Abstract
Alexithymia is a multifaceted personality trait linked to increased risk for psychological, psychosomatic, and physical health problems. One hypothesized mechanism through which alexithymia predisposes individuals to such problems is the interference of alexithymic characteristics in processing affective, particularly unpleasant content. This study aimed to investigate the relationship between alexithymia and biases in attentional processing of threatening vs. neutral pictorial stimuli, disentangling early (vigilance) from late (maintenance) attentional biases. One hundred participants (77 female; 18–35 years old) completed the Toronto Alexithymia Scale and underwent a free viewing task with picture pairs presenting illness, fear and neutral content, during which dwell time on each picture was recorded at time intervals of 0–500 ms, 501–1000 ms and 1001–6500 ms of exposure. Results from multilevel modeling showed that alexithymia interacted with time interval and picture type. Higher alexithymia scores were related to less dwell time towards fear pictures at 501 ms-1000 ms, but more dwell time at 1001 ms-6500 ms after stimulus onset. This effect was particularly observed for the externally oriented thinking and the difficulty in describing feelings facets of alexithymia, but not the difficulty in identifying feelings. There was no effect of alexithymia on early vigilance at 0–500 ms. This study provides evidence on the association between alexithymic traits and early avoidance, along with late maintenance bias to fear, which appears consistent with the view that alexithymia is associated with avoidant emotion regulation processes, but also greater requirements of cognitive resources for processing affective information.
PUBLIC INTEREST STATEMENT
Alexithymia is a personality trait characterized by difficulty in identifying and describing emotions. It is associated with higher risk for psychological and physical health problems. We investigated the relationship between alexithymia and biases in how healthy young adults allocate their attention while they process pictures with threatening (fear and illness-related) and neutral content. 100 participants completed the Toronto Alexithymia Scale and, went through an experimental task during which they had to view picture pairs while their eye movements were recorded to examine where they mostly focus their attention on. Results showed that higher scores in alexithymia were associated with avoidance (less time of attentional focus) of fear pictures during an early time interval of viewing picture pairs and maintenance of attention to fear during a later time interval. This is an indication of an effort by individuals with high alexithymia to avoid emotion at first, and of a greater need of attention towards the emotional information in order to process and understand it later on.
Alexithymia is a multifaceted personality trait characterized by a marked difficulty in identifying (DIF) and describing emotions (DDF) and an externally oriented thinking style (EOT; Luminet et al., Citation2018, Citation2021a). High levels of alexithymia have been associated with increased risk for psychological (Manninen et al., Citation2011), psychosomatic (Rief & Broadbent, Citation2007), and physical health problems (e.g., cardiovascular; Vanheule et al., Citation2010) and higher mortality rates (Kauhanen et al., Citation1996). One hypothesized mechanism through which alexithymic individuals are predisposed to such problems is the interference of alexithymic characteristics in processing affective content and in effective regulation of emotions (Panayiotou, Panteli, & Vlemincx, Citation2021; Panayiotou et al., Citation2018). A limited number of studies suggested the possibility that attention bias, a cognitive process that plays a role in many forms of psychopathology, may be associated with difficulties in processing emotional content in alexithymia (i.e., alexithymia as a deficit in emotional processing; Luminet et al., Citation2021a, Citation2021b; Taylor, Citation2000). Luminet and colleagues, in their recent review (Luminet et al., Citation2021a), identified a significant gap in knowledge regarding the role of attentional processes in alexithymic difficulties, and called for future research in this domain to clarify the type of bias that characterizes this trait and its facets, a need that the present study aims to address. Research to clarify this question is important, as biased attention to specific stimuli types, especially negative ones, may be related to perpetuated or frequent stress states (Koster et al., Citation2004), leading to physical and mental health risks.
There are three types of attention bias: vigilance to, delayed disengagement from and avoidance of affective stimuli (Cisler & Koster, Citation2010). In alexithymia, heightened early attention (vigilance) was indicated by altered neural activations in response to emotional stimuli, including faces and voices (Delle-Vigne et al., Citation2014). Conversely, Lundh and Simonsson-Sarnecki (Citation2002) using the affective Stroop task showed vigilance, only for illness-related, but not generally unpleasant stimuli in alexithymic individuals. Therefore, findings in support of a vigilance bias in early information processing stages in alexithymia are limited and inconsistent, especially with regard to the content of the emotional stimuli.
On the other hand, the second form of attention bias, disengagement difficulty in later processing stages, may lead to prolonged or enhanced stress through a vicious cycle, in which anxiety (or other negative emotion) increases as the person remains fixated on threatening/negative stimuli (Buckner et al., Citation2010; Koster et al., Citation2004). Various studies have shown that alexithymic individuals exhibit difficulties in disengagement from unpleasant (angry or disgust) facial stimuli (Mériau et al., Citation2006; Vermeulen et al., Citation2008) and slower attention shift toward new information (Patel & Azzam, Citation2005; Suwazono et al., Citation2000). Grynberg et al. (Citation2014) contributed evidence that slower disengagement was observed in alexithymia after the processing of fear and angry faces, but not after facial stimuli depicting pain. Overall, these findings suggest that unpleasant/threatening faces leave less attention resources available to process other stimuli, in support of a tendency for maintaining attention on negative emotional content in alexithymia. However, these deficits have been observed only for facial expression stimuli so far, and it is unclear if they extend to non-facial unpleasant stimuli, including unpleasant or illness-related information.
In addition to findings for vigilance and disengagement biases, the review of Donges et al. (Citation2014) presented evidence for reduced automatic attention allocation to emotional (negative or both negative and positive) lexical stimuli in alexithymia. In fact, a third proposed pathway linking alexithymia with health problems is attentional avoidance of threat. Avoidance may hinder adaptation to challenges (Davydov et al., Citation2010), and, when it comes to processing emotional facial expressions, may damage interpersonal relationships by alexithymic individuals appearing uninterested or bored (Chen et al., Citation2002). Evidence for this type of bias is derived from paradigms showing that less time or effort is devoted to processing emotional (Van der Velde et al., Citation2013), including bodily-symptom-related word stimuli (Mueller et al., Citation2006), relative to neutral. On the other hand, some studies have shown reduced processing for both emotional and neutral images in high relative to low alexithymic participants (Kraus et al., Citation2014; Pollatos & Gramann, Citation2012).
Of note, even though the majority of studies focused on the effects of total alexithymia on attention bias toward emotional material, findings support that the different facets of alexithymia are linked to different types of attentional biases (Luminet et al., Citation2021a). Prior work suggested that less preference and reduced processing of emotional content, either in early or in later processing stages, was differentially linked to the three alexithymia facets in different studies (with EOT after controlling for anxiety and depression (Wiebe et al., Citation2017); with DDF (Pollatos & Gramann, Citation2012); with dispositional attention to feelings, which is a construct similar to DIF (Bujanow et al., Citation2020)). Luminet et al. (Citation2021a) suggested that EOT is related to reduced interpretation and elaboration of emotional information and deficits in the ability to attend to emotion, i.e., avoidance. On the other hand, DIF, which reflects a deficit in the perception of emotion, may impact early emotion processing and, therefore, early attention allocation and emotion intensity. DDF reflects a deficit in expressing and communicating emotion to others, thus, it may impact later processing (memory, language, and interpersonal relationships). Research has, so far, not provided consistent evidence for each of the attention bias types in alexithymia and in relation to its specific facets (Luminet et al., Citation2021a), suggesting that this domain deserves further research attention
1. Current study
This study aimed to examine how alexithymia relates to different attention bias types (vigilance, attention maintenance and avoidance towards fearful, illness-related, and neutral stimuli). A second aim was to identify which facets of alexithymia (measured and analyzed as continuous dimensions), relate to such attention biases. Previous approaches to the study of attention biases, such as the use of reaction time to the dot-probe task or the Stroop task, have been criticized for their reliability and validity and have mostly focused on assessing early automatic attention orientation and priming (Luminet et al., Citation2021a). In order to combat some of these limitations, we used eye-tracking methodology to assess attention biases and track how they change over the duration of each type of stimulation. To this end, participants viewed picture pairs while eye gaze was continuously recorded over an exposure period of 6.5s, which was long enough to capture the three types of attention bias that possibly occur at different time-points during information processing, that is, in early automatic vs. later more conscious processing stages. Inclusion of fear and illness pictures allowed us to assess the effect of specific content of unpleasant stimuli on attention bias, given that alexithymia is related to both anxiety and various somatic illnesses and medically unexplained symptoms, and due to existing evidence for bias specific to illness-related stimuli (Lundh & Simonsson-Sarnecki, Citation2002; Mueller et al., Citation2006). Percent dwell time on each picture was calculated for three intervals: as an index for vigilance: 0–500 ms, 501–1000 ms, and as an index of attention maintenance/avoidance: 1001–6500 ms, since vigilance is expected to emerge early in the exposure duration and attention maintenance/avoidance are expected later (Wieser et al., Citation2009).
Considering existing evidence for attention bias in alexithymia for unpleasant and threatening stimuli (Luminet et al., Citation2021a), we expected a significant interaction effect between alexithymia, picture type and pair type on the percentage of dwell time during the free viewing of picture pairs. More specifically, we expected that higher alexithymia would be linked with either greater (i.e., vigilance and attention maintenance bias) or less (i.e., avoidance bias) dwell time towards threatening pictures (fear and illness) when paired with neutral pictures, than when two threatening pictures are presented in the same pair. Further hypotheses with regard to the specific type (vigilance, attention maintenance or avoidance) and time course (interaction with time interval) of the expected attention bias could not be made in light of the previous mixed findings. In addition, considering prior findings for associations of the three alexithymia facets with avoidance of attending to and processing emotional stimuli (Bujanow et al., Citation2020; Pollatos & Gramann, Citation2012; Wiebe et al., Citation2017), which comes into contrast with the evidence for attention bias towards negative stimuli, we further explored whether evidence for avoidance bias in alexithymia is driven by associations between avoidance and specific alexithymia facets.
2. Method
2.1. Sample
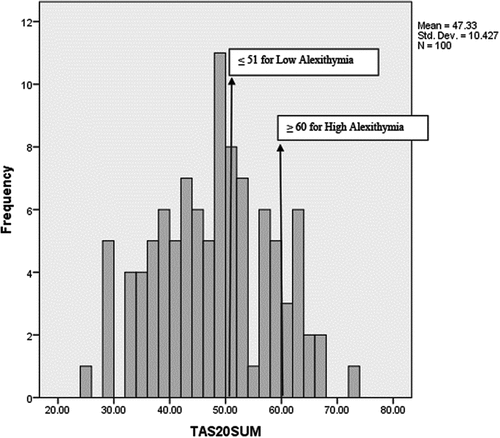
One hundred university students (77 female; 18–35 years old; M = 21.69, SD = 3.12) participated in this study receiving course credit for their participation. Students who reported vision or hearing disability that would influence their performance were excluded; students who reported corrected-to-normal vision were included. As the study was part of a large project on illness anxiety (initial sample: N = 616; see, Leonidou & Panayiotou, Citation2020), participants were selected to have a wide range of illness anxiety scores using the Illness Attitudes Scales (M = 42.32, SD = 14.73, Cronbach’s α = .833; Kellner, Citation1987; Leonidou & Panayiotou, Citation2017). Nevertheless, alexithymia scores were normally distributed in this sample (see, Figure ) and ranged between 25 and 73 for total alexithymia (M = 47.33, SD = 10.43), 7–32 for DIF (M = 16.09, SD = 5.54), 5–25 for DDF (M = 12.83, SD = 4.60), 11–27 for EOT (M = 18.41, SD = 3.86). Fourteen percent of participants scored high in alexithymia (above the widely used cutoff score of ≥60; Parker et al., Citation2003) and 67% scored low (≤51). Ten participants self-reported a medical diagnosis, which included allergies, arrhythmia, thyroid disorder, orthopedic problem, insulin resistance, high cholesterol levels, psoriasis, and pituitary gland prolactinoma.Footnote2
Figure 1. Distribution of alexithymia scores in our study with cutoff scores according to Parker et al. (Citation2003) noted.
Due to the well-evidenced association between alexithymia and depression and anxiety (Foran & O’Leary, Citation2013; Honkalampi et al., Citation2018); Panayiotou, Citation2018; Panayiotou et al., Citation2020; Picardi et al., Citation2011; Taylor & Bagby, Citation2004), participants were also screened using the Psychiatric Diagnostic Screening Questionnaire (PDSQ; Theodorou et al., Citation2013; Zimmerman & Mattia, Citation2001) to assess symptoms of depression (M = 3.87, SD = 3.68, α = .835) and four anxiety disorders: GAD, panic, agoraphobia and social anxiety a total anxiety symptom score was calculated for these subscales; (M = 8.37, SD = 7.63, α = .909). Participants were not excluded based on their PDSQ scores, rather their effects were controlled in separate analyses.
2.2. Experimental design
The experiment involved a free viewing task presenting an equal number of three picture pair types, distributed in 8 blocks with 6 trials each (48 trials in total): two blocks with illness-neutral (I-N) picture pairs, two blocks with illness-fear (I-F) pairs, two blocks with fear-neutral (F-N) pairs and two blocks with all types of pairs. Blocks were counterbalanced across participants and trials (picture pairs) were randomized within the block. In each trial, first, participants went through a drift correction (i.e., brief pre-trial calibration procedure), second, they focused on a fixation cross presented in the middle of the screen for 1.5s; then, they were instructed to look at the picture pair for 6.5s as they would look at a photograph album (Liossi et al., Citation2014). To alleviate participants’ fatigue, a blank screen was presented for 1.0/1.5/2.0s randomly after the end of each trial (Armstrong & Olatunji, Citation2012).
2.3. Material: experimental stimuli
Experimental stimuli were fear and neutral pictures from the International Affective Picture System (IAPS; Lang et al., Citation1997) and illness pictures from the IAPS, the picture set used by Lees et al. (Citation2005; personal communication in October 2016) and the Google Images search engine using the search terms: illness, symptoms, medical procedures. Thirty-two pictures of each type were selected based on ratings provided by an independent sample of 20 students prior to the experiment (see Supplementary Table 1). Pictures were presented in black and white and were matched in pairs based on complexity, i.e., number of objects, humans present, etc. Picture dimensions were 345 × 245 pixels and were presented in the middle left (256, 384) and right side of the screen (768, 384) on a white background. To control for the tendency of a left–right processing pattern, each picture type was equally presented on the left or the right side of the screen within each pair. To prevent habituation effects, pictures were presented once during the experiment.
2.4. Procedure
Ethical approval was obtained by the Cyprus National Bioethics Committee (Protocol Number: EEBK/EΠ/2017/12). Following informed consent, the Miles test (Miles, Citation1930) was applied to identify the participant’s dominant eye. Participants were seated in a dimly lit room in front of the eye-tracking equipment and a computer screen (BENQXL24IIT 24”, screen resolution: 1280x1024, display resolution: 1024 × 768). The display screen was approximately 70 cm from the participant’s eyes and the camera lens was about 60 cm from below the participant’s head. After a resting period of five minutes for participants to familiarize themselves with the setting, participants placed their head on the chin-rest and the eye-tracker calibration procedure was carried out. Experiment Builder (SR Research, Ottawa) was used to control the experiment. If tired, participants could take a break between blocks. They were informed that pupil dilation was the measure recorded during the task (Buckner et al., Citation2010). After the task and before debriefing, participants reported whether they paid more attention to one picture type over others (e.g., During the first phase of the experiment, I caught myself paying more attention to the pictures related with medical or health issues) on a 10-point scale (0 = statement totally non-representative to 9 = totally representative).
3. Measures
3.1. Self-reports
Alexithymia was assessed by the Greek translation of the Toronto Alexithymia Scale-20 (TAS20; (Bagby et al., Citation1994)). The scale consists of 20 statements rated on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree) and provides a total alexithymia score, and sub-scores on its three factors: DIF, DDF and EOT. Higher scores indicate increased alexithymia. It has well-established psychometric properties and the Greek version showed good reliabilities for most scales (total α = .79, DDF α = .79, DIF α = .74, EOT α = .58; Tsaousis et al., Citation2010). In this study, total α = .71, DDF α = .71, DIF α = .81, EOT α = .49.
3.2. Eye-tracking measurements and data reduction
EyeLink 1000 Plus Desktop Mount (SR Research, Ottawa) was used to record eye movements monocularly using the dominant eye (except in 2 cases where the non-dominant eye was better captured) at a sampling rate of 1000 Hz. A 9-point calibration template was applied; pupil threshold was >70 and <120 and corneal reflection was 200–240. Calibration was validated if the error rate was <.05 on average and no calibration was accepted with an error above 1. One rectangular interest area for each picture per pair was drawn and fixations within the interest areas were used to calculate dwell time scores. Fixation was defined as any fixation recorded after the first 100 ms from stimulus onset and lasted ≥100 ms (Wieser et al., Citation2009). Dwell time on each picture was calculated as the mean percent dwell time on each picture of the pair as a function of the total dwell time on both pictures, which was equal to 100%. The mean percent dwell time was calculated at three intervals: 0–500 ms, 501–1000 ms and 1001 ms-6500 ms. A 0.23% (1 trial missing in 2 participants, 9 trials missing in 1 participant) of the total number of trials was missing due to recording problems. Extreme outliers identified via boxplots (<3% of cases) were replaced with the sample minimum or maximum value per variable.
4. Data analysis
Main analyses consisted of linear mixed models using the nlme package in R, which examined the effects of negative Picture type (Illness vs. Fear), Pair Type (Paired with Neutral vs. Paired with another negative picture), Interval (0–500 ms, 501–1000 ms, 1001–6500 ms; the third interval was always used as the reference level in contrasts) and alexithymia scores (mean centered) and their interactions on percent dwell time. Models were run with the TAS-20 total score as a predictor, but also with each of the three subscales (DIF, DDF, EOT; mean centered).
To confirm that the effects on attention allocation were not linked to other psychological symptoms and were specific to alexithymia, depression, total anxiety score and illness anxiety were tested in separate models as predictors (instead of alexithymia). Briefly, these models showed no effects of depression and total anxiety on attentional bias and a different interaction pattern of illness anxiety on attention allocation, thus, the pattern of attention allocation presented below is specific to alexithymia. These models were not presented in detail here because it goes beyond the scope of this article.
In all cases, a top-down model selection was applied (Zuur et al., Citation2009) in which a full models was fitted first using REML method with Picture Type, Pair Type, Interval, TAS total score (mean centered) and all their interactions as fixed effects and maximal random effect structure (i.e., Participant as random intercept and random slopes for Pair Type, Picture Type and Interval and their interactions). The full model was then compared to simpler models in terms of random effects using the likelihood ratio test. Fixed effects were kept constant in all models as they are dictated by the experimental design and our hypotheses. Significant interactions were followed-up with separate models per condition. Estimated marginal means (emmean function) were calculated to explore differences between conditions.
5. Results
5.1. Experimental manipulation checks
On ratings after the end of the experimental task, participants reported that they mostly focused on generally fearful (M = 7.00, SD = 9.59) and illness pictures (M = 5.86, SD = 2.35) and they focused significantly less on neutral pictures (M = 4.20, SD = 2.20), F(2,98) = 10.72, p < .001, ηp2 = .18 (further details are reported in Leonidou & Panayiotou, Citation2020). Table presents the means and SDs for dwell time per experimental condition.
Table 1. Means and standard deviations (in parentheses) for dwell time (%) per picture-type perpicture pair
The full model on dwell time percentage with Picture Type, Pair Type, Interval and TAS total score and their interactions as fixed effects and Picture Type x Pair Type x Interval as random slopes and Participant as random intercept failed to converge. Thus, a simpler model was run removing the interactions from the random slopes. The random effects for this model included Participant as random intercept and random slopes for Picture Type, Pair Type and Interval (see, Table ). This model converged and model comparisons using likelihood ratio tests showed that simpler models (removing one of the three random slopes) did not increase the goodness-of-fit (Akaike Information Criterion (AIC): 8811.21 vs. 8823.99, 8889.12, 8792.14).
Table 2. Summary of linear mixed model examining the effects of picture type, pair type, interval and alexithymia total score on dwell time (percentage)
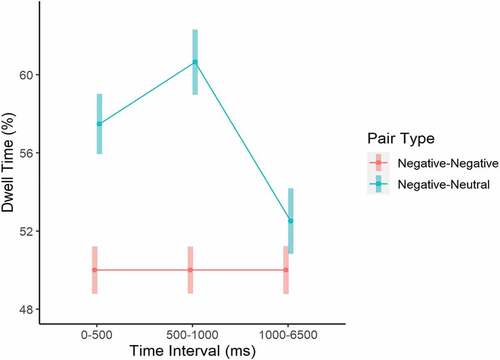
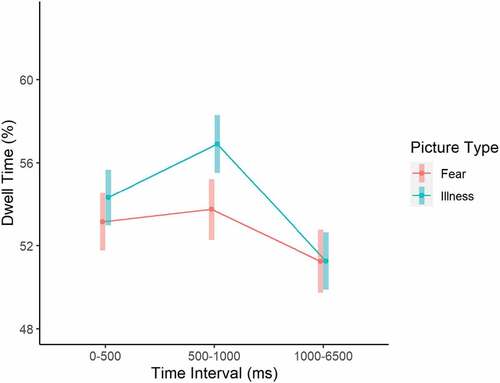
As for the fixed effects (explaining 18% of the variance based on the marginal R2 statistic; Table ), results showed a significant Picture Type x Pair Type interaction, with illness pictures receiving more dwell time (M = 58%, SE = 0.73) than fear pictures (M = 55.7%, SE = 0.82) when paired with neutral pictures. When paired with each other, illness and fear pictures received approximately 50% dwell time each (attention was split equally between them). A significant Pair Type x Interval interaction (Figure ) indicated that dwell time did not differ across intervals when illness and fear pictures were paired with each other (around 50% at all time intervals), but when a negative picture (either illness or fear) was paired with a neutral one, dwell time to the negative picture was significantly increased from the 0–500 ms to the 501–1000 ms interval (M = 57.5%, SE = 0.77 vs. M = 60.6%, SE = 0.84) and then significantly reduced during the 1001–6500 ms interval (M = 52.5%, SE = 0.84). Furthermore, a significant Picture Type x Interval (Figure ) interaction indicated that illness pictures regardless of the Pair Type received more dwell time than fear pictures only during the 501–1000 ms interval (M = 56.9%, SE = 0.70 vs. M = 53.7%, SE = 0.75, t(1078) = 3.24, p = .001). These findings point to a pattern of attentional vigilance towards negative pictures when paired with neutral ones across participants, especially at the early stages of attentional processing as measured in the current experimental paradigm, with illness pictures receiving even more attention at the 501–1000 ms interval (which replicates the experimental manipulation checks carried out by Leonidou & Panayiotou, Citation2020).
6. Effects of alexithymia on attentional processing
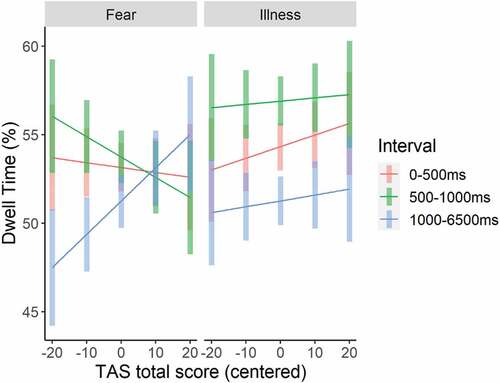
As for the effects of alexithymia on attentional processing of negative pictures, a significant Picture Type x Interval x TAS interaction was observed (see, Table ). Plotting the interaction (Figure ), suggested that higher alexithymia scores interact with interval mainly for the fear pictures, although separate models for each Pair Type showed that the TAS x Interval interaction did not reach significance for neither Fear (p = .14) nor Illness pictures (p = .13). Nevertheless, looking into the interaction for fear pictures in more detail by running separate models per interval, indicated that for fear pictures, the TAS effect, although non-significant at both the second and the third interval, was associated with attention bias in opposite directions (Estimate = −0.10, SE = 0.09, t(98) = −1.03, p = .30 and Estimate = 0.09, SE = 0.08, t(98) = 1.20, p = .23 respectively), with higher alexithymia related to less dwell time during the 501–1000 ms interval, but more during the 1001–6500 ms interval.
Figure 4. Picture type x interval x total alexithymia score (mean centered) interaction effect on the percentage dwell time. error bars represent 95% confidence intervals.
6.1. Effects of alexithymia facets on attentional processing
To examine which facets of alexithymia drive these interactions, three models were run with the three subscales of TAS20 (DIF, DDF, and EOT) as predictors instead of the total score. For the model using the EOT subscale, a significant Picture Type x Interval x EOT interaction emerged (0–500 ms vs. 1001–6500 ms: Estimate = 1.03, SE = 0.44, t(1078) = 2.35, p = .02; 501–1000 ms vs. 1001–6500 ms: Estimate = 0.92, SE = 0.44, t(1078) = 2.09, p = .04). Follow-up models per picture type showed a similar pattern as for the total alexithymia score, i.e., for the Fear pictures. EOT scores correlated positively with dwell time at the third interval, but negatively during the first and second intervals, although these separate effects did not reach significance. The same pattern was observed for the model using the DDF facet (Picture Type x Interval x DDF interaction), when the third interval was compared with the second (501–1000 ms vs. 1001–6500 ms: Estimate = 0.85, SE = 0.37, t(1078) = 2.31, p = .02). The DIF facet showed no significant effects on attentional processing.
7. Discussion
The current study responds to the call for research on the role of attention biases in the emotional difficulties experienced by alexithymic individuals, by investigating the relationship of alexithymia and its facets with attentional biases in the processing of threatening and non-threatening information. Prior work has established atypical attention allocation toward emotional stimuli in alexithymia (Luminet et al., Citation2021a), with incongruent findings regarding the type of deficits, i.e., vigilance (Delle-Vigne et al., Citation2014), disengagement difficulty (Vermeulen et al., Citation2008), or avoidance (Wiebe et al., Citation2017). To address the previous mixed findings with regard to the nature and time course of attention bias, we assessed all attention bias types, as indexed by gaze fixation at different time intervals after stimulus onset during a free viewing task. This was not previously possible when using behavioral tasks like the affective Stroop, or by analyzing dwell time on pictures over the whole exposure duration (as in Wiebe et al., Citation2017) because these methods focus on very specific or very widely set time points. Overall, findings supported our initial hypotheses. Alexithymia and its EOT and DDF facets interacted significantly with time interval and picture type, suggesting specific patterns of attention allocation linked to alexithymia. Findings have important implications for the relevant literature.
More specifically, results showed no evidence for vigilance during the early, first interval of processing linked to high alexithymia since all participants, regardless of alexithymia level, showed vigilance towards fear and illness pictures relative to neutral. This contradicts existing evidence for hypervigilance to threat in alexithymia (Delle-Vigne et al., Citation2014). However, the preferential allocation of attention to threat, which happens with less voluntary control and was observed for all participants in the first 500 ms of exposure, is in line with the theoretical premise for a general human predisposition to attend to stimuli perceived as threatening or having emotional content when other information within the environment is neutral/nonthreatening (Gerritsen et al., Citation2008; Kahneman & Triesman, Citation1984; LeDoux, Citation1995; Nummenmaa et al., Citation2006).
To the contrary, during the second interval (501–1000 ms), high alexithymia was linked to less dwell time on fear pictures, providing evidence for early attentional avoidance bias (Priebe et al., Citation2015; Wieser et al., Citation2009). Analyses further showed that the EOT and the DDF facets were specifically linked to this avoidant processing pattern during the second interval of exposure, but not the DIF facet. These findings fit with previous studies indicating avoidance bias (Pollatos & Gramann, Citation2012; Wiebe et al., Citation2017) and suggest that when alexithymic individuals start to process threatening information more consciously tend to implicitly direct their attention away from it. In support of our finding, DIF has not been previously associated with early emotional processing that requires attention allocation to the emotional stimuli. However, less dwell time, observed in eye tracking research, and reduced emotional priming have been linked to the deficit model of alexithymia and predominately to its EOT facet (Luminet et al., Citation2021a). DDF, as a deficit in verbalizing and communicating emotion, is, potentially, a direct outcome of avoidance since alexithymic individuals have less concrete emotional information to express to others. Indeed, existing findings support a strong association between DDF and experiential avoidance (Panayiotou et al., Citation2015). Behavioral inhibition and avoidance of unpleasant stimuli serve as protecting mechanisms from experiencing dysphoria in the short-term (Davydov et al., Citation2010) once a stimulus is initially perceived as potentially threatening (early vigilance). Findings of a bias towards attentional avoidance, are in line with theoretical accounts that avoidance of unwanted experiences plays a key role in alexithymia and its connections with health problems (Panayiotou et al., Citation2015; Panayiotou, Panteli, & Vlemincx, Citation2021).
The pattern of attentional avoidance observed here contradicts other findings showing difficulty in disengaging attention from threat in alexithymia (Vermeulen et al., Citation2008). This difference may reflect different timeframes of stimulus processing, as in our study, we found both avoidance and disengagement difficulty to co-occur at different time intervals, that is, the second vs. the third time intervals, respectively. Specifically, in addition to evidence of attentional avoidance during the 2nd interval, our findings indicate the presence of difficulty in disengaging attention from negative stimuli at later stages of processing (third interval) associated with more voluntary control and fuller awareness of the emotional content. This opposite pattern of avoidance-disengagement difficulty was specific to fear (Priebe et al., Citation2015; Wieser et al., Citation2009), a high arousing negative emotion (rated as more emotionally intense in the current study), which may point to stimulus saliency or intensity as driving the disengagement difficulty effect. Perhaps, after the more automatic stages of processing, individuals with alexithymic traits voluntarily try to recognize and understand the emotional content of stimuli initially perceived as potentially significant (threatening) in order to respond appropriately to environmental challenges. Great need to dwell on fearful (or other emotional stimuli) may be related to the difficulties in recognizing unpleasant affective states, noted previously (Grynberg et al., Citation2012), such that alexithymic individuals may require more attentional resources to effectively process the situation and describe the feelings triggered from it. In support of this study’s findings, prolonged attentional processing of threat linked to DDF and EOT has been previously reported by Grynberg et al. (Citation2014). In the long term, difficulty to disengage from negative stimuli may have negative consequences for health and adaptation (Panayiotou et al., Citation2018; Panayiotou, Panteli, & Vlemincx, Citation2021), via more persistent negative emotions and failed shutdown of emotional arousal (McEwen, Citation1998), resulting in affective dysregulation, and atypical physiological responses (Panayiotou et al., Citation2018). This form of chronic dysregulation may in turn contribute to the development of psychological, physical and psychosomatic difficulties in alexithymia (Panayiotou, Panteli, & Vlemincx, Citation2021).
Furthermore, the inclusion of illness and fear stimuli in the free viewing task allowed us to examine the hypothesis that alexithymic individuals may be highly sensitive to health-threatening information, which arises from the often-observed correlation between alexithymia and psychosomatic or medically unexplained symptoms (Panayiotou et al., Citation2015; Rief & Broadbent, Citation2007). Our results did not support this hypothesis, as attention processing patterns of illness-related information did not differ based on alexithymia level. A limitation that should be noted here is that illness stimuli were rated as less fearful and with lower emotionality, which may influence their comparability to the fear stimuli (see, Leonidou & Panayiotou, Citation2020).
Although offering novel findings that may help interpret previous mixed evidence, results of this study should be treated with caution due to a few limitations. First, the differences between the design of this study and previous studies make the comparison of findings difficult as previously noted (see Leonidou & Panayiotou, Citation2020; Leonidou & Panayiotou, Citation2018). This calls for further replication, and extension to other types of stimuli, or ways of presenting them, to verify the robustness and generalizability of current findings. Future research could particularly explore whether these biases are valence or intensity specific, i.e., whether they pertain only to intense negative emotions (fear), or extent to positive intense emotions as well. Second, the effects reported here are small. Although interactions were significant due to opposite directions of associations, the simple effects of alexithymia at separate time intervals did not reach significance, which may suggest limited effects of alexithymia levels on attention bias in a non-clinical sample, or that different types of stimuli, time frames (including analyzing dwell time per 500 ms intervals to track dynamic attentional changes in greater detail), or moderating conditions (e.g., presence of stress or state anxiety or social context), may be required to accentuate the biases associated with alexithymia. Although depression, anxiety and illness anxiety symptomatology were analyzed in follow-up models in this study to rule out their effect on attention bias, controlling for state-negative affect has been one of the major issues pointed out by Luminet et al. (Citation2021a) and should be assessed and analyzed in future studies for more confident conclusions. Finally, the lower internal consistency of the total TAS and DDF and EOT subscales in the present sample, although not uncommon in the literature (Bagby et al., Citation2020; Kooiman et al., Citation2002; Panayiotou, Panteli, & Vlemincx,; Tsaousis et al., Citation2010), should also be noted as a limitation, calling for replication of findings.
In spite of these limitations, this study adds significantly to our understanding of how alexithymic individuals process affective, and particularly threatening, stimuli in their environment, and yields tentative hypotheses regarding the way alexithymia is maintained and linked to increased distress and mental and physical health problems. Further replication and clarification of abnormal attention patterns, using methods like eye-tracking that permit the examination of dynamic patterns of attention over time, can point to ways to normalize the attention to, and processing of emotional stimuli, as a way to mitigate alexithymic emotional difficulties.Footnote1
Data Availability
Individual participant data used for the purpose of this study were not shared because the
authors did not have the necessary approval by the ethics committee and by participants.
Acknowledgements
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1. Currently works as a Clinical Psychologist at the Bank of Cyprus Oncology Centre.
2. There were no differences in eye tracking measures between the group of individuals who self-reported and the group who did not self-report medical diagnoses.
References
- Armstrong, T., & Olatunji, B. O. (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–16. https://doi.org/10.1016/j.cpr.2012.09.004
- Bagby, R. M., Parker, J. D. A., & Taylor, G. J. (1994). The twenty-item Toronto Alexithymia scale-I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. https://doi.org/10.1016/0022-3999(94)90005-1
- Bagby, R. M., Parker, J. D. A., & Taylor, G. J. (2020). Twenty-five years with the 20-item Toronto alexithymia scale. Journal of Psychosomatic Research, 131 (4) , 109940. https://doi.org/10.1016/j.jpsychores.2020.109940
- Buckner, J. D., Ae, J. K. M., Schmidt, N. B., Maner, J. K., & Schmidt, N. B. (2010). Difficulty disengaging attention from social threat in social anxiety. Cognitive Therapy and Research, 34(1), 99–105. https://doi.org/10.1007/s10608-008-9205-y
- Bujanow, A., Bodenschatz, C. M., Szymanska, M., Kersting, A., Vulliez-Coady, L., & Suslow, T. (2020). The relationship between dispositional attention to feelings and visual attention to emotion. Progress in Neuro-psychopharmacology & Biological Psychiatry, 100, 109882. https://doi.org/10.1016/j.pnpbp.2020.109882
- Chen, Y. P., Ehlers, A., Clark, D. M., & Mansell, W. (2002). Patients with generalized social phobia direct their attention away from faces. Behaviour Research and Therapy, 40(6), 677–687. https://doi.org/10.1016/S0005-7967(01)00086-9
- Cisler, J. M., & Koster, E. H. W. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30(2), 203–216. https://doi.org/10.1016/j.cpr.2009.11.003
- Davydov, D. M., Stewart, R., Ritchie, K., & Chaudieu, I. (2010). Resilience and mental health. Clinical Psychology Review, 30(5), 479–495. https://doi.org/10.1016/j.cpr.2010.03.003
- Delle-Vigne, D., Kornreich, C., Verbanck, P., & Campanella, S. (2014). Subclinical alexithymia modulates early audio-visual perceptive and attentional event-related potentials. Frontiers in Human Neuroscience, 8(MAR), 106. https://doi.org/10.3389/fnhum.2014.00106
- Donges, U. S., Kersting, A., & Suslow, T. (2014). Alexithymia and perception of emotional information: A review of experimental psychological findings. Universitas Psychologica, 13(2), 745–756. https://doi.org/10.11144/JAVERIANA.UPSY13-2.APEI
- Foran, H. M., & O’Leary, K. D. (2013). The role of relationships in understanding the alexithymia-depression link. European Journal of Personality, 27(5), 470–480. https://doi.org/10.1002/per.1887
- Gerritsen, C., Frischen, A., Blake, A., Smilek, D., & Eastwood, J. D. (2008). Visual search is not blind to emotion. Perception & Psychophysics, 70(6), 1047–1059. https://doi.org/10.3758/PP.70.6.1047
- Grynberg, D., Chang, B., Corneille, O., Maurage, P., Vermeulen, N., Berthoz, S., Luminet, O., & Fontenelle, L. (2012). Alexithymia and the processing of emotional facial expressions (EFEs): Systematic review, unanswered questions and further perspectives. PloS One, 7(8), e42429. https://doi.org/10.1371/JOURNAL.PONE.0042429
- Grynberg, D., Vermeulen, N., & Luminet, O. (2014). Amplification of attentional blink by distress-related facial expressions: relationships with alexithymia and affectivity. International Journal of Psychology, 49(5), 371–380. https://doi.org/10.1002/ijop.12006
- Honkalampi, K., De Berardis, D., Vellante, F., & Viinamäki, H. (2018) Relations between Alexithymia and Depressive and Anxiety Disorders and Personality Luminet, O., Bagby, M., Taylor, G. eds.). . Alexithymia: Advances in Research, Theory, and Clinical Practice (Cambridge: Cambridge University Press), 142–157. https://doi.org/10.1017/9781108241595.011
- Kahneman, D., & Triesman, A. (1984). Changing views of attention and automaticity. In R. Parasuraman & D. A. Davis (Eds.), Varieties of attention (pp. 28–61). Academic Press.
- Kauhanen, J., Kaplan, G. A., Cohen, R. D., Julkunen, J., & Salonen, J. T. (1996). Alexithymia and risk of death in middle-aged men. Journal of Psychosomatic Research, 41(6), 541–549. https://doi.org/10.1016/S0022-3999(96)00226-7
- Kellner, R. (1987). Abridged manual of the illness attitude scales. University of New Mexico, Department of Psychiatry, School of Medicine.
- Kooiman, C. G., Spinhoven, P., & Trijsburg, R. W. (2002). The assessment of alexithymia: A critical review of the literature and a psychometric study of the Toronto alexithymia scale-20. Journal of Psychosomatic Research, 53(6), 1083–1090. https://doi.org/10.1016/S0022-3999(02)00348-3
- Koster, E. H. W., Crombez, G., Van Damme, S., Verschuere, B., & De Houwer, J. (2004). Does imminent threat capture and hold attention? Emotion, 4(3), 312–317. https://doi.org/10.1037/1528-3542.4.3.312
- Kraus, C., Hahn, A., Pfabigan, D. M., Küblböck, M., Seidel, E. M., Sladky, R., Kasper, S., Windischberger, C., Lamm, C., & Lanzenbeger, R. (2014). Gray matter changes associated with factors of alexithymia in young adults. European Neuropsychopharmacology, Supplement, 2(24), S311–S312. https://doi.org/10.1016/S0924-977X(14)70494-8
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): technical manual and affective ratings. In NIMH Center for the Study of Emotion and Attention, Springer (pp. 39–58). https://www2.unifesp.br/dpsicobio/adap/instructions.pdf
- LeDoux, J. E. (1995). Emotion: Clues from the brain. Annual Review of Psychology, 46(1), 209–235. https://doi.org/10.1146/annurev.ps.46.020195.001233
- Lees, A., Mogg, K., & Bradley, B. P. (2005). Health anxiety, anxiety sensitivity, and attentional biases for pictorial and linguistic health-threat cues. Cognition & Emotion, 19(3), 453–462. https://doi.org/10.1080/02699930441000184
- Leonidou, C., & Panayiotou, G. (2017). Assessment of health anxiety: translation, validation and development of scales in the Greek language. Poster presentation at the 16th Conference of the hellenic psychological society, Thessaloniki, Greece.
- Leonidou, C., Panayiotou, G., & . (2018). How do illness-anxious individuals process health-threatening information? A systematic review of evidence for the cognitive-behavioral model. Journal of Psychosomatic Research, 111(June), 100–115. https://doi.org/10.1016/j.jpsychores.2018.06.001
- Leonidou, C., & Panayiotou, G. (2020). Attentional processing of information related to illness: Biases and associations with emotional response in young adults with different levels of illness anxiety. Journal of Health Psychology, 27(3), 726–742. https://doi.org/10.1177/1359105320967435
- Liossi, C., Schoth, D. E., Godwin, H. J., & Liversedge, S. P. (2014). Using eye movements to investigate selective attention in chronic daily headache. Pain, 155(3), 503–510. https://doi.org/10.1016/j.pain.2013.11.014
- Luminet, O., Bagby, M. R., & Taylor, G. J. (2018). Alexithymia: advances in research, theory, and clinical practice - Google Books. Cambridge University Press. https://books.google.com.cy/books?hl=en&lr=&id=QDdqDwAAQBAJ&oi=fnd&pg=PR9&ots=aHFbbut35R&sig=q3Pk1RlJnRLJRQvEO3UmHoHwAtw&redir_esc=y#v=onepage&q&f=false
- Luminet, O., Nielsen, K. A., & Ridout, N. (2021a). Cognitive-emotional processing in alexithymia: An integrative review. Cognition & Emotion, 35(3), 449–487. DOI:https://doi.org/10.1080/02699931.2021.1908231
- Luminet, O., Nielson, K. A., & Ridout, N. (2021b). Having no words for feelings: Alexithymia as a fundamental personality dimension at the interface of cognition and emotion. Cognition & Emotion, 35(3), 435–448. https://doi.org/10.1080/02699931.2021.1916442
- Lundh, L. G., & Simonsson-Sarnecki, M. (2002). Alexithymia and cognitive bias for emotional information. Personality and Individual Differences, 32(6), 1063–1075. https://doi.org/10.1016/S0191-8869(01)00110-6
- Manninen, M., Therman, S., Suvisaari, J., Ebeling, H., Moilanen, I., Huttunen, M., & Joukamaa, M. (2011). Alexithymia is common among adolescents with severe disruptive behavior. Journal of Nervous and Mental Disease, 199(7), 506–509. https://doi.org/10.1097/NMD.0b013e3182214281
- McEwen, B. M. (1998). Stress, adaptation, and disease allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
- Mériau, K., Wartenburger, I., Kazzer, P., Prehn, K., Lammers, C. H., van der Meer, E., Villringer, A., & Heekeren, H. R. (2006). A neural network reflecting individual differences in cognitive processing of emotions during perceptual decision making. NeuroImage, 33(3), 1016–1027. https://doi.org/10.1016/j.neuroimage.2006.07.031
- Miles, W. R. (1930). Ocular dominance in human adults. Journal of General Psychology, 3(3), 412–430. https://doi.org/10.1080/00221309.1930.9918218
- Mueller, J., Alpers, G. W., & Reim, N. (2006). Dissociation of rated emotional valence and Stroop interference in observer-rated alexithymia. Journal of Psychosomatic Research, 61(2), 261–269. https://doi.org/10.1016/j.jpsychores.2006.02.017
- Nummenmaa, L., Hyönä, J., & Calvo, M. G. (2006). Eye movement assessment of selective attentional capture by emotional pictures. Emotion, 6(2), 257–268. https://doi.org/10.1037/1528-3542.6.2.257
- Panayiotou, G. (2018). Alexithymia as a core trait in psychosomatic and other psychological disorders Charis, C., Panayiotou, D. eds . In Somatoform and other psychosomatic disorders (pp. 89–106). Springer.
- Panayiotou, G., Leonidou, C., Constantinou, E., Hart, J., Rinehart, K. L., Sy, J. T., & Bjorgvinsson, T. (2015). Do alexithymic individuals avoid their feelings? Experiential avoidance mediates the association between alexithymia, psychosomatic, and depressive symptoms in a community and a clinical sample. Comprehensive Psychiatry, 56, 206–216. https://doi.org/10.1016/j.comppsych.2014.09.006
- Panayiotou, G., Leonidou, C., Constantinou, E., & Michaelides, M. P. (2020). Self-Awareness in alexithymia and associations with social anxiety. Current Psychology, 39(5), 1600–1609. https://doi.org/10.1007/s12144-018-9855-1
- Panayiotou, G., Panteli, M., & Vlemincx, E. (2018). Processing emotions in alexithymia: A systematic review of physiological markers Luminet, O., Bagby, R. M., Taylor, G. J. eds . In Alexithymia: Advances in research, theory, and clinical practice (pp. 291–320). Cambridge University Press.https://doi.org/10.1017/9781108241595.018
- Panayiotou, G., Panteli, M., & Vlemincx, E. (2021). Adaptive and maladaptive emotion processing and regulation, and the case of alexithymia. Cognition & Emotion, 35(3), 488–499. https://doi.org/10.1080/02699931.2019.1671322
- Parker, J. D. A., Taylor, G. J., & Bagby, R. M. (2003). The 20-item Toronto alexithymia scale: III. reliability and factorial validity in a community population. Journal of Psychosomatic Research, 55(3), 269–275. https://doi.org/10.1016/S0022-3999(02)00578-0
- Patel, S. H., & Azzam, P. N. (2005). Characterization of N200 and P300: selected studies of the event-related potential. International Journal of Medical Sciences, 2(4), 147. https://doi.org/10.7150/ijms.2.147
- Picardi, A., Fagnani, C., Gigantesco, A., Toccaceli, V., Lega, I., & Stazi, M. A. (2011). Genetic influences on alexithymia and their relationship with depressive symptoms. Journal of Psychosomatic Research, 71(4), 256–263. https://doi.org/10.1016/j.jpsychores.2011.02.016
- Pollatos, O., & Gramann, K. (2012). Attenuated modulation of brain activity accompanies emotion regulation deficits in alexithymia. Psychophysiology, 49(5), 651–658. https://doi.org/10.1111/j.1469-8986.2011.01348.x
- Priebe, J. A., Messingschlager, M., & Lautenbacher, S. (2015). Gaze behavior when monitoring pain faces: an eye-tracking study. European Journal of Pain, 19(6), 817–825. https://doi.org/10.1002/ejp.608
- Rief, W., & Broadbent, E. (2007). Explaining medically unexplained symptoms: models and mechanisms. Clinical Psychology Review, 27(7), 821–841. https://doi.org/10.1016/j.cpr.2007.07.005
- Suwazono, S., MacHado, L., & Knight, R. T. (2000). Predictive value of novel stimuli modifies visual event-related potentials and behavior. Clinical Neurophysiology, 111(1), 29–39. https://doi.org/10.1016/S1388-2457(99)00186-8
- Taylor, G. J. (2000). Recent developments in alexithymia theory and research. The Canadian Journal of Psychiatry, 45(2), 134–142. https://doi.org/10.1177/070674370004500203
- Taylor, G. J., & Bagby, R. M. (2004). New trends in alexithymia research. Psychotherapy and Psychosomatics, 73(2), 68–77. https://doi.org/10.1159/000075537
- Theodorou, C., Ioannou, M., Karekla, M., Panayiotou, G., & Karekla, M. (2013). Examining the factors of the psychiatric diagnostic screening questionnaire (PDSQ). 3rd Annual Scientific Conference of the Center of Applied Neurosciences Nicosia, Cyprus.
- Tsaousis, I., Taylor, G., Quilty, L., Georgiades, S., Stavrogiannopoulos, M., & Bagby, R. M. (2010). Validation of a Greek adaptation of the 20-item Toronto Alexithymia Scale. Comprehensive Psychiatry, 51(4), 443–448. https://doi.org/10.1016/j.comppsych.2009.09.005
- Van der Velde, J., Servaas, M. N., Goerlich, K. S., Bruggeman, R., Horton, P., Costafreda, S. G., & Aleman, A. (2013). Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neuroscience and Biobehavioral Reviews, 37(8), 1774–1785. https://doi.org/10.1016/j.neubiorev.2013.07.008
- Vanheule, S., Inslegers, R., Meganck, R., Ooms, E., & Desmet, M. (2010 Interpersonal problems in alexithymia: A review Dimaggio, G., Lysaker, P.H. eds). . Metacognition in Severe Adult Disorders: From Research to Treatment, 161–176. http://hdl.handle.net/1854/LU-949147
- Vermeulen, N., Luminet, O., de Sousa, M. C., & Campanella, S. (2008). Categorical perception of anger is disrupted in alexithymia: Evidence from a visual ERP study Cognition and Emotion . 22(6), 1052–1067. https://doi.org/10.1080/02699930701597635
- Wiebe, A., Kersting, A., & Suslow, T. (2017). Deployment of attention to emotional pictures varies as a function of externally-oriented thinking: an eye tracking investigation. Journal of Behavior Therapy and Experimental Psychiatry, 55, 1–5. http://dx.doi.org/10.1016/j.jbtep.2016.11.001
- Wieser, M. J., Pauli, P., Weyers, P., Alpers, G. W., & Mühlberger, A. (2009). Fear of negative evaluation and the hypervigilance-avoidance hypothesis: An eye-tracking study. Journal of Neural Transmission, 116(6), 717–723. https://doi.org/10.1007/s00702-008-0101-0
- Zimmerman, M., & Mattia, J. I. (2001). The psychiatric diagnostic screening questionnaire: development, reliability and validity. Comprehensive Psychiatry, 42(3), 175–189. https://doi.org/10.1053/comp.2001.23126
- Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). Mixed Effects Modelling for Nested Data. In Mixed effects models and extensions in ecology with R (pp. 101–142). Springer. https://doi.org/10.1007/978-0-387-87458-6_5