, soils have been identified as the major sink for heavy metals released into the environment by anthropogenic activities (Wuana & Okieimen, Citation2011). Unlike organic contaminants which are oxidized to carbon (IV) oxide by microbial action, most metals do not undergo microbial or chemical degradation (Kirpichtchikova et al., Citation2006) and hence, accumulate in the environment (Abah et al., Citation2017).
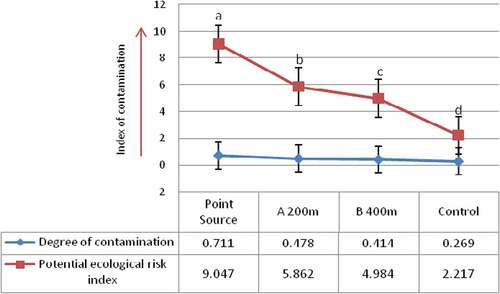
The degree of heavy metals contamination of the sampling sites (Figure ) revealed that the Point source > A 200 m > B 400 m > Control site. This trend is not surprising because the Point source is the Katima Mulilo urban open land wastewaters collection centre where diverse wastewaters generated from residential, business, school, office and industrial set ups are discharged directly without pre-treatment/segregation. Thus, the heavy metal residues discharged in such wastewaters will accumulate more at the point source.
Figure 4. Degree of contamination and potential ecological risk indices of the heavy metals at the sampling sites
In a review study on heavy metals contamination in the soil worldwide, Chao et al. (Citation2014) noted that with the development of the global economy, both type and content of heavy metals in the soil due to anthropogenic activities have gradually increased in recent years, which have resulted in serious environment deterioration. In addition, changes in demographics, water use patterns, economic status, public health issues and product diversity increase the range and concentration of novel and critical compounds in wastewater (Tjadraatmadja & Diaper, Citation2006), and this could impact meaningfully on the quality of receiving soil and its environs especially in the case of Katima Mulilo urban open land sewage collection centre. As noted by Tjadraatmadja and Diaper (Citation2006), the major input streams that characterise wastewater flows and quality include domestic wastewater from residential areas, small business and commercial dischargers such as health clinics, food establishments and other enterprises, trade waste which includes small, medium and large industries as well as infiltration and inflow which include contribution of groundwater infiltration and storm water inflow into the wastewater discharge system during dry and rainwater events. All these rightly constitute the major sources of wastewater generation in the study area. Even the study on the effect of polluted water on soil and plant contamination by heavy metals in El-Mahla El-Kobra, Egypt the authors reported that discharged water contains high levels of contaminants considered hazardous to the ecosystem (Mahmoud & Ghoneim, Citation2016). Thus, efforts such as frequent monitoring of the levels and sources of the heavy metals’ influx into the environment have become necessary preconditions for the protection of human and ecosystem’s health in the study area.
3.3. Ecological risk factors of the heavy metals
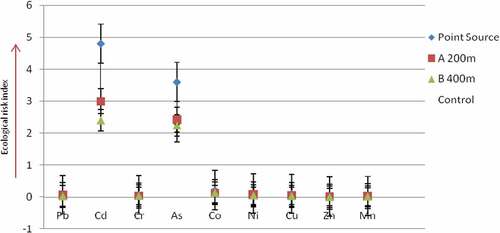
The results of ecological risk indices of the heavy metals (Figure ) showed consistent trend across the sampling points. The results obtained showed the following order of the metals’ ecological risk indices: Cd >As > Co > Ni > Cu >Pb> Cr >Mn> Zn. However, there is a general decrease in the value of a particular metal’s ecological risk index from the point source (Katima Mulilo urban open land wastewater collection centre) outward. For example, among Cd and As which present the highest ecological risk factor, their indices from the point source (PS), outward were Cd (4.8 at PS, 3.0 at point A 200 m away and 2.4 at point B 400 m away from PS), and As (3.6 at PS, 2.42 at point A 200 m away and 2.24 at point B 400 m away from PS). Based on the standard criteria for interpreting ecological risk factors (Table ), the present results generally suggest low ecological risk [Er< 40] (Hakanson, Citation1980) of the heavy metals across the sampling sites. The potential ecological risk indices (PEri) of the heavy metals across the sampling sites (Figure ) revealed similar pattern to the site’s contamination factor with the Point source > A 200 m > B 400 m > Control site. At the Point source, the risk index was 9.047 while at the sampling point A (200 m away from the point source), point B (400 m away from the point source) and control site (7.5 km away from the point source), risk indices of 5.862, 4.984 and 2.217 were recorded, respectively. These results also differed significantly (t-test paired mean: P < 0.05) between the sampling points. Based on the criteria for interpreting PEri of heavy metals in soils (Table ), all the present risk indices represent low level [PEri <150] (Hakanson, Citation1980). However, this finding does not preclude concern for the accumulation of the heavy metals in the soil since by their nature, metals are non-degradable and hence, environmentally persistent (Abah et al., Citation2017). In a study on the contamination and ecological risk assessment of heavy metals in surface soils of Esfarayen City Iran, Mohseni-Bandpei, Ashrafi, Kamani, and Paseban (Citation2016) held that contamination of urban surface soils with heavy metals is one of the worrying problems owing to their extensive causes, resistant to biodegradation, toxic and accumulative properties. Moreover, the presence of heavy metals in human environment has been associated with different adverse health effects in humans (Tchounwou, Yedjou, Patlolla, & Sutton, Citation2012). Even at low levels, some heavy metals such as cadmium and lead are dangerous to human health (Naghipour, Amouei, & Nazmara, Citation2014). For example, cadmium accumulation in human body causes malfunction of kidney, cancer and lead in body causes neurological disorders, anaemia and renal damage; and frequent presence of heavy metals in soil is reported as an indicator of the quality of the urban environment (Kamani, Hoseini, Safari, Jaafari, & Mahvi, Citation2014). Thus, the accumulative tendency and chronic effect of the heavy metals constitute environmental safety concern in the study area.
3.4. Geo-accumulation indices of the heavy metals
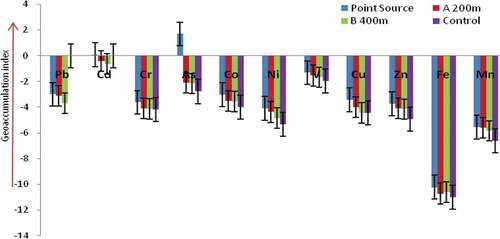
Figure presents the geo-accumulation indices (Igeo) of the heavy metals at the study site. The results obtained showed that at the point source—the Katima Mulilo urban open land wastewaters collection centre, As recorded the highest Igeo of 1.69. Based on class 2 category of the standard criteria for interpreting the Igeo of soil heavy metals (1< Igeo<2) (Barbieri, Citation2016), the surface soil at the point source is moderately contaminated with respect to As accumulation. The other results of the heavy metals’ Igeo at the point source revealed uncontaminated to moderately contaminated classification (0 < Igeo<1). At the sampling point A, 200 m away from the point source, Pb (−3.12), Cd (−0.41), Cr (−4.10), As (−2.09), Co (−3.50), Ni (−4.38), V (−1.54), Cu (−4.00), Zn (−4.08), Fe (−10.71) and Mn (−5.60), respectively, fall within the uncontaminated to moderately contaminated classification. The Igeo results for these metals at the sampling point B, 400 m away from the point source were Pb (−3.70), Cd (−0.63), Cr (−4.13), As (−2.16), Co (−3.55), Ni (−4.85), V (−1.67), Cu (−4.44), Zn (−4.15), Fe (−10.60) and Mn (−5.83) while at the control site the Igeo indices were Pb (−0.00), Cd (−0.00), Cr (−4.19), As (−2.78), Co (−4.00), Ni (−5.32), V (−1.97), Cu (−4.46), Zn (−4.92), Fe (−11.00) and Mn (−6.60). These values also fall within the uncontaminated to moderately contaminated classification. The Igeo of metals is one of the indices which identify numerically, the pollution level of soils because it represents the real bio-available fraction (Barbieri, Citation2016). According to this author, the bio-available metal content in soil exerts a decisive impact on soil quality (Barbieri, Citation2016). Thus, due to the expansion of human activities which are now encroaching into the vicinity of the open land sewage collection centre, the accumulation of these toxic metals in the surface soil post environment risk to the people operating within the sampling points due to possibility of inhaling and absorbing the contaminant-bearing dust emanating from the area.
3.5. Inter-elemental correlation between the heavy metals
The results (in Table ) show the inter-elemental correlation analysis between the heavy metals recorded across the sampling sites. Based on the guidance for interpreting the sizes of correlation coefficients proposed by Mukaka (Citation2012), the result obtained mostly revealed very high positive correlation (r > 0.9) and high positive correlation (r = 0.7 to 0.9). Only the correlation coefficients between Pb and Cr (r = 0.6839) and between Cr and Mn (r = 0.6463) fall to moderate positive classification. However, the positive inter-elemental correlations generally suggest that the heavy metals recorded at the sampling sites may have common sources of anthropogenic inputs (Salah et al., Citation2012). This is very likely since the point source (Katima Mulilo urban open land wastewater collection centre) is located within 200 m and 400 m of the sampling sites A and B, respectively. Due to the sloping topography of the open land wastewater collection centre, it is possible for trans-boundary transfer and deposition of metal particulates via surface overflow onto the adjacent areas.
Table 3. Inter-elemental correlation analysis between the heavy metals at the sampling sites
4. Conclusion
The laboratory analysis results of this study recorded varying concentrations of the following heavy metals: lead, cadmium, chromium, arsenic, cobalt, nickel, vanadium, copper, zinc, Iron, and manganese in the surface soil samples collected across the four-sampling points. At all the sampling areas, iron recorded the highest mean concentration while Cd recorded the least. Result of the analysis of variance between the heavy metals’ concentrations in the surface soil samples was statistically significant (p < 0.05). The inter-elemental correlation coefficients of the heavy metals mainly revealed very high positive correlation (r > 0.9) and high positive correlation (r = 0.7 to 0.9). These generally suggest common source of anthropogenic inputs of the heavy metals recorded at the study area. The assessment of the sampling sites’ contamination using different pollution assessment criteria revealed the following order: Point source > A200m > B400m > Control site. However, the current results of the potential ecological risk indices revealed environmental low-risk levels (Er <40). Additionally, the geo-accumulation indices of the heavy metals revealed uncontaminated to moderately contaminated levels, the point source—the Katima Mulilo urban open land wastewater disposal centre being the most contaminated. Although, the mean concentrations of the heavy metals recorded across the sampling sites were generally lower than their corresponding maximum permissible limits recommended by WHO for the protection of human and ecosystem’s health, the presence of the heavy metals in surface soils where humans and livestock traverse frequently constitutes health risk. Therefore, we recommend periodic monitoring of the heavy metals in the study area and advice precautionary measures to limit unintended human exposures to excessive metal contents. In addition, there is need for further study to determine the relative contributions of the different sources of wastewater to the heavy metals pollution load of the study area and devise segregation/pre-treatment measures to separate critical pollutants prior to disposal.
Acknowledgements
The authors greatly acknowledged the financial support provided by the University of Namibia, Katima Mulilo Campus to undertake this study under the campus' 2014 - 2018 Research Agenda project #3. We are also thankful to the Katima Mulilo Town Council for the approval granted for the study on the open land wastewater disposal centre. We thank the Analytical Services Laboratory Windhoek Namibia, for the laboratory preparations and analyses of the samples. Finally, we thank Mr. M. S. Lukubwe of the University of Namibia, Katima Mulilo campus for his assistance in plotting the map of the study area using the GPS coordinates of the sampling locations. God bless.
Additional information
Funding
Notes on contributors
Abah James
James Abah is a core analytical chemist and holds a Bachelor of Science (Honours), Chemistry of the Benue State University, Makurdi Nigeria, Master of Science - Analytical Chemistry and Doctor of Philosophy - Analytical Chemistry, both of the University of Maiduguri, Nigeria. He is a member of the Chemical Society of Nigeria (CSN) and the Institute of Chartered Chemists of Nigeria (ICCON). His research interests include environmental pollution and remediation studies as well as the impact of agrochemicals usages on soils and crops. In these areas, James Abah has done extensive research works and published widely in international reputable refereed journals. Specifically, the current study assessed the pollution status of environmentally concerned heavy metals in the surface soil within the vicinity of the Katima Mulilo urban open land wastewater disposal centre and provided important baseline reference data for future monitoring as well as advising precautionary mitigation measures in the study area.
References
- Abah, J., Mashebe, P., & Onjefu, S. A. (2014). Survey of the levels of some heavy metals in roadside dusts along KatimaMulilo urban road construction, Namibia. American Journal of Environmental Protection, 3, 19–16. doi:10.11648/j.ajep.20140301.13
- Abah, J., Mashebe, P., & Onjefu, S. A. (2017). Assessment of heavy metals pollution status of the pasture grass around KatimaMulilo municipal solid wastes dumpsite, Namibia. International Journal of Environmental Science and Development, 8(5), 372–377. doi:10.18178/ijesd.2017.8.5.980
- Abah, J., Mashebe, P., Onjefu, S. A., & Malu, S. P. (2015). Assessment of the in-situ concentrations of some heavy metals in surface soil dusts at the KatimaMulilo urban waste dumpsite, Namibia. International Journal of Advanced Scientific and Technical Research, 4, 88–100.
- Adriano, D. C. (2003). Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metals (2nd ed.). New York, NY: Springer.
- Amirah, M. N., Afiza, A. S., Faizal, W. I. W., Nurliyana, M. H., & Laili, S. (2013). Human health risk assessment of metal contamination through consumption of fish. Journal of Environment Pollution and Human Health, 1(1), 1–5.
- Anietie, O. V., & Labunmi, L. (2015). Surface soil pollution by heavy metals: A case study of two refuse dumpsites in Akure metropolis. International Journal of Scientific and Research, 4(3), 71–74.
- Barbieri, M. (2016). The importance of enrichment factor (EF) and geoaccumulation index (Igeo) to evaluate the soil contamination. Journal of Geology & Geophysics, 5, 237. doi:10.4172/2381-8719.1000237
- Basta, N. T., Ryan, J. A., & Chaney, R. L. (2005). Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. Journal of Environmental Quality, 34(1), 49–63. doi:10.2134/jeq2005.0049dup
- Brevik, E. C., Cerdà, A., Mataix-Solera, J., Pereg, L., Quinton, J. N., Six, J., & Van Oost, K. (2015). The interdisciplinary nature of SOIL.Soil, 1, 117–129. doi:10.5194/soil-1-117-2015
- Chao, S., LiQin, J., & WenJun, Z. (2014). A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environmental Skeptics and Critics, 3(2), 24–38.
- D’Amore, J. J., Al-Abed, S. R., Scheckel, K. G., & Ryan, J. A. (2005). Methods for speciation of metals in soils: A review. Journal of Environmental Quality, 34(5), 1707–1745. doi:10.2134/jeq2004.0014
- Dong, J., Yang, Q. W., Sun, L. N., Zeng, Q., Liu, S. J., & Pan, J. (2011). Assessing the concentration and potential dietary risk of heavy metals in vegetables at a Pb/Zn mine site, China. Environmental Earth Sciences, 64, 1317–1321. doi:10.1007/s12665-011-0992-1
- European Union. (2002). Heavy metals in wastes, European commission on environment. Retrieved from http://ec.europa.eu/environment/waste/studies/pdf/heavymetalsreport.pdf
- Ferner, D. J. (2001). Toxicity, heavy metals. eMedicine Journal, 2(5), 1.
- Hakanson, L. (1980). An ecological risk index for aquatic pollution control: A sedimentological approach. Water Research, 14, 975–1001. doi:10.1016/0043-1354(80)90143-8
- Janssen, M. P., Traas, T. P., Rila, J. P., & van Vlaardingen, P. L. A. (2004). Guidance for deriving dutch environmental risk limits from EU-risk assessment reports of existing substances. RIVM Report601501020/2004. Retrieved from https://rivm.openrepository.com/rivm/bitstream/10029/16457/1/601782001.pdf
- Jonathan, B. Y., Maina, H. M., & Maitera, O. N. (2016). Heavy metal pollution assessment in the sediments of Lake Chad, Nigeria sector. Bayero Journal of Pure and Applied Science, 9(1), 213–216. doi:10.4314/bajopas.v9i1.33
- Kabata-Pendias, A., & Pendias, H. (2001). Trace metals in soils and plants (2nd ed.). Boca Raton, FL: CRC Press.
- Kamani, H., Hoseini, M., Safari, G. H., Jaafari, J., & Mahvi, A. H. (2014). Study of trace elements in wet atmospheric precipitation in Tehran, Iran. Environmental Monitoring and Assessment, 186(8), PubMed:24718928, 5059–5067. doi: 10.1007/s10661-014-3759-9.
- Kasassi, A., Rakimbei, P., Karagiannidis, A., Zabaniotou, A., Tsiouvaras, K., Nastis, A., & Tzafeiropoulou, K. (2008). Soil contamination by heavy metals: Measurements from a closed unlined landfill. Bioresource Technology, 99, 8578–8584. doi:10.1016/j.biortech.2008.04.010
- Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z., & Zhu, Y. G. (2008). Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environmental Pollution, 152(3), 686–692. doi:10.1016/j.envpol.2007.06.056
- Kimani, N. G. (2007). Environmental pollution and impact to public health; implication of the dandora municipal dumping site in Nairobi. Kenya: United Nations Environment Programme.
- Kirpichtchikova, T. A., Manceau, A., Spadini, L., Panfili, F., Marcus, M. A., & Jacquet, T. (2006). Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochimica Et Cosmochimica Acta, 70(9), 2163–2190. doi:10.1016/j.gca.2006.02.006
- Li, X. Y., Liu, L. J., Wang, Y. G., Luo, G. P., Chen, X., Yang, X. L., … He, X. (2013). Heavy metal contamination of urban soil in an old industrial city (Shenyang) in Northeast China. Geoderma, 192, 50–58. doi:10.1016/j.geoderma.2012.08.011
- Lin, Y., Zhuang, L. Z., Ma, H., Wu, L. X., Huang, H. L., & Guo, H. H. (2016). Study on congenital cardiac anomalies induced by arsenic exposure before and during maternal pregnancy in fetal rats. Journal of Hygiene Research, 45, 93–97.
- Ling, W. Q., Shen, Y., Gao, X., Gu, Z., & Yang, Z. (2007). Use of bentonite to control the release of copper from contaminated soils. Australian Journal of Soil Research, 45(8), 618–623. doi:10.1071/SR07079
- Long, X. X., Yang, X. E., & Ni, W. Z. (2002). Current status and prospective on phytoremediation of heavy metal polluted soils. J Appl Ecol, 13, 757–62.
- Mahmoud, M. K., & Ghoneim, A. M. (2016). Effect of polluted water on soil and plant contamination by heavy metals in El-Mahla El-Kobra. Egypt.Solid Earth, 7, 703–711. doi:10.5194/se-7-703-
- Mapanda, F., Mangwayana, E. N., Nyamangara, J., & Giller, K. E. (2005). The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agriculture, Ecosystems and Environment, 107(2–3), 151–165. doi:10.1016/j.agee.2004.11.005
- McLaughlin, M. J., Hamon, R. E., McLaren, R. G., Speir, T. W., & Rogers, S. L. (2000). Review: A bioavailability-based rationale for controlling metal and metalloid contamination of agricultural land in Australia and New Zealand,”. Australian Journal of Soil Research, 38(6), 1037–1086. doi:10.1071/SR99128
- Mohseni-Bandpei, A., Ashrafi, S. D., Kamani, H., & Paseban, A. (2016). Contamination and ecological risk assessment of heavy metals in surface soils of Esfarayen city. Iran: Health Scope. doi:10.17795/jhealthscope-39703
- Mukaka, M. M. (2012). Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Medical Journal: the Journal of Medical Association of Malawi, 24(3), 69–71.
- Musoke, D., Ndejjo, R., Atusingwize, E., & Halage, A. A. (2016). The role of environmental health in one health: A Uganda perspective. One Health, 2, 157–160. Amsterdam, Netherlands. doi: 10.1016/j.onehlt.2016.10.003
- Naghipour, D., Amouei, A., & Nazmara, S. (2014). A comparative evaluation of heavy metals in the different breads in Iran: A case study of rasht city. Health Scope, 3(4), 181–185. doi:10.5812/jhealthscope.
- Pierzynski, G. M., Sims, J. T., & Vance, G. F. (2000). Soils and environmental quality (2nd ed.). London, UK: CRC Press.
- Qui, H. (2010). Studies on the potential ecological risk and homology correlation of heavy metal in the surface soil. JAS, 2, 194–201.
- Rahman, S. H., Khanam, D, Adyel, T. M., Islam, M. S., Mohammad Ahsan, M. A., & Akbor, M. A. (2012). Assessment of Heavy Metal Contamination of Agricultural Soil around Dhaka Export Processing Zone (Depz). Appl. Sci. 2, 584–601. Bangladesh: Implication of seasonal variation and indices. doi: 10.3390/app2030584
- Rashad, M., & Shalaby, E. A. (2007). Dispersion and deposition of heavy metals around two municipal solid waste (MSW) dumpsites, Alexandria, Egypt. American-Eurasian Journal of Agricultural & Environmental Sciences, 2(3), 204–212.
- Salah, E., Zaidan, T., & Al-Rawi, A. (2012). Assessment of heavy metals pollution in the sediments of euphrates river, Iraq. J Water Res Prot, 4, 1009–1023.
- Singh, A., Sharma, R. K., Agrawal, M., & Marshall, F. M. (2010). Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food ChemToxicol, 48(2), 611–619. doi:10.1016/j.fct.2009.11.041.
- Su, C., Jiang, L., & Zhang, W. (2014). A review on heavy metal contamination in the soil worldwide: Situation, impact and remediation techniques. Environmental Skeptics and Critics, 3(2), 24–38.
- Sun, Y., Zhou, Q., Xie, X., & Liu, R. (2010). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of Hazardous Materials, 174, 455–462. pmid:19825507. doi:10.1016/j.jhazmat.2009.09.074
- Taghipour, H., Mosaferi, M., Armanfar, F., & Gaemmagami, S. J. (2013). Heavy metals pollution in the soils of suburban areas in big cities: A case study. International Journal of Environmental Science and Technology, 10, 243–250. doi:10.1007/s13762-012-0143-6
- Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metals toxicity and the environment, EXS. Experientia Supplementum, 101, 133–164. doi:10.1007/978-3-7643-8340-4_6
- Tjadraatmadja, G., & Diaper, C. (2006). Sources of critical contaminants in domestic wastewater – A literature review. CSIRO: Water for a Healthy Country National Research Flagship. Australia.
- U.S. EPA. (1996). Method 3050B: Acid digestion of sediments, sludges, and soils, revision 2. Washington, DC: United States Environmental Protection Agency. Retrieved from https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils
- Vodyanitskii, A. Y. N. (2016). Standards for the contents of heavy metals in soils of some states. Annals of Agrarian Science, 14(3), 257–263. doi:10.1016/j.aasci.2016.08.011
- Wood, J. M. (1974). Biological cycles for toxic elements in the environment, science. 183, 1049–1052.
- Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology, 2011, 40264710.5402/2011/4.
- Xie, Y., Chen, T. B., Lei, M., Yang, J., Guo, Q. J., & Song, B. (2011). Spatial distribution of soil heavy metal pollution estimated by different interpolation methods: Accuracy and uncertainty analysis. pmid:20970158 Chemosphere, 82(3), 468–476. doi:10.1016/j.chemosphere.2010.09.053
- Zhang, M. K., Liu, Z. Y., & Wang, H. (2010). Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Communications in Soil Science and Plant Analysis, 41(7), 820–831. doi:10.1080/00103621003592341