Abstract
The aim of the study was to explore beliefs about pain and related coping strategies of individuals experiencing abdominal pain during remitted inflammatory bowel disease (IBD), and their perception of irritable bowel syndrome (IBS) in the context of IBD. In-depth semi-structured interviews were conducted with 23 participants who self-reported experiences of abdominal pain during remitted IBD. The study was embedded in the constructivism tradition and reflexive thematic analysis was used to analyse the interviews. Results encompass 1) How IBS is perceived; 2) How individuals monitor symptoms to distinguish active from quiescent IBD; 3) Coping strategies employed to navigate the pain; 4)How manageability of pain guides the distinction between active and quiescent disease; v. How context influences pain interpretation and management; 5) What role illness history and health literacy play in the meaning of ongoing pain. The IBS label was perceived by some as invalidating, although it helped some people to worry less about ongoing pain and symptoms during remitted IBD. However, even for the latter individuals, IBS did not bring a clear understanding of painful symptoms. Participants’ responses highlight a need for explanations that incorporate both the complexity of IBD and underlying causes of ongoing pain during remission. Communication would benefit from the appreciation of pain (and symptoms) in the wider context of illness history and health literacy.
PUBLIC INTEREST STATEMENT
Inflammatory bowel disease (IBD) is a chronic disease caused by inflammation of the gut. Abdominal (tummy) pain is a key symptom. Periods of remission occur where the inflammation settles down.
Some people with IBD continue to experience abdominal pain during periods of remission. Clinicians and scientists have argued that these people may also have irritable bowel syndrome (IBS). But how do patients with IBD view their pain during remission and how do they view the diagnosis of IBS?
We asked 23 people with IBD about their experiences of pain in remission. We learned that some people worried less about ongoing pain when they could attribute it to IBS. Others felt dismissed by their healthcare team when IBS was mentioned. Most people felt IBS did not help understand why they had pain in remission. More refined and detailed explanations of symptoms during remission are required.
1. Introduction
This article explores patients’ views on the dichotomy and overlap between inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), two conditions characterised by abdominal pain. It further explores the use of disease labels and their implications. IBD describes conditions where maladaptive immune responses, assumed to be caused by both genetic and environmental agents, culminate in long-lasting inflammation of the digestive system (Baumgart & Carding, Citation2007). The two main manifestations of IBD, Crohn’s disease (CD) and ulcerative colitis (UC), differ with regards to the distribution of affected areas and the depth of inflammation, but overlap in symptoms which typically involve abdominal pain, diarrhoea, fatigue, and weight loss. IBS refers to a disorder characterised by abdominal pain and alterations in stool consistency and frequency. There are no clear biomarkers for IBS. It is diagnosed based on the fulfilment of symptom-based validated diagnostic criteria and the refutation of other explanations (Drossman, Citation2016).
IBD is a relapsing remitting disease, with remission occurring when the inflammation dies down. While symptoms in IBD generally are presumed to indicate disease or inflammatory activity (Srinath et al., Citation2014), they do not always subside during disease remission (Minderhoud et al., Citation2004; Schirbel et al., Citation2010). Although there is no clear disease or inflammatory marker for IBS, factors including microbiota, low-grade inflammation, and alterations to gut functioning and sensitivity play a role in its pathophysiology (Barbara et al., Citation2014). Together the inconsistent association between subjective symptoms and inflammatory “biomarker” levels in IBD (Targownik et al., Citation2015) and the contribution of organic factors to IBS, have led to a more integrated model of abdominal pain (Drewes et al., Citation2020; Grover et al., Citation2006). This model advocates that abdominal pain is the result of complex reciprocal interactions between biological, psychological, and social factors executed through the brain-gut axis, which includes endocrine, immune, and neural pathways (Fichna & Storr, Citation2012; Jones et al., Citation2020).
Against this backdrop, discussions around the potential for patients with IBD to have comorbid IBS have arisen, with some arguing that IBD patients who experience abdominal pain during remission have co-existent IBS, and others contending that ongoing symptoms are an integral part of IBD (Aziz & Simrén, Citation2021; Quigley, Citation2016; Travis et al., Citation2009). Whilst this debate is important from a scientific and clinical point of view, understanding how helpful a secondary IBS label is for people with IBD has not been explored. This is important since mistaking IBD symptoms for IBS symptoms could have negative consequences if medication is needed to reduce disease. Without a clear explanation of what it means to have IBD and IBS, patients may feel very worried about ongoing symptoms despite a secondary IBS label. Furthermore, when people are diagnosed with IBS in IBD, this does not necessarily lead to any active treatment advise for the IBS, despite evidence based behavioural and dietary approaches for IBS (Black et al., Citation2021, Citation2020)
Understanding symptoms in remission and symptom-based illness labels can be challenging for patients. A recent study showed that young people found it challenging to make sense of everyday somatic symptoms after their cancer had gone into remission (Heathcote et al., Citation2020). Others found that patients respond ambiguously to labels that do not actually signal disease, but instead summarise a conglomerate of symptoms. Undeland and Malterud (Citation2007) reported that even though a fibromyalgia diagnosis initially led to relief in patients and was felt to legitimate symptoms, negative attributions followed as the diagnosis did not hold the status of a disease or exempt them from responsibility and stigmatisation. There have been numerous attempts to improve on the nomenclature and seek out labels that are minimally offensive (Picariello et al., Citation2015; Stone et al., Citation2002), but the act of diagnosing may be problematic in itself and it has been proposed that the objective of the patient-doctor encounter should not necessarily be diagnosis, but explanation (Jutel, Citation2010).
There are limited qualitative studies conducted on pain in IBD and as far as we are aware, none have explored pain during remission. A study by Sweeney et al. (Citation2019) on pain in IBD described several vicious cycles that people experience regarding symptoms, anxiety, inactivity, avoidance, communication and understanding. These appeared to negatively influence individuals’ pain perception and management strategies, and impact attitudes towards pain. Another study revealed that IBD inpatients feel frustrated, stigmatised, and misunderstood because of their pain (Bernhofer et al., Citation2017). Trivedi et al. (Citation2019) reported that patients understanding of flare and remission are largely symptom-based, but did not address uncertainty in the context of ongoing abdominal pain during remission or focus on patient perceptions of IBD-IBS diagnoses.
This interview study explored (1) how people with IBD experience and view abdominal pain during remission, (2) how they manage abdominal pain during remission, (3) how they manage uncertainty associated with abdominal pain, and (4) how they view IBS in relation to their IBD. Additionally, because the interviews for this study were conducted during the COVID-19 pandemic and this may have impacted on people’s experiences of symptoms, we asked if (and how) the pandemic affected abdominal pain levels.
2. Methods
This study is embedded in constructivism tradition, which upholds the principle that reality is relative and constructed by and between individuals and social experiences (Guba & Lincoln, Citation1994; Reeves et al., Citation2008). In line with this, we used reflexive thematic analysis which considers the subjectivity and reflexivity of researchers to be resources, rather than obstacles to analysis (Braun & Clarke, Citation2019a). We conducted in-depth semi-structured interviews posing open-ended questions to explore how IBD patients make sense of abdominal pain during remission and how this is related to disease labels, social-cultural context, and individual experiences. We also briefly explored how the COVID-19 pandemic influenced these perspectives. Ethical approval was obtained from the University Research Ethics Committee of King’s College London (MSRP-19/20-19284).
2.1. Participants
Participants were largely recruited though online dissemination to IBD communities via Crohn’s & Colitis UK and Twitter. To try and broaden the demographic characteristic of the sample, participants who had consented to contact for further research after participating in a laboratory-based study of pain in IBD were also sent an invitation. These patients were originally recruited through National Health Service (NHS) gastroenterology outpatient clinics in London. Inclusion criteria were a self-reported diagnosis of IBD and pain during remission. We aimed to recruit patients from a range of clinical and demographic backgrounds who either (1) were told by a clinician that they might have IBS and fulfilled the Rome-IV criteria, (2) were told that they might have IBS but did not fulfil the Rome-IV criteria, (3) were not told that they might have IBS but fulfilled the Rome-IV criteria, and (4) were not told that they might have IBS by their clinicians and did not fulfil the Rome-IV criteria (see, ). This was to capture a range of opinions and views. Patients did not need to be in remission during the interview as the interview could focus on past and/or present experiences of pain in remission. People who did not speak English were naturally excluded by our means of recruitment.
Table 1. Topic guide
2.2. Procedure
Interviews were conducted and recorded with a secure videoconference app (i.e., Microsoft Teams, Version 1.4.00.4167). The individual interviews were conducted by a female student (DH). A topic guide of core questions and probes was developed (see, ), agreed by the wider research team including IBD patient representatives. The interviewer followed the guide loosely; she would rephrase questions, adapt the order of questions, and diverge to explore topics brought up spontaneously by participants.
Table 2. Sample characteristics (n = 23) expressed in numbers and percentages unless stated otherwise
A participant information sheet was posted online and/or sent to patients who expressed an interest. Those who agreed to participate were sent a link to an online form generated in a password-protected programme (Qualtrics, Provo, UT). This contained an informed consent form and a brief screening questionnaire to make sure that people met the inclusion criteria—i.e., a diagnosis of IBD and self-reporting pain during remission—and to aid us in establishing a diverse research population. For instance, it allowed us to include both patients who had been told that they had secondary IBS and patients who had not (see, Table ). Audio records of the interviews were anonymised and transcribed verbatim by a professional transcriber. Transcripts were cross checked against the audio files, after which both audio and video files were destroyed. Findings were not returned to participants for member checking, as this is incongruent with the philosophy underlying our research paradigm—i.e., that reality is not fixed, but changeable and constructed by and between individuals within a certain context. Every time an experience is reconsidered its interpretation may shift (McConnell-Henry et al., Citation2011), which has its own limitations. Recruitment was stopped when each of the developed themes achieved sufficient depth and the researchers were confident that they had adequately approached thematic saturation (Braun & Clarke, Citation2019b; Saunders et al., Citation2018).
2.3. Analysis
Anonymised transcripts were entered into NVivo (2020 release). Analysis involved six recursive stages (Braun & Clark, Citation2006): (1) DH familiarised herself with the data by listening to the interviews and conducting a close reading of the transcripts and field notes; (2) she coded relevant parts of the transcripts to generate a rudimentary level of abstraction; (3) by exploring properties and dimensions of codes and merging codes that appeared to describe the same phenomena—both within and across cases—she generated initial themes; (4) she reviewed and further developed the themes by iteratively comparing codes and themes; (5) she consecutively refined, defined and named the themes; and (6) she wrote the results down. To add rigour to the analysis and make sure that the data set was properly reflected in the analysis, DH discussed codes, categories, themes, and supporting quotes with the wider research team. The constructivist paradigm views interviewers as actors in creating data and thus mandates that they reflect on their performance, biases, and feelings. Field notes were therefore taken after each interview both to aid reflection and to help interpretation during the analysis stage (Phillippi & Lauderdale, Citation2018).
3. Results
Demographic and clinical characteristics of participants (n = 23) are presented in Table . 21 participants were recruited via the CCUK website (91%), one participant had taken part in our earlier laboratory-based study (4%), and another came to us via word of mouth (4%). Interviews lasted 66 mins on average (between 41 and 93 minutes) and were conducted from July 2020 to June 2021. This meant interviews encapsulated different stages of the COVID-19 pandemic and patients were facing different levels of restrictions when they were interviewed. We identified three main themes from the data. Theme 1, Distinguishing and navigating pain during quiescent IBD, describes the experiences of participants with abdominal pain during remission. Notably, how people use physical cues to distinguish active from quiescent IBD, which coping strategies people use to navigate the pain and how these may aid the discernment of inflammation, and how context plays a role both in the discernment and navigation of pain during quiescent IBD. Theme 2, The meaning of pain during quiescent IBD, is described against the backdrop of the first theme and discusses illness history and health literacy. Themes 1 and 2 are summarised in a thematic map (Figure ). Theme 3, Consideration and helpfulness of the IBS label in IBD, relates to patients’ consideration of the IBS label in IBD, as well as its perceived helpfulness.
Figure 1. Schematic representation of the distinction, navigation, and meaning (of) pain during quiescent disease. Theme 1 = Distinguishing and navigating pain during quiescent IBD; Theme 2 = the meaning of pain during remission.
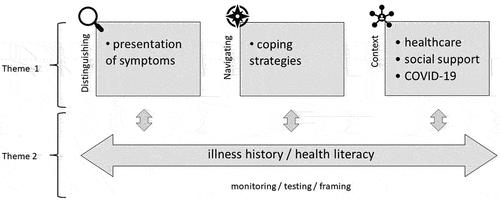
3.1. Theme 1—distinguishing and navigating pain during quiescent IBD
3.1.1. Distinguishing: presentation of symptoms
Most participants found it relatively easy to distinguish between active and remissive IBD, but the presence of pain alone was not driving that distinction. A combination of symptoms including pain, mucus, blood in stools, and urgency, usually indicated to participants that there was active disease; pain by and of itself would only prompt participants to seek out healthcare if it was severe and/or changes in pain lasted for a relatively long time.
FP01: [pain] happens far too frequently […] to be simply about active disease. Now that coupled with something else would probably be a red flag, but that on its own. No. I just take it as part of my new normal.
Some participants described active disease as an ”internal heat” [FP08] or feeling ”almost flu-like, without flu symptoms” [FP09]. Other reported markers were the intensity and presentation of the pain—i.e., stabbing and an aching body in active disease as opposed to bloating and localised pain in the gut in remitted disease. Not everybody found these markers to be clear-cut, however. Some people mentioned that comorbid conditions obstructed a clear interpretation of the symptoms as either active or quiescent.
FP36: I have three things that can cause fatigue […] I think it’s very hard to differentiate an inactive disease, but to have an active plight […] I think that you start with the pain and the diarrhoea and then that causes tiredness because you are just losing nutrients and goodness from your body.
Others described confusing midground periods when flares would build up slowly or patterns of symptoms changed.
FP40: I wasn’t in a full-blown flare, but I was not going to the toilet as I would hope to, and I was getting pain more often than I was not having pain. Then it would calm down a bit and then it would get bad again and then it would calm down a bit, so it was like it continued for about six months until I ended up when my bowel habits changed completely. At that point then I started basically demanding to see the IBD team to find out what was going on […] And they immediately gave me a colonoscopy and they immediately put me onto steroids which worked within 24 hours fortunately.
Taking it further, some described the danger of relating everything to IBD. This was considered to be problematic as it might obscure other serious conditions.
FP16: But then you automatically link everything back up to your Crohn’s diagnosis when actually it could be that you have something else or that you need to be investigated for something else and it can create anxiety because you think well am I avoiding getting a diagnosis for something that might be a bit more serious just because I’m assuming that it’s to do with the Crohn’s disease that I have.
Quiescent disease was often described in terms of the absence of symptoms that go along with a flare.
3.1.2. Navigating: coping strategies
Pain during active IBD was generally described as uncontrollable and incapacitating, whereas pain during quiescent disease was more often expressed in terms of cause and effect and considered more manageable.
FP46: I can tell the difference between flare and [remission] when it’s just, when I’m in a flare it’s just constant pain and when I’m in remission it tends to be after eating, before needing the toilet and then it does go away, and I do get some relief after.
As a result, decision models—based on which participants would distinguish between active and quiescent disease—often incorporated adaptive strategies.
FP36: And because I’m … my bloods are well monitored as well. The attitude was: this seems to be part of me in remission. And as long as it’s manageable, then I’m happy and they’re happy.
Participants described adapting their lifestyles to minimise the impact of symptoms. They altered their diets and eating patterns, made sure that they rested enough, adjusted their working circumstances, saw psychological therapists, exercised, practiced yoga, and meditated. Given these lifestyle changes it may be no surprise that food, stress, and physical exertion were most often mentioned as causing pain during remission. To decide whether a flare was likely participants would often (1) consider if their pain was congruent with any of these factors, and (2) apply strategies such as taking medication, adapting food intake, and lying down. If strategies did not work, a flare might be considered.
FP44: […] you just hold it, rub it, push it, get heat on it. That’s the only things that tend to work and that’s how I manage my pain in remission. It doesn’t work when you are in a flare-up […] There is no way in a flare-up you can control the pain just yourself. It’s just not physically possible.
While coping strategies helped participants to test whether their IBD was active, monitoring of the symptoms was still key. However, one participant who was told that her disease was active—based on inflammation markers in her blood—considered herself to be in remission as she could manage her symptoms by structuring her life, setting clear boundaries, and eating healthily.
FP37: […] on my profile it does say that the disease is active. How he [the gastroenterologist] quantifies that I don’t know exactly. I go for regular blood tests and things […] maybe it’s because there’s any kind of inflammation that is there that he deems that as active. Any colonoscopies that I’ve had has shown there is no active inflammation or ulcers […] So, I don’t know it’s kind of a … if it is in remission because genuinely, I can just get on and do my day-to-day life compared to when it definitely was active when it all started.
Most individuals work out for themselves if they are flaring and do so by relying on their own personal experience. However, how participants distinguished and navigated quiescent disease and ongoing pain was also impacted by contextual aspects.
3.1.3. The role of context: healthcare professionals (HCPs), social isolation, and social support
When symptoms were difficult to interpret, participants contacted their IBD teams for clarity, guidance, and/or reassurance. While this generally was provided, participants mentioned that their clinicians mainly focused on the disease and that little attention was paid to the burden of symptoms. Especially when it came to symptoms during remission participants thought that healthcare professionals were not that interested and explained little.
FP13: if they ask you how you are and you’d say well I have pain, they’re like yes but your bloods don’t show any inflammation so it’s fine. So that’s the way that I would find that it’s very dismissive of pain unless you’ve some other symptoms.
FP19: After he did the second endoscopy for me and had seen nothing, he just put me on a routine waiting listing, so all alarm bells were telling me he’s not caring.
This focus on disease occasionally caused clinicians to inadvertently invalidate a patient’s pain experience. One participant reported that her clinician had said that she was not one of his worst patients upon which she confided that she thought that her experience was rather awful.
FP40: I pity anybody that has worse than me I really do because I think it’s awful for me.
In contrast, other participants mentioned their IBD team interactions were supportive and validating.
FP44: They have said to me even if the markers don’t show it doesn’t mean you are not on the way to a relapse […] then if it’s not pulling back, they would go OK we’ll put you on a short course of steroids to see if we can just pull it back […] But they’re usually very good and you know your own body and they know you know it so.
Pain coping strategies were positively affected by working from home during the Covid-19 pandemic for some participants. They could be more flexible with their time which allowed them to manage their symptoms better—to take rests and go to the toilet whenever they needed. Lower pain levels during lockdown were also attributed to increased control over food—it was easier to eat healthily and plan and structure meals.
FP25: I know that after I’ve eaten, an hour later I’m going to start feeling bloated or having cramps. […] It doesn’t matter if I’m at home I can lie down, but especially if I’m at work or sitting in a train going back home, I often notice that that’s when […] I feel like my stomach is in pain. But it could be a little bit stress related as well because I’m rushing to pick up my daughter from school and I’m panicking will I make it there in time.
Other participants did not report any impact of the pandemic on their IBD or levels of pain.
3.2. Theme 2—the meaning of pain during quiescent IBD
Discerning pain as part of active or quiescent IBD, did not happen in a vacuum. Conversations often went beyond pain and symptoms during remission and sketched a background against which pain and symptoms were interpreted. Some elements of this background were dynamic and changed over time, represented in the subtheme illness history. Other elements were less dynamic and included social and cognitive skill to cope with disease and symptoms, described in the subtheme health literacy.
Both subthemes are relevant to the meaning that people ascribe to ongoing abdominal pain—i.e., as threatening or controllable—and drive how pain during quiescent IBD is distinguished and navigated (see, Figure ).
3.2.1. Illness history
In this study, illness history is defined as the ongoing experience and appraisal of illness-related events and, since every pain event is appraised in the context of prior experiences and simultaneously amalgamated with those experiences, it can be seen as a reality that feeds upon itself. The importance of illness history, the pain experience, and its definition in this way, can be illustrated by the formative role that IBD plays in the lives of some participants. A relatively high proportion of participants was admitted to hospital when they were first diagnosed with IBD, meaning that they had to attune themselves to the idea of living with a chronic disease (which includes persistent pain, a disease in its own right) while they were experiencing a difficult situation and/or severely suffering from their disease. This was reported to be distressing by most participants.
FP46: I don’t really want to go back to that place again when I was really ill […] I’ve written it all down […] that was my way of getting rid of the trauma.
How participants appraised pain seemed to be influenced by this first experience and/or other experiences with bad flares. While pain in and of itself was perceived to be part of life, it could signal more pain, bowel urgency, imminent incapacitation, and hospitalization—i.e., it could signal the downward spiral of active disease—and that prospect could feel threatening.
FP38: I always wonder if I’m going to end up in hospital […] when I start getting bad pain. I do get very, very scared and that probably exacerbates the pain as well. […] I’m scared that they are going to tell me that they have to take even more of my intestines away. I don’t really want to live with a stoma. That petrifies me. I would tend to think I would rather not be here. I’d rather not, sorry.
Illness history might influence both individuals’ appraisal of pain (as signifying either active or inactive disease) and coping strategies. This was evidenced by FP40 who reported that she had learned from the episode described above, when she found it hard to push the alarm button during a midground period when she wasn’t in a “full-blown flare”.
FP40: I don’t know if I was mentally denying what was going on, and maybe I should have reacted earlier, and I would have saved myself maybe about six months of the ups and downs. But I guess I also have at the back of my mind [now], I know that when I press that button, when I trigger that conversation […] I need something done with it. […] I guess I’ve learnt from those past experiences, and I don’t allow it to get to that point quite so severely anymore.
Furthermore, some participants described being misdiagnosed repeatedly and/or saw their treatment as a trial-and-error ordeal, which impacted how well they responded to the suggestion of IBS (see below). In sum, experiences with the disease accumulate and play an important role in someone's disease experience, shape coping strategies, and influence confidence in healthcare. These factors are all relevant to how pain is perceived and appraised, as shown in theme 1. Another principle impacting these aspects (but primarily coping) is health literacy.
3.2.2. Health literacy
As per description of the World Health Organization (WHO) “health literacy represents the cognitive and social skills which determine the motivation and ability of individuals to gain access to, understand and use information in ways which promote and maintain good health” (World Health Organisation, Citation1998, p. 10). The WHO also denotes that knowledge, personal skills, and a sense of agency are indispensable when the constraints of disease necessitate changes to lifestyles/living conditions.
3.2.2.1. Knowledge
One element of health literacy mentioned by participants was knowledge. This study distinguished two types of knowledge: literary knowledge and experiential knowledge. Regarding the former, most participants investigated the disease when they were first diagnosed (with interest generally tapering over time) and mainly searched for management strategies or information specific to their own case. One participant concluded that ”these diseases are so individual to a person” [FP14] that she needed to learn more about them.
FP14: I think with the knowledge of knowing what’s happening to your body it’s almost power. Knowledge is power and when you know what’s happening you plan better. Because you have to think in advance.
Some participants felt that information provision was lacking. One participant reported that she would have liked to have known more about the cause of symptoms during remission “why does it still happen. I don’t know if that is completely unknowable […] but that would be helpful [information]” [FP17] while another relayed that she would have liked to know more about her “future of living with IBD” [FP25]. HCPs may source such information; however, experiences of participants were mixed.
FP09: I have mentioned it [pain during remission] a couple of times and my consultant […] said oh it’s just IBS symptoms and then that was it kind of brushed off. […] I used to see students sometimes and I even asked them if I could have strong, what do you call them, probiotics and I was just told no, not unless you’ve had surgery. I mentioned it a couple of times to the specialist nurse, and she’s basically just said—because I’ve said to them as well do you think it’s the medication—oh no it’s not the medication it’s just part of the disease. So, whether it’s IBS part of IBD nobody has actually ever offered us anything.
While experiential knowledge is closely linked to illness history, it specifically relies on one’s cognitive ability to learn from experience. Some participants said that the experience of gruelling pain during active disease shaped how they appreciated and managed symptoms during quiescent disease. However, it usually took a while before participants could reap the benefits of experience—one participant reported that it took her about six years before she felt she had some grip on the disease. Even when people found a sense of mental equilibrium, this could be offset by unexpected events, such as COVID-19, or changes in symptom presentation.
Notably, pain during active disease was sometimes described as something that you undergo and as such could not be dealt with.
FP44: You get to a point that you think you are fine, and the medication is holding you, and then something happens, and then they have to change it or experiment it. […] Then you are experiencing the side effects of all the medications so you are trying to cope with your Crohn’s that have the medication side effects and then you don’t know which is causing what […] and then they basically turned around and said your body is starting to reject it so you can’t stay on it […] That’s when you go into panic mode because you are just like what else is there. […] But it is it’s just a rollercoaster […] experiment but it’s worth it because you have to try and find something that’s going to work. So, you have to go through the worst bit to get to the good bits.
3.2.2.2. Personal skills and agency
Personal skill and agency were aspects of health literacy (World Health Organisation, 1998) that specifically appeared to impact coping strategies. Accounts that participants gave highlighted their different ways of dealing with pain and ongoing symptoms: they might accept it, adapt to it, shrug it off, struggle with it, and/or try to defy it. This sometimes seemed to coincide with personal tendencies—i.e., to be ”stubborn” [MP03], to dismiss personal needs and not wanting to burden others, or to seek assurance.
MP03: So, I never give in to illness. I just, I just don’t, you know […] which is not always a good thing, sometimes you have to give in for your body to say, actually, I could do with half a day of just sitting doing nothing.
Valued activities, such as meeting up with friends, hobbies, and work, may drive people to assert agency over disease.
FP40: I said it’s not going to beat me, and I still have that in my head, and I want to live my life like that, I want to be able to live my life rather than it define me I guess […] I’ve got quite an interesting job and that mentally stimulates me. […] I’ve definitely enjoyed working at home though throughout [the pandemic]. Yes, I think it’s definitely had an impact on how I manage my IBD definitely.
Additionally, a sense of agency might also be driven by contexts and circumstance. Participant accounts suggested that they regained agency during the quarantine imposed by to COVID-19 as it helped them to recover some reign over their lives. This sentiment was echoed by someone who had recently retired and someone who had transitioned to a job where the management was more accommodating. It was also echoed in a relatively high proportion of self-employed participants. Ironically, a different experience of COVID-19 underlines the importance of agency as well. One participant had to come to terms with his IBD again during COVID-19. It had changed the rules and confronted him anew with the limitations posed by his disease, thereby impacting his sense of control and agency.
MP05: I was getting shielding letters and things like that it kind of brings home that you are a vulnerable person and that you do have a condition and […] a little bit more vulnerable than you thought you really were. […] It was like being diagnosed all over again. You get so used to it being part of who you are […] and then all of a sudden, it’s like you have to stay indoors and you have to be a lot more careful than all my friends, so it makes you feel a little bit different I suppose.
Finally, a sense of agency could be promoted or hampered by HCPs.
FP04: I’m fortunate with my consultant now. We sort of work hand in hand now, where previously … I feel if you don’t know your consultant and they don’t know you, it’s hard for them to understand … and he knows now that if I’m going there to see him and I’m telling him it’s really, really bad he sort of gets that […]. Where previously when I’ve not had that relationship with my consultant it’s really hard to convince them that it’s not just irritable bowel, I know my body, I know it now.
In general, changes in pain and symptoms would preoccupy participants, as they wondered whether they were heading for a flare. Personal tendencies, a need for agency, and the extent to which people were able to learn and adjust, guided how people responded to ongoing pain and symptoms and influenced the extent of interference.
3.3. Theme 3—Consideration and helpfulness of the IBS label in IBD
Participants generally perceived IBD and IBS to be distinct conditions because they feel different and are managed differently, pretty much in the same way that active and inactive disease feel different and are managed differently. However, even though the conditions were perceived to be distinct, participants saw some relation between IBD and IBS. This could simply be because the pain was in the same location or because participants had learned that they were at a higher risk of developing IBS than people without IBD. However, some were distinctly offended by an IBS diagnosis.
FP13: I felt it was just giving me another label for the sake of it.
In some cases, the disinclination to consider IBS was consistent with the assumption that IBD was previously mistaken for IBS. This was exemplified by one participant who presumed that he had fallen out of the system and did not want to go back to the GP; he reported to no longer have confidence in his GP, as the GP had misdiagnosed him with IBS before he ended up in hospital and his IBD was identified.
MP03: It kind of seems to be a collection of disparate symptoms that no one can put a label on, so they call it IBS. […] And I think this is where I lose faith in GPs to a certain extent. Because GPS don’t see IBD very often, but the symptoms are extremely similar, in the first instance, I think a lot of people have been through … they’ve been diagnosed with IBS in the first instance.
Other participants had had an earlier diagnosis of IBS, but they didn’t necessarily perceive this to be faulty and assumed they still had IBS as well. They might even report their previous experience with IBS to be helpful, as it provided a scale to measure their current symptoms against and assessseverity. Interestingly, one participant had decided she had IBS without it being mentioned by a healthcare professional.
FP02: […] before I was diagnosed with colitis I had IBS, so I think I’ve got both […] I’d say it’s IBS-like […] tummy pain now, and I maybe go to the toilet more often than other people. But I’d say I was in remission from the colitis and at the moment that’s being managed by the drugs that I’m on for sure.
Several participants considered heightened pain sensitivity to be part and parcel of IBD, some of whom considered their IBD intrinsically linked to other concurrent diseases as well. These participants tended to have a more holistic view of their disease and body, making an IBS diagnosis redundant in their perspectives.
FP44: When we talk about that midground that is probably when they would bring it [IBS] up. So, if all your markers show as normal or it doesn’t show in a flare that’s when they would probably have mentioned it a couple of times to say maybe it’s a bit of IBS. That’s when I usually pull my face and go how is that possible […] They’ve probably only done that once to me to be fair.
While pain was generally considered to be a logical consequence of the inflammation, complications, surgeries, and/or treatment, the resulting pain might still be judged to be IBS by some, though not by others.
FP04: I’ve had abdominal surgery, I’ve had a number of resections, strictureplasty and then I’ve had my … most abdominal pain now comes from adhesions due to the surgery of the Crohn’s. So, I have Crohn’s colitis and irritable bowel disease altogether.
Alternatively, one participant found it hard to envision that she might have pain due to her IBD when her IBD was in remission. She subscribed it to IBS instead.
FP41: A lot of people think that IBS is like something they tell you’ve got when they can’t find out what else it is. […] But I do think that IBS is very real, it’s a sensitivity […], it’s an intolerance to various things so sure that’s going to affect your body. I probably get more now I’ve got the other conditions, the IBS, I get more stomach cramps than I used to do […] I find it hard to believe that I can have pain and be in remission. I don’t understand why if I’m in remission I still have the pain.
Some participants responded negatively to the IBS label when this was first mentioned, but later found it to be helpful. One participant reported an initial sense of defeat when she learned that she had yet another condition. Another participant ended the interview, following an initial statement that he did not have IBS, by considering testing it out by taking Buscopan.
MP03: I suppose what would perhaps be helpful for me is if the clinicians say, “You know what, it could well be IBS, why don’t we try, why don’t we try and eliminate this early on?”. I mean, I’m gonna guess Buscopan doesn’t cost very much, wouldn’t really cost very much of a trial to figure out if you have got IBS going on as well as Crohn’s.
Overall, knowledge about IBS appeared limited, and participants reported that HCPs had not explained it in much detail.
4. Discussion
This is the first study to explore how people with IBD interpret and respond to abdominal pain during remission, and how they perceive the label of “secondary IBS”. Using reflective thematic analysis, we generated three themes that revealed how everybody’s pain experience is different, that a host of factors influence these differences, and that context is important. Most participants distinguished between symptoms during active and inactive disease by paying attention to symptoms (i.e., to their intensity, presentation, duration, and co-occurrence), deciding whether the symptoms can be controlled, and considering contextual factors (theme 1, distinguishing and navigating pain during quiescent IBD). However, the accumulation of disease-related experiences was found to play an important role in the appraisal of ongoing pain, the coping strategies that participants apply, and the degree to which people trust healthcare professionals. Furthermore, adaptability to new information and a sense of agency—i.e., health literacy—influenced the degree to which participants perceived ongoing pain and symptoms as either threatening or controllable, and thus influenced how they responded to them (theme 2: the meaning of pain during quiescent IBD). Finally, we found that secondary IBS labels have the potential of being perceived as invalidating and—even for those for whom the label helps—do not necessarily promote a clear understanding of ongoing pain and symptoms (theme 3—consideration and helpfulness of the IBS label in IBD). Together, these findings demonstrate that ongoing pain must be appreciated in the wider context of illness history and health literacy and that explanations of pain (and symptoms) during remission need to incorporate the complexity of IBD.
As participants generally adapted to symptoms over time and included a multitude of factors in their understanding of pain, we argue that a simple suggestion of secondary IBS may be too two-dimensional to explain ongoing abdominal pain to patients. It fails to acknowledge the complexity of IBD and the many mechanisms that underly symptoms in remission, which for a sizeable number are part of the IBD disease. Clinicians undoubtedly have the best interest of their patients at heart when they explain that symptoms are caused by IBS (Ford, Citation2020), but our results suggest that IBS does not necessarily provide a reassuring framework with which to understand and manage symptoms. While some participants did find the label beneficial, it elicited aversive or indifferent responses in others. The latter group may benefit more from an explanation which includes the brain-gut axis, central processing of pain, and/or hypersensitivity, either in the context of secondary IBS or simply as an explanation as to why symptoms can still be experienced during remission.
Aversive responses to the suggestion of secondary IBS when there is ongoing abdominal pain may be due to social factors. Recently, Karos et al. (Citation2018) suggested that pain should include a social dimension because it frustrates certain fundamental interpersonal needs and is, simultaneously, impacted by the frustrated expression of these needs. One such need is for justice and fairness (Karos et al., Citation2018). Feelings of injustice are considered to increase pain intensity and unpleasantness, and are often prompted by the actions and responses of others. They can for instance, be triggered by HCPs when the patient believes that the HCP has either contributed to the pain by assessing or treating the pain inadequately, or that the HCP has dismissed expressions of pain (Scott et al., Citation2016). Research has shown that HCPs generally rate disease activity and burden to be lower than their patients (Schreiber et al. (Citation2012) and it is not hard to imagine why IBD patients might find the suggestion of secondary IBS to be dismissive considering that people with IBS already often feel invalidated and stigmatised (Hearn et al., Citation1994).
That participants reported reduced stress and pain levels during periods of lockdown imposed by the COVID-19 pandemic, is also interesting in the context of stigma. Beneficial effects were commonly attributed to increased flexibility providing more control, but lockdown might also have reduced feelings of stigma by allowing our participants to retreat from a society that emphasises health, autonomy, functionality; one which denotes that pain should be short-lived, diagnosable, and fixable (Karos et al., Citation2018). Agency is important for how people experience and manage pain and HCPs can affirm or weaken it by not taking symptoms seriously and/or by suggesting secondary IBS.
The WHO stresses the importance of information and its potential for bringing about change and empowering people—i.e., bringing about agency (World Health Organisation, Citation1998). Our findings indicate that communication and information around symptoms need to be improved, something that has also been indicated by research in an IBS population (Masclee et al., Citation2021). Good information may facilitate coping; a notion that can be substantiated by the Common-Sense Model of Self-Regulation (CS-SRM; Leventhal et al., Citation2003, Citation1998, Citation2016). This framework puts illness representations—i.e., individuals’ intuitive appreciation of current and future threats to health—at the centre of how people manage disease. It stipulates that disturbances in homeostasis, including the experience of symptoms, signal health threat and activate illness representations. Illness representations in turn initiate coping strategies which are appraised for their effectiveness. If a coping strategy is appraised to be ineffective other strategies are put into operation or illness representations may be re-appraised. This was illustrated by our participants who switched from the representation quiescent disease to active disease when symptoms no longer responded to adaptive strategies. People with IBD may refine this process over time (Dorrian et al., Citation2009).
Illness identity is core to developing an illness representation; when patients experience symptoms they are motivated to find a label (or diagnosis), and when they are given an illness label they use this to integrate symptoms (Leventhal et al., Citation2016). A secondary IBS label may have helped some of our participants to frame ongoing symptoms as non-threatening and so aid coping. For others, who found that the label did not correctly represent the underlying condition, it may have impeded self-regulation. This latter group may benefit from explanations and information regarding ongoing pain in the wider context of illness history and health literacy.
It may be important to explicitly address uncertainty when explaining ongoing symptoms during remission. While unspecific, unfamiliar, inconsistent, numerous, and ill-defined symptoms may give rise to uncertainty (Mishel, Citation1984, Citation1988), initially causing distress in some patients, it may eventually drive patients to embrace complexity when conceptualising their condition (Mishel, Citation1990). Either because certainty and predictability prove to be unrealistic or, at least, unobtainable (Mishel, Citation1990), or because patients learn to expect the unpredictable (Zaman et al., Citation2021). Our participants seemed to try and reduce uncertainty either by strictly monitoring symptoms, informing themselves, and/or by contacting their care teams for tests. Addressing uncertainty may help patients to manage expectations and so aid the management of symptoms in remission and increase quality of life Citation(Graff et al., 2006). Making sure patients have access to good, evidenced-based information about possible complex mechanisms underlying symptoms may play a key role, and this is especially important from the onset of the disease.
4.1. Limitations
Our study had several limitations. The COVID-19 pandemic meant we had to change recruitment to largely online methods, so diagnosis of IBD was self-reported. However, based on the in-depth stories of our participants we have no reason to assume that their reports were false. Another limitation is that our study population was not as diverse as we planned. We had to rely on consenting volunteers and although we tried to recruit people from a range of ethnic groups, we were not successful. We had hoped to gain a more diverse research population because the views and experiences of our participants appeared to be context driven and we expect that a wider spread of background factors such as race, gender, and socio-economic status would have elucidated that even more. Straus et al. (Citation2000) previously reported that although IBD presents similarly in black and white people, healthcare utilisation and disease impact differed. They subscribed this to social and economic factors which influence tends to cut across ethnic groups (Smart & Harrison, Citation2017). The relatively high education of our population might also lead to an underestimation of the impact of health literacy on symptom burden, as low health literacy is reported to be more prevalent amongst racial and ethnic minorities and people with less formal education (Paasche-Orlow et al., Citation2005). Finally, due to the nature of our research, we could not verify what HCPs explained about the nature of IBD, abdominal pain, and symptoms during remission.
4.2. Future research and impact clinical practice
Whilst an IBS label aids some people in coping with ongoing pain, it is possible to explain the existence of ongoing pain and other symptoms in remission without the use of a secondary label, and this may be received more favourably by a wide group of people with IBD. Research in an IBS population has shown that communication and information about IBS, with a focus on scientific research, are crucial for optimised healthcare outcomes (Masclee et al., Citation2021). One option would be to provide patients with a mechanism-based explanation that describes how pain and IBD trigger and exacerbate one another, while having different underlaying drivers (Bakshi et al., Citation2021). How to provide clear explanations of ongoing symptoms across a range of patients is important. Tormey et al. (Citation2016) reported that HCPs currently do not have the tools necessary to meet the needs of people with low health literacy (approximately one-third of adults in the United States) and that education regarding how to communicate with them is important. They advise that gastroenterologists assume all patients have limited health literacy and approach them accordingly. We add that IBS in the context of IBD may sometimes be more confusing than helpful. Additionally, since information recall seems lower in people with low health literacy, we would stress that explanations may need to be an ongoing treatment plan where developing supporting resources that provide simple, to-the-point information focused on action and motivation is key (Bernstein et al., Citation2011; Davis et al., Citation2001; Wong et al., Citation2012). Increasing knowledge in this way might eventually lead to lower health care costs as well (Colombara et al., Citation2015). Furthermore, our finding that participants are less burdened by uncertainty over time is important information for patients, especially in the period immediately following diagnosis.
Future research should aim to build helpful explanations for IBD patients who report abdominal pain during remission, investigate what and how much is retained from these explanations, and whether they alter how people manage these symptoms and the distress associated with them.
5. Conclusion
While some attention has been paid to the role of psychological aspects of pain in IBD (Bielefeldt et al., Citation2009; Sweeney et al., Citation2020), our research demonstrates the importance of context too. We paint a picture of a complex reality leading us to conclude that, while IBS labels help some people to cope, they do not necessarily bring more clarity to patients who experience abdominal pain during quiescent IBD. Rather than relying on a diagnosis or label as the sole explanation for pain, individuals may benefit from considered explanations that address the multifactorial nature of their experiences.
Open science badges form
As this is a qualitative study, it was not pre-registered, and we have not deposited any materials and/or data due to possible issues with personal identifiers in interview data and because participants have not consented to this.
Acknowledgements
We would like to thank the participants to our study for giving us their time and for being so open during the interviews. Another thank you should be directed to Crohn’s & Colitis UK for helping in the dissemination of our study and helping to recruit participants. Finally, we would like to thank Ms Lara Tosunlar, MSc, and Ms Erin Walker, PhD, for their input at the start of this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Our data is not deposited in a community recognised repository due to issues with personal identifiers and because participants have not consented to this.
Additional information
Funding
Notes on contributors
Rona Moss-Morris
Professor Moss-Morris’s research group investigates psychological factors that affect symptom experience and adjusting to chronic medical conditions. This mixed-methods research is used to design cognitive behavioural based interventions, including digital interventions, for a range of patient groups. Patients are partners in this research providing input and feedback through-out. RCTs to test the clinical and cost-effectiveness of these interventions form a key component of her research. Danielle Huisman is a NIHR BRC funded PhD student in her group. She has conducted a series of studies on the experience of pain in inflammatory bowel diseases (IBD) during remission including any overlap with symptoms of irritable bowel syndrome (IBS). The core of her work is lab-based measurement of pain processing, but she also has an applied angle. She has conducted three qualitative studies aimed at gaining a better understanding of health care professional communication in this area, general information provision around abdominal pain during quiescent IBD, as well as this study on patients views of pain in IBD.
References
- Aziz, I., & Simrén, M. (2021). The overlap between irritable bowel syndrome and organic gastrointestinal diseases. The Lancet Gastroenterology & Hepatology, 6(2), 139–19. https://doi.org/10.1016/S2468-1253(20)30212-0
- Bakshi, N., Hart, A. L., Lee, M. C., Williams, A. C. C., Lackner, J. M., Norton, C., & Croft, P. (2021). Chronic pain in patients with inflammatory bowel disease. Pain, 162(10), 2466–2471. https://doi.org/10.1097/j.pain.0000000000002304
- Barbara, G., Cremon, C., & Stanghellini, V. (2014). Inflammatory bowel disease and irritable bowel syndrome: Similarities and differences. Current Opinion in Gastroenterology, 30(4), 352–358. https://doi.org/10.1097/MOG.0000000000000070
- Baumgart, D. C., & Carding, S. R. (2007). Inflammatory bowel disease: Cause and immunobiology. The Lancet, 369(9573), 1627–1640. https://doi.org/10.1016/S0140-6736(07)60750-8
- Bernhofer, E. I., Masina, V. M., Sorrell, J., & Modic, M. B. (2017). The pain experience of patients hospitalized with inflammatory bowel disease: A phenomenological study. Gastroenterology Nursing, 40(3), 200–207. https://doi.org/10.1097/SGA.0000000000000137
- Bernstein, K. I., Promislow, S., Carr, R., Rawsthorne, P., Walker, J. R., & Bernstein, C. N. (2011). Information needs and preferences of recently diagnosed patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 17(2), 590–598. https://doi.org/10.1002/ibd.21363
- Bielefeldt, K., Davis, B., & Binion, D. G. (2009). Pain and inflammatory bowel disease. Inflammatory Bowel Diseases, 15(5), 778–788. https://doi.org/10.1002/ibd.20848
- Black, C. J., Staudacher, H. M., & Ford, A. C. (2021). Efficacy of a low FODMAP diet in irritable bowel syndrome: Systematic review and network meta-analysis. Gut Published Online First: 10 Aug 2021. https://doi.org/10.1136/gutjnl-2021-325214
- Black, C. J., Thakur, E. R., Houghton, L. A., Quigley, E. M. M., Moayyedi, P., & Ford, A. C. (2020). Efficacy of psychological therapies for irritable bowel syndrome: Systematic review and network meta-analysis. Gut, 69(8), 1441–1451. https://doi.org/10.1136/gutjnl-2020-321191
- Braun, V., & Clark, V. (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. https://doi.org/10.1191/1478088706qp063oa
- Braun, V., & Clarke, V. (2019a). Reflecting on reflexive thematic analysis. Qualitative Research in Sport, Exercise and Health, 11(4), 589–597. https://doi.org/10.1080/2159676X.2019.1628806
- Braun, V., & Clarke, V. (2019b). To saturate or not to saturate? Questioning data saturation as a useful concept for thematic analysis and sample-size rationales. Qualitative Research in Sport, Exercise and Health, 13(2), 201–216. https://doi.org/10.1080/2159676x.2019.1704846
- Colombara, F., Martinato, M., Girardin, G., & Gregori, D. (2015). Higher levels of knowledge reduce health care costs in patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 21(3), 615–622. https://doi.org/10.1097/MIB.0000000000000304
- Davis, T. C., Dolan, N. C., Ferreira, M. R., Tomori, C., Green, K. W., Sipler, A. M., & Bennett, C. L. (2001). The role of inadequate health literacy skills in colorectal cancer screening. Cancer Investigation, 19(2), 193–200. https://doi.org/10.1081/cnv-100000154
- Dorrian, A., Dempster, M., & Adair, P. (2009). Adjustment to inflammatory bowel disease: The relative influence of illness perceptions and coping. Inflammatory Bowel Diseases, 15(1), 47–55. https://doi.org/10.1002/ibd.20583
- Drewes, A. M., Olesen, A. E., Farmer, A. D., Szigethy, E., Rebours, V., & Olesen, S. S. (2020). Gastrointestinal pain. Nature Reviews Disease Primers, 6(1), 1. https://doi.org/10.1038/s41572-019-0135-7
- Drossman, D. A. (2016). Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology, 150(6), 1262–1279.e2. https://doi.org/10.1053/j.gastro.2016.02.032
- Fichna, J., & Storr, M. A. (2012). Brain-gut interactions in IBS. Frontiers in Pharmacology, 5(3), 127. https://doi.org/10.3389/fphar.2012.00127
- Ford, A. C. (2020). Overlap between irritable bowel syndrome and inflammatory bowel disease. Gastroenterology & Hepatology, 16(4), 211–213.
- Graff L A et al . (2006). The Relationship of Inflammatory Bowel Disease Type and Activity to Psychological Functioning and Quality of Life. Clinical Gastroenterology and Hepatology, 4(12), 1491–1501.e1. https://doi.org/10.1016/j.cgh.2006.09.027
- Grover, M., Herfarth, H., & Drossman, D. A. (2009). The functional-organic dichotomy: Postinfectious irritable bowel syndrome and inflammatory bowel disease-irritable bowel syndrome. Clinical Gastroenterology and Hepatology, 7(1), 48–53. https://doi.org/10.1016/j.cgh.2008.08.032
- Guba, E. G., & Lincoln, Y. S. (1994). Competing paradigms in qualitative research. In N. K. Denzin & Y. S. Lincoln (Eds.), Handbook of qualitative research. (PP. 105–117). SAGE Publications Ltd.
- Hearn, M., Whorwell, P. J., & Vasant, D. H. (2020). Stigma and irritable bowel syndrome: A taboo subject? The Lancet Gastroenterology & Hepatology, 5(6), 607–615. https://doi.org/10.1016/S2468-1253(19)30348-6
- Heathcote, L. C., Loecher, N., Simon, P., Spunt, S. L., Jordan, A., Tutelman, P. R., Cunningham, S., Schapira, L., & Simons, L. E. (2020). Symptom appraisal in uncertainty: A theory-driven thematic analysis with survivors of childhood cancer. Psychology & Health, 36(10), 1182–1199. https://doi.org/10.1080/08870446.2020.1836180
- Jones, M. P., Dilley, J. B., Drossman, D., & Crowell, M. D. (2006). Brain-gut connections in functional GI disorders: Anatomic and physiologic relationships. Neurogastroenterology and Motility, 18(2), 91–103. https://doi.org/10.1111/j.1365-2982.2005.00730.x
- Jutel, A. (2010). Medically unexplained symptoms and the disease label. Social Theory & Health, 8(3), 229–245. https://doi.org/10.1057/sth.2009.21
- Karos, K., Williams, A. C. D. C., Meulders, A., & Vlaeyen, J. W. S. (2018). Pain as a threat to the social self: A motivational account. Pain, 159(9), 1690–1695. https://doi.org/10.1097/j.pain.0000000000001257
- Leventhal, H., Brissette, I., & Leventhal, E. A. (2003). The common-sense model of self-regulation of health and illness. In L. Cameron & H. Leventhal (Eds.), The self-regulation of health & illness behaviour (pp. 42–60). Routledge. https://doi.org/10.4324/9780203553220
- Leventhal, H., Leventhal, E. A., & Contrada, R. J. (1998). Self-regulation, health, and behavior: A perceptual-cognitive approach. Psychology & Health, 13(4), 717–733. https://doi.org/10.1080/08870449808407425
- Leventhal, H., Phillips, L. A., & Burns, E. (2016). The Common-Sense Model of Self-Regulation (CSM): A dynamic framework for understanding illness self-management. Journal of Behavioral Medicine, 39(6), 935–946. https://doi.org/10.1007/s10865-016-9782-2
- Masclee, G. M. C., Snijkers, J. T. W., Boersma, M., Masclee, A. A. M., & Keszthelyi, D. (2021). Patient preferences of healthcare delivery in irritable bowel syndrome: A focus group study. BMC Gastroenterology, 21(1), 438. https://doi.org/10.1186/s12876-021-02030-x
- McConnell-Henry, T., Chapman, Y., & Francis, K. (2011). Member checking and Heideggerian phenomenology: A redundant component. Nurse Researcher, 18(2), 28–37. https://doi.org/10.7748/nr2011.01.18.2.28.c8282
- Minderhoud, I. M., Oldenburg, B., Wismeijer, J. A., Van Berge Henegouwen, G. P., & Smout, A. J. P. M. (2004). IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of L. Digestive Diseases and Sciences, 9(3), 469–474. https://doi.org/10.1023/B:DDAS.0000020506.84248.f9
- Mishel, M. H. (1984). Perceived uncertainty and stress in illness. Research in Nursing & Health, 7(3), 163–171. https://doi.org/10.1002/nur.4770070304
- Mishel, M. H. (1988). Uncertainty in illness. Image: The Journal of Nursing Scholarship, 20(4), 225–232. https://doi.org/10.1111/j.1547-5069.1988.tb00082.x
- Mishel, M. H. (1990). Reconceptualization of the uncertainty in illness theory. Image: The Journal of Nursing Scholarship, 22(4), 256–262. https://doi.org/10.1111/j.1547-5069.1990.tb00225.x
- Paasche-Orlow, M. K., Parker, R. M., Gazmararian, J. A., Nielsen-Bohlman, L. T., & Rudd, R. R. (2005). The prevalence of limited health literacy. Journal of General Internal Medicine, 20(2), 175–184. https://doi.org/10.1111/j.1525-1497.2005.40245.x
- Phillippi, J., & Lauderdale, J. (2018). A guide to field notes for qualitative research: Context and conversation. Qualitative Health Research, 28(3), 381–388. https://doi.org/10.1177/1049732317697102
- Picariello, F., Ali, S., Moss-Morris, R., & Chalder, T. (2015). The most popular terms for medically unexplained symptoms: The views of CFS patients. Journal of Psychosomatic Research, 78(5), 420–426. https://doi.org/10.1016/j.jpsychores.2015.02.013
- Quigley, E. M. (2016). Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Therapeutic Advances in Gastroenterology, 9(2), 199–212. https://doi.org/10.1177/1756283X15621230
- Reeves, S., Albert, M., Kuper, A., & Hodges, B. D. (2008). Why use theories in qualitative research? BMJ, 337(aug07 3), a949. https://doi.org/10.1136/bmj.a949
- Saunders, B., Sim, J., Kingstone, T., Baker, S., Waterfield, J., Bartlam, B., Burroughs, H., & Jinks, C. (2018). Saturation in qualitative research: Exploring its conceptualization and operationalization. Quality & Quantity, 52(4), 1893–1907. https://doi.org/10.1007/s11135-017-0574-8
- Schirbel, A., Reichert, A., Roll, S., Baumgart, D. C., Buning, C., Wittig, B., Wiedenmann, B., Dignass, A., & Sturm, A. (2010). Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World Journal of Gastroenterology, 16(25), 3168–3177. https://doi.org/10.3748/wjg.v16.i25.3168
- Schreiber, S., Panés, J., Louis, E., Holley, D., Buch, M., & Paridaens, K. (2012). Perception gaps between patients with ulcerative colitis and healthcare professionals: An online survey. BMC Gastroenterology, 12(1), 108. https://doi.org/10.1186/1471-230X-12-108
- Scott, W., McEvoy, A., Garland, R., Bernier, E., Milioto, M., Trost, Z., & Sullivan, M. (2016). Sources of injustice among individuals with persistent pain following musculoskeletal injury. Psychological Injury and Law, 9(1), 6–15. https://doi.org/10.1007/s12207-015-9249-8
- Smart, A., & Harrison, E. (2017). The under-representation of minority ethnic groups in UK medical research. Ethnicity & Health, 22(1), 65–82. https://doi.org/10.1080/13557858.2016.1182126
- Srinath, A., Young, E., & Szigethy, E. (2014). Pain management in patients with inflammatory bowel disease: Translational approaches from bench to bedside. Inflammatory Bowel Diseases, 20(12), 2433–2449. https://doi.org/10.1097/MIB.0000000000000170
- Stone, J., Wojcik, W., Durrance, D., Carson, A., Lewis, S., MacKenzie, L., Warlow, C. P., & Sharpe, M. (2002). What should we say to patients with symptoms unexplained by disease? The “number needed to offend”. BMJ, 325(7378), 1449–1450. https://doi.org/10.1136/bmj.325.7378.1449
- Straus, W. L., Eisen, G. M., Sandler, R. S., Murray, S. C., & Sessions, J. T. (2000). Crohn’s Disease: Does Race Matter? American Journal of Gastroenterology, 95(2), 479–483. https://doi.org/10.1111/j.1572-0241.2000.t01-1-01531.x
- Sweeney, L., Moss-Morris, R., Czuber-Dochan, W., Belotti, L., Kabeli, Z., & Norton, C. (2019). ‘It’s about willpower in the end. You’ve got to keep going’: A qualitative study exploring the experience of pain in inflammatory bowel disease. British Journal of Pain, 13(4), 201–213. https://doi.org/10.1177/2049463719844539
- Sweeney, L., Moss-Morris, R., Czuber-Dochan, W., Murrells, T., & Norton, C. (2020). Developing a better biopsychosocial understanding of pain in inflammatory bowel disease: A cross-sectional study. European Journal of Gastroenterology & Hepatology, 32(3), 335–344. https://doi.org/10.1097/MEG.0000000000001615
- Targownik, L. E., Sexton, K. A., Bernstein, M. T., Beatie, B., Sargent, M., Walker, J. R., & Graff, L. A. The relationship among perceived stress, symptoms, and inflammation in Persons with inflammatory bowel disease. (2015). American Journal of Gastroenterology, 110(7), 1001–1012. quiz 1013. https://doi.org/10.1038/ajg.2015.147
- Tormey, L. K., Farraye, F. A., & Paasche-Orlow, M. K. (2016). Understanding health literacy and its impact on delivering care to patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 22(3), 745–751. https://doi.org/10.1097/MIB.0000000000000622
- Travis, S. P., Spiller, R. C., Holzer, P., & Colombel, J. F. (2009). IBD and IBS: Novel mechanisms and future practice. Digestive Diseases, 27(Suppl 1), 1–2. https://doi.org/10.1159/000268114
- Trivedi, I., Darguzas, E., Balbale, S. N., Bedell, A., Reddy, S., Rosh, J. R., & Keefer, L. (2019). Patient understanding of “flare” and “remission” of inflammatory bowel disease. Gastroenterology Nursing, 42(4), 375–385. https://doi.org/10.1097/SGA.0000000000000373
- Undeland, M., & Malterud, K. (2007). The fibromyalgia diagnosis: Hardly helpful for the patients? A qualitative focus group study. Scandinavian Journal of Primary Health Care, 25(4), 250–255. https://doi.org/10.1080/02813430701706568
- Wong, S., Walker, J. R., Carr, R., Graff, L. A., Clara, I., Promislow, S., Rogala, L., Miller, N., Rawsthorne, P., & Bernstein, C. N. (2012). The information needs and preferences of persons with longstanding inflammatory bowel disease. Canadian Journal of Gastroenterology, 26(8), 525–531. https://doi.org/10.1155/2012/735386
- World Health Organisation. (1998). Health promotion glossary. Health Promotion International, 13(4), 349–364. https://doi.org/10.1093/heapro/13.4.349
- Zaman, J., Van Oudenhove, L., & Vlaeyen, J. W. S. (2021). Uncertainty in a context of pain: Disliked but also more painful? Pain, 162(4), 995–998. https://doi.org/10.1097/j.pain.0000000000002106
