Abstract
Cognitive demands placed on university students are high and so is the prevalence of insomnia in this population. Neurocognitive functions, such as delayed recall, working memory, and flexibility are often impaired in patients with insomnia disorder. Therefore, this study investigates the development of neuropsychological functioning in students with insomnia symptoms over the course of a sleep training. In this pilot-study 54 university students with symptoms of insomnia were included and randomly assigned to a group intervention with cognitive behavioural therapy and hypnotherapy (n = 35) and a waiting-list control condition (n = 19). Sleep quality and objective measures of neurocognitive performance (attention, inhibition, flexibility, memory, and working memory) were assessed before and after the training or a waiting period of 6 weeks. Both the intervention condition and the waiting-list control condition improved with regards to sleep quality, sleep duration, sleep efficiency, and measures of higher cognitive functions. In addition, students in the intervention condition reported less subjective daytime impairments when compared to the waiting-list control condition. The subgroup of students with short sleep duration benefitted significantly from the intervention in flexibility and working memory tasks. In line with previous studies, improved sleep was associated with improved neuropsychological functioning. The additional improvements observed in students with short sleep duration indicate an association between insomnia severity and neuropsychological improvement.
PUBLIC INTEREST STATEMENT
Insomnia symptoms, such as long sleep onset latency, early awakening, awakenings after sleep onset and subjectively poor sleep quality, are very prevalent in university students and impair their academic performance. This study investigates the effect of a sleep intervention with cognitive behavioural and hypnotherapeutic elements on subjective and objective cognitive performance. Sleep and neurocognitive performances improved after the intervention and a waiting-control period. A severe insomnia phenotype with less than 6 hours of sleep per night benefitted the most. These are promising results for all people suffering from poor sleep quality and daytime impairments to improve their sleep and performance.
1. Introduction
Sleep problems are common among university students: Up to 60% report poor sleep quality Lund et al., Citation2010. Symptoms such as difficulty falling asleep (16%) and waking up at night (25.9%) are frequently reported and 7.7% fulfill diagnostic criteria for insomnia according to the International Classification for Sleep Disorders Schlarb et al., Citation2012. Sleep is essential for many aspects of human life, as the immune system. But also for neural connectivity and plasticity, which are necessary for acquiring, retaining, and integrating new information; Krueger et al., Citation2016. Every day, but especially during exam periods, university students face high cognitive demands. Correspondingly, sleep problems in university students are associated with an extended time of studying and significantly predict grade-point average; Schlarb, Claßen et al., Citation2017; Gaultney, Citation2010. This evidence is not surprising given the fact that insomnia appears to be associated with impairments in neuropsychological functioning; Fortier-Brochu et al., Citation2012; Shekleton et al., Citation2010. Not only patients with diagnosed insomnia disorder suffer from reduced neuropsychological functioning. In their review, Waters and Bucks investigated the neuropsychological effects of sleep loss; Waters & Bucks, Citation2011. While higher order cognitive functions are affected to a lesser extent, subjects with sleep loss consistently showed significantly reduced processing speed and attention/vigilance, Citation2011.
As commonly reported by patients, the consequences of insomnia are often subjective impairments of daytime functioning Wardle-Pinkston et al., Citation2019. Specifically, insomnia is known to appear jointly with various daytime cognitive impairments in working memory, episodic memory, and problem-solving as described in a meta-analysis by Fortier-Brochu and colleagues; Fortier-Brochu et al., Citation2012. Generally, psychomotor speed and alertness do not seem impaired in insomnia patients, but more complex processes of attention, such as inhibition and manipulation, might be negatively affected; Wardle-Pinkston et al., Citation2019. Zhao and colleagues found impaired stop signal reaction times but no difference in general inhibition tasks when comparing insomnia patients to healthy controls; Zhao et al., Citation2018. Overall, manipulation seemed to be impaired the most Wardle-Pinkston et al., Citation2019. Lower performance was reported in insomnia patients in switching attention tasks Shekleton et al., Citation2014. Several studies about memory deficits in insomnia indicate no deficits in initial learning, but impaired delayed recall and overnight consolidation; Nissen et al., Citation2011. Compared to healthy controls, patients with insomnia perform significantly worse in tasks assessing retention in working memory, manipulation of working memory, episodic memory, and problem-solving; Fortier-Brochu et al., Citation2012. Executive functions, in particular working memory, seem to be moderately impaired; Ballesio et al., Citation2019.
However, various reasons for inconsistencies have been discussed. Khassawneh, Bathgate, Tsai, and Edinger described insomnia patients with short sleep duration and hyperarousal to be more vulnerable to impairments in complex attentional and spatial working memory tasks; Khassawneh et al., Citation2018. In a large population-based sample, short sleep duration subtype seems to perform lower in processing-speed, switching attention, and visual memory, even when controlling for age, gender, education, physical, and mental health; Fernandez-Mendoza et al., Citation2010, Citation2021. Especially subjective insomnia, depression, and anxiety were associated with cognitive scores in patients with insomnia; Shekleton et al., Citation2014. Although subjective complaints exceed objectively measured daytime performance, patients with insomnia reporting subjective deficits perform significantly worse (Fortier-Brochu & Morin, Citation2014, Citation2010). A more recent meta-analysis showed that subjective impairment correlated positively with age; Wardle-Pinkston et al., Citation2019. In subjective impairment due to bad sleep, insomnia patients with longer and shorter sleep duration were equally impaired even though in objective measures patients with short sleep durations performed significantly worse Olaithe et al., Citation2021. If treatment of insomnia also affects neurocognitive functioning was reviewed by Herbert et al. and pointed out a lack of studies objectively measuring cognitive performance Herbert et al., Citation2018. A couple of earlier studies do however address this topic Herbert et al., Citation2018. Altena and colleagues tested elderly insomnia patients’ attention before and after sleep treatment compared to healthy controls and a waiting-list control condition Altena et al., Citation2008. Before cognitive behavioural therapy for insomnia (CBT-I), patients showed significantly impaired attention in complex vigilance tasks whereas after sleep therapy, participants performed as well as healthy controls. Also, patients in the intervention condition improved in the complex task compared to the waiting-list control condition confirming a reduction in complexity costs in reaction times Altena et al., Citation2008. Nevertheless, simple reaction time was equally impaired before and after treatment. In adolescents, the same effects were shown for executive functions, as phonological working memory and selective attention increased after online CBT-I, but declarative memory or general cognitive speed did not; De Bruin et al., Citation2015.
Sleep-improving CBT-I showed medium effect sizes in university students reporting insomnia; Friedrich & Schlarb, Citation2018. Relaxation training even had large effects on sleep-related variables and hypnotherapeutic elements were shown to be effective in adolescents and adults, Citation2018; Schlarb et al., Citation2010, Citation2018. The results mentioned above are promising for insomnia patients, but university students as late-adolescents are bound to high cognitive demands. Due to challenging university demands and commonly reported insomnia, it is crucial to investigate the improvement of cognitive function via insomnia treatment. The current study investigates the effect of cognitive-behavioural therapy with hypnotherapeutic elements compared to a waiting-list control condition on cognitive performance in university students with impaired sleep quality and symptoms of insomnia, nightmares, or irregular sleep-wake type. To our knowledge, this study is the first comparing an intervention group to a waiting-list control group in late-adolescent university students with insomnia complaints addressing both objective cognitive performance and subjective daytime complaints.
2. Objectives
The aim of the study was to (1) assess neuropsychological functioning and sleep in university students with symptoms of insomnia and investigate the improvement of objective and subjective cognitive functions by treating symptoms of insomnia with CBT-I and hypnotherapy for insomnia (HT-I). Furthermore, (2) we wanted to identify possible mediators for improvements.
3. Methods
3.1. Sample
The necessary sample size was computed with G*Power 3.1. With an effect of insomnia intervention of d = .85 for sleep quality, Koffel et al., Citation2015 alpha = .05 and power of .95 the sample size had to exceed 32 for repeated measures ANOVA.
In total, N = 54 participants with insomnia symptoms were included at pre-test (t1). Of those, n = 35 (62.9% female) were assigned to the intervention condition and n = 19 (68.4% female) to the waiting-list control condition (WLC). Mean age of M = 25.97 years (SD = 3.94; Md = 25; range 21 to 37 years) was observed in the SWIS condition (group intervention “study like you sleep”), whereas in the WLC condition mean age was M = 27.84 years (SD = 6.56; Md = 27; range 19 to 50 years). There was no significant difference in gender distribution or age between conditions (z = −1.146: p = .252). All participants in the WLC condition were diagnosed with primary insomnia (n = 19), whereas in the SWIS condition n = 29 were diagnosed with primary insomnia, n = 2 primarily with nightmare disorders, n = 2 with irregular sleep-wake type and two participants had sleep problems with PSQI score above 5.
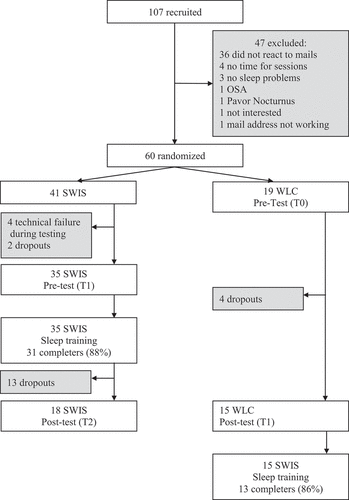
At post-measurement (t2), missing data was high with n = 13 missings in the intervention condition, mostly due to drop-out after completing the intervention. For a detailed flow chart see, . In post-measurement, the SWIS condition consisted of n = 18 and in the WLC group of n = 15.
Figure 1. Flow chart of the experimental design. After the waiting period, the WLC received the SWIS sleep training. All participants who completed at least four of the six sessions were counted as completers. Dropout rates aside from non-completers were 41% in the SWIS condition and 21% in the WLC condition.
3.2. Baseline differences
3.2.1. Sleep
At baseline, no significant differences between the conditions concerning sleep quality or self-reported concentration deficits were observed (F(8,55) = .977; p = .464; ηG² = .003).
3.2.2. Neuropsychological performance
Concerning objective measures no baseline-differences between SWIS and WLC conditions occurred in psychomotor speed, inhibition, and memory (p > .05). Participants of the WLC condition performed significantly better in flexibility (F(4,39) = 3.756; p = .011; ηG² = .007) and working memory (F(2,45) = 6.274; p = .004; ηG² = .001) at baseline.
3.2.3. Completer-analysis
No significant differences in age, gender, sleep variables, objective and subjective daytime performance at baseline between patients completing the study and patients dropping out were found (p > .05).
3.3. Inclusion criteria
Inclusion criteria for SWIS were symptoms of insomnia disorder, nightmare disorder, or an irregular sleep-wake type according to DSM-5 criteria and poor sleep quality reflected by a score above 5 in the Pittsburgh Sleep Quality Index. The inclusion and exclusion criteria were assessed using a self-developed diagnostic interview according to DSM-5 criteria. Participants were excluded when reporting symptoms of an organic sleep disorder (e.g. obstructive sleep apnea) and referred to a local sleep laboratory. Participants with previously diagnosed psychiatric disorders were included if the disorder was not the main cause of sleep problems determined in the diagnostic interview, and they did not receive additional psychological or psychotherapeutic treatment elsewhere for the duration of the study.
3.4. Measures
3.4.1. Sleep quality
The Pittsburgh Sleep Quality Index (PSQI) measures self-reported sleep quality with 19 items. Subscales of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication, and daytime sleepiness can be added up to a sum score with higher values indicating lower sleep quality. Cut-off scores are reported with values above 5 indicating a poor sleep quality and above 10 a clinically relevant sleep disorder Backhaus et al., Citation2002. Validity of the PSQI is good with specificity and sensitivity above 80%. Internal consistency has been confirmed (Cronbach’s alpha = .80).
Participants completed questionnaires to assess daytime sleepiness with the Epworth Sleepiness Scale, Johns, Citation1991 disbeliefs about sleep with the German version of the Disbeliefs about Sleep Scale Weingartz & Pillmann, Citation2009. Self-reported cognitive difficulties were measured with one item in a sleep-diary asking about difficulties with memory performance and concentration. The item was rated on a Likert scale from 1 to 5, with higher scores indicating more difficulties. A mean score was calculated for one week.
3.4.2. Neuropsychological tests
The d2 test of attention (Aufmerksamkeits-Belastungs-Test) is a widely used paper-pencil test to measure processing speed and accuracy. Participants are instructed to cross out all ds with two lines in 20 seconds per line Brickenkamp et al., Citation2010. In total, 14 lines are processed. An index of overall performance can be computed by summing up all correct characters minus wrongly crossed out characters and omitted ones (d2 concentration score). Norms are available for all age groups and standardized Z-scores are available. Reliability of α = .95 is high; Brickenkamp et al., Citation2010.
To assess attention, the German computer-based “test of attentional performance (TAP)” was used; Zimmermann & Fimm, Citation2002. Various subtests were selected. Alertness with cued reaction time and without cued reaction time relates to basic attentional processes, whereas the Go/noGo (1 of 2) subtest assesses inhibition. A third subtest was used to measure flexibility (verbal change). The tests are normed in various groups of age and clinical and non-clinical samples. Standardized T-values are provided and used in this study.
Verbal learning and memory performance were tested with the German Verbal Learning and memory test (VLMT); Helmstaedter et al., Citation2001. Participants learn a list of 15 unrelated nouns (List A) in five repetitions. Then they are presented with an interference list of 15 unrelated nouns (List B). Subsequently, they are asked to recall the first list immediately and 30 minutes later. Additionally, recognition is tested. Parallel versions are available for repeated measures. Working memory was extracted from the first round of learning the two lists (List A and B). Learning performance is calculated by summing up all correctly recalled words of List A in five repetitions (learning). Active recall, as well as recognition, were asked for after 30 minutes. Retest reliability is good (between rtt = .68 and rtt = .87 for separate parameters Helmstaedter et al., Citation2001.
To assess working memory, digit span forwards and backwards from the German version of the Wechsler Memory Scale (WMS-R) was used Wechsler & Härting, Citation2000. Participants are asked to recall a string of numbers from 3 to 8 in a row either in the same order or backwards. The test is validated for clinical neuropsychological testing. Reliability of these subscales is moderate (rtt = 0.73 to rtt = 0.83). Authors concluded a valid assessment of memory dysfunctions using the WSM-R.
4. Mental health
Various other questionnaires to measure mental health were used, such as the General Depression Scale (Allgemeine Depressionsskala, ADS) which is based on CES-D (Meyer & Hautzinger, Citation2001; Radloff, Citation1977) and stress and somatic complaints with the Patients Health Questionnaire Löwe et al., Citation2002. The CES-D assesses depressive symptoms and moodiness during the last week in 20 items. Higher scores indicate a higher tendency towards depressive symptoms. A good internal consistency was reported as well as a reliable cut-off score of 23 Meyer & Hautzinger, Citation2001. Since the results have been reported elsewhere we omitted depression, stress, and somatic complaints from the analysis of main effects Friedrich et al., Citation2018. We included depression for the analysis of possible factors influencing improvements of neuropsychological functioning.
4.1. Procedure
University students were recruited at Bielefeld University via leaflets, homepage, and informational events. All participants provided written informed consent and the study was approved by the ethics committee at Bielefeld University. The study conforms with the Declaration of Helsinki.
In a pre-assessment, all participants completed a diagnostic interview and completed questionnaires about sleep quality, daytime sleepiness, and depression online. In addition, participants were assessed with neuropsychological tests.
Participants reporting clinically relevant sleep disorder symptoms were randomly assigned to a group intervention (SWIS) or waiting-list control condition (WLC) in an experimental pre-post-design. When a patient with insomnia contacted the study group, six to eight patients were recruited to the intervention condition and the next six to eight patients were assigned to the WLC, the next again to the SWIS-condition and so forth.
For 6 weeks, groups of four to eight participants took part in the sleep intervention “Studieren wie im Schlaf” (SWIS), a manualized sleep intervention designed for university students suffering from insomnia, nightmares, and irregular-sleep-wake-disorder. All six sessions followed a structured procedure, starting with the students’ individual development, followed by CBT-I and hypnotherapy for insomnia (HT-I) respectively. Each session was conducted by two psychologists supervised by a psychotherapist. For a detailed description see Schlarb, Friedrich and Claßen Friedrich et al., Citation2018; Schlarb, Friedrich et al., Citation2017.
Participants assigned to the waiting-list control condition underwent pre-diagnostics, waited for 6 weeks, and completed measurements again. Afterwards, they received the SWIS intervention for ethical reasons because of their known sleep issues. Post-measurements were performed 1 week after the last session (SWIS) or 7 weeks after the first assessment (WLC).
4.2. Data analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 24 and 25 IBM Corp, Citation2017. All variables were tested for normal distribution (Shapiro-Wilk). Unless noted otherwise, variables were normally distributed and t-test or ANOVA for repeated measures were performed. Otherwise, Wilcoxon Signed Rank Tests were calculated for both conditions separately, comparing pre and post outcome variables.
Baseline differences were calculated using multivariate ANOVA for sleep variables and objective neuropsychological performance. The effect of SWIS intervention on sleep variables and neuropsychological performance was tested using repeated-measures ANOVA.
Further analyses in the intervention condition were performed to find predictors for improvements in neuropsychological performance. An ANCOVA with the post-score as the dependent variable and the pre-score, treatment group and subgroup as predictors was performed. Sleep-related variables, subjective impairment, and depression were considered in the analysis of predictors for improvement in neuropsychological functioning.
For further information on the development of sleep and mental health variables over the course of the treatment see Friedrich, Claßen and Schlarb Friedrich et al., Citation2018; Schlarb, Friedrich et al., Citation2017.
Due to the small sample size, missing data were not imputed, and alpha error accumulation was not corrected.
The significance level was set to α < .05. To report effect sizes generalized eta squared were reported accordingly with Olejnik and colleagues; Olejnik & Algina, Citation2003. ηG2 > .01 is considered a small effect, ηG2 > .06 a medium effect and ηG2 > .14 a large effect Rosenthal, Citation1991.
5. Results
5.1. Pre-Measurement
Participants in both conditions reported clinically relevant sleep problems (PSQI sum score >10, see, ). Furthermore, sleep latency in both conditions was longer than 30 minutes and sleep efficiency was low.
Table 1. Self-reported sleep qualit y at pre- and post-measurement according to PSQI scoring when not indicated otherwise
In the intervention condition, short sleep was reported by most participants (53.6% slept 6 hours or less), while 3.6% slept more than 9 hours. 78.6% reported sleep efficiency lower than 85%, around 50% PSQI-scores above 10. 78.6% of the participants in the intervention condition reported no intake of sleep medication, 14.3% less than once a week and 7.1% three times or more per week.
36.8% of the WLC condition reported a sleep duration of under 6 hours as well as low sleep efficiency likewise. 15.8% slept more than 9 hours. 31.6% had PSQI-scores above 10. 78.8% did not take any sleep medication, 10.5% reported taking sleep medication less than once a week and 5.3% once or twice or three times and more, respectively.
5.2. Effect of SWIS
5.2.1. Sleep
With regards to subjective sleep quality, both conditions reported significant changes for most subscales of the PSQI. In detail, sleep quality, sleep duration, sleep efficiency, and subjective sleep quality improved significantly over time (see, ). Daytime sleepiness showed a tendency to improve over time but failed to reach significance (p < .10). The subscales sleep latency and sleep disturbances did not differ significantly directly after treatment or waiting period (p > .05). Further analyses showed that participants who slept less than 6 hours before the treatment reported a sleep duration of longer than 6 hours after the treatment (Mpre = 5.37; Mpost = 6.08). However, the interaction of sleep duration and treatment condition failed to reach significance in the ANCOVA (p > .05).
Figure 2. Development of PSQI sum score, sleep duration and sleep efficiency in SWIS and WLC. Error bars for the standard error of the mean are displayed.Note: * p < .05; ** p < .01
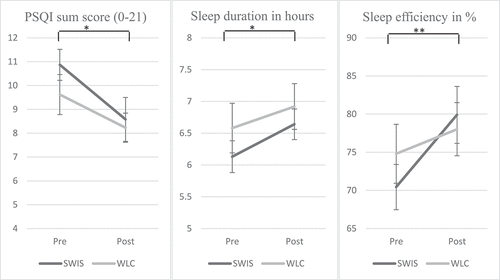
Self-reported cognitive difficulties including complaints about concentration, memory, and daytime functioning improved significantly over time in the SWIS condition with a medium effect size (Mpre = 3.19; Mpost = 2.83; Z = 2.030; p = .042; ηG2 = .072), but not in the WLC condition (p > .05).
5.2.2. Neuropsychological performance in general
As standardized T-values (or z-values) were mostly used for calculations and age was non-normally distributed, no covariates were inserted in the calculations.
Throughout the whole sample (participants with insomnia regardless of short or long sleep duration), time effects were observed in all objective measures of neuropsychological performance. Processing speed measured with d2 and flexibility in general improved significantly (see, ). The WLC group showed significantly better results than the SWIS group in simple reaction tasks (TAP) and the flexibility task (see, ).
Table 2. Summary of objective attention. Note. z = standardized z-value; T = standardized T-value, TAP = “test of attentional performance“
No time or group effects were observed in initial learning, delayed recall, or working memory. Descriptively, learning, and verbal working memory improved over time, but failed to reach statistical significance (p < .10; see, ).
Table 3. Summary of memory and working memory in both conditions before and after treatment. Note. z = standardized z-value; T = standardized T-Value, VLMT = verbal learning and memory test, D1 = first round of learning
Notably, a significant interaction of condition and short sleep duration showed that participants with short sleep duration (SWIS-SS) improved significantly more after sleep training concerning the recognition task mistakes compared to WLC with short sleep duration (WLC-SS) (F(1,26) = 6.901; p = .014; ηG2 = 0.101, number of mistakes after treatment (SWISS-SS = 0.88, SWIS = 1.60, WLC-SS = 2.13, WLC = 0.50). Additionally, the same patients improved more in a working memory (F(1,27) = 6.603; p = .016; ηG2 = 0.007, SWISS-SS = 7.78, SWIS = 7.13, WLC-SS = 6.40, WLC = 6.80).
5.3. Further factors predicting improvement in neuropsychological performance
To identify variables predicting improvements in neuropsychological functioning, ANCOVA was calculated with condition, sleep variables, subjective impairments, and depression scores before training as a predictor for post-scores in objective neuropsychological performance.
Sleep onset latency and wake after sleep onset were non-significantly associated with a change in all measures of neurocognitive performance (p > .05).
Attention performance after training was significantly predicted by sleep-efficiency and depression scores, as higher scores in alertness were associated with higher sleep-efficiency (F 1,17) =6.644, p = .020, ηG2 = 0.002), less subjective daytime-impairments (F 1,17) =5.101, p = .037, ηG2 = 0.002), and lower depression scores (F (1,18) = 8.040; p = .011, ηG2 = 0.003). Concerning working memory, more self-reported complaints in daytime functioning predicted lower performance in working memory (F(1,22) = 6.182; p = .021; ηG2 = 0.002).
The score before treatment always significantly predicted the performance after treatment (p < .05).
6. Discussion
The current study examined the effect of CBT-I and HT-I compared with a WLC condition on insomnia symptoms and neuropsychological functioning. Sleep and cognition such as attention, and flexibility were significantly improved after the training or waiting period in university students with insomnia. Relevant sleep variables like overall sleep quality and sleep efficiency also improved with statistical and clinical significance, correspondingly sleep-onset latency decreased over time in both conditions (CBT-I/HT-I and WLC). These significant effects were also observed in objective performance. Subjectively measured daytime functioning only increased significantly in those participants with insomnia receiving CBT-I and HT-I.
Nevertheless, some subgroups seemed to benefit from CBT-I and HT-I more than others. Patients with short sleep duration improved significantly more in the recognition task in this study. Since objective measures like sleep duration may be a biological marker for severity of the disorder these results are promising; Fernandez-Mendoza et al., Citation2010. In a study with young adults, insomnia patients with short sleep duration are more impaired in cognitive tasks, as well as objective sleep measurements (e.g. more N1, more N3, and less REM-sleep); Olaithe et al., Citation2021. The authors suggest a more severe phenotype due to hyperarousal, Citation2021. In line with this, the working group of Riemann reported, a severe subtype is often associated with high cognitive arousal and lower remission rates; Riemann et al., Citation2010. Furthermore, the hyperarousal concept and perfectionism were suggested to mask possible impairments in insomnia which might also be active in students; Shekleton et al., Citation2010. Thus, high cognitive arousal in students might lead to faster reaction times in simple attention tasks. In addition, patients with insomnia are reported to be more perfectionist and make more corrections when completing questionnaires; Regen et al., Citation2015. Due to these mechanisms, further effects in improvements due to sleep training might be masked, because insomnia patients react quite accurate beforehand.
In studies concerning older adults, as well as adolescents complex vigilance but not simple vigilance was improved; Altena et al., Citation2008; De Bruin et al., Citation2015 similarly only selective attention and flexibility improved over time directly after treatment in the current study.
In line with Shekleton’s study on self-reported cognitive impairments, some neurocognitive functions were mirrored in self-reports in our study; Shekleton et al., Citation2014. This underlines that widely reported deficits in daytime functioning are more than a mere cognitive bias, Citation2014. In another study treating university students with insomnia, subjective daytime performance was also enhanced after therapy Taylor et al., Citation2014. However, in this study, patients with self-reported daytime deficits performed worse before training, but caught up in the attentional task of inhibition. As sleep parameters usually need time to change, improvements in objective cognitive performance might also benefit from time as well; Okajima et al., Citation2011. Even though patients with short sleep duration (<6 h) seem to benefit significantly more from CBT-I and HT-I than patients with longer sleep duration, sleep duration remains short in other samples; Galbiati et al., Citation2018. In our sample, patients reporting short sleep duration were the only ones that improved. If objective deficits are due to chronically disturbed sleep, sleep needs to recover first and higher cognitive demands are particularly vulnerable to disturbed sleep; Fortier-Brochu et al., Citation2012. Therefore, we might have found no improvements in working memory performance directly after treatment. In conclusion, further and later post-measurements should be included in future studies.
In our study, clinical depression and prolonged sleep onset latency seemed to be associated with inhibition tasks, which is in line with results suggesting a lower performance in inhibition tasks significantly predicting the amount of rumination before sleep; Ballesio et al., Citation2018. Restoring abilities in inhibition might, therefore, be crucial to reduce rumination and along with it sleep-onset latency. These results underline the importance of neurocognitive functions in the maintenance of insomnia.
As the waiting control condition and the intervention group both showed improved sleep and objective neurocognitive functions, other factors besides treatment effects must be considered. Assessing sleep via sleep diaries and assessing objective performance might be enough for the patients to realize they need to change sleep habits. When given feedback on their sleep diaries, patients with insomnia showed less preoccupation about their sleep and assessed their sleep more accurately; Tang & Harvey, Citation2004. As university students are often young and well-educated, the conducted tests might be insensitive to small changes, because they are mostly used in the clinical context. For instance, training effects might be prevalent in d2-attention as the number of detected ds increases, compared to other measures of attention. In addition, most previous studies were conducted in middle-aged insomnia patients and it is proposed that the importance of different sleep stages shift over a lifetime; Edinger et al., Citation2000. Young insomnia patients like those in this sample might have a greater ability to compensate deficits in cognitive functioning. Nevertheless, it is an important goal to improve their quality of life and neurocognitive functioning to decrease impairments in later life.
6.1. Strength and limitations
The randomized controlled design and the wide variety of subjective and objective neurocognitive functions are an important strength of the current study. Objective measures to assess neuropsychological functions were used and compared to self-reported measures. Nevertheless, due to the high drop-out rate after training, sample sizes are small in post-measurements. A possible explanation for the high drop-out rate might be that the training was mostly administered during the beginning and near the end of the semester. Therefore, either exams or moving on to another university could cause participants to refuse the post-measurement, which is quite common in research in educational contexts; Pampaka et al., Citation2016. The waiting list control condition received treatment after t2. This might be another motivation for participants in the WLC to take the post measurements which is not prevalent in the SWIS condition. This might lead to a systematic selection of participants. In addition, the study is lacking objective measures of sleep quality which are often, but not always correlated with neurocognitive performance; Fortier-Brochu et al., Citation2012. In addition, university students with subclinical insomnia (nightmare disorder or irregular sleep-wake type primarily) were included in this study diminishing the effects of SWIS sleep training.
In the current study, the waiting-list control condition improved significantly over time concerning sleep and neurocognitive functioning. However, this effect is quite common, as other studies report improvements in active waiting-list control conditions too; Hofmann et al., Citation2012. A further study might, therefore, include Treatment as usual (TAU) or other control conditions.
7. Conclusion and future directions
This study showed that sleep, objective, and subjective daytime performance are associated and can be improved with sleep interventions in young adults. In the WLC sleep quality and neurocognitive performance enhanced as well due to a plausible effect of sleep diaries. A more severe phenotype with short sleep duration seems to benefit more from the intervention. The direction of the effect remains unclear, since the training of cognitive performance in older adults with insomnia improves sleep quality and enhancement in naming is associated with fewer awakenings after sleep onset and reduction of the number of awakenings Haimov et al., Citation2013. Inhibition and sleep onsetlatency are associated and improved, which might be due to reducing rumination. In addition, enhancing memory and learning improves treatment outcome in other disorders, therefore it is crucial to investigate if restored sleep quality enhances memory and learning, facilitating better therapy outcomes; Harvey et al., Citation2014.
Nevertheless, more research to deepen the insight into processes, long-term follow-ups and larger sample sizes are needed to investigate age-oriented types or changes concerning sleep (chronotype), subtypes of insomnia and their association with neurocognitive function. For instance, the role of hyperarousal in improvement was not assessed in this study. Our study revealed promising results concerning improvements but still, the severe phenotype seems to be associated with more improvements.
Abbreviations
CBT-I - cognitive behavioural therapy for insomnia
HT-I - hypnotherapy for insomnia
LLSQ - lower subjective sleep quality
LSOL - longer sleep onset latency
PSQI - Pittsburgh Sleep Quality Index
SSD - shorter sleep duration
SWIS - sleep intervention “Studieren wie im Schlaf”
SWIS- SS - Patients with short sleep duration
TAP - test of attentional performance
VMLT - Verbal Learning and memory test
WASO - awakenings after sleep onset
WLC - waiting-list control condition
WMS-R - Wechsler Memory Scale revised
Acknowledgements
We would like to thank Neele Busse for her help in the study. We further acknowledge support for the Article Processing Charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, MC, upon reasonable request.
Additional information
Funding
Notes on contributors
Merle Claßen
All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
References
- Altena, E., Werf, Y. D. V. D., Strijers, R. L. M., & Someren, E. J. W. V. (2008). Sleep loss affects vigilance: Effects of chronic insomnia and sleep therapy. Journal of Sleep Research, 17(3), 335–17. https://doi.org/10.1111/j.1365-2869.2008.00671.x
- Backhaus, J., Junghanns, K., Broocks, A., Riemann, D., & Hohagen, F. (2002). Test–retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. Journal of Psychosomatic Research, 53(3), 737–740. https://doi.org/10.1016/S0022-3999(02)00330-6
- Ballesio, A., Aquino, M.R.J.V., Kyle, S.D., Ferlazzo, F., and Lombardo, C. (2019). Executive Functions in Insomnia Disorder: A Systematic Review and Exploratory Meta-Analysis. Frontiers in Psychology, 10, 10. https://doi.org/10.3389/fpsyg.2019.001
- Ballesio, A., Ottaviani, C., & Lombardo, C. (2018). Poor cognitive inhibition predicts rumination about insomnia in a clinical sample. Behavioral Sleep Medicine, 1–10. https://doi.org/10.1080/15402002.2018.1461103
- Brickenkamp, R., Schmidt-Atzert, L., & Liepmann, D. Test D2-Revision: Aufmerksamkeits- Und Konzentrationstest. Hogrefe; 2010. Accessed August 31, 2018. http://library.mpib-berlin.mpg.de/toc/z2008_2431.pdf
- de Bruin, E. J., Dewald-Kaufmann, J. F., Oort, F. J., Bögels, S. M., & Meijer, A. M. (2015). Differential effects of online insomnia treatment on executive functions in adolescents. Sleep Medicine, 16(4), 510–520. https://doi.org/10.1016/j.sleep.2014.12.009
- Edinger, J. D., Fins, A. I., Glenn, D. M., Sullivan, R. J., Bastian, L. A., Marsh, G. R., Dailey, D., Hope, T. V., Young, M., Shaw, E., & Vasilas, D. (2000). Insomnia and the eye of the beholder: Are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? Journal of Consulting and Clinical Psychology, 68(4), 586. https://doi.org/10.1037/0022-006X.68.4.586
- Fernandez-Mendoza, J., Calhoun, S., Bixler, E. O., Pejovic, S., Karataraki, M., Liao, D., Vela-Bueno, A., Ramos-Platon, M. J., Sauder, K. A., & Vgontzas, A. N. (2010). Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: A general population study. Sleep, 33(4), 459–465. https://doi.org/10.1093/sleep/33.4.459
- Fernandez-Mendoza, J., He, F., Puzino, K., Amatrudo, G., Calhoun, S., Liao, D., Vgontzas, A. N., & Bixler, E. (2021). Insomnia with objective short sleep duration is associated with cognitive impairment: A first look at cardiometabolic contributors to brain health. Sleep, 44(1–9). https://doi.org/10.1093/sleep/zsaa150
- Fortier-Brochu, É., Beaulieu-Bonneau, S., Ivers, H., & Morin, C. M. (2012). Insomnia and daytime cognitive performance: A meta-analysis. Sleep Medicine Reviews, 16(1), 83–94. https://doi.org/10.1016/j.smrv.2011.03.008
- Fortier-Brochu, É., & Morin, C. M. (2014). Cognitive Impairment in Individuals with Insomnia: Clinical Significance and Correlates. Sleep, 37(11), 1787–1798. https://doi.org/10.5665/sleep.4172
- Friedrich, A., Claßen, M., & Schlarb, A. A. (2018). Sleep better, feel better? Effects of a CBT-I and HT-I sleep training on mental health, quality of life and stress coping in university students: A randomized pilot controlled trial. BMC Psychiatry, 18(1), 268. https://doi.org/10.1186/s12888-018-1860-2
- Friedrich, A., & Schlarb, A. A. (2018). Let’s talk about sleep: A systematic review of psychological interventions to improve sleep in college students. Journal of Sleep Research, 27(1), 4–22. https://doi.org/10.1111/jsr.12568
- Galbiati, A., Sforza, M., Poletti, M., Verga, L., Zucconi, M., Ferini-Strambi, L., and Castronovo, V. et al.(,2018) Insomnia patients with subjective short total sleep time have a boosted response to cognitive behavioral therapy for insomnia despite residual symptoms. Behavioral Sleep Medicine, 1–10.https://doi.org/10.1080/15402002.2018.1545650
- Gaultney, J. F. (2010). The prevalence of sleep disorders in college students: Impact on academic performance. Journal of American College Health, 59(2), 91–97. https://doi.org/10.1080/07448481.2010.483708
- Haimov, I., Shatil, E., & Laks, J. (2013). Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLOS ONE, 8(4), e61390. https://doi.org/10.1371/journal.pone.0061390
- Harvey, A. G., Lee, J., Williams, J., Hollon, S. D., Walker, M. P., Thompson, M. A., & Smith, R. (2014). Improving outcome of psychosocial treatments by enhancing memory and learning. Perspectives on Psychological Science, 9(2), 161–179. https://doi.org/10.1177/1745691614521781
- Helmstaedter, C., Lendt, M., & Lux, S. (2001). VLMT: Verbaler Lern- Und Merkfähigkeitstest. Beltz Test.
- Herbert, V., Kyle, S. D., & Pratt, D. (2018). Does cognitive behavioural therapy for insomnia improve cognitive performance? A systematic review and narrative synthesis. Sleep Medicine Reviews, 39, 37–51. https://doi.org/10.1016/j.smrv.2017.07.001
- Hofmann, S. G., Asnaani, A., Vonk, I. J. J., Sawyer, A. T., & Fang, A. (2012). The Efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Therapy and Research, 36(5), 427–440. https://doi.org/10.1007/s10608-012-9476-1
- IBM Corp. (2017). IBM SPSS statistics for windows.
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14(6), 540–545. https://doi.org/10.1093/sleep/14.6.540
- Khassawneh, B. Y., Bathgate, C. J., Tsai, S. C., & Edinger, J. D. (2018). Neurocognitive performance in insomnia disorder: The impact of hyperarousal and short sleep duration. Journal of Sleep Research, 27(6), e12747. https://doi.org/10.1111/jsr.12747
- Koffel, E., Koffel, J., & Gehrman, P. (2015). A Meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Medicine Reviews, 19, 6–16. https://doi.org/10.1016/j.smrv.2014.05.001
- Krueger, J. M., Frank, M. G., Wisor, J. P., & Roy, S. (2016). Sleep function: Toward elucidating an enigma. Sleep Medicine Reviews, 28, 46–54. https://doi.org/10.1016/j.smrv.2015.08.005
- Löwe, R., Spitzer, R. L., Zipfel, S., & Herzog, W. (2002). Gesundheitsfragebogen Für Patienten (PHQ D) (2 ed.). Auflage. Pfizer.
- Lund, H. G., Reider, B. D., Whiting, A. B., & Prichard, J. R. (2010). Sleep patterns and predictors of disturbed sleep in a large population of college students. Journal of Adolescent Health, 46(2), 124–132. https://doi.org/10.1016/j.jadohealth.2009.06.016
- Meyer, T. D., & Hautzinger, M. (2001). Allgemeine Depressions-Skala (ADS). Diagnostica, 47(4), 208–215. https://doi.org/10.1026//0012-1924.47.4.208
- Nissen, C., Kloepfer, C., Feige, B., Piosczyk, H., Spiegelhalder, K., Voderholzer, U., & Riemann, D. (2011). Sleep-related memory consolidation in primary insomnia. Journal of Sleep Research, 20(1pt2), 129–136. https://doi.org/10.1111/j.1365-2869.2010.00872.x
- Okajima, I., Komada, Y., & Inoue, Y. (2011). A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep and Biological Rhythms, 9(1), 24–34. https://doi.org/10.1111/j.1479-8425.2010.00481.x
- Olaithe, M., Ree, M., McArdle, N., Donaldson, S., Pushpanathan, M., Eastwood, P. R., & Bucks, R. S. (2021). Cognitive dysfunction in insomnia phenotypes: further evidence for different disorders. Frontiers in Psychiatry, 12, 1201. https://doi.org/10.3389/fpsyt.2021.688672
- Olejnik, S., & Algina, J. (2003). generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychological Methods, 8(4), 434–447. https://doi.org/10.1037/1082-989X.8.4.434
- Pampaka, M., Hutcheson, G., & Williams, J. (2016). Handling missing data: Analysis of a challenging data set using multiple imputation. International Journal of Research & Method in Education, 39(1), 19–37. https://doi.org/10.1080/1743727X.2014.979146
- Radloff, L. S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306
- Regen, W., Hertenstein, E., Weil, P., Kyle, S. D., Holz, J., Baglioni, C., Nissen, C., Feige, B., Riemann, D., & Spiegelhalder, K. (2015). Perfectionistic tendencies in insomnia patients‘ behavior during psychometric testing. Behavioral Sleep Medicine, 13(5), 387–394. https://doi.org/10.1080/15402002.2014.919918
- Riemann, D., Spiegelhalder, K., Feige, B., Voderholzer, U., Berger, M., Perlis, M., & Nissen, C. (2010). The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Medicine Reviews, 14(1), 19–31. https://doi.org/10.1016/j.smrv.2009.04.002
- Rosenthal, R. (1991). Meta-Analytic Procedures for Social Research. SAGE Publications, Inc. https://doi.org/10.4135/9781412984997
- Schlarb, A. A., Claßen, M., Grünwald, J., & Vögele, C. (2017). Sleep disturbances and mental strain in university students: Results from an online survey in Luxembourg and Germany. International Journal of Mental Health Systems, 11(1 1–10). https://doi.org/10.1186/s13033-017-0131-9
- Schlarb, A. A., Faber, J., & Hautzinger, M. (2018). CBT-I and HT-I group therapy for adults with insomnia in comparison to those with insomnia and comorbid depression – A pilot study. Neuropsychiatric Disease and Treatment, 14, 2429–2438. https://doi.org/10.2147/NDT.S164899
- Schlarb, A. A., Friedrich, A., & Claßen, M. (2017). Sleep problems in university students – An intervention. Neuropsychiatric Disease and Treatment, 13, 1989–2001. https://doi.org/10.2147/NDT.S142067
- Schlarb, A. A., Kulessa, K., & Gulewitsch, G. Sleep characteristics, sleep problems, and associations of self-efficacy among German university students. (2012). Nature and Science of Sleep 4 , 1. https://doi.org/10.2147/NSS.S27971
- Schlarb, A. A., Liddle, L., & Hautzinger, H. (2010). JuSt – A multimodal program for treatment of insomnia in adolescents: A pilot study. Nature and Science of Sleep, 3, 13–20. https://doi.org/10.2147/NSS.S14493
- Shekleton, J. A., Flynn-Evans, E. E., Miller, B., Epstein, L. J., Kirsch, D., Brogna, L. A., Burke, L. M., Bremer, E., Murray, J. M., Gehrman, P., Lockley, S. W., & Rajaratnam, S. M. W. (2014). Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep, 37(1), 107–116. https://doi.org/10.5665/sleep.3318
- Shekleton, J. A., Rogers, N. L., & Rajaratnam, S. M. W. (2010). Searching for the daytime impairments of primary insomnia. Sleep Medicine Reviews, 14(1), 47–60. https://doi.org/10.1016/j.smrv.2009.06.001
- Tang, N. K. Y., & Harvey, A. G. (2004). Correcting distorted perception of sleep in insomnia: A novel behavioural experiment? Behaviour Research and Therapy, 42(1), 27–39. https://doi.org/10.1016/S0005-7967(03)00068-8
- Taylor, D. J., Zimmerman, M. R., Gardner, C. E., Williams, J. M., Grieser, E. A., Tatum, J. I., Bramoweth, A. D., Francetich, J. M., & Ruggero, C. (2014). A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behavior Therapy, 45(3), 376–389. https://doi.org/10.1016/j.beth.2013.12.010
- Wardle-Pinkston, S., Slavish, D. C., & Taylor, D. J. (2019). Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Medicine Reviews, 48, 101205. https://doi.org/10.1016/j.smrv.2019.07.008
- Waters, F., & Bucks, R. S. (2011). neuropsychological effects of sleep loss: Implication for Neuropsychologists. Journal of the International Neuropsychological Society, 17(4), 571–586. https://doi.org/10.1017/S1355617711000610
- Wechsler, D., & Härting, C. (2000). Gedächtnistest-Revidierte Fassung: WMS-R; Manual; Deutsche Adaptation Der Revidierten Fassung Der Wechsler Memory Scale von David Wechsler. Huber.
- Weingartz, S., & Pillmann, F. (2009). The Dysfunctional Beliefs and Attitudes About Sleep scale. Somnologie - Schlafforschung und Schlafmedizin, 13(1), 29–36. https://doi.org/10.1007/s11818-008-0356-6
- Zhao, W., Gao, D., Yue, F., Wang, Y., Mao, D., Chen, X., and Lei, X. (2018). Response Inhibition Deficits in Insomnia Disorder: An Event-Related Potential Study With the Stop-Signal Task.Frontiers in Neurology, 9, 9. https://doi.org/10.3389/fneur.2018.00610
- Zimmermann, P., & Fimm, B. (2002). Testbatterie Zur Aufmerksamkeitsprüfung (TAP). Psytest.
