?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Thermoablation of cancerous cells using probe-based strategies has received a lot of attention because it provides a localised minimally invasive strategy that minimizes injuries to collateral tissues. Although radiofrequency ablation is the most widely used probe-based method, skin injuries at the site of the grounding pad are a major complication that limits its efficacy. This paper presents a novel plasmonic photo-thermal probe that has the potential to achieve reasonable lesion sizes without skin burns. First, the concept and its embodiment are presented. Then, its performance is investigated using a 3-D finite-element method (FEM) model. The FEM model was tested for its validity using an analytical model. Lesions are shown to have an ellipsoidal shape and their sizes controlled by the length of the active tip of the probe. The comparison with the analytical model showed reasonably good agreement to within 2%. Our predictions demonstrate the feasibility of our novel probe to achieve reasonable lesion sizes as well as show that generated heat is localised.
Public Interest Statement
The destruction of cancerous cells using heat delivered by needle-like devices has received a lot of attention due to its potential to localize treatment. Radiofrequency ablation is the most widely used technique, however, skin injury is a major issue that limits its efficacy. This work proposes and evaluates the feasibility of a novel needle-like device that can potentially eliminate these injuries and enhance the efficacy of treatment. The device is essentially a two-part cannula consisting of a distal active nanocomposite (gold nanoparticles (AuNPs) and polymer) tip and a proximal insulated shaft. Our predictions demonstrate the feasibility of our device to kill cancerous cells using heat converted from absorbed near-infrared (NIR) light by AuNPs in the tip. In the NIR region, light absorption by biological materials is minimal; therefore, skin injuries can be prevented. Furthermore, our device offers the possibility of combining hyperthermia and chemotherapy in one device to enhance treatment.
1. Introduction
The most frequently occurring cancer in women is breast cancer. It accounted for 6.8% of all cancer deaths among females and 14.6% of all cancer cases in the year 2012 (Torre et al., Citation2015). However, recent advances in diagnostic techniques have increased the detection of small breast carcinomas due to the increase in implementation of screening programmes (Jochelson, Citation2012). For such small breast cancers, mastectomy is an aggressive form of treatment. Therefore, treatment methods that can enhance the use of lumpectomy by eliminating residual cells or direct destruction of cancer cells are needed.
Externally controlled localized thermoablation techniques such as modern probe-based applicators hold significant promise for cancer therapy due to their potential to reduce injuries to surrounding healthy tissue that are associated with conventional techniques. Ablative techniques that have been reported in the literature include cryoablation (Chen, Lin, Xie, Zhang, & Li, Citation2015), laser ablation (Leuthardt, Citation2016), microwave ablation (Correa-Gallego et al., Citation2014), high focused ultrasound (Jolesz & Hynynen, Citation2002) and radiofrequency ablation (RFA) (de Baére et al., Citation2015), which is the most widely used technique due to its general availability and recent technical advances. However, issues such as the need for high-current RFA to increase heat generation leads to an increased risk of skin burns which in turn limits lesion sizes (Huffman, Huffman, Lewandowski, & Brown, Citation2011; Ibrahim, Finta, & Rind, Citation2016; Nguyen, Hattery, & Khatri, Citation2014). The incidence of skin burns after RFA ranges from 0.1–3.2% for second-/third degree skin burns and up to 33% for first-degree burns (Steinke, Gananadha, King, Zhao, & Morris, Citation2013).
In recent years nanomedicine, which involves the combination of nanotechnology-based techniques and conventional heat sources, has received a lot of attention (Krishnan, Daigaradjane, & Cho, Citation2010). Specifically, targeted laser heating of accumulated gold nanoparticles (AuNPs), so called plasmonic photothermal therapy (PPT), is one of such promising nanomedicine techniques (Krishnan et al., Citation2010). Different types of AuNPs, including gold nanospheres (El-Sayed, Huang, & El-Sayed, Citation2006), gold nanorods (Huang, Neretina, & El-Sayed, Citation2009), gold/silica nanoshells (Bardhan, Lal, & Joshi, Citation2011), and gold-silver nanocages (Chen et al., Citation2010) have been reported in the literature as suitable candidates for PPT. These nanoparticles have shown encouraging results due to properties such as low toxicity (Pan et al., Citation2007), ease of functionalization (Tiwari, Vig, Dennis, & Singh, Citation2011) and localized plasmon resonance (LSPR) (Amirjani, Bagheri, Heydari, & Hesaraki, Citation2016a,Citation2016b; mNehl & Hafner, Citation2008). LSPR, which is due to confinement of surface plasmons in noble metallic particles can be tuned to enable the strong absorption of light in the near infrared (NIR) region. Therefore, due to the high extinction coefficient of biological tissue in the NIR region, heating can be confined to region where AuNPs are contained. Furthermore, the strong absorption ensures the use of relatively lower laser energies to minimize injury to collateral tissue including the skin.
Recent advances in the nanomaterials science have motivated the development of multifunctional polymer nanocomposites, which are highly sought after in the field of biomedical sciences because of the opportunities they offer for the development of novel applicators. Specifically, the introduction of physical functionalities, such as magnetic and optical into polymeric matrix is desirable for many advanced techniques in thermometry. For example, the authors fabricated polymeric nanocomposite structures and investigated their potential for the treatment of post-operative breast cancer (Kan-Dapaah, Rahbar, & Soboyejo, Citation2014). Meenach and co-workers developed poly(amino ester) hydrogels and demonstrated their potential for multimodal treatment of cancer (Meenach & Chinedu, Citation2012). Kang and Ko reported the development of lipid-coated multifunctional nanocomposites composed of drugs and nanoparticles for cancer treatment. Their results showed that the nanocomposites caused significant cytotoxicity to breast-cancer and melanoma cell lines (Kang & Ko, Citation2015).
In this paper, a novel probe for localized thermoablation of cancer is presented, and its thermal performance is analysed using a 3D multiphysics finite element method (FEM) model (Reynoso, Lee, Cheong, & Cho, Citation2013). We hypothesize that our probe will potentially achieve reasonable lesion diameters and also eliminate skin burns associated with the RFA. Our ultimate research goal is to establish a novel treatment modality that reduces or completely prevents local recurrence in post-operative breast cancer cases.
2. Description of system
2.1. Concept of device
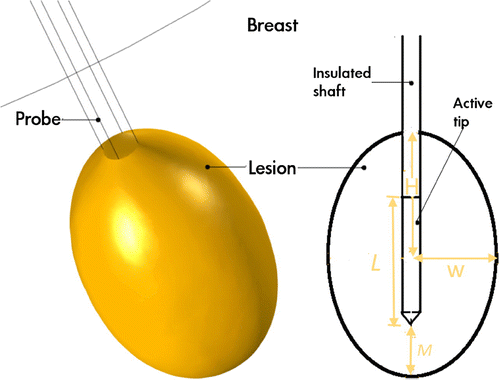
The probe exploits advances in nanocomposite science and PPT to develop a minimally invasive cancer treatment probe that can potentially enhance efficacy by eliminating issues such as skin burns associated with RFA. Figure presents a schematic of the probe. The probe is essentially a cannula with two main parts: a distal active tip made of a plasmonic nanocomposite and a proximal insulated shaft. The dimensions of the different parts of the cannula vary, depending on treatment requirements such as location and size of the affected area. Although, the probe can be used for traditional single thermotherapy (hyperthermia or ablation), the advantage of our probe design is the potential to use it for multimodal cancer therapy involving simultaneous hyperthermia and chemotherapy.
Figure 1. Schematic of plasmonic heating probe. The probe is a cannula with two main parts: a distal active tip made of a plasmonic nanocomposite (blue) and a proximal insulated shaft (pink). Therapeutic treatment is achieved when the active tip is brought in contact with the target area and exposed to laser source.
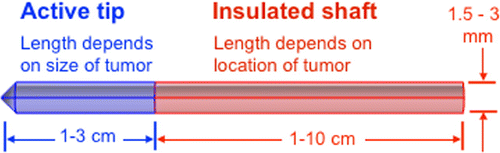
2.2. How system works
The probe is inserted into the tissue, using appropriate image guidance techniques, so that the active tip (nanocomposite) is located within the targeted area. Then, the patient is exposed to a therapeutic tumor irradiation protocol using a remote NIR light (650–900 nm). Au nanoparticles in the active tip absorb the NIR light and converts it into heat. The mode of heat transfer is primarily conduction. To achieve reasonable lesion sizes and avoid skin burns, NIR laser irradiation power density should not exceed the maximal permissible exposure to skin. For instance, for an 808 nm laser, the skin-permissible dosage is (American National Standard Institute, Citation2007).
Chemotherapeutic drugs can be simultaneously delivered to the target area through a fabricated pathway (within the probe) by connecting an automatically controlled syringe (drug holder) to the insulated shaft. The drugs can be delivered at preferred durations and rates.
3. Methods
3.1. In-vivo predictions
In an effort to evaluate the feasibility of the proposed system, we simulated the heating of post-operative breast tissue, using a finite element method (FEM) model, to explore the thermal damage.
3.1.1. Problem formulation
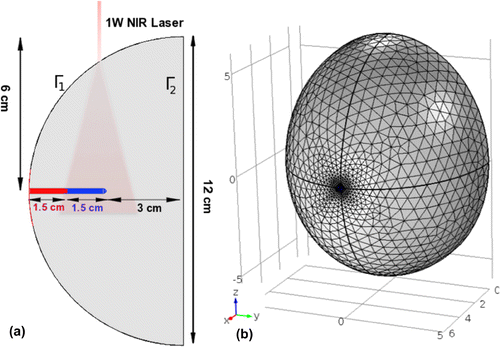
We assumed that the probe was inserted in a breast tissue phantom (see Figure ), such that only its active tip is located in the region of interest. The objective of the treatment was to maintain a uniform temperature distribution in the region of interest. The model takes into account laser illumination, thermal propagation and thermal damage. Characterization and prediction of tissue damage required a three-part model: (1) a transport theory model to estimate photon absorption, (2) a heat diffusion model to calculate temporal temperature distribution and (3) a cell death model to calculate the thermal dose, using the temperature history from part (2).
3.1.2. Geometry
The geometry of our FEM model is shown in Figure . The breast tissue phantom was modeled as a semi-ellipsoidal block with major and minor axes of 12 and 6 cm, respectively. Figure (a) shows that the probe was inserted into the semi-ellipsoidal breast tissue model. The distal active tip of the probe was assumed to be nanocomposite consisting of poly-methyl-methacrylate (PMMA) and AuNP, and the proximal shaft probe was assumed to be a pure PMMA. Figure (b) is the meshed model used for FEM modeling.
3.1.3. Photon transport
For a given medium, when scattering process dominates absorption process as is the case for biological systems the optical diffusion approximation can be used to describe photon transport. This is described by:(1)
(1)
(W m
) is the fluence rate,
(W m
) is the light sources term, and
(m
) is the absorption coefficients. The diffusion coefficient of the medium is then given by:
(2)
(2)
where = (
)
is the reduced scattering coefficient,
(m
) is the scattering coefficients and g is the anisotropy factor which takes into account the effects of directionally dependent scattering. For our analysis we assume a continuous wave Gaussian (e.g. full width at half maximum of 5 mm). When an NIR beam is incident onto a body, the light fluence rate within that body, the solution to Equation (1) is given by Reynoso et al. (Citation2013):
(3)
(3)
where is the effective attenuation coefficient,
is the direction of beam travel,
(W) is the laser power.
3.1.4. Heat diffusion
The heat distribution within the tissue is determined by the Pennes bioheat equation (Pennes, Citation1948):(4)
(4)
where (kg m
) is the density,
(J kg
K
) is the specific heat capacity at constant pressure,
(W m
K
) is the thermal conductivity and T (K) is temperature.
,
(s
),
and
are the density, blood perfusion rate, specific heat capacity and temperature of blood, respectively.
(W m
) is the metabolic heat. Q is the heat generation term, which varies for the different domains of the model. For the tissue and the insulated shaft domains,
(W m
), which corresponds to heat generated due to laser source. For the active tip (nanocomposite),
, where
(W m
). N is the number of AuNP per unit volume and
(m
) is the absorption cross-section of AuNP. The value of
was obtained from Hirsch et al. (Citation2003) as
, which represents a gold nanoshell with a diametre core of 110 nm and gold shell with a thickness of 10 nm. The boundary conditions for Equation (4) were: heat transfer by convection at
,
T) = h
(T
T); where the heat transfer coefficient h = 3.5 Wm
K
, T
= 20
C, at
; a prescribed temperature
at , T = 37
C, at
; and continuity,
T
T
) = 0 on all of interior boundaries. A temperature of 37
C (for the normal body) was used as the initial temperatures in all domains of the model. To predict thermal damage, an Arrhenius injury model (Rylander, Feng, Bass, & Diller, Citation2007) was used. The model is defined by:
(5)
(5)
where (J mol
) is the activation energy, A (s
) is a scaling factor and
(J mol
K
) is the gas constant. The values for
and A were obtained from Huang, Rege, and Heys (Citation2010) as 200 kJ mol
and 6
10
s
respectively. In this study,
= 1 is chosen to indicate that a sufficient irreversible damage has been achieved as reported in the literature (Marqa, Colin, Nevoux, Mordon, & Betrouni, Citation2011)
The details of the implementation are summarized in Appendix 1.
The values of parameters used in the simulation are summarized in Table . The effective thermal and physical properties of the nanocomposites were estimated using the rule of mixtures. Due to the pronounced changes in the blood perfusion (BP) and thermal conductivity of tissues, temperature-dependent values were considered for some parameters. To account for temperature dependence of BP, the second term on the right hand side of Equation (4) was multiplied by a scaling factor (SF). SF for breast tissue () can be expressed as (Dřížďal, Togni, Víšek, & Vrba, Citation2010):
(6)
(6)
Also, thermal conductivity as a function of temperature was obtained from Duck (Citation1990) to be:(7)
(7)
Table 1. Input parameters used in the simulation of bioheat transfer and thermal damage function
3.2. Model validation
We compared our predicted radial temperature distribution with results predicted by an analytical model developed by the authors in Andra, d’Ambly, Hergt, Hilger, and Kaiser (Citation1999) in an effort to validate the numerical model. The solution of the analytical model was implemented using a the technical software Matlab (The MathWorks, Inc, Natick, MA, USA). The details of the model are summarized in Appendix 2.
4. Results and discussion
4.1. In-vivo predictions
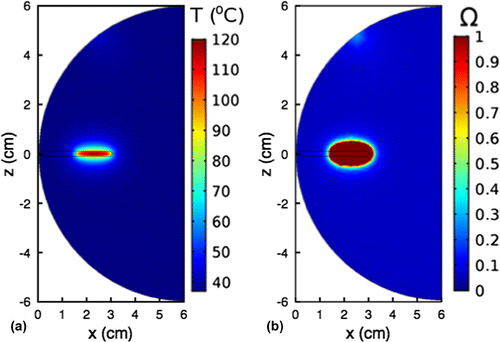
For all simulations, the laser power was set at 1 W unless otherwise stated. Figure (a) show the cross-sectional temperature distribution for the x-z plane, after the tissue is subjected to 15 min of heating at maximum probe-tissue interface temperature of 100C. AuNP concentration, N, was 81.7
10
nps/ml. The results shows that the temperature distribution is non-uniform. The maximum temperature occurs at the central part of the active probe tip. This maximum temperature does not directly affect the tissue, as it is located within the active tip. It can also be observed that the temperature is distributed symmetrically about the center of the probe tip and spreads radially away from the center of the active probe tip. This is consistent with the conduction heat transfer mode. Figure (b) shows that the volume where sufficient irreversible damage occurs is ellipsoidal and fairly symmetric about the probe tip. Figure and Table summarize the characteristics of the lesion as a function of the length of the active tip of the probe. The results show that the major axis, 2H, of the lesion increases with length, L of the active tip. For a given laser power of 1W, 2H increased from
13.8 mm (
mm) to
23.4 mm (
mm). Furthermore, the results show that distance between the distal end of the active tip and the boundary of the lesion (
) decreased slightly (
%) as L increased from 5 mm to 15 mm. Thus, the average value of M was
4.3 mm, which is a little over 40% more than the 3 mm margin needed to potentially avoid recurrence (Jardines et al., Citation1995).
Table 2. Effects of probe’s active tip length: L, length of the active tip; N, nanoparticle concentration; , maximum active tip temperature; W, radius of semi-minor axis of lesion; H, semi-major axis of lesion;
, represented size of the axial margin of the active tip.
4.2. Implications
The study gives insights that offer a context within which the performance of the probe can be discussed. However, it is important to state here that our analysis is based on a numerical model; thus, comprehensive experimental work is needed to obtain a realistic evaluation of the performance of the device.
Modern probe based treatment methods are good alternative for certain cancer cases where resection is not feasible for clinical or technical reasons (Rossi et al., Citation2011). These methods are considered minimally invasive due to factors such as reduced blood loss, less complication during the procedure and relatively shorter period of convalescene compared to surgery. Although RFA probes remain the most widely used ablative techniques worldwide (Campbell et al., Citation2009), increased risk of skin burns is a major concern because it leads to limited lesion sizes. The plasmonic heating based probe proposed in this study can potentially overcome this problem since it eliminates the need for skin contact electrodes (source of skin burns). Furthermore, since biological tissues have high extinction coefficient at NIR wavelength, the LSPR of AuNPs can be tuned to enable the strong absorption of light in the NIR region to minimize or prevent injury to collateral tissue. Also, RFA requires good contact between the RF probe tip and the biological media to generate heat, however, the heat generated by the plasmonic probe is not dependent on the biological media/probe contact but on the power of the NIR laser source and concentration of nanoparticle.
Finally, our predictions show that our probe can achieve negative margins required to prevent recurrence (Jardines et al., Citation1995), a potential advantage over existing RFA probes is the possibility of combining hyperthermia and chemotherapy in one probe. The enhancement of the antitumoral effect of various chemotherapeutic drugs during hyperthermia has been reported previously (Wust et al., Citation2002). This enhancement is explained by an increase in blood perfusion and the permeability of cell membrane as well as altered active drug transport and cell metabolism (Hahn & Shiu, Citation1983). Possible strategies that could be used to deliver drugs include the creation of a pathway within the probe to allow the injection of drugs or the use of thermo-sensitive smart hydrogel-based nanocomposites that will release the drug at a predetermined temperature. However, to investigate the feasibility of these concepts as well as the synergistic effects of the heat and drugs, further experimental studies are needed.
4.3. Model validation
The parameters used for the simulations were obtained from Amirjani et al. (Citation2016a). The following properties were used for the nanocomposite: W K
m
,
g cm
, c
J g
K
,
mm and a power density,
W cm
. The corresponding parameters of the surrounding breast tissue were:
W K
m
,
g cm
, c
J g
K
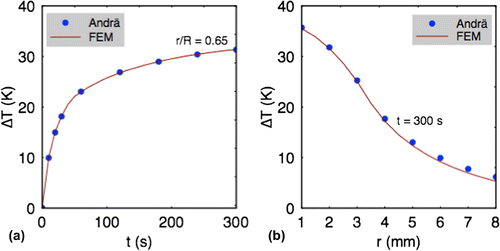
. Figure presents the comparisons of temperature change as function of time (a) and distance (b) from the composite center after 300 s of heating. The predict results from our numerical model shows about 1–2% divergence from the analytical results. This can be attributed to the effects of mesh size and the shape of the cylindrical geometry used.
Figure 5. Comparison of the temperature change as function of: (a) the radial spread and (b) time at a point from the center of the nanocomposite,
mm after 300 s of heating between analytical model (Andrä et al., Citation1999) and our numerical model.
5. Summary and concluding remarks
In this paper, a novel plasmonic heating probe was proposed and analyzed. In an effort to assess its performance, we explored thermal damage in biological tissue subjected to localized heating by the probe. Our predictions demonstrate the feasibility of our novel probe to achieve reasonable lesion sizes that are sufficient to potentially eliminate post-operative residual cancer cell. However, it is clear that systematic experimental studies (i.e. from in-vitro to in-vivo) are needed to obtain a realistic assessment of the actual performance of the probe.
Cover image
Source: Authors.
Additional information
Funding
Notes on contributors
Kwabena Kan-Dapaah
Kwabena Kan-Dapaah received a BSc in Computer Engineering in 2005 and a MSc in Biomedical Engineering in 2008 from the Kwame Nkrumah University of Science and Technology, Ghana; and the University of Lübeck, Germany; respectively and then a PhD in Materials Science and Engineering in 2015 from the African University of Science and Technology, Nigeria. He is currently a lecturer at the Department of Biomedical Engineering, University of Ghana. Kwabena’s research interest is focused on the physical and structural properties of nanocomposite materials for cancer theranostic application. By investigating nanocomposites, he explores the design and fabrication of structures made from the nanocomposite for localized single (thermotherapy only) or multimodal (thermotherapy and chemotherapy) cancer treatment.
Samuel Kojo Kwofie
Bernard Owusu Asimeng and Samuel Kojo Kwofie are lecturers at the Department of Biomedical Engineering, University of Ghana.
Abu Yaya
Abu Yaya is a senior lecturer at the Department of Materials Science and Engineering, University of Ghana.
References
- American National Standard Institute. (2007). American National safety standard for safe use of lasers. Orlando, FL: ANSI Z136.1.
- Amirjani, A., Bagheri, M., Heydari, M., & Hesaraki, S. (2016a). Colorimetric determination of Timolol concentration based on localized surface plasmon resonance of silver nanoparticles. Nanotechnology, 27, 375503.
- Amirjani, A., Bagheri, M., Heydari, M., & Hesaraki, S. (2016b). Label-free surface plasmon resonance detection of hydrogen peroxide; a bio-inspired approach. Sensors and Actuators B: Chemical, 227, 373–382.
- Andra, W., d’Ambly, G., Hergt, R., Hilger, I., & Kaiser, W. A. (1999). Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. Journal of Magnetism and Magnetic Materials, 194, 197–203.
- Bardhan, R., Lal, S., & Joshi, A. (2011). Theranostic nanoshells: from probe design to imaging and treatment of cancer. Accounts of Chemical Research, 44, 936–946.
- Campbell, S. C., Novick, A. C., Belldegrun, A., Blute, M. L., Chow, G. K., Derweesh, I. H., ... Uzzo, R. G. (2009). Guideline for management of the clinical T1 renal mass. The Journal of Urology, 182, 1271–1279.
- Chen, J., Glaus, C., Laforest, R., Zhang, Q., Yang, M., Gidding, M., ... Xia, Y. (2010). Gold nanocages as photothermal transducers for cancer treatment. Small, 6, 811–817.
- Chen, Y. H., Lin, H., Xie, C. L., Zhang, X. T., & Li, Y. G. (2015). Efficacy comparison between cryoablation and radiofrequency ablation for patients with cavotricuspid valve isthmus dependent atrial flutter: A meta-analysis. Scientific Reports, 5, 10910.
- Correa-Gallego, C., Fong, Y., Mithat Gonen, M., D’Angelica, M. I., Allen, P. J., DeMatteo, R. P., ... Kingham, T. P. (2014). A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Annals of Surgical Oncology, 21, 4278–4283.
- Daniels, C., & Rubinsky, B. (2009). Electrical field and temperature model of nonthermal irreversible electroporation in heterogeneous tissues. Journal of Biomechanical Engineering, 131, 071006.
- de Baére, T., Aupérin, A., Deschamps, F., Chevallier, P., Gaubert, Y., Boige, V., ... Palussiére, J. (2015). Radiofrequency ablation is a valid treatment option for lung metastases: Experience in 566 patients with 1037 metastases. Annals of Oncolog, 26, 987–91.
- Dříždal, T., Togni, P., Víšek, L., & Vrba, J. (2010). Comparison of constant and temperature dependent blood perfusion in temperature prediction for superficial hyperthermia. Radioengineering, 19, 281–289.
- Duck, F.A. (1990). Physical properties of tissue: A comprehensive reference book. London: Academic Press.
- El-Sayed, I. A., Huang, X., & El-Sayed, M. A. (2006). Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Letters, 239, 129–135.
- Hahn, G. M., & Shiu, E. C. (1983). Effect of pH and elevated temperature on the cytotoxicity of some chemotherapeutic agents in Chinese hampster cells in vitro. Cancer Research, 43, 5789–5791.
- Hirsch, L. R., Stafford, R. J., Bankson, J. A., Sershen, S. R., Rivera, B., Price, R. E., ... West, J. L. (2003). Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proceedings of the National Academy of Sciences, 100, 13549–554.
- Huang, X., Neretina, S., & El-Sayed, M. A. (2009). Gold nanorods: from synthesis and properties to biological and biomedical applications. Advanced Materials, 21, 4880.
- Huang, H., Rege, K., & Heys, J. J. (2010). Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano, 4, 2892–900.
- Huffman, S. D., Huffman, N. P., Lewandowski, R. J., & Brown, D. B. (2011). Radiofrequency ablation complicated by skin burn. Seminars in Interventional Radiology, 28, 179–82.
- Ibrahim, H., Finta, B., & Rind, J. (2016). Incidence and factors predicting skin burns at the site of indifferent electrode during radiofrequency catheter ablation of cardiac arrhythmias. Cardiology Research and Practice, 2016, 1.
- Jardines, L., Fowble, B., Schultz, D., Mackie, J., Daly, J., Weiss, M., ... Rosata, E. (1995). Factors associated with a positive reexcision after excisional biopsy for invasive breast cancer. Surgery, 118, 803–809.
- Jochelson, M. (2012). Advanced imaging techniques for the detection of breast cancer. American Society of Clinical Oncology educational book, 95–99.
- Jolesz, F. A., & Hynynen, K. (2002). Magnetic resonance image-guided focused ultrasound surgery. The Cancer Journal, 8, S100–S112.
- Kan-Dapaah, K., Rahbar, N., & Soboyejo, W. (2014). Implantable magnetic nanocomposites for the localized treatment of breast cancer. Journal of Applied Physics, 116, 233505.
- Kang, J. H., & Ko, Y. T. (2015). Lipid-coated gold nanocomposites for enhanced cancer therapy. International Journal of Nanomedicine., 10, 33–45.
- Krishnan, S., Daigaradjane, P., & Cho, S. H. (2010). Nanoparticle-mediated thermal therapy: evolving strategies for prostate cancer therapy. International Journal of Hyperthermia, 26, 775–89.
- Leuthardt, E. C., Duan, C., Kim, M. J., Campian, J. L., Kim, A. H., Miller-Thomas, M. M., ... Tran, D. D. (2016). Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS ONE, 11, e0148613.
- Marqa, M. F., Colin, P., Nevoux, P., Mordon, S. R., & Betrouni, N. (2011). Focal laser ablation of prostate cancer: Numerical simulation of temperature and damage distribution. Biomedical Engineering Online, 10, 45.
- Meenach, S. A., Chinedu, G., Otu, C. G., & Hilt, J. Z. (2012). Controlled synergistic delivery of paclitaxel and heat from poly(β-amino ester)/iron oxide-based hydrogel nanocomposites. International Journal of Pharmaceutics, 427, 177–184.
- Nehl, C. L., & Hafner, J. H. (2008). Shape-dependent plasmon resonances of gold nanoparticles. Journal of Materials Chemistry, 18, 2415–2419.
- Nguyen, T., Hattery, E., & Khatri, V. P. (2014). Radiofrequency ablation and breast cancer: A review. Gland Surgery, 3, 128–35.
- Pan, Y., Neuss, S., Leifert, A., Fischler, M., Wen, F., Simon, U., ... Jahnen-Dechent, W. (2007). Size-dependent cytotoxicity of gold nanoparticles. Small, 3, 1941–9.
- Pennes, H. H. (1948). Analysis of tissue and arterial blood temperatures in the resting human forearm. Journal of Applied Physiology, 1, 93–122.
- Reynoso, F. J., Lee, C. D., Cheong, S. K., & Cho, S. H. (2013). Implementation of a multisource model for gold nanoparticle-mediated plasmonic heating with near-infrared laser by the finite element method. Medical Physics, 40, 073301–1.
- Rossi, S., Ravetta, V., Rosa, L., Ghittoni, G., Viera, F.T., Garbagnati, F., ... Tinelli, C.(2011). Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: A long-term cohort study. Hepatology, 53, 136–147.
- Rylander, M. N., Feng, Y., Bass, J., & Diller, K. R. (2007). Heat shock protein expression and damage optimization for laser therapy design. Lasers in Surgery and Medicine, 39, 734–46.
- Steinke, K., Gananadha, S., King, J., Zhao, J., & Morris, D. L. (2013). Dispersive pad site burns with modern radiofrequency ablation equipment. Surgical Laparoscopy Endoscopy & Percutaneous Techniques, 13, 366–71.
- Tiwari, P., Vig, K., Dennis, V., & Singh, S. (2011). Functionalized gold nanoparticles and their biomedical applications. Nanomaterials, 1, 31–63.
- Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65, 87–108.
- Wust, P., Hildebrandt, B., Sreenivasa, G., Rau, B., Gellermann, J., Riess, H., ... Schlag, P. M. (2002). Hyperthermia in combined tratment of cancer. The Lancet Oncology, 3, 487–497.
Appendix 1
Implementation of FEM model
The COMSOL Multiphysics 4.3a software package (COMSOL Inc., Burlington MA, USA) was used to implement FEM model. All properties were added explicitly as a global definition or variable under the model node. The geometry was modeled in 3D.
The bioheat heat transfer application mode was added to the model to solve the Equation (4). Under the heat transfer application mode, the normal body temperature (37C) was used initial temperature of the phantoms. All boundary conditions were specified as those outlined in Section 3.1.4. The plasmonic heat source was inserted into bioheat transfer application mode as a user-defined heat source.
The geometry shape order of the mesh element was set to“quadratic”. The mesh consists of 119,429 elements and 162,113 degrees of freedom. The mesh used for the probe was dense compare to that used for the tissue. This was achieved by setting the element size of the mesh to “extra fine” and “fine” for the probe and tissue domains respectively. The numerical solutions were obtained using time-dependent solver “GMRES” with its default settings. The simulations were run on a mid-range workstation with Intel(R) Xeon(R) E5-1620 CPU and 8 GB of RAM (Intel, Santa Clara CA, USA). The solution time was approximately 4 min.
The simulation was divided into 3 steps: (i) The plasmonic heat generated was first calculated, (ii) The distribution of the time-dependent temperature was determined using the results from step (i) as heat generation term in Equation (4) and (iii) The thermal damage was calculated as a function of time using the temperature history, which was used as the input to Equation (5). A time-dependent study (10 s step) was used for all FEM analyses.
Appendix 2
Andrä Model
In an effort to study the temperature change, , in a spherical region embedded in a large inert region, Andrä et al. developed an analytical solution. The solution, which assumes heat transfer only by conduction, is given by:
(B1)
(B1)
(B2)
(B2)
where is density, c
is heat capacity, and
is thermal conductivity of the tissue-basde nanocomposite region, all approximated by the rule of mixtures.
, and
are the properties of the region surrounding the nanocomposite sample. Parameters
, and
are abbreviations,