Abstract
Cnr2 is one of two cannabinoid receptors known to regulate bone metabolism. Here, we compared the whole bone properties of femora and tibiae from three-month-old Cnr2−/- mice with wild-type controls using a C57BL/6 background. Bending stiffness was measured by three-point bending. The elastic modulus, density and mineral content were measured using ultrasound, Archimedes’ principle and ashing. Micro-CT was used to measure the second moment of area, inner and outer perimeters of the cortical shaft and trabecular parameters. Deleting Cnr2 increased the bending stiffness by increasing the second moment of area. Bone from affected male mice had a greater modulus than controls, although no difference was observed in females. The fractional volume of trabecular bone was greater in Cnr2−/- females than controls, while no difference was seen in males. These data indicate that inactivating Cnr2 increases the amount of cortical bone in both males and females at 3 months of age, but the effect on trabecular bone is different in the two sexes. These findings extend previous studies looking only at trabecular bone and provide further support for the possible use of Cnr2 antagonists for improving bone properties that may be of value in the treatment of bone disorders.
Public Interest Statement
The cannabinoid system has recently been shown to be involved in maintaining bone quality and two possible routes are available. The second route has been proposed as a potential target for treating bone diseases. There are two types of bone; dense, cortical bone that makes up the shaft of our long bones; and spongy (trabecular) bone, mostly found at the ends of long bones. Most studies look exclusively at trabecular bone but not measuring both could lead to problems, if the effects of treatment are different in both types. The results of this study show that deleting the second route for cannabinoid action improves the mechanical properties of both cortical bone in male and female mice but trabecular bone only in females. Because females more commonly suffer from osteoporosis, these results are encouraging that future treatments may help maintain cortical as well as trabecular bone quality.
1. Introduction
Recent studies have indicated a role for the cannabinoid receptors, Cnr1 and Cnr2, in bone metabolism (Bab, Citation2007; Idris & Ralston, Citation2010; Idris et al., Citation2009; Ofek et al., Citation2006) and shown that they play distinct roles in regulating bone mass and bone turnover. The cells that produce and maintain bone, osteoblasts, osteoclasts and osteocytes all express the Cnr2 receptor (Ofek et al., Citation2006), yet measurements of the bone phenotype are contradictory. Ofek et al. (Citation2006) reported that targeted inactivation of the Cnr2 receptor (Cnr2−/-) led to a reduction in the peak trabecular bone mass in both males and females, and resulted in trabecular osteoporosis and cortical expansion in 12 month-old mice. This, it was suggested, was due to an increase in osteoclast number and a high bone turnover. A study by Idris, Sophocleous, Landao-Bassonga, van’t Hof, and Ralston (Citation2008), however, found no difference in peak bone mass between Cnr2−/- mice and wild-type (WT) controls but showed that bone loss following ovariectomy (OVX) was reduced in Cnr2−/- mice, suggesting that inactivating Cnr2 mitigates the severe osteoporosis induced by OVX. They agreed, though, that this seemed to be due to a modulation of osteoclastic activity. In a later study, they again reported that bone mass and bone turnover were unchanged by inactivating Cnr2 in young (three-month-old) mice although they found an increased activity of bone-forming osteoblasts, and an increase in osteoclast number (Sophocleous, Landao-Bassonga, van’t Hof, Idris, & Ralston, Citation2011). By 12 months of age, however, the mice had developed osteoporosis due to high bone turnover and uncoupling of bone formation from resorption (Sophocleous et al., Citation2011).
These studies have resulted in suggestions that Cnr2 agonists might have considerable value as anabolic agents for treating osteoporosis (Ofek et al., Citation2006; Sophocleous et al., Citation2011), while an inverse agonist might protect against bone loss (Sophocleous et al., Citation2011). In this context, as animal models are crucial to our understanding of the mechanisms of action, it is important that we understand fully the phenotype of both cortical and trabecular bone in Cnr2−/- mice and whether there are differences related to the sex of the animals. To explore this further, we obtained Cnr2−/- mice on a C57BL/6 background and measured the mechanical and material properties of both cortical and trabecular bone in both males and females.
2. Materials and methods
2.1. Animals
A colony of C57BL/6 Cnr2−/- mice (KO) was generated for an unrelated study by homologous recombination, as described previously (Buckley et al., Citation2000) and kept under standard conditions in accordance with UK Home Office regulations. Mice were euthanised at the age of 12 weeks and 10 KO mice (five male, five female) were made available for this study. Age-matched WTs of the same strain were used as controls (four male, five female). The hind limbs were removed, and the femur and tibia separated and cleaned. The bones were wrapped in gauze soaked in phosphate-buffered saline, sealed in bags and stored at −20°C until measurements were made.
2.2. Mechanical and material properties
The properties of long bones were measured as described previously (Goodyear & Aspden, Citation2012; Huesa et al., Citation2011). Briefly, the mechanical properties of cortical bone were measured by subjecting each tibia and femur to three-point bending using an Instron 5564 materials testing machine (Instron, High Wycombe). The cross-head speed was 1.0 mm min-1 and the span was 9.93 mm. Bending stiffness, load to failure and strength were calculated from the resulting load-displacement graphs. The elastic modulus of the bone matrix was calculated from the product of the square of the speed of sound, measured ultrasonically, and the bone density, measured by Archimedes’ principle. The mineral content of the bone matrix was expressed as a fraction of the dry weight, and determined by ashing the dried bones at 600°C for 24 h (Mkukuma et al., Citation2003).
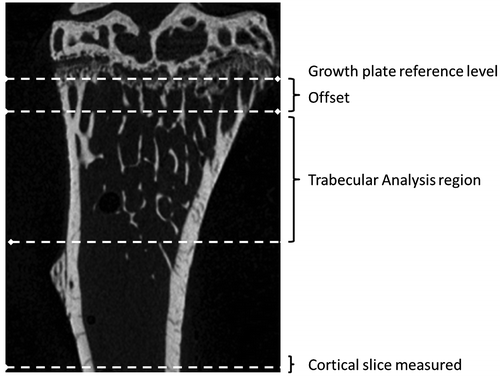
The proximal tibia and distal femur from each animal were imaged using a Skyscan 1072 X-ray Microtomograph (Skyscan, Aartselaar, Belgium) set at 50 kV/197 μA and using a 0.5 mm Al filter. Images were obtained at 5 μm pixel size (58x magnification) with a rotation step of 0.67° between each image. The 3D image stack was reconstructed using NRecon version 1.4.4 (Skyscan), and the trabecular and cortical parameters were measured using methods recommended by Skyscan (Salmon, Citation2009). First, a reference level was found at the level of the growth plate by scrolling down from the top of the data-set until the spots formed where the growth plate crossed the slice. Four spots were seen in femora and two in tibiae. The trabecular region was then defined as 200 slices offset by 50 slices from that reference level as recommended by Skyscan (Figure ), measured using CTan version 1.7.0.2 software (Skyscan). The segmentation process separating mineralised and non-mineralised structure was performed by drawing a region of interest (ROI) on one slice to include trabecular bone and exclude cortical bone. Around 7–8 ROI were drawn at regular intervals throughout the stack. The images were then scrolled down to ensure that ROI in the remaining slices, produced by interpolation, did not include cortical and exclude trabecular region while performing trabecular bone analysis. Images were thresholded to separate bone (pixels with higher grey level) and non-bone (pixels with a lower grey level) by adjusting the grey level until most of the image noise (speckle) was removed. The threshold levels finally used in all images were 74–255. Average volumetric bone mineral density (BMDv) values were calculated from primary micro-CT measurements for trabecular and cortical bone using calibration phantoms.
Figure 1. A section through a tibia showing the reference level (growth plate). Trabecular parameters were measured in region offset from the reference level by 50 slices. Cortical geometry was taken from the last complete slice above the fracture site in three-point bending (identified in 3D reconstructions). Femora were processed in a similar way.
2.3. Statistics
Data are presented as mean (standard deviation). Comparisons between KO and WT mice were made using SigmaPlot (version 11.0 Systat Software Inc., Hounslow). All data were checked for normality and equal variance and when these tests were passed pair-wise comparisons were made using Student’s t-test. Otherwise, a Mann–Whitney test was used. Significance was taken to be p < 0.05.
3. Results
3.1. Cortical parameters
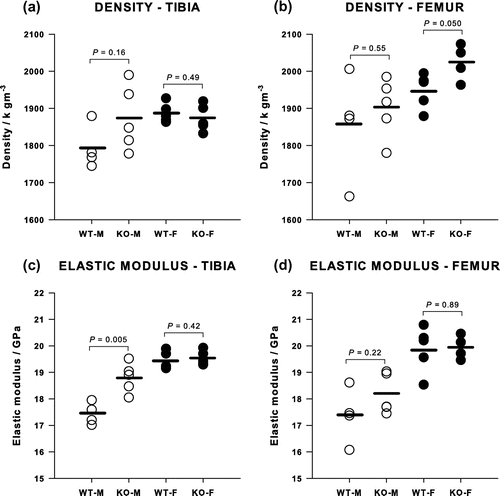
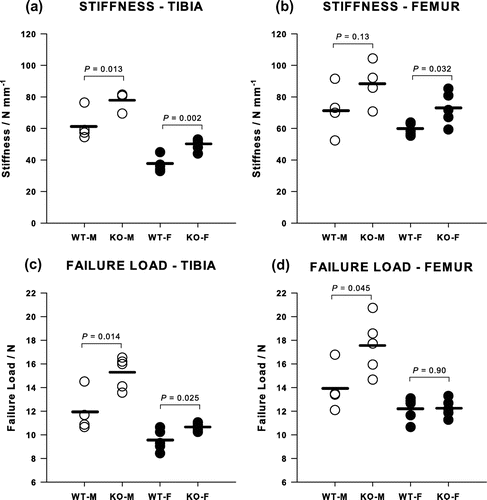
Deletion of the Cnr2 receptor increased the mechanical and material properties of cortical bone in both males and females, and the corresponding change in appearance can be seen in micro-CT images (Figure ). The bending stiffness of the tibia was increased by 27% in males (p = 0.013) and 34% in females (p = 0.002) (Figure (a)) and the failure load by 28% in males (p = 0.014) and 13% in females (p = 0.025) (Figure (c)). Equivalent figures for the femur showed significant increases of 22% for the bending stiffness in females (p = 0.032), although the same increase in stiffness for males failed to reach significance, and a 26% increase in the strength in males but no difference in females (Figure (b) and (d)).
Figure 2. 2D representative micro-CT images of trabecular bone from a tibia of (a) 12-week-old WT male mouse, (b) WT female, (c) Cnr2-/- male and (d) Cnr2-/- female.
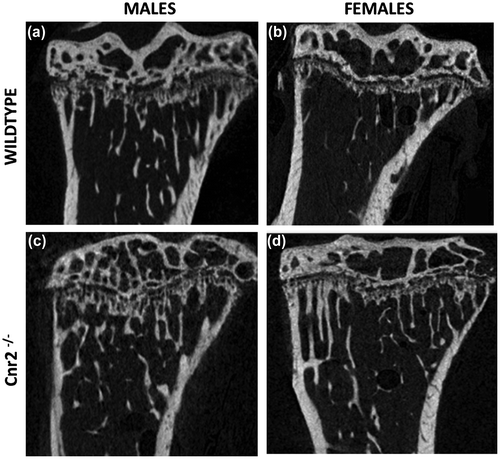
Figure 3. Mechanical properties of tibiae from Cnr2-/- mice measured by three-point bending compared with WT animals. The bending stiffnesses of (a) tibiae and (b) femora and the failure loads of (c) tibiae and (d) femora were generally greater in female KO animals although this was not always significant.
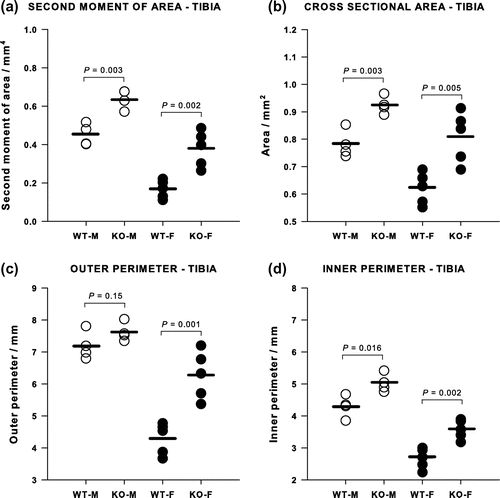
Micro-CT showed that KO mice had a greater axial second moment of area and cortical cross-sectional area than WT controls (Figure ). In females, the second moment of area in the KOs was more than double that in the WTs and this large difference arose from a significant increase in both the outer and inner perimeters (Figure (a)). In contrast, the axial second moment of area was only about 50% larger in the male KO than the WT and there was no difference in the outer perimeter, while the inner perimeter increased significantly (Figure (c) and (d)). No differences were found in the cortical thicknesses measured in the tibiae. A similar pattern was found in the femur, with an increase in the second moment of area in both males and females, but in this bone both the inner and outer perimeters increased and the cortices were significantly thinner, being reduced by 33% in the males (p = 0.024) and by 58% in the females (p = 0.003).
Figure 4. Cortical bone geometrical properties of tibiae measured by micro-CT. (a) Second moment of area and (b) cross-sectional area were significantly greater in both female and male Cnr2-/- mice tibiae than in the WT controls. The outer perimeter (c) was larger only in female knockouts, whereas the inner perimeter (d) was larger in both female and male knockouts.
Values for the modulus and density of cortical bone are shown in Figure . Deleting Cnr2 had no effect on the modulus of the cortical bone matrix in females, the average being 19.68 (0.54) GPa in both tibia and femur of KO and WT. In males, however, the modulus was significantly greater following deletion of Cnr2 by 8% in the tibia, from 17.44 (0.42) to 18.80 (0.56) GPa (p = 0.005) and by 4.5% in the femur, although this failed to reach significance. Archimedean densities of the cortical bone tended to be slightly higher in KO than WT although this only reached significance in the femur in females.
3.2. Trabecular parameters
Micro-CT measurements of BV/TV showed that deleting Cnr2 increased the amount of trabecular bone (Table ), by 42% in females, which was highly significant. The increase in male BV/TV was only 16% and this was not statistically significant. Values for BV/TV in females, however, were much smaller than in the males. The increase in BV/TV in the female mice corresponded to significant increases in the number of trabeculae, Tb·N, and their thickness, Tb·Th, and a decrease in their spacing, Tb·Sp. No significant differences were found in the thickness or separation in the males although the increase in Tb·N, came close (p = 0.07).
Table 1. Micro-CT measures of trabecular bone from the tibiae of male and female Cnr2-/- and WT mice
4. Discussion
In this study of cortical and trabecular bone from male and female C57BL/6 mice, from both tibia and femur, we have shown that deleting the Cnr2 cannabinoid receptor increases the resistance to bending in female mice in both tibia and femur although the strength was only increased in the tibia. In males, the changes in stiffness were similar but smaller, while the increase in strength was found in both bones. Only the female animals showed increases in the amount and organisation of trabecular bone. Previous studies have concentrated largely on trabecular bone from one location, the proximal tibia, and sometimes only in females, whereas with the mice made available to us here we were aiming to obtain a more comprehensive assessment of the effects of Cnr2 inactivation on the skeleton.
In the diaphysis, stiffness and strength were increased by enlarging the geometrical properties while leaving the material properties unchanged. For a beam in bending, the stiffness is proportional to the product of the modulus and the second moment of area; and in this study, we found that it was the second moment of area that was increased. Different contributions from inner and outer diaphyseal surfaces were found in males and females with much more pronounced effects on both inner and outer perimeter in females but greater effects on the inner, enodosteal, surface in males. This would reflect increased osteoclast function in both sexes, resorbing the endosteal surface as reported by Ofek et al. (Citation2006) and Sophocleous et al. (Citation2011), but only significantly increased osteoblastic activity forms new bone on the periosteal surface in females. Addition of material to the outside of a tubular structure, such as the diaphysis, has a greater effect than removing internal material due to the dependence of the second moment of area on the fourth power of the radius.
Previous studies were agreed about enhanced osteoporosis in elderly Cnr2−/- mice but disagreed about whether peak bone mass was unaffected at 3 months (Idris et al., Citation2008; Sophocleous et al., Citation2011) or reduced (Ofek et al., Citation2006). Sophocleous, Idris, and Ralston (Citation2014) have also recently reported different responses depending on the strain of mouse used, with deletion of Cnr2 in mice from a CD1 strain resulting in an increase in BV/TV. In this study, using C57BL/6 mice, we found that inactivating Cnr2 also led to an increased amount of trabecular bone (BV/TV) at three months. This was only significant in females although males showed a similar trend and larger numbers might have made this difference significant. Unfortunately, these were the only mice available to us, but they mark a stage at which bone growth is at its fastest. After 12 weeks-of-age, the rate of growth in C57BL/6 mice is starting to slow and skeletal maturity is reached at about 4 months (Somerville, Aspden, Armour, Armour, & Reid, Citation2004). Ideally, larger numbers at more time points are required to cover growth and maturity but could not be obtained for this study. Differences reported between studies could arise from a variety of sources, even within one mouse strain, including competing stimuli (Armour, van’t Hof, Grabowski, Reid, & Ralston, Citation1999), and even husbandry (Khalid, Citation2013; Reliene & Schiestl, Citation2006). The small numbers of animals are a limitation of this study but the data were normally distributed and we found many significant differences. Low power may mean that some subtle differences may have been missed but does not affect those that are significant. It is not unusual for femur and tibia to yield slightly different results (Ferguson, Ayers, Bateman, & Simske, Citation2003; Schriefer et al., Citation2005), and this is the reason why we wanted to measure both bones, given the small numbers of animals, in order not to miss anything of importance.
In conclusion, we have shown that preventing expression of the cannabinoid receptor Cnr2 in C57BL/6 mice up to 3 months of age enhanced bone formation in females, resulting in increased bending stiffness and bone volume in these animals. Results in males were less pronounced but still anabolic. This offers some support for suggestions that targeting CNR2 in humans might be therapeutically interesting as a treatment for osteopenic disorders (Sophocleous et al., Citation2011), especially if the effects are more pronounced in females as osteoporosis, for instance, is far more common in females. CNR2 is found in all three types of bone cell in greater amounts than the CNR1 receptor (Idris et al., Citation2005) and, unlike CNR1, it is not found in the brain, so targeting CNR2 should minimise psychotropic side-effects. In addition, activating CNR2 is reported to be a growth stimulator for haematopoietic cells leading to macrophages, B- and T-lymphocytes (Valk et al., Citation1997), to have pain-relieving effects (Anand et al., Citation2008), while inverse agonists of CNR2 inhibited IL-6-induced immunoglobulin (Feng, Milcarek, & Xie, Citation2014). Such a range of potentially beneficial effects of CNR2 is supported in this study.
Acknowledgements
We thank Dr J.S. Gregory for assistance with Image J and Mr K. Mackenzie for assistance with Micro-CT analysis.
Additional information
Funding
Notes on contributors
Richard M. Aspden
This study arose from a collaboration between Richard M. Aspden, who is interested in how our bones and joints are maintained, and Ruth A. Ross, whose speciality is the cannabinoid signalling systems our bodies use to regulate many processes. While neuropharmacological effects have dominated cannabinoid research, there is increasing interest in their ability to regulate other systems such as the skeleton and this may provide new therapeutic targets for common disorders such as osteoarthritis and osteoporosis. Combining our expertise in biomechanics and pharmacology provides a novel approach to understanding the complexity of how our musculoskeletal system functions and, sometimes, fails.
References
- Anand, U., Otto, W. R., Sanchez-Herrera, D., Facer, P., Yiangou, Y., Korchev, Y., … Anand, P. (2008). Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain, 138, 667–680. doi:10.1016/j.pain.2008.06.007
- Armour, K. E., van’t Hof, R. J., Grabowski, P. S., Reid, D. M., & Ralston, S. H. (1999). Evidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosis. Journal of Bone and Mineral Research, 14, 2137–2142. doi:10.1359/jbmr.1999.14.12.2137
- Bab, I. A. (2007). Regulation of skeletal remodeling by the endocannabinoid system. Annals of the New York Academy of Sciences, 1116, 414–422. doi:10.1196/annals.1402.014
- Buckley, N. E., McCoy, K. L., Mezey, É., Bonner, T., Zimmer, A., Felder, C.C., … Zimmer, A. (2000). Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. European Journal of Pharmacology, 396, 141–149. doi:10.1016/S0014-2999(00)00211-9
- Feng, R., Milcarek, C., & Xie, X.-Q. (2014). Antagonism of cannabinoid receptor 2 pathway suppresses IL-6-induced immunoglobulin IgM secretion. BMC Pharmacology and Toxicology, 15, 30. doi:10.1186/2050-6511-15-30
- Ferguson, V. L., Ayers, R. A., Bateman, T. A., & Simske, S. J. (2003). Bone development and age-related bone loss in male C57BL/6J mice. Bone, 33, 387–398. doi:10.1016/S8756-3282(03)00199-6
- Goodyear, S. R., & Aspden, R. M. (2012). Mechanical properties of bone ex vivo. Methods in Molecular Biology, 816, 555–571. doi:10.1007/978-1-61779-415-5_35
- Huesa, C., Yadav, M. C., Finnilä, M. A., Goodyear, S. R., Robins, S. P., Tanner, K. E., ... Farquharson, C. (2011). PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone, 48, 1066–1074. doi:10.1016/j.bone.2011.01.010
- Idris, A. I., & Ralston, S. H. (2010). Cannabinoids and bone: Friend or foe? Calcified Tissue International, 87, 285–297. doi:10.1007/s00223-010-9378-8
- Idris, A. I., Sophocleous, A., Landao-Bassonga, E., Canals, M., Milligan, G., Baker, D., … Ralston, S. H. (2009). Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metabolism, 10, 139–147. doi:10.1016/j.cmet.2009.07.006
- Idris, A. I., Sophocleous, A., Landao-Bassonga, E., van’t Hof, R. J., & Ralston, S. H. (2008). Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology, 149, 5619–5626.10.1210/en.2008-0150
- Idris, A. I., van ‘t Hof, R. J., Greig, I. R., Ridge, S. A., Baker, D., Ross, R. A., & Ralston, S. H. (2005). Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nature Medicine, 11, 774–779. doi:10.1038/nm1255
- Khalid, A. B. (2013). Regulation of bone by cannabinoid and cannabinoid-like receptors ( PhD thesis). University of Aberdeen, Aberdeen, SD.
- Mkukuma, L. D., Skakle, J. M. S., Gibson, I. R., Imrie, C. T., Aspden, R. M., & Hukins, D. W. L. (2003). Effect of the proportion of organic material in bone on thermal decomposition of bone mineral: An investigation of a variety of bones from different species using thermogravimetric analysis coupled to mass spectrometry, high temperature X-ray diffraction and Fourier transform infra-red spectroscopy. Calcified Tissue International, 75, 321–328. doi:10.1007/s00223-004-0199-5
- Ofek, O., Karsak, M., Leclerc, N., Fogel, M., Frenkel, B., Wright, K., … Bab, I. (2006). Peripheral cannabinoid receptor, CB2, regulates bone mass. Proceedings of the National Academy of Sciences, 103, 696–701. doi:10.1073/pnas.050418710
- Reliene, R., & Schiestl, R. H. (2006). Differences in animal housing facilities and diet may affect study outcomes—A plea for inclusion of such information in publications. DNA Repair, 5, 651–653. doi:10.1016/j.dnarep.2006.02.001
- Salmon, P. L. (2009). Morphometric parameters measured by Skyscan CT-analyser software. Kontich: Bruker MicroCT.
- Schriefer, J. L., Robling, A. G., Warden, S. J., Fournier, A. J., Mason, J. J., & Turner, C. H. (2005). A comparison of mechanical properties derived from multiple skeletal sites in mice. Journal of Biomechanics, 38, 467–475. doi:10.1016/j.jbiomech.2004.04.020
- Somerville, J. N., Aspden, R. M., Armour, K. E., Armour, K. J., & Reid, D. M. (2004). Growth of C57Bl/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcified Tissue International, 74, 469–475. doi:10.1007/s00223-003-0101-x
- Sophocleous, A., Idris, A. I., & Ralston, S. H. (2014). Genetic background modifies the effects of type 2 cannabinoid receptor deficiency on bone mass and bone turnover. Calcified Tissue International, 94, 259–268. doi:10.1007/s00223-013-9793-8
- Sophocleous, A., Landao-Bassonga, E., van’t Hof, R. J., Idris, A. I., & Ralston, S. H. (2011). The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology, 152, 2141–2149. doi:10.1210/en.2010-093
- Valk, P., Verbakel, S., Vankan, Y., Hol, S., Mancham, S., Ploemacher, R., … Delwel, R. (1997). Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood, 90, 1448–1457.