Abstract
Previous studies suggested that, compared to normal people, people who have compulsive sexual behaviours exhibit stronger brain activities in the ventral striatum, dorsal anterior cingulate, and amygdala. Activities at these brain areas in people with compulsive sexual behaviours may influence their cognitive functions such as working memory (WM). The present study investigated how sexual arousal and emotional arousal would affect WM performance using a 3-back task. Experiment 1 tested the effect of sexual arousal and emotional arousal (positive and negative) on WM performance using a 3-back task with English alphabets as stimuli. Experiment 2 examined whether the effect of sexual arousal on WM would be different in different types of 3-back tasks (using alphabetical, colour, and pictorial stimuli). Interestingly, people had better WM performance under the negative emotional arousal of fear and sexual arousal. Elevated attention and alertness are the possible causes of the better WM performance under such states. Correlation analyses showed that people with a higher tendency of compulsive sexual behaviour had worse performance in the 3-back tasks. Overall, this study provided new insights regarding the effect of emotional arousal and sexual arousal on cognitive functions.
PUBLIC INTEREST STATEMENT
Emotional arousal influences the way we think, perceive, and feel about the world. People behave very differently under different emotions. Emotion also influences the capacity of human mind to process information, including attention and memory functions. Working memory is the capacity of the mind to store and work on information that a person is currently thinking of. The present study investigated the effects of emotional arousal and sexual arousal on working memory performance. Participants performed better in a working memory task in conditions of negative emotional arousal and sexual arousal, when compared to conditions of neutral and position emotional arousal. These results give a hint on how states of emotional arousal might influence how we think and how good we perform a task in everyday life. In a broader sense, further research is needed to explore how emotion would affect other mental functions such as focusing attention and making decisions.
Competing interest
The authors declare no competing interests.
1. Introduction
1.1. Sexual behaviour and sexual arousal
Addiction is characterized by a pleasure-seeking and uncontainable urge to put something into practice, and involves the visceral overwhelming “hunger” that disregards both the risk and its negative effects (Goodman, Citation2008). A desire among people who are “addicts” of sexual behaviours stands on obsessive, impulsive, and compulsive factors which induce the individual to experience sexuality not as a symbolic function of the desire for fusion of bodies, but a realization of the urge to put something into practice in order to contain those intense desires (Laier, Schulte, & Brand, Citation2013). Compulsive sexual activities may interfere in a person’s daily life, and may also lead to emotional problems such as rumination, anxiety, and depression (Bancroft & Vukadinovic, Citation2004).
Studies in neuroscience have generated a wealth of knowledge about the brain regarding sexual behaviour. Compared to normal people, the brain of people who have compulsive sexual behaviours exhibit higher level of activities in the ventral striatum, dorsal anterior cingulate cortex, and amygdala (Sunderwirth, Milkman, & Jenks, Citation1996). Some neuroimaging studies have shown that seeing sexual stimuli is strongly associated with activation of the “reward system” of the brain which includes the prefrontal cortex, anterior cingulate cortex, insular cortex, hippocampus, dorsal and ventral striatum, substantia nigra, ventral tegmentum, thalamus, hypothalamus, subthalamic nucleus, globus pallidus, and amygdala (Berridge & Kringelbach, Citation2015; Grall-Bronnec & Sauvaget, Citation2014; Nestler, Hyman, & Malenka, Citation2009; Richard, Castro, DiFeliceantonio, Robinson, & Berridge, Citation2013; Yager, Garcia, Wunsch, & Ferguson, Citation2015).
Moreover, research has suggested that sexual arousal in healthy people would also interfere with working memory (WM) performance, due to the fact that sexual arousal processing in the brain is related to the regions (such as the prefrontal cortex) associated with emotion, arousal, and attention (Verdejo-Garcia, Perez-Garcia, & Bechara, Citation2006). The symptoms in people with compulsive sexual behaviours are described as impairments of the functional systems for motivation-reward, affect regulation, and behavioural inhibition (Goodman, Citation2008). Therefore, it can be reasoned that performance in these relevant cognitive processes might also be affected in these people.
1.2. Neurobiology of the cognitive processes of emotion and working memory
Emotion is related to the conscious experience, and is characterized by intense mental activities and degree of pleasure or displeasure (Damasio, Citation1998). It involves subjective experience, cognitive processing, expressive behaviours, and psychophysiological changes (Harmon-Jones, Vaughn-Scott, Mohr, Sigelman, & Harmon-Jones, Citation2004). According to the neurobiological perspective, pleasant and unpleasant mental states are organized in the limbic system that includes the cingulate cortex, hypothalamus, hippocampus, and amygdala (Damasio, Citation1998). Also, research has reported that memory can be improved when connected with emotion; however, extreme conditions such as intensive arousal or emotional arousal can actually impair memory (Cahill, Babinsky, Markowitsch, & McGaugh, Citation1995). The dorsolateral prefrontal cortex (DLPFC) is linked to the functions of short-term memory, such as working memory (Bechara, Damasio, & Damasio, Citation2000), while the amygdala was found to be an essential region when memory is connected with emotion (Bechara, Damasio, Tranel, & Anderson, Citation1998).
Working memory involves the processes of retaining, retrieving, and information processing after old information is stored in the brain (Curtis, Citation2006). It can be thought as a system with a limited capacity for temporary storage and manipulation of information in order to carry out a wide range of complex tasks, such as comprehension, learning, and reasoning (Owen, Citation1997). Previous research has suggested that DLPFC supports spatial working memory and might be more involved in tasks that require processing of memorized materials; the ventrolateral prefrontal cortex (VLPFC) supports non-spatial working memory and might be more involved in pure maintenance of information (Honey et al., Citation2002). Furthermore, involvement of the brain for WM is not only limited to the prefrontal cortex. Some studies have shown that activities scatter over a huge area of the cortex when people are performing WM tasks (Smith & Jonides, Citation1999). WM holds and processes much relevant information at one time, and has limited time span. When people need to make a decision or perform an action, the current and old information is both necessary and is processed in the WM. Therefore, it can be reasoned that people who have higher WM efficiency and capacity would have better performance and efficiency when performing a task that requires continuous monitoring, such as an “n-back” task (Morris & Jones, Citation1990).
1.3. Behavioural studies on the effects of emotional arousal and sexual arousal on working memory
In cognitive psychology, it has been well accepted that the cognitive functions and processes of emotion, attention and memory are highly interrelated. For instance, using the Stroop task and immediate memory tasks, emotional stimuli have been demonstrated to lead to longer naming time, better recall and recognition memory, as well as impairing recall for preceding and succeeding word items (MacKay et al., Citation2004). Regarding WM, Lindström and Bohlin (Citation2011) found that the processing of emotional pictures could facilitate WM task performance in terms of response accuracy and reaction time than low-arousal neutral stimuli. Attention has been proposed as one possible reason that accounts for enhanced memory performance on immediate memory for emotionally charged stimuli (Talmi & McGarry, Citation2012); and WM workload can attenuate the emotional memory enhancement effect (Miendlarzewska, van Elswijk, Cannistraci, & van Ee, Citation2013). Facilitation on interference resolution in WM has also been found when emotional stimuli were used, possibly by facilitating response selection (Levens & Phelps, Citation2008). However, the enhancement effect of emotional content on WM is not robust. In the experiments conducted by Kensinger and Corkin (Citation2003) to investigate the effect of negative emotional content on WM and long-term memory, in some instances emotional salience impeded WM performance (e.g. when faces were used as stimuli in an n-back task), but in others it did not (e.g. when words were used as stimuli). Some studies also reported that emotional arousal can impair inter-item binding (Bergmann, Rijpkema, Fernández, & Kessels, Citation2012) and feature binding (Mather et al., Citation2006). Other studies investigating psychological states such as psychosocial stress, anxiety, and negative emotional experience and rumination have found that these psychological states could be associated with impairments in WM capacity and performance (Curci, Lanciano, Soleti, & Rimé, Citation2013; Schoofs, Preuß, & Wolf, Citation2008; Shackman et al., Citation2006).
On the other hand, studies on the effect of sexual arousal on WM have been sparse in the literature. Using pictures of neutral, positive, negative, and sexual contents in a 4-back task, Laier et al. (Citation2013) found that pornographic pictures led to worse WM performance when compared to the other three conditions. But a conclusion can be difficult to draw because sexual interest in a stimulus may cause distraction leading to impairment in WM performance, and causing emotion-induced deficits in visual processing (Most, Smith, Cooter, Levy, & Zald, Citation2007), or it may facilitate memory on the item due to focused attention (Wright & Adams, Citation1999).
1.4. Objectives of the present study
Considering that cognitive processes are highly related in states of sexual arousal and emotional arousal, the present study examined two specific research issues. Experiment 1 investigated the effect of sexual arousal and emotional arousal (positive and negative) on the performance of a WM task (n-back task) using alphabets as the stimuli. Experiment 2 examined whether the effect of sexual arousal on WM performance would be modulated by the type of n-back task (when alphabetical, colour, and pictorial stimuli were used).
2. Experiment 1: the effect of emotional arousal and sexual arousal on WM performance
2.1. Background
In the previous section, we reviewed neuroscientific research that prefrontal cortex, limbic system, and amygdala play important roles in sexual arousal, emotional processing, and WM (Cools, Gibbs, Miyakawa, Jagust, & D’Esposito, Citation2008). Most previous studies focused on the impact of sexual arousal or emotional arousal on cognitive processing, such as choice reaction time or memory (Schaefer et al., Citation2006). Although some studies focused on the effects of sexual arousal on WM, very few focused on delineating between sexual arousal and emotion arousal. The present experiment thus focused on how emotion and sexual arousal would influence WM performance.
Emotional process can significantly influence WM efficiency and capacity; for instance, positive mood can lead to better performance in some WM tasks (Alvarez & Emory, Citation2006), while positive and negative stimulation can lead to capturing of attention which interferes WM performance (Bechara, Tranel, & Damasio, Citation2000). Since n-back task is an experimental task widely used in investigations of immediate memory, it is suitable for examining WM performance in this study.
2.2. Method
2.2.1. Participants
Participants of this experiment were males with heterosexual orientation and a minimum age of 18 years. A total of 24 participants were recruited from several public Internet forums in Hong Kong, such as “Hong Kong BDSM Club” and “Xocat.com”, and by advertisement in the campus of the institute of the authors. The age range of the participants was 19 to 27 years old (mean = 23.08, SD = 2.225). They were explained that during the experiment there would be a confrontation with explicit pornographic stimuli. All the participants signed a written consent form for participation before the experiment, and confirmed their sexual-orientation as “heterosexual” on a simple questionnaire asking for demographic information of age, education level, and sexual orientation (with options of “heterosexual”, “homosexual”, and “bisexual”). All of them did not have known drug abuse or recent drug taking at the time of the experiment.
2.2.2. Materials and procedure
2.2.2.1. Compulsive Sexual Behavior Inventory
The Compulsive Sexual Behavior Inventory (CSBI) includes 28 items (Coleman, Miner, & Ohlerking, & Raymond, Citation2001). The user rates on a 5-point scale to indicate how frequent he engages in the behaviours stated in each item from 1 (very frequently) to 5 (never), and the total score of the CSBI can range from 28 to 140. A lower score indicates a higher level of compulsive sexual behaviour of the person. The full inventory can be identified with three factors (13 items on “control”, 8 items on “abuse”, and 7 items on “violence”). The CSBI has been evaluated among heterosexual and homosexual people and was found to have a high level of reliability (Cronbach’s α = 0.96 for control, α = 0.91 for abuse, and α = 0.88 for violence; Coleman et al., Citation2001). It also demonstrated good test-retest reliability in English (r = 0.86) and Spanish (r = 0.93) samples in a previous study (Miner, Coleman, Center, Ross, & Rosser, Citation2007). From data of the present experiment, the reliability was found to be α = 0.83 for control, α = 0.56 for abuse, α = 0.90 for violence, and α = 0.71 for the full scale. Example items in the scale include: “How often have you had trouble controlling your sexual urges?”, “Were you sexually abused as a child?”, and “Have you ever hit, kicked, punched, slapped, thrown, choked, restrained, or beaten any of your sexual partners?” (Coleman et al., Citation2001).
2.2.2.2. Induction of emotional arousal and sexual arousal
In this experiment, the procedure included inducing neutral, positive emotion, negative emotion, and sexual arousal conditions by letting the participants watch videos (Martin, Citation1990) which were shown before the 3-back task. According to some previous studies on emotional induction, films clips have the capacity of efficiently inducing emotions and moods, especially for positive emotions (Heiman, Citation1980; Julien & Over, Citation1988).
In the present experiment, each condition comprised a 6-min exclusive neutral, positive emotion, negative emotion, or pornographic video. Each video was combined from three different movies of 2 min. All the movies were identified from the YouTube website. The Windows Movie Maker (Microsoft, Redmond, WA) was used to perform clipping and editing of the experimental videos.
In the neutral condition, the video content was composed of repeated and boring movements, which should not cause any positive or negative emotion in particular. It included moving scenes of a metronome at 80 BPM, a moving train, and a moving washing machine. In the positive emotion condition, the video comprised humorous and relaxing actions for causing people a happy and relaxed emotion. It included a short scene of a classical comedy and a scene of people interacting happily with an animal. In the negative emotion condition, the video included cruel and disturbing scenes such as murdering, abusing people, and a horrible clown for bringing up negative emotion. In the sexual arousal condition, a pornographic video displayed scenes of heterosexual vaginal intercourse between one or more men and one woman, with some elements of fetish, sadism, masochism, bondage, oral sex, and masturbating behaviours in different costumes, such as school uniform, nurse suit, office lady suit, and kimono. All the videos in the different conditions lasted for 6 min each, which were edited from three different video sources.
2.2.2.3. Self-reported sexual arousal ratings
For measuring the level of emotion and sexual urge induced by the video in each condition, physiological measurements and subjective report were employed. For the subjective report, the Discrete Emotions Questionnaire (DEQ) was used for the self-report of participant’s emotion level (Gross & Levenson, Citation1995). Participants were asked to rate on the 16 emotional feelings listed in the DEQ on a 9-point scale from 1 (“Not at all”) to 9 (“The most I have ever felt”), before and after they had watched the video in each condition. The 16 emotions included “fun”, “anger”, “excited”, “confusion”, “contempt”, “satisfaction”, “disgust”, “embarrassment”, “fear”, “happiness”, “interest”, “painfulness”, “relax”, “sadness”, “surprise”, and “nervous”. In the present experiment, DEQ was used for measuring the intensity of different emotions elicited in response to watching the videos, and also to ensure that the target emotion had been elicited by the procedure successfully (Demaree, Schemichel, Robinson, & Everhart, Citation2004).
In addition, before and after watching the movie in each condition, participants recorded their current sexual urge on a visual analogue scale (VAS), with a scoring range from 0 (“Not sexually aroused”) to 9 (“Very sexually aroused”). They also rated on their current need for masturbation using another VAS with a scoring range from 0 (“No need to masturbate”) to 9 (“Strongest need to masturbate”).
2.2.2.4. Physiological measurements
For physiological indicators that reflect changes in participant’s emotion and sexual desire, the experimenter measured the participant’s heart rate (HR) and blood pressure (BP) using a wrist sphygmomanometer (model: Panasonic EW-BW10), and body temperature using an infra-red thermometer (model: TSK DT-8806C), before and after they had watched the video in each condition in order to check the effect of emotional and sexual induction by the videos. In some previous studies, people showed responses of increased HR and BP when experiencing negative emotion (Campbell & Ehlert, Citation2012). In the present study, it was expected that when participants were induced with negative emotion such as anger, depression, or anxiety, these physiological indicators would increase. Moreover, in terms of sexual arousal, these indictors manifest the physiological indications of a person’s excitement (Janssen, Everaerd, Spiering, & Janssen, Citation2000). Excitement is associated with the physiological reactions to sexual desire; HR, BP, and breathing rate would increase in the stage of sexual excitement (Zuckerman, Citation1971).
2.2.2.5. The 3-back working memory task
To evaluate the participant’s WM performance, a 3-back task was used in this experiment. The experimental stimuli of the 3-back task were programmed in MATLAB R2017b (MathWorks, Natick, MA) using the Psychophysics Toolbox Version 3 extension (Brainard, Citation1997; Pelli, Citation1997). The stimuli were viewed on an LCD monitor at a refresh rate of 60 Hz (resolution: 1366 × 768 pixels), controlled by a personal computer running on the Windows 10 operating system. Participants viewed the screen at a distance of 45 cm in a bright and quiet room.
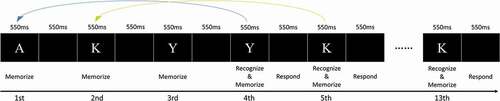
In the fundamental n-back task design, the participant is presented with a sequence of stimuli in order. For each stimulus, the participant must decide whether the current stimulus is the same as the stimulus which was presented n stimuli before. The n-back task has been adopted most extensively in cognitive neuroscience studies of WM, such as verbal WM and visuospatial WM (Morris & Jones, Citation1990). In this study, participants performed a 3-back task in each of the four different emotional/sexual arousal conditions. In each trial of the 3-back task (Figure ), English alphabets (A, K, or Y; size: 100 × 100 pixels) were presented in random order among a series of 13 stimuli (each lasting for 550 ms). Participants had to start responding at the fourth stimuli. For each stimuli from that point, participants had to press the “Yes” key on the keyboard if the same alphabet had been shown three stimuli ago, and the “No” key otherwise. Between each stimulus, a 550-ms blank screen was presented. Participants needed to perform the identification as quickly as possible and avoid making mistakes. There were a total of 20 trials in this experiment.
2.2.3. Procedure
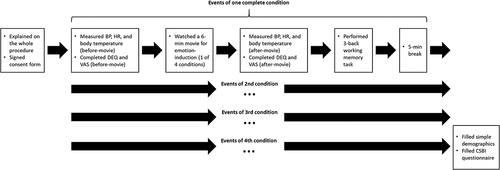
All participants were given clear instructions regarding the procedure in each experimental condition. The experimenter measured their BP, HR and body temperature first (and they also completed the DEQ and VAS), and then they watched the 6-min movie in the first condition, after which the experimenter measured their physiological responses again (also the DEQ and VAS), then they performed the 3-back task (Figure ). This procedure repeated four times for the four conditions, and 5-min breaks were given after the 3-back task in each condition in order to eliminate possible fatigue effect. Participants finally filled out the CSBI and the demographic questionnaire after all conditions were completed.
Participants performed the four conditions in counter-balanced order according to the Latin square design. They were allowed to ask any question for clarification on the experimental procedure and to quit the experiment if they had any concern. If a participant had certain abnormal effects after performing the experiment (such as maintaining a high level of sexual urge after the experiment), the experimenter would provide a 15-min video for comforting his emotion. This video contained landscape scenes accompanied with peaceful music. In total, the whole experiment took about 100 min to complete. All procedures involved in this experiment were reviewed and approved by the research ethics committee of the authors’ institution.
2.3. Results
IBM SPSS Statistics (version 24.0) was used to perform data analysis. Bonferroni correction was used to correct for all multiple comparisons in the analyses.
To investigate whether the movies in the four conditions (neutral, positive emotion, negative emotion, and sexual arousal) led to different magnitudes of change in physiological measurements, a 2 (Time: before/after movie) × 4 (Condition) repeated measures ANOVA was conducted with each of the physiological measurement as the dependent variable. Significant Time × Condition interaction was found for all the four physiological measurements: HR [F(3, 69) = 3.106, p = 0.032, effect size partial η2 = 0.119], systolic blood pressure (SBP) [F(3, 69) = 6.111, p = 0.001, partial η2 = 0.210], diastolic blood pressure (DBP) [F(3, 69) = 3.641, p = 0.017, partial η2 = 0.137], and body temperature [F(3, 69) = 10.512, p < 0.001, partial η2 = 0.314]. Simple main effect analysis was conducted along with the ANOVA to examine whether there was significant change (i.e. before vs. after) in each of the physiological measurement after watching the movie within each condition. For the neutral condition, significant change was found in HR [t(23) = 2.331, p = 0.029] and SBP [t(23) = 2.103, p = 0.047] only. For the positive emotion condition, significant change was found in SBP [t(23) = 5.635, p < 0.001] and body temperature [t(23) = 6.679, p < 0.001]. For the negative emotion condition, significant change was found in HR [t(23) = 9.122, p < 0.001], SBP [t(23) = 6.203, p < 0.001], DBP [t(23) = 6.815, p < 0.001], and body temperature [t(23) = 4.094, p < 0.001]. For the sexual arousal condition, significant change was found in HR [t(23) = 4.877, p < 0.001], SBP [t(23) = 5.571, p < 0.001], DBP [t(23) = 4.676, p < 0.001], and body temperature [t(23) = 6.582, p < 0.001] also.
Analyses for the effect of sexual arousal (measured by VAS on current sexual urge and desire to masturbate) and change in scores of emotions (measured by DEQ) after watching the videos in the four conditions were conducted in the same manner as above. The 2 (Time) × 4 (Condition) repeated measures ANOVA indicated significant interaction in VAS score for current sexual urge [F(3, 69) = 31.663, p < 0.001, partial η2 = 0.579] and VAS score for desire to masturbate [F(3, 69) = 48.802, p < 0.001, partial η2 = 0.680]. Simple main effect analysis was conducted to check whether significant change in sexual urge or desire to masturbate had occurred after watching the movie in each condition. For the neutral condition and the negative emotion condition, no significant change was found in both measurements (p > 0.05). For the positive emotion condition, significant change was found in sexual urge [t(23) = 3.11, p = 0.005] and desire to masturbate [t(23) = 3.19, p = 0.004]. For the sexual arousal condition, significant change was found in sexual urge [t(23) = 8.27, p < 0.001] and desire to masturbate [t(23) = 10.86, p < 0.001] too.
For the DEQ scores, the 2 (Time) × 4 (Condition) repeated measures ANOVA indicated significant interaction for the emotions of fun, excited, satisfaction, disgust, embarrassment, fear, happiness, interest, relax, sadness, and nervous (all p < 0.05, partial η2 ranging from 0.272 to 0.638). Simple main effect analysis revealed that in the neutral condition, significant change occurred for the emotions of fun and nervous only (p < 0.05). For the positive emotion condition, significant change in emotion was observed for fun, satisfaction, happiness, interest, and surprise (all p < 0.05). For the negative emotion condition, significant change was observed for nearly all the emotions included in the DEQ (all p < 0.05) except painfulness and surprise. For the sexual arousal condition, significant change was observed for fun, excited, confusion, satisfaction, embarrassment, surprise, and nervous (all p < 0.05).
For the performance of the 3-back WM task, the average accuracy rate and reaction time were calculated for each of the four conditions (Table ). One-way repeated measures ANOVA was performed to test whether significant difference existed in accuracy rate and reaction time between the four different conditions. The accuracy rate among the four conditions was significantly different [F(3, 69) = 81.698, p < 0.001, partial η2 = 0.780]. However, the reaction time was not significantly different among the four conditions [F(3, 69) = 0.078, p = 0.972, partial η2 = 0.003].
Table 1. The means and SDs of accuracy rate and reaction time in the 3-back task in the four conditions in Experiment 1
To further investigate the differences in accuracy rate of the 3-back task among the four conditions, post hoc pairwise comparisons were performed using paired-sample t-tests with Bonferroni correction for multiple comparisons. There were significant differences in accuracy rate between all pairs of the conditions (all p < 0.001).
Regarding the CSBI score, the range of score was 90–124 (mean = 108.38, SD = 9.23). To explore the relationship between tendency of compulsive sexual behaviours and WM performance, correlation analysis was performed between the CSBI score and accuracy rate of the 3-back task. The CSBI score and accuracy rate were all strongly correlated in the neutral condition (Pearson correlation coefficient r = 0.525, p < 0.01), positive emotion condition (r = 0.726, p < 0.01), negative emotion condition (r = 0.758, p < 0.01), and sexual arousal condition (r = 0.774, p < 0.01). In addition, to investigate whether a higher sexual tendency might be associated with a higher impulsivity (as reflected by shorter reaction time in the 3-back task), correlation analysis between CSBI score and reaction time of the 3-back task was performed. CSBI score was significantly correlated with the reaction time in the neutral condition (r = 0.420, p = 0.041) and sexual arousal condition (r = 0.413, p = 0.045). However, no significant correlation was found between CSBI score and reaction time in the positive emotion condition (r = 0.157, p = 0.464) and negative emotion condition (r = 0.251, p = 0.238). In conclusion, sexual tendency of the participants was strongly associated with WM performance, even in conditions of different emotional or sexual arousal.
In summary, using alphabets as the stimuli in a 3-back WM task, Experiment 1 demonstrated that brief and temporary emotional arousal and sexual arousal can influence cognitive performance of WM, with negative emotional arousal leading to the highest response accuracy in the 3-back task, followed by sexual arousal and positive emotional arousal, in comparison to neutral emotion. On the other hand, reaction time in the 3-back task seemed to be not influenced by emotional or sexual arousal.
2.4. Discussion
According to the results, the physiological responses of HR and BP changed significantly after participants watched the emotional/sexual arousal inducting movie in the different conditions. HR, BP, and body temperature showed slight increase after watching the videos in the positive emotion, negative emotion, and sexual arousal conditions. Since emotional videos would increase sympathetic activation of the cardiovascular system (Gross & Levenson, Citation1997), the body would exhibit these physiological changes if the videos could successfully induce the emotions.
Regarding the effects of emotional and sexual arousal on cognitive performance, the present results showed significant difference in performance of the 3-back task among the four conditions, implying that emotional and sexual arousal would affect WM process. Surprisingly, WM performance under the negative emotion and sexual arousal conditions were better than that in the neutral and positive emotion conditions. Based on their comparable WM performance, seemingly, sexual arousal might be exerting influences on a person’s cognitive processing in some ways similar to negative emotional arousal. Emotions and attention have an intimate relationship, as the state of emotion would influence the contents of consciousness and the performance of tasks which require selection among stimuli or intensive concentration (Matthews & Wells, Citation1999). Attention facilitates the processing of a target during the perceptual stage, and it also functions to maintain different kinds of information in the WM. Considering that the videos shown in this experiment was causing the negative emotion of disgust, stress, and fear (by scenes of murdering and a horrible clown in the dark), watching the movie induced the emotion of fear and anxiety. Here we first discuss about the effect of negative emotion of fear and anxiety on attention and WM.
According to Williams, Watts, MacLeod, and Mathews (Citation1988), depression and anxiety produce different influences on attention. They suggested that negative emotion (such as fear) facilitates greater attention than positive emotion (such as love, joy, or interest), and negative emotion would broaden the attentional focus. The central nucleus of the amygdala involves the control of emotional response elicited by negative emotion (such as fear and stress), and also the BP increase characteristics in the emotion of fear (Davis, Citation1992). Moreover, connections of the central nucleus of amygdala with the grey areas of the midbrain are also responsible for the startle response and the preparation for fight-or-flight behaviours (LeDoux, Citation2000) which refers to the physiological reaction of fear, harmful, or threat to survival. When animals react to stress or prepare for fighting or fleeing, the body reacts to discharge of sympathetic nervous system. This results in increased secretion of norepinephrine and a number of hormones such as oestrogen, testosterone, cortisol, and the neurotransmitters of dopamine and serotonin. As the result of increased secretion of norepinephrine, animals would stay focused and alert on the environment. In our study, after inducing the emotion of fear, participants would stay at a state of greater alertness and attention in the following WM task.
The ability of selectively process information (attention) and the ability to find the correct information in an accessible state (WM) have to interact. While WM occupies the ability of encoding and manipulating information, attention plays an important role on selectively encoding the information according to the current goal (Hollingworth, Citation2004). For instance, in this experiment, if a participant could not pay attention on encoding and memorizing the stimuli in the 3-back task, then he would not be able to respond by comparing the previous stimuli with the upcoming stimuli. The essentiality of attention for encoding is supported by research showing that attention affects performance in detection tasks (Irwin & Zelinsky, Citation2002). When information has been encoded into WM, it still has to be stored until it is retrieved again. This process in WM necessarily occupies the attention processes (Cowan, Citation2006). As supported by the fMRI study by Corbetta, Kincade, and Shulman (Citation2002), activation of WM storage is associated with visuospatial attention, suggesting the strong interconnection between the two in the cortex.
In our study, participants had better WM performance in the negative emotion and sexual arousal conditions than the in the neutral and positive emotion conditions. The evidence of successfully inducing the fear emotion was indicated by the significant change in physiological response (HR, BP) after watching the movies. Once the emotion has been induced, and the fight-or-flight mode has been turned on, the norepinephrine and dopamine levels in the brain would be increased for the person to stay at a state of high alertness and attention. During this moment, the WM task occupies the capacities of working memory and attention. Participants had better performance in the 3-back task when they were paying greater attention for encoding and retrieving information.
The correlation results showed that people with stronger tendency of compulsive sexual behaviour generally had poorer performance in the 3-back task. As discussed in Introduction, sexual arousal processing in the brain is associated with emotion and attention (amygdala and prefrontal cortex). People with a higher tendency of compulsive sexual behaviour would have more interference to the WM. The results showed that CSBI score was strongly correlated with WM performance in all conditions, implying that males with a higher tendency of sexual behaviours would have worse WM efficiency and capacity, even without any arousal (i.e., the neutral condition). Previous research showed that people with compulsion of watching pornography had higher risk of suffering memory loss. Sexual arousal has impact on various cognitive processes, and it would impair the ability of WM (Laier et al., Citation2013).
The results of the present experiment also showed that the conditions of negative emotion and sexual arousal shared similar performance in the 3-back task. The condition of negative emotion exhibited the best WM performance, which can be explained by the temporarily increased resources of attention. When people have higher alertness in the emotion of fear, they would have higher sensitivity towards the stimuli in the task. In the sexual arousal condition, people would also have higher alertness and attention on the task, due to that they had a higher desire for seeing more sexual pictures and movies again. And similar to the emotion of fear, the brain would release more dopamine, serotonin, and testosterone, along with the increased physiological responses (e.g. higher HR and BP) for getting the body ready to have sexual behaviour. These physiological responses are similar to those in the emotion of fear (ready to fight-or-flight), which would get the brain and the body ready for the next actions.
3. Experiment 2: the effect of different categories of 3-back tasks
3.1. Background
In Experiment 1, we discussed that negative emotion and sexual arousal would elicit greater level of attention and facilitate better WM performance. Since these two conditions share similarities in terms of their effects on physiological changes (HR, BP, body temperature) and WM performance, similar cognitive processes might be undergoing in the brain. The present experiment focused on how the effects of sexual arousal would exhibit in different categories of 3-back task, when the task utilizes colour, pictorial, or English alphabets as the stimuli.
According to Boyatzis and Varghese (Citation1994), “bright” colours such as yellow are associated with positive emotions, such as happiness, and “dark” colours such as blue are associated with negative emotions, such as anger and sadness. As there is evidence that colour influences emotion in people, the present experiment would investigate the performance in 3-back WM task using colour as stimuli after inducing sexual arousal among the participants. In addition, pictures were used as stimuli in the pictorial 3-back task of this experiment, since pictures are effective means to stimulate emotional change. Also, alphabets represent a standard set of letters which are used as basic written symbols. Since letters are used in daily language for communicating and writing, they thus might lead to weaker arousal than colour and pictures when used as stimuli in an n-back task. This experiment would be similar to the Experiment 1, except the arousal-inducing materials. And in this experiment, we only induced sexual arousal in the participants.
3.2. Method
3.2.1. Participants
Participants were 27 males with heterosexual orientation and a minimum age of 18 years. The age range of the participants was 18–31 years old (mean = 23, SD = 3.15). All of them signed a written consent form before the experiment and confirmed their sexual-orientation as “heterosexual” on the simple questionnaire on demographic information as in Experiment 1. All participants did not have known drug abuse or recent drug taking at the time of the experiment.
3.2.2. Materials and procedure
3.2.2.1. Induction of sexual arousal
In this experiment, a total of three videos were prepared for the three conditions of 3-back tasks (alphabetical, colour, and pictorial). Each condition comprised with a pornographic video which would display heterosexual vaginal intercourse between one or more men and one woman, and would include some elements of fetish, sadism, masochism, bondage, oral sex, and masturbating behaviours in various costumes. All the videos lasted for 6 min, which were edited from three different video sources (three different source videos × 2 min for each source video = 6 min for each experimental video).
3.2.2.2. The working memory task
The 3-back tasks were similar to that in Experiment 1, except the materials for the stimuli. For investigating the differences in WM performance between people with different tendency of compulsive sexual behaviour when processing different types of materials in the WM, three 3-back tasks using English alphabets, coloured circles, and pictures as the stimuli were employed. In the alphabetical task, three stimuli (the letters A, K, and Y) were presented in the stream of stimuli as in Experiment 1. For the colour task, coloured circles in red, yellow, and blue were presented as the stimuli. For the pictorial task, three sexually-arousing pictures were presented in the stimulus stream. All stimuli were presented in random orders.
3.2.2.3. Procedure
The procedure was also similar with Experiment 1. Each participant performed the three task conditions (i.e. alphabetical, colour, and pictorial) in counter-balanced orders according to the Latin square design. In addition, for each of the three orders, participants were also counter-balanced in the sexually-arousing movie they watch for each condition, resulting in 3 orders × 3 movies = 9 possible matchings in total. For measuring the level of arousal, the experimenter measured the participants’ physiological responses (HR, BP, body temperature) and then the participants completed the VAS (measuring sexual urge and desire to masturbate) and DEQ before and after viewing each video. Alphabetical, colour, and pictorial 3-back tasks were performed by the participant after the video for each corresponding task had been viewed. All participants were asked to complete the CSBI scale at the end of the experiment. From data of Experiment 2, reliability of CSBI was found to be α = 0.98 for control, α = 0.85 for abuse, α = 0.82 for violence, and α = 0.88 for the full scale. All procedures involved in this experiment were also reviewed and approved by the research ethics committee of the authors’ institution.
3.3. Results
Similar to Experiment 1, the data from Experiment 2 (n = 27) were analysed by IBM SPSS Statistics (version 24.0). All multiple comparisons in the analyses have been corrected using the Bonferroni correction. Experiment 2 was different from Experiment 1 in the way that the participants watched the same three sexually-arousing videos for each of the three task conditions (alphabetical, colour, and pictorial); therefore, the analyses exploring the effect of watching the movies on physiological measurements, VAS and DEQ focused on checking whether the three videos generally produced similar effects in these measurements.
Similar to analyses conducted in the previous experiment, 2 (Time) × 3 (Video) repeated measures ANOVA was conducted, with each of the physiological measurement as a dependent variable to investigate whether the three videos led to different magnitudes of physiological change. Significant Time × Video interaction was only found for the physiological measurement of SBP [F(2, 52) = 3.516, p = 0.037, partial η2 = 0.119]. Simple main effect analysis revealed significant change (i.e. before vs. after) in all the four physiological measurements (HR, SBP, DBP, and body temperature) for all of the three movies (all p < 0.001). These results generally suggest that the three sexually-arousing videos all had similar effects in terms of physiological change they brought to the participants.
The 2 (Time) × 3 (Video) repeated measures ANOVA conducted on the VAS scores indicated significant interaction in the scores of sexual urge [F(2, 52) = 17.048, p < 0.001, partial η2 = 0.396] and desire to masturbate [F(2, 52) = 20.039, p < 0.001, partial η2 = 0.435]. Simple main effect analysis revealed significant change in sexual urge and desire to masturbate for Video 1 [sexual urge: t(26) = 6.724, p < 0.001; desire to masturbate: t(26) = 8.293, p < 0.001] and Video 3 [sexual urge: t(26) = 4.149, p < 0.001; desire to masturbate: t(26) = 3.986, p < 0.001], but not for Video 2 [sexual urge: t(26) = 1.363, p = 0.185; desire to masturbate: t(26) = 1.870, p = 0.073].
For the DEQ scores, the 2 (Time) × 3 (Video) repeated measures ANOVA indicated significant interaction for the emotions of fun, anger, confusion, satisfaction, disgust, embarrassment, fear, interest, and sadness (all p < 0.05, partial η2 ranging from 0.118 to 0.309). Simple main effect analysis revealed that for Video 1, significant change occurred for the emotions of fun, excited, disgust, embarrassment, painfulness, relax, sadness, surprise, and nervous (all p < 0.05). For Video 2, significant change in emotion was observed for fun, anger, confusion, satisfaction, disgust, fear, happiness, interest, relax, sadness, and nervous (all p < 0.05). For Video 3, significant change in emotion was observed for surprise and nervous (p < 0.05). Overall, the emotion of “nervous” was commonly found to have significantly changed for all the three videos.
Regarding the performance in the 3-back WM tasks, the results of average accuracy rate and reaction time in each condition are presented in Table . To explore the effect of the three conditions of 3-back task (alphabetical, colour, and pictorial) on accuracy rate and reaction time, repeated measures ANOVA was performed using the Condition as the within-subject factor. The accuracy rate was found to be significantly different among the three conditions [F(2, 52) = 9.791, p < 0.001, partial η2 = 0.274], but not for reaction time [F(2, 52) = 0.641, p = 0.531, partial η2 = 0.024].
Table 2. The means and SDs of accuracy rate and reaction time in the three 3-back tasks using different types of stimuli in Experiment 2
To further investigate the significant differences in accuracy rate among the alphabetical, colour, and pictorial conditions of the 3-back task, post hoc pairwise comparisons were performed using paired-sample t-tests with Bonferroni correction. There was a significant difference in accuracy rate between the alphabetical task and the colour task [t(26) = −3.906, p < 0.001]. Also, the difference between the alphabetical task and the pictorial task was significant [t(26) = −2.569, p = 0.016]. Lastly, the difference between the colour task and the pictorial task was marginally significant [t(26) = −2.045, p = 0.051].
For tendency of sexual behaviour, the range of CSBI score of the participants was 81 to 130 (mean = 110.37, SD = 12.34). Correlation analysis was performed to explore the relationship between CSBI score and accuracy rate of the tasks (which reflects the relationship between tendency of compulsive sexual behaviour and efficiency for different types of WM processes). Significant positive correlation was found between CSBI score and accuracy rate in the alphabetical 3-back task (r = 0.454, p = 0.017), in the colour 3-back task (r = 0.535, p < 0.001), and also in the pictorial 3-back task (r = 0.500, p < 0.001). Moreover, the relationship between CSBI score and reaction time of the 3-back tasks was also investigated. Only in the colour 3-back task, there was a significant correlation between CSBI score and reaction time of the task (r = 0.392, p = 0.043). No significant correlation between CSBI score and reaction time was found for the alphabetical 3-back task (r = 0.317, p = 0.107) and the pictorial 3-back task (r = 0.159, p = 0.429).
In summary, using different types of 3-back WM task, Experiment 2 demonstrated that the effect of brief sexual arousal on WM performance can be influenced by the type of stimuli used in the task. Specifically, coloured stimuli and pictorial stimuli could lead to better response accuracy in the 3-back task than alphabetical stimuli, when a person is induced with sexual arousal temporarily, while no significant difference in response accuracy was found between coloured and pictorial stimuli. In addition, reaction time in the task was not influenced by the type of stimuli used.
3.4. Discussion
According to the results from Experiment 2, no significant difference in physiological effect was found among the three sexually-arousing videos, implying that these three videos had caused a similar level of arousal among the participants. Also, the experiment was designed to present combinations of videos and types of 3-back task in a counter-balanced order among the participants, so as to further minimize the influence of any possible difference in physiological effects and arousal levels brought by the three different videos.
As discussed in Experiment 1, the results of physiological change in HR, BP, and body temperature suggested successful emotional induction. Here in Experiment 2, significant change in the HR, SBP, DBP, and body temperature was found after watching the videos, indicating that all the videos could induce a similar level of arousal successfully. Since all the videos shared a similar level of induced arousal in the experiment, we could compare the results among the different categories of WM task by assuming the participants all had the same level of sexual arousal when performing each of the three 3-back tasks.
According to the results of the post hoc pairwise comparison comparing the accuracy rates among the three 3-back tasks, participants had a higher accuracy when performing the colour and pictorial 3-back tasks than the alphabetical 3-back task. This supported the idea that WM performance for emotionally- and sexually-arousing materials are processed more in-depth by the brain than neutral materials of English alphabets. This experiment demonstrated that, by just changing the type of materials used as the stimuli, the same participants could have quite different levels of performance in the same WM task. Together with the results from Experiment 1 that the negative emotion and the sexual arousal condition showed better performance than the neutral condition, the findings further support the notion that emotional processes can alter attention, and in turn, WM performance.
The post hoc comparison also showed that the difference in accuracy rate between the colour task and the pictorial task was not significant, implying that the categories of emotional materials (coloured circles) and sexually-arousing materials (sexually-arousing pictures) led to similar performance in WM. We may consider the degree of difficulty in these two tasks. Since the colour 3-back task involved identifying three filled colour circles on the screen, while the pictorial 3-back task involved identifying three similar sexually-arousing pictures, the difficulty of the colour task would be lower than that of the pictorial task due to lower visual complexity of the colour circles. The reaction time also appeared to exhibit a trend that participants needed more time to determine the pictures than the colours, although the results of the post hoc paired-sample t-test was not statistically significant. The colour 3-back task had the fastest reaction time among the three conditions (mean = 611.39 ms), while the pictorial task had the longest reaction time (mean = 620.69 ms). Previous studies have suggested that colour can improve object recognition, and that colour information would be processed in the brain prior to the processing of shape and structure of an object (Elliot & Maier, Citation2014). In the colour 3-back task, participants only had to encode and recognize the colour, with the shape of the object remained the same. On the other hand, in the pictorial 3-back task, participants had to memorize and recognize the pictures which showed similar dressing and postures. Furthermore, “bright” colours (such as red and yellow) would draw stronger attention than “dark” colours (black) (Boyatzis & Varghese, Citation1994). In the experiment, the colour task displayed red, yellow, and blue colours, which could attract stronger attention from the participants; in contrast, the pictorial task showed a girl who dressed up with black pantyhose, which would perceptually draw less attention than the colour task. Even though participants might be paying more attention on the sexually-arousing pictures while completing the task, it still had a higher difficulty than the colour task.
As reported in Experiment 1, a higher sexual tendency as measured by CSBI was associated with worse WM performance, under all the arousal conditions including neutral, positive emotion, negative emotion, and sexual arousal conditions. In Experiment 2, we also demonstrated this relationship in the three categories of WM tasks. According to the results of the correlation analysis, CSBI score had significant positive correlation with the accuracy rates in all three types of WM task, implying that people with a higher tendency of compulsive sexual behaviour would generally have poorer performance of WM, no matter to which type of materials. Sexual arousal is linked with regions responsible for processing emotion, arousal, and attention in the brain (e.g. amygdala); thus, it would be possible to interfere WM performance (Paul et al., Citation2008). People with a higher tendency of compulsive sexual behaviour would be more easily distracted by other stimuli (such as sexual pictures and videos). Since WM functions strongly demand for resources of attention and alertness, people with a higher sexual tendency would be more likely to fail to pay attention than people with a lower sexual tendency when aroused.
4. General discussion
4.1. Emotional processing and sexual arousal
In Experiment 1, we discussed about the physiological changes when people experience the emotion of fear and anxiety. Our experimental results suggested a possibility that similar processes might be involved when people are performing the WM tasks under the negative emotion of fear and sexual arousal.
The emotion of fear can activate the flight-or-fight response system (LeDoux, Citation2000). When people experience fear, the adrenal gland, which is a part of the endocrine system, would release hormones to activate and regulate the responses of anxiety, fear, and stress. The amygdala is the emotion-organizing centre in the brain, and significantly contributes to the emotion of fear (Cardinali, Citation2017; Silton et al., Citation2011). The amygdala controls the change of physiological responses, such as increasing heart rate and blood pressure. It also stores and contextualizes the memory of emotional experience, fear, and anxiety response (Goldstein & McEwen, Citation2002).
In addition to the amygdala, the hypothalamus and pituitary gland also form the feedback system which controls the emotion, mood, and stress reaction; and they are also parts of the brain that activate the fight-or-flight system (Julien et al., Citation1990). All these brain regions that regulate the emotion of fear are under the control of the autonomic nervous system which includes the sympathetic and parasympathetic nervous systems. The sympathetic nervous system plays the role of regulating the body to get ready to cope with stress and emergencies, by increasing the HR and BP, and regulating digestion (Mansour et al., Citation1998). The parasympathetic nervous system reacts to decrease the HR and BP, and returns the bodily functions to the normal state (Engels et al., Citation2007).
When people experience fear, involuntary attention is also involved. The function of attention scans the environment for relevant information, so that the brain encodes, interprets, and attends to all the information that would be regarded as important to the individual (Ohman, Flykt, & Esteves, Citation2001). Attention is known to be closely intertwined with WM, and influences WM at multiple processing stages (Awh, Vogel, & Oh, Citation2006; Gazzaley, Citation2011). These two central cognitive functions are the basis for humans to construct conscious experiences of temporal events (Marchetti, Citation2014). Emotional arousal and sexual arousal can lead to activation in the amygdala (Adolphs, Citation2002; Hamann, Ely, Hoffman, & Kilts, Citation2002; Hamann, Herman, Nolan, & Wallen, Citation2004; Mather et al., Citation2004), and increased activation in amygdala is associated with increased vigilance and attention (Davis & Whalen, Citation2001), especially under fearful and life-threatening situations (Löw, Lang, Smith, & Bradley; Citation2008; Whalen, Citation1998). At a state of increased attention, the body would increase its sensitivity to observe the environment (MacLeod, Mathews, & Tata, Citation1986). In our study, after watching the video that induced the emotion of fear, participants would have a higher level of attention and sensitivity to observe the computer screen, to get ready for any stimulus that might scare them in a sudden.
On the other hand, the state of sexual arousal also activates the regions of amygdala, hippocampus, and the autonomic nervous system in the brain. These areas are common to all mammals experiencing the sexual arousal state. Interestingly, these regions are also known to regulate emotion, and particularly in the emotion of fear. In addition, the amygdala has the ability to induce extreme pressure, and to motivate people to seek compulsive sexual behaviours to gain more pressure (Hamann et al., Citation2004).
People in the state of sexual arousal would have voluntary control of attention. In the experiment by Laws and Rubin (Citation1969), participants watched a sexually-arousing video, and they could either choose to masturbate or not. People who chose to masturbate would stay stronger focus and attention on the videos, and they would concentrate on the sexual thoughts. People who chose not to masturbate focused less on the movies. Even though they also would have distracting thoughts, they would choose to attend to other non-sexually-arousing materials. In the present study, participants were induced with sexual arousal by watching the pornographic videos, but they could not have masturbating behaviours during the experiment. Therefore, when they performed the 3-back task, they had to stay strong focus on the task for minimizing the sexual desire in the mind.
Based on our experimental results and theoretical discussions, similarities may be shared by the negative emotion of fear and sexual arousal. When people experience fear or sexual arousal, the amygdala and the autonomic nervous system would be activated to react and get ready for the relevant behaviours, such as flight, freeze, or sexual intercourse. Moreover, both fear and sexual arousal would occupy the attention capacity in order to react and stay alert, although they might involve different systems of attention. The emotion of fear demands the unconscious and involuntary attention for protection, observation, and alertness from danger; on the other hand, sexual arousal demands the voluntary attention for getting greater pleasure from sexual behaviours. In regard to the idea that fear and sexual arousal share similarities in processing in terms of biological and cognitive systems, the results of the present study were consistent. In Experiment 1, participants had better performance in the negative emotion and sexual arousal conditions, supporting that they had paid stronger attention to complete the 3-back tasks. Also, since the participants had to distract themselves from thoughts of sexual desire, their performance in the sexual arousal condition was worse than that in the negative emotion condition in which fear was triggered.
4.2. Tendency of compulsive sexual behaviour and cognitive performance
In the present study, we measured the tendency of compulsive sexual behaviour using the CSBI scale. People who had a lower score in the CSBI scale indicated a higher tendency of sexual behaviours. The results of Experiments 1 and 2 supported that the people with higher CSBI score had better performance on the WM tasks. A possible reason for the observation is that people with compulsive sexual behaviour might have more distracting thoughts in the mind during the task, as they would have a stronger need for masturbation after watching the pornographic videos despite they could not do so during the experiment. The stronger need for masturbation might have led to greater influence on the WM performance. As discussed in the previous section, the distraction about the sexual desire might interfere with the attention on the WM task, leading to distraction from the task. The interpretation is supported by previous research showing that the state of sexual arousal is linked with the brain structures associated with emotion, arousal, and attention (Cowan, Citation2010).
Compulsive sexual behaviour is associated with certain cognitive deficits, especially in WM and decision-making. Emotional processing is performed in the region of the prefrontal cortex (not only the limbic system), and sexual arousal is processed by the attention and emotion systems, and also the reward system of the brain. As the performing of WM task mainly relies on prefrontal areas, it would suggest that WM performance is interfered strongly by arousal-based attention and emotional stimuli (Davis & Whalen, Citation2001). Since the 3-back task in our study required the ability of monitoring, updating, and manipulating the presented stimuli, attention was highly demanded in the task. As sexual stimuli would distract attention in the task, people with a higher tendency in sexual behaviour would have a higher demand of sexual desire after watching the pornographic videos, which in turn also interferes their WM performance (Davis & Whalen, Citation2001).
Moreover, because decision-making tasks highly depends on WM capacity, such as updating the current information and using the top-down knowledge, it can be predicted that people with a higher tendency of compulsive sexual behaviours may also perform decision-making badly. This may explain why people with compulsive sexual behaviour may attempt risky sexual behaviours (e.g. cybersex with strangers, raping, insecure sexual intercourse) even when it brings severe consequences.
4.3. Limitations and implications for further studies
In the present study, the induction of emotional and sexual arousal was the most important manipulation on the participants to investigate their effects on cognitive performance. All the videos were identified on the YouTube website and some pornographic websites, and they might have caused different levels of emotional and sexual arousal in different people. To better control the strength of arousal induced by watching movies, future studies can perform a pilot test to rate the level of emotional and sexual arousal for different videos, and employ the most popular ones that people report to be the most arousing for the formal experiment. In addition, since there exists many kinds of positive and negative emotions, and the present study only focused on relaxation and humour as the positive emotion, and fear and stress as the negative emotion (due to the contents of the movies used), future studies may investigate the effects of a wider range of positive and negative emotions on cognitive performance.
To the best of the authors’ knowledge, there are very few studies in the literature which used the n-back task with manipulation on the type of stimuli presented. It could be difficult to have a direct comparison of task validity of the present experiments. Alphabets have been widely used in n-back task studies, and the validity of such task is well supported. However, n-back tasks using colours and sexual pictures as stimuli are dearth in the literature. Nevertheless, our experiments employed a within-subject design in which the same participant performed the different task conditions; this could help guarding the reliability of the data obtained from the colour and pictorial n-back tasks. Future studies can put more effort in investigating different research designs with such a classical experimental paradigm.
To measure the level of emotional and sexual arousal induced, we only used the physiological changes of HR, BP, and body temperature, and the self-reported VAS and DEQ scores. The reader should be cautious that these measures might have their limitations. Since physiological changes can be affected by other emotions (such as intense, nervous, or embarrassing), in future studies, it would be advisable to also measure genital response, such as using penile plethysmography, as an indicator of sexual arousal. This would allow monitoring of the duration of sexual arousal before and after viewing the videos and during the task.
Regarding the gender of the participants, the present study only focused on males, but it is unknown whether the effects of emotional arousal and sexual arousal on WM would be different in women and men, and also heterosexual and homosexual people, and by how much. Future studies can investigate people of different gender and sexual orientations in order to gain better understanding on the effects of emotional arousal and sexual arousal on cognitive performance.
Finally, the reader should be cautious that the sample size in the experiments of the present study was relatively small (n = 24 and 27). In view of the scales and measurements used to assess the dependent variables of subjective psychological feelings of sexual urge, desire to masturbate, and feelings of different emotions being put under a model of repeated measures ANOVA in data analysis, extraneous and potentially uncontrolled factors might influence the reliability of these measurements, since there might be considerable variations among individual participants due to the subjective nature of these measurements. The small sample size here might not offer enough statistical power for demonstrating the effects of the manipulations on the working memory variables. To avoid overstatements of the findings, the authors suggest that future studies using these kinds of scales for measuring subjective feelings should recruit a large sample in order to ensure good reliability of the measurements. Nevertheless, the present findings provided new insights regarding how emotional arousal and sexual arousal could affect cognitive functions.
Consent statement
All procedures performed in this study were in accordance to the ethical guidelines of the institution of the authors and the Declaration of Helsinki. Ethical approval was obtained from the research ethics committee of the institution of the authors.
Additional information
Notes on contributors
Ricky K. C. Au
Ricky K. C. Au received his academic training in the fields of experimental psychology and cognitive science. His research interests mainly lie on using methods of behavioural experiments to explore the explicit and implicit mechanisms of human perception, cognition, decision making, emotion, and the brain mechanisms related to these cognitive processes. Verity K. Y. Tang studied psychology and criminology, and her research interests include the cognitive psychology of emotion. Currently, the authors are working on exploring the effects of different types of emotional arousal on various cognitive functions and performance.
Verity K. Y. Tang
Ricky K. C. Au received his academic training in the fields of experimental psychology and cognitive science. His research interests mainly lie on using methods of behavioural experiments to explore the explicit and implicit mechanisms of human perception, cognition, decision making, emotion, and the brain mechanisms related to these cognitive processes. Verity K. Y. Tang studied psychology and criminology, and her research interests include the cognitive psychology of emotion. Currently, the authors are working on exploring the effects of different types of emotional arousal on various cognitive functions and performance.
References
- Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–20.
- Alvarez, J. A., & Emory, E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42.
- Awh, E., Vogel, E. K., & Oh, S.-H. (2006). Interactions between attention and working memory. Neuroscience, 139(1), 201–208.
- Bancroft, J., & Vukadinovic, Z. (2004). Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. Journal of Sex Research, 41(3), 225–234.
- Bechara, A., Damasio, H., & Damasio, A. R. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307.
- Bechara, A., Damasio, H., Tranel, D., & Anderson, S. W. (1998). Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience, 18(1), 428–437.
- Bechara, A., Tranel, D., & Damasio, H. (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain, 123(11), 2189–2202.
- Bergmann, H. C., Rijpkema, M., Fernández, G., & Kessels, R. P. (2012). The effects of valence and arousal on associative working memory and long-term memory. PLoS ONE, 7(12), e52616, 1–9. doi:10.1371/journal.pone.0052616
- Berridge, K. C., & Kringelbach, M. L. (2015). Pleasure systems in the brain. Neuron, 86(3), 646–664.
- Boyatzis, C. J., & Varghese, R. (1994). Children’s emotional associations with colors. Journal of Genetic Psychology, 155(1), 77–85.
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10(4), 433–436.
- Cahill, L., Babinsky, R., Markowitsch, H. J., & McGaugh, J. L. (1995). The amygdala and emotional memory. Nature, 377(6547), 295–296.
- Campbell, J., & Ehlert, U. (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134.
- Cardinali, D. P. (2017). Clinical implications of the timed autonomic nervous system. In D. P. Cardinali (Ed.), Autonomic nervous system (pp. pp. 313–373). New York: Springer.
- Coleman, E., Miner, M., Ohlerking, F., & Raymond, N. (2001). Compulsive Sexual Behavior Inventory: A preliminary study of reliability and validity. Journal of Sex and Marital Therapy, 27(4), 325–332.
- Cools, R., Gibbs, S. E., Miyakawa, A., Jagust, W., & D’Esposito, M. (2008). Working memory capacity predicts dopamine synthesis capacity in the human striatum. Journal of Neuroscience, 28(5), 1208–1212.
- Corbetta, M., Kincade, J. M., & Shulman, G. L. (2002). Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience, 14(3), 508–523.
- Cowan, N. (2006). Working memory capacity. New York: Psychology Press.
- Cowan, N. (2010). The magical mystery four: How is working memory capacity limited, and why? Current Directions in Psychological Science, 19(1), 51–57.
- Curci, A., Lanciano, T., Soleti, E., & Rimé, B. (2013). Negative emotional experiences arouse rumination and affect working memory capacity. Emotion, 13(5), 867–880.
- Curtis, C. E. (2006). Prefrontal and parietal contributions to spatial working memory. Neuroscience, 139(1), 173–180.
- Damasio, A. R. (1998). Emotion in the perspective of an integrated nervous system. Brain Research Reviews, 26(2–3), 83–86.
- Davis, M. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15(1), 353–375.
- Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6(1), 13–34.
- Demaree, H., Schmeichel, B., Robinson, J., & Everhart, D. E. (2004). Behavioural, affective, and physiological effects of negative and positive emotional exaggeration. Cognition and Emotion, 18(8), 1079–1097.
- Elliot, A. J., & Maier, M. A. (2014). Color psychology: Effects of perceiving color on psychological functioning in humans. Annual Review of Psychology, 65(1), 95–120.
- Engels, A. S., Heller, W., Mohanty, A., Harrington, J. D., Banich, M. T., Webb, A. G., & Miller, G. A. (2007). Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology, 44(3), 352–363.
- Gazzaley, A. (2011). Influence of early attentional modulation on working memory. Neuropsychologia, 49(6), 1410–1424.
- Goldstein, D. S., & McEwen, B. (2002). Allostasis, homeostats, and the nature of stress. Stress, 5(1), 55–58.
- Goodman, A. (2008). Neurobiology of addiction: An integrative review. Biochemical Pharmacology, 75(1), 266–322.
- Grall-Bronnec, M., & Sauvaget, A. (2014). The use of repetitive transcranial magnetic stimulation for modulating craving and addictive behaviours: A critical literature review of efficacy, technical and methodological considerations. Neuroscience and Biobehavioral Reviews, 47, 592–613.
- Gross, J. J., & Levenson, R. W. (1995). Emotion elicitation using films. Cognition and Emotion, 9(1), 87–108.
- Gross, J. J., & Levenson, R. W. (1997). Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology, 106(1), 95–103.
- Hamann, S., Herman, R. A., Nolan, C. L., & Wallen, K. (2004). Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience, 7(4), 411–416.
- Hamann, S. B., Ely, T. D., Hoffman, J. M., & Kilts, C. D. (2002). Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychological Science, 13(2), 135–141.
- Harmon-Jones, E., Vaughn-Scott, K., Mohr, S., Sigelman, J., & Harmon-Jones, C. (2004). The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion, 4(1), 95–101.
- Heiman, J. R. (1980). Female sexual response patterns: Interactions of physiological, affective, and contextual cues. Archives in General Psychiatry, 37(11), 1311–1316.
- Hollingworth, A. (2004). Constructing visual representations of natural scenes: The roles of short- and long-term visual memory. Journal of Experimental Psychology: Human Perception and Performance, 30(3), 519–537.
- Honey, G. D., Fu, C. H. Y., Kim, J., Brammer, M. J., Croudace, T. J., Suckling, J., … Bullmore, E. T. (2002). Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. NeuroImage, 17(2), 573–582.
- Irwin, D. E., & Zelinsky, G. J. (2002). Eye movements and scene perception: Memory for things observed. Perception and Psychophysics, 64(6), 882–895.
- Janssen, E., Everaerd, W., Spiering, M., & Janssen, J. (2000). Automatic processes and the appraisal of sexual stimuli: Toward an information processing model of sexual arousal. Journal of Sex Research, 37(1), 8–23.
- Julien, C., Kandza, P., Barres, C., Lo, M., Cerutti, C., & Sassard, J. (1990). Effects of sympathectomy on blood pressure and its variability in conscious rats. America Journal of Physiology, 259(5), H1337–H1342.
- Julien, E., & Over, R. (1988). Male sexual arousal across five modes of erotic stimulation. Archive Sexual Behavior, 17(2), 131–143.
- Kensinger, E. A., & Corkin, S. (2003). Effect of negative emotional content on working memory and long-term memory. Emotion, 3(4), 378–393.
- Laier, C., Schulte, F. P., & Brand, M. (2013). Pornographic picture processing interferes with working memory performance. Journal of Sex Research, 50(7), 642–652.
- Laws, D. R., & Rubin, H. B. (1969). Instructional control of an autonomic sexual response. Journal of Applied Behavioral Analysis, 2(2), 93–99.
- LeDoux, J. (2000). Cognition and emotion: What does the brain say? In R. D. Lane & L. Nadel (Eds.), Cognitive neuroscience of emotion (pp. pp. 129–155). New York: Oxford University Press.
- Levens, S. M., & Phelps, E. A. (2008). Emotion processing effects on interference resolution in working memory. Emotion, 8(2), 267–280.
- Lindström, B. R., & Bohlin, G. (2011). Emotion processing facilitates working memory performance. Cognition and Emotion, 25(7), 1196–1204.
- Löw, A., Lang, P. J., Smith, J. C., & Bradley, M. M. (2008). Both predator and prey: Emotional arousal in threat and reward. Psychological Science, 19(9), 865–873.
- MacKay, D. G., Shafto, M., Taylor, J. K., Marian, D. E., Abrams, L., & Dyer, J. R. (2004). Relations between emotion, memory, and attention: Evidence from taboo Stroop, lexical decision, and immediate memory tasks. Memory and Cognition, 32(3), 474–488.
- MacLeod, C., Mathews, A., & Tata, P. (1986). Attentional bias in emotional disorders. Journal in Abnormal Psychology, 95(1), 15–20.
- Mansour, V. M., Wilkinson, D. J. C., Jennings, G. L., Schwarz, R. G., Thompson, J. M., & Esler, M. D. (1998). Panic disorder: Coronary spasm as a basis for cardiac risk? Medical Journal of Australia, 168(8), 390–392.
- Marchetti, G. (2014). Attention and working memory: Two basic mechanisms for constructing temporal experiences. Frontiers in Psychology, 5(880), 1–15.
- Martin, M. (1990). On the induction of mood. Clinical Psychology Review, 10(6), 669–697.
- Mather, M., Canli, T., English, T., Whitfield, S., Wais, P., Ochsner, K., … Carstensen, L. L. (2004). Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science, 15(4), 259–263.
- Mather, M., Mitchell, K. J., Raye, C. L., Novak, D. L., Greene, E. J., & Johnson, M. K. (2006). Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience, 18(4), 614–625.
- Matthews, G., & Wells, A. (1999). The cognitive science of attention and emotion. In T. Dalgleish & M. J. Power (Eds.), Handbook of cognition and emotion (pp. pp. 171–192). Oxford, England: John Wiley & Sons.
- Miendlarzewska, E. A., van Elswijk, G., Cannistraci, C. V., & van Ee, R. (2013). Working memory load attenuates emotional enhancement in recognition memory. Frontiers in Psychology, 4(112), 1–10.
- Miner, M. H., Coleman, E., Center, B. A., Ross, M., & Rosser, B. R. S. (2007). The compulsive sexual behavior inventory: Psychometric properties. Archives of Sexual Behavior, 36(4), 579–587.
- Morris, N., & Jones, D. M. (1990). Memory updating in working memory: The role of the central executive. British Journal of Psychology, 81(2), 111–121.
- Most, S. B., Smith, S. D., Cooter, A. B., Levy, B. N., & Zald, D. H. (2007). The naked truth: Positive, arousing distractors impair rapid target perception. Cognition and Emotion, 21(5), 964–981.
- Nestler, E. J., Hyman, S. E., & Malenka, R. C. (2009). Molecular neuropharmacology: A foundation for clinical neuroscience (2nd ed.). New York: McGraw-Hill.
- Ohman, A., Flykt, A., & Esteves, F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130(3), 466–478.
- Owen, A. M. (1997). The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience, 9(7), 1329–1339.
- Paul, T., Schiffer, B., Zwarg, T., Krüger, T. H. C., Karama, S., Schedlowski, M., … Gizewski, E. R. (2008). Brain response to visual sexual stimuli in heterosexual and homosexual males. Human Brain Mapping, 29(6), 726–735.
- Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10(4), 437–442.
- Richard, J. M., Castro, D. C., DiFeliceantonio, A. G., Robinson, M. J. F., & Berridge, K. C. (2013). Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews, 37(9), 1919–1931. doi:10.1016/j.neubiorev.2012.12.008
- Schaefer, A., Braver, T. S., Reynolds, J. R., Burgess, G. C., Yarkoni, T., & Gray, J. R. (2006). Individual differences in amygdala activity predict response speed during working memory. Journal of Neuroscience, 26(40), 10120–10128.
- Schoofs, D., Preuß, D., & Wolf, O. T. (2008). Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology, 33(5), 643–653.
- Shackman, A. J., Sarinopoulos, I., Maxwell, J. S., Pizzagalli, D. A., Lavric, A., & Davidson, R. J. (2006). Anxiety selectively disrupts visuospatial working memory. Emotion, 6(1), 40–61.
- Silton, R. L., Heller, W., Engels, A. S., Towers, D. N., Spielberg, J. M., Edgar, J. C., … Miller, G. A. (2011). Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. Journal of Abnormal Psychology, 120(2), 272–285.
- Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661.
- Sunderwirth, S., Milkman, H., & Jenks, N. (1996). Neurochemistry and sexual addiction. Sexual Addiction and Compulsivity, 3(1), 22–32.
- Talmi, D., & McGarry, L. M. (2012). Accounting for immediate emotional memory enhancement. Journal of Memory and Language, 66(1), 93–108.
- Verdejo-Garcia, A., Perez-Garcia, M., & Bechara, A. (2006). Emotion, decision-making and substance dependence: A somatic-marker model of addiction. Current Neuropharmacology, 4(1), 17–31.
- Whalen, P. J. (1998). Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science, 7(6), 177–188.
- Williams, J. M. G., Watts, F. N., MacLeod, C., & Mathews, A. (1988). Cognitive psychology and emotional disorders. Oxford, England: John Wiley & Sons.
- Wright, L. W., Jr., & Adams, H. E. (1999). The effects of stimuli that vary in erotic content on cognitive processes. Journal of Sex Research, 36(2), 145–151.
- Yager, L. M., Garcia, A. F., Wunsch, A. M., & Ferguson, S. M. (2015). The ins and outs of the striatum: Role in drug addiction. Neuroscience, 301, 529–541.
- Zuckerman, M. (1971). Physiological measures of sexual arousal in the human. Psychological Bulletin, 75(5), 297–329.