Abstract
The labyrinth is a simple geometric form with one path leading to the center and out. It is often used in religious and health-related institutions for quiet walking and meditation. It is considered a convenient tool for decreasing psychological and physical stress. This study sought to better understand and characterize the short-term physiological responses of the autonomic nervous system associated with walking a projected light labyrinth by measuring respiratory sinus arrhythmia (RSA) and salivary alpha amylase (sAA) in 25 young adults and youth. Our objective was to examine the physiology of labyrinth walking as a potential clinical tool for use by individuals who are experiencing psychological stress. Three hypotheses were put forward: 1) walking the labyrinth would result in physiological arousal as indicated by a decrease in RSA and an increase in sAA; 2) physiological relaxation would be indicated by an increase in RSA after the labyrinth walk; and 3) participants would self-report calmness and relaxation following the labyrinth. Consistent with hypotheses, participants experienced immediate physiological arousal while walking the labyrinth, and heightened physiological and self-reported relaxation during and after the labyrinth walk.
PUBLIC INTEREST STATEMENT
Stress reduction techniques have become important to a public that experiences increasing demands on personal time and resources. This is especially true for those undergoing psychological and physical duress in a medical context where there may be few opportunities for stress reduction. Holistic activities that bring balance to body, mind, and spirit are becoming more accepted and available in medical settings. Our research team studied the stress response in 25 teens and young adults at a pediatrics hospital in an effort to understand the physiological and psychological benefits associated with walking a labyrinth. This research, like other research on meditative practices, supports the notion that a short, focused walk, using the labyrinth form, can induce physiological responses that are consistent with engagement and relaxation, and enhance a person’s experience of calmness and relaxation.
Competing Interests
The authors declare no competing interest.
1. Introduction
A labyrinth is a geometric form with earliest representations found as petroglyphs dating back more than 4,000 years (Saward, Citation2003). Throughout the centuries, the labyrinth form has been used as a tool for religious devotion and spiritual protection (Bloos & O’Connor, Citation2002). In present day, by using a simple unicursal design (i.e., one path leading to the center and out) the labyrinth is believed to serve a holistic function to promote a more balanced spiritual, psychological, emotional, and physical well-being (Harris, Citation1999). Sandor observed that most labyrinths in medical centers are primarily used to help reduce stress, manage grief, or relax (Sandor, Citation2005). Currently there are more than 4,000 labyrinths open to public use in the United States and Canada; most are located in churches and religious centers (42%) with an increasing number (13%) located in hospitals, prisons, and educational settings (Labyrinth Locator, Citation2017).1.1. Meditation and the physiological response
Meditative practices are presumed to relax the body, induce a lower metabolic state, and calm the mind (Lazar et al., Citation2000). Qualitative and anecdotal reports suggest that, like other meditative practices, walking the labyrinth is mentally calming. Individuals who walk the labyrinth report experiencing positive benefits, including feelings of calmness, mental focus and reduced anxiety, agitation, and stress (Peel, Citation2004). Less well understood are the effects that walking the labyrinth has on short-term physiological responses especially as they relate to anecdotal reports of calmness and relaxation.
Short term physiological responses (i.e., arousal or relaxation) are largely regulated by two divisions of the autonomic nervous system (ANS), although other systems are involved. The parasympathetic division (commonly referred to as “rest and digest” processes) predominates during quiet resting conditions and serves to reduce physiological arousal while the sympathetic division (commonly referred to as “fight or flight” processes) predominates during emergency reactions and promotes physiological arousal.
Indirect measures of the parasympathetic and sympathetic divisions of the ANS can be non-invasively assessed using respiratory sinus arrhythmia (RSA) and salivary α-amylase (sAA), respectively. RSA is a measure of variability in heart rate that occurs with respiration and reflects parasympathetic activity on the heart emanating from the vagus nerve (Berntson et al., Citation1997). According to Porges’ polyvagal theory (Porges, Citation2003) and the model of neurovisceral integration (Thayer, Hansen, Saus-Rose, & Johnsen, Citation2009), the vagus nerve provides the neurophysiological substrate for regulating arousal and relaxation. sAA is an enzyme produced by the saliva gland that is sensitive to sympathetic influences on stress-related changes in the body (Nater & Rohleder, Citation2009). The sympathetic and parasympathetic divisions of the ANS work in tandem to regulate stress responses, and data from HRV and sAA together provide a more complete picture of the short-term physiological responses than either system alone.
There is an extensive literature documenting the physiological effects of different forms of meditation (Balaji, Varne, & Ali, Citation2012; Barnes, Treiber, & Johnson, Citation2004; Delmonte, Citation1984; Ditto, Eclache, & Goldman, Citation2006; Peng et al., Citation2004; Tang et al., Citation2009; Travis, Olson, Egenes, & Gupta, Citation2001; Wallace, Citation1970). This research largely suggests that meditation is associated with a reduction in physiological arousal. For example, Anderson et al. reviewed nine randomized controlled trials (RCTs) and reported that transcendental meditation reduces systolic and diastolic blood pressure by 4.7 and 3.2 mmHg, respectively (Anderson, Liu, & Kryscio, Citation2008).
Delmonte et al. observed that, compared to rest, active meditation resulted in similar reductions in physiological arousal (i.e., reductions in blood pressure, heart rate, skin conductance, and electromyographic muscle tension) among 40 non-meditators and 12 experienced meditators (Delmonte, Citation1984). More recently, Tang et al recruited 86 undergraduate Chinese students and measured physiological and brain changes before, during, and after 5-days of integrative bodymind training (IBMT) relative to a relaxation only control condition (Tang et al., Citation2009). During and after training, the IBTM condition evidenced better physiological reaction in heart rate, respiration rate, and skin conductance relative to controls. Yet, not all results have been similarly consistent. In a series of two laboratory-based experiments, training in mindful bodyscan meditation resulted in an increase in cardiac sympathetic and parasympathetic function relative to listening to a popular novel, suggesting a mix of arousal and relaxation (Ditto et al., Citation2006). Moreover, different meditation practices have been reported to induce different profiles of physiological effects. For example, Peng et al. compared three forms of meditation and observed both similarities and differences in the physiological effects of three forms of meditation, concluding that some forms of meditation may, paradoxically, induce physiological arousal (Peng et al., Citation2004).
Recent evidence suggests that the physiological profile associated with various forms of meditative practices depend on characteristics such as the type of meditation and the level of meditation experience. For example, meditation practices that involved emotional (i.e., loving-kindness) or meta-cognitive (i.e., observing thoughts) demands may be less relaxing and accompanied by an increase in heart rate and a reduction in parasympathetic function compared to an attentive breathing meditation practice, suggesting heightened arousal (Lumma, Kok, & Singer, Citation2015). In a comprehensive and informative review, Britton et al. hypothesize that the effects of meditative practice unfold in a nonlinear multiphasic manner, with early phases being more effortful and producing greater arousal opposed to later stages which produce wakefulness and neuroplastic changes (Britton, Lindahl, Cahn, Davis, & Goldman, Citation2014). It is important to better understand the mental and physiological profiles of a labyrinth walk given that: 1) there is little research on the labyrinth, 2) different meditative practices induce different profiles of physiological reactivity, and 3) this information can be used to inform the selection of which techniques are best suited for use in various clinical populations.
1.2. Current study
To date there has been no research characterizing short-term changes in RSA and sAA during a walking labyrinth meditation practice, though anecdotal reports indicate that it is associated with a state of relaxation and mental calming. Our objective was to characterize the short-term physiological profile of autonomic arousal that occurs as a result of a walking labyrinth meditation practice. Given that there are emotional and cognitive demands associated with the labyrinth and that meditative practices may include dynamic changes in physiology, three hypotheses were put forward. First, we hypothesized that walking the labyrinth would result in physiological arousal as indicated by a decrease in RSA and an increase in sAA from baseline to the walking phase. Second, the labyrinth was hypothesized to result in greater relaxation, relative to baseline, as indicated by an increase in RSA after walking the labyrinth. Three, the labyrinth was hypothesized to result in greater mental clarity as indicated by self-report of calmness and relaxation.
2. Methods
2.1. Participants
Youth and young adults between the ages of 12 and 24 were recruited at a large Canadian Children’s Hospital to practice a walking labyrinth meditation. All recruits were volunteers at the hospital, many of whom were previous patients. Participants were English speaking and were excluded if their history included heart anomalies or defects. This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary, and all participants provided informed assent (in the case of minors whose parents provided informed consent) or informed consent prior to data collection.
2.2. Procedures
The study was conducted within the Sacred Space at the Alberta Children’s Hospital, which hosts a projected light labyrinth using a ceiling mounted projector (Buchanan Citation2007). The participants walked a classic pattern, five circuit, labyrinth of light, where the labyrinth pathway is projected white light. The “walls” of the labyrinth are indicated as dark (shadow) lines. Upon arrival, participants met with two research assistants in an office adjacent to the Sacred Space. Research assistants divided their tasks into data recorder and labyrinth walk guide. During the pre-walk phase, participants provided basic personal information, and provided a baseline saliva sample. Then they were outfitted with the heart rate monitoring device (Firstbeat Bodygaurd™) and asked to sit quietly for a period of three minutes to obtain a baseline recording. Prior to entering the Sacred Space they were given instructions with respect to labyrinth walking, which included picking up a small stone found in a bowl at the center of the labyrinth. They were asked to carry the stone out of the labyrinth reflecting upon how the stone might represent something good and hopeful in their life. A “guide” modeled the entire procedure for the participant.
Upon entering the Sacred Space, participants and the two research assistants were immediately seated for 3 min. This allowed for an acclimation period to the Sacred Space.
The research assistant guide then began walking the labyrinth as a model for the participant.
Once the research assistant guide reached the middle of the labyrinth, the participant stood and began the walk toward the middle, mimicking slow quiet walking (Path In). The research assistant guide followed the path out during this time. Once in the middle, the participant picked up a stone which they could use to think about something good and hopeful in their life. The participant then slowly walked out of the labyrinth holding the stone (Path Out). Once completed, the participant was seated for 3 min in the Sacred Space (Recovery). Finally, a second saliva sample was taken, the heart rate monitor was removed and a post walk interview asked three questions: “How would you describe your labyrinth walk?”, “How do you feel after walking the labyrinth”, and “What else would you like to say about your experience today”.
2.3. Measures
Cardiac vagal control (CVC) was indexed using Respiratory Sinus Arrhythmia (RSA)—variability in heart rate that fluctuates in phase with spontaneous respiration [see (Grossman & Taylor, Citation2007)]. RSA was measured using continuous heart period recordings of R-R intervals sampled at a rate of 1,000 Hz. Participants wore a Firstbeat Bodyguard recording device (Firstbeat Technologies, Oy Jyvaskyla, Finland) connected to two leads by pre-gelled (Ag/AgCl) disposable electrocardiograph (ECG) electrodes attached beneath the right clavicle and to the left ribcage.
Salivary α-amylase (sAA) was collected using whole saliva obtained from under the tongue with the Salimetrics Oral Swab (Salimetrics, State College, PA). Production of sAA is delayed by approximately 5-min relative to an experience (such as stress or the labyrinth walk) and therefore sAA concentrations in saliva reflect the physiological experience 5-min prior to the sample collection. For the current study, this means that the recovery saliva sample reflects the later phase of the labyrinth walk (i.e., the activity 5-min prior to sample collection). Saliva samples were stored at −80°C until assayed with the Salimetrics assay. The assay uses a chromogenic substrate, 2-chloro-ρ-nitrophenol linked to maltotriose. The enzymatic action of sAA yields 2-chloro-ρ-nitrophenol, which can be spectrophotometrically measured at 405 nm using a laboratory plate reader. The amount of sAA activity present in the sample is directly proportional to the increase (over a 2-min period) in absorbance at 405 nm. Results were computed in U/mL of sAA. Given that sAA concentrations may be affected by both salivary flow rate (mediated by the PNS) and protein secretion (mediated by the SNS) (Bosch, Veerman, De Geus, & Proctor, Citation2011), we controlled for parasympathetic influence on sAA by adjusting for flow rate using a previously described method (Beltzer et al., Citation2010). In brief, raw sAA concentration (U/mL) was multiplied by flow rate where flow rate was computed by dividing the sample volume (in mL) by the collection time (in minutes).
2.4. Data reduction
RSA was quantified using Nevrokard advanced Heart Rate Variability (aHRV) analysis software. R-R intervals during each phase were re-sampled into 70-s segments (2- segments for baseline, 2-segments for entry to the sacred space, 1-segment for path-in, 1- segment for path-out, and 2-segments for recovery). Heart period recordings for each segment was screened for artifacts according to recommendations (Berntson, Quigley, Jang, & Boysen, Citation1990). An algorithm was set to detect ectopic beats, defined as interbeat intervals below 200 ms, above 2,000 ms, and those that differed from the previous and subsequent 50 interbeat intervals by a value greater than 20%. Interpolation was used to correct ectopic and missing beats.
Segments requiring more than 10% data interpolation were deemed artifactual and excluded.
After scanning for artifacts, HRV was quantified using Fast Fourier Transformations (FFT).
RSA was quantified as the average power spectral density of R-R fluctuations occurring in the respiratory band (.15–.40 Hz) recommended for use with adults (Berntson et al., Citation1997; Camm et al., Citation1996). RSA for each phase was calculated as the mean RSA value occurring across the 70 s segments composing each phase. As is typical in this area of research, mean RSA values were then log transformed using the natural logarithm to produce normal distributions.
2.5. Statistical procedures
2.5.1. Data screening
Data from 26 participants were screened for potential outliers, (z-score > 3.29) and adjusted according to recommendation (Tabachnick & Fidell, Citation2013). No more than one value (4%) was adjusted for any variable. Multivariate outliers were assessed using Mahalanobis distances. One participant was a multivariate outlier, exceeding χ2(7) critical of 24.32, p < .001, and was removed from analyses resulting in a final sample of 25.
2.5.2. Effect of labyrinth on RSA
The effect of labyrinth on RSA was examined using a five condition (baseline, enter sacred space, path-in, path-out, recovery) repeated measured Analysis of Variance (ANOVA).
2.5.3. Qualitative measures
Qualitative data was analyzed using a general inductive approach commonly used in health and social science research and evaluation (Thomas, Citation2006). A coding framework of key words and phrases was devised after multiple readings and familiarity with the text. In this study, two researchers, independent of each other, read the text and coded the data. After discussion, common themes were identified, new codes were devised and the data was reassessed based upon the enhanced coding frame. In this way themes were developed and refined iteratively with each new reading and discussion of the data.
3. Results
The final sample consisted of 25 (21 female) youth and young adults with a mean age of 19 years (SD = 2.93). The majority of the sample (64%) reported no previous experience with meditation, seven participants (28%) described themselves as “occasional” meditators, while two participants (8%) reported being “active” meditators. Refer to Table for descriptive statistics of the sample.
Table 1. Descriptive statistics of the sample
3.1. Effect of labyrinth walk on physiological arousal
There was a significant main effect of phase on RSA, F(4, 96) = 30.30, SEM = 0.26, p < .01, ηp2 = .56, indicating significant change in RSA during the labyrinth walk. Follow-up tests were performed using pairwise comparisons after adjusting for inflation in Familywise error due to conducting multiple comparisons using Least Significant Difference. There was a significant increase in RSA from baseline to entry into the sacred space, Mdiff = 0.46 Ln(ms2/Hz), SE = 0.12, p < .01. RSA was significantly reduced during path-in, Mdiff = −0.76 Ln(ms2/Hz), SE = 0.18, p < .01, and path-out, Mdiff = 0.57 Ln(ms2/Hz), SE = 0.15, p < .01 relative to baseline. Figure presents a visual depiction of the change in RSA across the labyrinth task.
Figure 1. Change in respiratory sinus arrhythmia (RSA) and salivary α-amylase (sAA) across the labyrinth task. sAA was measured during recovery but plotted during path-out to reflect the 5-min delay between arousal and secretion of sAA in saliva. Error bars represent standard error of the mean
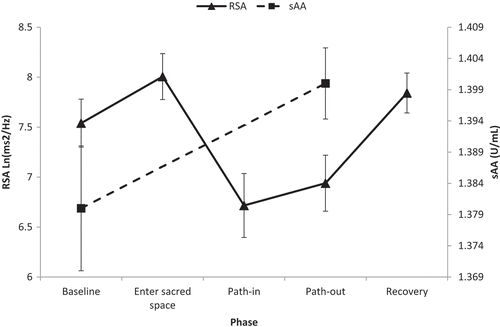
A concomitant increase in sAA was observed from baseline to the path-out phase of the labyrinth walk, Mdiff = −0.17 U/mL, t(24) = 2.04, SEM = 0.08, p = .05, d = 0.83, refer to Table for means. Note that although sAA was assessed using a post-walk saliva sample, this sample reflects sAA levels that are relevant to 5 min prior to sample collection.
3.2. Effect of labyrinth walk on physiological relaxation
Follow-up tests on the significant main effect of phase on RSA indicated that RSA increased significantly from labyrinth path-out to the recovery phase, Mdiff = 0.90 Ln(ms2/Hz), SE = 0.13, p < .01. Overall, RSA in the recovery phase was significantly higher than the baseline phase, Mdiff = 0.30 Ln(ms2/Hz), SE = 0.14, p < .05, refer to Figure .
3.3. Qualitative reports of calmness and relaxation
An analysis of qualitative data revealed several themes related to the labyrinth walk. Several participants described their experience as calming (n = 19/25, 76%) or relaxing (n = 10/25, 40%). Feeling peaceful and less stressed or less anxious was described by 11 participants (n = 11/25, 44%). Few participants described their participation at any point in the walk in negative terms. Only five participants (20%) felt an increase in stress, mostly during the initial phase of the walk. No participant spoke negatively about the overall labyrinth walk experience. One participant reported feeling tired following the walk. A key theme that emerged from the coded data related to the participant’s “increased awareness” during and following the labyrinth walk (n = 18/25, 72%). This was described in several ways that included shifts in the participant’s sensibilities related to: time “gives me time to think”, physical body “made my legs feel heavy”, emotion or felt sense “found it uplifting”, environment “noticing things around me”, and meaning “chance to think about just me”; as it pertained to the labyrinth walk activity. Participants described their thinking in terms that implied a shift in perspective that was positive overall.
4. Discussion
This study represents the first systematic investigation of the short-term physiological and self-reported effects of a walking labyrinth meditation in primarily novice meditators.
Autonomic nervous system activity of 25 youth and young adults that occurred in response to a walking labyrinth meditation practice was measured using RSA and sAA, and a qualitative account of participants’ personal experiences were gathered. Supporting our first hypothesis, walking the labyrinth was associated with RSA-withdrawal and an increase in sAA from baseline to the walking phase, suggesting the presence of emotional and cognitive demand. Supporting our second hypothesis, RSA-increased from path-out to recovery and ended at a level higher than baseline. Finally, the majority of participants described the experience as calming or relaxing, supporting our third hypothesis.
Consistent with a recent review on meditative practices performed by Britton et al., our results suggest that a walking labyrinth meditation practice is associated with a multiphasic trajectory (Britton et al., Citation2014). During the initial phases of the meditative practice participants are engaged and perhaps challenged by the emotional and cognitive goals of the task resulting in physiological arousal, which we observed as RSA withdrawal (i.e., a decrease in RSA) and an increase in sAA. The parasympathetic and sympathetic divisions of the ANS typically work in tight reciprocal control (Berntson, Cacioppo, & Quigley, Citation1991) and the observed pattern of response indicates increased physiological arousal during the labyrinth which is consistent with the hypothesis that the labyrinth is an emotionally and physiologically engaging meditative practice. Indeed, previous research suggests that RSA withdrawal is a good indicator of cognitive effort (Luft, Takase, & Darby, Citation2009). This pattern of physiological change is consistent with the results of a recent study by Lumma et al. (Citation2015) which suggested that meditative practices in which the training goals are to develop emotional or meta-cognitive awareness are paradoxically associated with greater levels of physiological arousal than are practices where the goal is attentive awareness. In other words, practices that attempt to move individuals toward acquiring increased positive affect require more effort than meditative processes that focus on holding attention, such as focussing on breath. It is also worth noting that the majority of participants in the study were novices (i.e., first or second time walkers) and the degree of arousal observed in this study may change as a result of experience and training (Britton et al., Citation2014).
While the act of walking the labyrinth appeared effortful, resulting in physiological arousal, our results suggest that the overall result of the labyrinth practice was greater relaxation and calmness both physiologically and mentally. Toward the end of the practice, participants began to experience physiological (i.e., increased parasympathetic activity) and mental (i.e., feeling calm and relaxed) benefits. The increase in RSA from path-out to recovery suggests reduced physiological arousal upon completing the labyrinth. Thus, the end result of the labyrinth appears to be a physical, mental, and emotional state that lends itself to deeper reflection, insight, problem solving, and healing (La Torre, Citation2004). This is a similar pattern of results to those observed during loving-kindness meditation and observing-thoughts meditation, but not breathing meditation (Lumma et al., Citation2015). It is interesting to speculate that meditative practices fall along a continuum of arousal with breathing meditation resulting in relaxation (Lumma et al., Citation2015), awareness building Bodyscan resulting in no net change in arousal due to simultaneous parasympathetic and sympathetic arousal (Ditto et al., Citation2006), and more emotional/cognitive forms of meditation (e.g., loving-kindness, observing-thought) resulting in arousal (Lumma et al., Citation2015).
Previous research with other meditative practices suggests that training and increased familiarity reduces the perceived cognitive effort of meditation (Tang, Rothbart, & Posner, Citation2012; Zanesco, King, Maclean, & Saron, Citation2013). Extrapolating from these results, the potential benefits associated with the labyrinth may become even stronger with time and practice. Nevertheless, the labyrinth walk is accessible to novice walkers who seem to achieve some physiological and psychological benefit. These findings support our clinical observations using the labyrinth walk with youth and families at the Alberta Children’s Hospital.
4.1. Limitations to the study
There are a number of limitations to this study. First, the lack of a control condition or group is a limitation. Without a control condition/group it cannot be determined whether the observed results were specific to walking the labyrinth or a result of nonspecific factors such as novelty or task engagement. Second, we did not measure sAA as a marker of sympathetic activity during recovery. Given that sAA has a temporal delay and peaks 5–10 min following a stressor (Nater & Rohleder, Citation2009), our measurement of sAA following the labyrinth practice was optimized to measure sympathetic arousal during the path-in/path-out phases. Thus, our data cannot speak to the recovery phase with evidence from the sympathetic nervous system. Future studies could include additional saliva samples at follow-up to determine sympathetic arousal in the post-walk period. Third, different phases of the labyrinth involved different degrees of mild physical activity (sitting versus slow walking) which may partially account for, or obscure, the observed results (Grossman, Wilhelm, & Spoerle, Citation2004). Similarly, respiration rate and depth influences RSA as a marker of parasympathetic function (Grossman & Taylor, Citation2007) and this influence was not measured or adjusted for. Finally, we cannot rule out expectancy effects as the cause of the physiological and psychological changes that we observed.
Although we were careful to not suggest to participants what they might experience, the dim lighting, quiet music, and demeanor of the research assistant guide may have generated expectations of what they should be experiencing. The Sacred Space itself may have an effect on participants apart from the labyrinth walk. Such effects could be addressed in future research in labyrinths that are located in more neutral spaces, such as a gymnasium.
4.2. Future direction
Although the overall effect of the labyrinth walk was positive, we cannot know the duration of the effect beyond the recovery phase of the study. Longitudinal studies on meditation lasting several weeks suggest increased parasympathetic resilience (Tang et al., Citation2009) with the implication that continued use of the labyrinth as walking mediation may have a similar effect. The physiological effect of the walking labyrinth on experienced meditators and older individuals or younger children are also unknown. Further, there are several variables that could influence the experience of labyrinth walking, including size, design, light, sound, scent, location, and color of the labyrinth, participant age, gender, and previous experience, all of which require systematic manipulation in order to gain a more complete picture of the potential benefits of the labyrinth walk in clinical practice.
5. Conclusion
Results of this study indicate that walking a projected light labyrinth was an emotionally and physiologically engaging practice for youth and young adults, with an overall effect of greater relaxation and calmness. Results suggest that the availability of stress reduction tools similar to, and including the labyrinth, could have wide benefits for those undergoing physical and mental duress.
Ethics and Disclosure Statements
All participants of the study provided written informed consent and the study was approved by the Conjoint Health Research Ethics Board, University of Calgary. All authors/disclose no actual or potential conflicts of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence (bias) their work.
Cover image
Source: Jim Buchanan, Artist and Labyrinth Designer, Scotland.
Additional information
Funding
Notes on contributors
Philip James Behman
Our research team is part of the Alberta Children’s Hospital Research Institute (ACHRI) where we collaborate to create opportunities for clinically relevant research. The primary focus of our team’s research is on early life with an objective to determine how the psychobiology of stress shapes development and what factors ameliorate the effects of stress. We are particularly interested in developmental switch points during which exposure to protective factors may shift developmental trajectories toward healthy outcomes. The labyrinth and its use in walking meditation is a complementary stress reduction practice that is gaining interest in both medical, educational and other contexts. We hope that the current study will spark interest in future research that tries to better understand the physiological effects of complementary medicine practices with the potential to improve outcomes for children and youth.
References
- Anderson, J. W., Liu, C., & Kryscio, R. J. (2008). Blood pressure response to transcendental meditation: A meta-analysis. American Journal of Hypertension, 21(3), 310–316. doi:10.1038/ajh.2007.65
- Balaji, P. A., Varne, S. R., & Ali, S. S. (2012). Physiological effects of yogic practices and transcendental meditation in health and disease. North American Journal of Medical Sciences, 4(10), 442–448. doi:10.4103/1947-2714.101980
- Barnes, V. A., Treiber, F. A., & Johnson, M. H. (2004). Impact of transcendental meditation on ambulatory blood pressure in African-American adolescents. American Journal of Hypertension, 17(4), 366–369. doi:10.1016/j.amjhyper.2003.12.008
- Beltzer, E. K., Fortunato, C. K., Guaderrama, M. M., Peckins, M. K., Garramone, B. M., & Granger, D. A. (2010). Salivary flow and alpha-amylase: Collection technique, duration, and oral fluid type. Physiology & Behavior, 101(2), 289–296. doi:10.1016/j.physbeh.2010.05.016
- Berntson, G. G., Bigger, J. T., Jr., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., … Van Der Molen, M. W. (1997). Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648.
- Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487.
- Berntson, G. G., Quigley, K. S., Jang, J. F., & Boysen, S. T. (1990). An approach to artifact identification: Application to heart period data. Psychophysiology, 27(5), 586–598.
- Bloos, I. D., & O’Connor, T. J. (2002). Ancient and medieval labyrinth and contemporary narrative therapy: How do they fit? Pastoral Psychology, 50(4), 219–230. doi:10.1023/A:1014097211429
- Bosch, J. A., Veerman, E. C., De Geus, E. J., & Proctor, G. B. (2011). alpha-Amylase as a reliable and convenient measure of sympathetic activity: Don’t start salivating just yet! Psychoneuroendocrinology, 36(4), 449–453. doi:10.1016/j.psyneuen.2010.12.019
- Britton, W. B., Lindahl, J. R., Cahn, B. R., Davis, J. H., & Goldman, R. E. (2014). Awakening is not a metaphor: The effects of Buddhist meditation practices on basic wakefulness. Annals of the New York Academy of Sciences, 1307, 64–81. doi:10.1111/nyas.12279
- Buchanan, J. (2007). Labyrinths for the spirit: How to create your own labyrinths for meditation and enlightenment. New York, NY: Sterling Publishing.
- Camm, A. J., Malik, M., Bigger, J. T., Breithardt, G., Cerutti, S., Cohen, R. J., … Lombardi, F. (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17(3), 354–381.
- Delmonte, M. M. (1984). Physiological responses during meditation and rest. Biofeedback Self-Regulation, 9(2), 181–200.
- Ditto, B., Eclache, M., & Goldman, N. (2006). Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine, 32(3), 227–234. doi:10.1207/s15324796abm3203_9
- Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychoogyl, 74(2), 263–285. doi:10.1016/j.biopsycho.2005.11.014
- Grossman, P., Wilhelm, F. H., & Spoerle, M. (2004). Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. American Journal of Physiology-Heart and Circulatory Physiology, 287(2), H728–H734. doi:10.1152/ajpheart.00825.2003
- Harris, N. (1999). Off the couch: An introduction to labyrinths and their therapeutic properties. Annals of the American Psychotherapy Association, 2(2), 7–8.
- La Torre, M. A. (2004). Walking: An important therapeutic tool. Perspectives in Psychiatric Care, 40(3), 120–122.
- Labyrinth Locator. (2017, April 1). The labyrinth society. Retrieved from http://labyrinthlocator.com
- Lazar, S. W., Bush, G., Gollub, R. L., Fricchione, G. L., Khalsa, G., & Benson, H. (2000). Functional brain mapping of the relaxation response and meditation. Neuroreport, 11(7), 1581–1585.
- Luft, C. D., Takase, E., & Darby, D. (2009). Heart rate variability and cognitive function: Effects of physical effort. Biological Psychology, 82(2), 164–168. doi:10.1016/j.biopsycho.2009.07.007
- Lumma, A. L., Kok, B. E., & Singer, T. (2015). Is meditation always relaxing? Investigating heart rate, heart rate variability, experienced effort and likeability during training of three types of meditation. International Journal of Psychophysiology, 97(1), 38–45. doi:10.1016/j.ijpsycho.2015.04.017
- Nater, U. M., & Rohleder, N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34(4), 486–496. doi:10.1016/j.psyneuen.2009.01.014
- Peel, J. M. (2004). The labyrinth: An innovative therapeutic tool for problem solving or achieving mental focus. The Family Journal, 12(3), 287–291. doi:10.1177/1066480704264349
- Peng, C. K., Henry, I. C., Mietus, J. E., Hausdorff, J. M., Khalsa, G., Benson, H., & Goldberger, A. L. (2004). Heart rate dynamics during three forms of meditation. International Journal of Cardiology, 95(1), 19–27. doi:10.1016/j.ijcard.2003.02.006
- Porges, S. W. (2003). The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. doi:10.1016/S0031-9384(03)00156-2
- Sandor, K. M. (2005). The labyrinth: A walking meditation for healing and self-care. Explore: the Journal of Science and Healing, 1(6), 480–483. doi:10.1016/j.explore.2005.08.013
- Saward, J. (2003). Labyrinths & mazes: A complete guide to magical paths of the world. New York, NY: Lark Books.
- Tabachnick, B. G., & Fidell, L. S. (2013). Using multivariate statistics (6th ed.). Thousand Oaks, CA: Sage Publications.
- Tang, Y. Y., Ma, Y., Fan, Y., Feng, H., Wang, J., Feng, S., … Fan, M. (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences, 106(22), 8865–8870. doi:10.1073/pnas.0904031106
- Tang, Y. Y., Rothbart, M. K., & Posner, M. I. (2012). Neural correlates of establishing, maintaining, and switching brain states. Trends in Cognitive Sciences, 16(6), 330–337. doi:10.1016/j.tics.2012.05.001
- Thayer, J. F., Hansen, A. L., Saus-Rose, E., & Johnsen, B. H. (2009). Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. doi:10.1007/s12160-009-9101-z
- Thomas, D. R. (2006). A general inductive approach for analyzing qualitative evaluation data. American Journal of Evaluation, 27(2), 237–246. doi:10.1177/1098214005283748
- Travis, F., Olson, T., Egenes, T., & Gupta, H. K. (2001). Physiological patterns during practice of the transcendental meditation technique compared with patterns while reading Sanskrit and a modern language. International Journal of Neuroscience, 109(1–2), 71–80.
- Wallace, R. K. (1970). Physiological effects of transcendental meditation. Science, 167(3926), 1751–1754.
- Zanesco, A. P., King, B. G., Maclean, K. A., & Saron, C. D. (2013). Executive control and felt concentrative engagement following intensive meditation training. Frontiers in Human Neuroscience, 7, 566. doi:10.3389/fnhum.2013.00566

