?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Metal concentrations in water, sediments and gills and edible stomach muscle tissues of two fish species, African Sharptooth mud catfish (Clarias gariepinus) and the Nile Tilapia (Oreochromis niloticus) in Eleyele Lake, Nigeria, were assessed in the rainy season (April), mid-dry spell time (July) and dry period (November) in 2017 to evaluate the potential ecological risks they pose to aquatic organisms. The concentrations of Fe, Co, Pb, Ni and Zn were significantly (p < 0.05) high in water and sediments and in some cases exceeded Nigerian Standards for Drinking Water Quality and World Health Organization guideline values. Study results highlight no significant spatial variation of all metals in the water and sediment phases and tissues for both fish species in the lake. Significant temporal variation in metals noted in the water and sediment phases most likely relate to the seasonal heterogeneity of catchment anthropogenic sources such as domestic sewer and fertilisers and pesticides from farming and backyard aquacultural enterprises. High Co, Cd and Zn concentrations recorded in tissues of the ecologically dichotomous two fish species corroborate with high metal levels in water and sediments. All pollution indices detected metal contamination in sediments whereas Ni posed a serious ecological risk to the fish (and possibly the fish consumers) in the lake indicating that nutrient retention especially within sediments is central to the pollution dynamics of Eleyele Lake and must inform management of the system.
PUBLIC INTEREST STATEMENT
Peri-urban freshwater impoundments such as Eleyele Lake in Nigeria face anthropogenic heavy metal pollution thus necessitating assessment of heavy metal pollution in the aquatic chain for effective remedial action. We detected intense metal pollution in water, sediments and fish tissues. Since humans utilise water and fishery resources, this implies a direct threat to their health. This should serve as a warning for immediate remedial action to the policymakers, fishers and humans utilising the lake. At most the simplest form of remedial action is to avoid loading heavy metal-laden effluent into the lake and recycle as much as possible domestic, industrial and agricultural effluent before it reaches the lake. Currently, there is no evidence to dissuade consumption of fish from the lake; however, for probity sake if there can be alternative cleaner fish resources it would be prudent to utilise them concurrently with those from Eleyele Lake.
Competing Interests
The authors declare no competing interests.
1. Introduction
Artificial lakes located within their catchments tend to be highly degraded, polluted and have reduced water ecosystem services to sustain livelihoods and aquatic biodiversity (Rosenberg et al., Citation2000). Leeward location of lakes within their catchments exposes them to high levels of pollution especially in highly populated and industrialised urban and peri-urban areas (Bernot & Dodds, Citation2005; Wetzel, Citation2001). Most urban and peri-urban anthropogenic activities such as manufacturing and agricultural industries discharge highly polluted effluents which pose a hazard to aquatic systems (Magadza, Citation2003; Thornton, Citation1990). Among such effluents are highly toxic, persistent and non-biodegradable heavy metals and radicals which tend to be cumulative within the aquatic ecosystem (Alloway, Citation1990). As most metals become toxic at elevated levels constant monitoring is vital to maintain the integrity of freshwater ecosystems (Liu et al., Citation2013).
The multipurpose Eleyele Lake, located in the densely populated and highly urbanised and industrialised metropolitan portion of Ibadan City in south-western Nigeria is degraded (Olanrewaju et al., Citation2017). The reservoir was constructed primarily to supply domestic water to Ibadan City through the Oyo State Water Corporation (Akinyemi et al., Citation2014). However, other multiple uses including artisanal fishery development, fish hatching and spawning refugia, flood control, dam side irrigation and water-based sports and recreational and religious activities have evolved and threatened water quality and quantity (Olanrewaju et al., Citation2017). Eleyele Lake is further exposed to pollution from the various anthropogenic activities such as cassava processing occurring in its watershed (Akinyemi et al., Citation2014).
In Eleyele Lake, the flood control gates at the dam wall are obsolete and dysfunctional increasing the hydraulic residence time, thus reducing the flushing period for contaminants in the process exposing aquatic organisms and the entire ecosystem to metal contamination. Research by Adeogun et al. (Citation2016) indicates that Eleyele Lake is contaminated with highly carcinogenic and mutagenic forms of interactive pollutants such as polychlorinated biphenyls (PCBs) and heavy metals especially cadmium, lead and mercury. Metal contamination of the lake is mostly attributed to various anthropogenic activities in the catchment. Moreso the elongated rainy season which mainly runs from April to October in Ibadan City, leads to metal-laden high runoff discharges into Eleyele Lake (Adeogun et al., Citation2016). Hence, ecosystem integrity of the Lake is constantly threatened which necessitates consistent and long-term monitoring efforts. This is buttressed by the fact that persistent and cumulative toxic metal pollutants pose environmental hazards and health problems such as reproductive, neurological and endocrinal defects in aquatic hydrobionts like fish, and even humans who constitute the end consumers of fish from polluted water bodies (Akinyemi et al., Citation2014; Luoma & Rainbow, Citation2005; Schantz et al., Citation2003; Van Dyk et al., Citation2009b; Wepener et al., Citation2001).
Several researches in highly degraded peri-urban and urban lakes indicate that heavy metals especially the more toxic and reactive ones, e.g. cadmium, lead and mercury bioaccumulate and biomagnify in aquatic organisms (Dietz et al., Citation2008; Viera et al., Citation2011). This accumulation can result in direct and indirect negative impacts on fish metabolism, physiology, behavior and ecology (Gbem et al., Citation2001). Most heavy metals are known to accumulate at varying concentrations in different tissues and organs of different fish species (Ciardullo et al., Citation2008; Viera et al., Citation2011). The patterns of bioaccumulation and biomagnification differ between tissues, organs (Ciardullo et al., Citation2008; Gbem et al., Citation2001; Rashed, Citation2001) and species (Kljaković-Gašpić et al., Citation2011, Viera et al., Citation2011). However, information on the spatiotemporal relationships of heavy metal concentration between different tissues within fish, and between fish tissues and the ambient environment such as water and sediments is limited within the scientific literature (Bevelhimer et al., Citation1997). This metal spatiotemporal data deficiency scenario is amplified for peri-urban and urban reservoirs facing various degradation threats and are located in resource-poor African countries such as Eleyele Lake in Nigeria (Van Dyk et al., Citation2009a, Citation2009b, Citation2012). Therefore, there is a need for continuous spatiotemporal monitoring and assessment of heavy metal concentrations in tissues of the most common fish species consumed in subtropical Sub Sahara African urban and peri-urban reservoirs.
The objectives of the present study were to i) assess the spatial and temporal variations in the concentrations of selected metals consisting of iron (Fe), copper (Cu), zinc (Zn), cobalt (Co), cadmium (Cd), lead (Pb), nickel (Ni) and mercury (Hg) in water, sediments and the gills and edible stomach muscle tissues of two commonly consumed fish species, African Sharptooth mud catfish (Clarias gariepinus) and the Nile Tilapia (Oreochromis niloticus), and ii) evaluate the potential ecological risks posed by metals to aquatic organisms in Eleyele Lake located in Ibadan City, Oyo State in south-western Nigeria. For this study, the functional hypothesis was that there were no significant spatiotemporal differences in the concentrations of metals in water, sediments and fish tissues regardless of the intermittent pollution in the highly degraded urban Eleyele reservoir.
2. Study area and methods
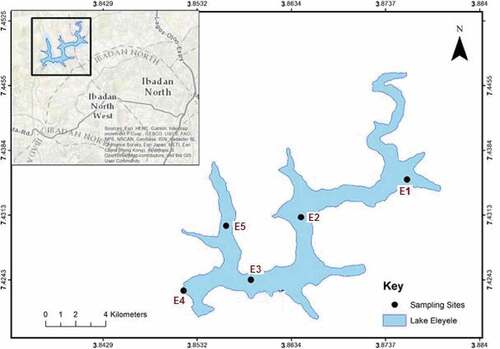
Eleyele Lake (Figure ), the second largest reservoir in Oyo State, Nigeria is located on the north-eastern part of Ibadan City within the Latitude 7°25ʹ30ʹ’-7°26ʹ30ʹ’ N and Longitude 30°51ʹ30ʹ’-3°52ʹ30ʹ’ E (Kareem et al., Citation1994). The lake was constructed in 1939–1942 by damming the Ona and some sections of the Alapata Rivers.
Eleyele Lake has a longitudinal width of 242 m across the dam, the catchment area is 323.7 km2, and covers an impoundment area of 156.2 ha with a storage capacity of 29.5 × 106 L of water. At the apex point, the lake lies 125 m above sea level with an average depth of 5.26.0 m. The lake and its catchment fall within a hydrological region which receives a mean annual rainfall of >1980.0 mm and has a mean annual temperature of 29.0ºC. The drainage system is controlled by the bedrock geology, with the characteristic dendritic pattern of structurally controlled streams and rivulets. Flash overland flow and drainage discharge are common during the wet season from April to October intermittently disturbed by the hilly nature of the surrounding terrain. The lake is located on the leeward side of the Ibadan metropolitan which is home to almost 15 million people and is characterised by numerous industries ranging from car manufacturing to agro-processing concerns mainly dealing with cassava, a staple plant. On the upward side, there are urban settlements and numerous industries which are served with open drainage sewer systems (pers. observation). In the catchment, litter dumps are rife and have the potential to leach metals during the heavy rainy season. Water quality and quantity concerns within the relatively shallow reservoir arise from the negative effects of pollution and excessive abstraction stemming from an exploding human population, expanding industrial and urban settlement, and overexploitation of the fishery resources (Akinyemi et al., Citation2014; Kareem et al., Citation1994). We selected sites that are normally utilised by fishermen and are located near to the Ibadan City, and which covered as much of the lake as was possible. We also tried to cover the main portion of the Lake where the Oyo State waterworks draw potable water for domestic and industrial use. This would enable us to obtain representative water and fish samples which are utilised by humans (Figure ).
2.1. Sample collection and quality assurance
Five sites across the breath of the Lake as indicated in Figure were sampled. Water, sediment and fish sampling were done once in April, July and November 2017. The sampling seasons were chosen so as to cover the rainy season which occurs from April to October, short-duration mid-season dry spell which occurs in late July in Ibadan, where the water levels are expected to drop considerably in Lake Eleyele, and in early November which represents the dry season where there are anticipated low dilution rates and high metal concentrations in the Lake. In essence, there is no drastic decrease in temperature (winter-spring dropdown) which would enforce partial or complete turnover of Lake Eleyele due to persistently high atmospheric temperatures in the equatorial catchment (Akinyemi et al., Citation2014). Metal concentration dynamics would most likely relate to stream flows, water level fluctuations and exogenous factors in the catchment (Kareem et al., Citation1994).
At each site three replicate water samples were collected using a 5 L Ruttner sampler at a maximum depth of 1 m for uniformity, and these were integrated into one sample in 500 m sterilised polyethene bottles. The 500 ml bottles were stored in iced cooler boxes for further analysis in the laboratory. In-situ measurements of pH, conductivity, turbidity, temperature and dissolved oxygen (DO) were done at each site using a pH, turbidimeter, Conductivity and DO meter (HACH, LDO, Germany). Water transparency (Secchi depth, SD) was measured using a 20 cm diameter Secchi disc having alternating black and white quadrants at each of the sampled sites. Bottom sediments were collected at the same site as water using a polypropylene coring device 1 m long with a 0.1 m internal diameter. Multiple 10 cm cores were collected at each site and were thoroughly mixed to create an integrated sample which was stored in sterilised polyethene bags. Sediment samples were stored at a temperature of 4°C in a refrigerator in the laboratory, for a maximum period of 24 hours, in preparation for heavy metal analysis.
The mud catfish and Nile tilapia were collected from the same sampling sites for water and sediments using gill nets, whilst some augment samples were collected from fishermen and fish traders at Apete market where fish from Eleyele Lake is processed and sold to consumers. At each sampling occasion, an effort was made to collect 15 specimens per species so as to obtain a truly representative sample which the fishermen catch and sell to consumers.
2.2. Extraction and analysis of metals in collected samples
The extraction and analysis of metals in fish, water and sediments were done following methods by Greenberg et al. (Citation1980) and Nhiwatiwa et al. (Citation2011). Water samples were filtered through 0.45 µm sized Whatman G/F filters into sterilised 100 ml flasks. This sample was acidified with nitric acid to a pH<2. Heavy metals were quantified using the FAAS. A multiple step acid digestion protocol to extract heavy metals was adopted for sediments and fish tissues following methods by Greenberg et al. (Citation1980). The procedure for heavy metals comprises of steps: Step 1. Digestion: sediments were oven-dried at 180–185°C in a hooded muffle furnace and large aggregates were broken into smaller granules. Step 2. 20 ml nitric acid and 5 ml perchloric acid were added to 5 g of the oven-dried sediment sample and the mixture was heated until fume production and cooled to room temperature of 22–24°C. Step 3. Acidification: 20 ml of 50% hydrochloric acid was added to the mixture from the first digestion. The acidified mixture was heated until boiling and then cooled to room temperature. This acidified mixture was filtered and distilled water added to a 100 ml mark. Step 4. Estimation of metals: Digested sediments were analysed for metals using the FAAS (Greenberg et al., Citation1980).
For fish samples, 3 g of wet weight of the gill and edible stomach muscle tissues was dried in separate clay crucibles at a temperature of 120°C until they reached a constant weight between 1–2.5 g. The dry gill and stomach muscle tissues were digested using 5 ml perchloric acid and 10 ml nitric acid in sterilised digestion flasks. The digestion flasks were heated in an oven at 130–145°C until all materials dissolved and then allowed to cool at room temperature. Metals in the digested fish tissues, water and sediments were estimated using the multicathode hollow lamp fast sequential AAS Spectra-AA 232 FS (Varian, Germany). Of note was that mercury was determined by the inductively coupled plasma-optical emission spectrometry (ICP-OES) method due to its low sensitivity in the FAAS.
2.3. Quality control
Calibration was performed using standard analytical solutions. Standard solutions were prepared by serial dilutions of 1000 mg/L PerkinElmer Pure single element-standards in 2% nitric acid (v/v). Before analysis, the detection method was validated with reference materials: Merck 100,473 for water, LKSD-1 for sediments and ERM-CE278 K for fish. The recovery rate was >95% for all elements with a 90% recovery rate for mercury with low RSD values<5%. Detection limits of the FAAS for the analysed metals were: 0.005 mg/L for Cu, Zn; 0.2 mg/L for Ni, Co; 0.04 mg/L for Cr, Pb; 0.03 mg/L for Fe and the ICP-OES had a sensitivity of 0.001 mg/L for Hg.
Every effort was ensured to have procedural blanks and standards for quality control of heavy metal estimation. At every stage of analysis after 10 samples of sediments and water, a reagent blank with concentration below detectable limits was prepared. All glassware used in this study was soaked for 12–24 hours in acid water (1% solution of hydrochloric or nitric acid) before being washed by distilled and deionised water in preparation for acid digestion procedures. All the water used for serial dilutions and preparation of blanks and topping up during acid digestion process was purified using a combination of technologies, such as reverse osmosis, ion exchange and ultraviolet photo-oxidation in the laboratory at the University of Ibadan Department of Chemistry. For repeatability and accuracy, each sample was analysed in triplicate with a standard deviation (SD<0.05) for all metals and (SD<0.001 for mercury). All acids used for digestion were standard analytical grade acids. The multiple-step acid analysis used for the FAAS was to ensure accuracy and precision in quantification and estimation of heavy metals in water, sediments and fish samples (except for mercury) as they are pertinent to the health of humans who use water and consume fish.
2.4. Statistical analysis
2.4.1. Physicochemical parameters in Eleyele Lake
From the raw values, the average (±standard deviation) values of selected physicochemical parameters, such as dissolved oxygen (DO), temperature, electrical conductivity (EC), turbidity, pH and Secchi Depth at the five sampled sites were calculated and compared to the locally approved Nigerian Standards for Drinking Water Quality (NSDQW) values and are summarised in Table . The calculated mean values for water quality parameters were nonparametric. Therefore, a non-parametric test, the Kruskal–Wallis Anova, was used to assess the spatial and temporal variations in the physicochemical parameters and results are summarised in Table .
Table 1. The mean ± SD values of water quality parameters in Eleyele Lake, Nigeria 2017. The NSDQW and WHO values are added for comparison
Table 2. Spatial and temporal analysis of water quality parameters in Eleyele Lake, Nigeria 2017. Significant values (p < 0.05) are marked*
2.4.2. Spatial-temporal metal concentrations in water, sediments and fish tissue
Metal concentrations were recorded in water, sediment and African Sharptooth mud catfish and Nile tilapia tissue samples from all the three sampling occasions in Eleyele Lake and their calculated mean values were used for further statistical analysis. The calculated mean (±standard deviation) data were non-parametric. Thus, differences in metal concentrations in water, sediments and fish tissues at the five sites in the two lakes were analysed using nonparametric Kruskal-Wallis Anova at p < 0.05 rejection level in SPSS version 21. The concentrations of metals in water from Eleyele Lake were compared to the Nigerian Standards for Drinking Water Quality (NSDQW) and the World Health Organisation standards (USEPA, Citation2000; NESREA, Citation2011; WHO, Citation2011; WHO/UNICEF, Citation2012). The concentration of metals in sediments in Eleyele Lake was compared to the USEPA sediment criteria (USEPA, Citation2000). Metal concentrations in fish were compared to the Food and Agriculture Organization (Citation1983) legal limits for hazardous substances in fish and fishery products.
2.4.3. Pollution status and potential ecological risks of metals in water and sediments in Eleyele Lake
For each sampling effort or month three replicate water and sediments were collected at each site and a composite sample was obtained for each site in the Eleyele Lake. The levels of each metal determined per site were then averaged and used for further statistical analysis. To determine the lake’s pollution status and potential ecological risk posed by each heavy metal, the pollution load index (PLI) and geological accumulation index method (Igeo) by Müller (Citation1969, Citation1981) and potential ecological risk evaluation method (PERI) by Hakanson (Citation1980) were used, respectively.
The pollution load index (PLI) in water is defined as the nth root of the multiplications of specific metal concentrations (CF metals):
Values of PLI > 1 imply that heavy metal pollution exists. Otherwise, if PLI < 1, there is no heavy metal pollution (Tomlinson et al., Citation1980).
The geo-accumulation index (Igeo) is defined by the following equation:
Where: Cn is concentration of metal n and Bn is background concentration of the metal (n) usually adopted from sediment quality guidelines (SDQ). Constant factor K is a background matrix correction factor due to lithospheric effects, which is usually defined as 1.5 (Müller, Citation1969, Citation1981). Classification of geo-accumulation index is presented in Appendix 1.
In the Potential Ecological Risk index (PERI) method by Hakanson (Citation1980), potential ecological risk coefficient Eir of a single element, and potential ecological risk index RI of the multielement are calculated using the following equations:
Where:
Cif is the pollution coefficient of a heavy metal of “i” or the monomial contamination factor; Cis is the measured level of sedimentary heavy metal;
Cin is the background level of sedimentary heavy metal;
Tir is the toxic response factor for the given heavy metal of “i”, which accounts for the toxic requirement and the sensitivity requirement;
RI is the sum of all risk factors for heavy metals in sediments.
The average shale background concentration of global sediments was selected as the reference baselines in this study. This study adopted the Hakanson (Citation1980), PERI classification criteria shown in Appendix 2. In order to obtain the sediment/water (S/W) ratio or bioconcentration factor (BCF) that is the ratio of metal concentrations in sediment phase to that in the water phase, the equation used was:
If the S/W Ratio or BCF is greater than 1000, it was considered as high and those under 250 were considered as low. BCF between 250–1000 was considered as moderately high. The sediment-water criterion for this study was derived from the Solids-water coefficient (Ksw) method used by Van Der Kooij et al. (Citation1991) and Shea (Citation1988).
3. Results
3.1. Water quality parameters
At most of the sites, DO levels were generally below the NSDQW and WHO values (Table ). Recorded surface water temperatures at most sites were within acceptable local and international limits (Table ). Turbidity values which were mostly <3 NTU fell within the acceptable standards. Electrical conductivity was high at E1 in April (733.23 ± 100.23 µS/cm) though all recorded values were within acceptable standards (Table ). High pH values were recorded at E1 in April (8.11 ± 0.53) and at E4 in November (8.01 ± 0.56), and the rest of pH values were within the acceptable limits (Table ). Water transparency was high at E5 in April (1.87 ± 0.11 m), July (1.77 ± 0.17 m) and November (1.63 ± 0.45 m) and lowest at E1 in July (0.17 ± 0.01 m). Spatial and temporal analysis of variations in water quality parameters reflected significant (Kruskal Anova, p < 0.05) spatial differences for DO (p = 0.009), temperature (p = 0.04) and turbidity (p = 0.026) in Eleyele Lake. There was no significant temporal variation for all water quality parameters tested except for turbidity (p = 0.02) as indicated in Table .
3.2. Metals in water and sediments
Concentrations of Fe in water were high at E2 (5.76 ± 1.36 mg/L) and E5 (5.44 ± 2.89 mg/L) in November (Table ). All recorded Fe values in water exceeded the NSDWQ and WHO limits (Table ). Co concentrations were on average 6.2 mg/L in Eleyele Lake although they exceeded the acceptable limits. Zn levels were within the acceptable limits and non-detectable at some sites for instance, at E3 and E5 in April (Table ). Low values of Cu and Cd were recorded in the Lake in all sampling months although some values exceeded acceptable standards (Table ). Significantly high values of Pb and Ni which in most cases exceeded acceptable thresholds were recorded in the water phase of the Lake in all the sampling months. Relatively low values of Hg were recorded in water in the Lake and all values fell within the acceptable standards (Table ). Analysis of the spatiotemporal variations of metals in water indicated significant temporal (Kruskal Anova, p < 0.05) variations in the concentrations of all metals except Co (Table ). There were no significant spatial variations in the concentration of all metals in water sampled in the Lake (Table ).
Table 3. The mean ± SD concentrations of heavy metals in water (mg/L) of Eleyele Lake, Nigeria 2017. The NSDWQ and WHO values are added for comparison
Table 4. The mean ± SD concentrations of heavy metals in sediments (mg/kg) of Eleyele Lake, Nigeria 2017. The USEPA values are added for normative comparison
Table 5. Spatiotemporal analysis of metals in water and sediments in Eleyele Lake, Nigeria 2017. *p < 0.05 indicate significant values
The Fe concentrations recorded in sediments were highest at all sites in November though they all fell within the acceptable international (USEPA) limits (Table ). Significantly high Co concentrations were detected in sediments in the Lake particularly at E1 in July (36.26 ± 11.19 mg/kg). Significantly high levels of Zn were recorded in sediments especially at E3 in April and July and at E5 in April and July which exceeded the acceptable limits (Table ). Low Cu and Cd values were recorded in the Lake and fell within the acceptable limits (Table ). Significantly high Pb values were detected at E3, E4 and E5 in sediments of Eleyele Lake. However, most Pb values in sediments fell within the acceptable limits (Table ). Significantly high Ni values which were above the acceptable limits were detected in sediments (Table ). Low Hg levels were detected in sediments and were within the acceptable thresholds (Table ). Analysis of the spatial and temporal variations in metal concentrations in sediments indicated significant (Kruskal Anova, p < 0.05) temporal variations in the levels of Cu (p = 0.007), Cd (p = 0.008) and Ni (p = 0.008). However, no significant spatial variations were calculated in the concentrations of all metals in sediments sampled in the Lake (Table ).
Bioconcentration factors for Fe, Cu, Cd, Hg and Pb for all sites except at E4 in November were low, i.e. <250 (Table ). Bioconcentration factor for Co in most cases was in the 250–1000 range and was considered moderately high for all sites except at E2 in April (Table ). However, Co bioconcentration factors at E1 of 1813 in July and E4 (1094.444) in November were high, i.e. >1000 (Table ). Bioconcentration factors for Zn were on average high >1000 across the lake (Table ). Bioconcentration factors for Ni at almost all sites were >250 particularly in November with a highlight value of 1246.714 at E3 (Table ). A pattern where BCF values tend to be high in November was noticeable for most of the metals with the exception of Cd and Ni (Table ).
Table 6. Bioconcentration factors of metals in Eleyele Lake, Nigeria 2017. Bioconcentration of metals classified as low<250, moderately high 250–1000 and high >1000
3.3. Pollution indices for metals in Eleyele Lake
The PLI indicated pollution, PLI>1, in sediments sampled at E1 in November, E2 in July and November and at E4 in November (Table ). The Geoaccumulation index indicated moderate heavy contamination (Igeo 2–3) for Fe, Zn and Pb (Table ). Cu showed extreme contamination in the sediments of Eleyele Lake (Table ). The potential ecological risk index (PERI) indicated significantly high ecological risk (RI≥600) for Ni with the rest of the metals reflecting low ecological risk in Eleyele Lake (Table ).
Table 7. Pollution Load Index (PLI) for metals in surface sediments of Eleyele Lake, Nigeria 2017. Polluted sites (PLI>1) are marked*
Table 8. Geoaccumulation Index (Igeo) for metals in surface sediments of Eleyele Lake, Nigeria 2017. Copper showing extreme contamination (Igeo) >5 is marked*
Table 9. Potential Ecological Risk Index (PERI) for metals in surface sediments of Eleyele Lake, Nigeria 2017. Nickel showing significantly high serious ecological risk RI≥600 is marked*
3.4. Metal concentrations in fish tissues in Eleyele Lake
The gills of mud catfish and Nile tilapia showed high levels of Fe with 1041 ± 103.30 mg/kg and 2303.8 ± 1578.68 mg/kg in April, respectively (Table ). High Co and Cd values which in most cases exceeded the FAO limit were recorded in gills and edible stomach muscle tissues of both fish species (Table ). High Zn levels of 56.8 ± 13.11 mg/kg were recorded in the gills of mud catfish. Analysis of the spatiotemporal variations in metal concentrations in fish tissues indicated significant temporal (Kruskal Anova, p < 0.05) variations for Co (p = 0.048), Cu (p = 0.037), Pb (p = 0.001) and Ni (p = 0.001) in the gills, and for Pb (p = 0.02) in the edible stomach muscles of both fish species (Table ). There were no significant spatial variations in the metal concentrations for the gills and edible stomach muscle tissues of both fish species in Eleyele Lake (Table ).
Table 10. Metal concentrations in tissues of the African Sharptooth mud catfish (N = 34; Mean weight = 0.845 ± 0.28 kg) and Nile Tilapia (N = 36; Mean weight = 0.437 ± 0.45 kg) sampled in Eleyele Lake 2017. Food and Agriculture Organization (Citation1983) limits are also shown for comparison
Table 11. Spatio-temporal analysis of metals in gills and stomach tissues of fish in Eleyele Lake, Nigeria 2017. *p < 0.05 indicate significant values
4. Discussion
Heavy metals tend to accumulate in lakes with slow flushing rates and pose a health risk to aquatic organisms and diminish the value of ecosystem services available (Alloway, Citation1990; Luoma & Rainbow, Citation2005; Tundisi et al., Citation2008; Wetzel, Citation2001). Concentrations of Fe, Co, Pb and Ni were high in water especially at E1 and E3, sites close to the urban areas and farming zones, respectively. Corresponding levels of Co, Ni and Zn were also very high in sediments at E1 and E3. This reflects the level and nature of pollutants released by the urban and industrial and agricultural zones in the predominantly metropolitan Eleyele catchment. Metals such as Ni, Co and Zn form the bases for most complex industrial organochemicals (Alloway, Citation1990).
Also, most organopesticides and fertilisers have Ni as a base, and Co, Cd and Zn as trace elements such that constant application on farmlands raises their concentrations in aquatic systems receiving the effluent (Tam & Wong, Citation2000).
Whilst it was expected to record low metal concentrations especially in water during the rainy season, as the dilution factor increases, this study actually recorded high concentrations of metals in water and sediments in the rainy period especially in April for some sites (Tables and ). However, concentrations of most metals were relatively lower in water and sediments in the mid-season dry period (i.e. in July). At the start of the dry season (i.e. from November onwards) the dilution factor decreases and metal concentration is expected to increase (Rosenberg et al., Citation2000; Wetzel, Citation2001). Besides the effects of water levels, seasonal anthropogenic activities reliant on rainfall like cassava farming and processing, and horticulture prevalent in the catchment, discharge metal-laden effluent which affects the temporal variation of metals in the Lake. In the main, it appears that significant temporal variation of some metals (e.g. Ni, Pb and Co) in water and sediments of Eleyele Lake is partly attributable to the intensity of the rainfall which tends to be high during March to May with peak periods in April (Akinyemi et al., Citation2014). Subtly, the significant temporal differences in specific metal concentrations as noted for Eleyele Lake indicate that even highly polluted and degraded and in most cases relatively shallow peri-urban and urban reservoirs have unique site-specific temporal differences in the levels of degradation and heavy metal pollution (Dierberg, Citation1992; Thornton, Citation1990). As such the temporal variation of each metal at each site may also be interlinked with the reaction, kinetics, adsorption and absorption dynamics of a specific metal species and the site-specific organic and inorganic covariates (Wetzel, Citation2001).
For Eleyele Lake it appears that the malfunctioning flood control gates at the dam wall which have been overwhelmed and are obsolete and dysfunctional may be increasing the hydraulic residence time and reducing the flushing period for contaminants as noted by the increased concentrations of metals in water and sediments at E5. Thus, metals tend to either directly desorb into sediments from the water phase or undergo lateral transportation across the whole lake (Qiao et al., Citation2013). The high metal retention capacity due to the low flushing rates of the lake may partly explain the non-significant spatial heterogeneity in metal concentrations in the sediments and water phases, though the focus must be on the actual implications of the magnitudes of the toxic metals which are hazardous even at very low levels (Alloway, Citation1990).
Bioconcentration factor, a reflection of intermetallic dynamics between the solid sediment phase and liquid water phase showed low values for toxic elements such as Fe, Cu, Cd, Hg and Pb. The BCFs for Co, Ni and Zn were either moderately high or high. A patchy pattern where BCF values tend to be high in November was noticeable for most of the metals with the exception of Cd and Ni and relates well to the increased metal concentrations in the sediments in the post-rainy period. Metals which tend to occur in high concentrations in sediments have a similar pattern in the water phase (Alloway, Citation1990). This phenomenon is attributable to reflux, resuspension and desorption dynamics of metal species in aquatic ecosystems under different physicochemical regimes mainly driven by water turbidity and temperature variations and presence of other reactive chemicals (Bernot & Dodds, Citation2005; Liu et al., Citation2013).
High Fe, Co, Zn and Cd concentrations were recorded in the gills and edible muscles of the mud catfish and Nile tilapia. In some cases, these metals, which tend to biomagnify and bioaccumulate in tissues, exceeded the FAO allowed safety limits for human health, thus, posing a health risk. The fact that most people consume both fish species in the Eleyele catchment, expose humans to health risks such as reproductive, neurological and endocrinal defects (Schantz et al., Citation2003; Van Dyk et al., Citation2009b). Analysis of the spatiotemporal variations in metal concentrations in fish tissues indicated significant temporal variations for Co, Cu, Pb and Ni in the gills, and for Pb in the edible stomach muscles of both fish species. There were no significant spatial variations in the metal concentrations for the gills and edible stomach muscle tissues of both fish species in Eleyele Lake. Temporal variation of metal concentrations in fish tissues can be linked to the seasonality in the bioavailability of metals in water and sediments which can be cross-linked to the seasonality in effluent discharging anthropogenic activities in the Eleyele catchment (Dietz et al., Citation2008).
Non-significant spatial variation in concentrations of metals in fish tissues dovetails with non-significant spatial variation of most metals in water and sediments in the Lake. Regardless, the implications to human health are dire since metals in exceedingly high concentrations such as Zn, Cd, Fe and Co are likely to be ubiquitous among fish catches irrespective of the fishing zones. This is further buttressed by the fact that fish are migratory and utilise almost all suitable zones in a lake system. Thus, humans are at a high risk of exposure to such metals (Nordberg et al., Citation2007; Szefer et al., Citation2003). However, other distinct factors such as varying depuration rates, age, reproductive status, feeding ecology and genetic traits unique among different species tend to affect metal accumulation in fish tissues (Andres et al., Citation2000; Kljaković-Gašpić et al., Citation2011; Odžak & Zvonaric, Citation1995; Sahrul et al., Citation1983). Hence, further studies must assess the human health risks associated with consuming different fish species from the Lake before conclusive statements.
From the pollution assessment indices assessed in this study, the PLI indicated pollution in sediments sampled at E1, E2 and E4 whereas notable metal contamination occurred in July and November. The Geoaccumulation index reflected varied responses with moderate-heavy contamination for Fe, Zn and Pb whilst Cu showed extreme contamination in the sediments of Eleyele Lake. The potential ecological risk index (PERI) indicated significantly serious ecological risk (RI≥600) for Ni with the rest of the metals reflecting low ecological risk in Eleyele Lake. These results reflect heavy metal pollution in the Lake particularly in the rainy season (i.e. March to October) and post-rainy season (i.e. November). This can be attributed to metal-laden effluent from run-off which tends to drain through highly polluted and often clogged open sewer drainage system downwards into the Lake. Leaching of nutrients from solid waste dump heaps which are rife in the Eleyele catchment also releases metals into the Lake (Akinyemi et al., Citation2014). Upon reaching the Lake, some metals due to other reactive dynamics tend to adsorb, desorb, precipitate and accumulate in bottom sediments (Müller, Citation1969, Citation1981; Wetzel, Citation2001). In as much PERI results highlight that some metals did not pose a serious ecological threat, their mere presence in elevated concentrations in water and sediments of the Lake implies they have potential to cause ecological risk in the lake as long as the hydraulic retention time is distorted mainly due to the dysfunctional flood control gates at the dam wall.
5. Conclusion
Results of this study indicated heavy metal contamination in water, sediments and tissues of the catfish and Nile tilapia in Lake Eleyele. Significant temporal variation in metal concentrations in water, sediment and fish tissues in the Lake may be related to the various anthropogenic activities in the highly urbanised metropolitan Ibadan City. Metal contamination poses a hazard to the aquatic organisms and humans who use the water and consume fish from Lake Eleyele. In the main, it appears nutrient retention in the sediments of Eleyele Lake is the key driver of metal pollution dynamics in the aquatic system with confounding factors such as the seasonality of the catchment human activities and the consequent effluent discharge as antecedents. Thus, controlling the nutrient retention capacity may be central to averting heavy metal pollution and its causal hazards in Eleyele Lake.
Acknowledgements
This research was supported by funding from the Department for International Development (DfID) under the Climate Impact Research Capacity and Leadership Enhancement (CIRCLE) programme implemented by the African Academy of Sciences (AAS) and the Association of Commonwealth Universities (ACU). Special gratitude goes to all staff at the Aquaculture and Fisheries Management at the University of Ibadan, Nigeria, for all the logistical help in field and laboratory work. Our sincere gratitude to Exeverino Chinoitezvi and Blessing Kavhu of P arks and Wildlife Zimbabwe (ZIMPARKS), and the Department of Wildlife Ecology and Conservation at the Chinhoyi University of Technology for assistance with the map.
Additional information
Funding
Notes on contributors
Beaven Utete
Dr Beaven Utete holds a PhD in Aquatic Ecology Conservation and had his post-graduate fellowship in the Department of Aquaculture and Fisheries Management at the University of Ibadan, Nigeria, under the Climate Impact Research Capacity and Leadership Enhancement (CIRCLE) programme funded by the Department for International Development (DfID-UK).
He is currently a senior lecturer and research fellow in the Department of Wildlife, Ecology and Conservation at the Chinhoyi University of Technology in Zimbabwe. His major interest lies in conserving vital but threatened global aquatic and fishery resources for the benefit of humanity.
References
- Adeogun, A. O., Adedara, I. A., & Farombi, E. O. (2016). Evidence of elevated levels of polychlorinated biphenyl congeners in commonly consumed fish from Eleyele Reservoir, Southwestern Nigeria. Toxicology Industrial Health, 32(1), 22–22. https://doi.org/10.1177/0748233713495585
- Akinyemi, L. P., Odunaike, R. K., Daniel, D. E., & Alausa, S. K. (2014). Physico-chemical parameters and heavy metals concentrations in Eleyele River in Oyo State, South-West of Nigeria. International Journal of Environment Science Toxicology Research, 2(1), 1–5.
- Alloway, B. J. (1990). Heavy metals in soils. John Wiley & Sons.
- Andres, H., Malm, O., Kinjo, Y., Harada, M., Branches, F., Pfeiffer, W., & Kato, H. (2000). Interspecific comparison of cadmium and zinc contamination in the organs of four fish species along a polymetallic pollution gradient (Lot River, France). Science of the Total Environment, 248(1), 11–25. https://doi.org/10.1016/S0048-9697(99)00477-5
- Bernot, M. J., & Dodds, W. K. (2005). Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems, 8(4), 442–453. https://doi.org/10.1007/s10021-003-0143-y
- Bevelhimer, M. S., Sample, B. E., Southworth, G. R., Beauchamp, J. J., & Peterson, M. J. (1997). Estimation of whole-fish contaminant concentrations from fish fillet data (ES/ER/TM202). Ridge National Laboratory.
- Ciardullo, S., Aureli, F., Coni, E., Guandalini, E., Iosi, F., Raggi, A., Rufo, G., & Cubadda, F. (2008). Bioaccumulation potential of dietary arsenic, cadmium, lead, mercury, and selenium in organs and tissues of rainbow trout (Oncorhynchus mykiss) as a function of fish growth. Journal of Agricultural Food Chemistry, 56(7), 2442–2451. https://doi.org/10.1021/jf703572t
- Dierberg, F. E. (1992). The littoral zone of Lake Okeechobee as a source of phosphorus after a drawdown. Environmental Management, 13, 729–742. https://doi.org/10.1007/BF01868312
- Dietz, R., Riget, F., Cleeman, M., Aarkrog, A., Johansen, P., & Hansen, J. C. (2008). Comparison of contaminants from different trophic levels and ecosystems. Science of the Total Environment, 245(1–3), 221–231. https://doi.org/10.1016/S0048-9697(99)00447-7
- Food and Agriculture Organization. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular, (464), 5–100.
- Gbem, T. T., Balogun, J. K., Lawal, F. A., & Annune, P. A. (2001). Trace metal accumulation in Clarias gariepinus (Teugels) exposed to sublethal levels of tannery effluent. Science of the Total Environment, 271(1–3), 1–9. https://doi.org/10.1016/S0048-9697(00)00773-7
- Greenberg, A. E., Connors, J. J., & Jenkin, D. (1980). Standard methods for the examination of water and wastewater (15th ed.). American Public Health Association.
- Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14(8), 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
- Kareem, O. K., Olanrewaju, A. N., Osho, E. F., Orisasona, O., & Akintunde, M. A. (1994). Growth patterns and condition factor of Hepsetus odoe (Bloch, 1794) Captured in Eleyele Lake, Southwest Nigeria. Fish Aquaculture Journal, 7, 178–184.
- Kljaković-Gašpić, Z., Zvonarić, T., Vrgoč, N., Odžak, N., & Barić, A. (2011). Cadmium and lead in selected tissues of two commercially important fish species from the Adriatic Sea. Water Research, 36(20), 5023–5028. https://doi.org/10.1016/S0043-1354(02)00111-2
- Liu, Q., Liang, L., Wang, F. Y., & Liu, F. (2013). Characteristics of heavy metals pollution in sediments of the hydro-fluctuation belt in the Liao River of Liaoning Province, Northeast China. China Environmental Science, 33(12), 2220–2227. http://www.zghjkx.com.cn
- Luoma, S. N., & Rainbow, P. S. (2005). Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environmental Science and Technology, 39(7), 1921–1931. https://doi.org/10.1021/es048947e
- Magadza, C. H. D. (2003). Lake Chivero: A management case study. Lakes and Reservoirs: Research and Management, 8(2), 69–81. https://doi.org/10.1046/j.1320-5331.2003.00214.x
- Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. Journal of Geology, 2(3), 108–118.
- Müller, G. (1981). Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chemistry Zeitung, 20(11), 157–164. https://doi.org/10.1002/anie.198109341
- Nhiwatiwa, T., Barson, M., Harrison, A. P., Utete, B., & Cooper, R. G. (2011). Metal concentrations in water, sediment and sharptooth catfish Clarias gariepinus from three peri-urban rivers in the upper Manyame catchment, Zimbabwe. African Journal of Aquatic Science, 3(3), 243–252. https://doi.org/10.2989/16085914.2011.636906
- Nigeria Environmental Standard and Regulatory Enforcement Agency (NESREA). (2011). Guidelines and standards for environmental pollution control in Nigeria.
- Nordberg, K., Nogawa, M., Nordberg, L., Friberg, T., Cadmium, G. F., Nordberg, B. A., Fowler, M., Nordberg, L., & Friberg, T. (2007). Handbook on the toxicology of metals (3rd ed.). Academic.Press.
- Odžak, N., & Zvonaric, T. (1995). Cadmium and lead uptake from food by the fish. Dicentrarchus Labrax. Water Science and Technology, 32(9–10), 49–55. https://doi.org/10.2166/wst.1995.0669
- Olanrewaju, A. N., Ajani, E. K., & Kareem, O. K. (2017). Physico-chemical status of Eleyele Reservoir, Ibadan, Nigeria. Journal of Aquac Res Development, 8(512), 1-8. https://doi.org/10.4172/21559546.1000512
- Qiao, M. M., Ji, H. B., Zhu, X. F., & Chen, Y. (2013). Fraction distribution and risk assessment of heavy metals in sediments of inflow rivers of Miyun Reservoir. Acta Scientiae Circumstantiae, 33(12), 3324–3333. http/doi.org10.1002Fs10661-012-3005-2
- Rashed, M. (2001). Monitoring of environmental heavy metals in fish from Nasser Lake. Environment International, 27(1), 27–33. https://doi.org/10.1016/S0160-4120(01)00050-2
- Rosenberg, D. M., McCully, P., & Pringle, C. M. (2000). Global-scale environmental effects of hydrological alterations: Introduction. BioScience, 50(9), 746–751. https://doi.org/10.1641/0006-3568(2000)050[0746:GSEEOH]2.0.CO;2
- Sahrul, M., Hidaka, H., & Tatsukawa, R. (1983). Organ and tissue distribution of heavy metals, and their growth-related changes in Antarctic fish. Agricultural and Biological Chemistry, 47(11), 2521–2532. https://doi.org/10.1080/00021369.1983.10865986
- Schantz, S. L., Widholm, J. J., & Rice, D. C. (2003). Effects of PCB exposure on neuropsychological function in children. Environmental Health Perspective, 111(3), 357–576. https://doi.org/10.1289/ehp.5461
- Shea, D. (1988). Developing national sediment quality criteria. Environmental Science & Technology, 22(11), 1256–1261. https://doi.org/10.1021/es00176a002
- Szefer, P., Domagała-Wieloszewska, M., Warzocha, J., Garbacik-Welosołowska, A., & Ciesielski, T. (2003). Distribution and relationships of mercury, lead, cadmium, copper and zinc in perch (Perca fluviatilis) from the Pomeranian Bay and Szczecin Lagoon, southern Baltic. Food Chemistry, 81(1), 73–83. https://doi.org/10.1016/S0308-8146(02)00380-1
- Tam, N. F. Y., & Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110(2), 195–205. https://doi.org/10.1016/S0269-7491(99)00310-3
- Thornton, K. W. (1990). Perspectives on reservoir limnology. In K. W. Thornton, B. L. Kimmel, & F. E. Payne (Eds.), Reservoir limnology: Ecological perspectives (pp. 1–14). John Wiley and Sons.
- Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgolaender. Meeresunter, 33(1–4), 566–575. https://doi.org/10.1007/BF02414780
- Tundisi, J. G., Matsumura-Tundisi, T., & Tundisi, J. E. M. (2008). Reservoirs and human well-being: New challenges for evaluating impacts and benefits in the neotropics. Brazil Journal of Biology, 68(4 suppl), 1133–1135. https://doi.org/10.1590/S1519-69842008000500020
- USEPA. (2000). National primary drinking water regulations.
- Van Der Kooij, L. A., Van De Meent, D., Van Leeuwen, C. J., & Bruggeman, W. A. (1991). Deriving quality criteria for water and sediment from the results of aquatic toxicity tests and product standards: Application of the equilibrium partitioning method. Water Research, 25(6), 679–705. https://doi.org/10.1016/0043-1354(91)90045-R
- van Dyk, J. C., Cochrane, M. J., & Wagenaar, G. M. (2012). Liver histopathology of the sharptooth catfish Clarias gariepinus as a biomarker of aquatic pollution. Chemosphere, 87(4), 301–311. https://doi.org/10.1016/j.chemosphere.2011.12.002
- Van Dyk, J. C., Marchand, M. J., Pieterse, G. M., Barnhoorn, I. E. J., & Bornman, M. S. (2009b). Histological changes in the gills of Clarias gariepinus (Teleostei: Clariidae) from a polluted South African urban aquatic system. African Journal of Aquatic Science, 34(3), 283–291. https://doi.org/10.2989/AJAS.2009.34.3.10.986
- Van Dyk, J. C., Marchand, M. J., Smit, N. J., & Pieterse, G. M. (2009a). A histology based fish health assessment of four commercially and ecologically important species from the Okavango Delta panhandle, Botswana. African Journal of Aquatic Science, 34(3), 273–282. https://doi.org/10.2989/AJAS.2009.34.3.9.985
- Viera, C., Morais, S., Ramos, S., Delerue-Matos, C., & Oliveira, M. B. P. P. (2011). Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food and Chemical Toxicology, 49(4), 923–932. https://doi.org/10.1016/j.fct.2010.12.016
- Wepener, W., van Vuren, J. H. J., & Du Preez, H. H. (2001). Uptake and distribution of a copper, iron and zinc mixture in gill, liver and plasma of a freshwater teleost. Tilapia Sparrmanii. Water SA, 27(1), 99–108. https://doi.org/10.4314/wsa.v27i1.5016
- Wetzel, R. G. (2001). Limnology: Lake and river ecosystems (3rd ed.). Academic Press.
- WHO. (2011). Guidelines for drinking water quality (4th ed.).
- WHO/UNICEF. (2012). Progress on drinking water sanitation. 2012 Update WHO, Geneva and UNICEF.
Appendices
Appendix 1. Muller classification for geo-accumulation index adopted from Müller (Citation1969) for heavy metals
Appendix 2
Hakanson (Citation1980) ecological risk classification criteria for metals in sediments Er = ecological risk, RI = multielement ecological risk