Abstract
There is growing evidence of the relationship between Metabolic Syndrome (MetS) and cognitive decline; however, this has not been conclusively established yet. This systematic review and meta-analysis address the most crucial cognitive performance findings, including those on global cognitive function, memory, attention, and executive functions, in adult people with MetS. Two electronic databases were searched (April–May 2020) using the terms “metabolic syndrome” and “cognition,” including publications from 2010 to 2020. Thirty-six studies were found. Among these, 17 reported negative associations in cognition with MetS, mainly in terms of verbal memory and executive functions, particularly in the middle-aged population. A meta-analysis of global cognitive function revealed that the MetS group had a lower score than the control group (25.26 and 25.6, respectively, 95% CI, −0.60 to −0.12, p = 0.004). MetS is related to the enhanced presentation of cognitive impairment and its evolution into a Major Neurocognitive Disorder (MND). Further research involving longitudinal studies, including assessments with similar instruments, correctly separated by age group and education, is required.
PUBLIC INTEREST STATEMENT
The presence of obesity, diabetes, hypertension and high blood triglycerides, metabolic alterations which in conjunction are called Metabolic Syndrome (MetS), represents an important public health problem nowadays. MetS is related with a worsening of not only physical health but also of mental health since it is a risk factor for the development of dementia, now called Major Neurocognitive Disorder (MND). A better understanding of the negative effects of metabolic disorders on mental functions opens the possibility to establish preventive measures to ensure their conservation throughout aging, and subsequently, to maintain the independence of elderly population, increasing their quality of life. For this reason, our aim is to actualize and strengthen the evidence of the association of MetS and cognitive deficit in mental health through a systematic review and meta-analysis. As conclusion, MetS is related to an enhanced presentation of cognitive impairment and its evolution to MND.
1. Introduction
In previous decades, the incidence of chronic noncommunicable diseases has increased due to lifestyle changes. Such is the case for Metabolic Syndrome (MetS), meaning that, in the future, it may overtake smoking as a leading risk factor for heart disease (National Heart, Lung and Blood Institute, Citation2019). This situation might worsen due to the increased life expectancy that has caused an increased risk for suffering from this kind of illness (Álvarez-Cisneros et al., Citation2017). MetS refers to a group of disorders including high blood pressure, abdominal obesity, glucose intolerance, insulin resistance, high serum triglyceride levels, and a low concentration of High-Density Lipoprotein (HDL) cholesterol (Yates et al., Citation2012). The importance of MetS in public health is that there is an association between this syndrome and an increased risk of developing cardiovascular disease, type 2 diabetes, certain types of cancer, and mortality in general (Zhang et al., Citation2019). This condition is made up of different pathological components with particular prevalence levels, and there are no precise data on the incidence and prevalence of MetS, since its diagnosis has not yet been fully agreed upon between specialists. Nevertheless, considering that MetS is about three times more common than diabetes, it is possible to estimate that it affects one-quarter of the world’s population or, put another way, over a billion people could have MetS (Saklayen, Citation2018). In recent years, there has been a growing interest in the association of metabolic ailments with cognitive dysfunction (Hao et al., Citation2011; Siervo et al., Citation2014) because cognitive deficit may be one of the consequences of MetS. There is evidence that this syndrome could be a predecessor to the development of mild cognitive impairment, such as Major Neurocognitive Disorder (MND), due to cerebrovascular disease (previously called vascular dementia), or even Alzheimer’s disease (Wang et al., Citation2019). Frisardi et al. (Citation2010) proposed the use of the term metabolic-cognitive syndrome. They suggested an insulin-resistant brain status as an additional feature of this compound syndrome, and also identified the molecular profiles of patients with an increased risk of developing MND. A recent study showed that participants who suffer from diabetes mellitus present with memory dysfunction and mild cognitive decline (Huang et al., Citation2019). The mechanisms that link this syndrome with cognitive impairment include insulin resistance (Ma et al., Citation2015), ischemic brain lesions (Bokura et al., Citation2011), and inflammation (Yaffe, Citation2007). There is a significant association between MetS and a higher C reactive protein concentration with cognitive impairment in the elderly population (Ghosh et al., Citation2015). Moreover, cardiometabolic risk factors can lead to hypersensitivity in the brain white matter, and something fundamental to consider is that the frontal brain cortex is especially vulnerable to cerebrovascular factors and vascular disease (Alcorn et al., Citation2017; Keage et al., Citation2015). However, if this is related to cognitive decline, it is uncertain, and some mechanism associated with MetS could be activated in order to compensate for the cognitive decline (Alkan et al., Citation2019). MetS is related to characteristics associated with motor dysfunction and neuropsychological decline (Li et al., Citation2016), such as impairment of short-term memory, cognitive slowdown, and executive dysfunction (Chang et al., Citation2015; Bonilla & Galindo-Aldana, Citation2017 & Yogi-Morren et al., Citation2014). Regarding other processes, such as language, minimal negative effects have been found, and this has been associated, in some cases, with education, which has a neuroprotective effect on this cognitive process (Alcorn et al., Citation2017; Philippou et al., Citation2018). Perception has not shown significant affection (Alcorn et al., Citation2019). Specific studies have found that expressive language naming skills do not differ among people with MetS (Cahana-Amitay et al., Citation2015). That is, the differences found in the multiple spheres of cognitive processing reveal have not yet revealed conclusive results. This could be due to the wide variety of methodologies and instruments that different authors have used to measure cognitive decline, as for a given instrument, different approaches may be used and different cognitive aspects measured. This situation makes it complicated to do comparisons.
There are some systematic reviews in the literature about this issue (Alcorn et al., Citation2019; Assuncao et al., Citation2018; Hao et al., Citation2011; Yates et al., Citation2012); however, it is important to reanalyze the existent evidence for two main reasons. First, some of the previous works incorporated studies in which samples consisted of teenagers or subjects who were already experiencing cognitive decline, had a neurological history, or even had MND. In these cases, it is essential to consider that the neuropsychological impairment found could have been associated with a previous history of cerebrovascular disease or even a genetic predisposition to accelerated neuronal death. Second, these previous revisions were mostly qualitative. In the last published meta-analysis conducted by Siervo et al. (Citation2014), a tendency for declined cognition was observed in MetS participants, but the findings were not statistically significant. It is noteworthy that new data have been published in recent years that may strengthen the conclusions reached until now. For these reasons, it is necessary to revise the existing evidence through a more in-depth analysis using quantitative methods. This analysis should only include studies in which the sample consisted exclusively of adult participants with no severe cognitive decline so that the effects of MetS in cognitive function can be appreciated in the subclinical stages and preventive measures can be proposed with the purpose of a developing a better understanding about what is known nowadays and which studies are necessary to conduct in the future. It is for these reasons that the present systematic review and meta-analysis aimed to evaluate the most critical findings associated with the actual research on global cognitive function in adults with MetS but with no previous clinical history using quantitative methods. The importance of carrying out this analysis is to present the latest findings in this area and strengthen the evidence regarding the relationship between MetS and cognitive impairment.
The Population/Intervention/Comparison/Outcome (PICO) question that will serve as a guide to this review is shown in .
Table 1. The PICO description
2. Methods
2.1. Search strategy and study selection
In April and May 2020, database searches were performed in PubMed and EBSCO using the following keywords “metabolic syndrome” AND “cognition.” Broad search terms were used to facilitate maximum coverage of the literature. These two words were the only ones used since the syndrome includes several independent components and diseases; however, the interest of the present review was the complete diagnosis and not only individual components. The cognition term includes a global sphere of the neuropsychological profile, rather than mental processes independently. Three researchers independently identified the studies through manual searches. No limits were added during the database search. By reading the abstract, the search was restricted to human studies reported in English. The method used to merge the articles identified in the two databases consisted of the results from the databases including the full names of the articles, their authors, and links to access them in a single word processor document. Later, using the match search tool included in the word processor document, duplicates were identified and eliminated from the list.
The bibliographies of relevant articles were reviewed. Only papers that met the following criteria were included: (a) the study referred to MetS as an organismic variable and not only its individual components; (b) neuropsychological assessments were made to define the general cognitive function measured by neuropsychological tests; (c) the study population was adults; and (d) studies published from January 2010 until May 2020 were considered. We took into consideration the fact that the first systematic review on this topic was made in 2011 and included studies up to 2009 (Hao et al., Citation2011).
Only original studies with abstracts and full-texts available were included. Articles were excluded accordingly if they met the following criteria: studies focused only on one component of MetS; neuropsychological assessments of just a single cognitive process, rather than considering the entire cognitive sphere; participants with a clinical psychiatric history such as previous diagnosis of depressive disorder, anxiety, or other mood disorders, as well as personality disorders such as schizophrenia; participants with a clinical history of MND; participants with a history of cerebrovascular or cardiovascular events; and studies involving individuals younger than 19 years old.
The titles and abstracts were selected, and relevant articles were retrieved and evaluated according to the criteria mentioned above. Discrepancies for the inclusion and selection of the articles between investigators were resolved by discussion.
2.2. Data extraction and quality assessment
The three investigators independently extracted data from the included studies using standardized tables. For each study, we recorded the title, the lead author’s last name, the year of publication, the country of origin, the demographic characteristics of the participants (range of age, mean age, and education mean), and the cognitive domains assessed with their respective neuropsychological tests. Additionally, we recorded the criteria that were used to consider (or not) the definition of MetS (see ). The search and review were conducted with adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Urrutia & Bonfill, Citation2010).
Table 2. General characteristics of the revised articles
2.3. Diagnostic criteria for MetS
Several variations exist depending on the organization that proposes the diagnostic criteria. The precise criteria used to define and diagnose MetS vary among organizations; however, in general, it is well accepted that the criteria comprise a set of abnormalities that include insulin resistance, elevated triglyceride levels, dyslipidemia, arterial hypertension, and central obesity (Zhang et al., Citation2019). The most commonly used definition for MetS is that described by the United States National Cholesterol Education Program (NCEP-ATP III), which considers that the presence of three or more of those criteria is required for diagnosis. However, other criteria, such as those proposed by the World Health Organization (WHO), include glucose intolerance, impaired glucose tolerance or diabetes mellitus, and insulin resistance together with two or more of the following components: impaired glucose regulation, insulin resistance, raised arterial pressure, raised plasma triglycerides, central obesity, and microalbuminuria. The WHO states that other components of MetS have been described, e.g., hyperuricemia and coagulation disorders, but they are not necessary for the diagnosis (World Health Organization, Citation1999). The International Diabetes Federation considers central obesity to be a necessary prerequisite (for men, this is considered a waist circumference greater than 90 cm and in women greater than 80 cm) as well as at least two of the following aspects: having a fasting glycemic score greater than 100 mg/dL or being in treatment for it, triglyceride values greater than 150 mg/dL or receiving drug treatment for this condition, high blood pressure (>130/85) or receiving drug treatment for it, and an HDL cholesterol level lower than 40 mg/dL for men or 50 mg/dL for women. Other criteria less frequently found in publications are those from the American Heart Association and the Harmonizing the Metabolic syndrome project (Pacheco-Armenta MC, Citation2017).
2.4. Neuropsychological assessment
The studies focused on measuring cognitive domains such as memory, attention, executive functions, language, and general cognitive function in a broad and general way. This last factor was measured through brief instruments that identified whether participants had a significant cognitive impairment and the degree of severity; however, a wide variety of instruments were used to measure the state of these mental processes. For this reason, in this review, we chose those used with the highest frequency to make comparisons. Tables considering these instruments corresponding to their quantitative descriptions were made and were prepared so that they could be analyzed in a more in-depth way.
2.5. Meta-analysis
The information extraction strategy was based on reviews by three independent researchers who extracted the quantitative data. The meta-analysis was conducted using Review Manager 5.4 software from the Cochrane Collaboration (Higgins et al., Citation2020). This software was used to analyze the data according to the random effect model, and for the generation of the forest plot, we opted to compare crude means and standardized them according to each case. Data are presented as mean scores and standard deviations for cognitive scores and 95% confidence intervals (95% CI). Studies that did not report means and standard deviations or compare participants with MetS against participants without MetS were not included in the meta-analysis. Forest plots were generated to provide graphical presentations of the individual and pooled effect size estimates. Statistical heterogeneity across studies was assessed using the Tau2, Chi2, and I2. Finally, to evaluate the risk of publication bias, a Funnel Plot was generated using the same software.
3. Results
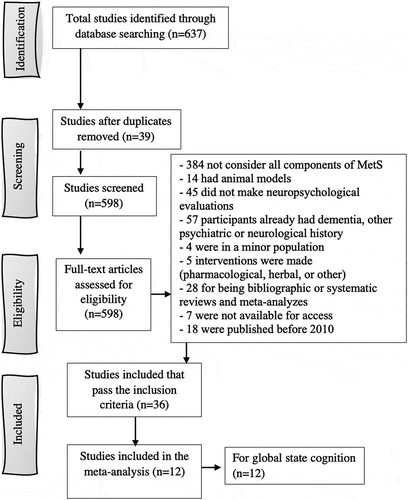
In the initial search, we identified 637 relevant articles. From these, we removed 39 duplicated documents, and 598 studies were screened. Finally, 562 were excluded (). Of the total that were eliminated, some did not consider all of the components of MetS (n = 384), some used animal models (n = 14), some did not do neuropsychological evaluations (n = 45), and some included participants who already had a psychiatric or neurological history or who had been diagnosed with MND (n = 57). Additionally, some had samples that included children or teenagers (n = 4), some included interventions (pharmacological or herbalist) (n = 5), and some articles were bibliographic or systematic reviews and meta-analyses (n = 28). Additionally, we could not access some documents (n = 7). Ultimately, 36 studies comprising 73,071 participants were included in the present systematic review and meta-analysis.
3.1. Characteristics of the included studies
The 36 studies were published from different continents (), especially America and Asia; some were from Europe and a couple from Oceania. The number of participants ranged from 32 to 16,415. Different methods were used to define the MetS criteria. The Mini-Mental State Examination (MMSE) was the most commonly used scale to assess general cognitive decline (n = 22), but other tests were used with a lower frequency, like the Montreal Cognitive Assessment (MoCa) (n = 2), for the same purpose. Verbal fluency tests were also used to examine executive functions through language skills (n = 12). The Trail Making Test part B (TMT-B) was used to evaluate executive function and information processing speed (n = 9), and digit symbol subtests (n = 7) were used to evaluate visual attention.
3.2. Characteristics of the results regarding MetS and cognition
Of the 36 studies selected, 17 found a clear association with adverse changes in cognition and the presence of the syndrome (M. Liu et al., Citation2015; Liu & Lippa, Citation2013; Raffaitin et al., Citation2011; Vieira et al., Citation2011). The combined risk of multiple factors in MetS was related to a lower overall performance on cognitive screeners and executive function (M. M. Y. Lai et al., Citation2020). On the other hand, adverse neurocognitive conditions were mainly identified in the middle-aged population (Akbaraly et al., Citation2010; González et al., Citation2018). According to the findings of Alfaro et al. (Citation2016), one of the most evident losses in patients with MetS was in the verbal memory cognitive domain, in addition to verbal fluency.
Likewise, Lamar et al. (Citation2015) found an association with a previously described relationship of the condition with the hippocampal structure. Some more specific findings were also reported regarding the syndrome components; Yao et al. (Citation2016) reported that particularly abdominal obesity and arterial hypertension were more significantly associated with the risk of mild cognitive impairment. Viscogliosi et al. (Citation2012) discovered that cognitive impairment was even assessable through the use of brief instruments, such as the MMSE, and that performance in these tests was lower in those even without symptomatic cognitive impairment. Coincidentally, Levin et al. (Citation2014) noted that blood pressure was significantly correlated with all cognitive domains, except memory. In addition to identifying this deterioration in patients with MetS, some authors were also able to identify neuroprotective factors, such as education (Collinson et al., Citation2014).
Some of these studies yielded inconclusive results; for example, Bangen et al. (Citation2019), despite finding a lower overall performance in cognitive screening tests in individuals of both middle and late age, did not find a significant change in the “cognitive trajectories.” Bae et al. (Citation2017) found that MetS was associated with mild cognitive impairment but only the nonamnestic type. There were also particular findings around the MetS indicators. Overman et al. (Citation2017) found that high blood glucose was significantly associated with visuo-constructional skills and processing speed, while Chen et al. (Citation2016) found that dyslipidemia was inversely associated with cognitive function. Del Brutto et al. (Citation2015) reported that only hypertriglyceridemia was associated with worse cognitive performance. Some findings suggest that the negative effect on cognition is secondary. According to Foong et al. (Citation2017) MetS increases an individual’s chance of suffering from other chronic conditions that can reduce cognition.
Other studies even considered the genotypes involved in cognitive impairment. Lai et al. (Citation2014) stated that MetS might play a role in subtle cognitive dysfunction in ε4 polymorphism carriers. Moreover, better performance in episodic memory tasks but not executive functions was found. Liu and Lippa (Citation2013) noted that central obesity is the most significant risk factor for cognitive decline. In contrast to the previously mentioned study, McEvoy et al. (Citation2012) stated that MetS is associated with better executive functions. With a decrease in long-term memory, and mainly when diabetes was present, MetS was associated with an accelerated decrease in executive functioning, but only in women. Katsumata et al. (Citation2012) noted that having a high level of glycosylated hemoglobin was associated with decreased memory function. It is possible to see that there is considerable variation in the general findings since some authors identified unfavorable changes, while others found them only under specific conditions. Finally, some studies did not report changes and even showed cognitive advantages in those with the syndrome (Lai et al., Citation2010; Tournoy et al., Citation2010).
3.3. Global cognitive function
As previously mentioned, the most widely used global cognitive assessment instrument (by 22 studies) was MMSE. Some of the authors reported the statistical means (except 6). Most authors used a 0–30 scale to rate the components of the instrument, and the corresponding standard deviations were given. It is noteworthy that, despite having some population adaptations, such as for the Chinese and Japanese versions of the MMSE, all of them used the same maximum value of 30. In 10 of the studies, conclusions suggested a more significant cognitive impairment associated with MetS, while the other nine studies had insignificant or suggestive findings, suggesting an unclear relationship between both variables. However, MMSE is not the only instrument available to assess general cognitive status. The MoCA test is also available. Like the MMSE, it assesses the main cognitive spheres of the global mental state. In the same way, it is scored on a scale of 0–30. Two studies (Chen et al., Citation2016; Gross et al., Citation2018) that assessed their participants with this instrument were included (see details below in ).
Table 3. Principal results of the twenty-four studies that used MMSE or MoCA for assessment of global cognition
3.4. Principal results of the twenty-four studies that used MMSE or MoCA for the assessment of general cognition
Of the twenty-four studies reviewed that used MMSE or MoCA to measure general cognitive status, 12 reached similar conclusions. There were discrete differences in performance between participants with MetS and controls. Given the nature of these tests, which assesses the general cognitive function, some reported generic changes in cognition (C. L. Liu et al., Citation2013; Lamar et al., Citation2015; Liu & Lippa, Citation2013; Raffaitin et al., Citation2011; Vieira et al., Citation2011; Viscogliosi et al., Citation2012).
On the other hand, other authors suggested that MetS is associated with cognitive impairment but only that of the nonamnestic type (Bae et al., Citation2017) or with general impairment in memory and executive functioning (Collinson et al., Citation2014). Similar findings related to mood, cognition, and hippocampal structure were described by Lamar et al. (Citation2015). Particularly, in younger older adults (C. L. Liu et al., Citation2013; Liu & Lippa, Citation2013), central obesity was identified as a significant risk factor for cognitive decline, but it was also shown to have a neuroprotective effect in late old age. Finally, some authors found no differences in the performance of those with the syndrome compared with controls (Alfaro et al., Citation2016; Harrison et al., Citation2015; Katsumata et al., Citation2012; Lai et al., Citation2014; M. M. Y. Lai et al., Citation2020; McEvoy et al., Citation2012; Shigaeff et al., Citation2013) or even stated that MetS could be associated with better cognitive function performance, especially in the elderly (Luo et al., Citation2013).
3.5. Cognitive domain: executive function
With respect to TMT, the current analysis is made from those who specifically mentioned having used part B, since this one measures executive functioning while part A is limited to processing speed tasks. Researchers who evaluated executive function and/or information processing speed through the TMT-B test had mixed results; of the eight studies reviewed, two did not find significant results regarding MetS having a negative influence on these mental functions (McEvoy et al., Citation2012 & Shigaeff et al., Citation2013). On the other hand, three studies reported a statistically significant lower performance in those with the syndrome (Bangen et al., Citation2019; Cavalieri et al., Citation2010; Philippou et al., Citation2018). Moreover, two more studies had inconclusive findings because they did not find a difference and also participants were not separated into those with and without the syndrome but rather by their levels of physical activity (Coll-Padrós et al., Citation2019; M. M. Y. Lai et al., Citation2020).
Other studies evaluated executive function through the verbal fluency task. It was found that in seven of the 11 studies (), the MetS groups showed a negative association between cardiometabolic risk factors and neuropsychological performance in this cognitive domain; however, a significant difference was not found (Katsumata et al., Citation2012; Levin et al., Citation2014; M. M. Y. Lai et al., Citation2020; McEvoy et al., Citation2012; Schuur et al., Citation2010; Shigaeff et al., Citation2013). Hassenstab et al. (Citation2010) found that MetS was associated with a lower level of performance, specifically for the executive function phonetic fluency. Alfaro et al. (Citation2016) found that hyperglycemia was the only component of MetS that was associated with lower cognitive performance in the domain of verbal fluency (), and González et al. pointed out that having MetS led to lower performance in verbal fluency (González et al., Citation2018).
Table 4. Principal results of the 12 studies that used verbal fluency for assessment
3.6. Cognitive domain: attention
Visual attention skills were assessed through the digit symbol test (the details of the obtained scores and the general results are described in (), in which data from seven studies were reported, five of which referred to the possible negative effects of MetS on this essential ability (Coll-Padrós et al., Citation2019; Foong et al., Citation2017; González et al., Citation2018; Philippou et al., Citation2018), especially in relation to elevated glucose levels (Overman et al., Citation2017). However, others concluded that causal interpretations could not be applied to the relationship of MetS with attentional impairment (C. L. Liu et al., Citation2013; Tournoy et al., Citation2010).
Table 5. Principal results of the seven studies that used digit symbol tests
3.7. Cognitive domain: memory
Of the articles reviewed, multiple studies measured this mental process, including Akbaraly et al. (Citation2010); Alfaro et al. (Citation2016); Alkan et al., Citation2019); Bae et al. (Citation2017); Bangen et al. (Citation2019); Cavalieri et al. (Citation2010); Collinson et al. (Citation2014); Foong et al. (Citation2017); Goh and Hart (Citation2014); González et al. (Citation2018); Harrison et al. (Citation2015); Hassenstab et al. (Citation2010); Lai et al. (Citation2010); Lai et al. (Citation2014); Lamar et al. (Citation2015); Levin et al. (Citation2014); Overman et al. (Citation2017); Philippou et al. (Citation2018); Schuur et al. (Citation2010); Shigaeff et al. (Citation2013); and Tournoy et al. (Citation2010). However, despite this recurrence in the assessment of that process, the studies differed in terms of the instruments used. Some used extensive scales with multiple subtests, and others used a single word list. Therefore, it was impossible to analyze all studies using the same quantitative comparison parameters. The expected averages differed markedly, as did the tests used. On the other hand, some studies reported significant differences; for example, González et al. (Citation2018) found that having a higher number of MetS components was consistently associated with more pronounced impairment in memory. Alfaro et al. (Citation2016) found that patients with the syndrome had lower memory performance, as did Lamar et al. (Citation2015) who found learning and memory alterations related to compromise in the hippocampal structures of the brain, and Collinson et al. (Citation2014) described this condition in middle-aged individuals and found that memory performance was worse in MetS participants. McEvoy et al. (Citation2012) reported that long-term memory was significantly affected in women with MetS. Raffaitin et al. (Citation2011) found an association of the syndrome with an increased risk of cognitive impairment, including a decline in memory. Other studies had inconclusive results about the association between the MetS and memory function. Bangen et al. (Citation2019) found that abdominal obesity was associated with lower cognitive performance in terms of memory but not the syndrome’s fundamental components. Philippou et al. (Citation2018) reported a significant negative coefficient for the body mass index for memory, but this is just one component of the syndrome. Katsumata et al. (Citation2012) described an association between memory impairment and glycated hemoglobin but not with all MetS components. Moreover, the study by Alkan et al. (Citation2019), in general, did not support the idea that MetS is reliably associated with memory deficits. Bae et al. (Citation2017) found no significant difference in memory performance between those with and without the syndrome. Overman et al. (Citation2017) found no evidence of a relationship between MetS and memory impairment. Harrison et al. (Citation2015) did not find a significant relationship between MetS and memory, these findings being consistent with those of Goh and Hart (Citation2014), Lai et al. (Citation2014), and Levin et al. (Citation2014). Despite finding a relationship between high blood pressure and other cognitive domains, particularly concerning memory, they did not find significant data. Furthermore, Lai et al. (Citation2014) did not report a significant relationship between memory and MetS, and Shigaeff et al. (Citation2013) found no differences in memory associated with MetS.
4. Meta-analysis
Due to the presentations of data, not all studies were candidates to be included in the meta-analysis of global cognitive function only in 12 were presented mean and standard deviations and comparisons between an MetS group and a control group shown.
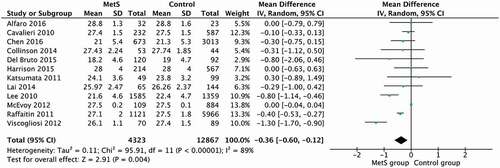
The pooled results of the 12 studies that evaluated global cognitive function revealed that the MetS group had a significantly lower score for this variable in comparison with the control group (25.26 and 25.6, respectively, 95% CI [−0.60 to −0.12] (p = 0.004). However, the level of heterogeneity was high (I2 = 89%; p < 0.00001) (see ). The studies were divided into groups based on the participants’ mean age (mean age inferior to 70 years old and superior to 70 years old) to reduce heterogeneity.
Figure 2. Forest Plot from the studies that measured global cognition by the application of MMSE and MoCA
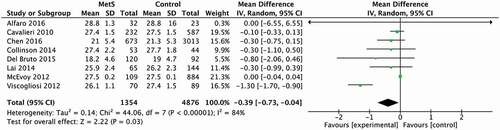
Studies involving participants younger than 70 years old (see ) were found to be significantly responsible for heterogeneity observed (I2 = 84%; p < 0.00001). Still, significant differences between MetS and control groups were found in this age group (25.28 and 25.67, respectively, 95% CI [−0.73 to −0.04] (p = 0.03).
Figure 3. Forest Plot from studies that measured global cognition by applying of the MMSE and MoCA in participants with a mean age inferior to 70 years old
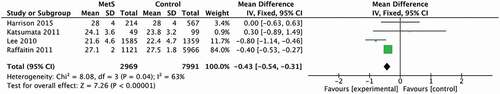
When only studies involving participants with a mean age superior to 70 years old were included in the analysis (see ), the level of heterogeneity decreased (I2 = 63%; p < 0.04). The MetS group had a significantly lower score for this variable in comparison with the control group (25.2 and 25.4, respectively, 95% CI [−0.54 to −0.31] (p = 0.00001).
Figure 4. Forest Plot from studies that measured global cognition by applying of the MMSE and MoCA in participants with a mean age superior to 70 years old
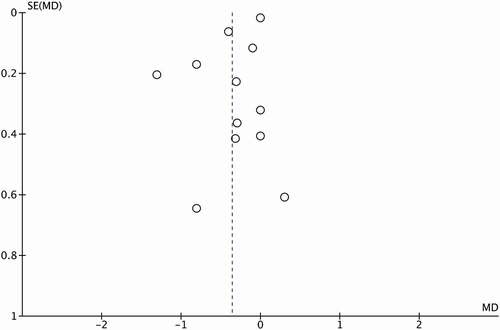
Additionally, to evaluate the risk of publication bias, a Funnel Plot was constructed using the Standardized Mean Difference (SMD) and 1/SE values obtained from studies that assessed global cognition (see ). It is noteworthy that a mild degree of asymmetry in the global cognitive domain was identified.
5. Discussion
5.1. Summary of the main results
One of the difficulties of doing this kind of analysis is that some studies report their data in a way that is not comparable, even when the same instruments are used to measure cognitive domains. One example of this is memory and this was the most commonly evaluated cognitive process. However, the wide variety of modalities involved in memory makes its assessment widely varied and hard to compare. In addition to this, some studies used instruments that were not common or were used with adaptations. It should be noted that each study had an individualized selection of instruments, which was chosen based on different aspects, such as the convenience in accessing the tests to be acquired, the materials already available in the corresponding institutions, and the feasibility and duration of application of the instruments, among other unknown factors. That raises questions about whether the variability in the results among studies is associated with the varied nature of instruments used to assess memory. Each type of study could measure different types of memory based on the instrument selected.
Some assessed short-term verbal memory, while others, by asking for information in a delayed way, measured a different phase of the memory process, for example, delayed recall involving the same type of verbal memory. The studies on the neuropsychology of the MetS carried out until now have shown ambivalent and inconclusive results regarding the specific cognitive processes affected as a result of metabolic conditions and those seem to resist the negative metabolic effects better. Regarding general cognitive function, which is evaluated by the MMSE or MoCA, it briefly and superficially seems to be affected by the syndrome even though there have been some inconclusive results with a tendency to indicate a negative impact of MetS on the general cognitive status. It is pertinent to emphasize that these tests assess cognitive status in a broad and shallow way since assessments with these instruments last for no more than 10 minutes. These screening tests are usually used to identify whether or not a participant is a candidate for a complete and in-depth assessment, so their use has several limitations, such as low sensitivity to mild cognitive impairment because only severe impairment would prevent the patient from answering most of the items in a correct way (Spencer et al., Citation2013). For this reason, when every study was evaluated individually, general cognitive impairment did not seem to be significantly affected; however, the joint analysis of means through a meta-analysis showed a significant effect of MetS on general cognitive function. However, it is noteworthy that there was a high level of heterogeneity in the meta-analysis. The explanation for this could be the variation in the different populations included in the studies with regard to educational level, socio-economic and cultural conditions, as well as genetic polymorphisms that affect brain neuroplasticity, which benefit from multiple factors throughout life, such as age, among others (Miskolczi et al., Citation2019; Phillips, Citation2017; Stewart & Cramer, Citation2017). Regarding this last topic, when a meta-analysis that involved subdividing by age mean groups was done, it was observed that the same low level of cognitive performance was present in both groups. This could imply that brain plasticity to adapt to the negative metabolic and vascular changes associated with MetS is lower, even from an earlier stage of life. After concentrating the data from multiple individual studies into a single meta-analytical study, it became apparent that the youngest and the oldest individuals were close in terms of the level of deterioration in cognitive performance, and the potential neuroprotective factor of age was less clear.
Evaluations of superior frontal brain function, made through assessing the executive function of verbal fluency, showed inconclusive results among the different studies, with some showing a statistically significant decrease (Alfaro et al., Citation2016; González et al., Citation2018 & Hassenstab et al., Citation2010) and others finding no difference (Katsumata et al., Citation2012; Levin et al., Citation2014; M. M. Y. Lai et al., Citation2020; McEvoy et al., Citation2012; Schuur et al., Citation2010; Shigaeff et al., Citation2013). A possible cause of the inconclusive results is that this superior function was assessed with tests of various modalities; some studies measured phonological verbal fluency and others measured semantic verbal fluency. Despite some researchers measuring phonological verbal fluency in the same way, the instructions could have involved different letters, but this was not specified in some of the studies. For this reason, it was difficult to determine the general findings regarding the maintenance or deterioration of this function. The performance in visual attentional skills and memory, unlike other cognitive processes, seems to have a more definite negative effect and more consistency in the findings. Since most of the studies reported a coincident deficit in participants with MetS, it is noteworthy that attention and memory are mainly regulated by cerebral prefrontal mechanisms. Some of the most basic processes involved in these cognitive functions (like visual analysis) are carried out in the posterior cerebral cortex structures, and these processes could be more susceptible to metabolic deterioration, especially under hyperlipidemic conditions (Friedman et al., Citation2014). Lastly, in terms of visual attention skills, findings on whether MetS may or may not negatively impact these cognitive functions have differed with regard to more basic sensory processes, such as sight.
5.2. Clinical Implications
There are multiple current implications of the present findings for clinical neuropsychological practice and mental health care in general, given that current therapeutic options are usually focused on when the patient already has the first neurological indicators of deterioration, such as decreased memory. However, it is crucial to keep in mind that preventive, therapeutic options focus on reducing the future presentation of such damage. However, these therapeutic options aim to modify lifestyle habits before the presentation of neurological damage and psychoeducational processes to inform the community about the importance of making these changes.
It is important to emphasize that the current relationship of MetS with health is not limited to the future presentation of higher cardiovascular risk but goes beyond the possible presence of an MND due to cerebrovascular disease or Alzheimer’s disease. As patients get older, therapeutic options become increasingly limited, as compensatory brain processes, such as brain neuroplasticity, decrease, and the cognitive reserve decreases. The cognitive reserve that individuals with MetS possess at various stages of life should be considered during neuropsychological exploration of general cognitive functioning and in terms of neural compensation and the brain reserve, as some cognitive functions can change both due to the passage of time as well as due to being fundamentally affected by the presence of the syndrome (Strong et al., Citation2020). Brain compensation can be compromised by nutritional aspects as well as by gradual deficits that force general neurological restructuring and, with it, a highly variable cognitive expression. A significant challenge for therapists is assessing the options of improvement for a patient and directing the treatment regarding the compensatory options for the family.
5.3. Practical Implications
MetS is significantly related to lifestyle aspects, such as low-quality diet, sedentarism, and smoking habits. That is why it is relatively feasible to treat MetS by making life habit changes that prevent its consequences. Due to this, studying how metabolic-related diseases are associated with the risk of developing cognitive impairment has significant relevance. Public health policies that are focused on mental illness may consider preventing and controlling metabolic-related diseases to hinder the subsequent development of cognitive ailments. Until now, no studies have evaluated how diet and nutritional status, as well as aspects of eating behavior and lifestyle, influence the effects of MetS on cognitive impairment; only one protocol proposed the evaluation of diet through a food frequency questionnaire, has been proposed (Mumme et al., Citation2019).
Another relevant piece of practical implication is that the assessment of an individual’s functionality in instrumental activities of daily life should be included since it is considered that minor defects from youth can gradually diminish a patient’s cognitive condition, leading to potentially less appropriate decisions about their health care in later adult years. Therefore, future studies should evaluate the effects of diet, level of independence, and lifestyle in patients with MetS and their associations with cognitive function.
5.4. Agreements and disagreements with previous results
Previous systematic reviews on this issue have some similarities and several differences with the present one. One of the first systematic reviews was conducted by Hao et al. (Citation2011). They found a relationship between MetS and cognitive impairment in studies that evaluated MND due to cerebrovascular disease, but the association was not found in participants with Alzheimer's Disease. On the other hand, in their review, Yates et al. (Citation2012) included samples of children, teenagers, and adults, and they also evaluated structural damage to the brain. With respect to Alcorn et. al. (Citation2017), one of the main conclusions was that participants with MetS only performed poorly in executive functioning tasks that were not adaptations of the verbal fluency task because in these verbal fluency adaptations, the results were less consistent. A central difference to the present review is that different instruments were considered to measure the diverse executive functions, as the TMT-B test assesses, in addition to the speed of information processing, cognitive flexibility to alternate focus attention, as well as working memory. On the other hand, Alcorn et al. (Citation2017) used the verbal fluency instead and found that this process seems to be maintained more than the other executive functions that are less dependent on language. It is important to note that this process usually has associated neuroprotective factors, such as a high level of education (Goñi Sarriés et al., Citation2015).
More recently, Assuncao et al. (Citation2018) carried out a systematic review to evaluate cognitive impairment in MetS; one of the differences with the present work is that they only included studies that assessed the elderly population with MND; it is probably for this reason that they concluded that the role of MetS in cognitive decline and the onset of MND showed heterogeneous results. Finally, Siervo et al. (Citation2014) carried out a meta-analysis that found that MetS is associated with a cognitive decline in an age-dependent manner. One of the most important differences to the present study, which is a major weakness, is that they mixed the different cognitive domains evaluated in the publications, including general cognitive assessment with MMSE, executive functions, and memory, in one analysis. They also only analyzed MMSE data, and unlike our review, they found a nonsignificant association of MetS with cognitive decline; the new evidence that has been published in recent years allowed the addition of new data into the present systematic review and meta-analysis, strengthening the evidence of the association of MetS with global cognition.
6. Limitations
Some of the limitations of the present study were the wide variability between the types of instruments used for neuropsychological assessments and the fact that there was no clear standardization between the expected parameters for that range of instruments. There is no clear differentiation between totally regular performances or differences between mild and severe impairment. On the other hand, studies have shown a difficulty with having heterogeneous populations, as this was the root problem of the syndrome itself. It is methodologically necessary to seek a new paradigm, where longitudinal studies are the key and cross-sectional comparison studies, where more heterogeneity is seen in a single moment, are avoided. In other words, if follow-up studies of patients where it is possible to find these discrete differences between one moment and another are carried out at a global level, it is possible that the slowed change process may lead to compensation factors intervening and having plenty of time for adaptation (Alkan et al., Citation2019). In a longitudinal study, the compensatory effects of the nervous system of subjects in metabolic crisis that is chronic and graduated over time could be seen. At the current moment, in terms of understanding the neuropsychological profile of MetS, it seems that differences are small and may be due to the cross-sectional measurements being conducted at different stages of illness for each participant, leaving doubt as to whether there is deterioration or not (Bonilla & Galindo-Aldana, Citation2017). A methodological implication lies in the fact that the aforementioned studies referred to the means of individuals of different educational levels; however, when they provided the results of neuropsychological tests, they usually did not explicitly report the means for those with a low educational level with and without the syndrome or for those with a high educational level with and without the syndrome. We emphasize that educational level functions as a significant neuroprotective factor, and some studies included samples with this more excellent neuroprotective factor. Although the meta-analysis allowed us to compare the group effect sizes, when they were combined, a methodological bias could have been generated when increasing the size of the global effect without correct division by educational level. Moreover, we found slight evidence of a publication bias through the Funnel Plot analysis. In the absence of a publication bias, such a plot is expected to have a shape resembling an inverted funnel. However, asymmetry is observed when studies are absent on one side of the axis, meaning that, for several reasons, they were not considered in the analysis, for example, studies that were not published or presented incomplete data. The risk with this kind of bias is that studies with positive results are more likely to be published and the conclusions reached are influenced by the nature and direction of the results because data from nonpublished studies are not available. The significance of this risk could not be analyzed in depth through statistics such as the Egger test, given the small number of studies that reported complete data on general cognitive function through means and standard deviations. Therefore, studies that report all types of information necessary to generate more complete meta-analyses are required. More research in this field is needed to increase the quality of data available to obtain more reliable results.
7. Conclusion
It is possible to conclude that there are key cognitive processes affected by MetS, such as attention, memory, executive functions, and the global mental state. Through a meta-analysis, it was found that global cognitive state is statistically significantly affected both in the early and later stages of aging. The present findings indicate that the syndrome has an anatomical–functional correlation, in which the metabolic state of the central nervous system has a significant influence on the brain’s ability to function, leading to specific mental abilities.
Neuropsychology teams working in the clinical field and in the research area must apply validated, standardized instruments for which there are normative data on the clinical population. Then, they must make adjustments based on language and culture. It is essential to avoid disparities in instrumentation that lead to the results obtained not being comparable. This process of standardizing neuropsychological work requires that the results are reported uniformly since there are publications in which the results obtained are transformed into z-values, which complicates their potential subsequent comparison. Future systematic reviews and meta-analyses should be conducted to deepen our understanding of the mental health effects of these highly prevalent diseases in our community. The importance of continuing to evaluate the neuropsychological profile of MetS is considered fundamental to elucidate the implications that lead the central nervous system to have neurological disorders secondary to metabolic diseases in the medium and long term.
Conflicts of interest
The authors have declared that they do not have a conflict of interest.
Acknowledgements
We are grateful to Dr. Gilberto Ochoa Ruíz for reviewing the document, to Dora María Ramírez for her contribution to the tables, and we give special thanks to Luis Pablo Zúñiga Torres for his technical help with the statistical analyses.
Additional information
Funding
Notes on contributors
Estefania Ochoa-Ruíz
The authors conform to a multidisciplinary research group that work in different areas of neuroscience, neuropsychology, behavior, and metabolism. Their research interest is the study of the causes and consequences of metabolic affection in different organism systems, in particular, the central nervous system, with experience in both animal model and human population.
References
- Akbaraly, T. N., Kivimaki, M., Shipley, M. J., Tabak, A. G., Jokela, M., Virtanen, M., Marmot, M. G., Ferrie, J. E., & Singh-Manoux, A. (2010). Metabolic syndrome over 10 years and cognitive functioning in late midlife: The Whitehall II study. Diabetes Care, 33(1), 84–33. https://doi.org/10.2337/dc09-1218
- Alcorn, T., Hart, E., Smith, A. E., Feuerriegel, D., Stephan, B. C. M., Siervo, M., & Keage, H. A. D. (2017). Cross-sectional associations between metabolic syndrome and performance across cognitive domains: A systematic review. Applied Neuropsychology: Adult, 26(2), 186–199. https://doi.org/10.1080/23279095.2017.1363039
- Alfaro, F. J., Lioutas, V. A., Pimentel, D. A., Chung, C. C., Bedoya, F., Yoo, W. K., & Novak, V. (2016). Cognitive decline in metabolic syndrome is linked to microstructural white matter abnormalities. Journal of Neurology, 263(12), 2505–2514. https://doi.org/10.1007/s00415-016-8292-z
- Álvarez-Cisneros, T., Torres-Castro, S., Mena-Montes, B., Magdalena, N., Montes, B. M., Castro, S. T., Cisneros, T. Á., Rabaneda, R., Magdalena, N., Carrillo, T., Arlet, P., Rojas, R., Martínez, A., Miguel, L., Robledo, G., Castro, S. T., Morales, P. K., & Alice, P. S. (2017). Alzheimer: Diferencias por género entre América Latina y otras regiones del mundo. Género y salud en cifras, 15(3), 5–11.
- Alkan, E., Taporoski, T. P., Sterr, A., Von Schantz, M., Vallada, H., Krieger, J. E., Pereira, A. C., Alvim, R., Horimoto, A. R. V. R., Pompéia, S., Negrão, A. B., & Evans, S. L. H. (2019). Metabolic syndrome alters relationships between cardiometabolic variables, cognition and white matter hyperintensity load. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-40630–6
- Assuncao, N., Sudo, F. K., Drummond, C., Guarino de Felice, F., & Mattos, P. (2018). Metabolic Syndrome and cognitive decline in the elderly: A systematic review. PLoS ONE, 13(3), 1–17. https://doi.org/10.1371/journal.pone.0194990
- Bae, S., Shimada, H., Lee, S., Makizako, H., Lee, S., Harada, K., Doi, T., Tsutsumimoto, K., Hotta, R., Nakakubo, S., Park, H., & Suzuki, T. (2017). The relationships between components of metabolic syndrome and mild cognitive impairment subtypes: A cross-sectional study of Japanese older adults. Journal of Alzheimer’s Disease, 60(3), 913–921. https://doi.org/10.3233/JAD-161230
- Bangen, K. J., Armstrong, N. M., Au, R., & Gross, A. L. (2019). Metabolic syndrome and cognitive trajectories in the Framingham offspring study. Journal of Alzheimer’s Disease,711, 931–943. https://doi.org/10.3233/JAD-190261
- Bokura, H., Nagai, A., Oguro, H., Kobayashi, S., & Yamaguchi, S. (2011). The association of metabolic syndrome and executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dementia & Geriatric Cognitive Disorders, 30(6), 479–485. https://doi.org/10.1159/000322057
- Bonilla, J., & Galindo-Aldana, G. M. (2017). Neuropsicología del síndrome metabólico. In M. A. Villa-Rodríguez, M. E. Navarro-Calvillo, & T. D. J. Villaseñor-Cabrera (Eds.), Neuropsicología clínica hospitalaria (pp. 192). Manual Moderno.
- Cahana-Amitay, D., Spiro, A., Cohen, J. A., Oveis, A. C., Ojo, E. A., Sayers, J. T., Obler, L. K., & Albert, M. L. (2015). Effects of metabolic syndrome on language functions in aging. Journal of the International Neuropsychological Society, 21(2), 116–125. https://doi.org/10.1017/S1355617715000028
- Cavalieri, M., Ropele, S., Petrovic, K., Pluta-Fuerst, A., Homayoon, N., Enzinger, C., Grazer, A., Katschnig, P., Schwingenschuh, P., Berghold, A., & Schmidt, R. (2010). Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care, 33(12), 2489–2495. https://doi.org/10.2337/dc10-0851
- Chang, T. T., Lung, F. W., & Yen, Y. C. (2015). Depressive symptoms, cognitive impairment, and metabolic syndrome in community-dwelling elderly in Southern Taiwan. Psychogeriatrics, 15(2), 109–115. https://doi.org/10.1111/psyg.12080
- Chen, B., Jin, X., Guo, R., Chen, Z., Hou, X., Gao, F., Zhang, Y., Zheng, S., Fu, C., Xue, F., Niu, H., & Zhang, Y. (2016). Metabolic syndrome and cognitive performance among Chinese ≥50 years: A cross-sectional study with 3988 participants. Metabolic Syndrome and Related Disorders,14(4), 222–227. https://doi.org/10.1089/met.2015.0094
- Collinson, S. L., Tong, S. J. F., Loh, S. S. K., Chionh, S. B., & Merchant, R. A. (2014). Midlife metabolic syndrome and neurocognitive function in a mixed Asian sample. International Psychogeriatrics, 26(8), 1305–1316. https://doi.org/10.1017/S104161021400057X
- Coll-Padrós, N., León, M., Valech, N., Ros, E., Vidal, J., Estruch, R., Fitó, M., Salas-Salvadó, J., Corella, D., Molinuevo, J. L., & Rami, L. (2019). Physical activity is associated with better global cognition and frontal function in overweight/obese older adults with metabolic syndrome. European Review of Aging and Physical Activity, 16(1), 1–8. https://doi.org/10.1186/s11556-019-0229-y
- Del Brutto, O. H., Mera, R. M., & Zambrano, M. (2015). Metabolic syndrome correlates poorly with cognitive performance in stroke-free community-dwelling older adults: A population-based, cross-sectional study in rural Ecuador. Aging Clinical and Experimental Research, 28(2), 321–325. https://doi.org/10.1007/s40520-015-0404–6
- Foong, H. F., Hamid, T. A., Ibrahim, R., Haron, S. A., & Shahar, S. (2017). Chronic condition as a mediator between metabolic syndrome and cognition among community-dwelling older adults: The moderating role of sex. Geriatrics and Gerontology International, 17(11), 1914–1920. https://doi.org/10.1111/ggi.12993
- Friedman, J. I., Tang, C. Y., De Haas, H. J., Changchien, L., Goliasch, G., Dabas, P., Wang, V., Fayad, Z. A., Fuster, V., & Narula, J. (2014). Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC: Cardiovascular Imaging, 7(10), 1039–1053. https://doi.org/10.1016/j.jcmg.2014.06.014
- Frisardi, V., Solfrizzi, V., Capurso, C., Imbimbo, B. P., Vendemiale, G., Seripa, D., Pilotto, A., & Panza, F. (2010). Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? Journal of Alzheimer’s Disease, 21(1), 57–63. https://doi.org/10.3233/JAD-2010-100015
- Ghosh, A., Biswas, A. K., & Banerjee, A. (2015). A study on cognitive decline with respect to metabolic syndrome and inflammation in elderly Indians. Neurology India, 63(4), 537–541. https://doi.org/10.4103/0028-3886.162037
- Goh, V. H. H., & Hart, W. G. (2014). The association of metabolic syndrome and aging with cognition in Asian men. Aging Male, 17(4), 216–222. https://doi.org/10.3109/13685538.2014.968772
- Goñi Sarriés, A., López-Goñi, J. J., Granados-Rodríguez, D., & González-Jiménez, Á. (2015). Age, schooling and verbal fluency tasks for the screening of Alzheimer’s disease patients. Anales de Psicología, 31(3), 773–781. https://doi.org/10.6018/analesps.31.3.168941
- González, H. M., Tarraf, W., Vásquez, P., Sanderlin, A. H., Rosenberg, N. I., Davis, S., Rodríguez, C. J., Gallo, L. C., Thyagarajan, B., Daviglus, M., Khambaty, T., Cai, J., & Schneiderman, N. (2018). Metabolic syndrome and neurocognition among diverse middle-aged and older Hispanics/Latinos: HCHS/SOL results. Diabetes Care, 41(7), 1501–1509. https://doi.org/10.2337/dc17-1896
- Gross, T. J., Araújo, R. B., Vale, F. A. C., Bessani, M., & Maciel, C. D. (2018). Dependence between cognitive impairment and metabolic syndrome applied to a Brazilian elderly dataset. Artificial Intelligence in Medicine, 90(January), 53–60. https://doi.org/10.1016/j.artmed.2018.07.003
- Hao, Z., Wu, B., Wang, D., & Liu, M. (2011). Association between metabolic syndrome and cognitive decline: A systematic review of prospective population-based studies. Acta Neuropsychiatrica, 23(2), 69–74. https://doi.org/10.1111/j.1601-5215.2011.00527.x
- Harrison, S. L., Stephan, B. C. M., Siervo, M., Granic, A., Davies, K., Wesnes, K. A., Kirkwood, T. B. L., Robinson, L., & Jagger, C. (2015). Is there an association between metabolic syndrome and cognitive function in very old adults? The Newcastle 85+ study. Journal of the American Geriatrics Society, 63(4), 667–675. https://doi.org/10.1111/jgs.13358
- Hassenstab, J. J., Sweat, V., Bruehl, H., & Convit, A. (2010). Metabolic syndrome is associated with learning and recall impairment in middle age. Dementia and Geriatric Cognitive Disorders, 29(4), 356–362. https://doi.org/10.1159/000296071
- Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., & Welch, V. A., editors. Cochrane handbook for systematic reviews of interventions versión 6.1 (updated (September 2020). 2020. Available from. www.training.cochrane.org/handbook
- Huang, X., Wang, C., Tian, S., Huang, R., Guo, D., Zhang, H., Shi, J., & Wang, S. (2019). Higher plasma level of nampt presaging memory dysfunction in Chinese type 2 diabetes patients with mild cognitive impairment. Journal of Alzheimer’s Disease, 70(1), 303–314. https://doi.org/10.3233/JAD-190269
- Institute, N. H. L. and B. (2019). Metabolic Syndrome. https://www.nhlbi.nih.gov/health-topics/metabolic-syndrome
- Katsumata, Y., Todoriki, H., Higashiuesato, Y., Yasura, S., Willcox, D. C., Ohya, Y., Willcox, B. J., & Dodge, H. H. (2011). Metabolic syndrome and cognitive decline among the oldest old in Okinawa: In search of a mechanism. The KOCOA project. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 67A(2), 126–134. https://doi.org/10.1093/gerona/glr189
- Katsumata, Y., Todoriki, H., Higashiuesato, Y., Yasura, S., Willcox, D. C., Ohya, Y., Willcox, B. J., & Dodge, H. H. (2012). Metabolic syndrome and cognitive decline among the oldest old in Okinawa: In search of a mechanism. The KOCOA project. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 67 A(2), 126–134. https://doi.org/10.1093/gerona/glr189
- Keage, H. A. D., Kurylowicz, L., Lavrencic, L. M., Churches, O. F., Flitton, A., Hofmann, J., Kohler, M., & Badcock, N. A. (2015). Cerebrovascular function associated with fluid, not crystallized, abilities in older adults: A transcranial Droppler study. Psychology and Aging, 30(3), 613–623. https://doi.org/10.1037/pag0000026
- Lai, C. L., Lin, R. T., Liou, L. M., Hsu, C. Y., Hsieh, H. Y., & Liu, C. K. (2010). The effects of metabolic syndrome and apolipoprotein E4 on cognitive event-related potentials. Biological Psychology, 83(1), 56–61. https://doi.org/10.1016/j.biopsycho.2009.10.004
- Lai, C. L., Liou, L. M., Liu, C. K., Yang, Y. H., & Lin, R. T. (2014). Effects of metabolic syndrome, apolipoprotein E, and CYP46 on cognition among Taiwanese Chinese. Kaohsiung Journal of Medical Sciences, 30(7), 343–349. https://doi.org/10.1016/j.kjms.2014.03.005
- Lai, M. M. Y., Ames, D. J., Cox, K. L., Ellis, K. A., Sharman, M. J., Hepworth, G., Desmond, P., Cyarto, E. V., Szoeke, C., Martins, R., Masters, C. L., & Lautenschlager, N. T. (2020). Association between cognitive function and clustered cardiovascular risk of metabolic syndrome in older adults at risk of cognitive decline. Journal of Nutrition, Health and Aging, 24(3), 300–304. https://doi.org/10.1007/s12603-020-1333-4
- Lamar, M., Leah, R., Ajilore, O., Charlton, R., Zhang, A., Yang, S., Cohen, J., & Kumar, A. (2015). What metabolic syndrome contributes to brain outcomes in African American & Caucasian cohorts. Current Alzheimer Research, 12(7), 640–647. https://doi.org/10.2174/1567205012666150701102325
- Lee, K. S., Jang, Y., Chung, Y. K., Chung, J. H., Oh, B. H., & Hong, C. H. (2010). Relationship between the diagnostic components of metabolic syndrome (MS) and cognition by ApoE genotype in the elderly. Archives of Gerontology and Geriatrics, 50(1), 69–72. https://doi.org/10.1016/j.archger.2009.01.014
- Levin, B. E., Llabre, M. M., Dong, C., Elkind, M. S. V., Stern, Y., Rundek, T., Sacco, R. L., & Wright, C. B. (2014). Modeling metabolic syndrome and its association with cognition: The northern Manhattan study. Journal of the International Neuropsychological Society, 20(10), 951–960. https://doi.org/10.1017/S1355617714000861
- Li, P., Quan, W., Lu, D., Wang, Y., Zhang, H., & Liu, S. (2016). Association between metabolic syndrome and cognitive impairment after acute ischemic stroke : A cross-sectional study in a Chinese population. PLoS ONE, 11(12), 1–14. https://doi.org/10.1371/journal.pone.0167327
- Liu, C. L., Lin, M. H., Peng, L. N., Chen, L. K., Su, C. T., Liu, L. K., & Chen, L. Y. (2013). Late-life metabolic syndrome prevents cognitive decline among older men aged 75 years and over: One-year prospective cohort study. Journal of Nutrition, Health and Aging, 17(6), 523–526. https://doi.org/10.1007/s12603-013-0010-2
- Liu, M., He, Y., Jiang, B., Wu, L., Wang, J., Yang, S., & Wang, Y. (2015). Association between metabolic syndrome and mild cognitive impairment and its age difference in a Chinese community elderly population. Clinical Endocrinology, 82(6), 844–853. https://doi.org/10.1111/cen.12734
- Liu, Z., & Lippa, C. F. (2013). Association of metabolic syndrome and inflammation with cognitive decline in adults aged 60 years and older: findings from a national health survey in the United States. Neuroscience Journal, 2013(2013), 1–7. https://doi.org/10.1155/2013/846027
- Luo, L., Yang, M., Hao, Q., Yue, J., & Dong, B. (2013). Cross-sectional study examining the association between metabolic syndrome and cognitive function among the oldest old. Journal of the American Medical Directors Association, 14(2), 105–108. https://doi.org/10.1016/j.jamda.2012.10.001
- Ma, L., Wang, J., & Li, Y. (2015). Insulin resistance an cognitive dysfunction. Clinica Chimica Acta, 444, 18–23. https://doi.org/10.1016/j.cca.2015.01.027
- McEvoy, L. K., Laughlin, G. A., Barrett-Connor, E., Bergstrom, J., Kritz-Silverstein, D., Der-Martirosian, C., & Von Mühlen, D. (2012). Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Annals of Epidemiology, 22(5), 310–317. https://doi.org/10.1016/j.annepidem.2011.12.003
- Miskolczi, C., Halász, J., & Mikics, É. (2019). Changes in neuroplasticity following early-life social adversities: The possible role of brain-derived neurotrophic factor. Pediatric Research, 85(2), 225–233. https://doi.org/10.1038/s41390-018-0205-7
- Mumme, K. D., Von Hurst, P. R., Conlon, C. A., Jones, B., Haskell-Ramsay, C. F., Stonehouse, W., Heath, A. L. M., Coad, J., & Beck, K. L. (2019). Study protocol: Associations between dietary patterns, cognitive function and metabolic syndrome in older adults - A cross-sectional study. BMC Public Health, 19(1), 1–8. https://doi.org/10.1186/s12889-019-6900-4
- National Heart Lung and Blood Institute. (2019). Metabolic Syndrome. nhlbi.nih.gov/health-topics/metabolic-syndrome
- Overman, M. J., Pendleton, N., O’Neill, T. W., Bartfai, G., Casanueva, F. F., Forti, G., Rastrelli, G., Giwercman, A., Han, T. S., Huhtaniemi, I. T., Kula, K., Lean, M. E. J., Punab, M., Lee, D. M., Correa, E. S., Ahern, T., Laurent, M. R., Verschueren, S. M. P., Antonio, L., & Tournoy, J. (2017). Glycemia but not the metabolic syndrome is associated with cognitive decline: Findings from the European male ageing study. American Journal of Geriatric Psychiatry, 25(6), 662–671. https://doi.org/10.1016/j.jagp.2017.02.004
- Pacheco-Armenta MC, J.-T. J. (2017). Prevalencia de síndrome metabólico en la consulta externa. Revista de Sanidad Militar, 71(3), 264–275. http://libcon.rec.uabc.mx:3079/eds/pdfviewer/pdfviewer?vid=8&sid=0e323533-3397-4067-8fea-6562f6b80961%40sessionmgr4006
- Philippou, E., Michaelides, M. P., & Constantinidou, F. (2018). The role of metabolic syndrome factors on cognition using latent variable modeling: The neurocognitive study on aging. Journal of Clinical and Experimental Neuropsychology, 40(10), 1030–1043. https://doi.org/10.1080/13803395.2018.1483487
- Phillips, C. (2017). Lifestyle modulators of neuroplasticity: How physical activity, mental engagement, and diet promote cognitive health during aging. Neural Plasticity, 2017, 1–22. https://doi.org/10.1155/2017/3589271
- Raffaitin, C., Féart, C., Le Goff, M., Amieva, H., Helmer, C., Akbaraly, T. N., Tzourio, C., Gin, H., & Barberger-Gateau, P. (2011). Metabolic syndrome and cognitive decline in French elders: The three-city study. Neurology, 76(6), 518–525. https://doi.org/10.1212/WNL.0b013e31820b7656
- Saklayen, M. G. (2018). The global epidemic of the metabolic syndrome. Current Hypertension Reports, 20(2), 1–8. https://doi.org/10.1007/s11906-018-0812-z
- Schuur, M., Henneman, P., Van Swieten, J. C., Zillikens, M. C., De Koning, I., Janssens, A. C. J. W., Witteman, J. C. M., Aulchenko, Y. S., Frants, R. R., Oostra, B. A., Van Dijk, K. W., & Van Duijn, C. M. (2010). Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. European Journal of Epidemiology, 25(8), 561–568. https://doi.org/10.1007/s10654-010-9476-y
- Shigaeff, N., Jacinto, A. F., Franco, F. G. D. M., Chiochetta, G., Cendoroglo, M. S., & Cítero, V. D. A. (2013). Cognitive assessment in an elderly population with metabolic syndrome in Brazil. Dementia & Neuropsychologia, 7(2), 206–209. https://doi.org/10.1590/s1980-57642013dn70200011
- Siervo, M., Harrison, S. L., Jagger, C., Robinson, L., & Stephan, B. C. M. (2014). Metabolic syndrome and longitudinal changes in cognitive function: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 41(1), 151–161. https://doi.org/10.3233/JAD-132279
- Spencer, R. J., Wendell, C. R., Giggey, P. P., Katzel, L. I., Lefkowitz, D. M., Siegel, E. L., & Waldstein, S. R. (2013). Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates. Experimental Aging Research, 39(4), 382–397. https://doi.org/10.1080/0361073X.2013.808109
- Stewart, J. C., & Cramer, S. C. (2017). Genetic variation and neuroplasticity: Role in rehabilitation after stroke. Journal of Neurologic Physical Therapy: JNPT, 41( Suppl3 IV STEP Spec Iss), S17. https://doi.org/10.1097/NPT.0000000000000180
- Strong, J., Fonda, J. R., Grande, L., Milberg, W., McGlinchey, R., & Leritz, E. (2020). The role of cognitive reserve in the relationship between metabolic syndrome and cognitive functioning. Aging, Neuropsychology, and Cognition, 1–16. https://doi.org/10.1080/13825585.2020.1817304
- Tournoy, J., Lee, D. M., Pendleton, N., O’Neill, T. W., O’Connor, D. B., Bartfai, G., Casanueva, F. F., Finn, J. D., Forti, G., Giwercman, A., Han, T. S., Huhtaniemi, I. T., Kula, K., Lean, M. E. J., Moseley, C. M., Punab, M., Silman, A. J., Vanderschueren, D., Wu, F. C. W., & Group, E. (2010). Association of cognitive performance with metabolic syndrome and with glycaemia in middle-aged and older European men: The European male ageing study. Diabetes/Metabolism Research and Reviews, 26(1), 668–676. https://doi.org/10.1002/dmrr.1144
- Urrutia, G., & Bonfill, X. (2010). PRISMA_Spanish.pdf. Medicina Clínica, 135(11), 507–511. https://doi.org/10.1016/j.medcli.2010.01.015
- Vieira, J. R., Elkind, M. S. V., Moon, Y. P., Rundek, T., Boden-Albala, B., Paik, M. C., Sacco, R. L., & Wright, C. B. (2011). The metabolic syndrome and cognitive performance: The northern Manhattan study. Neuroepidemiology, 37(3–4), 153–159. https://doi.org/10.1159/000332208
- Viscogliosi, G., Andreozzi, P., Chiriac, I. M., Cipriani, E., Servello, A., Ettorre, E., & Marigliano, V. (2012). Screening cognition in the elderly with metabolic syndrome. Metabolic Syndrome and Related Disorders, 10(5), 358–362. https://doi.org/10.1089/met.2012.0043
- Wang, X., Luan, D., Xin, S., Liu, Y., & Gao, Q. (2019). association between individual components of metabolic syndrome and cognitive function in northeast rural China. American Journal of Alzheimer’s Disease & Other Dementias, 7(8), 507. https://doi.org/10.1177/1533317519865428
- World Health Organization. (1999). Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1, diagnosis and classification of diabetes mellitus. World Health Organization. https://apps.who.int/iris/handle/10665/66040
- Yaffe, K. (2007). Metabolic syndrome and cognitive disorders: Is the sum greater that its parts? Alzheimer Disease & Associated Disorders, 21(2), 167–171. https://doi.org/10.1097/WAD.0b013e318065bfd6
- Yao, Q., Jiang, G. X., Zhou, Z. M., Chen, J. M., & Cheng, Q. (2016). Metabolic syndrome and mild cognitive impairment: A case-control study among elderly in a Shanghai suburb. Journal of Alzheimer’s Disease, 51(4), 1175–1182. https://doi.org/10.3233/JAD-150920
- Yates, K. F., Sweat, V., Yau, P. L., Turchiano, M. M., & Convit, A. (2012). Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(9), 2060–2067. https://doi.org/10.1161/ATVBAHA.112.252759
- Yogi-Morren, D., Galioto, R., Strandjord, S. E., Kennedy, L., Manroa, P., Kirwan, J. P., Kashyap, S., & Gunstad, J. (2014). Duration of type 2 diabetes and very low density lipoprotein levels are associated with cognitive dysfunction in metabolic syndrome. Cardiovascular Psychiatry and Neurology, 2014(31)2014. https://doi.org/10.1155/2014/656341
- Zhang, J. Y., Jiang, Y. T., Liu, Y. S., Chang, Q., Zhao, Y. H., & Wu, Q. J. (2019). The association between glycemic index, glycemic load, and metabolic syndrome: A systematic review and dose–response meta-analysis of observational studies. European Journal of Nutrition, 59(2), 451–463. https://doi.org/10.1007/s00394-019-02124-z