Abstract
Chemical contaminants that migrate into food may affect the safety and quality of the food depending on the nature and composition of the packaging material. The introduction of different packaging materials and designs have increased the specific hazards that humans are exposed to due to chemical migration into food. In Ghana, these food contact materials are not only used for food storage or transportation but also, foods such as kenkey are cooked with these food contact materials at very high temperatures for longer periods hence increasing the possibility of the migration rate and thereby posing hazard to consumers. The purpose of this study is to assess the quality and safety of polyethylene food contact materials used in the Ghanaian food industry in terms of phthalate migration. Five different polyethylene food contact materials specifically black polyethylene bags (BPB), plain polyethylene bags (TAB), thick plain polyethylene films/bags (TPB), polyethylene food containers (PFC) and polyethylene plastic bottles (PPB) were purchased from three different markets namely; Madina, Makola and Kwame Nkrumah circle within the Accra Metropolitan Assembly. The samples were analysed using a GC-MS after extraction with aqueous and fatty foods simulants.
PUBLIC INTEREST STATEMENT
The use of plastics in food handling and processing in the packaging industry has increased significantly in recent times.
Polyethylene for example, is widely used as a packaging material to wrap all kinds of food. These plastics have the tendency of presenting a source of contamination through the leaching of toxic chemicals such as phthalates, used as plasticizers during the manufacturing process, which can be deleterious to human health. These chemicals such as di-(2-ethyl hexyl) phthalates are toxic and can cause damage to reproduction and development, alter liver and kidney functions and cause damage to the heart and lungs. The purpose of this study is to assess the quality and safety of polyethylene food contact materials used in the Ghanaian food industry in terms of phthalate migration.
The concentration of the phthalates detected in the aqueous food simulant was below the Specific Migration Limit (SML) of the current EC regulation for the migration of phthalates in food contact materials (FCM) whiles the migrated concentrations of the phthalates in the fatty food simulant; however, had some of its concentrations exceeding the SML stated in the regulation (EU) 10/2011.
Competing interests
The authors declares no competing interests.
1. Introduction
Before the use of modern forms of packaging as a means of preservation and transportation of food, other forms of packaging existed. The use of animal skins and other forms of plant materials for example, was used by earlier civilization as a means of food preservation and transportation (Mercer, Citation1990). These old forms of packaging do still exist in Ghana and other parts of the world (Mercer, Citation1990). A clear example is the use of dried leaves of musa paradisiaca and leaves of the katemfe plant (Thaumatococcus daniellii) in the packaging and sale of kenkey and cooked rice and beans (waakye) respectively.
In the last decade, however, due to increase in the consumption of snacks and street foods, the packaging of food prior to retail and consumption has become increasingly important (Barnes et al., Citation2007). Different forms and designs of modern packaging are used for packaging different kinds of everyday products (Barnes et al., Citation2007). As a result, the packaging market has grown and this expansion can be clearly seen on the shelves of any modern supermarket. The use of plastics as packaging materials has increased at a greater rate than any other form of packaging materials such as papers and glasses (Lule et al., Citation2012). Plastic packaging materials are widely used in the food industry in different designs and shapes and are able to retain product integrity, beneficial effects of processing, extend shell-life and maintain or increase the quality or safety of food (Marsh & Bugusu, Citation2007).
In Ghana, polyethylene has become popular in wrapping all kinds of raw and ready to eat foods such as cooked rice and beans (waakye) and porridge. These foods hitherto were served in plant leaves and calabashes, respectively. Foods are normally packaged in these polyethylene packages regardless of the temperature. Foods such as kenkey and rice sold on the streets and in local restaurants are not only cooked at high temperatures with these polyethylene food contact materials but are also stored in them for longer periods.
The goal of packaging materials is to contain food in a cost-effective way that satisfies industry requirements, consumers’ requirements, maintains food safety and quality (Brody et al., Citation2002). Polyethylene food contact materials used in the food industry are therefore supposed to make food packaging convenient and provide greater safety assurance from biological and chemical contamination. However, these food contact materials have the tendency of contaminating the food through the migration of pollutants such as phthalates which can be deleterious to human health (Brody et al., Citation2002). These phthalates, which are incorporated within the polymeric packaging material to improve functionality, may interact with food components during processing or storage (Bhunia et al., Citation2013). Polyethylene and other forms of plastic materials according to a report by (Barnes et al., Citation2007) may contain a wide range of potential industrial chemical migrants such as phthalates (Barnes et al., Citation2007). Humans are frequently exposed to these chemicals directly or indirectly through sources such as food, cosmetics, air, water, or pharmaceutical products (Digangi et al., Citation2002). Many of these industrial chemicals are toxic at certain concentrations and under certain conditions of exposure (Digangi et al., Citation2002). Phthalates are covalently bonded to the polymers hence are able to leach from the plastic materials into food when heated or stored with food for a longer period (Alin, Citation2012). These migratory chemical species have raised several concerns owing to their potential carcinogenic effects in rodents (Barnes et al., Citation2007).
Phthalates are described as endocrine disruptors and therefore have been linked to adverse reproductive effects in male rodents by the National Toxicology Program (NTP) and Breast Cancer & the Environment Research Centers (BCERC) after various researches (Bonini et al., Citation2008). Though there are limited number of studies on humans, the wealth of research data on animal studies shows that some of these phthalates such as Di-(2-ethylhexyl) phthalate (DEHP) are toxic and can impair reproduction and development, alter liver and kidney functions, damage the heart and lungs and also have adverse effects on blood clotting (Heise & Litz, Citation2004). Several studies have also shown that phthalates exposure increases the growth of breast cells in vitro (Cancer & Environment, Citation1988).
2. Materials and methods
2.1. Samples collection and preparation
Five different kinds of polyethylene food contact materials specifically black polyethylene bags (BPB), plain polyethylene bags (TAB), thick plain polyethylene films/bags (TPB), polyethylene food containers (PFC) and polyethylene plastic bottles (PPB) were purchased from three different local open markets: Madina, Makola and Kwame Nkrumah circle, all within the Accra Metropolitan Assembly. These food contact materials were each cut accurately into 3 × 3 cm pieces and thoroughly mixed to give a representative sample.
2.2. Chemicals and reagents
Ethyl acetate, acetone, acetonitrile and n-hexane, Di-(2-ethylhexyl) phthalate [99.8%], Benzyl butyl phthalate [99.7%], Diethyl phthalate [99.9%] and Dibutyl phthalate [99.8%] were procured from Paska Fine Chemical Industries (India), Riedel-de Haen (Germany), VWR, Prolab (France) and Wako Pure Chemical Industries Ltd (Japan) respectively. Olive oil in glass bottles was procured from the Madina market. Distilled water was obtained from the General chemistry laboratory of Ghana Standards Authority. All glasswares used were washed with detergent, rinsed thoroughly with distilled water and dried in an oven. The dried glassware was each rinsed thoroughly with n-hexane and acetone before each use.
2.3. Determination of phthalates migration in aqueous foods
To evaluate the migration of phthalates from food contact materials into aqueous foods, ultrapure water was used as a food simulant according to the European commission regulation No.10/2011.
These food contact materials included three different types of polyethylene bags/films, plastic bottles and plastic food containers. Each of the samples was cut into 3 × 3 cm pieces and 2.0 g each of the samples accurately weighed and immersed in 100 mL distilled water. The samples were heated for 30 min, 1, 2 and 4 h at 20°C, 40°C and 80°C using a water bath with a temperature regulator. Another set of twenty (20) samples were accurately weighed (2.0 g) into beakers of 100 mL distilled water and kept in a fridge at 5°C for 30 min, 1, 2 and 4 h with the maximum and minimum temperatures set at 5°C ± 4°C to regulate the temperature of the fridge. The temperature conditions and contact times were chosen based on conditions that are commonly used in everyday life, where the higher temperature is used for cooking food, the medium temperature is for thawing food or handling hot foods and the lower temperature is for frozen foods. The samples after attaining the contact times and temperatures assigned, were removed, allowed to cool and the aqueous solution for each sample was extracted with 50 mL ethyl acetate using 250 mL separatory funnel. The extraction with ethyl acetate was repeated two more times and the organic layers combined and dried over 1 g of magnesium sulfate, filtered and evaporated in vacuo to dryness using a rotary evaporator. The extract was reconstituted with 2 mL ethyl acetate and subjected to a Gas Chromatography-Mass Spectrometer (GC-MS) analysis after ultra-sonication for 1 min.
The analysis of each batch of samples contained a sample blank and a spiked sample.
2.4. Determination of phthalates migration in fatty foods
The analysis of phthalates migration from polyethylene contact materials was carried out using olive oil as a fatty food simulant according to European Commission regulation No. 10/2011. The analysis was carried out with two out of the five polyethylene food contact materials commonly found in the food industry in Ghana. These were the take away polyethylene bags (TAB) and the polyethylene food containers (PFC).
Each sample was cut into 3 × 3 cm pieces and 2.0 g accurately weighed into clean 100 mL beakers containing 20 mL of olive oil. The samples were then heated in a water bath for 30 min, 1, 2 and 4 h at 20°C, 40°C and 80°C. Another set of samples were kept in a fridge at a temperature of 5 ± 4°C for the same different contact times. Each batch contained a sample blank and a spiked sample. 10 mL acetonitrile was added to each sample including the sample blanks and the spiked samples after the required temperatures and contact times were reached and the samples allowed to attain room temperature. The samples were vortexed for 1 min and centrifuged for 5 min at 3000rpm. The samples were left overnight in a freezer. Subsequently, the frozen samples were centrifuged for 5 min at 3000rpm and 6 mL each of the samples extract picked for cleanup.
For cleanup, 150 mg of Primary Secondary Amines (PSA) was accurately weighed into a glass centrifuge tube prior to the addition of 900 mg MgSO4. The 6 mL extract of the acetonitrile layer was added and centrifuged for 5 min at 3000rpm after 1-min vortex. 4 mL of the clean sample was carefully transferred into a pear-shaped flask and 40 μL of 1% formic acid in acetonitrile added. The samples were then concentrated to dryness using a rotary evaporator and reconstituted with 2 mL ethyl acetate. The 2 mL ethyl acetate extract was then picked into vials after ultra-sonication for 1 min and analysed with a GC-MS.
2.5. Preparation of calibration standards
A bulk mixed phthalates standard of 100 mg/L was prepared by accurately weighing 0.01 g (10 mg) of each of the stock standards solutions into 20 mL clean-dried beakers. The standards were then dissolved in 10 mL ethyl acetate in each of the beakers and combined into a 100 mL volumetric flask. The beakers were rinsed thoroughly with ethyl acetate and added to the 100 mL volumetric flask and made up to the 100 mL mark with ethyl acetate. A mixed working phthalates standard of 100 μg/L was then prepared from the bulk phthalates standard by taking 0.1 mL of the bulk standard into a clean 100 mL volumetric flask and topping up with ethyl acetate to the 100 mL mark. Calibration standards of 1 μg/L, 5 μg/L, 10 μg/L and 20 μg/L for the migration test were prepared from the mixed working standard by taking 0.2 mL, 1 mL, 2 mL and 4 mL, respectively from the working phthalates standard into 20 mL volumetric flasks and making it up to the 20 mL mark for each of the calibration standards. The bulk and working standards were stored in a fridge at 5°C and the calibration standards freshly prepared from the working standards for each analysis.
2.6. Phthalate determination
The determination of phthalates migration in polyethylene food contact materials was conducted using a Varian CP 3800 Gas Chromatograph (GC) coupled to a Saturn 2200 mass spectrometer (MS). The GC was fitted with a programmable splitless injector; the injector-port temperature was maintained at 280°C. The injector line was packed with glass wool to improve vaporization and also to provide a surface for the collection of any dissolved plastic. A varian HP-5 (30 m × 0.25 mm × 0.25 μm) GC column temperature was programmed from 100°C to 260°C at 8°C/min, then to 300°C at 35°C/min and held for 8.86 min. The carrier gas, helium was set at a constant flow of 1 mL/min. A volume of 2 μL was injected at a normal speed with ethyl acetate as the rinsing solvent. A Saturn 2200 mass spectrometer equipped with a Varian CP8400 autosampler was operated in full scan mode for this analysis. The scanning parameters were across a range of m/z 45–300. The heated zones were set at 80°C for the manifold, 210°C for the ion trap and 260°C for the transfer line to prevent condensation of the analytes. Qualitative analysis of the phthalates was carried out by a Varian MS station software version 6.9
3. Results and discussion
3.1. Quality control analysis
The analysis of phthalates migration in polyethylene food contact materials was carried out with extreme care due to the ubiquitous nature of phthalates and its high affinity for fats hence the tendency of cross contamination of the samples. Each batch of samples was analysed with a spiked and a blank sample in duplicates. The percentage recovery of the method was evaluated by spiking extract samples in each batch by 5 μg/L of phthalate standard solutions. The mean concentrations of the spiked samples with the percentage recovery are shown in . The percentage recovery of the mean concentrations ranged from 88.34% to 116.98%.
3.2. Migration of phthalates from polyethylene contact materials at constant temperature into aqueous food simulant
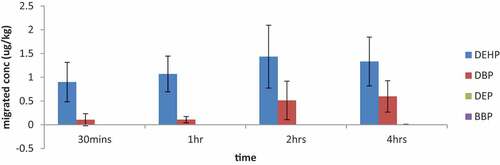
From the results in Figures –, it was observed that the phthalates migrated from the food contact materials into the food simulant (water) irrespective of the contact time. The average concentrations of DEHP that migrated from BPB, TAB, TPB, PPB and PFC were 1.01 μg/kg, 2.02 μg/kg, 0.56 μg/kg, 0.85 μg/kg and 1.07 μg/kg, respectively, after 30 minutes contact time at 5°C. The migrated concentrations of DEHP after the 4h contact time at 5°C also recorded; 2.29 μg/kg, 1.45 μg/kg, 1.11 μg/kg, 0.52 μg/kg and 1.54 μg/kg for BPB, TAB, TPB, PPB and PFC, respectively. Though there was phthalates migration observed in Figure , there was no linear correlation between the migrated concentrations of DEHP and the varied contact times. For instance, the highest average migrated concentration of DEHP was detected at the 2 h contact time whilst the highest average migrated concentration of DBP was detected at the 4 h contact time. From Figure , both di-2 (ethyl hexyl) phthalates (DEHP) and dibutyl phthalates (DBP) showed significant levels of migration in all the varied contact times whereas di ethyl phthalate (DEP) was detected at very low concentrations at the 4 h contact time. The benzyl butyl phthalates (BBP) however was not detected in any of the contact times. DEP according to (Heise & Litz, Citation2004) is used as a plasticizer in various cosmetics and personal care products and other cosmetic ingredients including medical treatment tubing. This might have accounted for the low concentrations of migrated DEP from the food contact materials.
A research by (Evelina et al., 2012) on the migration of phthalates, alkylphenols, bisphenols A and di (2-ethylhexyl) adipates from different food packaging materials revealed that the migrated concentrations of DBP, BBP and DEHP detected were similar to what was detected in this study. Additionally, another research by (Moreira et al., 2014) on plastic containers used in microwave oven reported migrated concentrations of DBP from <LOD to 2.0 μg/L and <LOD to 7.5 μg/L for new and used containers, respectively, which are not much different from the migrated concentration of DBP reported in this work.
A research study by (U.S. E.P.A, Citation2007) stated that DEHP, when absorbed into the body, increases liver weight which affects its functions such as impairing bile excretion by the liver. The study further listed the effects of DEHP to include causing testicular atrophy and infertility in male organs when accumulated in the male human body.
Di benzyl Phthalate (DBP), one of the common phthalates mostly found in food packaging materials as shown in this work and other studies have serious adverse effects on humans when consumed in greater quantities. For instance (Digangi et al., Citation2002) opined that DBP causes infertility in the male organism by interfering with the male reproductive tract development when accumulated in the system. This is because of its toxicity to cells in the testes that are responsible for normal sperm and hormone production.
3.3. Migration of phthalates from polyethylene contact materials at varied temperature into aqueous food simulant
The migration of phthalates from polyethylene packaging materials into food using distilled water as food simulant was analysed at constant contact times with varying temperatures. The packaging materials were exposed to the food simulant at 30 min, 1, 2 and 4 h at varying temperatures of 5, 20, 40°C and 80°C.
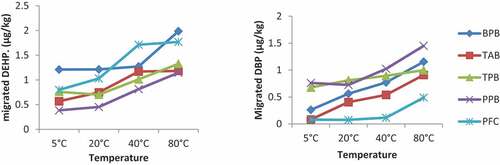
Though the concentrations of migrated DBP from the packaging materials into the food simulant were detected at lower concentrations compared to DEHP, there was a linear correlation between temperature and the migrated phthalate concentrations. The concentrations of the migrated DBP in the TAB increased gradually as the temperature of the contact time increases from 0.02 μg/kg at the 5°C to 0.41 μg/kg at the 80°C as shown in Figure . The migration of DEHP as indicated below shows a continuous increase in the migrated concentration from the 5°C to the 80°C in all the packaging materials shown in Figure ,b). For example, the migrated concentration of DEHP for the Black Polyethylene Bags (BPB) increased from 0.83 μg/kg at the 5°C continuously to a concentration of 1.21 μg/kg at 80°C. This trend of continuous increase in the concentration of the migrated DEHP as temperature increase was observed in all the migration analysis of phthalates in the polyethylene packaging materials. The average mean concentration of the migrated DBP was lower in concentration than the average-migrated concentration of the DEHP. This may be due to the difference in molecular structure, thus phthalates with long chains such as DEHP are weakly bonded to the packaging materials hence would easily migrate when the heat is applied.
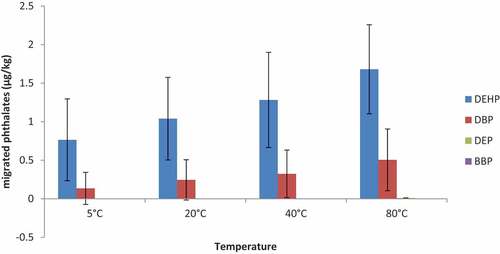
The migration of phthalates from polyethylene food contact materials into food or food simulant as observed in the migration trend has a linear correlation with increasing temperature. The migration of phthalates is dependent on temperature hence the concentrations of migrated phthalates increases as the temperature of contact between the food contact material and the food or food simulant increases. This is graphically shown in Figures and . As shown in the graph, the average concentrations of the migrated phthalates, i.e. DEHP and DBP increases gradually from the lowest temperature of 5°C to the highest temperature of 80°C. A study by Nerin et al. according to (Miriany et al., Citation2014) indicated that temperature increase leads to an upsurge in the decomposition of additives and breakdown of polymer chains which causes the release of chemicals from the hot plastic surface and perhaps this might have accounted for the increase in migrated phthalate concentrations as the temperature increases.
The migrated DEP concentration was detected in minute concentrations averaging 0.01 μg/kg at the 4 h contact time similar to that detected at the constant temperature with varying contact times as shown in Figure . BBP migration was not detected in any of the food contact materials at varying temperatures. As indicated above, the average concentrations of the migrated phthalates detected were 0.76 μg/kg, 1.04 μg/kg, 1.28 μg/kg, 1.68gμ/kg and 0.13 μg/kg, 0.24 μg/kg, 0.32 μg/kg, 0.50 μg/kg, respectively, for DEHP and DBP at 5°C, 20°C, 40°C and 80°C respectively.
3.4. Migration of phthalates from polyethylene food contact materials into fatty food simulant (olive oil)
With respect to the phthalates migration from food contact materials into olive oil as a food simulant, two of the food contact materials namely; polyethylene food containers (PFC) and the plain polyethylene bags/films commonly called take away bags (TAB) were considered. Table summarizes the various detected concentrations of the migrated phthalates from the food contact packaging material (PFC). DEHP which was detected in trace amounts in all the blank samples was the predominant phthalate detected in the fatty food simulant followed by DEP and then DBP. Whereas DEP was detected in minute concentrations at the highest temperature (80°C) and the highest contact time (4hrs) in the aqueous food simulant, it was however detected in almost all the samples with significant concentrations in the olive oil. The migrated concentrations of DEHP ranged from 0.69 mg/kg to 1.95 mg/kg at the highest temperature and highest contact time. DEP migrated concentrations however ranged from “not detected” to 1.77 mg/kg. DBP which was one of the predominant migrated phthalates detected in the aqueous food simulant was also detected in significant concentrations in the fatty food simulant (olive oil) from “not detected” to 1.43 mg/kg.
Table 1. Analytical recovery % of spiked samples at 5ppb
Table 2. Average concentrations of migrated phthalates in polyethylene food container (PFC)
BBP was not detected in any of the polyethylene packaging materials. The same was observed in the phthalate migration into the aqueous food simulant. The migrated phthalate concentrations detected in the olive oil, however, showed very high concentrations compared to the migrated phthalate concentrations detected in the aqueous food simulant. This may be due to the high affinity for fats by phthalates as reported by (Lacoste, Citation2014).
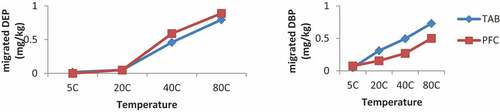
From Figure (a,b), it is observed that the migrated phthalates concentration increased as the contact time increased from 30 min to 4 h. The concentration of migrated DEHP increased from 0.69 mg/kg at the 30 min contact time to 1.22 mg/kg at the 4 h contact time for the 5°C constant temperature and also from 0.59 mg/kg at 30 min contact time to 1.55 mg/kg at the 4 h contact time for the 40°C constant temperature for the DEP. Thus, the rate of migration increased with increasing contact time, indicating a correlation between the migrated phthalates concentrations and the contact times. There was also a corresponding increase in the migrated concentrations with increasing temperature. This trend of phthalate migration with both contact time and temperature was observed in all the migrated phthalates in all the packaging materials. A study on the migration of plasticizers from PVC films into food by (Mercer, Citation1990) reported that the levels of phthalates found in retail foods wrapped in phthalate-plasticized films contained 0.1ppm—14.1ppm DBP and 0.06ppm—2.4ppm DEHP. The migrated phthalate concentrations reported in this work for DEHP and DBP, therefore, were consistent with what was reported by Mercer.
Average concentrations of the various phthalates migrated from the take away polyethylene bags are shown in Table . Take away polyethylene bags are the common plastics used to wrap most raw and ready to eat foods on the Ghanaian market. Similar to what was observed in the migration of phthalates in the polyethylene food containers, the predominant migrated phthalate was DEHP followed by DEP and then DBP whereas migrated BBP was not detected in any of the samples.
Table 3. Average concentrations of migrated phthalates in take away bags (TAB)
From Table , it is observed that the migrated concentrations of the phthalates increased as temperature and contact time increased from 50C to 80°C and from 30 min to 4 h, respectively. For example, the migrated concentration of DEP at 5°C increased from 0.02 mg/kg to 0.80 mg/kg at 80°C for the 30 min contact time and also from 0.02 mg/kg from the 30 min contact time to 0.52 mg/kg for 4 h at the 5°C.
Figure 5. a Migration of DEP into olive oil for 30 mins, b Migration of DBP into olive oil for 2hrs.
As observed in Tables and , the concentrations of migrated phthalates in the olive oil are not only higher in the fatty food simulant than the aqueous food simulant but also increases with increasing temperature and contact time.
The migration of phthalates to food/food simulants from packaging materials under different storage conditions for the past decade has been reported by several researchers such as castle et al. (1990), Lau and wong (1996), Goulas et al. (2000). A study by Xu et al. (2010) evaluated the migration of eight (8) phthalates from plastics in cooking oil and mineral water under different storage conditions and reported that the content of the eight (8) phthalates was always higher in the cooking oil than the mineral water. DEHP and DBP according to Xu et al. were the dominant phthalates in the cooking oil migration whilst DBP and DINP were dominant in the mineral water migration. The results, therefore, reported in this work are similar to what was reported by Xu et al.
4. Conclusion
The concentration of all the phthalates detected in the aqueous food simulant in this study was in accordance with the current EC regulation for the migration of phthalates in food contact materials (FCM). The regulation (EU) No. 10/2011 states 1.5 mg/kg as the specific migration limit (SML) of food simulant for DEHP, 0.3 mg/kg for DBP and 30.0 mg/kg for BBP of products intended for food contact. The concentrations of all the migrated phthalates obtained from the polyethylene contact materials in the distilled water were below the SML. DEHP which was the dominant phthalates migrated in the distilled water had its highest migrated concentrations of 1.68 μg/kg at the highest temperature and 1.43 μg/kg at the 2 h contact time. DBP on the other hand had its highest migrated concentration of 0.60 μg/kg after 4 h contact time and 0.50 μg/kg at the highest temperature of 80°C. The migrated concentrations of the phthalates in the olive oil however, had some of its concentrations exceeding the SML stated in the regulation (EU) 10/2011. Di-2 (ethyl hexyl) phthalates (DEHP) and di benzyl phthalate (DBP) for example, had some of the migrated concentrations exceeding the SML stated above for food contact materials.
There was a correlation between the increase in phthalate migration and increasing temperature in both food simulants. Though the migration of phthalates during the various contact times in the aqueous food simulant was observed, there was no linear correlation between the migrated phthalate concentrations and the contact times observed whereas a linear correlation was observed between the various migrated phthalates and the contact times in the olive oil migration.
The migrated phthalate concentrations found in the fatty food simulant coupled with the ubiquitous nature of these phthalates in the environment and several exposure routes, there is the need for more research work to be carried out on phthalates to ascertain the adverse health effects and the possible accumulation of these phthalates at different exposure routes so that regulatory bodies can enact legislation to help curb its use in the manufacturing process especially in the production of food contact materials.
Acknowledgements
We are grateful to Dr Paul Osei-Fosu and the staff of the Pesticide Residue Laboratory – Ghana Standards Authority, for their support in providing the chemicals and glassware used in this project. Our appreciation also goes to Dr Samuel Frimpong, Kumasi Regional Officer- Ghana Standards Authority for his assistance and guidance in the use of the GC-MS. We would also like to thank staff of the Metallic Contaminants Laboratory-GSA for their support and encouragement throughout this project.
Additional information
Funding
Notes on contributors
Adongo Abdul-Malik Ayamba
The Author, Ayamba A. Abdul-Malik is an analytical chemist with several years of experience in metallic Contaminants analysis in both food and environmental Samples using the Atomic Absorption Spectrophotometer (AAS) and the Inductively Coupled Plasma Mass Spectrometer (ICP-MS).
To have abetter understanding of food safety with respect to food contaminants, the author also has research interest in other food contaminants such as Aflatoxins, Histamine and pesticide residue. Mr Ayamba is currently aSenior Scientific Officer (SSO) of the Food and Agriculture Department of Ghana Standards Authority and holds amaster of philosophy degree in analytical chemistry. He is passionate about research since that is the only way to have abetter understanding of the world.
References
- Alin, J. (2012). Migration from plastic food packaging during microwave heating, American chemical society. Journal of Agricultural and Food Chemistry, 59(10), 1405–11.
- Barnes, K. A., Richard Sinclair, C., & Watson, D. H. (2007). Chemical migration and food contact materials. Woodhead publishing ltd.
- Bhunia, K., Sablani, S. S., Tang, J., & Rasco, B. (2013). Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Comprehensive Reviews in Food Science and Food Safety, 12(5), 523–545. https://doi.org/10.1111/1541-4337.12028
- Bonini, M., Errani, E., Zerbinati, G., Ferri, E., & Girotti, S. (2008). Extraction and gas chromatographic evaluation of plasticizers content in food packaging films. Microchemical Journal, 90(1), 31–36. https://doi.org/10.1016/j.microc.2008.03.002
- Brody, A. L., Strupinsky, E. R., & Klime, L. R. (2002). Active packaging for food applications. CRC press LLC.
- Cancer, B., & Environment, T. H. E. (1988). Early Life Exposure to Phenols and Breast Cancer Risk in Later Years fact sheet on phenols. 1988(8), 9–20. www.bcerc.org/cotc.htm.
- Digangi, J., Schettler, T., Cobbing, M., & Rossi, M. (2002). Aggregate exposures to phthalates in humans. CRC press.
- Heise, S., & Litz, N. (2004). Phthalates. German Federal Environmental Agency.
- Lacoste, F. (2014). Undesirable substances in vegetable oils: Anything to declare? OCL, 21 (1),A103. EDP Sciences. http://www.ocl-journal.org/articles/ocl/full_html/2014/01/ocl130041/ocl130041.html
- Lule, F., Kigozi, J. B., Ssempala, C., & Banadda, N. (2012). Development and evaluation of models for predicting chemical contaminant migration in foods. Makerere university press, Uganda.
- Marsh, K., & Bugusu, B. (2007). Food packaging–roles, materials, and environmental issues. Journal of Food Science, 72(3), R39–R55.
- Mercer, A. (1990). Migration studies of plasticizers from PVC film into food. De Montfort University. http://hdl.handle.net/2086/4319
- Miriany, A., André, L. C., & Cardeal, Z. L. (2014). Analysis of phthalate migration to food simulants in plastic containers during microwave operations. International Journal of Environmental Research and Public Health, 11, 507–526.
- U.S. E.P.A. (2007). Toxicity and exposure assessment for children’s health, phthalates. U.S.E.P.A